Introduction
Lung cancer has the second highest incidence and the
highest mortality rate among all cancers worldwide. Non-small cell
lung cancer (NSCLC) is the most common type of lung cancer,
accounting for ~80–85% of all lung cancers, and lung adenocarcinoma
is a diverse form of NSCLC (1,2). While
molecularly targeted drugs and immune-based therapies have recently
been developed, chemotherapy remains the primary method of
treatment for patients with advanced lung adenocarcinoma (3). Cisplatin, one of the most commonly
used chemotherapeutic agents in clinical practice, often leads to
chemotherapy failure due to inherent or acquired cisplatin
resistance (4). Therefore,
understanding how tumors grow and identifying the causes of tumor
drug resistance is necessary to effectively treat lung
adenocarcinoma.
Ribonucleotide reductases are a family of enzymes
that perform vital biological functions by catalyzing the
conversion of four common nucleotides into deoxy-ribonucleoside
triphosphate (dNTP), which is required for DNA replication and
repair (5). Ribonucleotide
reductase M2 (RRM2) is a component of ribonucleotide reductase. It
has been reported that tumor cells express RRM2 in the late G1 and
early S phases of the cell cycle, and regulate DNA replication and
repair by controlling the synthesis of dNTPs (6). RRM2 is an oncogene that is highly
expressed in cancers such as hepatocellular carcinoma (7) and colorectal cancer (8,9). It is
not only associated with cancer cell proliferation, migration,
invasion and apoptosis, but it also has a role in the chemotherapy
resistance of cancer cells. For instance, inhibition of RRM2
expression not only enhances the sensitivity of pancreatic cancer
cells (10,11) and squamous cell carcinoma cells
(12) to gemcitabine, but also
enhances the sensitivity of ovarian cancer cells (13–15) to
cisplatin. RRM2 may be used as a biomarker in NSCLC (16–18) to
detect chemotherapy sensitivity and predict the prognosis of
patients, and understanding the role of RRM2 in lung adenocarcinoma
and how it works is critical to developing more effective
treatments for this disease.
The Wnt signaling pathway is a complex regulatory
network with three major components: Wnt/β-catenin, Wnt/planar cell
polarity and Wnt/Ca2+. The Wnt/β-catenin signaling
pathway transports accumulated cytoplasmic β-catenin to the nucleus
and has a key role in embryonic development, stem cell self-renewal
and tumorigenesis by activating downstream target genes (19,20).
Previous studies have reported that RRM2 is overexpressed in
hepatocellular carcinoma and promotes the proliferation and
migration ability of hepatocellular carcinoma cells through the
Wnt/β-catenin signaling pathway (21). RRM2 may also affect cell
proliferation and apoptosis through the Wnt/β-catenin signaling
pathway when overexpressed in multiple myeloma cells (22). However, research on RRM2 in lung
adenocarcinoma is currently limited. Therefore, the present study
used a bioinformatics analysis of lung adenocarcinoma data from The
Cancer Genome Atlas (TCGA) database to investigate the role of RRM2
in lung adenocarcinoma proliferation, migration, apoptosis and
cisplatin resistance formation using A549 and cisplatin-resistant
A549 (A549/DDP) cells.
Materials and methods
Bioinformatics analysis
Lung adenocarcinoma data were obtained from the TCGA
website (https://portal.gdc.cancer.gov/) and processed using
Perl (v5.30.1) and the R software (v4.1.3). Gene expression
analysis and clinical characterization were performed using the
limma, ggplot2 and ggpubr packages. Survival analysis was performed
with the survival and survminer packages. Receiver operating
characteristic (ROC) curves were plotted using the TimeROC package.
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway prediction
and gene set enrichment analysis (GSEA) were performed with filter
conditions set at |log2 fold change|>1 and false discovery rate
<0.05. Differential analysis of the tumor microenvironment (TME)
was performed using the estimate package and immune cell
infiltration analysis was performed using the cibersort
algorithm.
Cell culture and cell
transfection
The lung adenocarcinoma cell lines A549 and A549/DDP
were cultured in Ham's F-12K medium supplemented with 10% FBS (100
U/ml penicillin, 100 mg/l streptomycin) and incubated at 37°C, 5%
CO2 and 95% humidity. A549/DDP cells were cultured with
a final concentration of 1 µg/ml DDP. Cell lines and reagents were
purchased from Pricella. RRM2 knockdown was performed using a
lentiviral vector (shRRM2) obtained from GenePharma. The sense
sequence of the vector is
5′-GATCCGTAGAGAGAACCCATTTGACTTTATTCAAGAGATAAAGTCAAATGGGGTTCTCTATTTTTTG-3′,
while the antisense sequence is
5′-AATTCAAAAAATAGAGAGAACCCATTTGACTTTATCTCTTGAATAAAGTCAAATGGGTTCTCTACG-3′.
As for the negative control (NC), it possesses a sense sequence of
5′-GATCCGTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAACTTTTTTG-3′
and an antisense sequence of
5′-AATTCAAAAAAGTTCTCCGAACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAACG-3′.
Before lentiviral transfection, cells were seeded in 6-well plates
and transfected with the virus upon reaching 60–70% confluence.
After transfection, cells were selected in complete medium
containing 4 µg/ml puromycin. Remaining cells after one week were
considered to have stable knockdown of RRM2 and were cultured in
complete medium with 2 µg/ml puromycin for long-term culture. The
overexpression plasmid of β-catenin and its negative control
pcDNA3.1 were from Hanbio (cat. no. KWC20221122CDNWH-PC01). Before
plasmid transfection, cells were seeded in 6-well plates. When the
cells reached 60–70% confluence, 4 µg of the overexpression plasmid
or its control were transfected into corresponding cells using 10
µl LipoFiter 3.0 (Hanbio). After 8 h, the old medium of the cells
was replaced with fresh medium.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using the TRIzol (Aidlab
Biotechnologies Co., Ltd) method, followed by RT using the
RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions, to
generate complementary DNA. Real-time qPCR was performed using the
MagicSYBR Mixture (CoWin Biotech) following the manufacturer's
instructions. Real-time qPCR was conducted on a QuantStudio™ 6 Flex
instrument (Thermo Fisher Scientific, Inc.) with the following
thermocycling conditions: Initial denaturation at 95°C for 30 sec;
denaturation at 95°C for 5 sec, annealing/extension at 60°C for 30
sec, for a total of 40 cycles; and melting curve analysis at 95°C
for 15 sec, 60°C for 1 min, 95°C for 15 sec and 50°C for 30 sec.
The gene primer sequences used were as follows: GAPDH forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′; RRM2 forward,
5′-AGTGGAAGGCATTTTCTTTTCC-3′ and reverse,
5′-GCAAAATCACAGTGTAAACCCT-3′; β-catenin forward,
5′-GGCTCTTGTGCGTACTGTCCTTC-3′ and reverse,
5′-GCTTCTTGGTGTCGGCTGGTC-3′. The 2−ΔΔCq formula
(23) is used to calculate the
relative expression levels of the target gene.
Western blot analysis
Each group of cells was lysed and denatured using
Cell Lysis Buffer for Western and IP (Beyotime Institute of
Biotechnology), and protein quantification was performed by the BCA
method. A total of 25 µg of protein sample per lane was denatured
and electrophoresed using 10% SDS-PAGE, followed by semi-dry
transfer of protein onto a PVDF membrane (Immobilion-P; EMD
Millipore) and protein blocking with Protein Free Rapid Blocking
Buffer (Epizyme) at room temperature for 30 min. The membrane was
then incubated with the following primary antibodies at 4°C for 15
h: GAPDH (1:2,000 dilution; cat. no. AF1186; Beyotime Institute of
Biotechnology), β-actin (1:2,000 dilution; cat. no. AF1186;
Beyotime Institute of Biotechnology), RRM2 (1:1,000 dilution; cat.
no. 11661-1-AP; Proteintech), β-catenin (1:1,000 dilution; cat. no.
