Introduction
Head and neck squamous cell carcinoma (HNSCC) is
currently the sixth most common tumor entity worldwide and
represents a heterogeneous group of malignancies arising from the
oral cavity, pharynx, and larynx (1). Tumors are related to habitual alcohol
drinking and smoking (2).
Furthermore, there is an increasing number of high-risk human
papillomavirus-associated cancers especially within the oropharynx
(3). Multidisciplinary treatment
comprises surgery and adjuvant radiotherapy with or without
conventional chemotherapy, or primary chemoradiation. In cases of
treatment failure prognosis is dismal and therapeutic options are
limited to salvage surgery, radiotherapy and medical therapy. There
was hardly any improvement for survival in recurrent or metastatic
HNSCC (R/M-HNSCC) during the last decades (4) until the rise of immune checkpoint
inhibition (ICI) targeting programmed cell death protein 1 (PD-1).
With the approval of the two PD-1 inhibitors nivolumab and
pembrolizumab in R/M-HNSCC, patients were for the first time able
to benefit from a better survival accompanied by a stable quality
of life compared to standard of care (5,6).
However, the objective response rate remains modest with only 13.3%
for nivolumab (5) and 17% for
pembrolizumab, depending on the tumor programmed death ligand 1
(PD-L1) status (6). Nonetheless, in
case of a response to therapy it can potentially be durable and
even complete remission (CR) is possible. In cases of a durable CR
the question arises whether treatment can ever be stopped without
the risk of recurrence. In particular, it remains unclear whether
ICI in R/M-HNSCC patients who achieve a durable CR can be
discontinued after a certain treatment duration. As clinical
experience on this issue is limited, the current manuscript reports
