Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most
common type of non-Hodgkin lymphoma (NHL), with 40% incidence in
patients aged >70 years (1). The
chimeric anti-CD20 monoclonal antibody rituximab plus doxorubicin,
cyclophosphamide, vincristine and prednisone chemotherapy (R-CHOP)
has markedly improved the overall survival rate in patients with
DLBCL in the last two decades. Full dose R-CHOP was initially
preferred in adult patients and elderly patients aged 60–80 years
(2,3). An attenuated immunochemotherapy
regimen [rituximab combined with low-dose CHOP (R-miniCHOP)] was
identified, and it exhibited favorable efficacy and safety in
patients >80 years of age (4).
Although the chemotherapy dose was reduced in older patients, the
presence of comorbidities often led to reduced tolerability to
treatment-related toxicities, resulting in treatment
discontinuation and treatment failure. Moreover, a previous study
revealed that treatment failure of R-miniCHOP in older patients
(>80 years) was associated with treatment-related toxicities and
pre-existing medical comorbidities, other than advanced age and
relatively-low dose intensity (5).
Thus, more efficacious and low-toxicity regimens need to be
explored.
Bendamustine is a unique bifunctional alkylating
agent with antimetabolic properties and antitumor effects (6). The rebirth of bendamustine was based
on the fact that it exhibited synergistic antitumor effects
combined with rituximab for the treatment of lymphoid malignancies
(7,8). Previous clinical trials have reported
that bendamustine plus rituximab (BR) improved the survival outcome
in patients with indolent NHL and relapsed/refractory DLBCL
(9,10). However, limited clinical data are
currently available to rationalize the therapeutic regimens for
older patients with newly diagnosed DLBCL.
In lymphoma, the cut-off age of 65 years was defined
as a watershed between younger and older patients (11). Generally, R-miniCHOP was recommended
when the patient was ≥80 years of age. However, age alone is not
enough to determine the treatment plan; treatment should be
individualized while taking individual life expectancy, functional
reserve and social support into consideration (12). Comprehensive Geriatric Assessment
(CGA) divides older patients into the following three categories:
Fit, unfit and frail, according to age, comorbidities and
functional abilities of daily living (13). CGA has proved to be an efficacious
tool for identifying fit patients who can benefit from an intensive
curative approach (14). However,
there are few studies on the therapeutic regimens used in unfit
patients. In the present prospective study, the efficacy and safety
between BR and R-miniCHOP as first-line treatment in unfit patients
with newly diagnosed DLBCL or follicular lymphoma grade 3B (FL3B)
in China were compared.
Materials and methods
Study design and patient
eligibility
The present randomized, controlled, two-center study
(Xiangyang No. 1 People's Hospital, Hubei, China; and Wuhan Union
Hospital, Hubei, China) compared the efficacy and safety of a BR
regimen with R-miniCHOP in unfit patients with newly diagnosed
DLBCL and FL3B. In the present study, patients aged >70 years,
with newly diagnosed DLBCL or FL3B were enrolled from January 2020
to December 2021. Diagnosis of DLBCL or FL3B was based on the
histopathological morphology reviewed by hematopathologists,
immunohistology, and clinical features recommended by WHO 2016
(15).
Patients classified as ‘unfit’ according to simple
CGA were included in this two-center study. The inclusion criteria
for this study is listed in Table I
(14). Patients considered unfit
who were included in this study met the following criteria: i) Age
≥70 years; ii) white blood cells ≥3.0×109/l or
granulocytes ≥1.5×109/l, hemoglobin
≥90.0×1012/l and platelets ≥100.0×109/l; iii)
normal sinus rhythm and ejection fraction 50–70% on
echocardiography; iv) alanine aminotransferase and aspartate
aminotransferase levels below the 2× upper limit of normal, serum
albumin ≥30.0 g/l and serum creatinine below the 1.5× upper limit
of normal; and v) negative (−) human immunodeficiency virus.
 | Table I.Definition of unfit according to
CGA. |
Table I.
Definition of unfit according to
CGA.
| Age (years) | ADL score | IADL score | CIRS-G |
|---|
| >79 | 6 | 8 | No grade-3/4
comorbidities and <5 grade-2 comorbidities |
| <80 | 5 | 6-7 | No grade-3/4
comorbidities and 5–8 grade-2 comorbidities |
Clinical staging was based on the modified Lugano
2014 staging criteria. Risk stratification was identified using the
International Prognostic Index (16). BR or R-miniCHOP chemotherapy was
randomly assigned to the patients based on a randomization schedule
generated by SAS programming (version 9.4; SAS Institute Inc.). In
patients presenting with potentially worsening cardiac function,
such as myocardial infarction ≤5 years ago, abnormal stress test,
previous percutaneous coronary intervention or coronary artery
bypass grafting and marked activity restriction secondary to the
cardiac status, doxorubicin was replaced with the liposomal
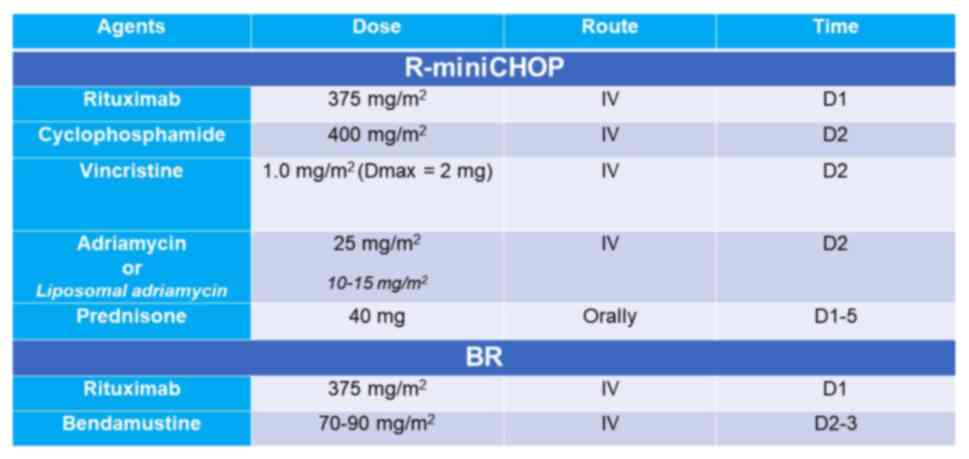
Adriamycin. The chemotherapy regimens used in the present study are
presented in Fig. 1.