AC106; Beyotime Institute of Biotechnology), cyclin D1 (1:2,000
dilution; cat. no. AF0126; Beyotime Institute of Biotechnology) and
c-MYC (1:2,000 dilution; cat. no. 10828-1-AP; Proteintech).
Subsequently, it was incubated with HRP-labeled Goat Anti-Rabbit
IgG (1:1,000 dilution; cat. no. A0208; Beyotime Institute of
Biotechnology) for 1 h at room temperature and final visualization
was performed using a GelDoc XR System (Bio-Rad Laboratories,
Inc.). The experimental results were analyzed in grayscale using
Image J (v1.8.0; National Institutes of Health).
CCK-8 assay
Cells were harvested and cultured in 96-well plates
(Corning, Inc.) at a density of 3×103 cells per 100 µl
of medium, with five replicate wells per group. Upon cell adhesion,
the time was set to 0 h. Subsequently, cells were incubated in a
constant temperature chamber at 37°C and the medium was replaced
with complete medium supplemented with 10 µl CCK-8 (Abbkine) per
100 µl at 0, 24, 48 or 72 h. After 1 h, the optical density (OD) at
450 nm was measured using a Varioskan LUX Multimode Reader (Thermo
Fisher Scientific, Inc.).
Colony-formation assay
The experimental cells were harvested and seeded in
a 6-well plate (Corning, Inc.) at a density of 600 cells per 2 ml
of culture medium. A medium change was performed on the 6th day. On
the 12th day, the 6-well plate was analyzed as follows: Cells were
washed twice with PBS, fixed with 4% paraformaldehyde for 15 min,
washed twice with PBS, stained with crystal 1% violet for 15 min
and washed twice with PBS before analysis; all steps were conducted
at room temperature. The wells were photographed using an optical
microscope and colonies were manually counted. In this study,
clusters of cells containing >50 cells were considered
colonies.
Chemotherapy sensitivity assay
Experimental cells were harvested and seeded onto a
96-well plate at a density of 5×103 cells per 100 µl of
medium. For each group, six different concentrations of cisplatin
were prepared and five wells were used for each concentration.
After overnight cell culture, the medium was replaced with complete
medium containing 0, 1, 2, 4, 8 and 16 µg/ml concentrations of
cisplatin for the transfected A549 cells, and with 0, 4, 8, 16, 32
and 64 µg/ml concentrations of cisplatin for the transfected
A549/DDP cells, followed by continuous cultivation for 24 h. The
old medium was discarded and replaced with 100 µl complete medium
containing 10 µl of CCK-8 (Abbkine). Following incubation at 37°C
for 2 h, the OD at 450 nm was measured using a Varioskan LUX
Multimode Reader (Thermo Fisher Scientific, Inc.). The cell
inhibition rate (%) was calculated as follows: (OD of control
group-OD of experimental group)/(OD of control group-OD of blank
group) ×100%.
Transwell assays
Experiments were performed using 24-well plates
(Corning, Inc.) and 8-µm pore size Transwell cell culture chambers
(Falcon; Corning Life Science). Cells were collected by preparing a
suspension of 5×104 cells in 200 µl serum-free medium,
which was added to the upper chamber. The lower chamber was filled
with 600 µl complete medium containing 10% FBS. After 24 h of
incubation, the Transwell chambers were removed and washed twice
with PBS. The cells were then fixed with 4% paraformaldehyde for 15
min, washed twice with PBS, stained with 1% crystal violet for 15
min and washed twice with PBS. Fixing, washing and staining steps
were conducted at room temperature. The cells in the upper chamber
were removed with cotton swabs and three randomly selected fields
of view were counted using an inverted fluorescence microscope
(Leica Microsystems GmbH) at ×400 magnification.
Cell cycle analysis and apoptosis
assay
For cell cycle experiments, the experimental cells
were seeded in 6-well plates at a density of 3×105 cells
per well, and were collected after 48 h of incubation. The cells
were washed once with PBS and then fixed with 70% ethanol at 4°C
for 12 h. Following fixation, the cells were stained using the Cell
Cycle and Apoptosis Analysis Kit (Beyotime Institute of
Biotechnology) according to the manufacturer's instructions.
Initially, 500 µl of staining buffer was added, followed by the
addition of 25 µl propidium iodide staining solution and 10 µl
RNase A. The cells were then incubated for 30 min at room
temperature while being protected from light, and then analyzed
using a flow cytometer.
For the apoptosis experiments, cells were seeded in
6-well plates at a density of 3×105 cells per well and
incubated for 24 h. The old medium was then removed and replaced
with complete medium containing or lacking 2 µg/ml DDP for A549
cells, and complete medium containing or lacking 8 µg/ml DDP for
A549/DDP cells. After 24 h of incubation, the cells were collected.
Staining was performed using the Annexin V-APC/7-AAD Apoptosis
Detection Kit (KeyGEN Biotech) according to the manufacturer's
instructions. First, 500 µl of Binding Buffer was added, followed
by 5 µl AnnexinV-APC and 5 µl 7-AAD, which were mixed thoroughly
while avoiding light. The cells were then incubated for 10 min and
analyzed using a flow cytometer.
Confocal microscopy
Complete medium (1 ml) containing 1.5×105
cells was added to the BeyoGold™ 35 mm Confocal Dishes (Beyotime
Institute of Biotechnology) and cells were allowed to attach by
culture for 24 h. Following this, the medium in the confocal dish
was replaced with complete medium containing 0 or 2 µg/ml cisplatin
and incubation was continued for another 24 h. Subsequently, the
cells were fixed with 4% paraformaldehyde for 15 min, Immunol
Staining Blocking Buffer (Beyotime Institute of Biotechnology) was
applied for 60 min, incubation with RRM2 primary antibody (cat. no.
11661-1-AP; 1:500 dilution; Proteintech) was performed for 1 h at
room temperature and incubation with a fluorescent-labeled
secondary antibody (cat. no. A0468; 1:500 dilution; Beyotime
Institute of Biotechnology) for 1 h. All experiments were conducted
at room temperature. Finally, fluorescence images were captured
using a confocal microscope (FV3000; Olympus Corporation) after
adding Antifade Mounting Medium with DAPI (Beyotime Institute of
Biotechnology).
