Introduction
Hepatocellular carcinoma (HCC) is the sixth most
common type of cancer and third leading cause of cancer-related
deaths worldwide in 2020 (1). HCC
has emerged as one of the top five cancer types with regards to
incidence, mortality and disability-adjusted life year of cancer in
China; HCC is ranked tenth and fourteenth in the US and UK,
respectively, although the disability-adjusted life year burden of
HCC has decreased by 41.5%, and its ranking dropped from second to
fifth between 1990 and 2019 (2).
Currently, a number of options are recommended as treatments for
HCC including surgical resection, liver transplantation and
radiofrequency ablation (3).
However, ~66% of patients with HCC are already at intermediate or
advanced stage of disease at the time of diagnosis, and qualify for
non-curative types of treatment, such as transarterial
chemoembolization (TACE) and tyrosine kinase inhibitors (TKIs)
(4).
The Barcelona Clinical Liver Cancer (BCLC) staging
system is classified based on tumor load (tumor size, tumor number,
vascular invasion and extrahepatic metastasis), liver function
status (Child-Pugh class) and performance status (Eastern
Cooperative Oncology Group-performance status), and is widely used
for HCC staging (5). According to
current recommendations, TACE is the global standard treatment for
BCLC B stage HCC (5). However, due
to the presence of heterogeneity with regards to tumor burden,
liver function and clinical characteristics, as well as the
recommendation for this treatment for a wide range of patients with
intermediate stage HCC, there are several limitations associated
with this type of treatment. Based on varying liver function and
tumor burden, Bolondi et al (6) reclassified BCLC B stage HCC into four
substages. Subsequently, various staging systems have since
emerged, and appropriate treatments for different subgroups have
been recommended (7). Based on the
criteria recommended by Bolondi et al (6), Kudo et al (8) modified and improved the Kinki
criteria, which subclassified intermediate HCC into three
substages. The Kinki criteria are based on the Child-Pugh score,
Milan criteria (solitary tumor ≤5 cm, or two or three nodules ≤3
cm) and up-to-seven criteria (the sum of the size in cm, and the
number of tumors ≤7). The B2 substage includes patients with HCC
with a Child-Pugh score of 5–7, beyond Milan criteria and exceeding
the up-to-seven criteria. In 2018, Kudo (9) proposed the Kindai criteria (Table I), which were modified on the basis
of the original version of the Kinki criteria in order to provide
more suitable treatment decision-making in patients with
intermediate-stage HCC.
 | Table I.Subclassification of BCLC B stage
hepatocellular carcinoma [Kindai criteria (9)]. |
Table I.
Subclassification of BCLC B stage
hepatocellular carcinoma [Kindai criteria (9)].
|
|
Subclassification |
|---|
|
|
|
|---|
| Criteria or
clinical strategy | B1 | B2 | B3a | B3b |
|---|
| Milan criteria | Beyond | Beyond | Beyond | Beyond |
| Up-to-seven
criteria | In | Out | In | Out |
| Child-Pugh
score | 5-7 | 5-7 | 8-9 | 8-9 |
| Concept of
treatment strategy | Curative | Non-curative,
palliative | Curative if within
up-to-7 | Palliative, no
treatment |
| Treatment
option | Resection,
ablation, superselective c-TACE |
Lenvatiniba | Transplantation,
ablation, superselective c-TACE | HAIC, selective
DEB-TACE, BSC |
| Alternative |
DEB-TACEb, B-TACEc |
Sorafeniba, TACE + sorafenib,
DEB-TACEd, bland
TAE4 followed by MTA | DEB-TACE, B-TACE,
HAIC | BSC |
According to both the Bolondi and Kinki criteria,
TACE and sorafenib are recommended as the first and/or alternative
treatment options for patients with B2 stage HCC. However,
>66.6% of patients with HCC exhibit resistance and a high rate
of recurrence after TACE. Thus, repeated TACE is needed for such
patients, and resistance to this treatment may lead to a poor
prognosis. Previous studies suggested that TACE causes tumor cells
to be surrounded by a hypoxic environment, which may elevate
expression levels of vascular endothelial growth factor (VEGF) and
basic fibroblast growth factor (FGF), and ultimately lead to tumor
angiogenesis (10–12). TKIs targeting VEGF receptor (VEGFR)
and other related receptors, inhibit receptor activity and the
activation of downstream signaling pathways, and achieve
anti-angiogenesis by blocking multiple signaling pathways (13,14).
Previous randomized controlled trials in patients with HCC reported
that combination therapy with TACE and sorafenib significantly
prolonged progression-free survival time compared with TACE
monotherapy (15,16). A recent meta-analysis reported a
significantly increased efficacy of TACE plus sorafenib for
patients with unresectable HCC (uHCC) compared with TACE alone
(17). However, a multicenter
retrospective observational study enrolled 1,719 patients with uHCC
and divided these patients into three groups, namely low, moderate
and high tumor burden, based on tumor size and number, and reported
that TACE plus sorafenib provided notable survival benefits
compared with TACE alone, only in patients with a moderate tumor
burden (18).
As a novel member of multi-kinase inhibitor agents,
targeting VEGF, FGF and platelet-derived growth factor receptors,
lenvatinib (Len) was reported to show non-inferiority or
superiority in both efficacy and safety measured compared with
sorafenib, and has been recommended as a first-line treatment for
patients with uHCC in Japan, America, China and other countries
(19–22). In addition, TACE plus Len also
demonstrated improved efficacy compared with that of TACE plus
sorafenib in patients with HCC (23). Kudo et al (24) recommended Len-TACE sequential
therapy as the first option for patients with uHCC deemed
unsuitable for TACE treatment. Recently, a number of studies made a
comparison between TACE plus Len, and Len alone for patients with
uHCC, and the combination therapy achieved improved clinical
outcomes (25,26). However, to the best of our
knowledge, no studies focused on comparing the efficacy of the two
treatments in subgroups of patients, especially those with moderate
tumor burden.
Therefore, the present retrospective study was
conducted to compare the efficacy and safety of combination therapy
with TACE and Len against TACE monotherapy in patients with BCLC B2
stage HCC.
Materials and methods
Study design and patient
population
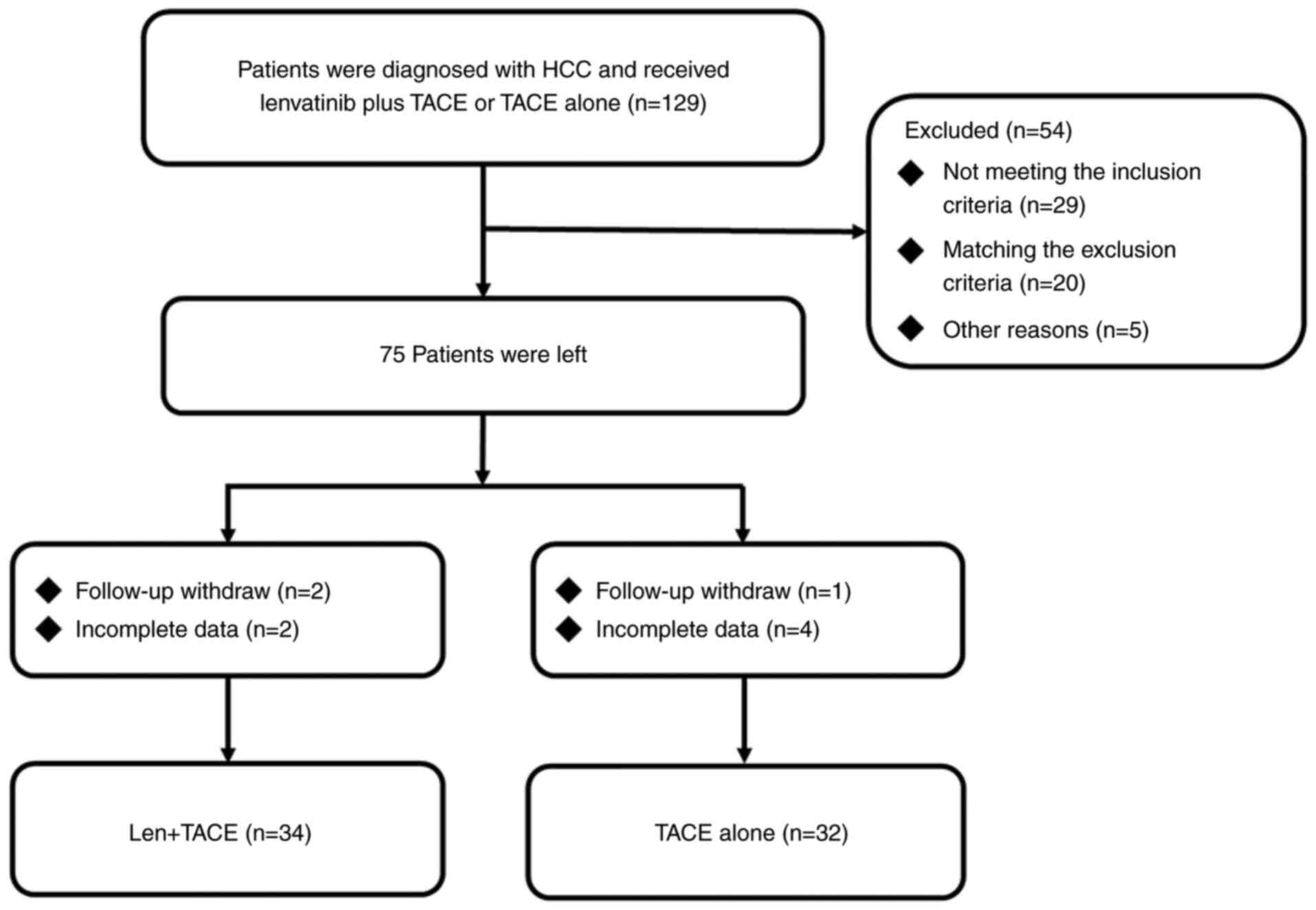
A total of 66 patients with BCLC B2 stage HCC who
received Len + TACE or TACE alone at the Affiliated Hospital of
North Sichuan Medical College between May 2018 and May 2020 were
retrospectively reviewed (Fig. 1).
