Introduction
Giant cell tumor of bone (GCTB) comprises primarily
intramedullary bone tumors and accounts for 4–5% of all bone tumors
based on a multicenter study including 103 patients between 1980
and 2008 (1). GCTBs are benign
lesions; however, several published reports of pulmonary metastasis
exist (2). Pulmonary metastasis is
detected in ~2–5% of cases of GCTB and is associated with poor
treatment outcomes (3). The
formation of an ossified rim is a prominent finding in both
recurrent and metastatic GCTBs (4).
These types of tumors are composed of reactive multinuclear cells
expressing receptor activators of nuclear factor-kappa B (RANK)
(5), resembling osteoclasts.
Previous reports suggest there are overlapping markers for GCTB
that are similar to those of osteoclasts, such as
tartrate-resistant acid phosphatase (6), cathepsin K (7), carbonic anhydrase II (8) and calcitonin receptor (9).
A population-based study reported that the
prevalence of GCTB is 80% in patients aged 20–40 years (10). Although GCTBs occur at multiple
sites in the body, the predominant site is the end of a long bone
and around the knee, which account for over half of the total
number of reported cases (11–14).
The clinical manifestations of GCTB comprise swelling, pain and
pathological fracture (15). The
first-line therapy for GCTB comprises curettage using a high-speed
burr to reduce GCTB recurrence (16,17).
Patients with GCTB must undergo long-term follow-up as recurrence
and metastasis may occur up to 20 years postoperatively (18).
The diagnosis of GCTB comprises clinical
observation, radiographs and histopathological analysis (19). The typical clinical manifestations
of GCTB are swelling, local pain and pathological fracture
(20). Dynamic contrast-enhanced
MRI has increased the accuracy of diagnosing GCTBs (21). Additionally, molecular research has
provided insights into diagnostic markers, such as p53, p63,
kinectin 1, rho-associated, coiled-coil-containing protein kinase
1, nebulin and sterile alpha motif and leucine zipper containing
kinase AZK, among others, related to GCTBs (22–24).
Pulmonary metastasis is difficult to diagnose in early-stage GCTBs
and is more likely to be discovered in recurrent cases (25). The development of pulmonary
metastasis from primary lesions may take months to years (26,27).
The aim of the present study was to evaluate patient
outcomes and identify any influencing factors in GCTB-induced
pulmonary metastasis.
Materials and methods
Patient selection
The medical records of 50 patients with GCTB treated
in Khon Kaen Hospital (Khon Kaen, Thailand) from January 2016 to
December 2021 were retrospectively analyzed. Patients with
incomplete medical records were excluded from the study. Ethical
approval from The Institute Review Board in Human Research of Khon
Kaen Hospital was obtained prior to the initiation of the study
(approval no. KEXP65041).
Pulmonary metastasis was confirmed by considering
the tumor characteristics, namely single or multiple pulmonary
nodules differentiated from developing abnormal lesions, with
rounded, well-defined opacities on chest computed tomography (CT).
Metastasis was confirmed with subsequent CT if the lesions had
increased in number and size.
The collected data comprised the patients'
demographic and clinical data, namely the location(s) of the
primary lesions, local recurrence history and metastasis. Follow-up
durations were defined as the time from the first evaluation for
primary treatment to local recurrence and from diagnosis of the
primary tumor to pulmonary metastasis. Additional parameters were
the treatment type for local recurrence, metastasis treatment,
treatment course and follow-up events.