on a case of discontinued ICI after durable CR of R/M-HNSCC and
gives an overview on the current literature.
Case report
A 62-year-old male patient was referred to our
Department of Head and Neck Surgery in November 2016 with a new
lesion of his left oropharynx and bilateral masses of the neck.
Histological confirmation of squamous cell carcinoma has already
been performed by fine needle aspiration cytology at the referring
hospital. He had a history of a pT2 pN1 M0 G2 hypopharyngeal cancer
of the left side, which was successfully treated in curative
intention by surgery and adjuvant radiotherapy at another hospital
in 2006.
At the time of presentation at our institution,
clinical examination revealed an ulcerating tumor of the left
oropharynx. 18-Fluoro-deoxyglucose positron emission tomography
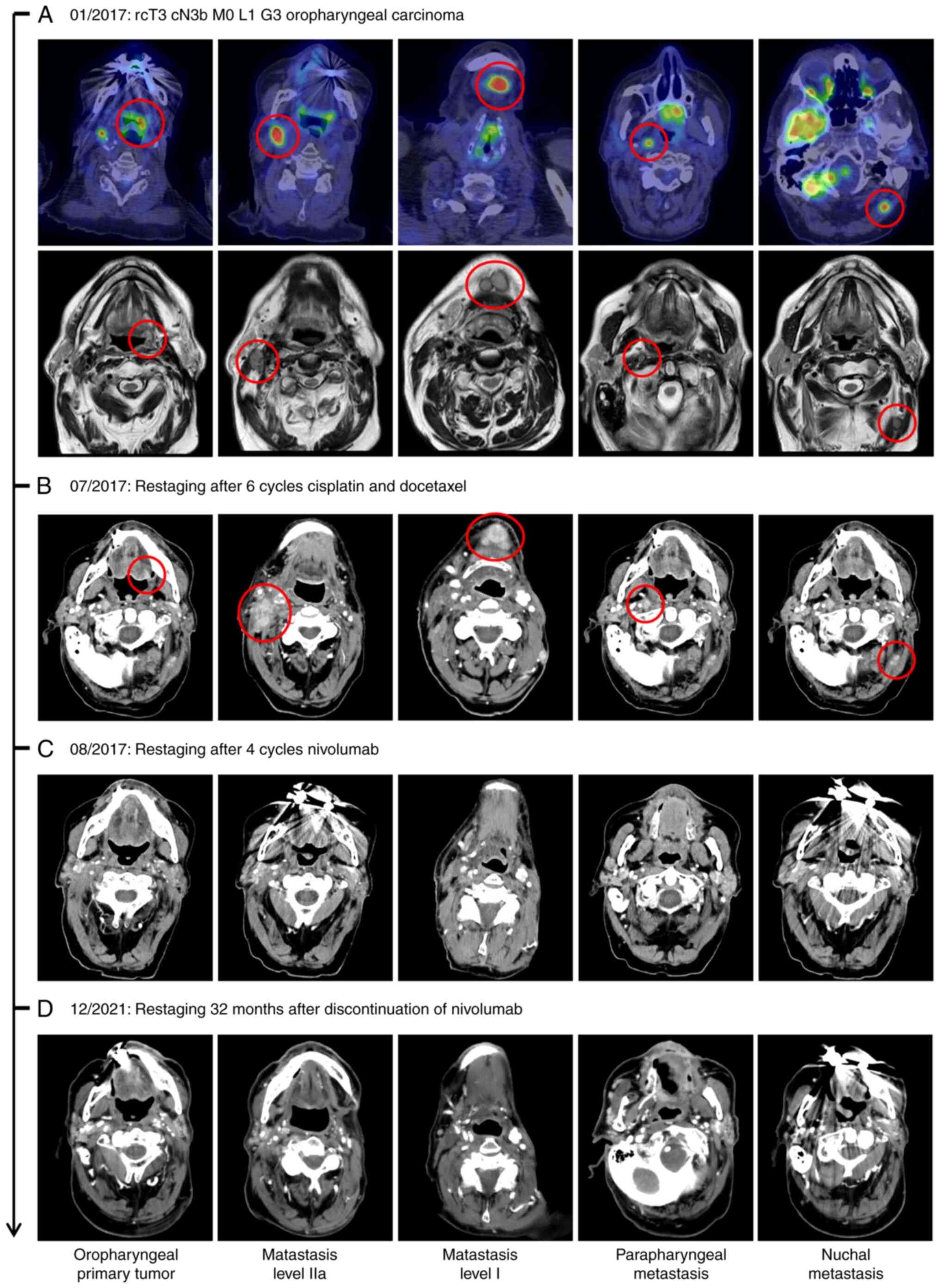
(FDG-PET) computed tomography (CT) showed a hypermetabolic lesion
of the left oropharynx as well as bilateral lesions of the neck in
level Ib of the left side and IIa of the right side. Furthermore,
there were parapharyngeal and nuchal lesions, which was also
confirmed in MRI (Fig. 1A).
Endoscopic examination was performed, which showed a tumor
extending from the tonsil to the base of the tongue with
infiltration of the midline. The biopsy confirmed the diagnosis of
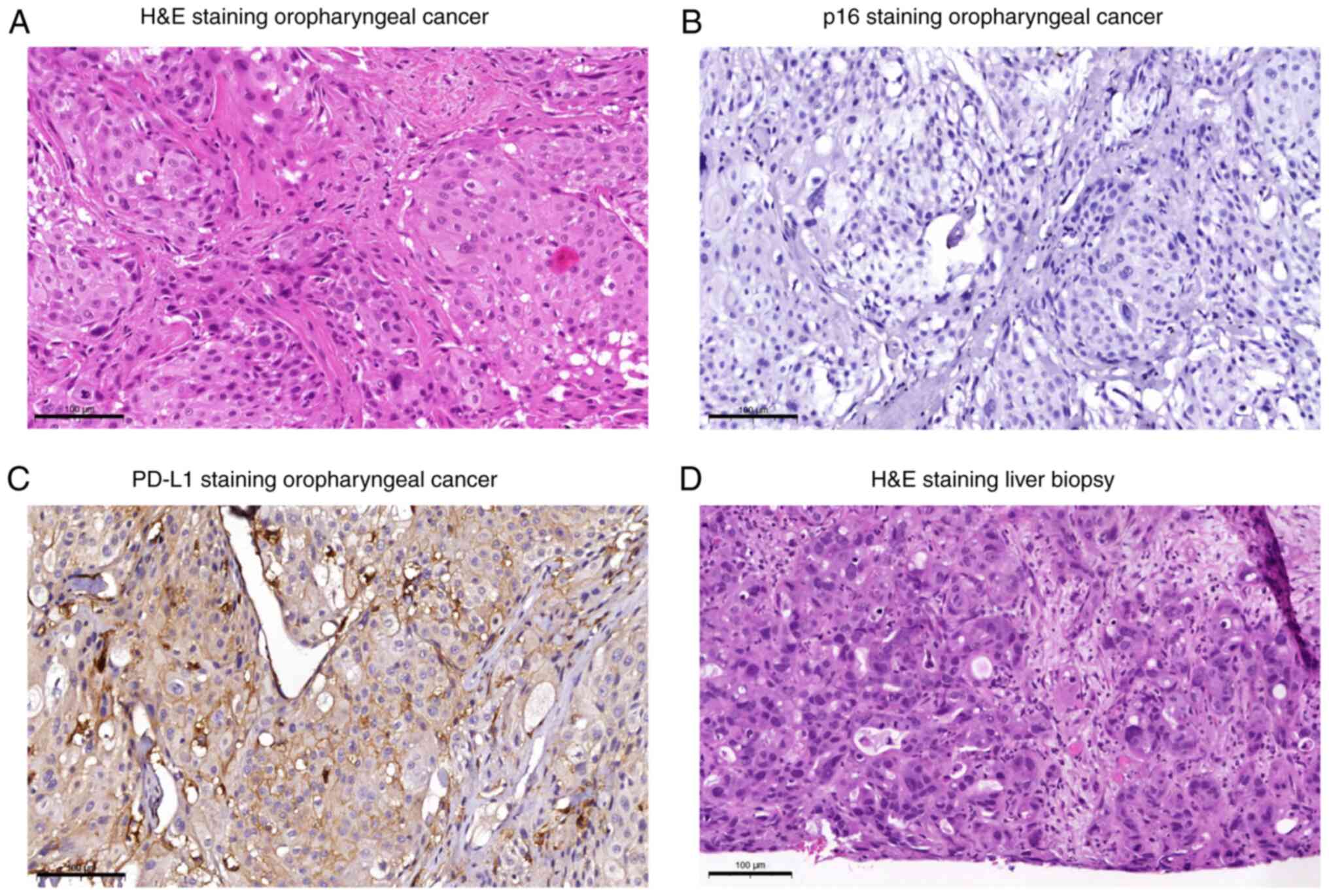
a rcT3 cN3b M0 L1 G3 p16 negative squamous cell carcinoma of the
oropharynx (Fig. 2A, B). Due to the
tumor extension, salvage surgery was not feasible. Also,
re-irradiation was not possible. A palliative chemotherapy with
cisplatin (75 mg/m2) and docetaxel (75 mg/m2)
was started in February 2017. CT scan after 6 cycles showed
progression of the cervical metastases (Fig. 1B). Therefore, an ICI with nivolumab
(3 mg per kilogram of body weight) every two weeks was initiated in