Statistical analysis
GraphPad Prism 9.0.0 (GraphPad Software; Dotmatics)
was used for graphic representation of the data. The measurement
data were expressed as the mean ± standard deviation. Statistical
analysis was performed using SPSS 25.0 (IBM Corporation). The
unpaired Student's t-test was used for comparisons between two
groups, and one-way analysis of variance (ANOVA) followed by the
Least Significant Difference (LSD) post-hoc test was used for
comparisons between more than two groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
RRM2 is highly expressed in lung
adenocarcinoma and is associated with patient prognosis and
clinical features
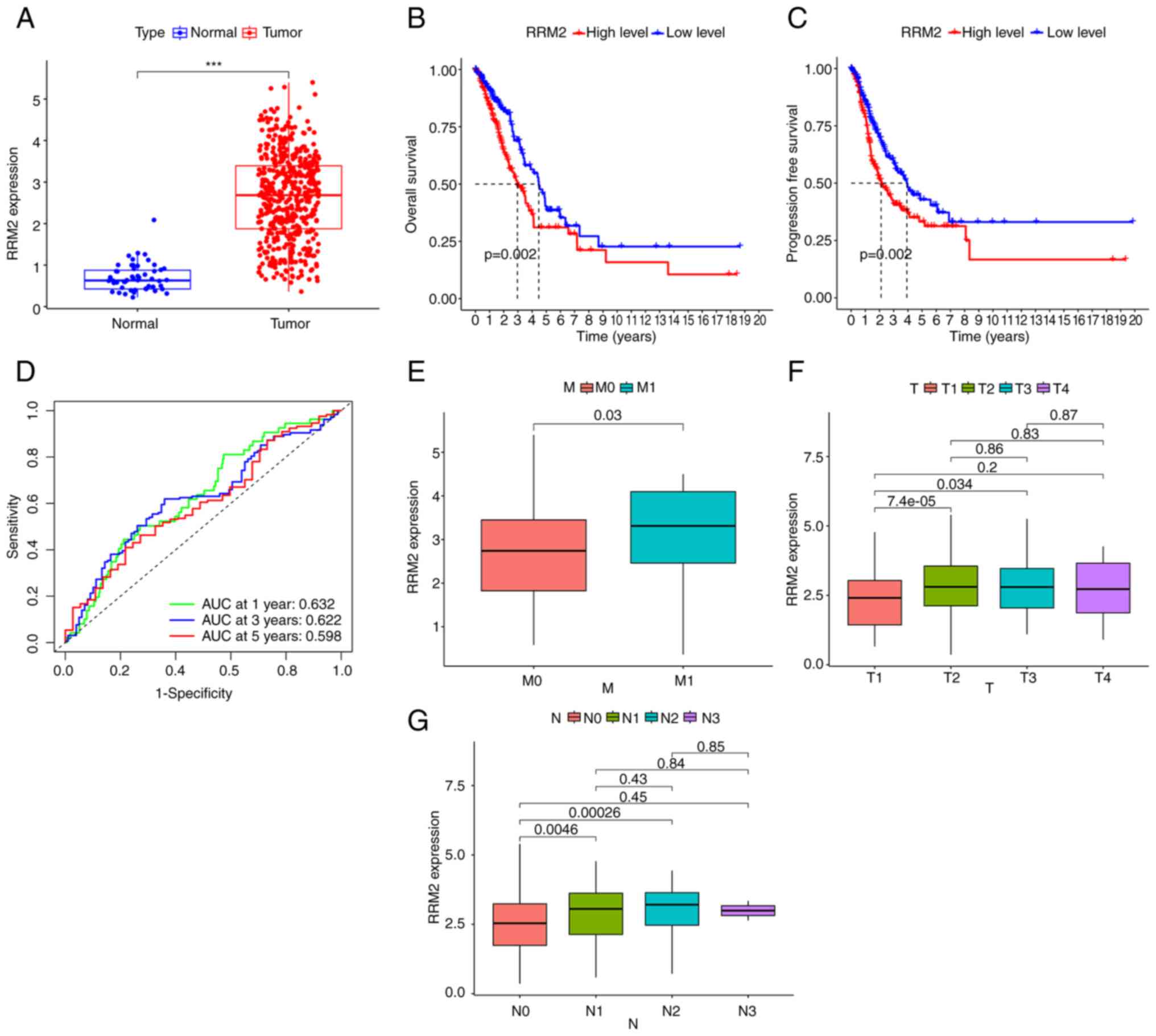
By analyzing data from the TCGA database, it was
found that RRM2 expression was elevated in lung adenocarcinoma
compared to normal lung samples (Fig.
1A). Furthermore, patients with low RRM2 expression had longer
overall and progression-free survival than those with high RRM2
expression (Fig. 1B and C). Using
RRM2 to predict patient survival, the area under the curve was
0.632, 0.622 and 0.598 for 1, 2 and 5 years, respectively (Fig. 1D). In the TNM classification of lung
adenocarcinoma, stage M1 with distant metastasis exhibited higher
RRM2 expression levels than stage M0 without metastasis (Fig. 1E). In addition, stages T2 and T3,
which represent larger tumor sizes, showed elevated RRM2 expression
compared to stage T1 (Fig. 1F).
Similarly, stages N1 and N2, involving lymph node metastasis,
displayed higher RRM2 expression levels than stage N0 without lymph
node involvement (Fig. 1G).
However, no statistically significant difference was observed in
RRM2 expression levels between patients at T4 and T1, and N3 and N1
stages, possibly due to the limited number of patients at stages T4
and N3 in the database.
Results of enrichment analysis and
immune infiltration analysis
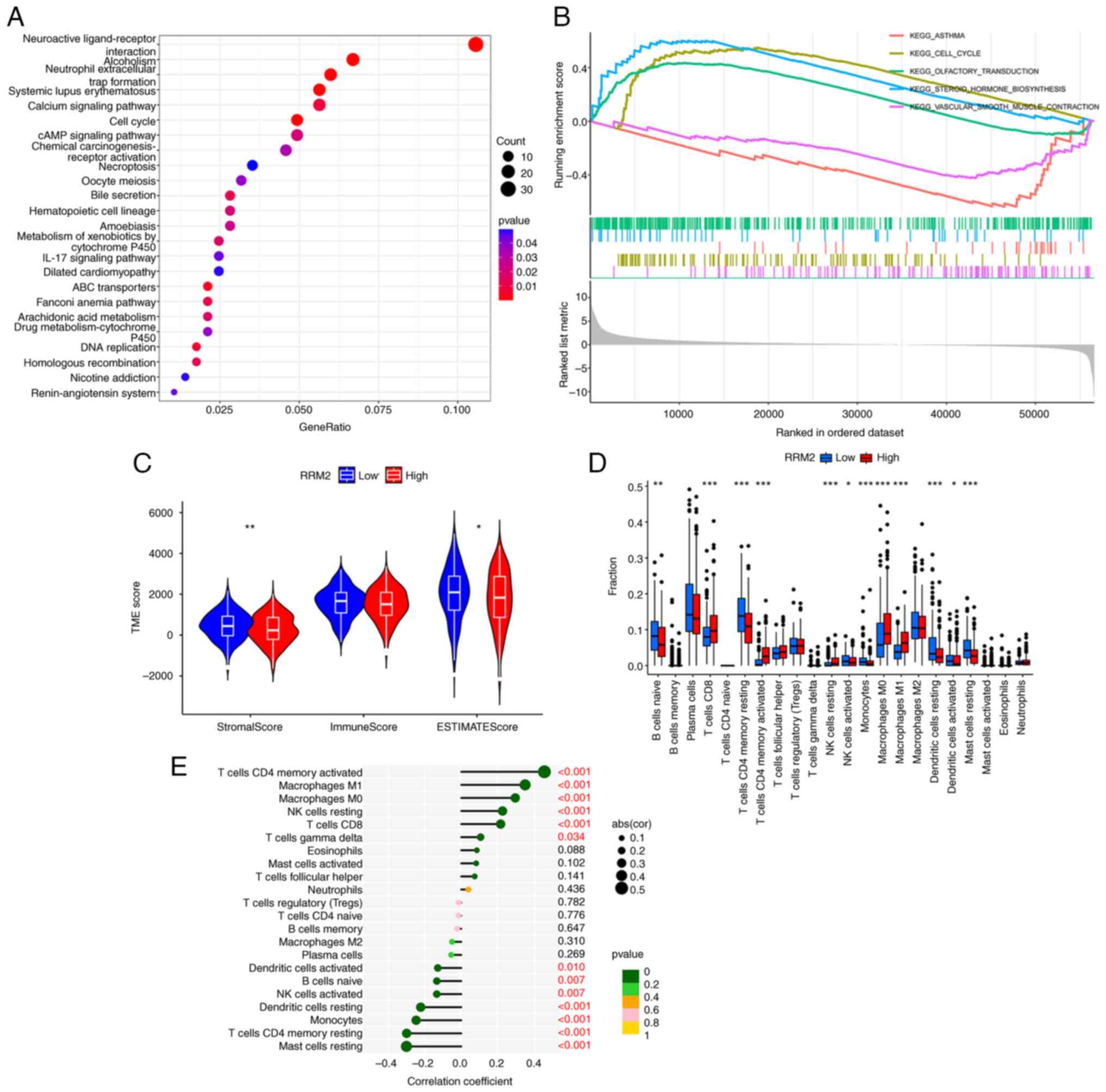
The KEGG enrichment analysis and GSEA revealed that
genes associated with RRM2 were significantly enriched in the cell
cycle in tumor samples, particularly in lung adenocarcinoma tissues
with high RRM2 expression (Fig. 2A and
B). Furthermore, analysis of the TME indicated significant
differences in the StromalScore and ESTIMATEScore between patients
with high and low RRM2 expression, but no difference in the
ImmuneScore (Fig. 2C). The results
of the immune cell differential and correlation analysis in the
tumor tissues of patients with lung adenocarcinoma indicated that
there were differences in the levels of 12 immune cells, namely B
cells naive, T cells CD8, T cells CD4 memory resting, T cells CD4
memory activated, natural killer (NK) cells resting, NK cells
activated, monocytes, macrophages M0, macrophages M1, dendritic
cells resting, dendritic cells activated and mast cells resting,
between patients expressing different levels of RRM2. In addition,
the expression levels of T cells CD4 memory activated, macrophages
M1, macrophages M0, NK cells resting, T cells CD8 and T cells gamma
delta were positively associated with RRM2 expression, while
dendritic cells activated, B cells naive, NK cells activated,
dendritic cells resting, monocytes, T cells CD4 memory resting and
mast cells resting were negatively correlated with RRM2 expression
levels (Fig. 2D and E).
