Lung cancer is the leading cause of cancer deaths.
In the last decade, patients with lung cancer have demonstrated
continuously improved overall survival rates, mainly due to the
development of effective advanced treatment regimens and precision
medicine in the field of oncology (1). Precision medicine can treat tumors by
matching a patient's unique clinical and biological characteristics
with an optimal treatment or combination of treatments, with the
intent to maximize clinical benefit with minimal side effects and
ultimately achieve an effective and long-lasting impact on immune
response and tumor cell escape (2–4).
Advances in immunotherapy have demonstrated that certain beneficial
immune responses are triggered in patients with cancer. For
example, in patients with non-small cell lung cancer (NSCLC) who
demonstrate overexpression of programmed cell death ligand-1
(PD-L1), treatment regimens using immune checkpoint inhibitors
(ICIs) are currently one of the mainstays of immunotherapy
(3,4). The detection of certain biomarkers,
including tumor mutational burden (TMB) (5) and microsatellite instability (MSI)
(6), can improve the outcomes of
checkpoint blockade-based immunotherapy by identifying patients
with the best response. Nonetheless, the majority of patients with
relatively high MSIs or TMBs fail to respond to immunotherapy or
will develop a mechanism for adaptive resistance (7). Therefore, there is an urgent need for
advanced treatment sensitivity predictors that can guide
therapeutic regimens and prevent the unnecessary exposure of
patients to ineffective treatments. Static biomarkers detected
using genomic (8), transcriptome
(8) or proteomic (9) analysis can support the selection of a
suitable choice among treatment regimens. However, analysis of
certain biomarkers, such as TMB and PD-L1 expression (5) and the content of certain immune cells,
such as CD8+ cells, fails to adequately capture inter- and
intra-tumor heterogeneity alongside the variation demonstrated
between individuals (10). Compared
with the aforementioned static tumor biomarkers, in vivo
animal models can directly evaluate how immunotherapy may affect
patient-derived materials (PDMs), thereby providing a functional
readout of tumor response to certain treatments. While murine
models are a powerful tool for the investigation of the efficacy of
classical drugs, they are not applicable to all types of
immunotherapy, as the murine immune system differs from the human
immune system (11). Developing
alternative models that can replicate human tumors and preserve the
characteristics of the human immune system is therefore necessary
to improve immunotherapy research. Currently, the interactions of
cancer cells with their environment have been successfully modeled
using patient-derived tumor xenografts (PDTX) and human cell lines.
PDTX cost, productivity and comprehensive immuno-compatibility are
currently issues with this form of model (10,11).
The construction of effective PDTX models is laborious, taking 4–8
months to develop. As a consequence, these models are not currently
a feasible option for targeted cancer therapy research (12). In previous years, both tumor
organoids and complex tumor-immune organoids have been considered
promising models which mimic the human TME and migration,
extravasation, and Angiogenesis) of the human immune system. In the
present article, the current developments in lung cancer organoid
(LCO) technology will be reviewed and its application in precision
immunotherapy approaches will be discussed. Comparison of different
types of lung cancer models were list in Table I.
Organoids are multicellular spheroids originally
derived from healthy organ tissue with the aim of reconstructing
and miniaturizing the multicellular architecture of the organ
(13). Organoids are three
dimensional (3D) structures which can be cultured in embedded 3D
matrices to imitate original tissues (14–16).
In 2008, Eiraku et al (17)
reported the production of one self-organized formation of
apico-basally polarized cortical tissues from embryonic stem cells
using an efficient 3D aggregation culture, the cortical neurons
were both functional and transplantable. In 2009, the creation of
organoids from mouse intestinal stem cells was first described by
Sato et al (18) and this
work served as the basis for subsequent organoid cultivation
techniques in other murine and human epithelial tissues. Organoids
have the capacity to self-organize and can be produced from human
stem cells to simulate disease progression or tissue homeostasis,
or from pluripotent embryonic stem cells or induced pluripotent
stem cells to imitate embryonic development.
To evaluate the application of organoids in lung
diseases, numerous studies first explored the use of lung
organoids. Lung organoids have the potential to aid in the
development of advanced treatments for a number of lung diseases,
such as lung cancer (19,20), idiopathic pulmonary fibrosis
(21), cystic fibrosis (22) and asthma (23). Notably, in vitro organoid
models for human distal pulmonary infectious diseases, such as
coronavirus disease 2019, have also previously been established
(24). Since organoids are derived
from cells with progenitor potential, adult lung epithelial stem or
progenitor cells, including basal cells, alveolar type II cells and
airway secretory cells can be used as the required primary cell
source to establish lung organoids (25). Rock et al (26) cultured airway basal cells in a 3D
air-liquid interface system and reported that basal cells could
differentiate into tracheal spheroids in the absence of mesenchymal
stem cells (MSCs). The differentiation of lung progenitor cells
into airway and alveolar structures can be promoted when
co-cultured with MSCs (27,28). Moreover, the ability to alter human
pluripotent stem cells (hPSCs) using the CRISPR-Cas9 system enables
researchers to modify and investigate human genes linked to lung
organoid development and disease, is an additional benefit of this
type of organoid model (21).
In a previous study, certain techniques used for
creating human or mouse lung-derived organoids failed to achieve
the long-term goal of constructing single lung organoids derived
from lung basal cells cultured in culture for 2 weeks shows
abnormal differentiation occurs) (25). However, with development of lung
organoid culture technology, this may no longer be a limitation in
the future. Sachs et al (29) previously described the conditions
required for long-term lung organoid culture. In the aforementioned
study, human lung-derived organoids were passaged every 2 weeks for
>1 year, maintaining similar proportions of basal, rod,
multiciliated and secretory cells. Furthermore, hPSC-derived lung
organoids can be cultivated for up to 170 days (28). Notably, Salahudeen et al
(24) reported distal human lung
progenitors as organoids derived clonally from single adult human
alveolar epithelial type II (AT2) or KRT5+ basal cells) for the
long-term growth of human distal airway and alveolar organoids.
Similar to organs, tumors are composed of numerous
cell types with the addition of cancer cells (5). The clonal heterogeneity and mutational
status of donor tumors can be substantially retained in organoids
(30). The development of
patient-derived tumor organoid (PDTO) cultures presents a novel
form of in vitro model to effectively mimic human tumors
(31). To date, tumor tissues of
certain types of cancer have been successfully cultured into PDTOs,
most of which are derived from epithelial carcinoma (32), including lung adenocarcinoma. LCOs
are 3D structures derived from processed lung tumor tissue that
contain different cell types and grow in a standardized manner
(33). Numerous lung cell types,
including stromal cells, as well as cancer cells at various stages
of disease, can be cultured in lung cancer cell lines derived from
patient tumor tissue (34).
Furthermore, cancer cells at the most advanced stage of disease can
be modeled by short-term cultured cells (PDX/PDO), which may serve
as a genetic depiction of the primary tumor (33). Although certain types of
patient-derived lung cell lines can be cultured in monolayers, the
original 3D organ architecture and heterogeneity of a cancerous
organ cannot be retained in a monolayer differentiated environment.
The cooperative interplay of numerous cell types that are dispersed
and arranged in a 3D structure is necessary for human organs to
operate in a disease-free state. Originating from healthy organs,
lung tumors are a complex cell community. The intricate
extracellular matrix and tumor microenvironment (TME) also aid in
the formation of tumors (35).