June 2017. Immunohistochemical analysis of the tumor tissue was
performed prior to therapy initiation. This showed a dense
lymphocytic infiltrate and a PD-L1 tumor cell score (Cologne Score
(7)) of 3. The combined positive
score (CPS) was introduced into the clinical assessment of PD-L1
expression only at a later stage (6). Therefore, a retrospective evaluation
was performed, which revealed a CPS of 23 (Fig. 2C). During CT re-staging after four
doses of nivolumab, a complete response was noted. Therapy with
nivolumab was continued following the international
recommendations. Further follow-up examinations and restaging (CT
scan of the neck, chest and abdomen every 3 months) confirmed an
ongoing complete response. After a total of 46 cycles of nivolumab
the patient asked for a break from therapy as he found the regular
clinic visits too demanding. The Patient was informed about the
risk of a possible relapse after a break in therapy. As to the
patients wish, ICI was finally terminated in April 2019. No
immune-related adverse events (irAE) occurred during and after
therapy. Under continuous clinical and radiological control (CT
scan of the neck, chest and abdomen every 3 months during the first
year, followed by controls twice a year), there were no signs of
tumor recurrence. Due to a post-therapeutic laryngeal chondritis
the patient presented with acute decompensating dyspnea.
Re-tracheostomy was necessary. Repeated endoscopies with biopsy did
not show any signs of local recurrence. In December 2021, 32 months
after nivolumab therapy was stopped, there was still no evidence of
HNSCC tumor recurrence. However, two suspicious masses of the
pancreas and multiple new lesions of the liver were found in
re-staging. Sonographic puncture of the liver revealed hepatic
metastases of a pancreatic cancer (Fig.