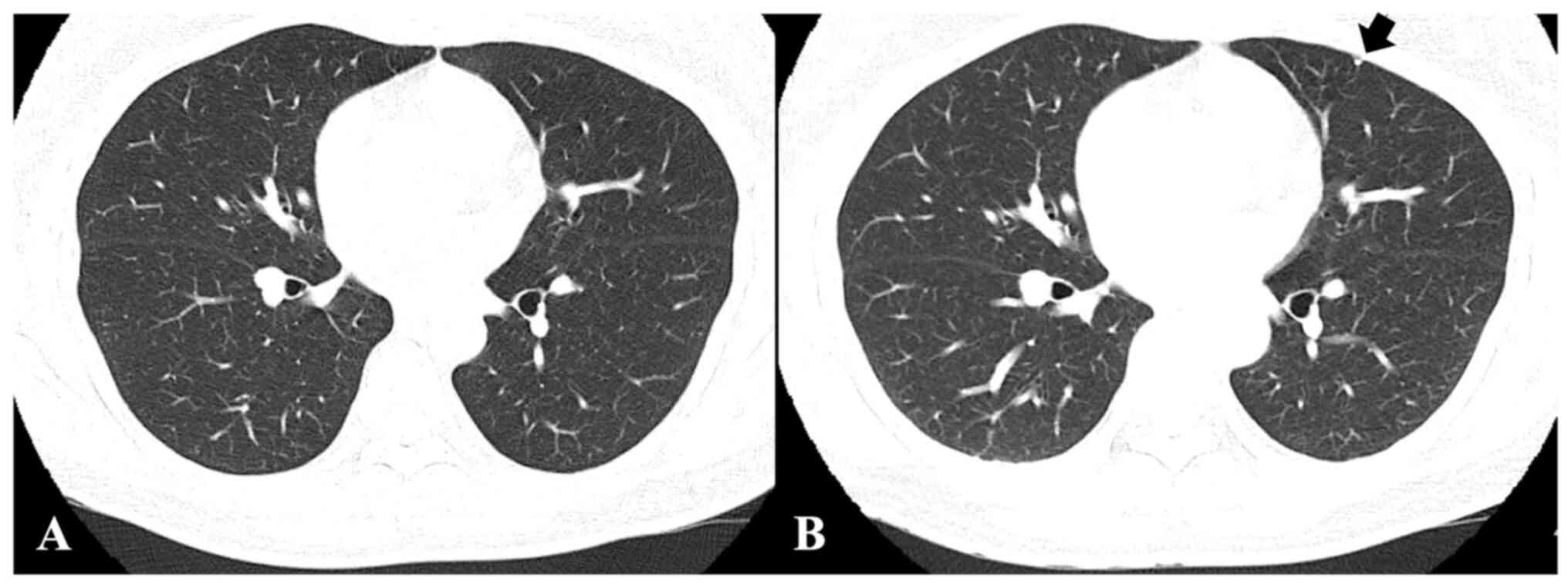
To meet the study purpose, radiographic images,
namely CT, X-rays and magnetic resonance images were reviewed. CT
images and chest X-rays were evaluated to confirm pulmonary
metastasis. Follow-up chest CT images were screened for metastatic
nodule development and progression to evaluate treatment efficacy.
Follow-up chest CT images were analyzed to measure and evaluate the
course of metastatic nodules receiving treatment. The chest CT
evaluations determined whether the metastatic nodules were
advanced, stationary or reduced (Fig.
1).
Statistical analysis
Data analysis was conducted using Microsoft Excel
version 16.76 (Microsoft Corporation) for editing, sorting and
coding. The final Excel file was subsequently imported into SPSS
software (version 27; IBM Corp.) for statistical analysis.