The cohort of patients in the present study was not included in
other clinical trial studies. HCC was diagnosed using
histopathological and/or imaging examinations, and was classified
using the BCLC staging system (5).
BCLC B2 stage HCC was defined as intermediate-stage HCC with
Child-Pugh scores of 5–7, beyond the Milan criteria and up-to-seven
criteria (8,9).
The inclusion criteria were as follows: i) Diagnosis
of BCLC B2 stage HCC; ii) age ≥18 but <75 years; and iii) no
other history of malignant tumors. The exclusion criteria were as
follows: i) Received previous local or systemic therapy, such as
TACE, TKIs or programmed death-1(PD-1); and iii) incomplete data or
loss of follow-up.
Baseline data were collected including age, sex,
hepatitis B virus (HBV) status, α-fetoprotein (AFP) level,
Child-Pugh score, liver cirrhosis, largest tumor size, tumor number
and distribution.
TACE
After the assessment of routine blood tests, liver
and kidney function, and PS, a 5-F infusion catheter was
selectively inserted into the tumor-feeding hepatic arteries. An
injection of an emulsion of epirubicin (20–40 mg;
Pharmorubicin®; Pfizer, Inc.) and lipiodol (2–10 ml;
Guerbet Laboratories Ltd.) into the intrahepatic arterial was
performed, and small gelatin sponge particles were used for
embolization. Biochemical indicators such as AFP, bilirubin,
albumin and others were reviewed on day 3 post-operation, and
patients were evaluated for changes in the tumor using CT or MRI
scans 4 weeks after TACE. TACE was repeated until progression to
either TACE-refractory criteria (27,28),
unacceptable toxicity or withdrawal of consent.
Len + TACE
In principle, doctors recommended Len + TACE
combination therapy for all patients with BCLC B2 stage HCC.
However, patients who refused Len accepted TACE monotherapy for
either economic or personal reasons, such as a fear of
complications or disagreement with the doctor's decisions. If no
obvious abnormalities in biochemical indicators, such as abnormal
elevation of liver transaminase, and other symptoms, such as severe
nausea and anaphylaxis, were observed on day 3 post operation, Len
was administered on the same day, otherwise, Len was administered
after symptoms had ceased. Len (Eisai Co., Ltd) was administered
orally at 8 mg once per day in patients with weight <60 kg or at
12 mg per day in patients with weight ≥60 kg based on the
recommended doses published by the REFLECT trial (20). If serious adverse events (AEs; grade
≥3) or any unacceptable treatment-related AEs occurred, the dose of
Len was reduced, delayed or discontinued according to the
manufacturer's instructions.
Follow-up and assessment
The first follow-up was conducted 4 weeks after TACE
and included analysis of related biochemical indicators and CT or
MRI. Follow-up was repeated every 4–8 weeks to detect any
recurrence or metastasis. Follow-up was censored in April 2022.
Overall survival (OS) was defined as the period from
the date of initial TACE to death or last follow-up, whereas
progression-free survival (PFS) was defined as the period from the
date of initial TACE to the time of disease progression or last
follow-up. Tumor response was assessed every 4–8 weeks according to
the modified response evaluation criteria for solid tumors
(mRECIST) (29), including complete
response (CR), partial response (PR), stable disease (SD) and
progressive disease (PD). The objective response rate (ORR) was
defined as the proportion of patients who achieved CR or PR, and
the disease control rate (DCR) was defined as the proportion of
patients who achieved either CR, PR or SD. Treatment-related AEs
were evaluated according to the Common Terminology Criteria for
Adverse Events (version 5) (30).
Statistical analysis
Continuous data with normal distribution were
expressed as the mean and standard deviation and skewed
distributions were expressed as medians and interquartile ranges.
Categorical data are expressed as frequencies and percentages.
Categorical variables were compared using the χ2 or
Fisher's exact tests and continuous variables were comparing using
the unpaired Student's t-test. PFS and OS were calculated using the
Kaplan-Meier method and the log-rank test was used to compare PFS
and OS between the groups. Hazard ratios (HRs) and confidence
intervals (CIs) were estimated using the Cox proportional hazards
model. Statistical analysis was performed using the SPSS software
(version 21; IBM Corp.) and RStudio (version 4.2.1; RStudio, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
In total, 66 patients were included in the present
study with 34 patients receiving Len + TACE, and 32 patients
receiving TACE alone (Table II).
Most of the patients were male [n=58 (87.9%)] and had an HBV
infection [n=57 (86.4%)], >3 tumors [n=56 (84.8%)], a bilobar
distribution [n=53 (80.3%)] and a mean age of 52.55±13.45 years. In
the Len + TACE group, the number of patients with higher AFP levels
>400 ng/l [n=21 (61.8%)] was significantly higher compared with
that in the TACE group [n=10 (31.2%)] (P=0.013). Other patient
characteristics of note included hepatitis C virus (HCV) infection
[n=1 (1.5%)], alcohol abuse [n=1 (1.5%)], non-alcoholic fatty liver
disease [n=2 (3.0%)] and other unknown background of liver damage
[n=5 (7.5%)]. All patients infected with HBV were treated with 0.5
mg entecavir (Chia Tai Tianqing Pharmaceutical Group Co., Ltd.)
once daily, while the patient infected with HCV received 400/100 mg
sofosbuvir and velpatasvir (Gilead Sciences, Inc.) once daily.
 | Table II.Baseline patient characteristics. |
Table II.