2D). The patient did not wish any further therapy in a
palliative overall setting, was switched to best supportive care
and deceased March 2022 due to his metastasized pancreatic
cancer.
Discussion
Durable complete responses can occasionally be seen
during ICI in R/M-HNSCC. However, they are not specific to ICI but
can also be observed during targeted therapy (8), radiotherapy (9) and chemotherapy (10). Still, durable responses and
especially a durable complete response is a rare event during
palliative therapy in R/M-HNSCC.
Despite the breakthrough in the treatment of
R/M-HNSCC by ICI, most patients do not benefit. Multiple clinical
and molecular factors have been suggested to be associated with a
favorable prognosis during ICI. Nevertheless, it remains unclear,
which exact factors influence a response to therapy. A predictive
role of PD-L1 expression is recognized. However, PD-L1 based assays
inherent several crucial limitations, such as a lack of
standardization (11) or
intratumoral heterogeneity of PD-L1 (12). Human papillomavirus (HPV) positivity
is another factor which is proposed to correlate with a favorable
clinical outcome. The phase I/II CheckMate 358 trial evaluated
neoadjuvant nivolumab in HNSCC patients with previously untreated,
resectable HPV positive or negative tumors. Here, radiologic and
pathologic response were seen more frequently in HPV positive
tumors (13). In contrast, a
meta-analysis of clinical trials using ICI in HNSCC including 732
patients with reported HPV status, failed to identify any
statistically significant advantages in tumor response and overall
survival for HPV positive patients (14). In another meta-analysis of five
randomized controlled trials including 2015 patients with reported
HPV status, anti-PD-1-based ICI seemed to be more efficient in HPV
negative tumors while anti-PD-L1-based ICI seemed to be more
efficient in HPV positive tumors (15). Interestingly, there is experimental
and clinical data indicating a role of HPV E5 oncoprotein in
mediating resistance to anti-PD-1/PD-L1 ICI by downregulation of
major histocompatibility complex expression in HNSCC tumors
(16). Furthermore, HPV is a
potential target for therapeutic vaccination in HPV positive HNSCC.
Combining ICI with HPV vaccination might be a promising therapeutic
option to improve clinical outcome (17). Immunohistochemical positivity of p16
is a surrogate marker for HPV association in oropharyngeal cancer,
which is negative in our case. However, discrepancy between p16 and
HPV DNA or RNA status exists in some patients with oropharyngeal
cancer (18). In our case, further
molecular tests like RNA in situ hybridization were not
performed in addition to p16 status to detect HPV infection.
Therefore, a limitation of the case we present is that there is no
information about the HPV status of the tumor. Alternative
biomarkers that may correlate with a response to ICI including
tumor immune cell infiltrate, blood-based markers, tumor metabolic
profile and mutational burden are under investigation (19).
In the case of a response to ICI, the identification
of predictive factors for prolonged disease control could be of
great utility to identify long-term responder and to guide their
follow-up. Furthermore, in the case of a patients wish to
discontinue treatment such factors could contribute to estimate the
risk of relapse upon therapy discontinuation. In various tumor
entities including R/M-HNSCC irAE were shown to be associated with
an improved PFS, as well as ORR and OS (20,21).
Furthermore, in other tumor entities such as non-small cell lung
cancer (NSCLC) (22) and malignant
melanoma (23) PFS and OS seems to
be improved in case of a CR before ICI discontinuation.
In cases of persistent stable disease under ICI a
metabolic response evaluation by FDG-PET-CT might help to improve
response evaluation by determine whether vital tumor tissue is
still present or whether there is a complete metabolic response
(CMR), defined as complete resolution of FDG uptake within the
target lesion (24). There is data
suggesting that a CMR may be associated with a favorable outcome
upon ICI discontinuation in NSCLC and malignant melanoma (22,25).
However, there is a lack of data for HNSCC. Nevertheless, it might
be worth to consider an additional FDG-PET-CT scan before ICI
discontinuation to identify patients with a CMR. Whether these
patients have a lower risk for tumor progression upon ICI
discontinuation needs to be evaluated in future studies. In our
case, PET-CT was performed only prior to ICI initiation, but was
not repeated at a later stage.
A durable response after ICI discontinuation has
also been reported for various tumor entities (22,26–29).