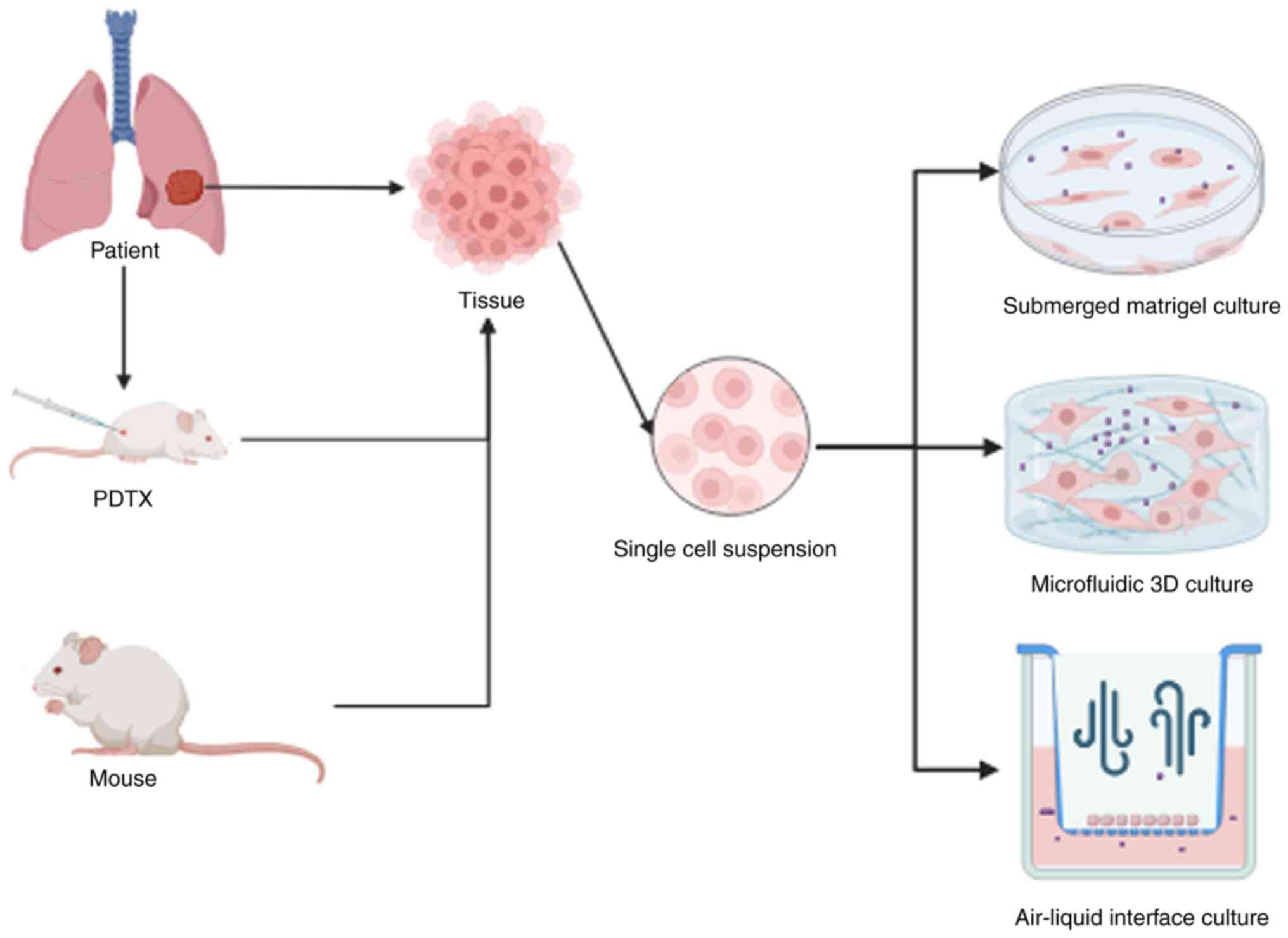
Certain LCO culture methods have been used to model
suitable TMEs for the testing of immunotherapy, including
reconstructive methods. Reconstructive methods involve the culture
of organoids composed solely of epithelial cells in submerged
Matrigel (36). Then, exogenous
immune cells and various stromal cells are added for the
investigation of immunotherapy and TME that rely on this method
(33). Holistic approaches, such as
microfluidic 3D culture and air-liquid interface (ALI), have also
been used to model TMEs suitable for immunotherapy research. This
can involve production of aggregates of 3D spheroids, in suspension
or implanted in 3D matrices (Fig.
1) (34). Notably, ALI can be
applied through explant culture, which can be used to preserve
tissue structures. With these techniques, small fragments of native
TME and tumor tissue are preserved as a complete unit (35).
The submerged Matrigel technique is used to culture
tumor cells isolated from tumor biopsies in tissue culture media
mixed with 3D Matrigel in domed or flat gels (36–38).
This technique requires growth factor supplementation and the use
of small-molecule inhibitors, such as Y27632 and Rho-associated
kinase, or activators, such as smoothened agonist (SAG), the use of
which may differ between laboratories (39,40).
Small-molecule activators and inhibitors can promote or block
multiple biological responses, which is helpful to maintain
organoid growth and phenotype. For instance, a previous study by
Huo et al (41) used common
media formulations such as DMEM/F12, HEPES, penicillin-streptomycin
and Glutamax, with N-acetyl-L-cysteine and B-27 supplements for the
maintenance of certain properties (genetic markers such as
EGFR)/protein markers (e.g., TTF-1, p63, cytokeratin 5)) of lung
cancer cells derived from stem cells or patients. A number of small
molecule activators or inhibitors, including A83-01, CHIR 99021,
Noggin, Y-27632 and SAG were also added to the culture medium. With
this tailor-made ‘cocktail’, which also included certain growth
factors such as epidermal growth factor (EGF), fibroblast growth
factor (FGF) 4 and FGF10, the successful organoid culture period
exceeded 3 months (19). By
contrast, another previous study produced a simpler organoid
culture medium with fewer components (18). This medium consisted of EGF, βFGF,
Y-27632, N2 and B-27 supplements and basic cell culture components
such as penicillin-streptomycin and DMEM/F12. In the aforementioned
study, organoids derived from lung tumors could be effectively
cultured for >6 months. However, the addition of media
components is only part of the reason for the success of organoid
cultures.
During LCO culture, issues with healthy epithelial
cell overgrowth have been documented (40). The genetic instability and high
mortality rate of cancer cells may be one reason why healthy lung
cells display a growth advantage during organoid culture compared
with cancer cells (42). Another
reason may be a large amount of stem cells in the medium
formulation (43). Bleijs et
al (32)supplemented growth
medium with a variety of small-molecule signal regulators and
growth factors, including A83-01, Noggin, SB 202190, R-spondin,
Y-27632, FGF7 and FGF10 and also added Nutlin-3α to induce the
senescence or apoptosis of TP53 wild-type cells, which can slow the
increased proliferation rate observed in healthy epithelial cells.
Utilizing the Nutlin-3a selection method, the aforementioned study
enlarged the selected LCOS carrying p53 mutations and produced pure
LCOs derived from several tumor tissues belonging to different
histological subtypes (mucinous/acinar/lepidic). However, the
effective incubation time of these organoids was not detailed in
the study.
The aim of the aforementioned organoid culture
strategies is to construct pure LCOs while largely inhibiting the
growth of healthy lung cells. Other culture techniques, including
ALI, have also used these supplements (44). It should be noted that the
traditional method of submerged Matrigel enriches only epithelial
cancer cells but does not maintain components
(lymphocyte/tumor-associated macrophages) (17). Therefore, co-culture of
patient-derived organoids (PDOs) with exogenous immune cells is
necessary for temporal modeling in these methodologies. A pure
tumor organoid was produced by Dijkstra et al (45) using Nutlin-3a, a mouse double minute
2 homolog inhibitor, to culture NSCLC organoids with p53 mutations,
which was compared with patient autologous peripheral blood
mononuclear cells (PBMCs) in Geltrex basement membrane. PBMCs were
co-cultured with colorectal cancer cell and induced tumor-reactive
CD8+ population expansion and showed specific anti-tumor
responsiveness in the TME. A previous study by Takahashi et
al (46) also used this method
to culture LCOs called ‘Fukushima’ organoids and assess the
efficacy of lung cancer immunotherapy by simulating the complex
interaction between immune cells and malignant cells. However, the
TME of the cultivated organoid is still relatively monolithic,
which is a drawback of this strategy, but this problem can be
solved by producing a comprehensive model of native TME.