Baseline patient characteristics.
| Patient
characteristic | Lenvatinib + TACE
(n=34) | TACE (n=32) | Total (n=66) | P-value |
|---|
| Age,
yearsa | 51.79±12.30 | 53.34±14.72 | 52.55±13.45 | 0.643 |
| Sex, n (%) |
|
|
| 0.710 |
|
Female | 5 (14.7) | 3 (9.4) | 8 (12.1) |
|
|
Male | 29 (85.3) | 29 (90.6) | 58 (87.9) |
|
| HBV, n (%) |
|
|
| >0.999 |
|
Negative | 5 (14.7) | 4 (12.5) | 9 (13.6) |
|
|
Positive | 29 (85.3) | 28 (87.5) | 57 (86.4) |
|
| AFP (ng/l), n
(%) |
|
|
| 0.013 |
|
≤400 | 13 (38.2) | 22 (68.8) | 35 (53.0) |
|
|
>400 | 21 (61.8) | 10 (31.2) | 31 (47.0) |
|
| Child-Pugh score, n
(%) |
|
|
| 0.977 |
| 5 | 13 (38.2) | 13 (40.6) | 26 (39.4) |
|
| 6 | 13 (38.2) | 12 (37.5) | 25 (37.9) |
|
| 7 | 8 (23.5) | 7 (21.9) | 15 (22.7) |
|
| Liver cirrhosis, n
(%) |
|
|
| 0.709 |
| No | 11 (32.4) | 9 (28.1) | 20 (30.3) |
|
|
Yes | 23 (67.6) | 23 (71.9) | 46 (69.7) |
|
| Tumor number, n
(%) |
|
|
| 0.654 |
| ≤3 | 4 (11.8) | 6 (18.8) | 10 (15.2) |
|
|
>3 | 30 (88.2) | 26 (81.3) | 56 (84.8) |
|
| Largest tumor size
(cm), n (%) |
|
|
| 0.622 |
| ≤4 | 18 (52.9) | 15 (46.9) | 33 (50.0) |
|
|
>4 | 16 (47.1) | 17 (53.1) | 33 (50.0) |
|
| Tumor distribution,
n (%) |
|
|
| 0.420 |
|
Unilobar | 8 (23.5) | 5 (15.6) | 13 (19.7) |
|
|
Bilobar | 26 (76.5) | 27 (84.4) | 53 (80.3) |
|
Overall survival
As the number of deaths was so small that >50% of
the patients still survived at the end of the follow-up, causing
the inability to calculate the median value, the median OS time was
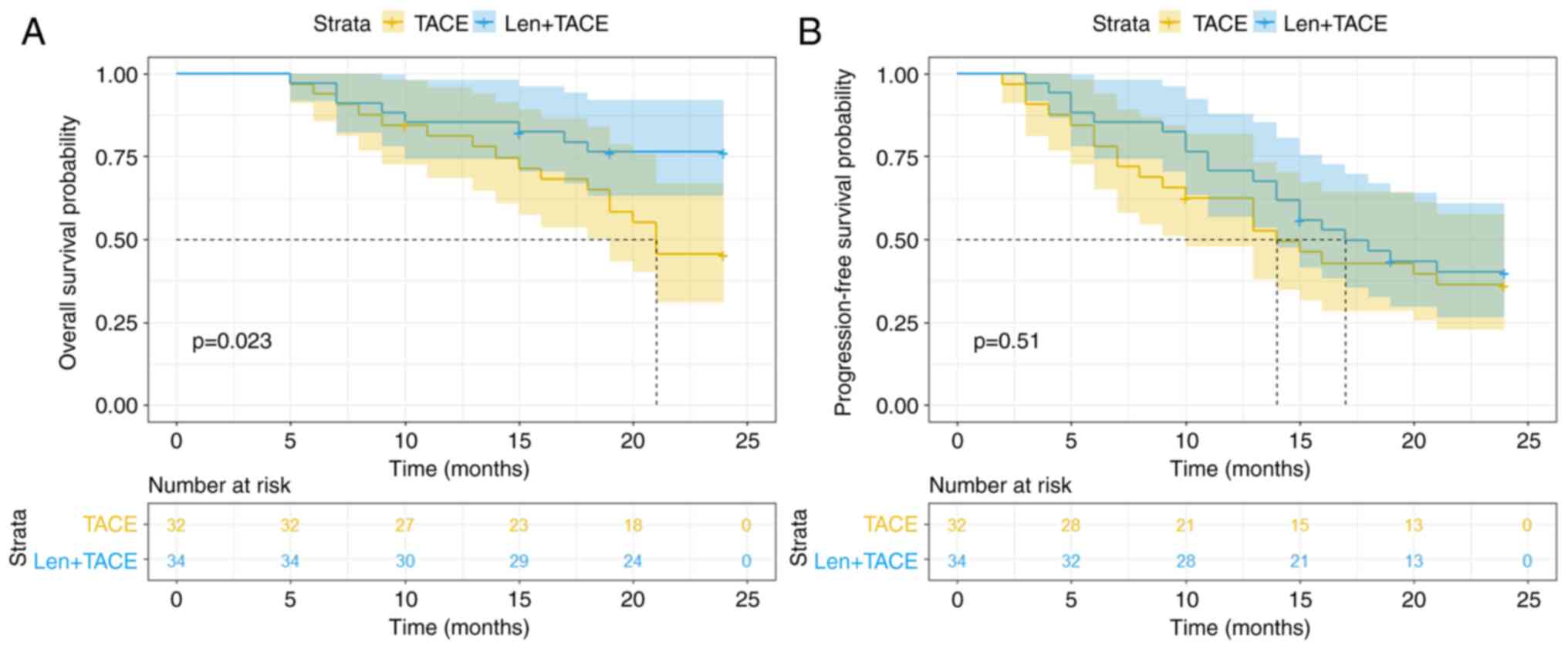
not reached in the two treatment groups (Fig. 2A). The OS time was significantly
longer in the Len + TACE group compared with that in the TACE group
(HR, 0.395; 95% CI, 0.180–0.867; P=0.023). The 6-month, 1- and 2-
year OS rates in the Len + TACE group were 97.1, 85.3 and 76.3%,
respectively. The 6-month, 1- and 2- year OS rates in the TACE
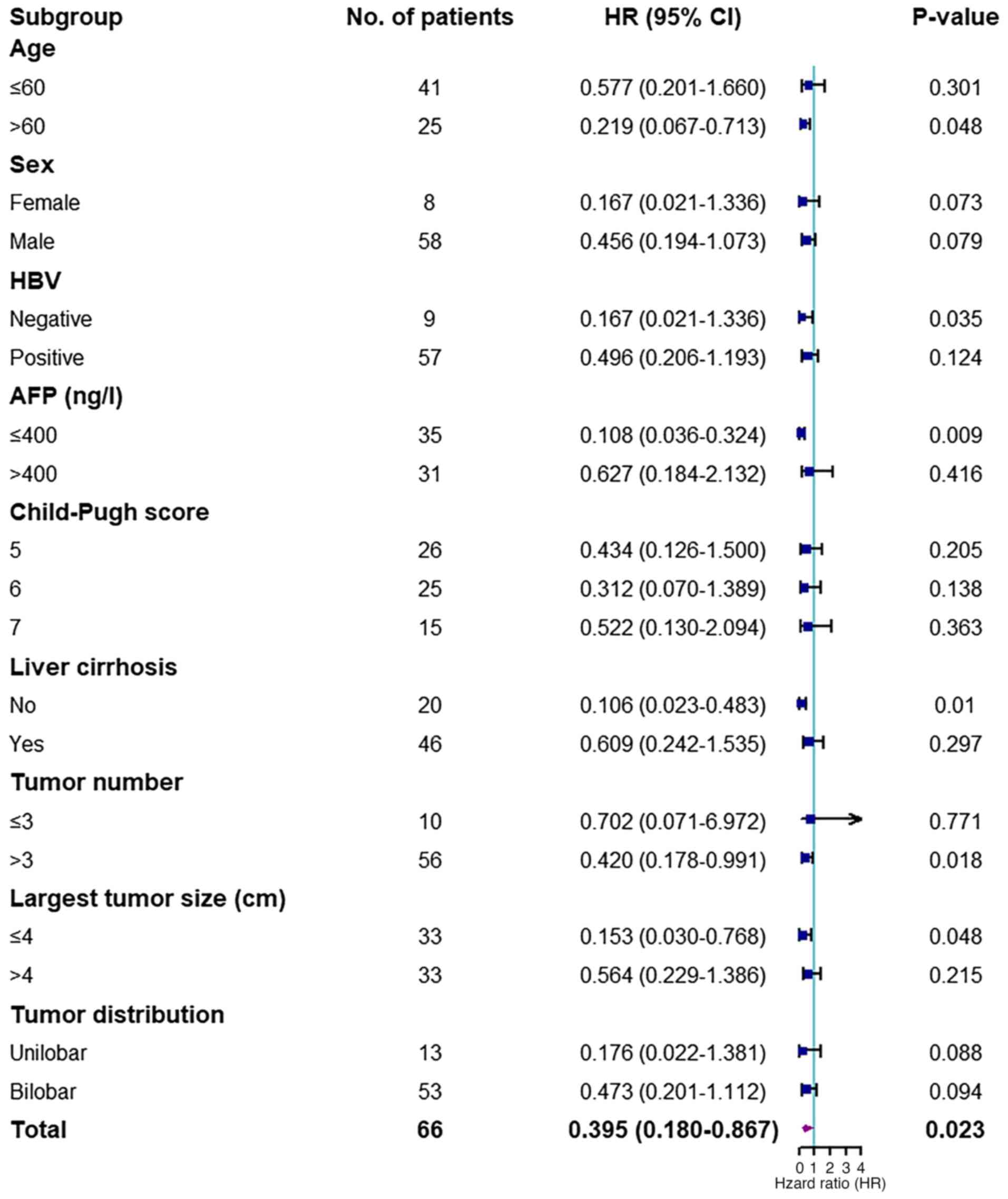
group were 93.8, 81.1 and 45.4%, respectively. The subgroup
analysis demonstrated that patients aged >60 years, with lower
levels of AFP (≤400 ng/ml), >3 tumors, largest tumor size ≤4 cm
and without HBV infection and liver cirrhosis, benefited more from
Len + TACE compared with TACE monotherapy (P<0.05; Fig. 3).