The present study was approved by the Ethics and
Scientific Committee of Hubei University of Medicine, Xiangyang No.
1 People's Hospital (Xiangyang, China; approval no. 2022PR-H002).
All patients provided their written informed consent prior to
enrollment in the present study and data were collected from
electronic medical records.
Response evaluation
Treatment responses were evaluated by computed
tomography scans or positron emission tomography scan after the
completion of immunochemotherapy. Bone marrow aspiration and
immunotyping were also routinely performed to determine bone marrow
invasion at the initial diagnosis. Therapeutic evaluation was based
on Lugano 2014 classification, and it was divided into imaging
remission and metabolic remission, including complete remission
(CR), partial remission (PR), stable disease, and progressive
disease (16).
Adverse reactions assessment
Routine physical examination, hematological and
biochemical tests and an electrocardiogram were performed before
and after each cycle of immunochemotherapy. Adverse effects were
graded according to the National Cancer Institute Common
Terminology Criteria for Adverse Events v4.0 (17).
Statistical analysis
Data is presented as the mean ± standard deviation.
The primary endpoint was progression-free survival (PFS). PFS was
defined as the interval from date of the beginning of first
treatment to the date of disease progression or date of death.
Secondary endpoints included objective remission rate (ORR), CR, PR
and safety. The endpoints for DLBCL and FL3B were the same. The
laboratory and clinical data, response rates and adverse reactions
were analyzed using the chi-square test and Mann Whitney U test.
PFS was evaluated using the Kaplan-Meier curve, and the log-rank
test was used to calculate the significance of differences.
Prognostic risk factors were estimated using univariate analysis.
Statistical analyses were performed using GraphPad 7.0 software
(GraphPad Software, Inc.; Dotmatics) and the Statistical Package