For R/M-HNSCC the broadly accepted concept is an ICI treatment
until disease progression or unacceptable toxicity such as irAE.
Interruption of therapy due to a durable complete response
challenges this concept. For patients, a long-lasting ICI therapy
is associated with regular visits to the oncology outpatient
clinic, the need of transportation and often significant financial
expenses. Likewise, an enduring therapy has a significant financial
impact on the health care system (30). Thus, in the rare cases of a durable
CR, the question as to the definitive duration of therapy or a
possible break in therapy arises. However, the effect of a
discontinued ICI after CR on disease control and prognosis in
R/M-HNSCC is unclear.
While CR is defined as disappearance of all
detectable evidence of cancer, the literature lacks a clear
definition of a durable response. Various definitions exist,
defining long responders as patients with a treatment response that
has lasted at least one or two years (31). Another definition for a durable
response to treatment is a progression-free survival that exceeds
three times the median progression-free survival of the whole
population (32). Several cases of
therapy discontinuation after durable CR can be found in the
literature (Table I). In that
context, the most common reasons for therapy interruption are
irAEs. Sekido et al presented a case of oral squamous cell
carcinoma with lung metastasis, who was treated with nivolumab.
After 33 cycles, ICI had to be stopped due to interstitial lung
disease as a pulmonary irAE that was treated with prednisone.
During follow-up no tumor recurrence was detected for six more
months (33). Yasumasu et al
presented a case series of 14 R/M-HNSCC patients with interrupted
nivolumab therapy due to irAEs. Five patients had a CR before
discontinuation of the therapy. Interestingly, these patients
showed no signs of tumor recurrence during a follow-up of up to 20
months after therapy discontinuation. In contrast, progression
occurred in three patients with previous partial response and in
one patient with previous stable disease. The authors suggest, that
CR before therapy discontinuation is a positive prognostic factor
for a durable disease control upon discontinuation due to irAE in
R/M-HNSCC (34). Gauci et al
also consider CR before therapy discontinuation to be a positive
factor for a durable response upon ICI discontinuation in different
kinds of cancer. In 39 analyzed patients who responded to ICI,
therapy was interrupted due to a prolonged response, adverse events
or per protocol. Interestingly, relapse was seen in 87% of patients
with stable disease or partial remission. Whereas, 88% of patients
with a prior CR showed an ongoing response (31). Similar observations could be made in
another case series of 14 patients with discontinued ICI after CR
in metastatic solid tumors, including one HNSCC patient. In none of
the cases a relapse was detected during a median follow-up of over
20 months from the end of treatment. The authors suggested, that
ICI discontinuation in patients who achieved CR appears feasible
(35).
 | Table I.Overview of published cases on
discontinuation of anti-programmed death receptor 1 therapy after
complete remission in recurrent or metastatic head and neck
squamous cell carcinoma. |
Table I.
Overview of published cases on
discontinuation of anti-programmed death receptor 1 therapy after
complete remission in recurrent or metastatic head and neck
squamous cell carcinoma.
| Authors | Cancer type | Patients with
discontinued ICI | Patients with CR | Relapse after ICI
discontinuation despite CR | (Refs.) |
|---|
| Sekido et
al | R/M-HNSCC | 1 | 1 | 0 | (33) |
| Yasumatsu et
al | R/M-HNSCC | 14 | 5 | 0 | (34) |
| Gauci et
al |
Miscellaneousa | 39 | 17 | 2 | (31) |
| Lopez-Flores et
al |
Miscellaneousa | 14 | 14 | 0 | (35) |
Whether or not immunotherapy discontinuation should
be considered after CR is currently discussed for various tumor
entities such as melanoma (29),
renal cell carcinoma (36) or NSCLC
(22). However, the issue remains
unsolved. In line, there is no broad consent on the duration of ICI
therapy in R/M-HNSCC patients with durable CR. As in our case, the
decision process on whether to pause immunotherapy is primarily
based on the patient's wish. Should the patient seek an
interruption of therapy, several factors should be considered when
counseling the patient. A CR appears to be a favorable factor for a
durable response. In the case of persistent stable disease, a
complementary FDG-PET-CT scan may be considered. In this way, a
complete metabolic response may be detected. However, there is a
lack of data regarding the prognostic value of a CMR in ICI of
R/M-HNSCC. Other factors that should be considered when counselling
the patient regarding a break in therapy include the duration of
response, the presence of irAE, the patient's age and
comorbidities.