Progression-free survival
The median PFS time of the entire patient cohort was
16.00 months (95% CI, 11.60–20.41 months). In the Len + TACE
combination therapy group and the TACE monotherapy group, the
median PFS time was 17.00 months (95% CI, 11.55–22.45 months) and
14.00 months (95% CI, 8.68–19.32 months), respectively, and no
significant difference was observed between the two treatment
groups (HR, 0.815; 95% CI, 0.437–1.520; P=0.510; Fig. 2B). However, the PFS rates at 6
months, 1 and 2 years were higher in patients who received
combination therapy (85.3, 70.6 and 40.1%, respectively) compared
with those in patients who received monotherapy (78.1, 62.5 and
36.2%, respectively), however the difference was not statistically
significant. A total of seven and two patients with disease
progression received additional PD-1 treatment in the Len + TACE
group and the TACE group, respectively. In total, ~70% of patients
chose to maintain their original treatment strategy after disease
progression because of either economic or personal reasons.
Tumor response
Based on the mRECIST criteria, four and 18 patients
exhibited CR and PR, respectively after receiving Len + TACE, and
two and nine patients, respectively after TACE (Table III). The ORR in the Len + TACE
group was significantly higher compared with that in the TACE group
(64.7 vs. 34.4%; P=0.014). Furthermore, DCR was markedly increased
in the Len + TACE group compared with the TACE group (79.4 vs.
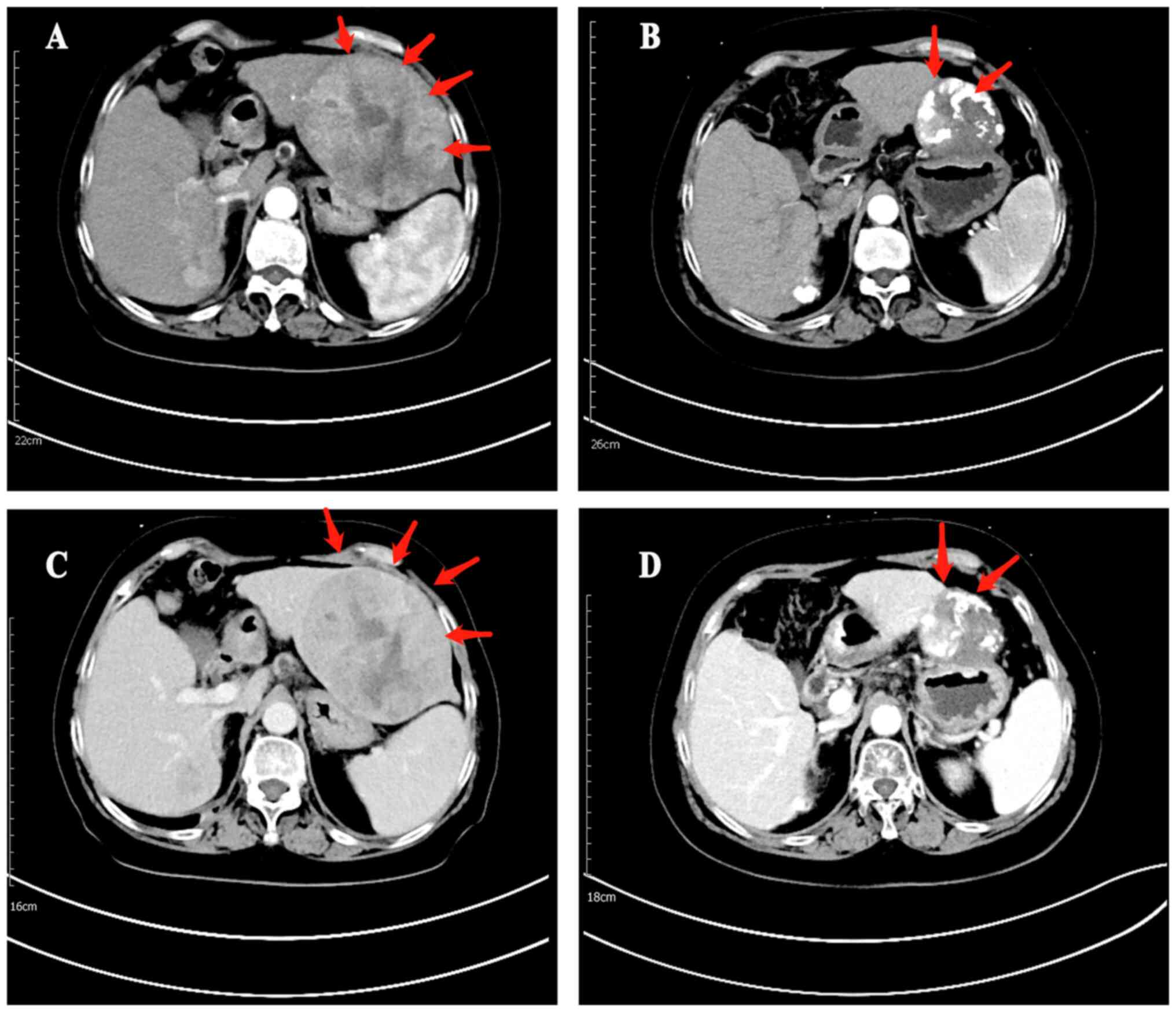
59.4%; P=0.066). Representative images of necrosis and regression
of tumor lesion are shown in Fig.
4.
 | Table III.Tumor response in the combination and
monotherapy treatment groups of patients. |
Table III.
Tumor response in the combination and
monotherapy treatment groups of patients.
| Tumor response | Lenvatinib + TACE,
n (%) | TACE, n (%) | P-value |
|---|
| CR | 4 (11.8) | 2 (6.3) | 0.673 |
| PR | 18 (52.9) | 9 (28.1) | 0.040 |
| SD | 5 (14.7) | 10 (31.3) | 0.109 |
| PD | 7 (20.6) | 11 (34.4) | 0.209 |
| ORR | 22 (64.7) | 11 (34.4) | 0.014 |
| DCR | 27 (79.4) | 19 (59.4) | 0.066 |
Adverse events
Occurrences of treatment-emergent AEs were recorded
(Table IV). In the Len + TACE
group, the most common AEs elevated were alanine aminotransferase
(ALT)/aspartate transaminase (AST) (52.9%), fever (47.1%), pain
(35.3%), hypertension (32.4%) and decreased white blood cells
(WBCs; 23.5%), while grade 3/4 AEs included elevated ALT/AST
(23.5%), hypertension (11.8%), decreased WBCs (5.9%), fever (2.9%)
and diarrhea (2.9%). In the TACE group, fever was the most frequent
event (46.9%), followed by elevated ALT/AST (43.8%), pain (25.0%),
decreased WBCs (18.8%), diarrhea (9.4%) and rash (3.1%) and grade
3/4 AEs were reported in this patient group, including elevated
ALT/AST (18.8%), fever (3.1%) and decreased WBCs (3.1%). In the Len
+ TACE group, three patients reduced the dose of Len, and one
patient temporarily withdrew Len because of intolerance to AEs,
which led to the AEs becoming manageable for the patient.
 | Table IV.Adverse events in the combination and
monotherapy treatment groups of patients. |
Table IV.