for the Social Sciences version 24.0 software (IBM Corp.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Baseline characteristics of
patients
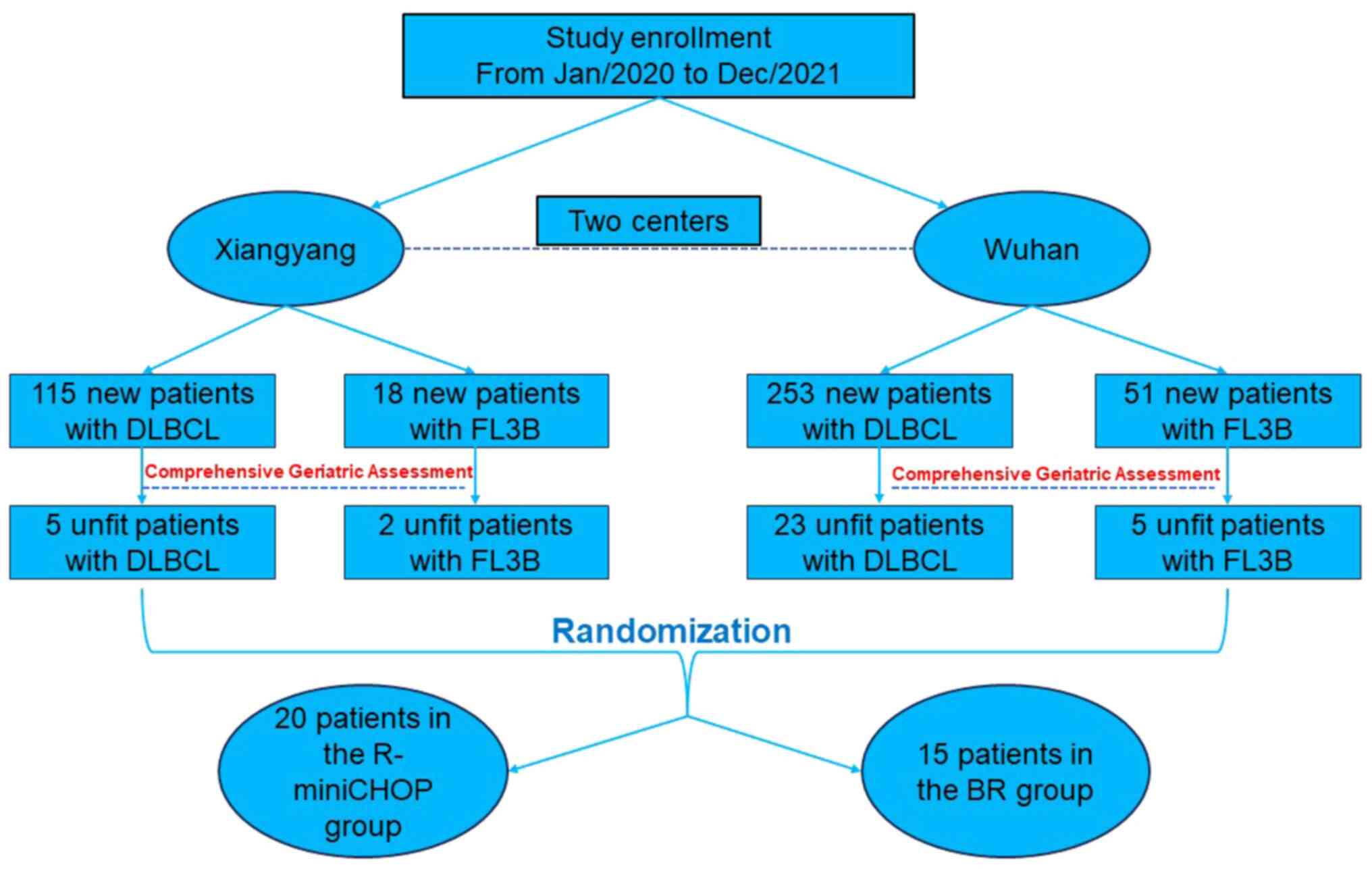
In the present study, a total of 35 patients were
recruited between January 2020 and November 2021, and the detailed
screening and inclusion process is revealed in Fig. 2. The median age of the patients was
74 years (range, 70 to 82 years), and the male to female ratio was
0.94:1. In total, 28 patients were diagnosed with DLBCL, and 7
patients were diagnosed with FL3B. Stage IV disease was diagnosed
in 18 patients (56.25%). In total, 20 patients were treated with
R-miniCHOP, while 15 patients received the BR regimen. There was no
significant difference in age, sex, stage and comorbidities between
the two groups. The baseline characteristics are listed in Table II.
 | Table II.Characteristics of patients enrolled
in the present study. |
Table II.
Characteristics of patients enrolled
in the present study.
|
| Number of patients
(n=35) |
|
|---|
|
|
|
|
|---|
| Variables | R-miniCHOP
(n=20) | BR (n=15) | P-value |
|---|
| Age (years) |
|
| 0.064 |
| ≥70 and
<80 | 19 | 10 |
|
|
>80 | 1 | 5 |
|
| Sex |
|
| 0.845 |
|
Male | 10 | 7 |
|
|
Female | 10 | 8 |
|
| Pathology |
|
| 0.660 |
|
FL3B | 4 | 3 |
|
|
DLBCL | 16 | 12 |
|
| Stage (Lugano) |
|
| 0.991 |
| I and
II | 8 | 5 |
|
| III and
IV | 12 | 10 |
|
| IPI |
|
| 0.737 |
| 1 | 2 | 4 |
|
| 2 | 6 | 5 |
|
| 3 or
more | 12 | 6 |
|
| ECOG |
|
| 0.738 |
|
0-1 | 9 | 8 |
|
| 2 | 11 | 7 |
|
| Grade-2
comorbidities |
|
| 0.833 |
|
<5 | 7 | 5 |
|
|
5-8 | 13 | 10 |
|
Treatment and response
In total, 2 patients received 4 cycles of R-miniCHOP
and 1 patient received 4 cycles of BR due to tumor progression and
severe infection caused by myelosuppression. The remaining patients
received 6 cycles of immunochemotherapy. All patients were alive
after the median follow-up of 13 months. The CR rate was similar
between the two groups (35 vs. 33.3%). The ORR in the BR group was
slightly lower compared with that in the R-miniCHOP group (73.3 vs.