In a microfluidic 3D device, a patient- or
murine-derived organotypic tumor spheroid (PDOTS/MDOTS) is
cultivated in collagen gel (type IV collagenase and HEPES)
(47). In PDOTS/MDOTS cultures,
tumor tissue specimens are obtained from patients and separated
enzymatically and mechanically (48). This process ultimately yields a
heterogeneous mixture of spheroids, single cells and macroscopic
tumor fragments. Next, this mixture is filtered via 100 and 40 mm
aperture filters in order to retain 40–100 mm diameter spheres.
These spheres are then combined with collagen gel, pelleted in
ultra-low attachment plates and seeded into the middle of regional
microfluidic device. The medium is injected into the medium
channels flanking the central channel to nurture the spheroids. In
addition, Jung et al (49)
developed an all-in-one microfluidic system that continuously flows
medicated medium through the system to deliver nutrients and oxygen
to LCOs and can also deliver drugs to LCOs for drug susceptibility
testing. The aforementioned study also reported that after
induction using cisplatin and etoposide, cells in the peripheral
region of LCOs died, while cells in the core region continued to
survive for 72 h. This suggested that the core region of LCOs
contained chemo-resistant cells, which indicated that this system
could aid in predicting the chemotherapy response of lung cancer
cells and could be used to choose the most effective treatment
plan. Notably, in this approach, spheroids preserve the cellular
diversity and complexity of native cancer tissues, such as
autologous bone marrow cell populations (dendritic cells,
myeloid-derived suppressor cells, monocytes and tumor-associated
macrophages), lymphocytes (T and B cells) and non-reconstituted
cancer cells (50). By adding
exogenous T cells to the media channels, Kitajima et al
(51) evaluated T cell infiltration
into LCOs and LCO interactions and crosstalk with immune cells in
these devices.
The ALI culture method relies on a set of inner and
outer disks, with the inner disk being made of two layers (52). First, a collagen gel matrix is added
to the inner disk to prepare the bottom layer (53). After obtaining primary tissue
samples, samples are immediately placed into ice-cold culture
medium for further preparation (53). Next, the tissue is rinsed,
physically broken down into small pieces, then incubated and mixed
into collagen gel. This mixture is then poured onto the underlying
gel matrix of the inner dish, to produce the top layer inside the
inner dish (53). The gel in the
inner dish is allowed to harden by being placed into the outer dish
and moved to a 37°C incubator (53). The culture medium is subsequently
poured into the outer disk, where it can permeate into the inner
disk via the porous membrane. Tissues and organs may efficiently
receive oxygen from the top layer as the culture is directly
exposed to the air (53–55). In contrast to submerged Matrigel
cultures, ALI enables the development of larger multicellular
fragments that maintain original tissue structure, such as
resection-sourced cancer cells co-cultured with non-reconstituted
immune cells and indigenous stroma (56). According to a previously published
study by Finnberg et al (57), endogenous CD45+ immune cells of
human colorectal organoids and LCOs can persist for up to 10 days
using the ALI culture technique, despite a significant reduction in
the number of CD3+ cells. Additionally, the PDO cultured with ALI
can retain native tumor genetic changes in addition to the TME's
complex cellular components and structural organization (44). Compared with submerged Matrigel
cultures, ALI's characteristics make this technique an appropriate
TME model (58). The ALI technique
was utilized to develop PDOs from several types of surgically
removed tumors, including lung cancer (44). Cultures retain tumor epithelium and
its stromal microenvironment for 30 days along with fibroblasts and
immune cells, such as tumor-associated T helper cells, B cells,
cytotoxic T lymphocytes, natural killer (NK) cells, NK T cells and
macrophages. Furthermore, the T cell receptor (TCR) heterogeneity
present in the initial tumor is retained in these cultures
(59).
ALI-cultured organoids derived from murine or human
tumors are notably different in many aspects. The doubling times
and serial passaging of cell line-derived murine organoids are
rapid and reproducible (60).
Comparatively, the growth and reproduction rates of PDOs are highly
variable and are associated with the initial conditions present
during tumor biopsy, such as sampling condition, preservation
duration, tumor viability, pre- or post-treatment and tumor
histology grade (high or low) (61). Thus, in the culture plate where
organoids are grown, necrotic tissue is various proportions of the
organoids. In immunogenic murine-derived organotypic tumor spheroid
(MDOTS), cytotoxic responses as well as activation and expansion of
tumor infiltrating lymphocytes (TILs) are fixed, but a very
different picture exists in human PDOs, given the patient-intrinsic
differences, and many well-documented tumors and immune components
are resistant to checkpoint inhibition to various extents. Immunity
declined and fibroblast stroma in murine- and human-derived
organoids deteriorated during a 1 year period. Though
anti-CD3/anti-CD28 or IL-2 supplementation can slow TIL loss,
further optimization is required to preserve TIL above the current
60 day limit (40).
In the field of lung cancer treatment, particularly
for NSCLC, a transformation occurred following the identification
of immunological checkpoints and the discovery of ICIs, work which
was recognized with a Nobel Prize (62). Among several types of tumors that
are known to use immune checkpoints to evade the host immune
system, cytotoxic T lymphocyte antigen-4 (CTLA-4) and programmed
cell death protein-1 (PD-1)/PD-L1 are the most widely clinically
used (63). Once these pathways are
inhibited, cytotoxic T-cell priming and antitumor activity can
occur. Successful breakthroughs with ICIs in the immunotherapeutic
treatment of NSCLC include PD-1 inhibitors, such as nivolumab
(64) and pembrolizumab (65), PD-L1 inhibitors durvalumab (66) and atezolizumab (67) and the CTLA-4 inhibitor ipilimumab
(68). Compared with the increased
application of ICIs in the treatment of NSCLC, the frequency of the
use of this type of treatment in small cell lung cancer (SCLC)
remains low (69). Over the past 30
years, first-line therapy that combines platinum-based chemotherapy
with a PD-L1 inhibitor (atezolizumab or durvalumab) has been the
sole advancement in the treatment of extensive-stage SCLC (70). However, though ICIs have achieved
unprecedented success in clinical trial sites in the treatment of
lung cancer, breast cancer, ovarian cancer and other fields, the
in vivo mechanisms of action and possible drug resistance
factors are still unclear. First, ICI-based treatment does not
provide long-term benefits for ~70% of patients with advanced NSCLC
and ~80% of patients with SCLC (71). Secondly, efforts are currently
underway to identify a suitable biomarker of the ICI response.
Finally, SCLC is difficult to treat and the addition of existing
ICIs to therapeutic modalities has seen limited success due to the
immunosuppressive TME (59). In
order to overcome these obstacles and combat the propensity for
SCLC to persist, new strategies for harnessing the potential of the
immune system must be developed.
Tumor immunity arises from the synergy of active
communication between peripheral and intratumoral components
(72–74). However, current in vivo
systems are unable to break down the distinct contributions made by
peripheral immune cells to intra-tumor immunological responses,
rather than immune cells that are inherent residents of the TME
(75). The recognition of such
local events is currently enabled by the basic mechanism of
existing PDOs. It remains unclear whether anti-PD-1 antibodies can
enlarge intratumorally depleted CD8+ T cells through acting on
peripheral and tumor-infiltrating populations (76) and also increase the proliferation of
peripheral blood PD-1+ CD8+ T cells (77,78).
Therefore, PD-1 axis inhibition inside the TME is effective for the
induction of growth and activation of TILs, as anti-PD-1 and
anti-PD-L1 can activate TILs in human and murine PDOs. According to
a previous study reported by Neal et al (79), expanded clear cell renal cell
carcinoma tumor infiltrating lymphocytes(ccRCC TIL) TCR clonotypes
are enriched in depleted T cells, which may aid in the development
of tumors.
