Introduction
Pancreatic cancer is a particularly deadly type of
gastrointestinal malignancy. Due to the difficulty of early
diagnosis and the limited effectiveness of treatment with surgery,
radiotherapy and chemotherapy, patients with pancreatic cancer have
a poor prognosis and high mortality rate, with an overall survival
rate of only 8% at 5 years (1).
Ductal adenocarcinoma of the pancreas is the most common type,
accounting for 95% of all cases of pancreatic cancer (2). Most patients with pancreatic cancer
are diagnosed at an advanced stage, so only 15–20% of patients with
pancreatic cancer can undergo surgery. In addition, pancreatic
cancer has a high rate of recurrence even after radical resection
(3). Studies have shown that the
malignant progression, treatment resistance and poor prognosis of
pancreatic cancer are significantly linked to the immunosuppressive
nature of the tumor microenvironment (TME) of pancreatic cancer
(4–6). Although immunotherapy has achieved
significant results in tumors, such as breast, lung and ovarian
cancer, immunotherapy has not been effective in pancreatic cancer
due to the highly suppressive nature of the tumor immune
microenvironment (7,8). Therefore, it is crucial to research
the immunological microenvironment of pancreatic cancer. By
focusing on its constituents and inhibitory characteristics,
researchers are expected to provide new ideas and directions for
immunotherapy of pancreatic cancer.
Over 10 cell types have been reported to routinely
express eukaryotic translation initiation factor 2α kinase 2
(EIF2AK2) and it may be activated by a variety of cellular
stresses, such as viral infections, hypoxia and nutritional
shortages (9,10). The significance of EIF2AK2 in cancer
remains controversial and complex. In general, EIF2AK2 is
considered to have tumor suppressive functions (11–14).
Several studies have demonstrated a link between EIF2AK2
suppression or inactivation and poor prognosis in a variety of
malignancies, including breast, lung and colorectal cancer
(15,16). Invasive ductal carcinoma cells have
been shown to express higher levels of EIF2AK2 compared with normal
breast tissue (17). In addition,
the expression and activity levels of EIF2AK2 are linked to the
probability of breast cancer cells spreading (18). The activation of EIF2AK2 by
double-stranded RNA (dsRNA) has also been documented to be involved
in the management of breast cancer cell mobility (19). These results indicated that EIF2AK2
may have a crucial role in suppressing cancer metastasis. However,
EIF2AK2 has also been reported to be associated with the
proliferation and migration of hepatocellular carcinoma, and the
metastasis of gastric cancer (20,21).
Therefore, it is possible that its antitumor or oncogenic function
of EIF2AK2 depends on the type of cancer cells. Notably, to the
best of our knowledge, the relationship between EIF2AK2 expression
and pancreatic cancer, and its predictive value, has not been
investigated.
The present study aimed to investigate the
relationship between EIF2AK2 expression and survival outcomes in
patients with pancreatic cancer using The Cancer Genome Atlas
(TCGA), Genomic Tumor Expression Atlas (GTEx) and Gene Expression
Omnibus (GEO) datasets. The present study examined whether there is
a correlation between EIF2AK2 mRNA levels and the presence of
immune cells in tumors. The results of the present study highlight
the potential importance of EIF2AK2 in pancreatic cancer and shed
light on the methods by which EIF2AK2 may interact with the tumor
immune system.
Materials and methods
EIF2AK2 gene expression analysis
UCSC XENA (https://xenabrowser.net/datapages/) Functional Genome
Browser, with 1098 public datasets from 91 cohorts including TCGA,
ICGC, TARGET, GTEx and CCLE, is a next-generation online data
analysis and visualization platform that integrates analysis,
visualization, and Galaxy. Therefore, the pancancer analysis and
the prognostic outcome relied on RNA sequencing data from TCGA
(dataset no. TCGA-PAAD.htseq_fpkm.tsv; 178 human PAAD tumors and 4
non-malignant pancreas samples) and the GTEx (dataset no.
gtex_gene_expected_count, 167 non-malignant pancreas samples)
databases, both of which were available from UCSC XENA (22–24).
Additionally, EIF2AK2 expression data from normal and tumor tissues
were obtained from GEO datasets (https://www.ncbi.nlm.nih.gov/geo/) (25,26):
GSE15471 (pairs of normal and tumor tissue samples were obtained at
the time of surgery from resected pancreas of 36 pancreatic cancer
patients) (27), GSE16515 (this
consists of 36 tumor samples and 16 normal samples; a total of 52
samples; 16 samples consist of both tumor and normal expression
data, whereas 20 samples consist of only tumor data) (28), GSE32676 (42 human PDAC tumors and 7
non-malignant pancreas samples snap-frozen at the time of surgery
were chosen)(29) and GSE62165 (118
human PDAC tumors and 13 non-malignant pancreas samples snap-frozen
at the time of surgery were chosen) (30), in order to assess their expression
differences. UALCAN online analysis software (http://ualcan.path.uab.edu/index.html)
was additionally employed to examine EIF2AK2 protein levels.
Ultimately, the protein expression levels of EIF2AK2 in tumors and
normal tissues were verified using The Human Protein Atlas database
(THPA; http://www.proteinatlas.org/).
Cells and reagents
The hTERT-HPNE, MIA PaCa-2, PANC-1, AsPC-1 and
SW1990 cell lines were purchased from The Cell Bank of Type Culture
Collection of The Chinese Academy of Sciences. E.Z.N.A. Total RNA
Kit I kit was purchased from Omega Bio-Tek Co., Ltd. (cat. no.:
R6834-01). Evo M-MLV Reverse Transcription Premix Kit (cat. no.
AG11728), SYBR Green Pro Taq HS Premix qPCR Kit (cat. no. AG11701)
and ROX Reference Dye (cat. no. AG11703) were purchased from
Accurate Biology Co., Ltd. LV-EIF2AK2-RNAi Lentivirus and the
corresponding RNAi-negative lentivirus were purchased from Shanghai
GeneChem Co., Ltd. (cat. no. GIEL0368481. The generation system was
a second generation self-inactivating lentiviral packaging system
and the supplier of the interim cell line used (293T cells) was
Shanghai GeneChem, Co., Ltd. The GV quantity of lentiviral plasmid
was vector Plasmid: 20 µg, pHelper 1.0 vector plasmid: 15 µg,
pHelper 2.0 vector plasmid: 10 µg). Anti-EIF2AK2 (1:1,000; cat. no.
18244-1-AP; Proteintech Group, Inc.), anti-AKT (1:1,000; cat. no.
BS-2720R; BIOSS), anti-phosphorylated (p)-AKT (1:1,000; cat. no.
GTX121937; GeneTex, Inc.) and anti-GAPDH (1:1,000; cat. no.
ab128915; Abcam) were used in the present study. The 5X protein
loading buffer, electrophoresis solution and Tris-Glycine Transfer
Buffer were purchased from Wuhan Servicebio Technology Co., Ltd.
The PVDF membrane was purchased from Millipore Sigma.
Cell culture and infection
Human normal pancreatic cell lines (hTERT-HPNE) and
human pancreatic cancer cell lines (MIA PaCa-2, PANC-1 and SW1990)
were cultured in DMEM (cat. no. SH30243.01; Cytiva) with 10% fetal
bovine serum (cat. no. AB-FBS-0500; ABW) and the AsPC-1 pancreatic
cancer cell line was cultured with 1640 medium (Cytiva; cat. no.
SH30809.01) containing 10% fetal bovine serum. All cell lines were
grown in a 37°C and 5% CO2 constant-temperature
incubator. The cells were digested with trypsin and 2 ml complete
medium was aspirated and mixed to make a single-cell suspension.
Then, 200–300 µl single-cell suspension was added to a 6-well cell
culture plate and the medium was replenished to 2 ml. The 6-well
cell culture plate was removed from the constant-temperature
incubator on alternate days, and the cell status and density were
observed under an inverted microscope.
Lentiviral infections was performed when the cell
confluence was 70%. The virus was infected with the cells at a
concentration of 3.0×109 TU/ml, and the lentiviral
transfection reagent HiTrans G P/A was also added in 25 µl of each
reagent. After 12h, fluorescence expression was observed under a
biological inverted microscope (RCX41; Ningbo Sunny Precision
Industry Co., Ltd). When the cell density reached 90%, 4 ml of DMEM
medium containing 2 µg/ml (PANC-1; MOI=2) puromycin was added, and
the culture continued to be incubated at 37°C for 24 h. After 48 h
of incubation, the expression of the green fluorescent protein was
then observed under a fluorescence microscope to ensure a stable
infection. By using RT-quantitative PCR (RT-qPCR), highly efficient
transfected cells were obtained for further experiments. The
primers used for the assay were as follows: EIF2AK2 forward,
5′-GGCATTCAGCTCCACACTTG-3′and reverse, 5′-ACAGACGAGTGATACCAGCG-3′;
GAPDH forward, 5′-AGGGCTGCTTTTAACTCTGGT-3′ and reverse,
5′-CCCCACTTGATTTTGGAGGGA-3′; EIF2AK2-RNAi (115396–2): 5′-GAAGGTGAAGGTAGATCAAAG-3′;
EIF2AK2-RNAi (115397–1):
5′-GGAATTACATAGGCCTTATCA-3′; EIF2AK2-RNAi (115398–1): 5′GACAGTTTAAACAGTTCTTCG-3′;
RNAi-negative control: 5′-TTCTCCGAACGTGTCACGT-3′.
RT-qPCR
RT-qPCR was used to examine the mRNA expression
levels of EIF2AK2 in pancreatic cell lines and to assess knockdown
efficiency after infection of PANC-1 cells for 48 h. Total RNA was
extracted from cells with E.Z.N.A. Total RNA Kit I and cDNA was
synthesized according to the Evo M-MLV Reverse Transcription
Premixed Kit. qPCR was performed to amplify the cDNA using the Evo
M-MLV Reverse Transcription Premix Kit with the primers listed in
Table SI. The following
thermocycling conditions were used: Stage 1, 95°C for 30 sec; Stage
2, 95°C for 5 sec and 60°C for 30 sec, 40 cycles; Stage 3
(dissociation curve), 95°C for 15 sec, 60°C for 1 min and 95°C for
15 sec. Relative gene expression was calculated using the
2−ΔΔCq method (31) and
GAPDH was used as the control gene.
Western blotting
Cells were lysed by radioprecipitation with lysis
buffer containing RIPA lysate (cat. no. G2002; Wuhan Servicebio
Technology Co., Ltd.) and 1% phenylmethylsulfonyl fluoride
(MilliporeSigma) for 30 min at 4°C. The total protein lysate was
then collected and the concentration determined using a BCA protein
assay kit (cat. no. PC0020; Wuhan Servicebio Technology Co., Ltd.).
Subsequently, denatured proteins (30 µg/lane) were separated on a
12% SDS-PAGE gel and transferred to a PVDF membrane (cat. no.
IRVH00010; MilliporeSigma). After being blocked with 5% skimmed
milk (cat. no. D8340, Solarbio) for 1.5 h at room temperature, the
membranes were incubated with EIF2AK2 primary antibody, AKT and
p-AKT overnight at 4°C, followed by goat anti-rabbit (dilution
1:3,000; cat. no. RS0002; ImmunoWay Biotechnology Company)
secondary antibody conjugated to horseradish peroxidase for 1 h at
room temperature. The densities of the specific protein bands were
visualized and captured using Image J (National Institutes of
Health, v.1.8.0).
Clinical sample collection
A total of 48 paraffin-embedded tumor tissue samples
and 48 paraffin-embedded adjacent tissues collected between
September 2020 and August 2022 were obtained from The First
Hospital of Lanzhou University (Gansu, China). The
clinicopathological data of 48 patients with pancreatic cancer from
the First Hospital of Lanzhou University were extracted, which
showed a total of 23 males (47.9%) and 25 females (52.1%), with a
mean age of 62 years and an age range of 37–83 years. All patients
had a postoperative pathological diagnosis of pancreatic ductal
cell carcinoma, and none received chemotherapy or radiation
therapy. All patients provided written informed consent and the
present study was approved by the Ethics Committee of the First
Hospital of Lanzhou University (approval no. LDYYLL2023-304).
Immunohistochemical staining
Pathological specimens (paraffin-embedded sections
on glass slides) were collected, and underwent dewaxing, hydration
(dewaxing and hydration of paraffin sections: Xylene I and II for
25 min each, different gradients of ethanol 100, 95, 90, 85, 80,
70% for 10 min each) and antigen retrieval (sodium citrate at 95°C
twice for 5 min each, followed by 3 washes with PBS for 5 min
each). Subsequently, 3% hydrogen peroxide was incubated for 15 min
followed by dropwise closure with normal goat serum and incubation
at 37°C for 30 min. The sections were incubated with EIF2AK2
primary antibody (1:100, cat. no. 18244-1-AP; Proteintech Group,
Inc.) overnight at 4°C. Then the sections were incubated with the
EIF2AK2 secondary antibody (1:100, cat. no. 18244-1-AP; Proteintech
Group, Inc.) at 37°C for 30 min, followed by the addition of a
tertiary antibody (horseradish peroxidase labelled streptavidin
working solution, cat. no. SP-9001; Broad Spectrum.) and incubation
at 37°C for 30 min. DAB (1:20) color development was carried out
for 8 min and observed under the microscope. Hematoxylin
re-staining was carried out at room temperature for 60 sec,
followed by gradient alcohol dehydration (70% for 5 min, 80% for 5
min, 85% for 5 min, 90% for 5 min, 95% for 10 min, and 100% for 10
min). Finally, the slices were cleared with xylene (xylene I and II
for 25 min each) before being sealed with neutral resin. The
results were observed and analyzed: Three fields were randomly
selected under the fluorescence microscope (Leica DM2500; Leica
Microsystems GmbH) and the images were analyzed by Image-Pro Plus
software (Media Cybernetics, Inc. v.6.0). SP Kit (Broad Spectrum,
cat. no. SP-9001) and DAB Substrate kit (cat. no. ZLI-9018) were
purchased from OriGene Technologies, Inc. Hematoxylin (cat. no.
G1080), neutral gum (cat. no. G8590) and 0.01 M sodium citrate
buffer (cat. no. C1010) were purchased from Beijing Solarbio
Science & Technology Co., Ltd.
Cell Counting Kit-8 (CCK-8) assay
After trypsin digestion of the cells, 100 µl
(~2×103 cells/100 µl) cell suspension was added to each
well of a 96-well plate. The plates were incubated in a 37°C
incubator for 3–4 h until the cells were fully attached to the
plates. After incubation at 37°C for 12, 24, 48 and 72 h, 10 µl of
CCK-8 solution (cat. no. CA1210; Beijing Solarbio Science &
Technology Co., Ltd.) was added to each well and incubated for a
further 3 h. Absorbance at 450 nm was measured with a
spectrophotometer (Epoch; BioTek Instruments, Inc.).
Wound-healing assay
When the cell density of the 6-well plate reached
~90%, the state of the cells was observed using an inverted
microscope (RCX41; Ningbo Sunny Precision Industry Co., Ltd). The
cells were then scratched using a 100-µl pipette tip, were washed
three times with PBS to remove the scratched cells and incubated at
37°C in a 5% CO2 incubator with 1% serum-containing medium
(32). Images of the experimental
and control groups were captured at 0 and 24 h. Image-Pro Plus 6.0
(Media Cybernetics) was used for assessing the relative width of
the wound. Wound healing area was calculated as Final width/Initial
width.