Adverse events in the combination and
monotherapy treatment groups of patients.
|
| Lenvatinib + TACE
(n=34) | TACE (n=32) |
|---|
|
|
|
|
|---|
| Adverse events | All grades, n
(%) | Grade 3/4, n
(%) | All grades, n
(%) | Grade 3/4, n
(%) |
|---|
| Elevated
ALT/AST | 18 (52.9) | 8 (23.5) | 14 (43.8) | 6 (18.8) |
| Fever | 16 (47.1) | 1 (2.9) | 15 (46.9) | 1 (3.1) |
| Pain | 12 (35.3) | 0 (0.0) | 8 (25.0) | 0 (0.0) |
| Hypertension | 11 (32.4) | 4 (11.8) | 0 (0.0) | 0 (0.0) |
| Decreased white
blood cell count | 8 (23.5) | 2 (5.9) | 6 (18.8) | 1 (3.1) |
| Diarrhea | 5 (14.7) | 1 (2.9) | 3 (9.4) | 0 (0.0) |
| HFSR | 3 (8.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Rash | 2 (5.9) | 0 (0.0) | 1 (3.1) | 0 (0.0) |
Univariate and multivariate
analyses
Univariate and multivariate Cox regression analyses
of factors influencing OS and PFS were performed (Table V). Univariate analysis demonstrated
that the use of Len + TACE and largest tumor size ≤4 cm were shown
to be significant prognostic factors for favorable OS, but the
largest tumor size was the only significant prognostic factor for
PFS. Multivariate Cox proportional hazards model for OS identified
the largest tumor size (>4 vs. ≤4 cm; HR, 4.086; 95% CI,
1.623–10.289; P=0.003) and treatment strategy (Len + TACE vs. TACE;
HR, 0.426, 95% CI, 0.184–0.990; P=0.047) as independent risk
factors. Similarly, for PFS, patients with largest tumor size ≤4 cm
had significantly improved prognosis (HR, 2.548; 95% CI,
1.334–4.870; P=0.005).
 | Table V.Univariate and multivariate analysis
of overall survival and progression-free survival of patients
treated with combination Len + TACE and TACE monotherapy. |
Table V.
Univariate and multivariate analysis
of overall survival and progression-free survival of patients
treated with combination Len + TACE and TACE monotherapy.
|
| Overall
survival | Progression-free
survival |
|---|
|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Patient
characteristic | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years | 0.998
(0.970–1.027) | 0.892 | N/A | N/A | 1.020
(0.996–1.044) | 0.107 | N/A | N/A |
| Sex |
|
|
|
|
|
|
|
|
| Male vs.
female | 0.643
(0.220–1.879) | 0.419 | N/A | N/A | 0.774
(0.303–1.977) | 0.592 | N/A | N/A |
| HBV |
|
|
|
|
|
|
|
|
| Positive vs.
negative | 0.756
(0.259–2.203) | 0.608 | N/A | N/A | 0.726
(0.304–1.731) | 0.470 | N/A | N/A |
| AFP, ng/l |
|
|
|
|
|
|
|
|
| >400 vs.
≤400 | 1.192
(0.544–2.613) | 0.661 | N/A | N/A | 1.123
(0.604–2.091) | 0.714 | N/A | N/A |
| Child-Pugh
score |
|
|
|
|
|
|
|
|
| 6 vs. 5 | 0.697
(0.265–1.833) | 0.465 | N/A | N/A | 0.672
(0.326–1.385) | 0.282 | N/A | N/A |
| 7 vs. 5 | 1.620
(0.638–4.111) | 0.310 | N/A | N/A | 0.982
(0.449–2.150) | 0.964 | N/A | N/A |
| Liver
cirrhosis |
|
|
|
|
|
|
|
|
| Yes vs. no | 1.201
(0.502–2.876) | 0.681 | N/A | N/A | 0.816
(0.420–1.585) | 0.548 | N/A | N/A |
| Tumor number |
|
|
|
|
|
|
|
|
| >3 vs. ≤3 | 1.437
(0.430–4.801) | 0.556 | N/A | N/A | 1.664
(0.651–4.252) | 0.287 | N/A | N/A |
| Largest tumor size,
cm |
|
|
|
|
|
|
|
|
| >4 vs. ≤4 | 4.297
(1.709–10.806) | 0.002 | 4.086
(1.623–10.289) | 0.003
(1.334–4.870) | 2.548 | 0.005
(1.334–4.870) | 2.548 | 0.005 |
| Tumor
distribution |
|
|
|
|
|
|
|
|
| Bilobar vs.
Unilobar | 1.394
(0.478–4.066) | 0.543 | N/A | N/A | 1.099
(0.506–2.387) | 0.811 | N/A | N/A |
| Treatment |
|
|
|
|
|
|
|
|
| Len + TACE vs.
TACE | 0.394
(0.170–0.914) | 0.030 | 0.426
(0.184–0.990) | 0.047
(0.438–1.515) | 0.815 | 0.517 | N/A | N/A |
Discussion
According to the updated BCLC guidelines that take
into consideration the extensive heterogeneity of tumor burden,
intermediate stage HCC was divided into three groups (5). Among these groups, the BCLC B2
subgroup was defined as patients with defined tumor burden,
preserved portal flow and feasibility of selective access to
feeding tumor arteries who did not meet the extended liver
transplant criteria, and TACE was recommended as the first line
treatment option for this group (5). However, the cut-off for the division
into subgroups is still not sufficiently specific, and
heterogeneity and limitations still exist. Based on the Bolondi
criteria, Kudo (9) developed the
Kindai criteria. Len was recommended as the first-line treatment,
and either sorafenib alone or sorafenib plus TACE were recommended
as alternative options for patients categorized into the BCLC B2
substage, according to the Kindai criteria (8,9). As
previously reported by Wang et al (18), no notable differences were observed
in patient groups treated with TACE plus sorafenib and sorafenib
alone in the BCLC B1 substage, as patients with low tumor burden
responded favorably to TACE monotherapy. Although tumor burden was
approved as an independent risk factor, TACE may cause a larger
area of embolization and necrosis, and the addition of sorafenib
cannot offset these side effects for patients with a high tumor
burden, which leads to not marked clinical effects of treatment
(18,31). In the TACTICS clinical trial, TACE
plus sorafenib notably prolonged PFS than TACE alone for whole
patient population, but did not for those within up-to-seven
criteria (low tumor burden) (32).
In a previous study by Kudo et al (24), it was reported that Len-treated
patients with B2 stage disease had a significantly improved ORR
(73.3 vs. 33.3%; P<0.001) and DCR (100 vs. 53.3%; P<0.001)
compared with TACE alone. Additionally, four patients no longer
received treatment with lenvatinib, achieving drug-free status,
after CR in the Len group, of which three patients received
additional selective TACE. It could be suggested that Len-TACE
sequential therapy can achieve improved CR, but additional
comparisons of specific survival data between Len-TACE sequential
therapy and TACE alone were lacking (24). Previous retrospective studies
demonstrated that Len + TACE combination therapy notably improved
treatment efficacy compared to TACE monotherapy for patients with
uHCC, however, Fu et al (26) reported that TACE combined with Len
failed to prolong OS in patients with BCLC B or C stage (P=0.070
and P=0.328, respectively) (25).
Chen et al (25) further
studied patients with BCLC B and C stage and Len + TACE combination
therapy notably contributed to increased survival in this
population.