RRM2 is highly expressed in
cisplatin-resistant lung adenocarcinoma cells and cisplatin induces
RRM2 expression in lung adenocarcinoma cells
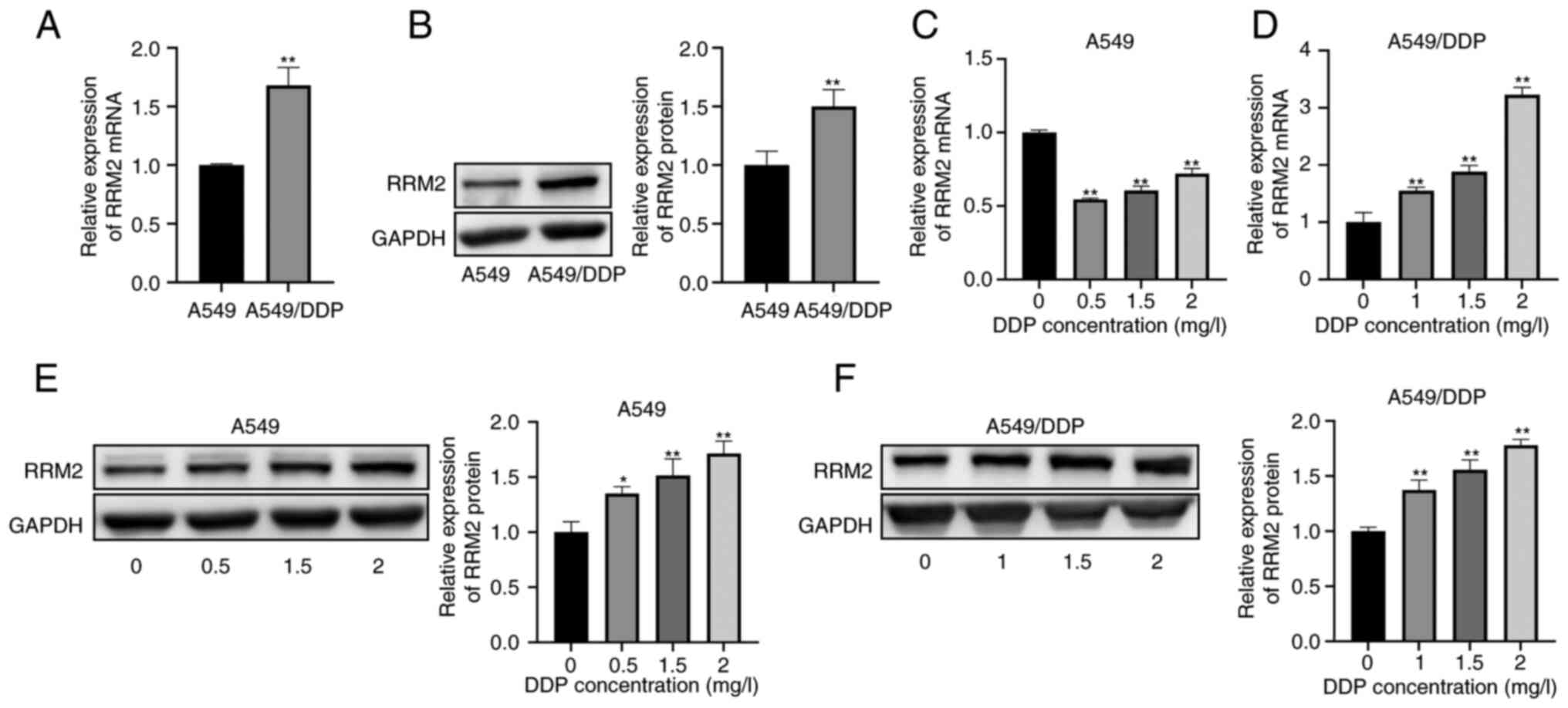
RT-qPCR and western blot analyses demonstrated that
RRM2 expression was significantly upregulated in
cisplatin-resistant A549/DDP cells compared to A549 cells (Fig. 3A and B). Subsequently, A549 cells
were treated with cisplatin concentrations of 0, 0.5, 1.5, and 2
µg/ml for 48 h, while A549/DDP cells were treated with cisplatin
concentrations of 0, 1, 1.5 and 2 µg/ml for the same period. The
results suggested that cisplatin treatment led to a decrease in
RRM2 mRNA expression levels in A549 cells (Fig. 3C). However, a gradual increase in
RRM2 mRNA expression was observed with higher concentrations of
cisplatin (Fig. 3C). In contrast to
the mRNA expression levels, the protein expression of RRM2 in A549
cells did not decrease upon cisplatin treatment but showed a
progressive increase throughout the experiment (Fig. 3E). In A549/DDP cells, both RT-qPCR
and western blot analysis revealed an increase in RRM2 expression
that correlated with the concentration of cisplatin used, both at
the mRNA and protein levels (Fig. 3D
and F).