Flow cytometric analysis of the cell
cycle and apoptosis
To assess apoptosis, PANC-1 cells were collected
after 48 h of infection. PANC-1 cells were washed with PBS,
followed by suspension in 1.5 ml centrifuge tube, centrifuged at
100 × g at room temperature for 5 min. After centrifugation, cells
were washed with 1 ml PBS, resuspended with 100 µl 1X binding
buffer, and filtered through a 70 µm cell sieve. PE staining
solution (5 µl) was added and incubated at room temperature for 5
min, then 7-AAD staining solution (10 µl) added and incubated at
room temperature for 20 min. Finally, the apoptosis rate
(percentage of early apoptotic + late apoptotic cells) was examined
using flow cytometry (CytoFLEX; Beckman Coulter, Inc.) and analysis
with CytExpert software v.2.4 (Beckman Coulter, Inc.). Annexin-V
PE/7-AAD/Apoptosis Detection Kit (cat. no. CA1030) was purchased
from Beijing Solarbio Science & Technology Co., Ltd.
To assess the cell cycle, following infection PANC-1
cells were washed with PBS to collect cell suspension in 1.5 ml
centrifuge tube, centrifuged at 100 × g at room temperature for 5
min. Cells were collected by adding 1 ml PBS to be washed again,
and 500 µl 70% ethanol was added to fix the cells at room
temperature for 2 h. Centrifugation was carried out at 100 × g at
room temperature for 5 min. PBS (1 ml) was added to wash off the
residual fixation solution. RNase A solution (100 µl) was added to
resuspend the cells at 37°C for 30 min. PI staining solution (400
µl) was added, mixed well and incubated at 4°C in the dark for 30
min. Finally, the cell cycle was detected with a flow cytometer
(CytoFLEX, Beckman Coulter, Inc.) and analyzed with CytExpert
software v.2.4 (Beckman Coulter, Inc.). DNA Content Quantitation
Assay (Cell Cycle; cat. no.: CA1510) purchased from Beijing
Solarbio Science & Technology Co., Ltd.
Prognostic analysis
First, survival studies of overall survival (OS) and
disease-specific survival (DSS) were performed to elucidate the
prognostic value of EIF2AK2. In TCGA dataset, RNA sequencing data
and accompanying clinical data were gathered and visualized using
receiver operating characteristic (ROC) and Kaplan-Meier curves.
Pancreatic cancer patients were categorized into low-risk and
high-risk groups based on the median expression of EIF2AK2.
P-values and hazard ratios (HR) and 95% confidence intervals (CI)
were derived by log-rank test and univariate Cox proportional
hazards regression. The association between EIF2AK2 expression, and
OS and DSS rates in patients with pancreatic carcinoma from TCGA
database was also assessed using univariate and multivariate
regression models. Finally, a personalized nomogram was drawn up to
predict the OS and DSS of patients with malignant neoplasms of the
pancreas, which comprises calibration plots and critical clinical
data.
Functional enrichment analysis
The differential expression of EIF2AK2 in pancreatic
cancer data in the TCGA database was assessed using the limma
package (https://bioconductor.org/packages/release/bioc/html/limma.html,
v.4.3). To account for false-positive results, adjusted P-values
were used. Adjusted P<0.05 and |log2 (fold change)|>1 were
established as criteria for distinguishing differentially expressed
genes (DEGs). The results of this analysis were analyzed using the
ClusterProfiler package (https://bioconductor.org/packages/clusterProfiler/;
v.3.14.3) for Gene Ontology (GO) and the Kyoto Encyclopedia of
Genes and Genomes (KEGG) to further determine the crucial
biological functions of EIF2AK2.
Gene set enrichment analysis
(GSEA)
To examine functional and pathway differences
between the EIF2AK2 high and low expression groups (33), GSEA analyses of GSE15471 in the GEO
database were performed using ClusterProfiler. According to the
median expression of EIF2AK2, samples were classified as high or
low EIF2AK2 levels. The gene sets were sorted 1,000 times for each
analysis to obtain more accurate results. Adjusted P-values
<0.05 and FDR values <0.25 were considered statistically
significant.
Immune cell infiltration and immune
checkpoints correlated with EIF2AK2
TIMER (https://cistrome.shinyapps.io/timer/; v.2.0) was used
to examine the correlation of EIF2AK2 expression with immune cell
infiltration and immune cell biomarkers. Immunological infiltration
analysis of EIF2AK2 was performed by single-sample gene set
enrichment analysis (ssGSEA) using the GSVA (https://github.com/rcastelo/GSVA; v.4.3) package in R.
A total of 24 infiltrating immune cells were analyzed for
correlation with EIF2AK2. Finally, the association between EIF2AK2
expression and pancreatic cancer immune checkpoints was evaluated.
P<0.05 was considered to indicate a statistically significant
difference.
Statistical methods
R (https://www.r-project.org/; v.3.6.3) and SPSS (IBM
Corp.; v.23.0.) were used to perform statistical analysis.
Experimental data from three replicates are presented as the mean ±
standard deviation. Comparisons between two groups were made using
paired two-tailed Student's t-test, and comparisons between
multiple groups were made using one-way ANOVA with Dunnett's
post-hoc test. Pearson χ2 test was used to analyze the
association between EIF2AK2 expression levels and
clinicopathological characteristics. Cox regression and
Kaplan-Meier analyses were used to evaluate prognostic factors.
Multi-factorial Cox analysis was used to compare the effect of
EIF2AK2 expression and other clinical characteristics on survival.
Median EIF2AK2 expression was used as the cut-off value. In
addition, ROC analysis was performed using the pROC package
(https://www.rdocumentation.org/packages/pROC/versions/1.17.0.1;
v.1.17.0.1) to assess the effectiveness of EIF2AK2 transcript
expression in differentiating between pancreatic cancer and healthy
samples. Area under the curve (AUC) values were calculated between
0.5-1.0, indicating an identification capacity of 50–100%.
Spearman's test was used to analyze the correlation between EIF2AK2
expression levels and immune cell infiltration. P<0.05 was
considered to indicate a statistically significant difference in
all tests.
Results
EIF2AK2 expression is elevated in
pancreatic cancer
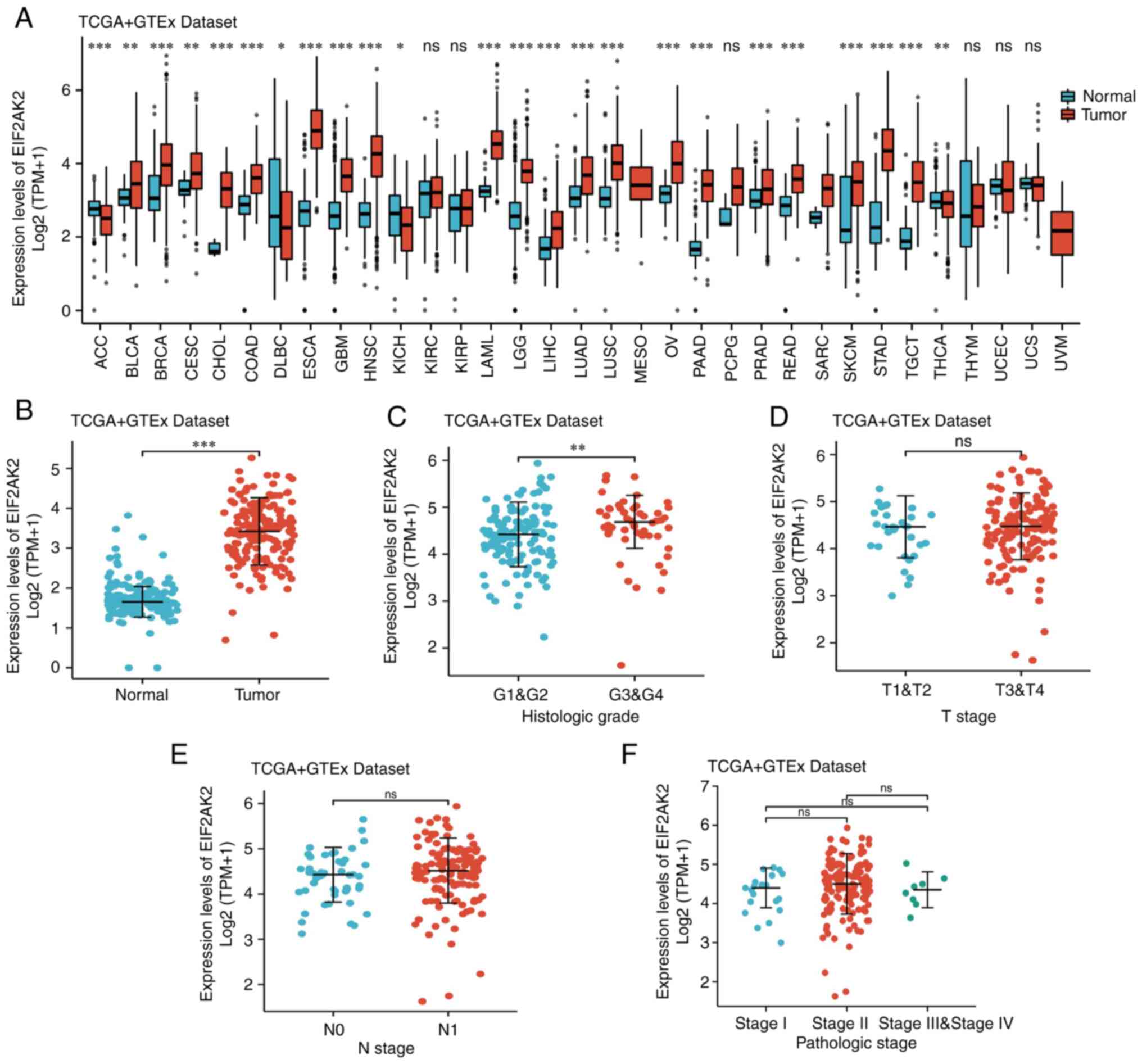
The results of pancancer analysis of EIF2AK2
revealed that the mRNA expression levels of EIF2AK2 were increased
in adrenocortical carcinoma, bladder urothelial carcinoma, breast
invasive carcinoma, cholangiocarcinoma, colon adenocarcinoma/rectal
adenocarcinoma, esophageal carcinoma, glioblastoma multiforme,
liver hepatocellular carcinoma, lung squamous cell carcinoma,
rectal adenocarcinoma, thyroid carcinoma, lung adenocarcinoma,
prostate adenocarcinoma and pancreatic adenocarcinoma (Fig. 1A). After removing the samples with
zero expression value, combined with pancreatic cancer samples from
the TCGA database and normal pancreatic tissue samples from the
GTEx database, the results showed that the expression level of
EIF2AK2 was significantly elevated in pancreatic cancer tissues
when compared to normal pancreatic tissues (tumor: 3.21±0.85,
normal: 1.20±0.55, P<0.0001; Fig.
1B). Further analysis indicated statistically significant
differences in EIF2AK2 expression between histological grades
(P<0.05), but not between T, N or pathological stages
(P>0.05; Fig. 1C-F).
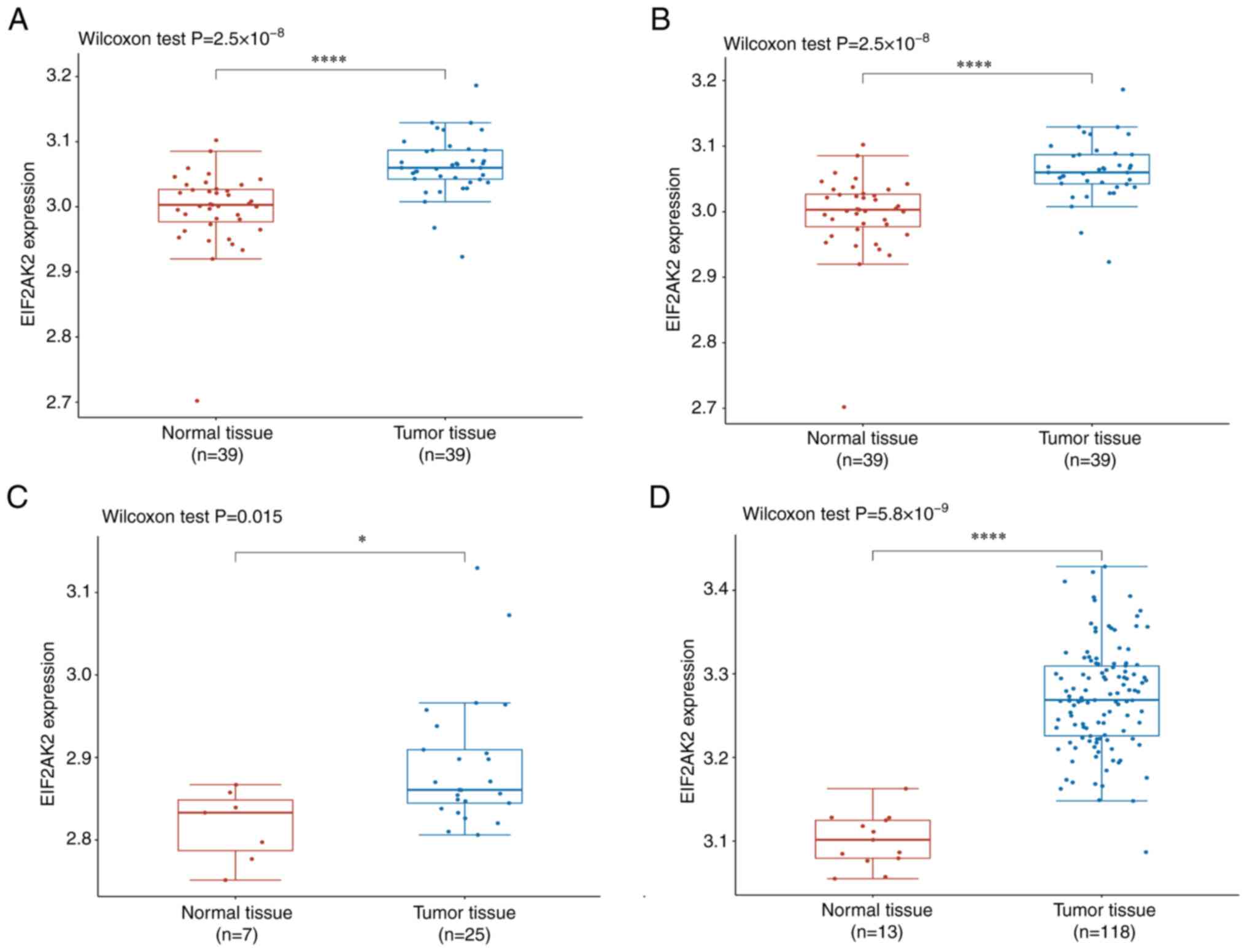
The higher expression of EIF2AK2 in pancreatic tumor
tissues compared with normal pancreatic tissues was verified in the
GSE15471, GSE16515, GSE32676 and GSE62165 datasets. (P<0.05;
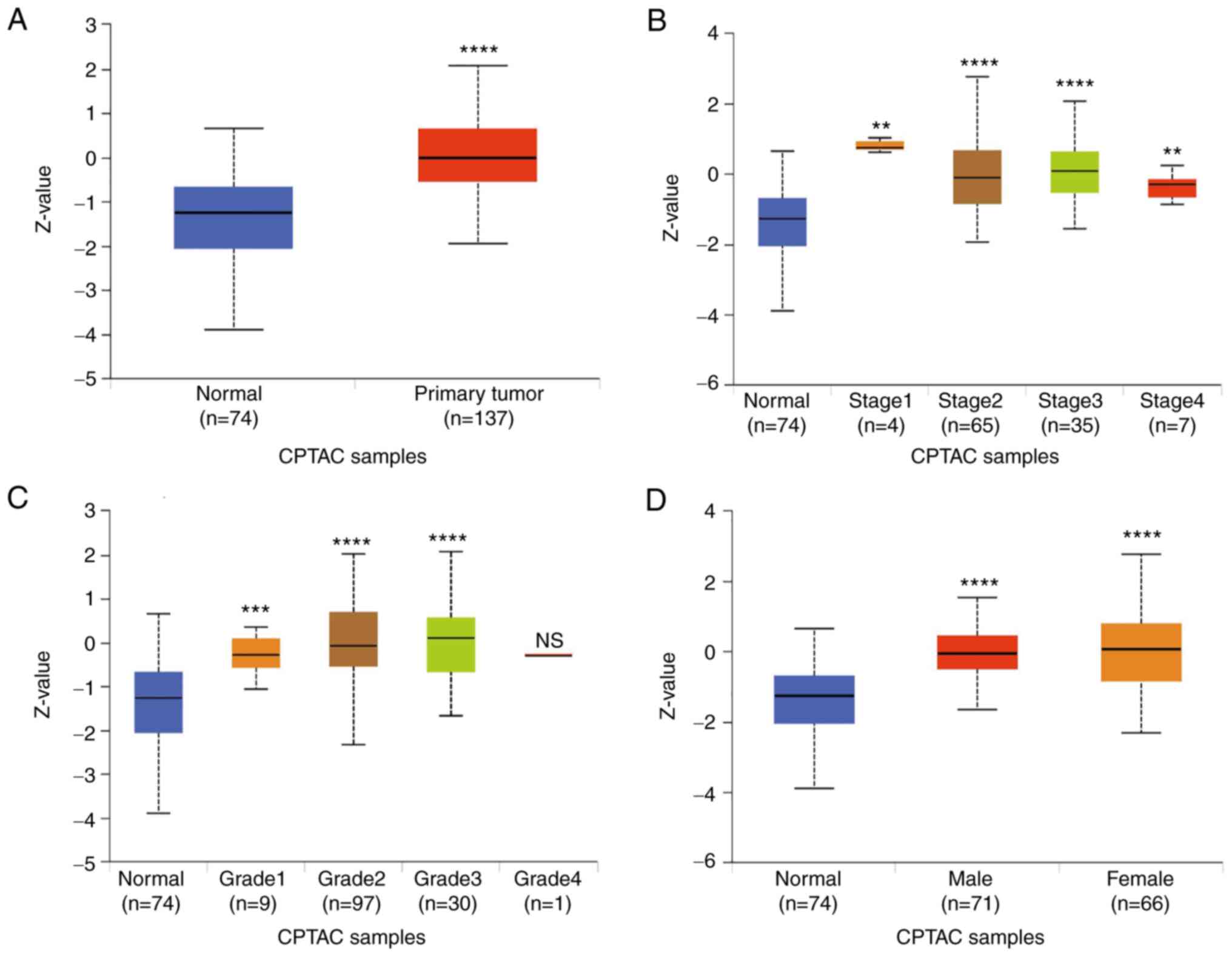
Fig. 2). In addition, the
expression of EIF2AK2 was analyzed in the UALCAN online tumor
database website, where high EIF2AK2 expression was associated with
gene expression, tumor grade, stage and gender in patients with
pancreatic cancer (Fig. 3).