75.0%), but with no statistical significance (P=0.606), as revealed
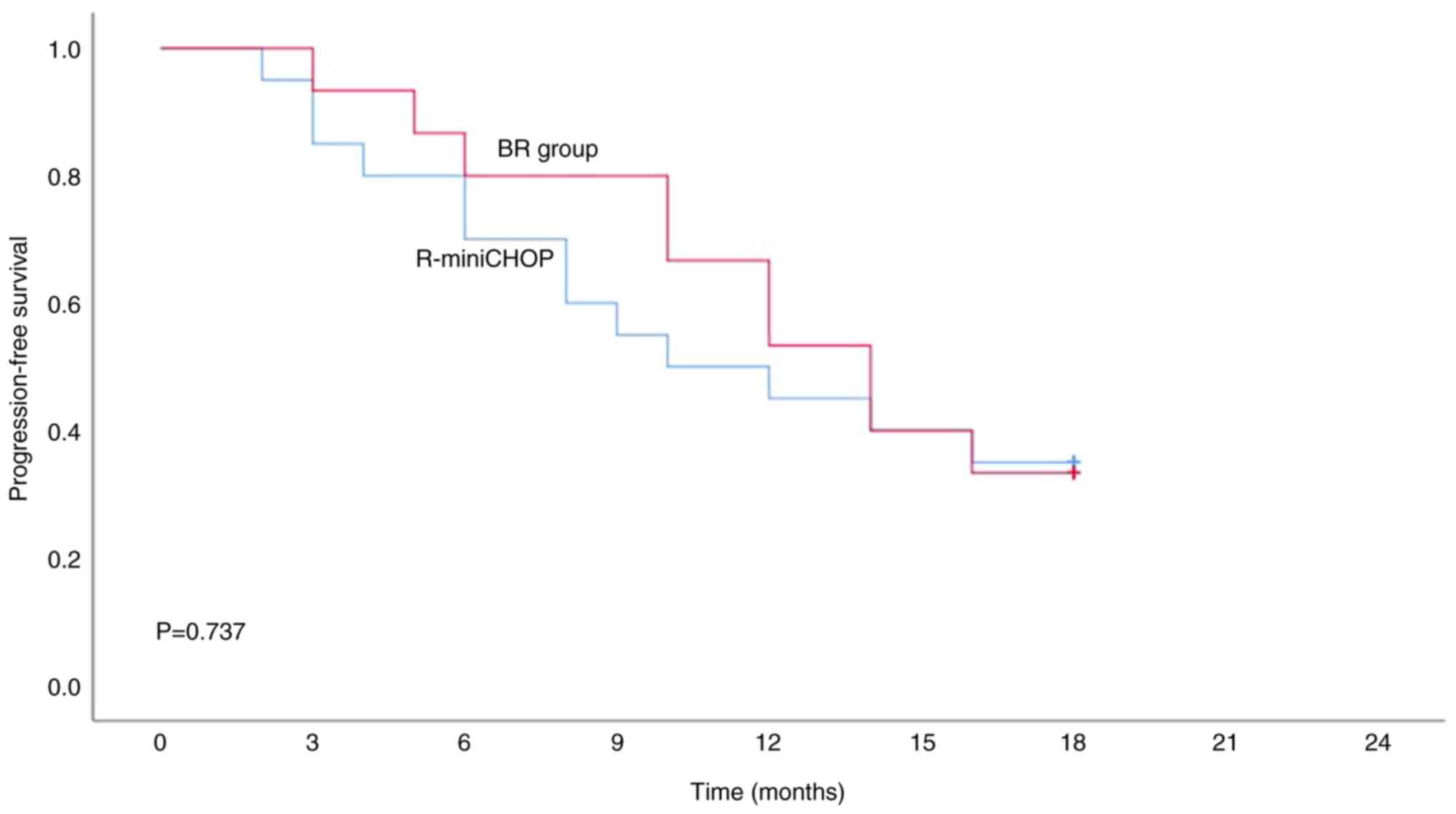
in Table III. The median 1.5-year
PFS in the BR group and R-miniCHOP were 12.8 and 11.3 months,
respectively (Fig. 3).
 | Table III.Overall response rates of R-miniCHOP
and BR regimens. |
Table III.
Overall response rates of R-miniCHOP
and BR regimens.
| Response | R-miniCHOP
(n=20) | BR (n=15) | P-value |
|---|
| Complete remission
rate, % (n/total n) | 35 (7/20) | 33 (5/15) | 0.918 |
| Partial remission
rate, % (n/total n) | 40 (8/20) | 40 (6/15) | 0.635 |
| Stable disease
rate, % (n/total n) | 10 (2/20) | 20 (3/15) | 0.448 |
| Progressive disease
rate, % (n/total n) | 15 (3/20) | 7 (1/15) | 0.419 |
| Objective remission
rate, % (n/total n) | 75 (15/20) | 73 (11/15) | 0.606 |
Adverse reactions
Adverse events were classified as hematological and
non-hematologic adverse reactions (Table IV). The R-miniCHOP group had a
higher incidence of leukopenia compared with the BR group. A total
of 4 patients reported cardiac events in the R-miniCHOP group.
Furthermore, the BR group had a higher incidence of transient
fever, but with no statistical significance (P=0.199).
 | Table IV.Hematological and extra-hematological
adverse events between R-miniCHOP and BR groups. |
Table IV.
Hematological and extra-hematological
adverse events between R-miniCHOP and BR groups.
|
| Grade of adverse
reaction, n (%) |
|
|---|
|
|
|
|
|---|
| Toxicity | R-miniCHOP
(n=20) | BR (n=15) | P-value |
|---|
| Hematological |
|
|
|
|
Leukopenia | 12 (60) | 3 (20) | 0.037a |
|
Anemia | 13 (65) | 6 (40) | 0.182 |
|
Thrombocytopenia | 6 (30) | 2 (13) | 0.419 |
|
Non-hematological |
|
|
|
| Nausea
and vomiting | 8 (40) | 6 (40) | 0.635 |
| ALT/AST
elevation | 6 (30) | 2 (13.) | 0.419 |
|
Hypoalbuminemia | 3 (15) | 2 (13) | 0.640 |
| Cardiac
disorders | 4 (20) | 1 (7) | 0.365 |
| Nervous
system disorders | 3 (15) | 1 (7) | 0.619 |
|
Allergy | 5 (25) | 2 (13) | 0.672 |
|
Transient fever | 2 (10) | 5 (33) | 0.199 |
|
Electrolyte imbalance | 8 (40) | 7 (47) | 0.741 |
|
Infection | 6 (30) | 2 (13) | 0.419 |
Sensitivity analysis
Univariate analyses were performed to clarify the
association between prognostic factors and ORR in these 35
patients. In the univariate analysis, lactate dehydrogenase and
erythrocyte sedimentation rate levels, Eastern Cooperative Oncology
Group performance status (0–1), no extranodal sites and tumor mass
(<10 cm) were not associated with the ORR. However, β2
microglobulin <3.0 mg/l may be predictive of a higher ORR
(P=0.014; Table V).