Continuous variables are expressed as the mean ± standard
deviation. The normality of the data was tested using the
Kolmogorov-Smirnov test. Unpaired Student's t-test was used to
analyze statistical differences between group means. Categorical
variables were presented as percentages and the chi-square test was
used to compare categorical variables, including patients' clinical
characteristics. If the expected value was <5 for >20% of the
total cells, Fisher's exact test was used. P<0.05 was considered
to indicate a statistically significant difference. Univariate and
multivariable logistic regression analyses were conducted to
evaluate the risk factors for GCTB-induced pulmonary metastasis. An
alternative method, namely the continuity correction, was used for
logistic regression analysis on contingency tables containing zero
cell counts. The number of events and non-events in studies with
zero cell counts was increased by 0.5. Variables with a P<0.10
in the univariate logistic regression analyses and other variables
of known clinical relevance were included in the multivariable
logistic regression analyses. The regression was performed with 95%
confidence intervals (CIs). Univariate logistic regression models
with 95% CI were used to conduct univariate [odds ratio (OR)] and
multivariable analysis [adjusted odds ratio (AOR)]. An AOR is a
statistical measure that has been modified to accommodate the
influence of additional predictor variables, including age at
diagnosis, axial location of GCTB, fracture and neurological
deficit, stage III, and 1 and >1 local recurrences within a
model. This measure is particularly valuable in illustrating the
impact of a specific predictor variable on the likelihood of an
event occurring, while controlling for the influence of other
predictor variables. The logistic regression model is prone to
being affected by small-sample bias (28). In medical literature, a commonly
adopted lower limit for developing prediction models that forecast
a binary outcome is an events per variable (EPV) of 10 (29,30).
The EPV, representing the smaller count between the number of
subjects who experienced the outcome and those who did not, is
calculated by dividing it by the number of predictor variables
utilized in building the prediction model. In the present study,
with 50 events (cases) and six predictor variables including age at
diagnosis, location of GCTB, clinical presentation, Campanacci
stage, no. of local recurrences and treatment for local recurrence,
the resulting EPV is ~8, falling below the recommended threshold of
10. The Firth method (Firth's Bias-Reduced Logistic Regression),
named after its creator, utilizes a penalized likelihood approach
to mitigate the impact of small-sample bias in maximum likelihood
estimation (31). The Firth method
was used for small-sample analysis in STATA version 16.0 (StataCorp
LP). The variance inflation factor (VIF) was also calculated to
confirm multicollinearity. All methods were performed in accordance
with the relevant guidelines described by Altman et al
(32).
Results
Patient demographics and clinical
data
Of the 50 patients with GCTB, 26 (52.0%) were female
and 24 (48.0%) were male, with a female-to-male ratio of 1.08:1.0
(Table I). The primary tumor sites
in an extremity were the proximal humerus (n=2), distal radius
(n=4), distal humerus (n=0), distal ulna (n=5), distal femur
(n=17), proximal femur (n=3), proximal tibia (n=7), proximal fibula
(n=3), distal tibia (n=2), talus (n=0), calcaneus (n=1), hand (n=2)
and foot (n=1). Additionally, primary tumors were located in the
axial locations in the spine (n=0), sacrum (n=2) and pelvis (n=1).
Primary GCTBs occurred most often in the distal femur (n=17),
accounting for 34% of all patients. Of the 50 patients evaluated in
this study, 47 (94%) were primary cases and 3 (6%) were recurrent
cases.
 | Table I.Patient demographic and clinical
data. |
Table I.
Patient demographic and clinical
data.