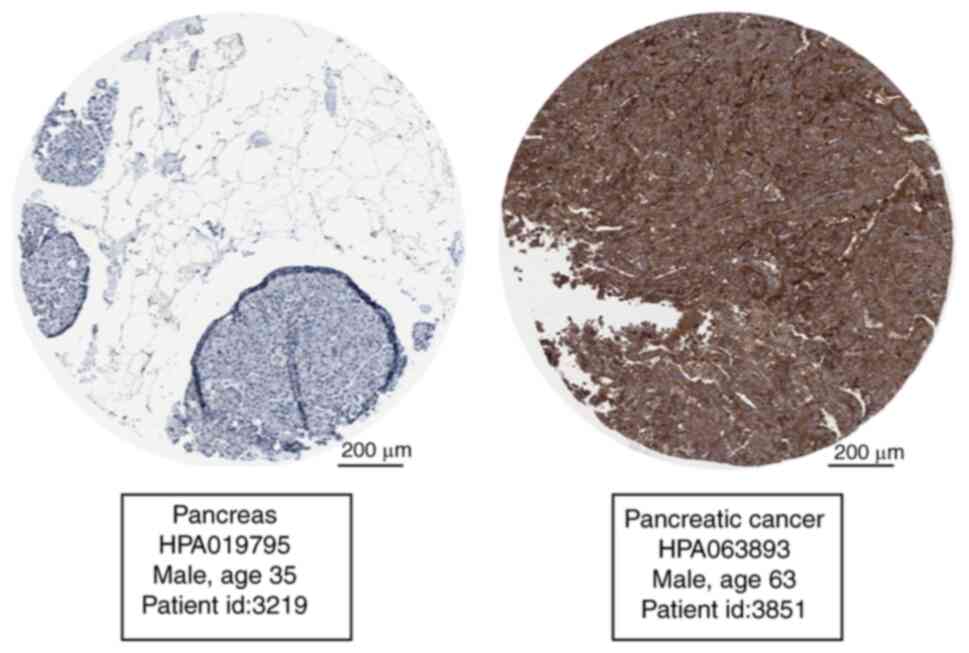
Subsequently, THPA database was used to verify EIF2AK2 expression
in pancreatic cancer and normal tissues. The expression levels of
EIF2AK2 in pancreatic cancer tissues were substantially higher
compared with those in normal tissues (Fig. 4).
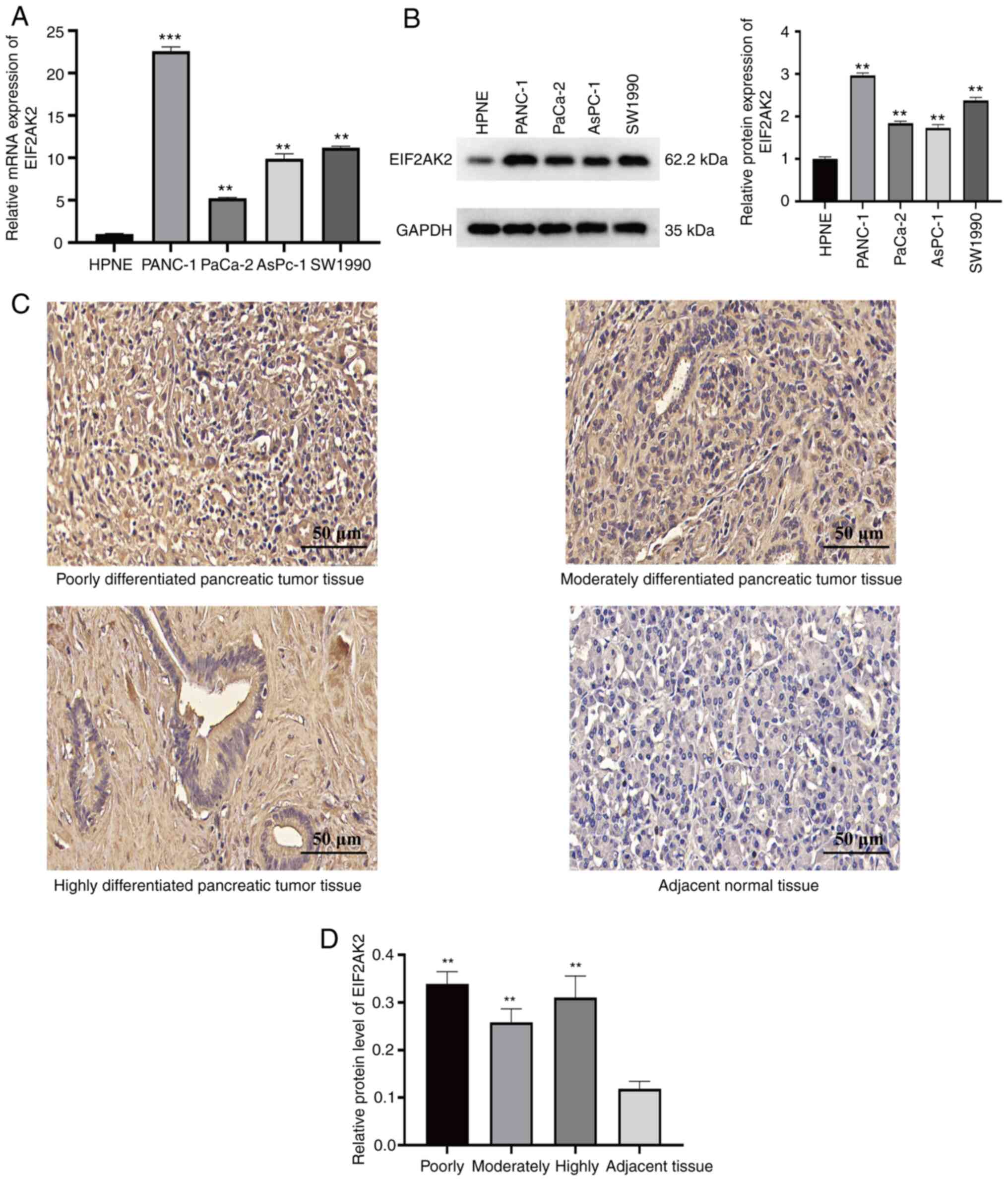
To validate EIF2AK2 expression in pancreatic cancer
cells in vitro, normal pancreatic cells were compared with
four distinct pancreatic cancer cell lines. RT-qPCR and western
blotting results indicated that the pancreatic cancer cell lines
had significantly higher mRNA and protein expression levels of
EIF2AK2 compared with those in normal pancreatic cells (Fig. 5A and B). To further examine the
expression characteristics of EIF2AK2 in pancreatic carcinoma,
immunohistochemical staining of pancreatic tumor tissues and
adjacent normal tissues from 48 patients was performed. Notably,
EIF2AK2 was revealed to be localized in the nucleus. The results
showed that EIF2AK2 was weakly positive in paracancerous tissues,
but was highly expressed in pancreatic cancer tissues (Fig. 5C). Furthermore, as shown in Fig. 5D, EIF2AK2 expression was
considerably higher in highly, moderately and poorly differentiated
pancreatic cancer compared with that in adjacent pancreatic
tissues. These findings indicated that EIF2AK2 may be highly
expressed in pancreatic cancer.
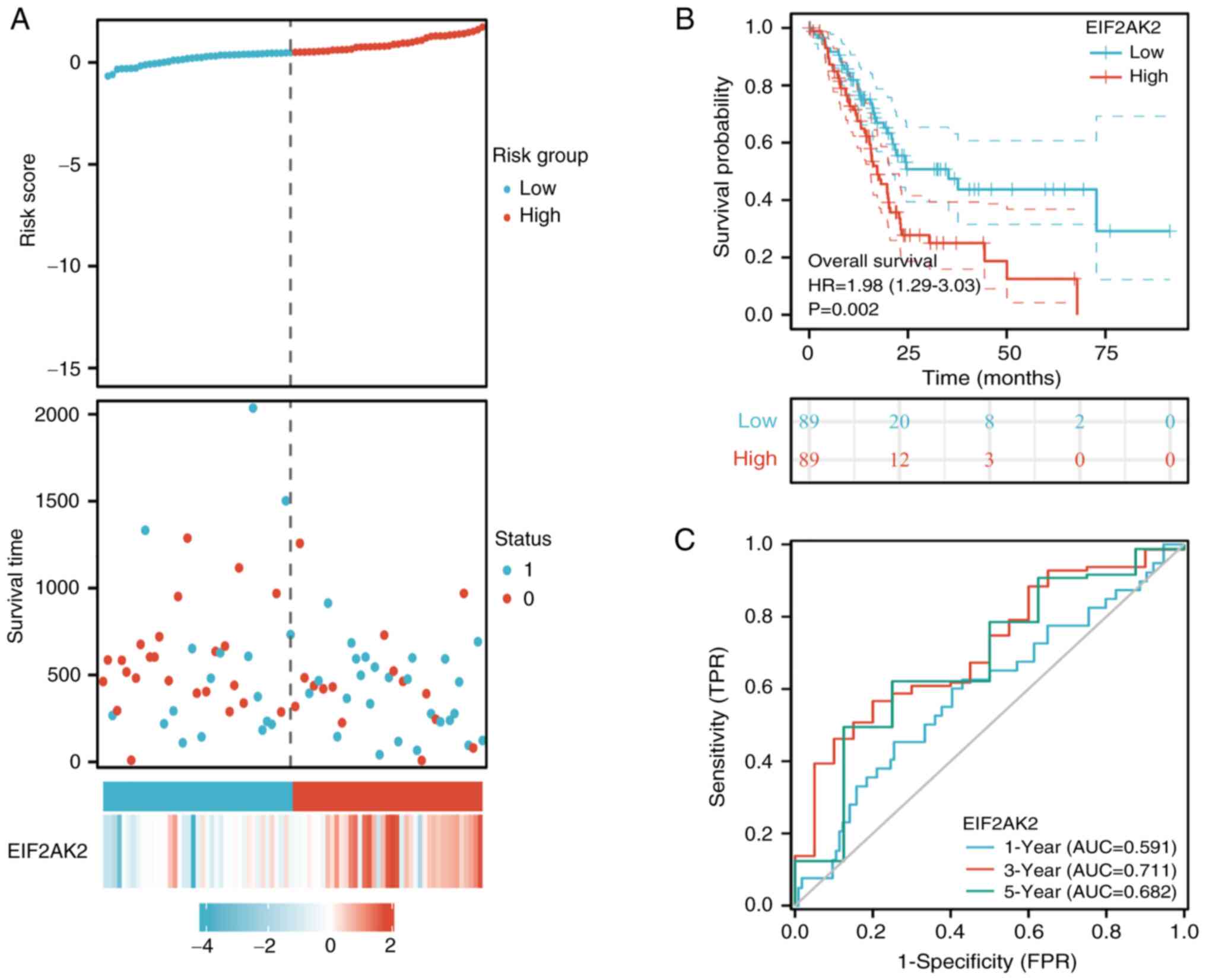
Prognostic relevance of EIF2AK2
To predict the relationship between EIF2AK2
expression level and survival in patients with pancreatic cancer,
the link between EIF2AK2 expression and the prognosis of patients
with pancreatic cancer was evaluated. Notably, the expression of
EIF2AK2 was substantially linked with OS in patients with
pancreatic cancer. Based on median EIF2AK2 expression, patients
were separated into high expression and low expression groups.
Combining the risk profile and survival status, it was found that
the fatality rate was considerably greater in the EIF2AK2
high-expression group compared with that in the low-expression
group. Considering the risk profile and survival together, the
mortality rate in the EIF2AK2 high-expression group was
significantly higher than that in the low-expression group
(Fig. 6A). High expression of
EIF2AK2 was substantially linked with a poor prognosis, based on
the Kaplan-Meier survival analysis (HR=1.98, 95% CI=1.29-3.03,
P=0.002; Fig. 6B). To observe the
predictive value of EIF2AK2 mRNA expression in prognosis, EIF2AK2
expression was assessed using ROC curves to distinguish between
EIF2AK2-high and EIF2AK2-low patients. It was determined that
evaluating the area under the curve (AUC) under the ROC curve to
estimate the risk of patients with pancreatic cancer at 1, 3 and 5
years was the most effective measure (1-year AUC, 0.591; 3-year
AUC, 0.711; 5-year AUC, 0.682) (Fig.