RRM2 knockdown reduces lung
adenocarcinoma cell proliferation and migration, promotes apoptosis
and partially restores their sensitivity to cisplatin
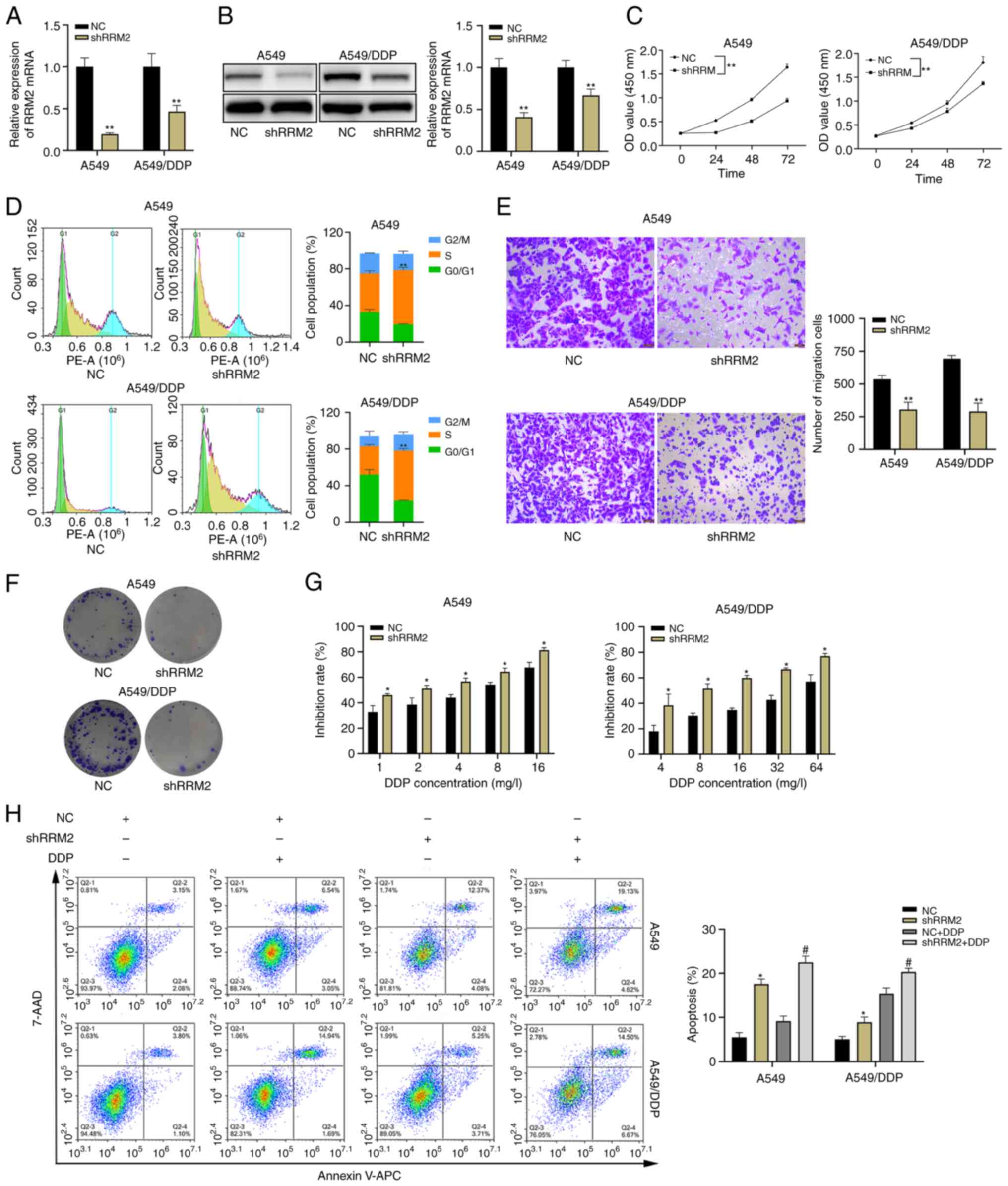
To investigate the role of RRM2 in lung
adenocarcinoma cells, lentivirus was used to knock down RRM2 in
lung adenocarcinoma cells (Fig. 4A and
B). The results indicated that RRM2 knockdown inhibited the
proliferation (Fig. 4C and F) and
migration (Fig. 4E) of A549 and
A549/DDP cells, and also increased the number of cells arrested in
the S phase of the cell cycle (Fig.
4D). The CCK-8 toxicity assay showed that RRM2 knockdown
resulted in increased inhibition of cell growth by cisplatin
(Fig. 4G). In addition, flow
cytometry experiments indicated that RRM2 knockdown not only
increased apoptosis in A549 and A549/DDP cells, but also enhanced
the effect of cisplatin on these cells (Fig. 4H). Importantly, during the apoptosis
experiments, different concentrations of DDP were used for treating
the native A549 cell line and the DDP-resistant cell line.
Specifically, the native A549 cell line was treated with DDP at a
concentration of 2 µg/ml, whereas the DDP-resistant cell line was
exposed to DDP at a concentration of 8 µg/ml. This discrepancy in
DDP concentrations contributed to the higher apoptosis rate
observed in the DDP-resistant cell line compared to the native A549
cell line in the NC + DDP group (Fig.
4H).
RRM2 promotes the development of lung
adenocarcinoma through the Wnt/β-catenin signaling pathway
Camptothecin facilitates the translocation of RRM2
into the nucleus. Knockdown of RRM2 enhances the effect of
camptothecin on DNA damage in the cell (24). To investigate the precise mechanism
of action of RRM2 in lung adenocarcinoma, A549 and A549/DDP cells
were treated with or without 2 µg/ml cisplatin for 24 h, and they
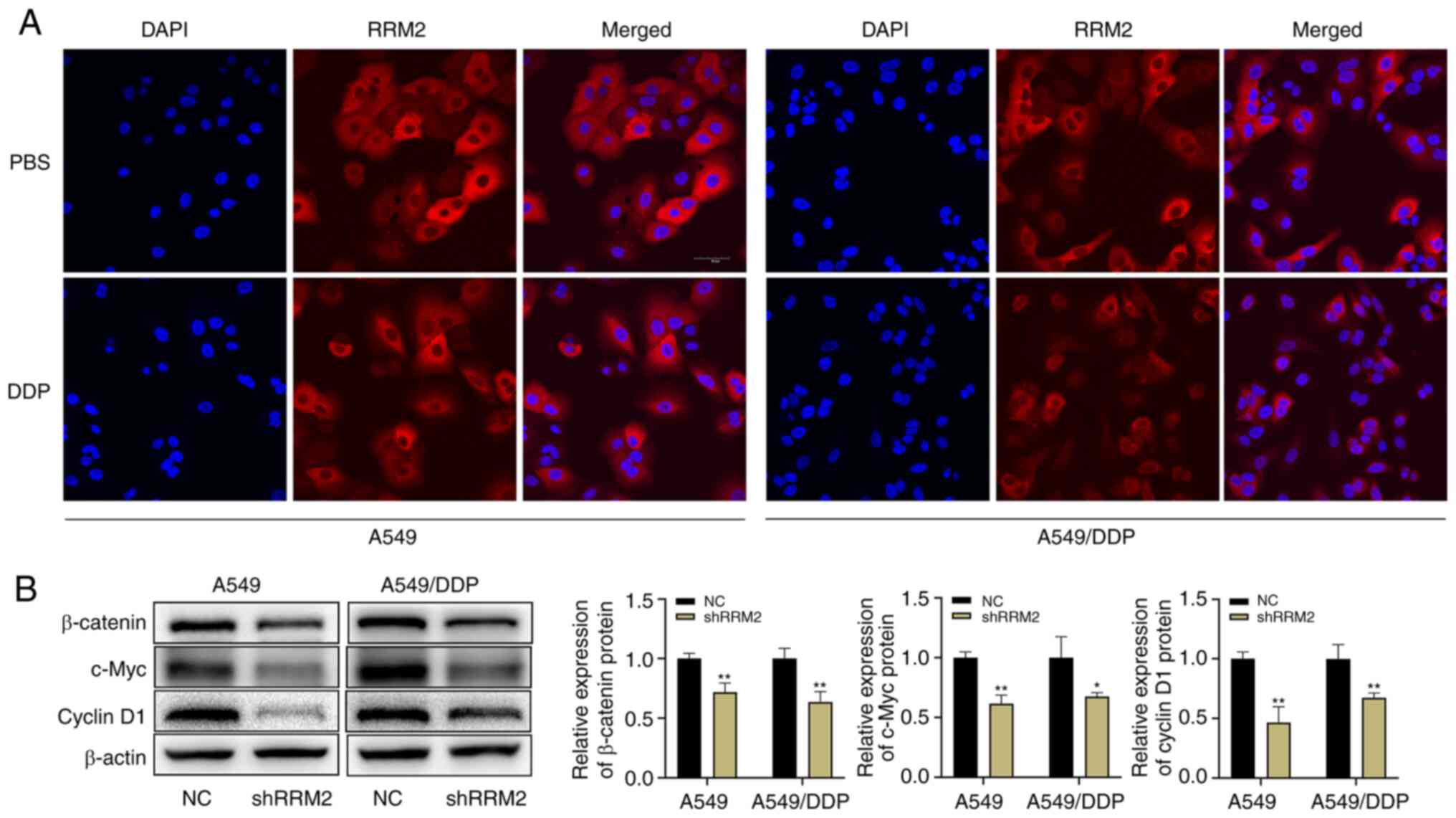
were examined by laser confocal microscopy. The results suggested
that cisplatin treatment did not induce nuclear translocation of
RRM2 in either A549 or A549/DDP cells (Fig. 5A). Therefore, it was postulated that
the influence of RRM2 in promoting cellular resistance to cisplatin
was not exerted through nuclear translocation. Subsequently, it was
found that the expression levels of β-catenin, c-Myc and cyclin D1
were reduced in A549 and A549/DDP cells after RRM2 knockdown, as
demonstrated by western blot analysis (Fig. 5B). In conclusion, knockdown of RRM2
had an inhibitory effect on lung adenocarcinoma via the
Wnt/β-catenin signaling pathway.