| Patient
characteristic | Result |
|---|
| Mean patient age,
years (mean ± SD) | 36.0±17.2 |
| Follow-up duration,
months (mean ± SD) | 26.3±18.4 |
| Patient sex, n
(%) |
|
|
Female | 26 (52.0) |
|
Male | 24 (48.0) |
| Duration from first
treatment to first appearance of recurrence, (mean ± SD) | 13.5±12.5 |
| Primary GCTB
diagnosis to first appearance of pulmonary metastasis, (mean ±
SD) | 11.7±9.4 |
| Primary tumor
location, n (%) |
|
|
Extremity |
|
|
Proximal humerus | 2 (4.0) |
| Distal
humerus | 0 (0.0) |
| Distal
radius | 4 (8.0) |
| Distal
ulna | 5 (10.0) |
|
Proximal femur | 3 (6.0) |
| Distal
femur | 17 (34.0) |
|
Proximal tibia | 7 (14.0) |
|
Proximal fibula | 3 (6.0) |
| Distal
tibia | 2 (4.0) |
|
Talus | 0 (0.0) |
|
Calcaneus | 1 (2.0) |
|
Hand | 2 (4.0) |
|
Foot | 1 (2.0) |
| Axial |
|
|
Spine | 0 (0.0) |
|
Sacrum | 2 (4.0) |
|
Pelvis | 1 (2.0) |
| Tumor type, n
(%) |
|
|
Primary | 47 (94.0) |
|
Recurrence | 3 (6.0) |
| Clinical
presentation, n (%) |
|
|
Incidental finding | 0 (0.0) |
|
Pain | 27 (54.0) |
|
Mass | 12 (24.0) |
|
Pathological fracture | 10 (20.0) |
|
Neurological deficit | 1 (2.0) |
| Campanacci
radiographic stage, n (%) |
|
| I | 1 (2.0) |
| II | 11 (22.0) |
|
III | 38 (76.0) |
| Mode of treatment
for the primary tumor, n (%) |
|
| Simple
curettage (without local adjuvant therapy) | 3 (6.0) |
|
Extended curettage (with local
adjuvant therapy) | 35 (70.0) |
| Wide
resection | 11 (22.0) |
|
Amputation/disarticulation | 1 (2.0) |
| Local recurrence
(number of tumors), n (%) |
|
| 0 | 37 (74.0) |
| 1 | 12 (24.0) |
|
>1 | 1 (2.0) |
| Treatment of local
recurrence, n (%) |
|
| None
(no local recurrence) | 38 (76.0) |
| Simple
curettage (without local adjuvant therapy) | 0 (0.0) |
|
Extended curettage (with local
adjuvant therapy) | 4 (8.0) |
| Wide
resection | 3 (6.0) |
|
Amputation/disarticulation | 2 (5.0) |
|
Adjuvant therapy | 2 (5.0) |
| Mixed
(curettage with adjuvant therapy and wide resection) | 1 (2.0) |
| Location/extension
of pulmonary metastasis |
|
| Single
lesion |
|
|
None | 41 (82.0) |
|
Right upper
lung | 1 (2.0) |
|
Right middle
lung | 1 (2.0) |
|
Right lower
lung | 0 (0.0) |
|
Left upper
lung | 1 (2.0) |
|
Left lower
lung | 0 (0.0) |
|
Multiple lesions |
|
|
Right lung | 2 (4.0) |
|
Left lung | 1 (2.0) |
|
Both lungs | 3 (6.0) |
| Treatment of
pulmonary metastasis, n (%) |
|
| None/no
metastasis | 41 (82.0) |
|
Observation | 6 (12.0) |
|
Bisphosphonate | 0 (0.0) |
|
Denosumab | 3 (6.0) |
|
Chemotherapy | 0 (0.0) |
|
Resection | 0 (0.0) |
|
Radiation | 0 (0.0) |
Clinical characteristics
Pain, the presence of a mass, pathological fracture
and neurological deficit were significant parameters in identifying
and diagnosing the possibility of a tumor (Table I). Pain in 27 patients and
pathological fracture in 10 patients accounted for two notable
contributors at 54.0 and 20.0% of the cases, respectively.
According to the Campanacci grading system (17), Campanacci grade I tumors were
reported in 2% of the patients (n=1), Campanacci grade II tumors
were reported in 22% of patients (n=11) and Campanacci grade III
was most frequent being reported in 76% of patients (n=38). The
average period from treatment to recurrence was 13.5±12.5 months.
The mean time from primary GCTB to pulmonary metastasis diagnosis
was 11.7±9.4 months.
Mode of treatment
Of the 50 patients with GCTB, 3 patients (6%)
received simple curettage without local adjuvant therapy and 35
(70%) received extended curettage with local adjuvant therapy
(Table I). Additionally, 11
patients (22%) underwent wide resection, and amputation or
disarticulation was necessary for 1 patient (2%).