6C).
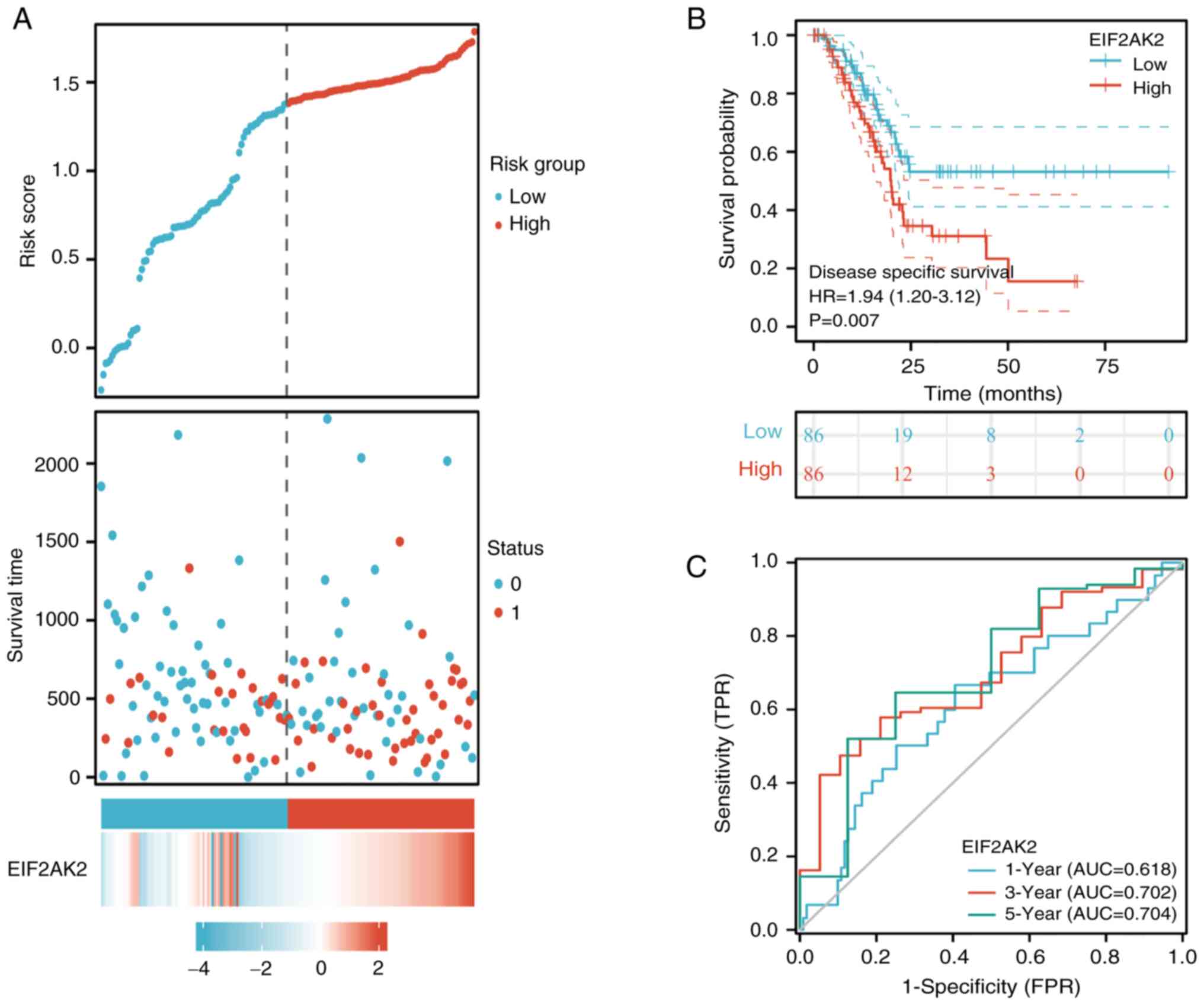
In addition, the relationship between EIF2AK2
expression and DSS in patients with pancreatic cancer was analyzed.
Considering the risk profile and survival together, the mortality
rate in the EIF2AK2 high-expression group was significantly higher
than that in the low-expression group (Fig. 7A). The assessment of the connection
between EIF2AK2 expression and DSS illustrated that EIF2AK2
expression not only affected the DSS of patients with pancreatic
cancer (HR=1.94, 95% CI=1.20-3.12, P=0.007; Fig. 7B) but also predicted overall risk
(1-year AUC, 0.618; 3-years AUC, 0.702; 5-year AUC,=0.704; Fig. 7C. Taken together, these consistent
OS and DSS outcomes strongly indicated that the EIF2AK2 gene is
related to the prognosis of patients with pancreatic cancer.
In addition, as shown in Table I, the univariate Cox analysis
revealed that high EIF2AK2 levels, and high T, N and pathological
stages were associated with OS (P<0.05). In the multivariate Cox
analysis, N stage and EIF2AK2 expression represented independent
components associated with OS in patients with pancreatic cancer.
Similarly, as shown in Table II,
the univariate Cox analysis revealed that high EIF2AK2 levels, and
high T, N and pathological stages were associated with DSS events
(P<0.05). In the multivariate Cox analysis, only N stage was an
independent factor associated with DSS in patients with pancreatic
cancer.
 | Table I.Association of EIF2AK2 expression and
other clinicopathological factors with OS calculated via univariate
and multivariate Cox regression analyses. |
Table I.
Association of EIF2AK2 expression and
other clinicopathological factors with OS calculated via univariate
and multivariate Cox regression analyses.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Characteristic | N | HR (95% CI) | P-value | N | HR (95% CI) | P-value |
|---|
| T stage | 176 |
| 0.03 | 176 |
| 0.249 |
| T1 and
T2 | 31 | Reference |
| 31 | Reference |
|
| T3 and
T4 | 145 | 2.023
(1.072-3.816) |
| 145 | 1.798
(0.663-4.877) |
|
| N stage | 173 |
| 0.004 | 173 |
| 0.04 |
| N0 | 50 | Reference |
| 50 | Reference |
|
| N1 | 123 | 2.154
(1.282-3.618) |
| 123 | 1.969
(1.033-3.753) |
|
| Pathological
stage | 175 |
| 0.037 | 175 |
| 0.307 |
| Stage
I | 21 | Reference |
| 21 | Reference |
|
| Stage
II, Stage III and Stage IV | 154 | 2.291
(1.051-4.997) |
| 154 | 0.491
(0.125-1.926) |
|
| Sex | 178 |
| 0.311 |
|
|
|
|
Female | 80 | Reference |
|
|
|
|
|
Male | 98 | 0.809
(0.537-1.219) |
|
|
|
|
| Age | 178 |
| 0.227 |
|
|
|
| ≤65
years | 93 | Reference |
|
|
|
|
| >65
years | 85 | 1.290
(0.854-1.948) |
|
|
|
|
| Histological
grade | 176 |
| 0.052 |
|
|
|
| G1 and
G2 | 126 | Reference |
|
|
|
|
| G3 and
G4 | 50 | 1.538
(0.996-2.376) |
|
|
|
|
| EIF2AK2 | 178 |
| 0.002 | 178 |
| 0.042 |
|
Low | 89 | Reference |
| 89 | Reference |
|
|
High | 89 | 1.981
(1.294-3.032) |
| 89 | 1.585
(1.017-2.470) |
|
 | Table II.Association of EIF2AK2 expression and
other clinicopathological factors with DSS in pancreatic cancer
calculated via univariate and multivariate Cox regression
analyses. |
Table II.
Association of EIF2AK2 expression and
other clinicopathological factors with DSS in pancreatic cancer
calculated via univariate and multivariate Cox regression
analyses.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Characteristic | N | HR (95% CI) | P-value | N | HR (95% CI) | P-value |
|---|
| T stage | 170 |
| 0.008 | 170 |
| 0.1 |
| T1 and
T2 | 30 | Reference |
| 30 | Reference |
|
| T3 and
T4 | 140 | 3.119
(1.346-7.229) |
| 140 | 3.210
(0.800-12.875) |
|
| N stage | 167 |
| 0.001 | 167 |
| 0.024 |
| N0 | 48 | Reference |
| 48 | Reference |
|
| N1 | 119 | 2.746
(1.473-5.121) |
| 119 | 2.368
(1.120-5.004) |
|
| Pathological
stage | 169 |
| 0.023 | 169 |
| 0.297 |
| Stage
I | 20 | Reference |
| 20 | Reference |
|
| Stage
II, Stage III and Stage IV | 149 | 3.249
(1.175-8.979) |
| 149 | 0.379
(0.061-2.347) |
|
| Sex | 172 |
| 0.227 |
|
|
|
|
Female | 76 | Reference |
|
|
|
|
|
Male | 96 | 0.715
(0.473-1.194) |
|
|
|
|
| Age | 172 |
| 0.784 |
|
|
|
| ≤65
years | 92 | Reference |
|
|
|
|
| >65
years | 80 | 1.067
(0.670-1.701) |
|
|
|
|
| Histological
grade | 170 |
| 0.053 |
|
|
|
| G1 and
G2 | 122 | Reference |
|
|
|
|
| G3 and
G4 | 48 | 1.616
(0.994-2.628) |
|
|
|
|
| EIF2AK2 | 172 |
| 0.007 | 172 |
| 0.157 |
|
Low | 86 | Reference |
| 86 | Reference |
|
|
High | 86 | 1.935
(1.202-3.115) |
| 86 | 1.425
(0.872-2.329) |
|
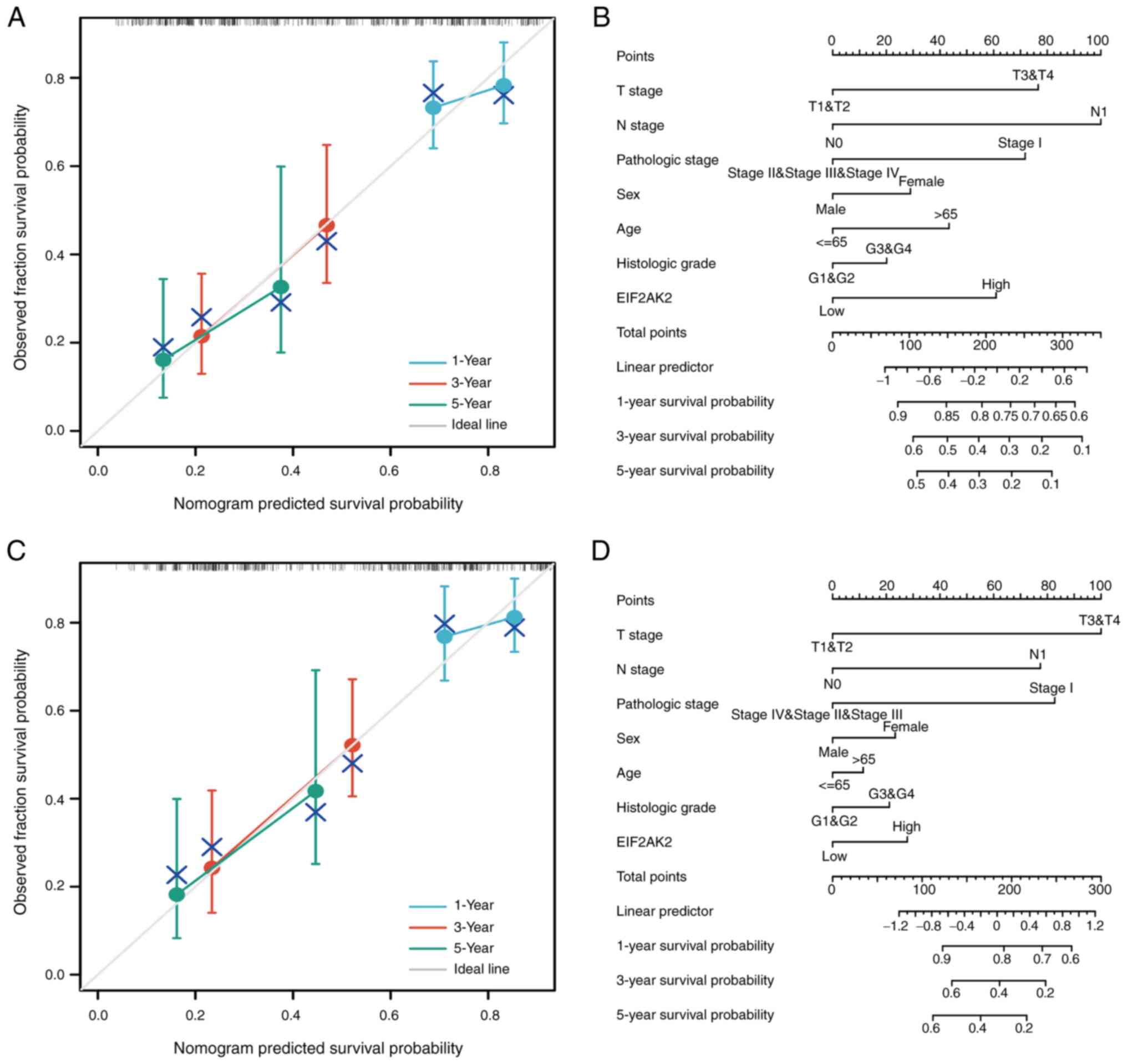
Through the integration of clinicopathological
factors (including T stage, N stage, pathological stage and EIF2AK2
expression), a nomogram model was generated from the results of the
OS and DSS analyses, which can be employed to accurately measure
the survival probabilities at 1-, 3- and 5-years in clinical
settings (Fig. 8).
Functional inference of EIF2AK2
To further confirm the putative biological roles of
the EIF2AK24 gene, a functional enrichment analysis was performed
using TCGA transcriptome data. Based on the degree of EIF2AK2
expression, pancreatic cancer samples were categorized as
EIF2AK2high or EIF2AK2low. Next, the DEGs
analyzed in the EIF2AK2high and EIF2AK2low
groups were identified using the following criteria: |log2FC|>1,
adjusted P<0.05. A total of 1,318 genes exhibited different
expression levels, 209 upregulated genes and 1,109 downregulated
genes, as indicated by the volcano plot (Fig. 9A). These degrees were analyzed using
a heatmap for hierarchical clustering (Fig. 9B). To determine the possible
function of EIF2AK2, a range of enrichment analyses were performed,
including GO and KEGG. The GO enrichment analysis included three
main functions, namely, biological process, cellular components,
and molecular functions. The biological process mainly included fat
digestion and absorption, Pancreatic secretion, protein digestion
and absorption, salivary secretion, digestion antimicrobial humoral
response, response to food. Molecular functions mainly included
antigen binding, receptor ligand activity, immunoglobulin receptor
binding, humoral immune response, humoral immune response mediated
by circulating immunoglobulin, serine hydrolase activity,
serine-type peptidase activity, serine-type endopeptidase activity.
Cellular components mainly included immunoglobulin complex,
external side of plasma membrane, immunoglobulin complex,
circulating, neuron projection membrane, dendrite membrane, AMPA
glutamate receptor complex. KEGG analysis demonstrated that EIF2AK2
might regulate the process of complement activation, classical
pathway. Obviously, GO and the KEGG results can be concluded to
provide a new direction for tumor immunotherapy research (Fig. 9C and D). Fig. 9E shows the GSEA of all genes
significantly coexpressed with EIF2AK2. Notably, the functions of
these genes significantly coexpressed with EIF2AK2 were mainly
enriched in the cell cycle, degradation of extracellular matrix,
complement system and activation of extracellular goblet B cells by
Sarscov2.