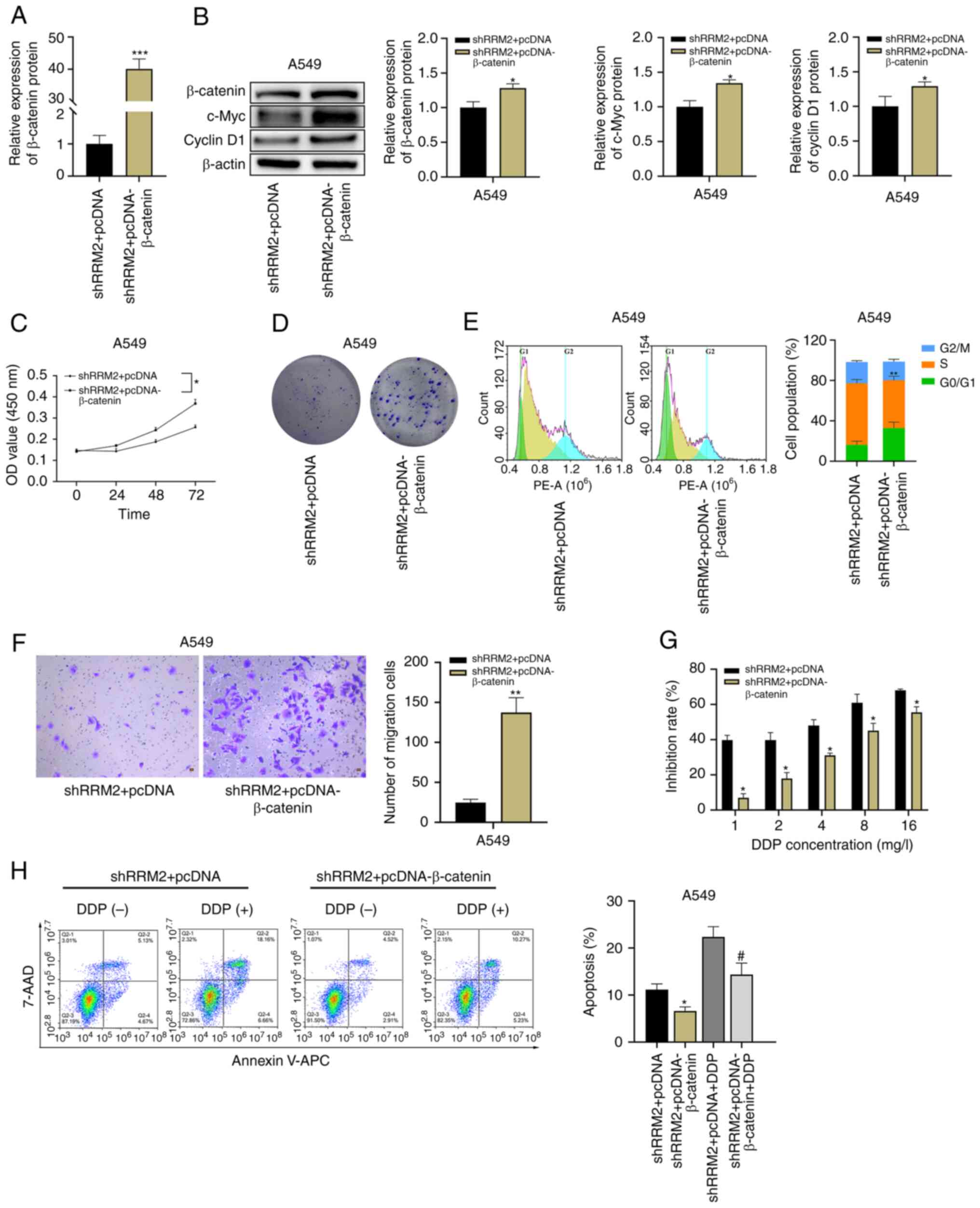
β-Catenin overexpression partially
reverses the effect of RRM2 knockdown on lung adenocarcinoma
cells
In a rescue experiment, pcDNA was used to
overexpress β-catenin in A549 cells with RRM2 knockdown (Fig. 6A and B). Through western blot
analysis of proteins involved in the Wnt/β-catenin signaling
pathway, it was found that β-catenin overexpression restored the
expression of c-Myc and cyclin D1 in A549 cells (Fig. 6B). β-Catenin overexpression not only
accelerated the proliferation (Fig. 6C
and D) and migration (Fig. 6F)
of A549 cells, but it also reduced the number of cells originally
blocked in the S phase (Fig. 6E).
In CCK-8 toxicity assays, β-catenin overexpression attenuated the
inhibitory effect of cisplatin on cells (Fig. 6G). Furthermore, flow cytometry
demonstrated that treatment of A549 cells overexpressing β-catenin
with or without 2 µg/ml of cisplatin resulted in the finding that
overexpression of β-catenin reduced the effect of RRM2 knockdown on
A549 cells (Fig. 6H).
Discussion
Cancer cells are known for their ability to grow
uncontrollably, which may lead to damage to surrounding tissues and
impaired organ function. In addition, cancer cells with abnormal
expression of MMP2, MMP9, N-cadherin, E-cadherin and other proteins
are more likely to metastasize by breaking through the basement
membrane (25,26). Cisplatin is a widely used
chemotherapeutic drug for the treatment of advanced tumors, as it
works by forming DNA cross-links in cancer cells, which leads to
inhibition of DNA replication and transcription, ultimately causing
cell cycle arrest and apoptosis (27). However, in cancer cells, DNA damage
repair processes, such as nucleotide excision repair,
post-replication repair and homologous recombination repair, may
counteract the effects of cisplatin, resulting in cisplatin
resistance (28). Recent studies
have indicated that RRM2, an oncogene, plays a critical role in the
proliferation, migration, invasion, and drug resistance of breast
cancer cells, small cell lung cancer cells, renal clear cell
carcinoma cells, and pancreatic cancer cells (29–32).
In lung adenocarcinoma, suppression of RRM2 also inhibits the
proliferation, migration and invasive ability of lung
adenocarcinoma cells (33–36). However, there is still limited
research on the specific mechanism of action of RRM2 in lung
adenocarcinoma. The present study aimed to investigate RRM2
expression, immune infiltration and its mechanism of action in lung
adenocarcinoma.