In summary, R/M-HNSCC can experience durable
responses and in rare cases even a durable CR during ICI. Reports
in the literature suggest that CR might be a positive prognostic
factor for a durable response upon ICI discontinuation. However,
there is still a lack of data to make a clear recommendation.
Therefore, decisions about when to stop a successful immunotherapy
have to be discussed for every patient individually.
Acknowledgements
Not applicable.
Funding
This research was funded by the Interdisciplinary Centre for
Clinical Science (IZKF) at the University of Würzburg (grant no.
Z-2/81).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MSt, TM, TG, RH, MSc, SH and AS conceptualized the
study. MSt, TM and AS contributed to data collection and
interpretation. MSt and TG participated in data visualization. RH,
MS, SH and AS contributed to supervision. MSt, MS, SH and AS
drafted the manuscript. MSt, TM, TG, RH, MSc, SH and AS
participated in revising the manuscript. MSt, TM and AS confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
This study protocol was reviewed and approved by the
Ethics Committee of the Medical Faculty of the
Julius-Maximilians-University Würzburg (approval no. 2023062901).
Patient informed consent was waived due to the study's
retrospective nature by the ethic committee of the medical faculty
of the Julius-Maximilians-University Würzburg, taking into account
the national legal bases. Data collection and publication were
carried out in compliance with the guidelines for ensuring good
scientific practice. Only data generated in compliance with
relevant legal requirements and professional ethics were evaluated.
The applicable data protection regulations and the institute's
internal guidelines for handling patient data were observed.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CR
|
complete remission
|
|
CT
|
computed tomography
|
|
ICI
|
immune checkpoint inhibition
|
|
FDG-PET
|
18-Fluoro-deoxyglucose positron
emission tomography
|
|
HPV
|
human papillomavirus
|
|
irAE
|
immune related adverse events
|
|
PD-L1
|
programmed death ligand 1
|
|
PD-1
|
programmed cell death protein 1
|
|
R/M-HNSCC
|
recurrent or metastatic head and neck
squamous cell carcinoma
|
References
|
1
|
Johnson DE, Burtness B, Leemans CR, Lui
VWY, Bauman JE and Grandis JR: Head and neck squamous cell
carcinoma. Nat Rev Dis Primers. 6:922020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Decker J and Goldstein JC: Risk factors in
head and neck cancer. N Engl J Med. 306:1151–1155. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
zur Hausen H: Papillomaviruses in the
causation of human cancers-a brief historical account. Virology.
384:260–265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Forastiere A, Koch W, Trotti A and
Sidransky D: Head and neck cancer. N Engl J Med. 345:1890–1900.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferris RL, Blumenschein G Jr, Fayette J,
Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE,
Even C, et al: Nivolumab for recurrent squamous-cell carcinoma of
the head and neck. N Engl J Med. 375:1856–1867. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Burtness B, Harrington KJ, Greil R,
Soulières D, Tahara M, de Castro G Jr, Psyrri A, Basté N, Neupane
P, Bratland Å, et al: Pembrolizumab alone or with chemotherapy
versus cetuximab with chemotherapy for recurrent or metastatic
squamous cell carcinoma of the head and neck (KEYNOTE-048): A
randomised, open-label, phase 3 study. Lancet. 394:1915–1928. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scheel AH, Dietel M, Heukamp LC, Jöhrens
K, Kirchner T, Reu S, Rüschoff J, Schildhaus HU, Schirmacher P,
Tiemann M, et al: Predictive PD-L1 immunohistochemistry for
non-small cell lung cancer: Current state of the art and
experiences of the first German harmonization study. Pathologe.