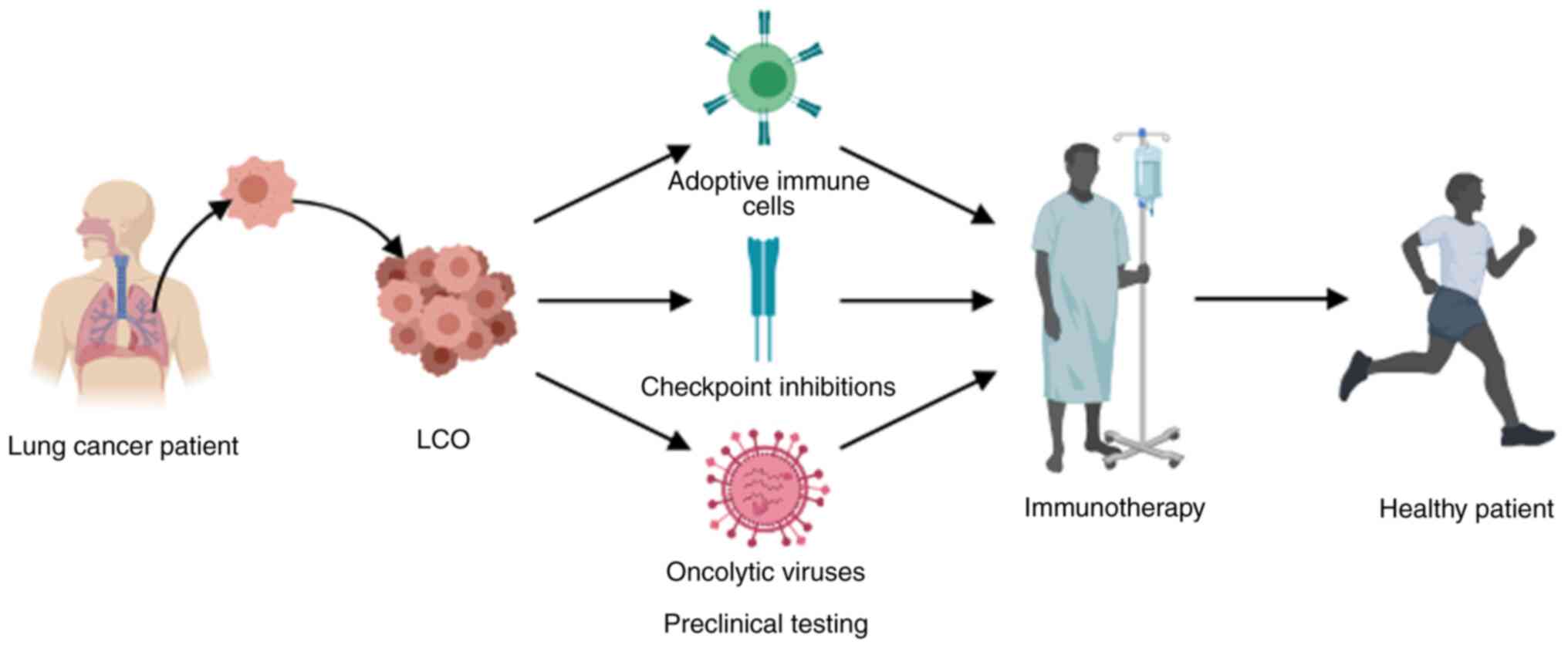
Recently, immune cell-based treatment has been used
as a potential immunotherapy strategy to combat lung cancer
(87). Building on the success of
previously published oncology studies, the aforementioned study
harnessed the innate ability of immune cells to destroy cancer
cells and generate a robust immune response by bringing in
additional cells to the TME. The genetic engineering of T or NK
cells enables these cells to target certain antigens produced on
lung cancer cells and reprogram the behavior of immune cells to
enhance their function, increasing the specificity of immune
detection of cancer cells.
Most immune cell-based lung cancer targeting studies
have reported the use of chimeric antigen receptor (CAR)-T cells,
although a growing body of research is using the allogenic nature
of NK cells to provide infused CAR-T cells, which is a potentially
safer alternative (NK cells have a limited life span in circulation
and less produce memory cells) (88,89).
Despite the success of cell-based approaches to treat hematological
malignancies, solid tumors, such as lung cancers, consistently
demonstrate poor response rates to these treatments (90). This may be related to immune
tolerance and TME heterogeneity in the presence of immune cells in
adoptive cell transfer therapy. In lung cancer, the TME forms a
complex barrier to immune cell activity, often leading to
resistance to therapy. In the lung cancer TME, CAR-T cells display
poor ability to aggregate (91). In
addition, unfavorable intra-tumoral immunometabolic conditions
(enhanced glycolysis and lactate production, reduced mitochondrial
respiration, and alterations in lipid and amino acid metabolism)
can lead to the dysfunction of infiltrating immune cells. CAR-T
cell dysfunction can involve fatigue, senescence or weakness
(92). Systemic drug toxicity
caused by CAR-T cells is also a possibility (93). Adoptive cell transfer immunotherapy
involves the collection of circulating lymphocytes or TILs and the
selection or genetic engineering of high-affinity TCRs that detect
tumor antigens. These cells are subsequently activated and expanded
in vitro before infusion into patients (94,95).
CAR-T cells produce synthetic TCRs that target certain antigens on
the tumor cell surface and can overcome major histocompatibility
complex (MHC) limitations such as polymorphism (96). Previous studies have reported that
PDO is an effective platform to assess the cytotoxicity of T cells
(TCR or CAR-T cells) for specific tumors (97,98).
PDO was utilized to assess the outcome of CAR-T cell therapy
combined with the apoptosis antagonist birinapant in a study by
Michie et al (99). The
aforementioned study reported that CAR-T cells alone were
insufficient, whereas a combination of birinapant and CAR-T cells
inhibits PDO development in a manner which relied on tumor necrosis
factor. A preclinical model (luciferase-based measurement), 3D PDO,
was created by Schnalzger et al (100) to enable the identification of
CAR-mediated cytotoxicity in a natural TIME model. Additionally,
the aforementioned study developed a procedure for confocal
live-cell imaging to dynamically monitor cytotoxic activity against
individual organoids. In co-cultures of NK cells with regular
cancer organoids on colorectal cancer (CRC) or extracellular matrix
layers, co-cultures demonstrated durable effector-target cell
connections. Additionally, tumor antigen-specific cytotoxicity of
FRIZZLED or EGFRvIII receptor-targeted NK-92 cells engineered by
CAR was monitored using CRC organoids. In conclusion, a framework
was developed to analyze CAR effectiveness and tumor selectivity in
an individualized manner (101).
In addition, although epithelial-only PDO is devoid of
immunological and stromal components, it can be utilized to select
T cells that are reactive to tumors (45). Tumor-reactive lymphocyte enrichment,
stimulation and efficacy assessments may be performed using this
co-culture method (45). To produce
tumor-reactive CD8+ communities, autologous circulating T cells
(PBMCs) and CRC or NSCLC organoids were co-cultured in medium
containing IL-2, anti-CD28 and anti-PD1. MHC-dependent cytotoxicity
and T cell-mediated killing effects on autologous tumor organoids
were observed after 2 weeks of co-culture, along with an elevation
in the production of IFNg and CD107a in CD8+ T cells. However, the
survival of matching healthy organoids was unaffected by CD8+ T
cells that could react to tumor cells. As a result, a previous
study created a platform for developing tumor-reactive T cells and
testing the sensitivity and precision of autologous T cells to
eradicate cancer cells at the individual level (102). In addition, it is possible to
extract tumor-reactive T cells from TILs and re-infuse them into
patients, which is a more targeted treatment than the use of
non-infiltrating lymphocytes (103).
The cytotoxicity and infectivity of oncolytic
viruses alone or in conjunction with chemotherapy can be studied
using PDO (104). Oncolytic
adenovirus demonstrated high replication selectivity in PDAC
organoids in a study by Raimondi et al (104) but not in organoids from healthy
pancreatic tissue. Additionally, patient-specific responses were
observed, which indicated that PDO was a useful in vitro
tumor model for evaluating early oncolytic viral responses. The
efficacy and specificity of antibody-based immunotherapies can also
be studied using tumor organoids. Previous studies focused on
antibody-based ICB treatment have utilized organoid models
(42,50,105).