However, to the best of our knowledge, there are
still no studies for patients with BCLC B2 substage HCC comparing
the efficacy of Len + TACE combination therapy and TACE
monotherapy. Therefore, in the present retrospective study, the
efficacy and safety between combination therapy and monotherapy in
patients with BCLC B2 substage HCC were compared. The present study
demonstrated that patients receiving Len + TACE had significantly
longer OS compared with those receiving TACE alone, meanwhile, a
similar tendency of PFS was also observed. In addition, patients in
the combination therapy group had improved ORR and treatment safety
compared with those the monotherapy group. Currently, multiple
signaling pathways such as the β-catenin signaling pathway and the
epidermal growth factor receptor (EGFR) system, have been reported
to be involved in the tumorigenesis and progression of HCC, and
crosstalk among various signal transduction pathways also exists
(33). Therefore, combination
therapy may circumvent these signal transduction pathways and
possibly cause tumor suppression more effectively than single
treatment agents limited to targeting single molecules or pathways
(33). Previous studies have
reported that TACE can induce tumor angiogenesis, which leads to a
risk of tumor recurrence and metastasis, while TKIs serve a role in
suppressing cell proliferation and tumor angiogenesis by targeting
VEGFR and other related receptors (12–14,34).
Based on these findings, a combination of TKIs and TACE has
previously been used by researchers. Qu et al (35) treated 45 patients with advanced HCC
with sorafenib in combination with TACE or TACE alone and
combination therapy significantly prolonged the median OS time
compared with monotherapy. Thereafter, a series of studies
comparing the two treatment types were performed and reported the
same outcomes in patients with intermediate-advanced HCC (36). The results of these studies provide
a preliminary basis for the application of combination therapy.
The combination therapy of TACE and Len has also
been used. Compared with the combination of TACE with sorafenib,
Len possesses a stronger affinity for VEGFR2 and inhibits more
targets, including FGF, which results in a difference in its higher
efficacy in combination with TACE (19,37–39).
Moreover, Len targets more signaling pathways, including FGFR-MAPK,
ERK/MAPK and EGFR-PI3K-AKT compared with sorafenib, which results
in a greater therapeutic advantage compared sorafenib (40–42).
Previous studies reported the increased efficacy of Len + TACE
combination therapy for patients with uHCC compared with TACE
monotherapy (25,26). However, the diagnosis of uHCC has a
wide range of presentations and characteristics, and combination
therapy or monotherapy is not the best option for all patients. For
example, Kim et al (43)
studied 277 patients BCLC B stage disease treated with surgical
resection or TACE, and reclassified patients into four subgroups on
a basis of characteristics and estimated HRs. The aforementioned
study reported a significantly increased survival in the surgical
resection group compared with the TACE group (5-year survival rate,
53.6 vs. 26.1%; P=0.021) at the B2 substage which was characterized
as ‘oligo’ tumors (2–4 nodules) with intermediate size and low AFP
level, or with small to intermediate size and high AFP level.
According to the Kinki criteria, Kudo (44) recommended curative treatment such as
surgical resection and ablation, as a first-line option for
patients with BCLC B1 stage HCC. In the B2 substage, tumor size and
number may affect survival in patients with HCC and patients
treated with TACE who had >6 tumors had a significantly shorter
median time to progression than those with ≤6 tumors (10.4 vs. 14.0
months; P=0.002) (45). Thus, the
present study focused only on the comparison between Len + TACE
combination therapy and TACE monotherapy for BCLC B2 stage HCC. The
Len + TACE group exhibited significantly improved OS and tumor
response compared with the TACE group, which was in accordance with
previous studies in which the same treatment comparisons were
performed in patients with uHCC (25,26).
Although a significant difference in PFS was not observed between
the two treatment groups, with increasing sample size, this
difference may be statistically significant. A recent network
meta-analysis by Zhang et al (46) compared the combination therapy of
TACE plus TKIs with TACE or TKIs monotherapy for patients with uHCC
and these results showed that Len + TACE combination therapy ranked
the highest in terms of OS (rank probability, 0.7559), PFS (rank
probability, 0.8595) and DCR (rank probability, 0.3857) and was
considered the optimal treatment for patients with uHCC.
The present multivariate analysis demonstrated that
the largest tumor size >4 cm was an independent risk factor and
was associated with OS and PFS. Additionally, a large tumor size
and multiple tumors were regarded as poor prognostic factors for
the survival of patients with HCC. A previous cohort study of 362
patients treated with TACE and reported that a maximal tumor size
>4 cm (OR, 1.66; 95% CI, 1.29–2.30; P=0.002) and >5 tumors
(OR, 1.92; 95% CI, 1.44–2.55; P<0.001) were significant
prognostic risk factors and a lager sample size study with 8,410
patients also reported similar results (47,48).
In addition, the subgroup analysis from the present study
demonstrated that patients with >3 tumors benefited more from
Len + TACE compared with monotherapy. Patients with a higher number
of tumors are less likely to respond to TACE and achieve a limited
effect from this treatment (47,49).
Therefore, combination therapy may provide additional benefits to
patients with multiple tumors. However, the sample size should be
larger to further compare Len + TACE with TACE treatment alone in
other subgroups, such as those of tumor distribution and Child-Pugh
class. In subgroup analysis, it was demonstrated that patients aged
>60 years benefited more from Len + TACE compared with patients
aged ≤60 years. Mosconi et al (50) compared the efficacy and safety of
TACE between patients aged greater and less than 70 years and no
significant difference was observed in survival efficacy and
complications between these age groups. In an additional
retrospective study, patients aged >70 years old who received
sorafenib had longer OS (16 vs. 12 months) and PFS (12 vs. 8
months) compared with those aged ≤70 years, although these results
were not statistically significant (51). In addition, the same phenomenon was
also observed in a CELESTIAL trial as patients aged >65 years
benefited from cabozantinib, a type of TKI, in regard to increased
OS, but younger patients did not (52). This may indicate that TKI improve
the survival of the elderly population more effectively compared
with younger patients. However, the phase III RESORCE trial yielded
opposite results to the aforementioned study (53). Elderly patients were more likely to
report comorbidities compared with younger patients, which may
increase the risk of complications after receiving systemic
therapy. In the present study, on the one hand, the follow-up time
was not long enough to observe such complications, so the
occurrence of complications in patients was not been fully
explored, which leads to the possibility of exaggerating the
treatment effect in the elderly patient population. On the other
hand, only 25 patients ages >60 years were included in the
subgroup analysis, which may introduce bias and potentially lead to
inaccurate results. Thus, these results should be interpreted
cautiously. Given that the prevalence and incidence of HCC is
increasing in the elderly patient population (54), more randomized trials and prediction
models concerning the treatment of HCC based on age factors are
needed to explore suitable treatment strategies that balance
efficacy and safety for the elderly patient population.
As presented in the current study, although AEs in
the Len + TACE group were more frequent, amongst which mild to
moderate AEs were the most predominant, AEs associated with
combination therapy were classed as manageable. In addition,
hand-foot-skin reactions and hypertension were observed only in the
Len + TACE group, which were most likely attributable to Len. Due
to the significantly increased survival benefit of combination
therapy of TACE + TKIs compared with either TACE or TKIs alone, if
serious AEs occur after using Len, sorafenib or other TKI drugs are
a viable alternative option, rather than discontinuation of Len
(45). In addition, previous
studies have reported that the incidence of treatment-related AEs
in the sorafenib plus TACE group were comparable to, or lower than,
those in the Len + TACE group during the combination treatment
(55–57).
The present study has several limitations. First,
this was a retrospective study, therefore, selection biases were
unavoidable. Second, the sample size was small, and the observation
period was not long enough for the median OS time to be observed,
which may have led to masking of the true therapeutic effects.
Therefore, large-scale, multicenter, randomized controlled studies
are needed to confirm these results and apply these findings to
further research.
In conclusion, Len + TACE combination therapy was
associated with increased OS and tumor response compared with TACE
monotherapy in patients with BCLC B2 stage HCC. Combination therapy
and monotherapy were safe and manageable. Tumor number can be used
as an independent risk factor for both OS and PFS.
Acknowledgements
Not applicable.