37:557–567. 2016.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thinn MM and Hsueh CT and Hsueh CT:
Sustained complete response to erlotinib in squamous cell carcinoma
of the head and neck: A case report. World J Clin Cases. 7:616–622.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roh KW, Jang JS, Kim MS, Sun DI, Kim BS,
Jung SL, Kang JH, Yoo EJ, Yoon SC, Jang HS, et al: Fractionated
stereotactic radiotherapy as reirradiation for locally recurrent
head and neck cancer. Int J Radiat Oncol Biol Phys. 74:1348–1355.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Albers AE, Grabow R, Qian X, Jumah MD,
Hofmann VM, Krannich A and Pecher G: Efficacy and toxicity of
docetaxel combination chemotherapy for advanced squamous cell
cancer of the head and neck. Mol Clin Oncol. 7:151–157. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de Ruiter EJ, Mulder FJ, Koomen BM, Speel
EJ, van den Hout MFCM, de Roest RH, Bloemena E, Devriese LA and
Willems SM: Comparison of three PD-L1 immunohistochemical assays in
head and neck squamous cell carcinoma (HNSCC). Mod Pathol.
34:1125–1132. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rasmussen JH, Lelkaitis G, Hakansson K,
Vogelius IR, Johannesen HH, Fischer BM, Bentzen SM, Specht L,
Kristensen CA, von Buchwald C, et al: Intratumor heterogeneity of
PD-L1 expression in head and neck squamous cell carcinoma. Br J
Cancer. 120:1003–1006. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ferris RL, Spanos WC, Leidner R, Gonçalves
A, Martens UM, Kyi C, Sharfman W, Chung CH, Devriese LA, Gauthier
H, et al: Neoadjuvant nivolumab for patients with resectable
HPV-positive and HPV-negative squamous cell carcinomas of the head
and neck in the CheckMate 358 trial. J Immunother Cancer.
9:e0025682021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Patel JJ, Levy DA, Nguyen SA, Knochelmann
HM and Day TA: Impact of PD-L1 expression and human papillomavirus
status in anti-PD1/PDL1 immunotherapy for head and neck squamous
cell carcinoma-Systematic review and meta-analysis. Head Neck.
42:774–786. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Botticelli A, Cirillo A, Strigari L,
Valentini F, Cerbelli B, Scagnoli S, Cerbelli E, Zizzari IG, Rocca
CD, D'Amati G, et al: Anti-PD-1 and Anti-PD-L1 in head and neck
cancer: A network meta-analysis. Front Immunol. 12:7050962021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miyauchi S, Sanders PD, Guram K, Kim SS,
Paolini F, Venuti A, Cohen EEW, Gutkind JS, Califano JA and Sharabi
AB: HPV16 E5 mediates resistance to PD-L1 blockade and can be
targeted with rimantadine in head and neck cancer. Cancer Res.
80:732–746. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Massarelli E, William W, Johnson F, Kies
M, Ferrarotto R, Guo M, Feng L, Lee JJ, Tran H, Kim YU, et al:
Combining immune checkpoint blockade and tumor-specific vaccine for
patients with incurable human papillomavirus 16-related cancer: A
phase 2 clinical trial. JAMA Oncol. 5:67–73. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mehanna H, Taberna M, von Buchwald C, Tous
S, Brooks J, Mena M, Morey F, Grønhøj C, Rasmussen JH,
Garset-Zamani M, et al: Prognostic implications of p16 and HPV
discordance in oropharyngeal cancer (HNCIG-EPIC-OPC): A
multicentre, multinational, individual patient data analysis.
Lancet Oncol. 24:239–251. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park JC, Krishnakumar HN and Saladi SV:
Current and future biomarkers for immune checkpoint inhibitors in
head and neck squamous cell carcinoma. Curr Oncol. 29:4185–4198.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsuki T, Okamoto I, Fushimi C, Takahashi
H, Okada T, Kondo T, Sato H, Ito T, Tokashiki K, Tsukahara K, et
al: Real-World, long-term outcomes of nivolumab therapy for
recurrent or metastatic squamous cell carcinoma of the head and
neck and impact of the magnitude of best overall response: A
retrospective multicenter study of 88 patients. Cancers (Basel).