 | Table V.Analysis of the association between
prognostic factors and ORR in 35 patients with DLBCL. |
Table V.
Analysis of the association between
prognostic factors and ORR in 35 patients with DLBCL.
| Prognostic
factor | ORR rate, % | 95% CI | P-value |
|---|
| ECOG 0–1 | 48.57 vs.
71.43 | 0.31–0.66 | 0.152 |
| β2 microglobulin
<3.0 mg/l | 57.14 vs.
89.47 | 0.40–0.74 | 0.014 |
| No extranodal
sites | 51.42 vs.
72.22 | 0.34–0.69 | 0.151 |
| No LDH
elevation | 65.71 vs.
72.72 | 0.49–0.82 | 0.851 |
| ESR <50
mm/h | 62.85 vs.
79.16 | 0.46–0.80 | 0.954 |
| Tumor mass <10
cm | 80.00 vs.
92.85 | 0.66–0.94 | 0.833 |
Discussion
The Surveillance, Epidemiology and End Results
program predicted that the estimated incidence of NHL would be ~4%
of all cancer cases worldwide in 2024, with a median age of 66
years (18). DLBCL accounts for
~31% of NHL cases, with the majority of DLBCL patients >60 years
of age (11,19). Therefore, measures to improve
patient outcomes, individual life expectancy and survival of
elderly patients with DLBCL should be.
In the past two decades, R-CHOP was the recommended
standard regimen for the treatment of DLBCL in older patients
<80 years of age. Older patients aged 60–80 years with DLBCL
could achieve a relatively higher CR rate and prolonged median
survival after 4–6 cycles of R-CHOP (3). R-miniCHOP offered an improved
compromise between safety and efficiency in patients aged >80
years as a substantial number of older patients could be cured
(4). However, increases in
complications and adverse treatment reactions were reported in
patients aged ≥70 years (20).
Several risk factors, including comorbidity, functional impairment,
cognitive decline, poor performance score and social isolation,
contributed to the treatment-related toxicity (21). Despite the use of R-CHOP or
R-miniCHOP as treatment options in older patients, the presence of
comorbidities and treatment-related toxicity, to some extent,
contribute to limited available therapeutic options in real-world
practice. Furthermore, when the dose of CHOP was reduced to 7/12,
patients had a relatively low overall survival and event-free
survival (22).
Several studies have demonstrated that the BR
regimen is a promising prospect in both indolent and aggressive
lymphomas due to its modest activity and manageable toxicity
profile. The BRIGHT study reported that the BR regimen had a better
long-term disease control than the R-CHOP regimen, and therefore,
it should be recommended as the first-line treatment in indolent
and mantle cell lymphoma (23).
Furthermore, a multicenter, retrospective study reported that the
BR regimen was less toxic and more efficient compared with the
R-CHOP regimen in patients with FL grade 3A and this regimen
produced a low rate of non-hematological adverse events in older
patients with chronic lymphocytic leukemia (24,25).
Moreover, the BR regimen was well tolerated and safe in patients
with indolent NHL who had renal impairment (26).
To date, a few studies have demonstrated that BR is
a feasible option for the first-line treatment of DLBCL in elderly
patients (27–30). Table
VI summarizes the clinical data of elderly patients treated
with BR from different studies. The ORR and median PFS in the BR
group was similar to the results obtained in the R-miniCHOP group
(4). The present study further
reverified this finding; the ORRs in the BR group and R-miniCHOP
group were 73.3 and 75%, respectively.
 | Table VI.Summary of prospective studies of BR
as frontline treatment in elderly patients with newly diagnosed
DLBCL. |
Table VI.
Summary of prospective studies of BR
as frontline treatment in elderly patients with newly diagnosed
DLBCL.
| First author,
year | Region | Patients | Median age in years
(limits) | Bendamustine | ORR (%) | CRR (%) | Survival
(months) | (Refs.) |
|---|
| Weidmann et
al, 2011 | Germany | 13 | 85 (80–95) | 120
mg/m2/q3w | 69 | 54 | mPFS 7.7 | (31) |
| Horn et al,
2012 | Germany | 20 | 72 (51–86) | 90
mg/m2/q4w | 55 | 20 | mPFS 8.3; mOS
19.4 | (27) |
| Park et al,
2016 | USA | 23 | 80 (>65) | 120
mg/m2/q3w | 78 | 52 | mPFS 5.4; mOS
10.2 | (30) |
| Storti et
al, 2018 | Italy | 49 | 81 (>70) | 90
mg/m2/q4w | 62 | 53 | mOS 10.0 | (29) |
| Cheng et al,
2018 | Taiwan | 26 | 81 (75–93) | 90
mg/m2/q3w | 50 | 42.3 | mOS 11.2 | (28) |
| Present study | China | 15 | 70 (70–82) | 70-90
mg/m2/q3w | 73.3 | 33.3 | mPFS 12.8 | - |
In a previous study (24), nausea and vomiting were frequently
reported in older patients after BR treatment; the incidence of
this adverse reaction was consistent with another literature study.