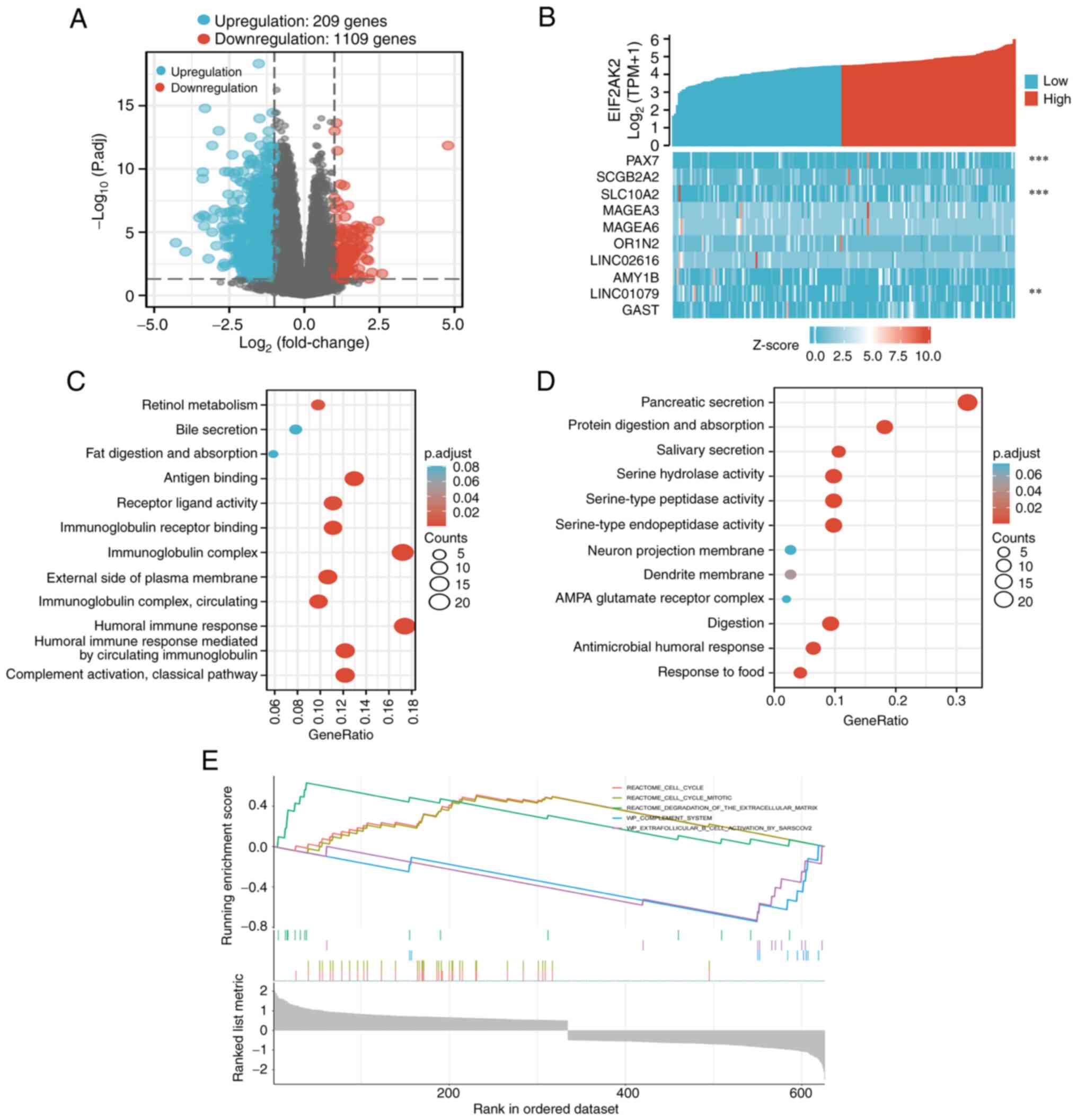 | Figure 9.A total of 1,318 DEGs were identified
as being statistically significant between EIF2AK2 high-expression
and low-expression groups. (A) Volcano plot of DEGs, including 209
upregulated and 1,109 downregulated genes. Normalized expression
levels were shown in descending order from blue to red. (B) Heatmap
of the 10 DEGs, including five upregulated genes and five
downregulated genes. The x-axis represents the samples, while the
y-axis denotes the DEGs. Blue and red represent downregulated and
upregulated genes, respectively. **P<0.01, ***P<0.001 vs.
EIF2AK2. The X-axis represents the samples, while the Y-axis
denotes the differentially expressed RNAs. Blue and red tones
represented down-regulated and up-regulated genes, respectively.
(C) KEGG enrichment and GO enrichment analysis of EIF2AK2
coexpressed upregulated DEGs. (D) KEGG enrichment and GO enrichment
analysis of EIF2AK2 coexpressed downregulated DEGs. (E) Enrichment
plots from the gene set enrichment analysis. DEGs, differentially
expressed genes; EIF2AK2, eukaryotic translation initiation factor
2α kinase 2; KEGG, Kyoto Encyclopedia of Genes and Genomes; GO,
Gene Ontology. |
Association between EIF2AK2 expression
and biomarkers of immune cells
To investigate the relevance of EIF2AK2 in the tumor
immune microenvironment, correlations were established between
EIF2AK2 expression and immune cell biomarkers. As listed in
Table III, EIF2AK2 was positively
correlated with B-cell biomarkers (CD19, CD20 and CD38),
CD8+ T-cell biomarkers (CD8A and CD8B), other T-cell
subsets [follicular helper T cells, T helper (Th)1, Th2, Th9, Th17,
Th22 and regulatory T cells (Tregs)], M1 macrophage biomarkers
(IRF5 and PTGS2), M2 macrophage biomarkers (CD115),
tumor-associated macrophage (TAM) biomarkers (PDCD1LG2, CD80, CD40
and TLR7), natural killer cell biomarkers (CD7 and XCL1),
neutrophil biomarkers (ITGAM and FUT4) and dendritic cell (DC)
biomarkers (CD1C and ITGAX) in pancreatic cancer. These findings
indicated the existence of a direct relationship between EIF2AK2
and immune cell invasion.
 | Table III.Correlation analysis between EIF2AK2
expression and immune cell markers. |
Table III.
Correlation analysis between EIF2AK2
expression and immune cell markers.
| Immune cell | Biomarker | Spearman's
rs value | P-value |
|---|
| TAM | PDCD1LG2 | 0.510 | <0.001 |
|
| CD80 | 0.490 | <0.001 |
|
| CD40 | 0.296 | <0.001 |
|
| TLR7 | 0.394 | <0.001 |
| Natural killer
cell | CD7 | 0.159 | 0.034 |
|
| KIR3DL1 | −0.040 | 0.593 |
|
| XCL1 | 0.265 | <0.001 |
| Neutrophil | CD11b (ITGAM) | 0.366 | <0.001 |
|
| CD15 (FUT4) | 0.326 | <0.001 |
|
| CD66b
(CEACAM8) | 0.185 | 0.013 |
| Dendritic cell | CD1C | 0.201 | 0.007 |
|
| CD11c (ITGAX) | 0.243 | 0.001 |
|
| CD141 (THBD) | 0.339 | <0.001 |
| M1 macrophage | COX2 (PTGS2) | 0.424 | <0.001 |
|
| INOS (NOS2) | 0.207 | 0.006 |
|
| IRF5 | 0.285 | <0.001 |
| M2 macrophage | ARG1 | 0.014 | 0.851 |
|
| CD206 (MRC1) | 0.353 | <0.001 |
|
| CD115 (CSF1R) | 0.373 | <0.001 |
| B cell | CD19 | 0.180 | 0.017 |
|
| CD20 (KRT20) | 0.185 | 0.014 |
|
| CD38 | 0.363 | <0.001 |
| CD8+ T
cell | CD8A | 0.323 | <0.001 |
|
| CD8B | 0.301 | <0.001 |
| Tfh | BCL6 | 0.468 | <0.001 |
|
| ICOS | 0.359 | <0.001 |
|
| CXCR5 | 0.180 | 0.016 |
| Th1 | T-bet (TBX21) | 0.191 | 0.011 |
|
| STAT1 | 0.809 | <0.001 |
|
| STAT4 | 0.230 | 0.002 |
|
| IL12RB2 | 0.130 | 0.085 |
|
| WSX1 (IL27RA) | 0.274 | <0.001 |
|
| IFN-γ (IFNG) | 0.265 | <0.001 |
|
| TNF-a (TNF) | 0.173 | 0.021 |
| Th2 | CCR3 | 0.364 | <0.001 |
|
| GATA3 | 0.296 | <0.001 |
|
| STAT5A | 0.391 | <0.001 |
|
| STAT6 | 0.484 | <0.001 |
| Th9 | IRF4 | 0.296 | <0.001 |
|
| PU.1 (SPI1) | 0.204 | 0.006 |
|
| TGFBR2 | 0.583 | <0.001 |
| Th17 | IL-17A | 0.128 | 0.089 |
|
| IL-21R | 0.356 | <0.001 |
|
| IL-23R | 0.202 | 0.007 |
|
| STAT3 | 0.597 | <0.001 |
| Th22 | AHR | 0.614 | <0.001 |
|
| CCR10 | −0.051 | 0.496 |
| Treg | CCR8 | 0.480 | <0.001 |
|
| CD25 (IL2RA) | 0.429 | <0.001 |
|
| FOXP3 | 0.335 | <0.001 |
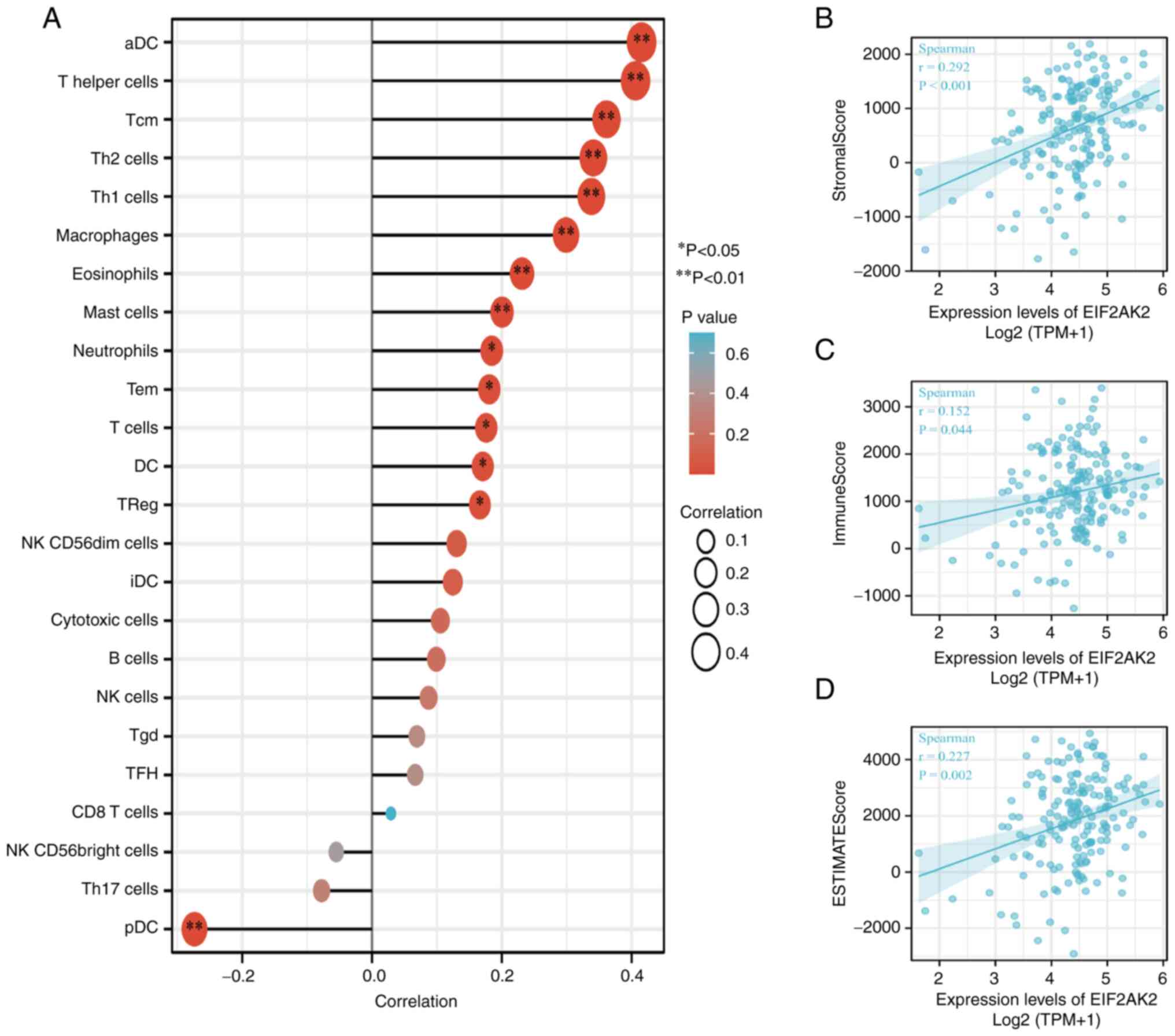
Immune infiltration analysis
The correlation of EIF2AK2 expression with immune
cell infiltration into the microenvironment of pancreatic cancer
tumors was quantified by ssGSEA, as calculated by Spearman
correlation analysis. EIF2AK2 expression was weakly associated with
the StromalScore, ImmuneScore and the ESTIMATE score (Fig. 10B-D). Notably, EIF2AK2 was
correlated with activated DCs (aDCs), T cells, CD4+
T-cell subsets (Th1 cells, Th2 cells, Tregs, Th17 cells, follicular
helper T cells), CD8+ T cells, γδ T cells, memory T-cell
subsets [central memory T (Tcm) cells, effective memory T cells], T
helper cells, B cells, macrophages, eosinophils, mast cells,
neutrophils, DCs, immature DCs, plasmacytoid DCs (pDCs), natural
killer (NK) cells, NK cell subsets (NK CD56dim cells and NK
CD56bright cells) and cytotoxic cells (Fig. 10A).