12:34272020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Foster CC, Couey MA, Kochanny SE, Khattri
A, Acharya RK, Tan YC, Brisson RJ, Leidner RS and Seiwert TY:
Immune-related adverse events are associated with improved
response, progression-free survival, and overall survival for
patients with head and neck cancer receiving immune checkpoint
inhibitors. Cancer. 127:4565–4573. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bilger G, Girard N, Doubre H, Levra MG,
Giroux-Leprieur E, Giraud F, Decroisette C, Carton M and Massiani
MA: Discontinuation of immune checkpoint inhibitor (ICI) above 18
months of treatment in real-life patients with advanced non-small
cell lung cancer (NSCLC): INTEPI, a multicentric retrospective
study. Cancer Immunol Immunother. 71:1719–1731. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Perez L, Samlowski W and Lopez-Flores R:
Outcome of elective checkpoint inhibitor discontinuation in
patients with metastatic melanoma who achieved a complete
Remission: Real-World data. Biomedicines. 10:11442022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wahl RL, Jacene H, Kasamon Y and Lodge MA:
From RECIST to PERCIST: Evolving considerations for PET response
criteria in solid tumors. J Nucl Med. 50 (Suppl 1):122S–150S. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tan AC, Emmett L, Lo S, Liu V, Kapoor R,
Carlino MS, Guminski AD, Long GV and Menzies AM: FDG-PET response
and outcome from anti-PD-1 therapy in metastatic melanoma. Ann
Oncol. 29:2115–2120. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sato K, Akamatsu H, Murakami E, Sasaki S,
Kanai K, Hayata A, Tokudome N, Akamatsu K, Koh Y, Ueda H, et al:
Correlation between immune-related adverse events and efficacy in
non-small cell lung cancer treated with nivolumab. Lung Cancer.
115:71–74. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martini DJ, Hamieh L, McKay RR, Harshman
LC, Brandao R, Norton CK, Steinharter JA, Krajewski KM, Gao X,
Schutz FA, et al: Durable clinical benefit in metastatic renal cell
carcinoma patients who discontinue PD-1/PD-L1 therapy for
immune-related adverse events. Cancer Immunol Res. 6:402–408. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yatsuda Y, Hirose S, Ito Y, Onoda T,
Sugiyama Y, Nagafuchi M, Suzuki H, Niisato Y, Tange Y, Ikeda T, et
al: A durable response after the discontinuation of nivolumab in an
advanced gastric cancer patient. Intern Med. 60:1011–1017. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Davies MA: Is it safe to stop Anti-PD-1
immunotherapy in patients with metastatic melanoma who achieve a
complete response? J Clin Oncol. 38:1645–1647. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Andrews A: Treating with checkpoint
inhibitors-figure $1 million per patient. Am Health Drug Benefits.
8((Spec Issue)): 92015.PubMed/NCBI
|
|
31
|
Gauci ML, Lanoy E, Champiat S, Caramella
C, Ammari S, Aspeslagh S, Varga A, Baldini C, Bahleda R, Gazzah A,
et al: Long-Term survival in patients responding to Anti-PD-1/PD-L1
therapy and disease outcome upon treatment discontinuation. Clin
Cancer Res. 25:946–956. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pons-Tostivint E, Latouche A, Vaflard P,
Ricci F, Loirat D, Hescot S, Sablin MP, Rouzier R, Kamal M, Morel
C, et al: Comparative analysis of durable responses on immune
checkpoint inhibitors versus other systemic therapies: A pooled
analysis of phase III trials. JCO Precis Oncol. 3:1–10. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sekido K, Imaue S, Tomihara K, Tachinami
H, Yamagishi K, Okazawa S, Ikeda A, Fujiwara K and Noguchi M:
Durable complete response to immunotherapy with anti-PD-1 antibody
nivolumab in a patient with oral squamous cell carcinoma presenting
with lung metastasis: A case report. Clin Case Rep. 9:e045452021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yasumatsu R, Matsuo M, Wakasaki T, Masuda
M, Takeuchi T, Manako T, Jiromaru R, Uchi R, Hashimoto K and
Nakagawa T: Clinical outcome in recurrent and/or metastatic head
and neck cancer patients after discontinuation of nivolumab
monotherapy due to immune-related adverse events. Acta Otolaryngol.
140:1043–1048. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lopez-Flores R, Samlowski W and Perez L:
Elective checkpoint inhibitor discontinuation in metastatic solid
tumor patients: A case series. Ann Case Rep. 7:8942022.PubMed/NCBI
|
|
36
|
Zambrana F, Carril-Ajuria L, Gomez de
Liano A, Martinez Chanza N, Manneh R, Castellano D and de Velasco
G: Complete response and renal cell carcinoma in the immunotherapy
era: The paradox of good news. Cancer Treat Rev. 99:1022392021.
View Article : Google Scholar : PubMed/NCBI
|