Courau et al (106)
reported that the infiltration of activated/memory T and NK cells
into organoids involves both the NK Group 2A-Human Leukocyte
Antigen E (NKG2A-HLA-E) and NKG2D-Major Histocompatibility Complex
Class I Chain-Related Molecule A/B (MICA/B) pathways and these
activated cells may subsequently destroy 3D structures and kill
cancer cells. The aforementioned study demonstrated that during
co-culture with autologous TIL, anti-MICA/B antibodies and an
antibody cocktail consisting of anti-MICA/B and anti-NKG2A might
trigger immune-mediated death in colorectal tumor organoids.
Gonzalez-Exposito et al (107) constructed eight PDOs, seven of
which were derived from refractory metastatic CRC, while one was
derived from untreated primary CRC tumor to study the mechanisms of
resistance and sensitivity to cibisatamab, a compound which binds
tumor cells and CD3 Carcinoembryonic antigen (CEA) bispecific
monoclonal antibody on T cells. In order to assess the efficacy of
cibisatamab, co-cultures of organoids and allogeneic CD8+ T cells
were produced. This method demonstrated that CEA-low PDO is
resistant to cibisatamab whereas CEA-high PDO is vulnerable to this
treatment. CEA-low cells support tumor cell proliferation and
through the use of RNA sequencing, it was reported that CEA-low
cells demonstrate increased WNT/β-catenin pathway activity
(108). To increase the efficacy
of this therapy, the aforementioned study proposed the use of a
possible combination of cibisatamab with an inhibitor of the
WNT/β-catenin pathway.
The study of the dynamic interplay between the tumor
and immune system using PDO has gained increasing attention over
recent years. Moreover, advances in TME modeling could facilitate
the testing of novel immunotherapies in preclinical settings.
Organoid-focused techniques have limitations when used to analyze
the effects of TIME on the behavior of immunotherapy medications in
cancer, due to the absence of stroma and a vascular network.
Complex organoids can be created by co-culturing source or
progenitor cells with cancer-associated fibroblasts, mesodermal
progenitor cells and immune cells to negate these constraints.
Furthermore, the cancer-immune cycle, composed of effector T cell
initiation or activation, T cell trafficking or infiltration into
cancer tissue and T cell killing or recognition of cancer cells,
may be mimicked through organoid co-culture with additional immune
cells sourced from lymph nodes or PBMCs. IL-2, anti-CD3 and
anti-CD28 antibodies are among the extra supplements that are
advised for the long-term protection of immune cells. The
composition of the growth medium should also be adjusted such that
it promotes the development of all clones equally rather than
favoring the growth of any particular colony. The modeling of
native TME can be enhanced by the reproduction of mechanical
stressors, such as physiological shear flow. The repeatability of
drug screening findings can be enhanced by the use of scaffolds
with a certain shape, cell quantity, regulated size, relative
arrangement and customized composition of the various cell types
inside the organoid. Animal models require more time to develop a
research platform than organoid models, whereas effective human
organoid cultures may be established in weeks or months while
enabling high-throughput screening and circumventing possible
ethical implications. question. Thus, PDO can be utilized in
precision medicine to provide reliable data about individual drug
responses and mutation profiles (109). At present, researchers have begun
to use LCO models to evaluate the efficacy of chemotherapy and
targeted drug therapy in a real clinical scenario (110). Certain clinical studies, such as
NCT03778814, NCT04951115 and NCT05332925, have been registered to
explore the effect of PDO in different stages of lung cancer
immunotherapy, so as to reduce the current treatment time spent in
the clinical-laboratory-clinical cycle(mainly for the time of drug
screening model construction). Immunotherapy may also be used for
additional practical applications (tumor vaccine) in the
future.
Not applicable.
The present study was supported by the National Natural Science
Foundation of China (grant no. 81603438), the Natural Science
Foundation of Tianjin (grant no. 19JCZDJC37000) and the Scientific
Research Plan project of Tianjin Municipal Education Commission
(grant no. 2020KJ163).
Not applicable.
YJ and HT designed the project and wrote, reviewed
and edited the manuscript. JR and RM constructed figures and
tables. All authors read and approved the final version of the
manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Howlader N, Forjaz G, Mooradian MJ, Meza
R, Kong CY, Cronin KA, Mariotto AB, Lowy DR and Feuer EJ: The
effect of advances in lung-cancer treatment on population
mortality. N Engl J Med. 383:640–649. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kucherlapati R: Impact of precision
medicine in oncology. Cancer J. 29:1–2. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen DS and Mellman I: Elements of cancer
immunity and the cancer-immune set point. Nature. 541:321–330.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lahiri A, Maji A, Potdar PD, Singh N,
Parikh P, Bisht B, Mukherjee A and Paul MK: Lung cancer
immunotherapy: Progress, pitfalls, and promises. Mol Cancer.
22:402023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hellmann MD, Nathanson T, Rizvi H, Creelan
BC, Sanchez-Vega F, Ahuja A, Ni A, Novik JB, Mangarin LMB,
Abu-Akeel M, et al: Genomic features of response to combination
immunotherapy in patients with advanced non-small-cell lung cancer.
Cancer Cell. 33:843–852.e4. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Overman MJ, Lonardi S, Wong KYM, Lenz HJ,
Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill
A, et al: Durable clinical benefit with Nivolumab plus Ipilimumab
in DNA mismatch repair-deficient/microsatellite instability-high
metastatic colorectal cancer. J Clin Oncol. 36:773–779. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jenkins RW, Aref AR, Lizotte PH, Ivanova
E, Stinson S, Zhou CW, Bowden M, Deng J, Liu H, Miao D, et al: Ex
Vivo profiling of PD-1 blockade using Organotypic tumor spheroids.
Cancer Discov. 8:196–215. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hugo W, Zaretsky JM, Sun L, Song C, Moreno
BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G,
et al: Genomic and transcriptomic features of response to Anti-PD-1
therapy in metastatic melanoma. Cell. 165:35–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harel M, Ortenberg R, Varanasi SK,
Mangalhara KC, Mardamshina M, Markovits E, Baruch EN, Tripple V,
Arama-Chayoth M, Greenberg E, et al: Proteomics of melanoma
response to immunotherapy reveals mitochondrial dependence. Cell.
179:236–250.e18. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jamal-Hanjani M, Quezada SA, Larkin J and
Swanton C: Translational implications of tumor heterogeneity. Clin
Cancer Res. 21:1258–1266. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mestas J and Hughes CC: Of mice and not
men: Differences between mouse and human immunology. J Immunol.
172:2731–2738. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jespersen H, Lindberg MF, Donia M,
Söderberg EMV, Andersen R, Keller U, Ny L, Svane IM, Nilsson LM and
Nilsson JA: Clinical responses to adoptive T-cell transfer can be
modeled in an autologous immune-humanized mouse model. Nat Commun.
8:7072017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao Y, Shuen TWH, Toh TB, Chan XY, Liu M,
Tan SY, Fan Y, Yang H, Lyer SG, Bonney GK, et al: Development of a
new patient-derived xenograft humanised mouse model to study
human-specific tumour microenvironment and immunotherapy. Gut.
67:1845–1854. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hidalgo M, Amant F, Biankin AV, Budinská
E, Byrne AT, Caldas C, Clarke RB, de Jong S, Jonkers J, Mælandsmo
GM, et al: Patient-derived xenograft models: An emerging platform
for translational cancer research. Cancer Discov. 4:998–1013. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wan ACA: Recapitulating Cell-cell
interactions for Organoid Construction-are biomaterials
dispensable? Trends Biotechnol. 34:711–721. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Clevers H: Modeling development and
disease with organoids. Cell. 165:1586–1597. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eiraku M, Watanabe K, Matsuo-Takasaki M,
Kawada M, Yonemura S, Matsumura M, Wataya T, Nishiyama A, Muguruma
K and Sasai Y: Self-organized formation of polarized cortical
tissues from ESCs and its active manipulation by extrinsic signals.