Importantly, the BR group had a lower rate of leukopenia compared
with the R-miniCHOP group. This result showed that BR is likely to
reduce the risk of infection and febrile neutropenia. Additionally,
BR had a relative low rate of cardiac events due to the absence of
anthracyclines, demonstrating that BR is safer and tolerable for
unfit older patients, especially for those with ventricular
dysfunction (31). It is worth
noting that BR was associated with a higher incidence of transient
fever, which could be attributed to drug-induced fever, as the
inflammatory indicators, such as C-reactive protein and
procalcitonin, were maintained in the normal range. Generally, the
body temperature could be quickly reduced to normal after auxiliary
antipyretic treatment. However, when this symptom arises on the
initiation of treatment, it is difficult for physicians to
determine the cause of fever, which may preclude treatment.
The limitation of the present study was its small
cohort size and short follow-up. Bendamustine was introduced in the
market, in China, in May 2019. It is relatively expensive, and as
the cost of bendamustine is not reimbursed by the national health
insurance system in China, only a small percentage of patients
prefer to use this agent. Furthermore, the number of elderly
patients newly diagnosed with DLBCL and defined as unfit was small.
Thus, it is challenging to recruit an adequate number of unfit
participants in such a short time. Hence, overall survival and
median PFS could not be analyzed in the present study. The authors
of the present study conclude that a large-scale, long-term
follow-up prospective study will provide better insight into
treatment options for DLBCL, in the future.
The emergence of new targeted drugs also provides
more options for the treatment of DLBCL. A recent clinical trial
(ClinicalTrials.gov number, NCT03274492)
showed that the anti-CD79b antibody polatuzumab vedotin combined
with BR could reduce the risk of mortality in relapsed/refractory
DLBCL (32). The novel histone
deacetylase inhibitor chidamide could synergize with rituximab by
upregulating CD20 expression in DLBCL, and it significantly
inhibited tumor growth in vitro and in vivo (33). The phosphoinositide 3-kinase (PI3K)
inhibitor, copanlisib, exhibited high cytotoxicity in vivo
and could improve survival in the DLBCL model (34). Moreover, the Bcl-2 inhibitor,
venetoclax, and Bruton's tyrosine kinase inhibitor, ibrutinib,
could enhance the sensitivity of the PI3K inhibitor in activated
B-cell like DLBCL (34,35). It is considered that the treatment
for unfit patients with DLBCL will move towards a chemotherapy-free
era in the future.
In conclusion, the current treatment for unfit
patients with DLBCL remains a daunting challenge for physicians.
The choice of treatment should be individualized, and an accurate
assessment of the risk-benefit ratio should be performed for each
patient before treatment. CGA is a validated tool to assess the
patient fitness status before the initiation of treatment.
Considering the results of the present study, BR is a promising
regimen with lesser toxicity and it may be recommended as an
alternative regimen to R-miniCHOP for unfit patients with DLBCL or
FL3B. However, further studies with larger sample sizes are
required to evaluate its efficacy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 82200214), the Key Research and
Development Project of Hubei (grant no. 2022BCE028), the Innovative
Research Program of Xiangyang No. 1 People's Hospital (grant no.
XYY2021Q02), the Platform Special Fund for Scientific Research of
Xiangyang No. 1 People's Hospital (grant no. XYY2022P05), and the
Key projects of Xiangyang Science and Technology Bureau (grant no.
2021YL26).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
LZ provided the project direction, designed the
study and performed the statistical analysis. DZ and YL conceived
and performed the study, analyzed the data, and wrote the
manuscript. YD contributed to data acquisition and revised the
manuscript. DZ and LZ confirm the authenticity of all the raw data.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Ethical approval was obtained from the Ethics and
Scientific Committee of Hubei University of Medicine, Xiangyang
No.1 People's Hospital, Xiangyang, China (approval no.
2022PR-H002). All methods were carried out in accordance with the
recommendations of the Ethics and Scientific Committee of Hubei
University of Medicine and the Declaration of Helsinki. All
patients provided their written informed consent prior to
enrollment in the present study and data were collected from
electronic medical records.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
d'Amore F, Brincker H, Christensen BE,
Thorling K, Pedersen M, Nielsen JL, Sandberg E, Pedersen NT and
Sørensen E: Non-Hodgkin's lymphoma in the elderly. A study of 602
patients aged 70 or older from a Danish population-based registry.