In the present study, a bioinformatics analysis was
conducted, which found that RRM2 was highly expressed in lung
adenocarcinoma, and there was an association of RRM2 with tumor
size and metastasis. Patients with high RRM2 expression in lung
adenocarcinoma had poor prognosis, and RRM2 was indicated to hold
significant predictive value for the 5-year survival of patients.
The results of enrichment analyses suggested that the mechanism of
action of RRM2 in lung adenocarcinoma may involve cell cycle
regulation. TME analysis showed that patients with high RRM2
expression had fewer stromal cells and lower tumor purity, which
may contribute to their poor prognosis. However, the ImmuneScore
did not significantly differ between patients with varying levels
of RRM2. In the present study, differential and correlation
analyses of several types of immune cell in the tumor tissue of
patients with lung adenocarcinoma were performed and it was
indicated that the levels of 12 immune cells differed between
patients expressing different levels of RRM2. Furthermore, six
immune cell types exhibited a positive correlation with RRM2
expression levels, while seven immune cell types showed a negative
correlation with RRM2 expression levels. In cellular experiments,
the expression of RRM2 was found to be higher in
cisplatin-resistant A549 cells than in A549 cells. The expression
level of RRM2 in the cells increased with increasing cisplatin
concentration. However, a noteworthy phenomenon emerged: Although
cisplatin induced protein expression of RRM2, the mRNA expression
level of RRM2 decreased in cisplatin-treated A549 cells compared to
that in untreated A549 cells. This decrease in RRM2 mRNA expression
in cisplatin-treated A549 cells is likely due to the inhibitory
effect of cisplatin on tumor cell DNA replication and transcription
by causing DNA cross-linking in tumor cells. However, other
mechanisms appear to affect RRM2 mRNA translation and
post-translational modifications, resulting in no decrease in RRM2
protein expression. It may be suggested that RRM2 has a role in the
development of lung adenocarcinoma and is associated with the
development of cisplatin resistance.
Based on the results of the previous analyses, the
present study aimed to investigate the specific mechanisms of
action of RRM2 in the cell cycle and cisplatin resistance of lung
adenocarcinoma. Lentivirus was used to stably knock down RRM2 in
A549 and A549/DDP cells and it was found that knockdown of RRM2
slowed down cell proliferation and migration, increased their
sensitivity to cisplatin and promoted apoptosis. These findings
align with the discoveries made by Liu et al (33). Furthermore, the present study
demonstrated, through cell cycle experiments, that the depletion of
RRM2 in lung adenocarcinoma cells led to an increased population of
cells with cell cycle arrest in S phase. This phenomenon may
potentially account for the observed inhibition of cancer cell
proliferation, restoration of cisplatin sensitivity in lung
adenocarcinoma cells and facilitation of apoptosis. The cell cycle
analysis results suggested that RRM2 knockdown inhibited cell
proliferation and activated programmed cell death by blocking the
cell cycle in S phase. Previous studies suggested that RRM2 may be
involved in cancer development by regulating the activation of the
Wnt/β-catenin signaling pathway. In the present study, the
expression of related proteins in this pathway was examined and it
was found that the expression levels of β-catenin, c-Myc and cyclin
D1 were decreased after RRM2 knockdown. This suggests that RRM2 may
be involved in the regulation of the Wnt/β-catenin signaling
pathway in lung adenocarcinoma. To further clarify the relationship
between RRM2 and the Wnt/β-catenin signaling pathway, β-catenin was
overexpressed using pcDNA3.1 in cells with knockdown RRM2. The
results showed that overexpression of β-catenin attenuated the
effect of RRM2 knockdown on A549 cells. However, despite attempts
to overexpress β-catenin in A549/DDP cells, successful transfection
could only be achieved in A549 cells. Therefore, only A549 cells
were used for the subsequent overexpression experiments. In
addition, only one cell line, A549, and its cisplatin-resistant
variant, A549/DDP, was used for experimental validation throughout
the study. This is one of the limitations of the present study, as
the use of a single cell line does not eliminate the influence of a
single genetic background on the obtained results.
Regarding the downstream mechanisms of RRM2 in lung
adenocarcinoma, Ma et al (34) discovered that RRM2 may influence the
sensitivity of A549 cells to gemcitabine by modulating the
phosphorylated phosphatase and tensin homolog/PI3K/AKT signaling
pathway. Another study demonstrated that suppression of RRM2
expression not only inhibits the malignant behavior of lung
adenocarcinoma cells but also synergistically enhances the efficacy
of radiotherapy in inducing cell death (35). This synergistic effect is achieved
through the activation of the GMP-AMP synthase/stimulator of
interferon genes signaling pathway. A separate study by Cao et
al (36) showed that RRM2 may
be regulated by microRNA-202-3p. Downregulation of RRM2 results in
inhibited proliferation, migration and invasion of lung
adenocarcinoma cells, possibly through the Notch signaling pathway.
However, Cao et al (36)
solely employed KEGG enrichment analysis to suggest the involvement
of the Notch signaling pathway in the RRM2-mediated effects on lung
adenocarcinoma, without conducting experimental validation.
In conclusion, the present study demonstrated that
RRM2 is highly expressed in lung adenocarcinoma tissues and that
its expression is higher in cisplatin-resistant lung adenocarcinoma
cells compared to non-cisplatin-resistant cells. Cisplatin also
induces RRM2 expression in a dose-dependent manner. Knockdown of
RRM2 inhibited the malignant behavior of lung adenocarcinoma cells.
On the other hand, overexpression of β-catenin attenuated the
effects of RRM2 knockdown, suggesting that RRM2 may affect lung
adenocarcinoma development through the Wnt/β-catenin signaling
pathway. The present study provided further insight into the
mechanism of RRM2 action in lung adenocarcinoma and suggests that
RRM2 may be a promising therapeutic target for the treatment of
this disease.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YJ, XH and LJ designed the study, performed the
experiments and wrote the manuscript. BR, YH and MP performed the
statistical analysis. BR and LH collected the mRNA transcriptome
data from public databases and conducted the data analysis. LH and
LJ critically revised the manuscript and confirmed the authenticity
of the data before submission. All authors have read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu X, Liu B, Li R, Wang F, Wang N, Zhang
M, Bai Y, Wu J, Liu L, Han D, et al: miR-146a-5p plays an oncogenic
role in NSCLC via suppression of TRAF6. Front Cell Dev Biol.
8:8472020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hellmann MD, Li BT, Chaft JE and Kris MG:
Chemotherapy remains an essential element of personalized care for
persons with lung cancers. Ann Oncol. 27:1829–1835. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sarin N, Engel F, Kalayda GV, Mannewitz M,
Cinatl J Jr, Rothweiler F, Michaelis M, Saafan H, Ritter CA, Jaehde
U and Frötschl R: Cisplatin resistance in non-small cell lung
cancer cells is associated with an abrogation of cisplatin-induced
G2/M cell cycle arrest. PLoS One. 12:e1810812017. View Article : Google Scholar
|
|
5
|
Mazzu YZ, Armenia J, Chakraborty G,
Yoshikawa Y, Coggins SA, Nandakumar S, Gerke TA, Pomerantz MM, Qiu
X, Zhao H, et al: A novel mechanism driving poor-prognosis prostate
cancer: Overexpression of the DNA repair gene, ribonucleotide
reductase small subunit M2 (RRM2). Clin Cancer Res. 25:4480–4492.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duxbury MS, Ito H, Zinner MJ, Ashley SW
and Whang EE: RNA interference targeting the M2 subunit of
ribonucleotide reductase enhances pancreatic adenocarcinoma
chemosensitivity to gemcitabine. Oncogene. 23:1539–1548. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peng S, Yi L, Liao L, Bin Y, Qu W and Hu
H: Circ_0008285 knockdown represses tumor development by
miR-384/RRM2 axis in hepatocellular carcinoma. Ann Hepatol.