Recurrence and metastasis
The average follow-up duration was 26.3 months,
during which 74% of the patients (n=37) were without local
recurrence (Table I). However, 12
patients (24%) developed recurrence once and 1 patient (2%)
developed recurrence more than once.
A total of 41 patients (n=82) did not develop
metastasis. From the cohort of 9 patients (18%) who developed
pulmonary metastasis, three had single lesions as follows: Right
upper lung (n=1; 2%); right middle lung (n=1; 2%); and left upper
lung (n=1; 2%). Of the remaining 6 patients, 3 patients (6%)
developed multiple lesions in both lungs, 2 patients (4%) developed
multiple lesions in the right lung and 1 patient (2%) developed
multiple lesions in the left lung.
Of the 9 pulmonary metastasis cases, 5 reported
fractures (Table II). Within this
group of patients, 2 individuals developed tumor recurrence whereas
the remaining 7 patients developed new primary tumors. Of the 2
cases with local recurrence, 1 underwent extended curettage and 1
underwent wide resection. None of the patients died from pulmonary
metastasis in the present study.
 | Table II.Clinical data of patients with
pulmonary metastasis. |
Table II.
Clinical data of patients with
pulmonary metastasis.
| Patient number | Age, years | Location of
GCTB | Clinical
presentation | Tumor type | Campanacci
radiographic stage | Treatment of
GCTB | No. of local
recurrences | Treatment of local
recurrence | Time from diagnosis
of the primary GCTB to diagnosis of pulmonary metastasis,
months | Treatment for
pulmonary metastasis | Follow-up time,
months |
|---|
| 1 | 46 | Distal femur | Fracture | Primary | III | Curettage | >1 | Wide resection | 15 | Denosumab | 20 |
| 2 | 68 | Distal femur | Fracture | Primary | III | Curettage | 1 | Amputation | 0 | Observation | 6 |
| 3 | 58 | Distal femur | No fracture | Primary | III | Wide resection | No recurrence | None | 26 | Observation | 26 |
| 4 | 39 | Distal femur | No fracture | Primary | III | Wide resection | No recurrence | None | 14 | Observation | 31 |
| 5 | 42 | Distal femur | Fracture | Primary | III | Wide resection | No recurrence | None | 2 | Observation | 6 |
| 6 | 42 | Distal femur | Fracture | Primary | III | Curettage | 1 | Mixed, curettage
with adjuvant therapy and wide resection | 14 | Denosumab | 29 |
| 7 | 39 | Proximal tibia | No fracture | Recurrent | III | Curettage | 1 | Extended
curettage | 3 | Denosumab | 7 |
| 8 | 65 | Proximal femur | Fracture | Primary | II | Curettage | No recurrence | None | 7 | Observation | 8 |
| 9 | 18 | Distal femur | No fracture | Recurrent | II | Curettage | 1 | Wide resection | 24 | Observation | 8 |
Risk factors for pulmonary
metastasis
In the present study, pulmonary metastasis occurred
in 9 of the 50 patients with GCTB. GCTB occurrence was more
frequent in patients aged ≥35 years compared with those aged <35
years in patients with Campanacci grade III tumors (8 vs. 1
patient, respectively; Table II).
There was a statistically significant association between GCTB
occurrence and age (P=0.045; Table
III). The pulmonary metastasis incidence rate among patients
with fractures was 55.5% (5/9), which was statistically significant
(P=0.024). Similarly, in patients with >1 local tumor
recurrence, the pulmonary metastasis incidence rate was 11.11%
(1/9) and was statistically significant (P=0.038). Local treatment
was demonstrated to be a significant risk factor for developing
pulmonary metastasis (P=0.035; Table
III). In the present study, sex, location of GCTB, tumor type,
Campanacci stage, treatment for GCTB and mean time from treatment
to local recurrence were not significantly associated with
pulmonary metastasis (P=0.142, P=0.560, P=0.080, P=0.818, P=0.577
and P=0.251, respectively).
 | Table III.Characteristics of the patients with
and without pulmonary metastasis. |
Table III.