The Danish LYEO-study group. Ann Oncol. 3:379–386. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feugier P, Van Hoof A, Sebban C,
Solal-Celigny P, Bouabdallah R, Fermé C, Christian B, Lepage E,
Tilly H, Morschhauser F, et al: Long-term results of the R-CHOP
study in the treatment of elderly patients with diffuse large
B-cell lymphoma: A study by the Groupe d'Etude des Lymphomes de
l'Adulte. J Clin Oncol. 23:4117–4126. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coiffier B, Thieblemont C, Van Den Neste
E, Lepeu G, Plantier I, Castaigne S, Lefort S, Marit G, Macro M,
Sebban C, et al: Long-term outcome of patients in the LNH-98.5
trial, the first randomized study comparing rituximab-CHOP to
standard CHOP chemotherapy in DLBCL patients: A study by the Groupe
d'Etudes des Lymphomes de l'Adulte. Blood. 116:2040–2045. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peyrade F, Jardin F, Thieblemont C, Thyss
A, Emile JF, Castaigne S, Coiffier B, Haioun C, Bologna S, Fitoussi
O, et al: Attenuated immunochemotherapy regimen (R-miniCHOP) in
elderly patients older than 80 years with diffuse large B-cell
lymphoma: A multicentre, single-arm, phase 2 trial. Lancet Oncol.
12:460–468. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kobayashi Y, Miura K, Hojo A, Hatta Y,
Tanaka T, Kurita D, Iriyama N, Kobayashi S and Takeuchi J: Charlson
comorbidity index is an independent prognostic factor among elderly
patients with diffuse large B-cell lymphoma. J Cancer Res Clin
Oncol. 137:1079–1084. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheson BD and Rummel MJ: Bendamustine:
Rebirth of an old drug. J Clin Oncol. 27:1492–1501. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rummel MJ, Niederle N, Maschmeyer G, Banat
GA, von Grünhagen U, Losem C, Kofahl-Krause D, Heil G, Welslau M,
Balser C, et al: Bendamustine plus rituximab versus CHOP plus
rituximab as first-line treatment for patients with indolent and
mantle-cell lymphomas: An open-label, multicentre, randomised,
phase 3 non-inferiority trial. Lancet. 381:1203–1210. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Flinn IW, van der Jagt R, Kahl BS, Wood P,
Hawkins TE, Macdonald D, Hertzberg M, Kwan YL, Simpson D, Craig M,
et al: Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP
in first-line treatment of indolent NHL or MCL: The BRIGHT study.
Blood. 123:2944–2952. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hong JY, Yoon DH, Suh C, Kim WS, Kim SJ,
Jo JC, Kim JS, Lee WS, Oh SY, Park Y, et al: Bendamustine plus
rituximab for relapsed or refractory diffuse large B cell lymphoma:
A multicenter retrospective analysis. Ann Hematol. 97:1437–1443.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ohmachi K, Niitsu N, Uchida T, Kim SJ,
Ando K, Takahashi N, Takahashi N, Uike N, Eom HS, Chae YS, et al:
Multicenter phase II study of bendamustine plus rituximab in
patients with relapsed or refractory diffuse large B-cell lymphoma.
J Clin Oncol. 31:2103–2109. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fields PA and Linch DC: Treatment of the
elderly patient with diffuse large B cell lymphoma. Br J Haematol.
157:159–170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Balducci L and Extermann M: Management of
cancer in the older person: A practical approach. Oncologist.
5:224–237. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Extermann M, Aapro M, Bernabei R, Cohen
HJ, Droz JP, Lichtman S, Mor V, Monfardini S, Repetto L, Sørbye L,
et al: Use of comprehensive geriatric assessment in older cancer
patients: Recommendations from the task force on CGA of the
international society of geriatric oncology (SIOG). Crit Rev Oncol
Hematol. 55:241–252. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tucci A, Martelli M, Rigacci L, Riccomagno
P, Cabras MG, Salvi F, Stelitano C, Fabbri A, Storti S, Fogazzi S,
et al: Comprehensive geriatric assessment is an essential tool to
support treatment decisions in elderly patients with diffuse large
B-cell lymphoma: A prospective multicenter evaluation in 173
patients by the lymphoma Italian foundation (FIL). Leuk Lymphoma.
56:921–926. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cazzola M: Introduction to a review
series: the 2016 revision of the WHO classification of tumors of
hematopoietic and lymphoid tissues. Blood. 127:2361–2364. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheson BD, Fisher RI, Barrington SF,
Cavalli F, Schwartz LH, Zucca E, Lister TA; Alliance, Australasian
Leukaemia; Lymphoma Group and Eastern Cooperative Oncology Group, ;
et al: Recommendations for initial evaluation, staging, and
response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano
classification. J Clin Oncol. 32:3059–3068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dueck AC, Mendoza TR, Mitchell SA, Reeve
BB, Castro KM, Rogak LJ, Atkinson TM, Bennett AV, Denicoff AM,
O'Mara AM, et al: Validity and reliability of the US national
cancer institute's patient-reported outcomes version of the common
terminology criteria for adverse events (PRO-CTCAE). JAMA Oncol.