Funding
This work was supported by the Bureau of Science and Technology
Nanchong City (grant no. 22SXQT0052).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL and PY conceived and designed the study. JL, SY,
GZ and PY collected patient data. GZ, SW and LY analyzed and
interpreted the data. JL, SY and PY drafted and revised the
manuscript. All authors read and approved the final version of the
manuscript. JL and PY confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Affiliated Hospital of North Sichuan Medical College
(approval no. 2023ER059-1) according to the retrospective protocol
and all procedures for the cohort in the present study were carried
out in agreement with the Declaration of Helsinki. Written,
informed consent was obtained from all subjects and/or their legal
guardian(s).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qiu H, Cao S and Xu R: Cancer incidence,
mortality, and burden in China: A time-trend analysis and
comparison with the United States and United Kingdom based on the
global epidemiological data released in 2020. Cancer Commun (Lond).
41:1037–1048. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grandhi MS, Kim AK, Ronnekleiv-Kelly SM,
Kamel IR, Ghasebeh MA and Pawlik TM: Hepatocellular carcinoma: From
diagnosis to treatment. Surg Oncol. 25:74–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moon H, Choi JE, Lee IJ, Kim TH, Kim SH,
Ko YH, Kim HB, Nam BH and Park JW: All-treatment array of
hepatocellular carcinoma from initial diagnosis to death
Observation of cumulative treatments. J Cancer Res Clin Oncol.
143:2327–2339. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reig M, Forner A, Rimola J, Ferrer-Fàbrega
J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V,
Salem R, et al: BCLC strategy for prognosis prediction and
treatment recommendation: The 2022 update. J Hepatol. 76:681–693.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bolondi L, Burroughs A, Dufour JF, Galle
PR, Mazzaferro V, Piscaglia F, Raoul JL and Sangro B: Heterogeneity
of patients with intermediate (BCLC B) Hepatocellular Carcinoma:
proposal for a subclassification to facilitate treatment decisions.
Semin Liver Dis. 32:348–359. 2012.PubMed/NCBI
|
|
7
|
Yi PS, Wang H and Li JS: Evolution and
current status of the subclassification of intermediate
hepatocellular carcinoma. World J Gastrointest Surg. 12:85–92.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kudo M, Arizumi T, Ueshima K, Sakurai T,
Kitano M and Nishida N: Subclassification of BCLC B stage
hepatocellular carcinoma and treatment strategies: Proposal of
modified bolondi's subclassification (Kinki Criteria). Dig Dis.
33:751–758. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kudo M: Extremely High Objective Response
Rate of Lenvatinib: Its Clinical Relevance and Changing the
Treatment Paradigm in Hepatocellular Carcinoma. Liver cancer.
7:215–224. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li X, Feng GS, Zheng CS, Zhuo CK and Liu
X: Influence of transarterial chemoembolization on angiogenesis and
expression of vascular endothelial growth factor and basic
fibroblast growth factor in rat with Walker-256 transplanted
hepatoma: An experimental study. World J Gastroenterol.
9:2445–2449. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shim JH, Park JW, Kim JH, An M, Kong SY,
Nam BH, Choi JI, Kim HB, Lee WJ and Kim CM: Association between
increment of serum VEGF level and prognosis after transcatheter
arterial chemoembolization in hepatocellular carcinoma patients.
Cancer Sci. 99:2037–2044. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sergio A, Cristofori C, Cardin R, Pivetta
G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A and
Farinati F: Transcatheter arterial chemoembolization (TACE) in
hepatocellular carcinoma (HCC): The role of angiogenesis and
invasiveness. Am J Gastroenterol. 103:914–921. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kalbasi A and Ribas A: Tumour-intrinsic
resistance to immune checkpoint blockade. Nat Rev Immunol.
20:25–39. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rahma OE and Hodi FS: The intersection
between tumor angiogenesis and immune suppression. Clin Cancer Res.
25:5449–5457. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kudo M, Ueshima K, Ikeda M, Torimura T,
Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, et al:
Randomised, multicentre prospective trial of transarterial
chemoembolisation (TACE) plus sorafenib as compared with TACE alone
in patients with hepatocellular carcinoma: TACTICS trial. Gut.
69:1492–1501. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park JW, Kim YJ, Kim DY, Bae SH, Paik SW,
Lee YJ, Kim HY, Lee HC, Han SY, Cheong JY, et al: Sorafenib with or
without concurrent transarterial chemoembolization in patients with
advanced hepatocellular carcinoma: The phase III STAH trial. J
Hepatol. 70:684–691. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li H, Li S, Geng J, Zhao S, Tan K, Yang Z,
Feng D and Liu L: Efficacy evaluation of the combination therapy of
sorafenib and transarterial chemoembolization for unresectable HCC:
A systematic review and meta-analysis of comparative studies. Ann
Transl Med. 8:5402020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Z, Wang E, Bai W, Xia D, Ding R, Li
J, Wang Q, Liu L, Sun J, Mu W, et al: Exploratory analysis to
identify candidates benefitting from combination therapy of
transarterial chemoembolization and sorafenib for first-line
treatment of unresectable hepatocellular carcinoma: A multicenter
retrospective observational study. Liver Cancer. 9:308–325. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Okamoto K, Ikemori-Kawada M, Jestel A, von
König K, Funahashi Y, Matsushima T, Tsuruoka A, Inoue A and Matsui
J: Distinct binding mode of multikinase inhibitor lenvatinib
revealed by biochemical characterization. ACS Med Chem Lett.
6:89–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vogel A, Qin S, Kudo M, Su Y, Hudgens S,
Yamashita T, Yoon JH, Fartoux L, Simon K, López C, et al:
Lenvatinib versus sorafenib for first-line treatment of
unresectable hepatocellular carcinoma: Patient-reported outcomes
from a randomised, open-label, non-inferiority, phase 3 trial.
Lancet Gastroenterol Hepatol. 6:649–658. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Facciorusso A, Tartaglia N, Villani R,
Serviddio G, Ramai D, Mohan BP, Chandan S, Abd El Aziz MA,
Evangelista J, Cotsoglou C and Ambrosi A: Lenvatinib versus
sorafenib as first-line therapy of advanced hepatocellular
carcinoma: A systematic review and meta-analysis. Am J Transl Res.
13:2379–2387. 2021.PubMed/NCBI
|
|
23
|
Liu JN, Li JJ, Yan S, Zhang GN and Yi PS:
Transarterial chemoembolization combined with lenvatinib versus
transarterial chemoembolization combined with sorafenib for
unresectable hepatocellular carcinoma: A systematic review and
meta-analysis. Front Oncol. 13:10747932023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kudo M, Ueshima K, Chan S, Minami T,
Chishina H, Aoki T, Takita M, Hagiwara S, Minami Y, Ida H, et al:
Lenvatinib as an initial treatment in patients with
intermediate-stage hepatocellular carcinoma beyond Up-To-Seven
Criteria and Child-Pugh A Liver Function: A Proof-Of-Concept Study.
Cancers (Basel). 11:10842019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen YX, Zhang JX, Zhou CG, Liu J, Liu S,
Shi HB and Zu QQ: Comparison of the efficacy and safety of
transarterial chemoembolization with or without lenvatinib for
unresectable hepatocellular carcinoma: A retrospective propensity
score-matched analysis. J Hepatocell Carcinoma. 9:685–694. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fu Z, Li X, Zhong J, Chen X, Cao K, Ding
N, Liu L, Zhang X, Zhai J and Qu Z: Lenvatinib in combination with
transarterial chemoembolization for treatment of unresectable
hepatocellular carcinoma (uHCC): A retrospective controlled study.
Hepatol Int. 15:663–675. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kudo M, Matsui O, Izumi N, Kadoya M,
Okusaka T, Miyayama S, Yamakado K, Tsuchiya K, Ueshima K, Hiraoka
A, et al: Transarterial chemoembolization failure/refractoriness:
JSH-LCSGJ criteria 2014 update. Oncology. 87 (Suppl 1):S22–S31.
2014. View Article : Google Scholar
|
|
28
|
Lee JS, Kim BK, Kim SU, Park JY, Ahn SH,
Seong JS, Han KH and Kim DY: A survey on transarterial
chemoembolization refractoriness and a real-world treatment pattern
for hepatocellular carcinoma in Korea. Clin Mol Hepatol. 26:24–32.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
National Cancer Institute (NCI), . Common
terminology criteria for adverse events (CTCAE) version 5.0. NCI;
Bethesda, MD: 2017
|
|
31
|
Hsu C, Po-Ching-Liang, Morita S, Hu FC and
Cheng AL: Perspectives on the design of clinical trials combining
transarterial chemoembolization and molecular targeted therapy.