Characteristics of the patients with
and without pulmonary metastasis.
| Factor | Without lung
metastasis (N=41) | With lung
metastasis (N=9) | P-value |
|---|
| Mean age ± SD,
years | 33.8±16.8 | 46.3±15.4 | 0.045a |
| Sex, n (%) |
|
|
|
|
Female | 19 (73.1) | 7 (26.9) | 0.142b |
|
Male | 22 (91.7) | 2 (8.3) |
|
| Location of
GCTB |
|
|
|
|
Extremity | 38 (82.6) | 8 (19.1) | 0.560b |
|
Axial | 3 (75.0) | 1 (25.0) |
|
| Clinical
presentation |
|
|
|
| No
fracture | 35 (89.7) | 4 (10.3) | 0.024b |
|
Fracture | 5 (50.0) | 5 (50.0) |
|
|
Neurological deficit | 1 (100.0) | 0 (0.0) |
|
| Tumor type |
|
|
|
|
Primary | 40 (85.1) | 7 (14.9) | 0.080b |
|
Recurrence | 1 (33.3) | 2 (66.7) |
|
| Campanacci
stage |
|
|
|
| I | 1 (100.0) | 0 (0.0) | 0.818b |
| II | 9 (81.8) | 2 (18.2) |
|
|
III | 31 (81.6) | 7 (18.4) |
|
| Treatment for
GCTB |
|
|
|
|
Curettage | 32 (84.2) | 6 (15.8) | 0.577b |
| Wide
resection | 8 (72.7) | 3 (27.3) |
|
|
Mixed | 1 (100) | 0 (0.0) |
|
| No. of local
recurrences |
|
|
|
| No
recurrence | 33 (89.2) | 4 (10.8) | 0.038b |
| 1 | 8 (66.7) | 4 (33.3) |
|
|
>1 | 0 (0.0) | 1 (100.0) |
|
| Mean time from
treatment to local recurrence ± SD, months | 16.4±14.7 | 9.0±7.1 | 0.251a |
| Treatment for local
recurrence |
|
|
|
|
None | 34 (89.5) | 4 (10.5) | 0.035b |
|
Curettage | 3 (75.0) | 1 (25.0) |
|
| Wide
resection | 2 (40.0) | 3 (60.0) |
|
|
Adjuvant therapy | 2 (100.0) | 0 (0.0) |
|
|
Mixed | 0 (0.0) | 1 (100.0) |
|
Univariate analysis using Firth's Bias-Reduced
Logistic Regression revealed a statistically significant
association between GCTB-induced pulmonary metastasis and fractures
(OR, 7.89; 95% CI, 1.69–36.65, P=0.008; Table IV). Local treatment requiring
curettage with wide resection emerged as a significant risk factor
for developing pulmonary metastasis (OR, 10.73; 95% CI, 1.61–71.56,
P=0.014; Table IV). Multivariable
analysis revealed two independent risk factors for developing
pulmonary metastasis. Patients who presented with pathological
fractures had an increased risk of developing pulmonary metastasis
that was 6.107 times (AOR, 6.107; 95% CI, 1.075–34.70) higher than
that of patients without pathological fractures (P=0.041). Local
recurrence increased the risk of pulmonary metastasis by 6.480
times (AOR, 6.480; 95% CI, 1.027–40.87; P=0.047; Table V). Multicollinearity tests conducted
on the model did not detect a significant level of
multicollinearity among any of the included covariates with VIF
<1.20.
 | Table IV.Univariate analysis of the factors
associated with pulmonary metastasis from GCTB using the Firth's
Bias-Reduced Logistic Regression method. |
Table IV.