1:1051–1059. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Martelli M, Ferreri AJ, Agostinelli C, Di
Rocco A, Pfreundschuh M and Pileri SA: Diffuse large B-cell
lymphoma. Crit Rev Oncol Hematol. 87:146–171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Advani RH, Chen H, Habermann TM, Morrison
VA, Weller EA, Fisher RI, Peterson BA, Gascoyne RD, Horning SJ;
Eastern Cooperative Oncology Group, ; et al: Comparison of
conventional prognostic indices in patients older than 60 years
with diffuse large B-cell lymphoma treated with R-CHOP in the US
intergroup study (ECOG 4494, CALGB 9793): Consideration of age
greater than 70 years in an elderly prognostic index (E-IPI). Br J
Haematol. 151:143–151. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pal SK, Katheria V and Hurria A:
Evaluating the older patient with cancer: Understanding frailty and
the geriatric assessment. CA Cancer J Clin. 60:120–132. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kayamori K, Shono K, Onoda M and Yokota A:
Efficacy and tolerability of rituximab and reduced-dose
cyclophosphamide, doxorubicin, vincristine, and prednisolone
therapy for elderly patient with diffuse large B-cell lymphoma.
Hematology. 24:52–59. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Flinn IW, van der Jagt R, Kahl B, Wood P,
Hawkins T, MacDonald D, Simpson D, Kolibaba K, Issa S, Chang J, et
al: First-line treatment of patients with indolent non-Hodgkin
lymphoma or mantle-cell lymphoma with bendamustine plus rituximab
versus R-CHOP or R-CVP: Results of the BRIGHT 5-year follow-up
study. J Clin Oncol. 37:984–991. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mondello P, Steiner N, Willenbacher W,
Cerchione C, Nappi D, Mauro E, Ferrero S, Cuzzocrea S and Mian M:
Bendamustine plus rituximab versus R-CHOP as first-line treatment
for patients with follicular lymphoma grade 3A: Evidence from a
multicenter, retrospective study. Oncologist. 23:454–460. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Woyach JA, Ruppert AS, Heerema NA, Zhao W,
Booth AM, Ding W, Bartlett NL, Brander DM, Barr PM, Rogers KA, et
al: Ibrutinib regimens versus chemoimmunotherapy in older patients
with untreated CLL. N Engl J Med. 379:2517–2528. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ribes D, Hachem HEL, Oberic L, Vergez F,
Delas A, Belliere J, Protin C, Kamar N, Ferrandiz I, Tavitian S, et
al: Bendamustine plus rituximab for indolent B-cell lymphoma of
renal significance. Am J Hematol. 93:356–362. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Horn J, Kleber M, Hieke S, Schmitt-Gräff
A, Wäsch R and Engelhardt M: Treatment option of bendamustine in
combination with rituximab in elderly and frail patients with
aggressive B-non-Hodgkin lymphoma: Rational, efficacy, and
tolerance. Ann Hematol. 91:1579–1586. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng CL, Liu JH, Chou SC, Yao M, Tang JL
and Tien HF: Retrospective analysis of frontline treatment efficacy
in elderly patients with diffuse large B-cell lymphoma. Eur J
Haematol. 101:28–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Storti S, Spina M, Pesce EA, Salvi F,
Merli M, Ruffini A, Cabras G, Chiappella A, Angelucci E, Fabbri A,
et al: Rituximab plus bendamustine as front-line treatment in frail
elderly (>70 years) patients with diffuse large B-cell
non-Hodgkin lymphoma: A phase II multicenter study of the
Fondazione Italiana Linfomi. Haematologica. 103:1345–1350. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park SI, Grover NS, Olajide O, Asch AS,
Wall JG, Richards KL, Sobol AL, Deal AM, Ivanova A, Foster MC, et
al: A phase II trial of bendamustine in combination with rituximab
in older patients with previously untreated diffuse large B-cell
lymphoma. Br J Haematol. 175:281–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Weidmann E, Neumann A, Fauth F, Atmaca A,
Al-Batran SE, Pauligk C and Jäger E: Phase II study of bendamustine
in combination with rituximab as first-line treatment in patients
80 years or older with aggressive B-cell lymphomas. Ann Oncol.
22:1839–1844. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sehn LH, Herrera AF, Flowers CR, Kamdar
MK, McMillan A, Hertzberg M, Assouline S, Kim TM, Kim WS, Ozcan M,
et al: Polatuzumab vedotin in relapsed or refractory diffuse large
B-cell lymphoma. J Clin Oncol. 38:155–165. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guan XW, Wang HQ, Ban WW, Chang Z, Chen
HZ, Jia L and Liu FT: Novel HDAC inhibitor chidamide synergizes
with rituximab to inhibit diffuse large B-cell lymphoma tumour
growth by upregulating CD20. Cell Death Dis. 11:202020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bojarczuk K, Wienand K, Ryan JA, Chen L,
Villalobos-Ortiz M, Mandato E, Stachura J, Letai A, Lawton LN,
Chapuy B and Shipp MA: Targeted inhibition of PI3Kα/δ is
synergistic with BCL-2 blockade in genetically defined subtypes of
DLBCL. Blood. 133:70–80. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sasi BK, Martines C, Xerxa E, Porro F,
Kalkan H, Fazio R, Turkalj S, Bojnik E, Pyrzynska B, Stachura J, et
al: Inhibition of SYK or BTK augments venetoclax sensitivity in
SHP1-negative/BCL-2-positive diffuse large B-cell lymphoma.
Leukemia. 33:2416–2428. 2019. View Article : Google Scholar : PubMed/NCBI
|