Relationship between EIF2AK2
expression and immune checkpoints
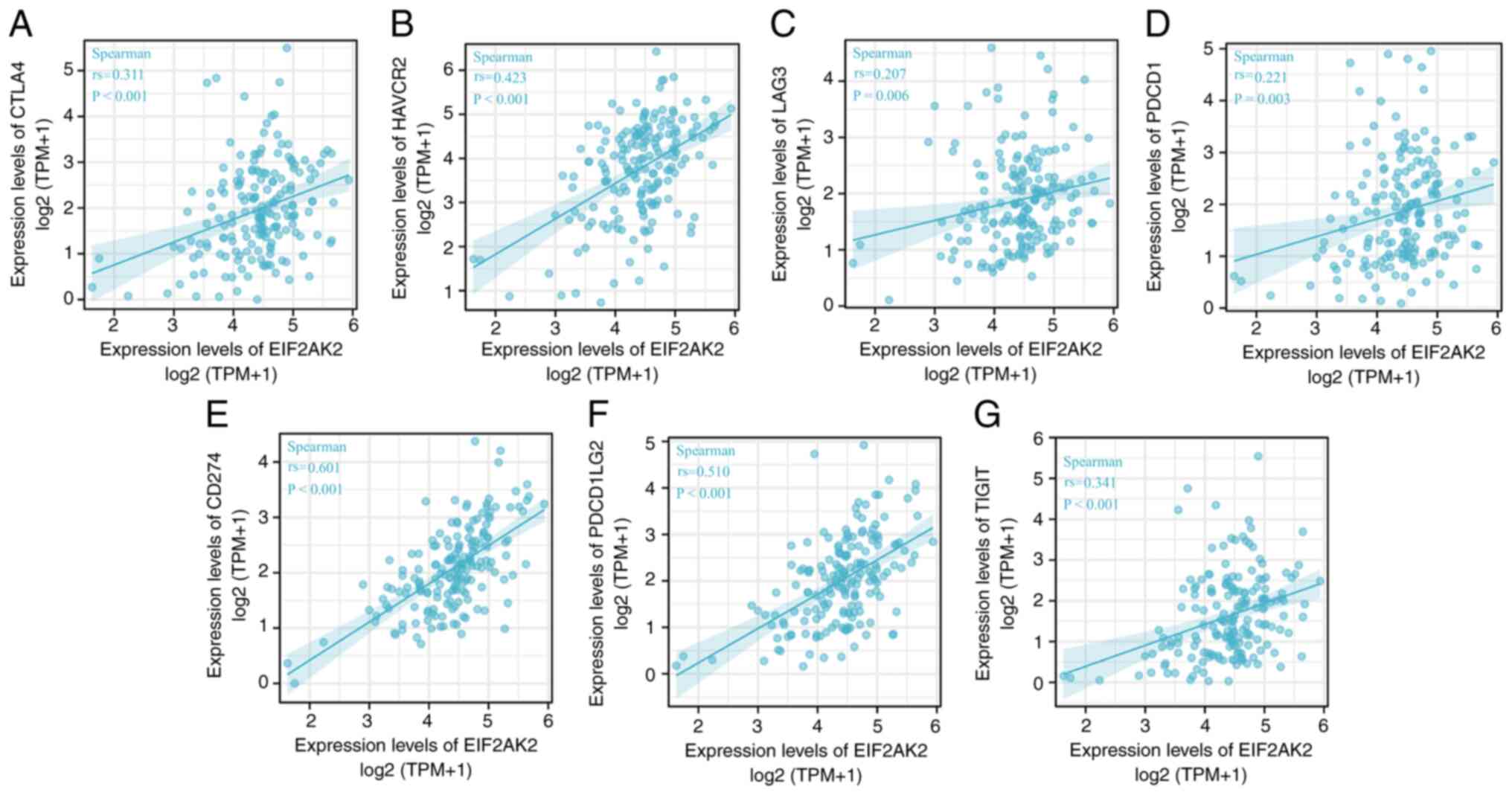
To determine the relationship between EIF2AK2
expression and immune cell, the interactions between EIF2AK2 and
chemokines and chemokine receptors were studied. It was revealed
that there was a significant correlation between the expression of
EIF2AK2 and immune cell-associated chemokines, as well as chemokine
receptors, such as CTLA4 (rs=0.311, P<0.001), HAVCR2 (rs=0.423,
P<0.001), LAG3 (rs=0.207, P=0.006), PDCD1 (rs=0.221, P=0.003),
CD274 (rs=0.601, P ≤0.001), PDCD1LG2 (rs=0.501, P <0.001) and
TIGIT (rs=0.341, P<0.001) (Fig.
11). Since the expression of these chemokines and chemokine
receptors appears to be correlated with EIF2AK2 expression, it is
possible that high EIF2AK2 expression is implicated in the
migration of immune cells to the tumor microenvironment. Spearman
correlation analysis showed that LAG3 and PDCD1 were weakly
correlated with EIF2AK2 and the others were moderately correlated
with it.
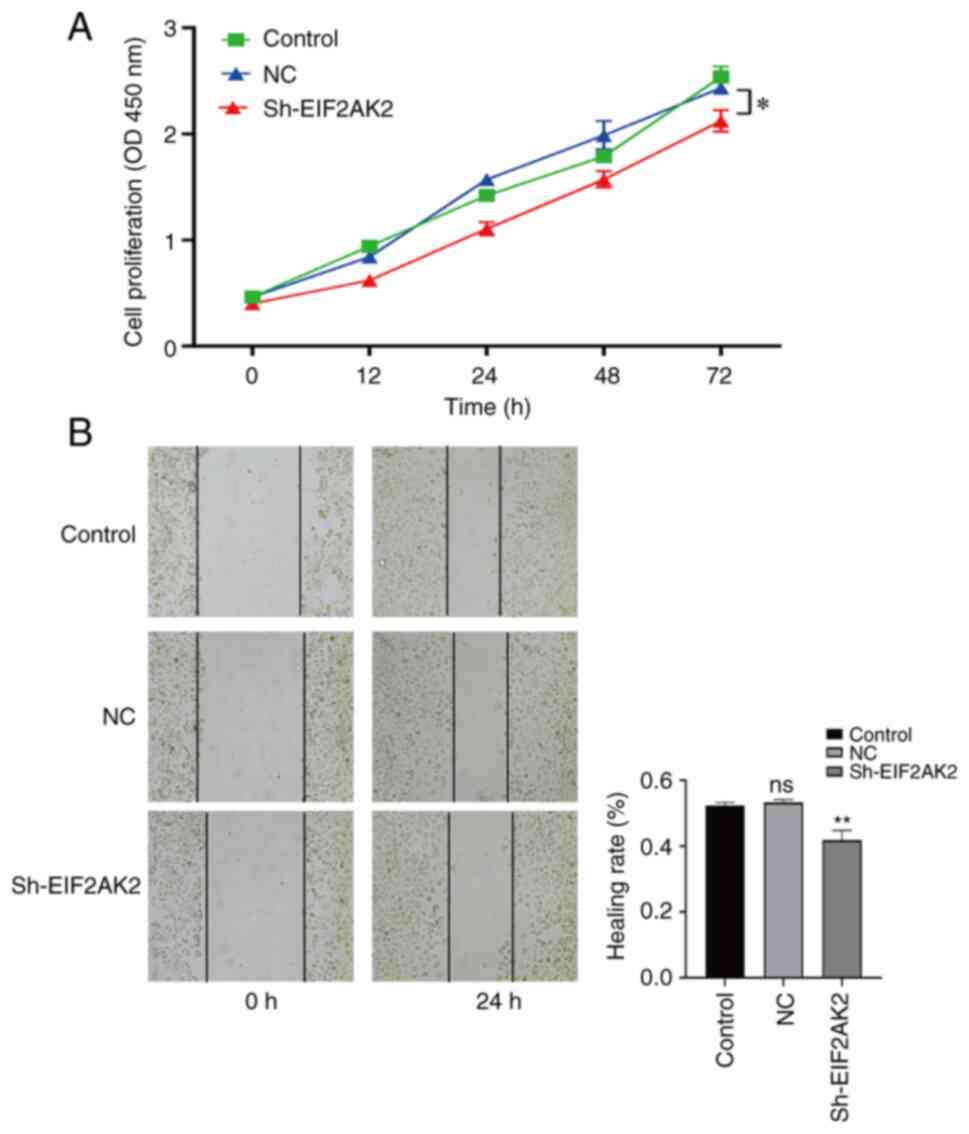
EIF2AK2 knockdown inhibits the
migration and proliferation of PANC-1 cells
The present study revealed that EIF2AK2 was
upregulated in pancreatic cancer and that it was negatively
associated with the survival of patients with pancreatic cancer;
however, the role of EIF2AK2 in pancreatic carcinogenesis requires
further investigation. Three EIF2AK2 shRNA knockdown vectors were
constructed and transfected into PANC-1 cells. The effect of
lentiviral infection is shown in Fig.
S1A. RT-qPCR results showed that sh-EIF2AK2-397 (Shanghai
GeneChem Co., Ltd. cat. no. 115397-1) was more efficient than
sh-EIF2AK2-396 (Shanghai GeneChem Co., Ltd. cat. no. 115396-2) and
sh-EIF2AK2-398 (Shanghai GeneChem Co., Ltd. cat. no. 115398-1)
(Fig. S1B). Therefore, subsequent
in vitro cellular experiments were performed using
sh-EIF2AK2-397. To investigate whether EIF2AK2 affects the
proliferation and migration of PANC-1 cells, CCK-8 and
wound-healing assays were performed. As shown in Fig. 12A and B, the knockdown of EIF2AK2
markedly diminished PANC-1 cell proliferation and migration. These
results confirm that EIF2AK2 promotes PANC-1 cell proliferation and
migration
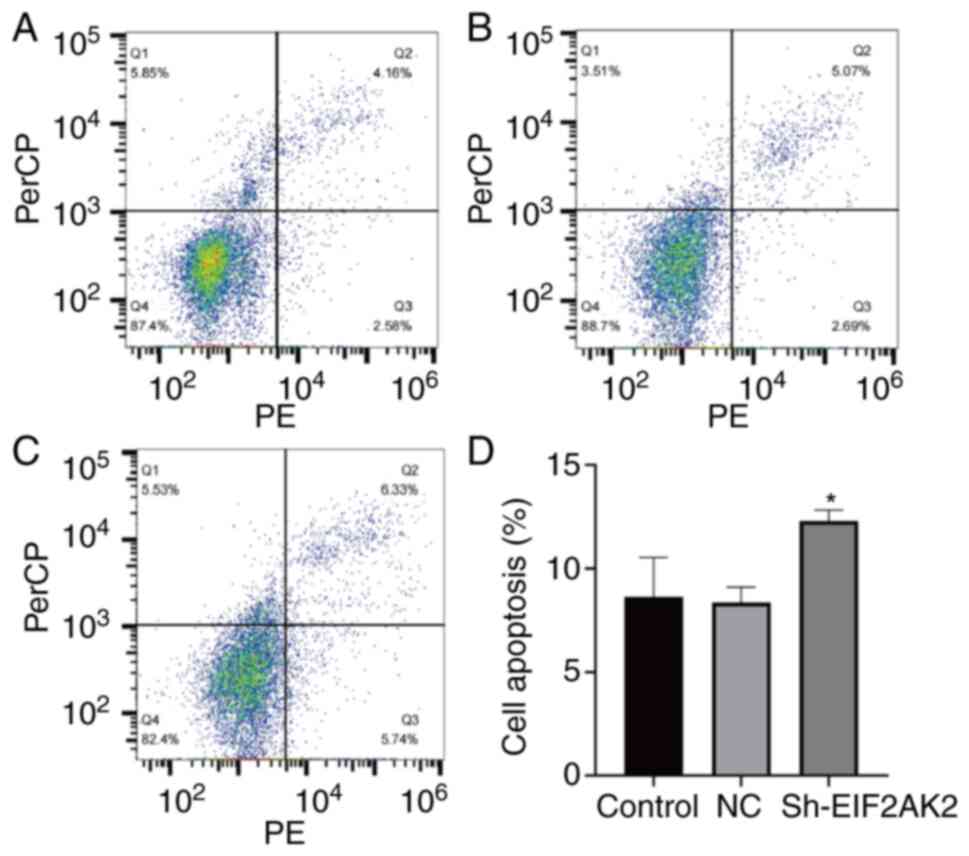
Effect of EIF2AK2 knockdown on PANC-1
cell cycle progression and apoptosis
The present study further assessed the effect of
EIF2AK2 expression on apoptosis and cell cycle progression in
PANC-1 cells. As shown in Fig. 13,
the apoptotic rate was significantly higher in the sh-EIF2AK2 group
compared with that in the control and NC groups. Furthermore, the
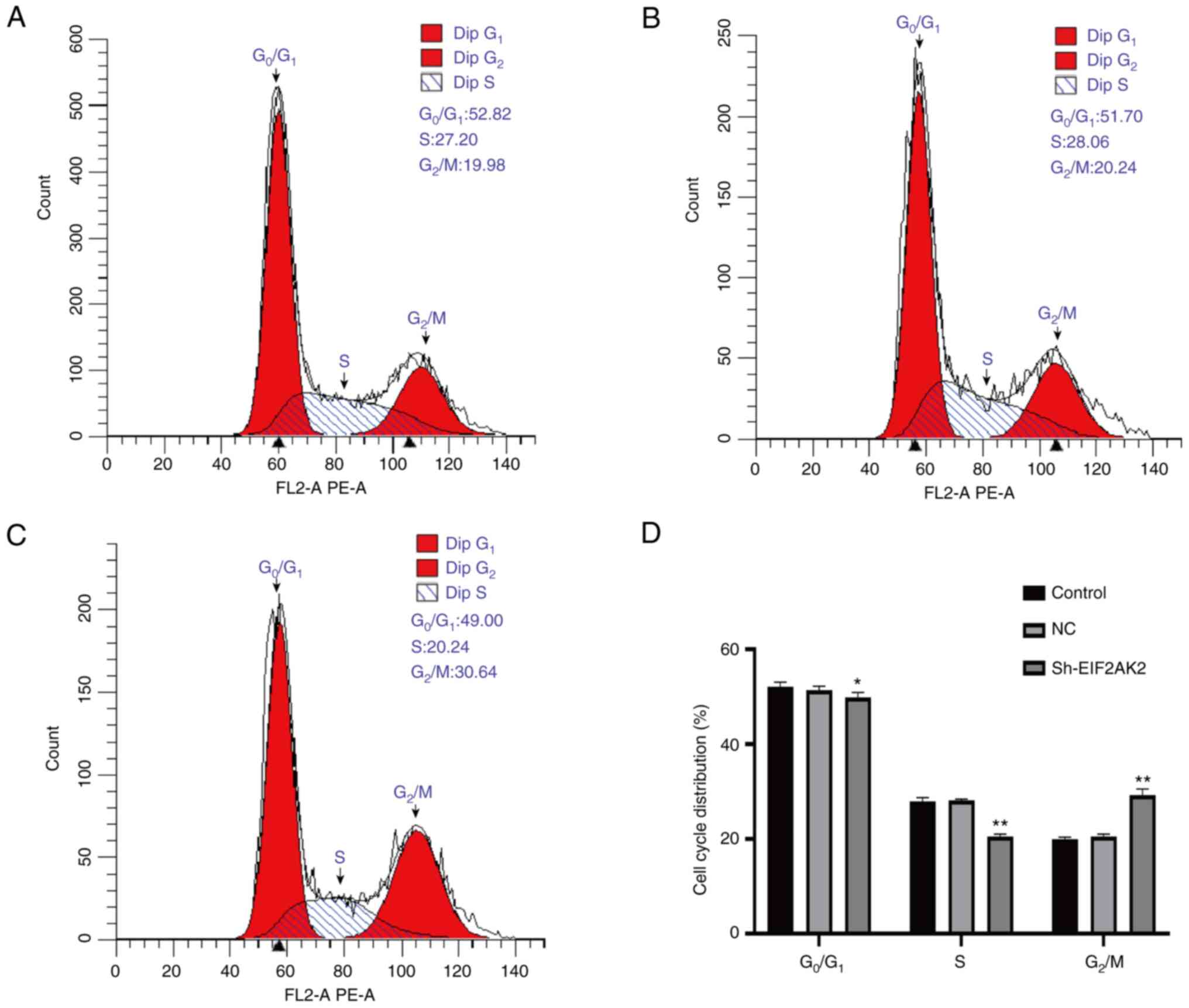
effect of EIF2AK2 knockdown on the cell cycle progression of PANC-1
cells indicated that low EIF2AK2 expression in PANC-1 cells could
inhibit the cell cycle transition from G1 to S phase and
blocked it in G2/M phase (Fig. 14).