Cell Stem Cell. 3:519–532. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sato T, Vries RG, Snippert HJ, van de
Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters
PJ and Clevers H: Single Lgr5 stem cells build crypt-villus
structures in vitro without a mesenchymal niche. Nature.
459:262–265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim M, Mun H, Sung CO, Cho EJ, Jeon HJ,
Chun SM, Jung DJ, Shin TH, Jeong GS, Kim DK, et al: Patient-derived
lung cancer organoids as in vitro cancer models for therapeutic
screening. Nat Commun. 10:39912019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi R, Radulovich N, Ng C, Liu N, Notsuda
H, Cabanero M, Martins-Filho SN, Raghavan V, Li Q, Mer AS, et al:
Organoid cultures as preclinical models of non-small cell lung
cancer. Clin Cancer Res. 26:1162–174. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Strikoudis A, Cieślak A, Loffredo L, Chen
YW, Patel N, Saqi A, Lederer DJ and Snoeck HW: Modeling of fibrotic
lung disease using 3D organoids derived from human pluripotent stem
cells. Cell Rep. 27:3709–3723.e5. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
De Poel E, Lefferts JW and Beekman JM:
Intestinal organoids for Cystic Fibrosis research. J Cyst Fibros.
19:S60–S64. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Paolicelli G, Luca A, Jose SS, Antonini M,
Teloni I, Fric J and Zelante T: Using lung organoids to investigate
epithelial barrier complexity and IL-17 signaling during
respiratory infection. Front Immunol. 10:3232019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Salahudeen AA, Choi SS, Rustagi A, Zhu J,
de la O SM, Flynn RA, Margalef-Català M, Santos AJM, Ju J, Batish
A, et al: Progenitor identification and SARS-CoV-2 infection in
long-term human distal lung organoid cultures. Preprint. bioRxiv.
Jul 27–2020.doi: 10.1101/2020.07.27.212076. PubMed/NCBI
|
|
25
|
Barkauskas CE, Chung MI, Fioret B, Gao X,
Katsura H and Hogan BL: Lung organoids: Current uses and future
promise. Development. 144:986–997. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rock JR, Onaitis MW, Rawlins EL, Lu Y,
Clark CP, Xue Y, Randell SH and Hogan BL: Basal cells as stem cells
of the mouse trachea and human airway epithelium. Proc Natl Acad
Sci USA. 106:12771–12775. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McQualter JL, Yuen K, Williams B and
Bertoncello I: Evidence of an epithelial stem/progenitor cell
hierarchy in the adult mouse lung. Proc Natl Acad Sci USA.
107:1414–9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen YW, Huang SX, de Carvalho ALRT, Ho
SH, Islam MN, Volpi S, Notarangelo LD, Ciancanelli M, Casanova JL,
Bhattacharya J, et al: A three-dimensional model of human lung
development and disease from pluripotent stem cells. Nat Cell Biol.
19:542–549. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sachs N, Papaspyropoulos A, Zomer-van
Ommen DD, Heo I, Böttinger L, Klay D, Weeber F, Huelsz-Prince G,
Iakobachvili N, Amatngalim GD, et al: Long-term expanding human
airway organoids for disease modeling. EMBO J. 38:e1003002019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roerink SF, Sasaki N, Lee-Six H, Young MD,
Alexandrov LB, Behjati S, Mitchell TJ, Grossmann S, Lightfoot H,
Egan DA, et al: Intra-tumour diversification in colorectal cancer
at the single-cell level. Nature. 556:457–62. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Subtil B, Iyer KK, Poel D, Bakkerus L,
Gorris MAJ, Escalona JC, van den Dries K, Cambi A, Verheul HMW, de
Vries IJM and Tauriello DVF: Dendritic cell phenotype and function
in a 3D co-culture model of patient-derived metastatic colorectal
cancer organoids. Front Immunol. 14:11052442023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bleijs M, van de Wetering M, Clevers H and
Drost J: Xenograft and organoid model systems in cancer research.
EMBO J. 38:e1016542019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Weiswald LB, Bellet D and Dangles-Marie V:
Spherical cancer models in tumor biology. Neoplasia. 17:1–15. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang R, Zhang J, Chen S, Lu M, Luo X, Yao
S, Liu S, Qin Y and Chen H: Tumor-associated macrophages provide a
suitable microenvironment for non-small lung cancer invasion and
progression. Lung Cancer. 4:188–196. 2011. View Article : Google Scholar
|
|
35
|
de Visser KE and Joyce JA: The evolving
tumor microenvironment: From cancer initiation to metastatic
outgrowth. Cancer Cell. 41:374–403. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Luckett KA and Ganesh K: Engineering the
immune microenvironment into organoid models. Ann Rev Cancer Biol.
7:171–187. 2023. View Article : Google Scholar
|
|
37
|
Yuki K, Cheng N, Nakano M and Kuo CJ:
Organoid models of tumor immunology. Trends Immunol. 41:652–664.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Powley IR, Patel M, Miles G, Pringle H,
Howells L, Thomas A, Kettleborough C, Bryans J, Hammonds T,
MacFarlane M and Pritchard C: Patient-derived explants (PDEs) as a
powerful preclinical platform for anti-cancer drug and biomarker
discovery. Br J Cancer. 122:735–744. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ouchi T, Morimura S, Dow LE, Miyoshi H and
Udey MC: EpCAM (CD326) regulates intestinal epithelial integrity
and stem cells via Rho-associated kinase. Cells. 10:2562021.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Watanabe K, Ueno M, Kamiya D, Nishiyama A,
Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S,
Muguruma K and Sasai Y: A ROCK inhibitor permits survival of
dissociated human embryonic stem cells. Nat Biotechnol. 25:681–686.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huo KG, D'Arcangelo E and Tsao MS:
Patient-derived cell line, xenograft and organoid models in lung
cancer therapy. Transl Lung Cancer Res. 9:2214–2232. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Karthaus WR, Iaquinta PJ, Drost J,
Gracanin A, van Boxtel R, Wongvipat J, Dowling CM, Gao D, Begthel
H, Sachs N, et al: Identification of multipotent luminal progenitor
cells in human prostate organoid cultures. Cell. 159:163–175. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pamarthy S and Sabaawy HE: Patient derived
organoids in prostate cancer: Improving therapeutic efficacy in
precision medicine. Mol Cancer. 20:1252021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Weeber F, Ooft SN, Dijkstra KK and Voest
EE: Tumor organoids as a Pre-clinical cancer model for drug
discovery. Cell Chem Biol. 24:1092–1100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dijkstra KK, Cattaneo CM, Weeber F,
Chalabi M, van de Haar J, Fanchi LF, Slagter M, van der Velden DL,
Kaing S, Kelderman S, et al: Generation of tumor-reactive T cells
by Co-culture of peripheral blood lymphocytes and tumor organoids.
Cell. 174:1586–1598.e12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Takahashi N, Hoshi H, Higa A, Hiyama G,
Tamura H, Ogawa M, Takagi K, Goda K, Okabe N, Muto S, et al: An in
vitro system for evaluating molecular targeted drugs using lung
patient-derived tumor organoids. Cells. 8:4812019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sontheimer-Phelps A, Hassell BA and Ingber
DE: Modelling cancer in microfluidic human organs-on-chips. Nat Rev
Cancer. 19:65–81. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Aref AR, Campisi M, Ivanova E, Portell A,
Larios D, Piel BP, Mathur N, Zhou C, Coakley RV, Bartels A, et al:
3D microfluidic ex vivo culture of organotypic tumor spheroids to
model immune checkpoint blockade. Lab Chip. 18:3129–3143. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jung DJ, Shin TH, Kim M, Sung CO, Jang SJ
and Jeong GS: A one-stop microfluidic-based lung cancer organoid
culture platform for testing drug sensitivity. Lab Chip.