27:1007432022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Q, Guo L, Qi H, Lou M, Wang R, Hai B,
Xu K, Zhu L, Ding Y, Li C, et al: A MYBL2 complex for RRM2
transactivation and the synthetic effect of MYBL2 knockdown with
WEE1 inhibition against colorectal cancer. Cell Death Dis.
12:6832021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang CC, Lin CC, Wang CH, Huang CC, Ke
TW, Wei PL, Yeh KT, Hsu KC, Hsu NY and Cheng YW: MiR-211 regulates
the expression of RRM2 in tumoral metastasis and recurrence in
colorectal cancer patients with a k-ras gene mutation. Oncol Lett.
15:8107–8117. 2018.PubMed/NCBI
|
|
10
|
Liu Q, Song C, Li J, Liu M, Fu L, Jiang J,
Zeng Z and Zhu H: E2F2 enhances the chemoresistance of pancreatic
cancer to gemcitabine by regulating the cell cycle and upregulating
the expression of RRM2. Med Oncol. 39:1242022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu H, Lu S, Yang D, Zhang L, Ye J, Li M
and Hu W: MiR-20a-5p regulates gemcitabine chemosensitivity by
targeting RRM2 in pancreatic cancer cells and serves as a predictor
for gemcitabine-based chemotherapy. Biosci Rep. 39:BSR201813742019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen P, Wu JN, Shu Y, Jiang HG, Zhao XH,
Qian H, Chen K, Lan T, Chen CG and Li J: Gemcitabine resistance
mediated by ribonucleotide reductase M2 in lung squamous cell
carcinoma is reversed by GW8510 through autophagy induction. Clin
Sci (Lond). 132:1417–1433. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen CW, Li Y, Hu S, Zhou W, Meng Y, Li Z,
Zhang Y, Sun J, Bo Z, DePamphilis ML, et al: DHS
(trans-4,4′-dihydroxystilbene) suppresses DNA replication and tumor
growth by inhibiting RRM2 (ribonucleotide reductase regulatory
subunit M2). Oncogene. 38:2364–2379. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xue T, Wang L, Li Y, Song H, Chu H, Yang
H, Guo A and Jiao J: SiRNA-mediated RRM2 gene silencing combined
with cisplatin in the treatment of epithelial ovarian cancer in
vivo: An experimental study of nude mice. Int J Med Sci.
16:1510–1516. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang M, Wang J, Yao R and Wang L: Small
interfering RNA (siRNA)-mediated silencing of the M2 subunit of
ribonucleotide reductase: A novel therapeutic strategy in ovarian
cancer. Int J Gynecol Cancer. 23:659–666. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang L, Meng L, Wang XW, Ma GY and Chen
JH: Expression of RRM1 and RRM2 as a novel prognostic marker in
advanced non-small cell lung cancer receiving chemotherapy. Tumour
Biol. 35:1899–1906. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deng Y, Chen X, Huang C, Song J, Feng S,
Chen X and Zhou R: Screening and validation of significant genes
with poor prognosis in pathologic stage-I lung adenocarcinoma. J
Oncol. 2022:37940212022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grossi F, Dal Bello MG, Salvi S, Puzone R,
Pfeffer U, Fontana V, Alama A, Rijavec E, Barletta G, Genova C, et
al: Expression of ribonucleotide reductase subunit-2 and
thymidylate synthase correlates with poor prognosis in patients
with resected stages I–III non-small cell lung cancer. Dis Markers.
2015:3026492015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
DasGupta R, Kaykas A, Moon RT and Perrimon
N: Functional genomic analysis of the Wnt-wingless signaling
pathway. Science. 308:826–833. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brown AM: Canonical Wnt signaling:
High-throughput RNAi widens the path. Genome Biol. 6:2312005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu H and Li B: MicroRNA-582-3p targeting
ribonucleotide reductase regulatory subunit M2 inhibits the
tumorigenesis of hepatocellular carcinoma by regulating the
Wnt/β-catenin signaling pathway. Bioengineered. 13:12876–12887.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu X, Peng J, Zhou Y, Xie B and Wang J:
Silencing RRM2 inhibits multiple myeloma by targeting the
Wnt/beta-catenin signaling pathway. Mol Med Rep. 20:2159–2166.
2019.PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang YW, Jones TL, Martin SE, Caplen NJ
and Pommier Y: Implication of checkpoint kinase-dependent
up-regulation of ribonucleotide reductase R2 in DNA damage
response. J Biol Chem. 284:18085–18095. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cabral-Pacheco GA, Garza-Veloz I,
Castruita-De LRC, Ramirez-Acuna JM, Perez-Romero BA,
Guerrero-Rodriguez JF, Martinez-Avila N and Martinez-Fierro ML: The
roles of matrix metalloproteinases and their inhibitors in human
diseases. Int J Mol Sci. 21:97392020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sisto M, Ribatti D and Lisi S: Cadherin
signaling in cancer and autoimmune diseases. Int J Mol Sci.
22:133582021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kryczka J, Kryczka J, Czarnecka-Chrebelska
KH and Brzezianska-Lasota E: Molecular mechanisms of
chemoresistance induced by cisplatin in NSCLC cancer therapy. Int J
Mol Sci. 22:88852021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li S, Mai H, Zhu Y, Li G, Sun J, Li G,
Liang B and Chen S: MicroRNA-4500 inhibits migration, invasion, and
angiogenesis of breast cancer cells via RRM2-dependent MAPK
signaling pathway. Mol Ther Nucleic Acids. 21:278–289. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Khan P, Siddiqui JA, Kshirsagar PG,
Venkata RC, Maurya SK, Mirzapoiazova T, Perumal N, Chaudhary S,
Kanchan RK, Fatima M, et al: MicroRNA-1 attenuates the growth and
metastasis of small cell lung cancer through CXCR4/FOXM1/RRM2 axis.
Mol Cancer. 22:12023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zou Y, Zhou J, Xu B, Li W and Wang Z:
Ribonucleotide reductase subunit M2 as a novel target for
clear-cell renal cell carcinoma. Onco Targets Ther. 12:3267–3275.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shan J, Wang Z, Mo Q, Long J, Fan Y, Cheng
L, Zhang T, Liu X and Wang X: Ribonucleotide reductase M2 subunit
silencing suppresses tumorigenesis in pancreatic cancer via
inactivation of PI3K/AKT/mTOR pathway. Pancreatology. 22:401–413.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Y, Zhang Y, Li Q, Xu R and Huang N:
MiR-200c-3p and miR-485-5p overexpression elevates cisplatin
sensitivity and suppresses the malignant phenotypes of non-small
cell lung cancer cells through targeting RRM2. Thorac Cancer.
13:1974–1985. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ma X, Fu T, Ke ZY, Du SL, Wang XC, Zhou N,
Zhong MY, Liu YJ and Liang AL: MiR-17- 5p/RRM2 regulated
gemcitabine resistance in lung cancer A549 cells. Cell Cycle.
22:1367–1379. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jiang X, Li Y, Zhang N, Gao Y, Han L, Li
S, Li J, Liu X, Gong Y and Xie C: RRM2 silencing suppresses
malignant phenotype and enhances radiosensitivity via activating
cGAS/STING signaling pathway in lung adenocarcinoma. Cell Biosci.
11:742021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cao X, Xue F, Chen H, Shen L, Yuan X, Yu
Y, Zong Y, Zhong L and Huang F: MiR-202-3p inhibits the
proliferation and metastasis of lung adenocarcinoma cells by
targeting RRM2. Ann Transl Med. 10:13742022. View Article : Google Scholar : PubMed/NCBI
|