Liver Cancer. 1:168–176. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kudo M, Ueshima K, Ikeda M, Torimura T,
Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, et al:
Final Results of TACTICS: A randomized, prospective trial comparing
transarterial chemoembolization plus sorafenib to transarterial
chemoembolization alone in patients with unresectable
hepatocellular carcinoma. Liver Cancer. 11:354–367. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mir IH, Guha S, Behera J and
Thirunavukkarasu C: Targeting molecular signal transduction
pathways in hepatocellular carcinoma and its implications for
cancer therapy. Cell Biol Int. 45:2161–2177. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schicho A, Hellerbrand C, Krüger K, Beyer
LP, Wohlgemuth W, Niessen C, Hohenstein E, Stroszczynski C, Pereira
PL and Wiggermann P: Impact of different embolic agents for
transarterial chemoembolization (TACE) procedures on systemic
vascular endothelial growth factor (VEGF) levels. J Clin Transl
Hepatol. 4:288–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qu XD, Chen CS, Wang JH, Yan ZP, Chen JM,
Gong GQ, Liu QX, Luo JJ, Liu LX, Liu R and Qian S: The efficacy of
TACE combined sorafenib in advanced stages hepatocellullar
carcinoma. BMC Cancer. 12:2632012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xie Y, Tian H, Xiang B, Zhang Y, Liu J,
Cai Z and Xiang H: Transarterial chemoembolization plus sorafenib
versus sorafenib for intermediate-advanced hepatocellular
carcinoma: A meta-analysis comparing clinical outcomes. Medicine
(Baltimore). 100:e269582021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xue M, Wu Y, Zhu B, Zou X, Fan W and Li J:
Advanced hepatocellular carcinoma treated by transcatheter arterial
chemoembolization with drug-eluting beads plus lenvatinib versus
sorafenib, a propensity score matching retrospective study. Am J
Cancer Res. 11:6107–6118. 2021.PubMed/NCBI
|
|
38
|
Matsuki M, Hoshi T, Yamamoto Y,
Ikemori-Kawada M, Minoshima Y, Funahashi Y and Matsui J: Lenvatinib
inhibits angiogenesis and tumor fibroblast growth factor signaling
pathways in human hepatocellular carcinoma models. Cancer Med.
7:2641–2653. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yamamoto Y, Matsui J, Matsushima T,
Obaishi H, Miyazaki K, Nakamura K, Tohyama O, Semba T, Yamaguchi A,
Hoshi SS, et al: Lenvatinib, an angiogenesis inhibitor targeting
VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft
models associated with microvessel density and pericyte coverage.
Vasc Cell. 6:182014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hoshi T, Watanabe Miyano S, Watanabe H,
Sonobe RMK, Seki Y, Ohta E, Nomoto K, Matsui J and Funahashi Y:
Lenvatinib induces death of human hepatocellular carcinoma cells
harboring an activated FGF signaling pathway through inhibition of
FGFR-MAPK cascades. Biochem Biophys Res Commun. 513:1–7. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
He X, Hikiba Y, Suzuki Y, Nakamori Y,
Kanemaru Y, Sugimori M, Sato T, Nozaki A, Chuma M and Maeda S: EGFR
inhibition reverses resistance to lenvatinib in hepatocellular
carcinoma cells. Sci Rep. 12:80072022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Romei C, Ciampi R and Elisei R: A
comprehensive overview of the role of the RET proto-oncogene in
thyroid carcinoma. Nat Rev Endocrinol. 12:192–202. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim JY, Sinn DH, Gwak GY, Choi GS, Saleh
AM, Joh JW, Cho SK, Shin SW, Carriere KC, Ahn JH, et al:
Transarterial chemoembolization versus resection for
intermediate-stage (BCLC B) hepatocellular carcinoma. Clin Mol
Hepatol. 22:250–258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kudo M: A new treatment option for
intermediate-stage hepatocellular carcinoma with high tumor Burden:
Initial lenvatinib therapy with subsequent selective TACE. Liver
Cancer. 8:299–311. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Arizumi T, Minami T, Chishina H, Kono M,
Takita M, Yada N, Hagiwara S, Minami Y, Ida H, Ueshima K, et al:
Impact of tumor factors on survival in patients with hepatocellular
carcinoma classified based on kinki criteria stage B2. Dig Dis.
35:583–588. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang Z, Wu Y, Zheng T, Chen X, Chen G,
Chen H, Guo X, Zheng S, Xie X and Zhang B: Efficacy of
transarterial chemoembolization combined with molecular targeted
agents for unresectable hepatocellular carcinoma: A network
meta-analysis. Cancers (Basel). 14:37102022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hu HT, Kim JH, Lee LS, Kim KA, Ko GY, Yoon
HK, Sung KB, Gwon DI, Shin JH and Song HY: Chemoembolization for
hepatocellular carcinoma: Multivariate analysis of predicting
factors for tumor response and survival in a 362-patient cohort. J
Vasc Interv Radiol. 22:917–923. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Takayasu K, Arii S, Ikai I, Omata M, Okita
K, Ichida T, Matsuyama Y, Nakanuma Y, Kojiro M, Makuuchi M, et al:
Prospective cohort study of transarterial chemoembolization for
unresectable hepatocellular carcinoma in 8510 patients.
Gastroenterology. 131:461–469. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Miyayama S, Kikuchi Y, Yoshida M,
Yamashiro M, Sugimori N, Ikeda R, Okimura K, Sakuragawa N, Ueda T,
Sanada T, et al: Outcomes of conventional transarterial
chemoembolization for hepatocellular carcinoma ≥10 cm. Hepatol Res.
49:787–798. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mosconi C, Gramenzi A, Biselli M, Cappelli
A, Bruno A, De Benedittis C, Cucchetti A, Modestino F, Peta G,
Bianchi G, et al: Survival and Tolerability of Transarterial
Chemoembolization in Greater Versus less than 70 Years of Age
Patients with Unresectable Hepatocellular Carcinoma: A Propensity
Score Analysis. Cardiovasc Intervent Radiol. 43:1015–1024. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Di Costanzo GG, Tortora R, De Luca M,
Galeota Lanza A, Lampasi F, Tartaglione MT, Picciotto FP, Imparato
M, Mattera S, Cordone G and Ascione A: Impact of age on toxicity
and efficacy of sorafenib-targeted therapy in cirrhotic patients
with hepatocellular carcinoma. Med Oncol. 30:4462013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Abou-Alfa GK, Meyer T, Cheng AL,
El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park
JW, et al: Cabozantinib in patients with advanced and progressing
hepatocellular carcinoma. N Engl J Med. 379:54–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bruix J, Qin S, Merle P, Granito A, Huang
YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al:
Regorafenib for patients with hepatocellular carcinoma who
progressed on sorafenib treatment (RESORCE): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet. 389:56–66.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang X, El-Serag HB and Thrift AP: Sex
and race disparities in the incidence of hepatocellular carcinoma
in the united states examined through age-period-cohort analysis.
Cancer Epidemiol Biomarkers Prev. 29:88–94. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang JX, Chen YX, Zhou CG, Liu J, Liu S,
Shi HB and Zu QQ: Transarterial chemoembolization combined with
lenvatinib versus transarterial chemoembolization combined with
sorafenib for unresectable hepatocellular carcinoma: A comparative
retrospective study. Hepatol Res. 52:794–803. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yang B, Jie L, Yang T, Chen M, Gao Y,
Zhang T, Zhang Y, Wu H and Liao Z: TACE plus lenvatinib versus TACE
plus sorafenib for unresectable hepatocellular carcinoma with
portal vein tumor thrombus: A prospective cohort study. Front
Oncol. 11:8215992021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ding X, Sun W, Li W, Shen Y, Guo X, Teng
Y, Liu X, Zheng L, Li W and Chen J: Transarterial chemoembolization
plus lenvatinib versus transarterial chemoembolization plus
sorafenib as first-line treatment for hepatocellular carcinoma with
portal vein tumor thrombus: A prospective randomized study. Cancer.
127:3782–3793. 2021. View Article : Google Scholar : PubMed/NCBI
|