19:2854–2865. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jenkins RW, Barbie DA and Flaherty KT:
Mechanisms of resistance to immune checkpoint inhibitors. Br J
Cancer. 118:9–16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kitajima S, Ivanova E, Guo S, Yoshida R,
Campisi M, Sundararaman SK, Tange S, Mitsuishi Y, Thai TC, Masuda
S, et al: Suppression of STING associated with LKB1 loss in
KRAS-driven lung cancer. Cancer Discov. 9:34–45. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Öhlinger K, Kolesnik T, Meindl C, Gallé B,
Absenger-Novak M, Kolb-Lenz D and Fröhlich E: Air-liquid interface
culture changes surface properties of A549 cells. Toxicol In Vitro.
60:369–382. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li X, Ootani A and Kuo C: An Air-liquid
interface culture system for 3D organoid culture of diverse primary
gastrointestinal tissues. Methods Mol Biol. 1422:33–40. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ootani A, Li X, Sangiorgi E, Ho QT, Ueno
H, Toda S, Sugihara H, Fujimoto K, Weissman IL, Capecchi MR and Kuo
CJ: Sustained in vitro intestinal epithelial culture within a
Wnt-dependent stem cell niche. Nat Med. 15:701–706. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li X, Nadauld L, Ootani A, Corney DC, Pai
RK, Gevaert O, Cantrell MA, Rack PG, Neal JT, Chan CW, et al:
Oncogenic transformation of diverse gastrointestinal tissues in
primary organoid culture. Nat Med. 20:769–777. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xia T, Du WL, Chen XY and Zhang YN:
Organoid models of the tumor microenvironment and their
applications. J Cell Mol Med. 25:5829–5841. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Finnberg NK, Gokare P, Lev A, Grivennikov
SI, MacFarlane AW IV, Campbell KS, Winters RM, Kaputa K, Farma JM,
Abbas AE, et al: Application of 3D tumoroid systems to define
immune and cytotoxic therapeutic responses based on tumoroid and
tissue slice culture molecular signatures. Oncotarget.
8:66747–66757. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sachs N, de Ligt J, Kopper O, Gogola E,
Bounova G, Weeber F, Balgobind AV, Wind K, Gracanin A, Begthel H,
et al: A living Biobank of breast cancer organoids captures disease
heterogeneity. Cell. 172:373–386.e10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mu P, Zhou S, Lv T, Xia F, Shen L, Wan J,
Wang Y, Zhang H, Cai S, Peng J, et al: Newly developed 3D in vitro
models to study tumor-immune interaction. J Exp Clin Cancer Res.
42:812023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Koehler KR and Hashino E: 3D mouse
embryonic stem cell culture for generating inner ear organoids. Nat
Protocols. 9:1229–1244. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
El Harane S, Zidi B, El Harane N, Krause
KH, Matthes T and Preynat-Seauve O: Cancer spheroids and organoids
as novel tools for research and therapy: State of the art and
challenges to guide precision medicine. Cells. 12:10012023.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho
BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK,
Bondarenko I, et al: Pembrolizumab versus chemotherapy for
previously untreated, PD-L1-expressing, locally advanced or
metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised,
open-label, controlled, phase 3 trial. Lancet. 393:1819–1830. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang H, Dai Z, Wu W, Wang Z, Zhang N,
Zhang L, Zeng WJ, Liu Z and Cheng Q: Regulatory mechanisms of
immune checkpoints PD-L1 and CTLA-4 in cancer. J Exp Clin Cancer
Res. 40:1842021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Paz-Ares L, Ciuleanu TE, Cobo M, Schenker
M, Zurawski B, Menezes J, Richardet E, Bennouna J, Felip E,
Juan-Vidal O, et al: First-line nivolumab plus ipilimumab combined
with two cycles of chemotherapy in patients with non-small-cell
lung cancer (CheckMate 9LA): An international, randomised,
open-label, phase 3 trial. Lancet Oncol. 22:198–211. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Rudin CM, Awad MM, Navarro A, Gottfried M,
Peters S, Csőszi T, Cheema PK, Rodriguez-Abreu D, Wollner M, Yang
JC, et al: Pembrolizumab or placebo plus etoposide and platinum as
first-line therapy for extensive-stage small-cell lung cancer:
Randomized, double-blind, phase III KEYNOTE-604 study. J Clin
Oncol. 38:2369–2379. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Rizvi H, Sanchez-Vega F, La K, Chatila W,
Jonsson P, Halpenny D, Plodkowski A, Long N, Sauter JL, Rekhtman N,
et al: Molecular determinants of response to Anti-programmed cell
death (PD)-1 and Anti-programmed Death-Ligand 1 (PD-L1) blockade in
patients with non-small-cell lung cancer profiled with targeted
next-generation sequencing. J Clin Oncol. 36:633–641. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Felip E, Altorki N, Zhou C, Csőszi T,
Vynnychenko I, Goloborodko O, Luft A, Akopov A, Martinez-Marti A,
Kenmotsu H, et al: Adjuvant atezolizumab after adjuvant
chemotherapy in resected stage IB-IIIA non-small-cell lung cancer
(IMpower010): A randomised, multicentre, open-label, phase 3 trial.
Lancet. 398:1344–1357. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wong DJ, Bauer TM, Gordon MS, Bene-Tchaleu
F, Zhu J, Zhang X and Cha E: Safety and clinical activity of
atezolizumab plus ipilimumab in locally advanced or metastatic
non-small cell lung cancer: Results from a phase 1b trial. Clin
Lung Cancer. 23:273–281. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wong SK and Iams WT: Front line
applications and future directions of immunotherapy in small-cell
lung cancer. Cancers. 13:5062021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Iams WT, Porter J and Horn L:
Immunotherapeutic approaches for small-cell lung cancer. Nat Rev
Clin Oncol. 17:300–312. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Mamdani H, Matosevic S, Khalid AB, Durm G
and Jalal SI: Immunotherapy in lung cancer: Current landscape and
future directions. Front Immunol. 13:8236182022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Spitzer MH, Carmi Y, Reticker-Flynn NE,
Kwek SS, Madhireddy D, Martins MM, Gherardini PF, Prestwood TR,
Chabon J, Bendall SC, et al: Systemic immunity is required for
effective cancer immunotherapy. Cell. 168:487–502.e15. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wei SC, Levine JH, Cogdill AP, Zhao Y,
Anang NAS, Andrews MC, Sharma P, Wang J, Wargo JA, Pe'er D and
Allison JP: Distinct cellular mechanisms underlie Anti-CTLA-4 and
Anti-PD-1 checkpoint blockade. Cell. 170:1120–33.e17. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Huang AC, Postow MA, Orlowski RJ, Mick R,
Bengsch B, Manne S, Xu W, Harmon S, Giles JR, Wenz B, et al: T-cell
invigoration to tumour burden ratio associated with anti-PD-1
response. Nature. 545:60–65. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kamphorst AO, Pillai RN, Yang S, Nasti TH,
Akondy RS, Wieland A, Sica GL, Yu K, Koenig L, Patel NT, et al:
Proliferation of PD-1+ CD8 T cells in peripheral blood after
PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci
USA. 114:4993–4998. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: Nivolumab versus Everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1803–1813. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Migden MR, Rischin D, Schmults CD,
Guminski A, Hauschild A, Lewis KD, Chung CH, Hernandez-Aya L, Lim
AM, Chang ALS, et al: PD-1 blockade with cemiplimab in advanced
cutaneous squamous-cell carcinoma. N Engl J Med. 379:341–351. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Neal JT, Li X, Zhu J, Giangarra V,
Grzeskowiak CL, Ju J, Liu IH, Chiou SH, Salahudeen AA, Smith AR, et
al: Organoid modeling of the tumor immune microenvironment. Cell.
175:1972–1988.e16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Larkin J, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J,
Dummer R, et al: Five-year survival with combined nivolumab and
ipilimumab in advanced melanoma. N Engl J Med. 381:1535–1546. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
González-Rodríguez E, Rodríguez-Abreu D
and Boronat M: Nivolumab for Squamous-cell cancer of head and neck.