Activation of the AKT signaling
pathway by EIF2AK2
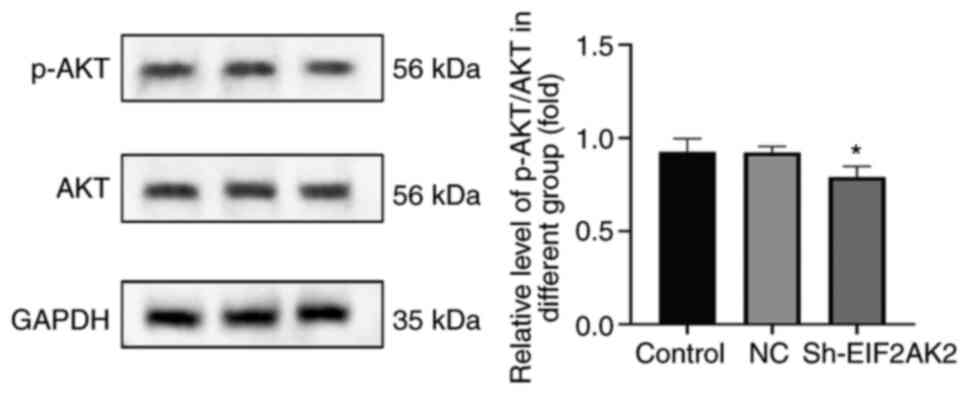
To elucidate the relationship between EIF2AK2 and
pancreatic cancer development, the expression of AKT and p-AKT in
cells following knockdown of EIF2AK2 was determined by western
blotting. The results showed that the p-AKT/AKT ratio was decreased
following knockdown of EIF2AK2 (Fig.
15). This finding suggested that EIF2AK2 may activate the AKT
signaling pathway in vitro.
Discussion
EIF2AK2 is a serine/threonine kinase that is
normally activated by dsRNA. In addition to dsRNA, EIF2AK2 can be
activated by other non-self RNAs (34). EIF2AK2 was originally associated
with cellular innate immunity; dsRNA binds dsRBM1 and 2 (two dsRNA
binding motifs of EIF2AK2) with high affinity (KD=~4 nM) to induce
conformational changes upon viral invasion, resulting in the
release of a kinase domain from dsRBM2 leading to apoptosis in
virus-infected cells (35). In
addition, EIF2AK2 was considered to be a tumor suppressor based on
its proapoptotic activity. The first experimental evidence of
EIF2AK2 tumor-suppressing activity was provided by a catalytically
inactivated EIF2AK2 mutant that inhibited endogenous EIF2AK2
activity in a dominantly negative manner (36). Its low expression level is
considered to be associated with cancer phenotypes, such as active
cell proliferation, poor pathological differentiation or poor
patient prognosis, including in head and neck squamous cell
carcinoma, breast cancer, hepatocellular carcinoma, colon cancer,
bile duct cancer and primary lung cancer (37–42).
Conversely, EIF2AK2 is elevated in other cancer cell subsets
relative to the corresponding normal cells or tissues, and is
associated with tumor aggressiveness or poor patient prognosis,
including breast cancer cell lines, melanoma cells, colon cancer,
thyroid cancer, hepatocellular carcinoma, bile duct cancer, acute
myeloid leukemia and lung adenocarcinoma (17,40,42–46).
In addition, EIF2AK2 expression shows a mixed pro-tumor and
antitumor profile in a number of clinical specimens. Examples of
these include breast cancer, cholangiocarcinoma and hepatocellular
carcinoma samples (47).
Given the limited research conducted on the EIF2AK2
gene in cancer, to identify its biological activities and relevant
regulatory mechanisms in pancreatic cancer, the present study
carried out comprehensive and integrative bioinformatics research.
Based on public databases and clinical samples, the first attempt
to validate the expression of EIF2AK2 in pancreatic cancer tissues
and its prognostic value revealed that EIF2AK2 expression was
elevated in pancreatic cancer tissues compared with that in normal
tissues, and that high expression was strongly associated with
poorer OS and DSS in pancreatic cancer. Notably, the most
clinically relevant finding was that high EIF2AK2 expression was
associated with poor OS. Multivariate Cox regression analysis
revealed that high EIF2AK2 expression was another independent
prognostic factor in addition to N stage. Nomogram prediction
modelling further confirmed the predictive role of EIF2AK2
expression in prognosis. These findings suggested that it could be
a potentially valuable diagnostic and prognostic biomarker for
pancreatic cancer.
Patients with pancreatic cancer have a poor
prognosis, and the standard treatment combines surgery with
chemotherapy and radiation therapy. During the past several years,
immunotherapy has transformed the paradigm for cancer treatment,
gaining recognition as a potential technique for treating certain
malignancies (48). With the
emergence of immunotherapy, several clinical studies have appeared
to verify the efficacy of immunotherapy (49–51).
These experiments explore the use of cellular transfer, immune
checkpoint inhibitors (ICIs), cancer vaccines, and combinations of
chemoradiotherapy and other molecular therapeutic approaches
(52). Notably, due to their broad
applicability across a broad spectrum of tumor types and excellent
clinical response when treatment is successful, ICIs, a novel
treatment approach comprised of anti-programmed cell death
protein-1 (PD-1) and anti-programmed cell death protein ligand 1
(PD-L1) antibody treatments, have assumed the lead in the area of
cancer immunotherapy (53,54). The majority of these experiments,
however, have had uniformly poor outcomes. Pancreatic cancer is a
tumor with low immunogenicity, which is attributable to a low tumor
mutational burden. In light of this, it remains of the utmost
importance to identify a potential biomarker of immune infiltration
that may also predict the prognosis of a patient, as well as to
determine potential molecular pathways that drive immunotherapeutic
responses. In recent years, the incorporation of immunotherapy into
the treatment of a number of solid tumors has led to a renaissance
in oncology treatment (55).
Taking into account the potential oncogenic role of
EIF2AK2 in pancreatic cancer, the present study assessed the
relationship of EIF2AK2 with PD-1 (PDCD1), PD-L1 (CD274), PD-L2
(PDCD1LG2), CTLA4, LAG3, HAVCR2 and TIGIT. The use of monoclonal
antibodies to inhibit CTLA4, PD-1 and PD-L1, thereby targeting
immune checkpoint molecules, has led to a paradigm shift in the
treatment of melanoma, lung, kidney, uroepithelial, head and neck
cancer, and other malignancies (56). CTLA4 is an inhibitory receptor that
regulates the initial phase of T-cell activation by competitively
inhibiting the binding of B7 ligands to the costimulatory receptor
CD28, preventing immune overactivation. PD-1 protein is another
T-cell coinhibitory receptor with a more unique biological function
than CTLA4; it binds PD-L1 and PD-L2 ligands, and blocks the
activity of T-cell peripheral tissues. PD-L1 is the primary PD-1
ligand upregulated and detected in a variety of solid cancer types,
including pancreatic, esophageal, gastric, colon, lung and breast
cancer (57). A single phase II
study, in which patients with locally advanced or metastatic
pancreatic cancer were treated with ipilimumab monotherapy,
revealed that of the 27 patients included, 74% had received prior
gemcitabine-based chemotherapy (chemotherapy followed by
immunotherapy can avoid damage to lymphocytes, improve immunity and
enhance the advantages of chemotherapy and immunotherapy.). No
responders were observed according to the assessment criteria, but
a delayed response was reported in one subject whose initial
progression was followed by regression of the primary tumor and
metastases. The patient had significant new lesions in the
mesentery of the small intestine after 9 months on Ipilimumab and
eventually succumbed due to progression of liver metastases. The
median OS was 4.5 months (58).
Regarding PD-1/PD-L1 inhibitor monotherapy, Brahmer et al
(59) tested the anti-PD-1 antibody
BMS-936559 in a phase I trial involving 207 patients with different
types of advanced cancer. Preliminary results from another
randomized phase II trial were obtained from 65 patients with
metastatic pancreatic cancer who had failed first-line 5-FU or
gemcitabine therapy. Patients were randomized to receive durvalumab
monotherapy or durvalumab in combination with tremelimumab. The
median OS was 3.6 and 3.1 months, with disease control rates
(60) (defined as stable disease +
partial response + complete response) of 6 and 9%, respectively
(59). Therefore, the relationship
between EIF2AK2 and immune checkpoints was also assessed in the
present study. In addition, antagonizing the CD155/TIGIT axis or
LAG3 can induce profound antitumor responses as demonstrated by
in vitro experiments (61,62).
The aforementioned findings indicated that tumor immune escape may
be involved in the EIF2AK2-mediated pancreatic cancer progression.
In addition to monotherapy, combination therapy with
immunosuppressants or with other antitumor agents is rapidly
emerging. This phenomenon is due to the fact that single-agent
checkpoint inhibitors have shown disappointingly limited activity
in pancreatic cancer. The few studies of combination therapy have
shown promise in terms of response (63–66).
Patients with pancreatic cancer treated with checkpoint inhibitors
have not shown improved response rates or OS. However,
immunotherapy and its impact are topics of interest and possible
ways to improve the prognosis of pancreatic cancer (67). This area of research includes both
targeted therapies and antitumor vaccination.
Cancer-associated fibroblasts, tumor-infiltrating
immune cells, endothelial cells and neurons are only a few of the
nontumor components that comprise the tumor microenvironment
(68). Additionally, numerous
extracellular matrix components play a vital role in the creation
of an energetic TME with complex interactions between different
internal components that promote tumor growth and treatment
resistance (69). With the
development of checkpoint inhibitor immunotherapy, tumor
immunotherapy has evolved from an adjuvant therapy to an essential
therapeutic strategy (70). The
results of the present study indicated a link between EIF2AK2
expression and tumor-infiltrating immune cells in the TME. In
addition, EIF2AK2 expression was associated with the StromalScore,
ImmuneScore and the ESTIMATE score. EIF2AK2 was also revealed to be
positively correlated with various immune cells, including aDCs, Th
cells, Tcm cells, Th1/Th2 cells, macrophages and pDCs. DCs are
antigen-presenting cells that serve crucial roles in the
establishment of adaptive and advanced immunity. aDCs can present
antigens in vivo or in vitro by a variety of
mechanisms, including an antibody-conjugated antigen unique to aDCs
and tumor-specific antigen capture. A substantial number of aDCs
can travel to the region of the tumor and remain there for an
extended period of time, hence augmenting the antitumor immune
response (71,72). Since both Th1 and Th2 cells secrete
cytokines to promote their own proliferation and inhibit the
proliferation of the other, Th1 and Th2 cells are normally in a
somewhat balanced state within the body (73). However, when the body has functional
problems, the balance is frequently skewed to one side, a condition
known as ‘Th1/Th2 drift’. Once the equilibrium between Th1 cells
and Th2 cells is disrupted, the dynamic equilibrium of the human
cytokine network is likely to be compromised, leading to the
emergence and development of numerous illnesses (74).
Based on the analysis of the enrichment results, it
can be concluded that knockdown of the EIF2AK2 gene reduces the
proliferation and migration of PANC-1 cells, and can promote their
apoptosis and inhibit the cell cycle. The experimental results that
knockdown of EIF2AK2 promotes apoptosis in PANC-1 cells are clearly
inconsistent with the findings reported above in the literature
(36). Therefore it is necessary to
review the basic principles of the EIF2AK2 pathway. When EIF2AK2 is
activated, two representative downstream branches are stimulated:
Pro-proliferative NF-κB (pro-tumor effect) and pro-apoptotic eIF2α
(tumor suppressor effect). In addition to these two pathways,
EIF2AK2 has several other downstream pathways, including PI3K, p38,
p53 and PP2A (75). AKT, a PI3K
downstream regulator, can phosphorylate target proteins through
multiple downstream pathways and thus exert apoptosis-inhibiting
effects (76). The present
experimental results indicated that EIF2AK2 knockdown reduced
p-AKT/AKT expression but increased apoptosis in PANC-1 cells.
Therefore, whether EIF2AK2 has a biological function in promoting
or inhibiting apoptosis in pancreatic tumor cells needs further
investigation.
Unfortunately, there are some limitations in the
present study. For example, the specific molecular pathway of
EIF2AK2-mediated proliferation and migration remains to be
elucidated. The function of EIF2AK2 in vivo is also not yet
clear. We aim to conduct relevant in vivo studies in the
future to address these deficiencies, such as colony formation,
spheroid formation and tumorigenesis assays. However, the present
study confirmed that EIF2AK2 is upregulated in pancreatic cancer
and can effectively distinguish the degree of differentiation in
patients with pancreatic cancer.
In conclusion, the present study demonstrated that
EIF2AK2 may play a prognostic role and could be associated with
immune infiltration in pancreatic cancer. As a direct consequence,
EIF2AK2 has potential as a novel prognostic marker and as a
prospective biomarker of new immunotherapeutic strategies.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural Science
Foundation of China (grant no. 81960516).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HXD and KXZ conceived and designed the study and
methodology. HXD, HW and HC performed biological experiments, data
analysis, statistical work and manuscript preparation. HXD, ABD and
XPM gathered clinical samples. KXZ and HXD confirmed the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All patients provided written informed consent and
the present study was approved by the Ethics Committee of the First
Hospital of Lanzhou University (Gansu, China; approval no.
LDYYLL2023-304).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mizrahi JD, Surana R, Valle JW and Shroff
RT: Pancreatic cancer. Lancet. 395:2008–2020. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Noë M, Hong SM, Wood LD, Thompson ED,
Roberts NJ, Goggins MG, Klein AP, Eshleman JR, Kern SE and Hruban
RH: Pancreatic cancer pathology viewed in the light of evolution.
Cancer Metastasis Rev. 40:661–674. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cicenas J, Kvederaviciute K, Meskinyte I,
Meskinyte-Kausiliene E, Skeberdyte A and Cicenas J: KRAS, TP53,
CDKN2A, SMAD4, BRCA1, and BRCA2 Mutations in Pancreatic Cancer.
Cancers (Basel). 9:422017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mao X, Xu J, Wang W, Liang C, Hua J, Liu
J, Zhang B, Meng Q, Yu X and Shi S: Crosstalk between
cancer-associated fibroblasts and immune cells in the tumor
microenvironment: New findings and future perspectives. Mol Cancer.
20:1312021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang M, Wu M, Liu X, Shao S, Huang J, Liu
B and Liang T: Pyroptosis remodeling tumor microenvironment to
enhance pancreatic cancer immunotherapy driven by membrane
anchoring photosensitizer. Adv Sci (Weinh). 9:e22029142022.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Looi CK, Chung FF, Leong CO, Wong SF,
Rosli R and Mai CW: Therapeutic challenges and current
immunomodulatory strategies in targeting the immunosuppressive
pancreatic tumor microenvironment. J Exp Clin Cancer Res.
38:1622022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Balachandran VP, Beatty GL and Dougan SK:
Broadening the impact of immunotherapy to pancreatic cancer:
Challenges and opportunities. Gastroenterology. 156:2056–2072.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li HB, Yang ZH and Guo QQ: Immune
checkpoint inhibition for pancreatic ductal adenocarcinoma:
Limitations and prospects: A systematic review. Cell Commun Signal.
19:1172021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lou X, Gao D, Yang L, Wang Y and Hou Y:
Endoplasmic reticulum stress mediates the myeloid-derived immune
suppression associated with cancer and infectious disease. J Transl
Med. 21:12023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smyth R and Sun J: Protein Kinase R in
Bacterial Infections: Friend or Foe? Front Immunol. 12:7021422021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meurs EF, Galabru J, Barber GN, Katze MG
and Hovanessian AG: Tumor suppressor function of the
interferon-induced double-stranded RNA-activated protein kinase.
Proc Natl Acad Sci USA. 90:232–236. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu B, Song M, Dong Q, Xiang G, Li J, Ma X
and Wei F: UBR5 promotes tumor immune evasion through enhancing
IFN-γ-induced PDL1 transcription in triple negative breast
cancer. Theranostics. 12:5086–5102. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shir A and Levitzki A: Inhibition of
glioma growth by tumor-specific activation of double-stranded
RNA-dependent protein kinase PKR. Nat Biotechnol. 20:895–900. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang X and Chan C: Repression of PKR
mediates palmitate-induced apoptosis in HepG2 cells through
regulation of Bcl-2. Cell Res. 19:469–486. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Minnee E and Faller WJ: Translation
initiation and its relevance in colorectal cancer. FEBS J.
288:6635–6651. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Watanabe T, Ninomiya H, Saitou T,
Takanezawa S, Yamamoto S, Imai Y, Yoshida O, Kawakami R, Hirooka M,
Abe M, et al: Therapeutic effects of the PKR inhibitor C16
suppressing tumor proliferation and angiogenesis in hepatocellular
carcinoma in vitro and in vivo. Sci Rep. 10:51332020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim SH, Forman AP, Mathews MB and Gunnery
S: Human breast cancer cells contain elevated levels and activity
of the protein kinase, PKR. Oncogene. 19:3086–3094. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakamura K, Aizawa K, Aung KH, Yamauchi J
and Tanoue A: Zebularine upregulates expression of CYP genes
through inhibition of DNMT1 and PKR in HepG2 cells. Sci Rep.
7:410932017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Luo X, Lin J, Fu S, Feng P, Su H,
He X, Liang X, Liu K and Deng W: Gelsolin promotes cancer
progression by regulating epithelial-mesenchymal transition in
hepatocellular carcinoma and correlates with a poor prognosis. J
Oncol. 2020:19803682020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Watanabe T, Imamura T and Hiasa Y: Roles
of protein kinase R in cancer: Potential as a therapeutic target.
Cancer Sci. 109:919–925. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song H, Tian D, Sun J, Mao X, Kong W, Xu
D, Ji Y, Qiu B, Zhan M and Wang J: circFAM120B functions as a tumor
suppressor in esophageal squamous cell carcinoma via the
miR-661/PPM1L axis and the PKR/p38 MAPK/EMT pathway. Cell Death
Dis. 13:3612022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vivian J, Rao AA, Nothaft FA, Ketchum C,
Armstrong J, Novak A, Pfeil J, Narkizian J, Deran AD,
Musselman-Brown A, et al: Toil enables reproducible, open source,
big biomedical data analyses. Nat Biotechnol. 35:314–316. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goldman MJ, Craft B, Hastie M, Repečka K,
McDade F, Kamath A, Banerjee A, Luo Y, Rogers D, Brooks AN, et al:
Visualizing and interpreting cancer genomics data via the Xena
platform. Nat Biotechnol. 38:675–678. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li K, Luo H, Luo H and Zhu X: Clinical and
prognostic pan-cancer analysis of m6A RNA methylation regulators in
four types of endocrine system tumors. Aging (Albany NY).
12:23931–23944. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barrett T, Wilhite SE, Ledoux P,
Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH,
Sherman PM, Holko M, et al: NCBI GEO: Archive for functional
genomics data sets-update. Nucleic Acids Res. 41:D991–D995. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barrett T, Troup DB, Wilhite SE, Ledoux P,
Rudnev D, Evangelista C, Kim IF, Soboleva A, Tomashevsky M,
Marshall KA, et al: NCBI GEO: Archive for high-throughput
functional genomic data. Nucleic Acids Res. 37:D885–D890. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Badea L, Herlea V, Dima SO, Dumitrascu T
and Popescu I: Combined gene expression analysis of whole-tissue
and microdissected pancreatic ductal adenocarcinoma identifies
genes specifically overexpressed in tumor epithelia.
Hepatogastroenterology. 55:2016–2027. 2008.PubMed/NCBI
|
|
28
|
Pei H, Li L, Fridley BL, Jenkins GD,
Kalari KR, Lingle W, Petersen G, Lou Z and Wang L: FKBP51 affects
cancer cell response to chemotherapy by negatively regulating Akt.
Cancer Cell. 16:259–266. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Donahue TR, Tran LM, Hill R, Li Y,
Kovochich A, Calvopina JH, Patel SG, Wu N, Hindoyan A, Farrell JJ,
et al: Integrative survival-based molecular profiling of human
pancreatic cancer. Clin Cancer Res. 18:1352–1363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Janky R, Binda MM, Allemeersch J, Van den
Broeck A, Govaere O, Swinnen JV, Roskams T, Aerts S and Topal B:
Prognostic relevance of molecular subtypes and master regulators in
pancreatic ductal adenocarcinoma. BMC Cancer. 16:6322016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nallagatla SR, Hwang J, Toroney R, Zheng
X, Cameron CE and Bevilacqua PC: 5′-triphosphate-dependent
activation of PKR by RNAs with short stem-loops. Science.
318:1455–1458. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu W, Zhou C, Jing Q, Li Y, Yang J, Yang
C, Wang L, Hu J, Li H, Wang H, et al: FTH promotes the
proliferation and renders the HCC cells specifically resist to
ferroptosis by maintaining iron homeostasis. Cancer Cell Int.
21:7092021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu G, Wang LG, Han Y and He QY:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Holcik M: Could the eIF2α-independent
translation Be the achilles heel of cancer? Front Oncol. 5:2642015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Karnam K, Sedmaki K, Sharma P, Venuganti
VVK and Kulkarni OP: Selective inhibition of PKR by C16 accelerates
diabetic wound healing by inhibiting NALP3 expression in mice.
Inflamm Res. 72:221–236. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee YS, Kunkeaw N and Lee YS: Protein
kinase R and its cellular regulators in cancer: An active player or
a surveillant? Wiley Interdiscip Rev RNA. 11:e15582020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Haines GK III, Panos RJ, Bak PM, Brown T,
Zielinski M, Leyland J and Radosevich JA: Interferon-responsive
protein kinase (p68) and proliferating cell nuclear antigen are
inversely distributed in head and neck squamous cell carcinoma.
Tumour Biol. 19:52–59. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Haines GK, Cajulis R, Hayden R, Duda R,
Talamonti M and Radosevich JA: Expression of the double-stranded
RNA-dependent protein kinase (p68) in human breast tissues. Tumour
Biol. 17:5–12. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shimada A, Shiota G, Miyata H, Kamahora T,
Kawasaki H, Shiraki K, Hino S and Terada T: Aberrant expression of
double-stranded RNA-dependent protein kinase in hepatocytes of
chronic hepatitis and differentiated hepatocellular carcinoma.
Cancer Res. 58:4434–4438. 1998.PubMed/NCBI
|
|
41
|
Singh C, Haines GK, Talamonti MS and
Radosevich JA: Expression of p68 in human colon cancer. Tumour
Biol. 16:281–289. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Terada T, Ueyama J, Ukita Y and Ohta T:
Protein expression of double-stranded RNA-activated protein kinase
(PKR) in intrahepatic bile ducts in normal adult livers, fetal
livers, primary biliary cirrhosis, hepatolithiasis and intrahepatic
cholangiocarcinoma. Liver. 20:450–457. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
He Y, Correa AM, Raso MG, Hofstetter WL,
Fang B, Behrens C, Roth JA, Zhou Y, Yu L, Wistuba II, et al: The
role of PKR/eIF2α signaling pathway in prognosis of non-small cell
lung cancer. PLoS One. 6:e248552011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim SH, Gunnery S, Choe JK and Mathews MB:
Neoplastic progression in melanoma and colon cancer is associated
with increased expression and activity of the interferon-inducible
protein kinase, PKR. Oncogene. 21:8741–8748. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Terada T, Maeta H, Endo K and Ohta T:
Protein expression of double-stranded RNA-activated protein kinase
in thyroid carcinomas: Correlations with histologic types,
pathologic parameters, and Ki-67 labeling. Hum Pathol. 31:817–821.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Blalock WL, Grimaldi C, Fala F, Follo M,
Horn S, Basecke J, Martinelli G, Cocco L and Martelli AM: PKR
activity is required for acute leukemic cell maintenance and
growth: A role for PKR-mediated phosphatase activity to regulate
GSK-3 phosphorylation. J Cell Physiol. 221:232–241. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Roh MS, Kwak JY, Kim SJ, Lee HW, Kwon HC,
Hwang TH, Choi PJ and Hong YS: Expression of double-stranded
RNA-activated protein kinase in small-size peripheral
adenocarcinoma of the lung. Pathol Int. 55:688–693. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Riley RS, June CH, Langer R and Mitchell
MJ: Delivery technologies for cancer immunotherapy. Nat Rev Drug
Discov. 18:175–196. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Immunotherapy shows promise in pancreatic
cancer. Cancer Discov. 9:13302019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
O'Hara MH, O'Reilly EM, Varadhachary G,
Wolff RA, Wainberg ZA, Ko AH, Fisher G, Rahma O, Lyman JP, Cabanski
CR, et al: CD40 agonistic monoclonal antibody APX005M (sotigalimab)
and chemotherapy, with or without nivolumab, for the treatment of
metastatic pancreatic adenocarcinoma: An open-label, multicentre,
phase 1b study. Lancet Oncol. 22:118–131. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rech AJ, Mick R, Martin S, Recio A, Aqui
NA, Powell DJ Jr, Colligon TA, Trosko JA, Leinbach LI, Pletcher CH,
et al: CD25 blockade depletes and selectively reprograms regulatory
T cells in concert with immunotherapy in cancer patients. Sci
Transl Med. 4:134ra622012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Reap EA, Suryadevara CM, Batich KA,
Sanchez-Perez L, Archer GE, Schmittling RJ, Norberg PK, Herndon JE
II, Healy P, Congdon KL, et al: Dendritic cells enhance
polyfunctionality of adoptively transferred T cells that target
cytomegalovirus in glioblastoma. Cancer Res. 78:256–264. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
He X and Xu C: Immune checkpoint signaling
and cancer immunotherapy. Cell Res. 30:660–669. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bagchi S, Yuan R and Engleman EG: Immune
Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact
and Mechanisms of Response and Resistance. Annu Rev Pathol.
16:223–249. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Herzberg B, Campo MJ and Gainor JF: Immune
checkpoint inhibitors in non-small cell lung cancer. Oncologist.
22:81–88. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Brower V: Checkpoint blockade
immunotherapy for cancer comes of age. J Natl Cancer Inst.
107:djv0692015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Farasati Far B, Safaei M, Mokhtari F,
Fallahi MS and Naimi-Jamal MR: Fundamental concepts of protein
therapeutics and spacing in oncology: An updated comprehensive
review. Med Oncol. 40:1662023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Royal RE, Levy C, Turner K, Mathur A,
Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I and
Rosenberg SA: Phase 2 trial of single agent Ipilimumab
(anti-CTLA-4) for locally advanced or metastatic pancreatic
adenocarcinoma. J Immunother. 33:828–833. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Brink GJ, Groeneweg JW, Hooft L, Zweemer
RP and Witteveen PO: Response to systemic therapies in ovarian
adult granulosa cell tumors: A literature review. Cancers (Basel).
14:29982022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gulhati P, Schalck A, Jiang S, Shang X, Wu
CJ, Hou P, Ruiz SH, Soto LS, Parra E, Ying H, et al: Targeting T
cell checkpoints 41BB and LAG3 and myeloid cell CXCR1/CXCR2 results
in antitumor immunity and durable response in pancreatic cancer.
Nat Cancer. 4:62–80. 2023.PubMed/NCBI
|
|
62
|
Freed-Pastor WA, Lambert LJ, Ely ZA,
Pattada NB, Bhutkar A, Eng G, Mercer KL, Garcia AP, Lin L, Rideout
WM III, et al: The CD155/TIGIT axis promotes and maintains immune
evasion in neoantigen-expressing pancreatic cancer. Cancer Cell.
39:1342–1360.e14. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Qian Y, Gong Y, Fan Z, Luo G, Huang Q,
Deng S, Cheng H, Jin K, Ni Q, Yu X and Liu C: Molecular alterations
and targeted therapy in pancreatic ductal adenocarcinoma. J Hematol
Oncol. 13:1302020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Conroy T, Castan F, Lopez A, Turpin A, Ben
Abdelghani M, Wei AC, Mitry E, Biagi JJ, Evesque L, Artru P, et al:
Five-Year outcomes of FOLFIRINOX vs gemcitabine as adjuvant therapy
for pancreatic cancer: A randomized clinical trial. JAMA Oncol.
8:1571–1578. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Mahalingam D, Wilkinson GA, Eng KH, Fields
P, Raber P, Moseley JL, Cheetham K, Coffey M, Nuovo G, Kalinski P,
et al: Pembrolizumab in combination with the oncolytic virus
pelareorep and chemotherapy in patients with advanced pancreatic
adenocarcinoma: A phase Ib study. Clin Cancer Res. 26:71–81. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Conroy T, Hammel P, Hebbar M, Ben
Abdelghani M, Wei AC, Raoul JL, Choné L, Francois E, Artru P, Biagi
JJ, et al: FOLFIRINOX or gemcitabine as adjuvant therapy for
pancreatic cancer. N Engl J Med. 379:2395–2406. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Schizas D, Charalampakis N, Kole C,
Economopoulou P, Koustas E, Gkotsis E, Ziogas D, Psyrri A and
Karamouzis MV: Immunotherapy for pancreatic cancer: A 2020 update.
Cancer Treat Rev. 86:1020162020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hessmann E, Buchholz SM, Demir IE, Singh
SK, Gress TM, Ellenrieder V and Neesse A: Microenvironmental
determinants of pancreatic cancer. Physiol Rev. 100:1707–1751.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Neesse A, Bauer CA, Öhlund D, Lauth M,
Buchholz M, Michl P, Tuveson DA and Gress TM: Stromal biology and
therapy in pancreatic cancer: ready for clinical translation? Gut.
68:159–171. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Binnewies M, Roberts EW, Kersten K, Chan
V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI,
Ostrand-Rosenberg S, Hedrick CC, et al: Understanding the tumor
immune microenvironment (TIME) for effective therapy. Nat Med.
24:541–550. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Murphy TL and Murphy KM: Dendritic cells
in cancer immunology. Cell Mol Immunol. 19:3–13. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yin X, Chen S and Eisenbarth SC: Dendritic
cell regulation of T helper cells. Annu Rev Immunol. 39:759–790.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Sanders VM: Epigenetic regulation of Th1
and Th2 cell development. Brain Behav Immun. 20:317–3124. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Dong C: Cytokine regulation and function
in T cells. Annu Rev Immunol. 39:51–76. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Garcia-Ortega MB, Lopez GJ, Jimenez G,
Garcia-Garcia JA, Conde V, Boulaiz H, Carrillo E, Perán M, Marchal
JA and Garcia MA: Clinical and therapeutic potential of protein
kinase PKR in cancer and metabolism. Expert Rev Mol Med. 19:e92017.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signaling
pathway and cancer. Cancer Treat Rev Apr. 30:193–204. 2004.
View Article : Google Scholar : PubMed/NCBI
|