N Engl J Med. 376:5952017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Socinski MA, Jotte RM, Cappuzzo F, Orlandi
F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D,
Thomas CA, Barlesi F, et al: Atezolizumab for first-line treatment
of metastatic nonsquamous NSCLC. N Engl J Med. 378:2288–2301. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zhou Z, Cong L and Cong X: Patient-derived
organoids in precision medicine: Drug screening, organoid-on-a-chip
and living organoid biobank. Front Oncol. 11:7621842021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kong JCH, Guerra GR, Millen RM, Roth S, Xu
H, Neeson PJ, Darcy PK, Kershaw MH, Sampurno S, Malaterre J, et al:
Tumor-infiltrating lymphocyte function predicts response to
neoadjuvant chemoradiotherapy in locally advanced rectal cancer.
JCO Precis Oncol. 2:1–15. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Bhattacharya S, Calar K and de la Puente
P: Mimicking tumor hypoxia and tumor-immune interactions employing
three-dimensional in vitro models. J Exp Clin Cancer Res. 39:1–16.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Halldorsson S, Lucumi E, Gómez-Sjöberg R
and Fleming RMT: Advantages and challenges of microfluidic cell
culture in polydimethylsiloxane devices. Biosens Bioelectron.
63:218–231. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Budczies J, Kirchner M, Kluck K, Kazdal D,
Glade J, Allgäuer M, Kriegsmann M, Heußel CP, Herth FJ, Winter H,
et al: A gene expression signature associated with B cells predicts
benefit from immune checkpoint blockade in lung adenocarcinoma.
Oncoimmunology. 10:18605862021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Yi M, Li A, Zhou L, Chu Q, Luo S and Wu K:
Immune signature-based risk stratification and prediction of immune
checkpoint Inhibitor's efficacy for lung adenocarcinoma. Cancer
Immunol Immunother. 70:1705–1719. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Yilmaz A, Cui H, Caligiuri MA and Yu J:
Chimeric antigen receptor-engineered natural killer cells for
cancer immunotherapy. J Hematol Oncol. 13:1682021. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Dagar G, Gupta A, Masoodi T, Nisar S,
Merhi M, Hashem S, Chauhan R, Dagar M, Mirza S, Bagga P, et al:
Harnessing the potential of CAR-T cell therapy: Progress,
challenges, and future directions in hematological and solid tumor
treatments. J Transl Med. 21:4492023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Wen Q, Yang Z, Dai H, Feng A and Li Q:
Radiomics study for predicting the expression of PD-L1 and tumor
mutation burden in non-small cell lung cancer based on CT images
and Clinicopathological features. Front Oncol. 11:6202462021.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Chen Q, Zhang L, Mo X, You J, Chen L, Fang
J, Wang F, Jin Z, Zhang B and Zhang S: Current status and quality
of radiomic studies for predicting immunotherapy response and
outcome in patients with non-small cell lung cancer: A systematic
review and meta-analysis. Eur J Nucl Med Mol Imaging. 49:345–360.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Khorrami M, Prasanna P, Gupta A, Patil P,
Velu PD, Thawani R, Corredor G, Alilou M, Bera K, Fu P, et al:
Changes in CT radiomic features associated with lymphocyte
distribution predict overall survival and response to immunotherapy
in non-small cell lung cancer. Cancer Immunol Res. 8:108–19. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Rosenberg SA and Restifo NP: Adoptive cell
transfer as personalized immunotherapy for human cancer. Science.
348:62–68. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Waldman AD, Fritz JM and Lenardo MJ: A
guide to cancer immunotherapy: From T cell basic science to
clinical practice. Nat Rev Immunol. 20:651–668. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Majzner RG and Mackall CL: Clinical
lessons learned from the first leg of the CAR T cell journey. Nat
Med. 25:1341–1355. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Kaushik G, Venkatesha S, Verma B,
Vishwakarma B, Zhang AH and Wesa A: Preclinical in vitro and in
vivo models for adoptive cell therapy of cancer. Cancer J.
28:257–262. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Dekkers JF, Alieva M, Cleven A, Keramati
F, Wezenaar AKL, van Vliet EJ, Puschhof J, Brazda P, Johanna I,
Meringa AD, et al: Uncovering the mode of action of engineered T
cells in patient cancer organoids. Nat Biotechnol. 41:60–69. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Michie J, Beavis PA, Freeman AJ, Vervoort
SJ, Ramsbottom KM, Narasimhan V, Lelliott EJ, Lalaoui N, Ramsay RG,
Johnstone RW, et al: Antagonism of IAPs enhances CAR T-cell
efficacy. Cancer Immunol Res. 7:183–192. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Schnalzger TE, de Groot MH, Zhang C, Mosa
MH, Michels BE, Röder J, Darvishi T, Wels WS and Farin HF: 3D model
for CAR-mediated cytotoxicity using patient-derived colorectal
cancer organoids. EMBO J. 38:e1009282019. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Badalamenti G, Fanale D, Incorvaia L,
Barraco N, Listì A, Maragliano R, Vincenzi B, Calò V, Iovanna JL,
Bazan V and Russo A: Role of tumor-infiltrating lymphocytes in
patients with solid tumors: Can a drop dig a stone. Cell Immunol.
343:1037532019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Cattaneo CM, Dijkstra KK, Fanchi LF,
Kelderman S, Kaing S, van Rooij N, van den Brink S, Schumacher TN
and Voest EE: Tumor organoid-T-cell coculture systems. Nat Protoc.
15:15–39. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Islam SMR, Maeda T, Tamaoki N, Good ML,
Kishton RJ, Paria BC, Yu Z, Bosch-Marce M, Bedanova NM, Liu C, et
al: Reprogramming of Tumor-reactive Tumor-infiltrating Lymphocytes
to Human-induced pluripotent stem cells. Cancer Res Commun.
3:917–932. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Raimondi G, Mato-Berciano A,
Pascual-Sabater S, Rovira-Rigau M, Cuatrecasas M, Fondevila C,
Sánchez-Cabús S, Begthel H, Boj SF, Clevers H and Fillat C:
Patient-derived pancreatic tumour organoids identify therapeutic
responses to oncolytic adenoviruses. EBioMedicine. 56:1027862020.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Deng J, Wang ES, Jenkins RW, Li S, Dries
R, Yates K, Chhabra S, Huang W, Liu H, Aref AR, et al: CDK4/6
inhibition augments antitumor immunity by enhancing T-cell
activation. Cancer Discov. 8:216–233. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Courau T, Bonnereau J, Chicoteau J,
Bottois H, Remark R, Assante Miranda L, Toubert A, Blery M,
Aparicio T, Allez M and Le Bourhis L: Cocultures of human
colorectal tumor spheroids with immune cells reveal the therapeutic
potential of MICA/B and NKG2A targeting for cancer treatment. J
Immunother Cancer. 7:742019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Gonzalez-Exposito R, Semiannikova M,
Griffiths B, Khan K, Barber LJ, Woolston A, Spain G, von Loga K,
Challoner B, Patel R, et al: CEA expression heterogeneity and
plasticity confer resistance to the CEA-targeting bispecific
immunotherapy antibody cibisatamab (CEA-TCB) in patient-derived
colorectal cancer organoids. J Immunother Cancer. 7:1012019.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Sun CP, Lan HR, Fang XL, Yang XY and Jin
KT: Organoid models for precision cancer immunotherapy. Front
Immunol. 13:7704652022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Kim J, Koo BK and Knoblich JA: Human
organoids: Model systems for human biology and medicine. Nat Rev
Mol Cell Biol. 21:571–584. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Wang HM, Zhang CY, Peng KC, Chen ZX, Su
JW, Li YF, Li WF, Gao QY, Zhang SL, Chen YQ, et al: Using
patient-derived organoids to predict locally advanced or metastatic
lung cancer tumor response: A real-world study. Cell Rep Med.
4:1009112023. View Article : Google Scholar : PubMed/NCBI
|