Univariate analysis of the factors
associated with pulmonary metastasis from GCTB using the Firth's
Bias-Reduced Logistic Regression method.
| Factor | Without lung
metastasis (n=41) | With lung
metastasis (N=9) | Odd ratio | 95% CI | P-value |
|---|
| Mean age ± SD | 33.8±16.8 | 46.3±15.4 | 1.04 | 0.99–1.08 | 0.058 |
| Sex |
|
|
|
|
|
| Female
(ref.) | 19 (73.1) | 7 (26.9) | 1 | - | - |
|
Male | 22 (91.7) | 2 (8.3) | 0.29 | 0.06–1.37 | 0.117 |
| Location of
GCTB |
|
|
|
|
|
|
Extremity (ref.) | 38 (82.6) | 8 (19.1) | 1 | - | - |
|
Axial | 3 (75.0) | 1 (25.0) | 1.96 | 0.25–15.11 | 0.526 |
| Clinical
presentation |
|
|
|
|
|
| No
fracture (ref.) | 35 (89.7) | 4 (10.3) | 1 | - | - |
|
Fracture | 5 (50.0) | 5 (50.0) | 7.89 | 1.69–36.65 | 0.008 |
|
Neurological deficit | 1 (100.0) | 0 (0.0) | 2.63a | 0.09–74.76 | 0.571 |
| Tumor type |
|
|
|
|
|
| Primary
(ref.) | 40 (85.1) | 7 (14.9) | 1 | - | - |
|
Recurrence | 1 (33.3) | 2 (66.7) | 9.00 | 1.03–78.75 | 0.050 |
| Campanacci
stage |
|
|
|
|
|
| I
(ref.) | 1 (100.0) | 0 (0.0) | 1 | - | - |
| II | 9 (81.8) | 2 (18.2) | 0.79a | 0.02–25.90 | 0.894 |
|
III | 31 (81.6) | 7 (18.4) | 0.71a | 0.03–19.33 | 0.842 |
| Treatment for
GCTB |
|
|
|
|
|
|
Curettage (ref.) | 32 (84.2) | 6 (15.8) | 1 | - | - |
| Wide
resection | 8 (72.7) | 3 (27.3) | 2.06 | 0.46–9.25 | 0.346 |
|
Mixed | 1 (100) | 0 (0.0) | 1.66a | 0.06–45.62 | 0.762 |
| No. of local
recurrences |
|
|
|
|
|
| No
recurrence (ref.) | 33 (89.2) | 4 (10.8) | 1 | - | - |
| 1 | 8 (66.7) | 4 (33.3) | 3.94 | 0.87–17.80 | 0.075 |
|
>1 | 0 (0.0) | 1 (100.0) | 22.33a | 0.78–635.58 | 0.069 |
| Mean time from
treatment to local recurrence ± SD, months | 16.4±14.7 | 9.0±7.1 | 0.96 | 0.87–1.05 | 0.373 |
| Treatment for local
recurrence |
|
|
|
|
|
| None
(ref.) | 34 (89.5) | 4 (10.5) | 1 | - | - |
|
Curettage | 3 (75.0) | 1 (25.0) | 3.28 | 0.38−28.21 | 0.278 |
| Wide
resection | 2 (40.0) | 3 (60.0) | 10.73 | 1.61–71.56 | 0.014 |
|
Adjuvant therapy | 2 (100.0) | 0 (0.0) | 1.53a | 0.06–37.29 | 0.793 |
|
Mixed | 0 (0.0) | 1 (100.0) | 23.00a | 0.81–654.23 | 0.066 |
 | Table V.Multivariable analysis of the factors
associated with pulmonary metastasis from GCTB using the Firth's
Bias-Reduced Logistic Regression method. |
Table V.
Multivariable analysis of the factors
associated with pulmonary metastasis from GCTB using the Firth's
Bias-Reduced Logistic Regression method.
| Factor | P-value | Adjusted odds
ratio | 95% CI |
|---|
| Age at
diagnosis | 0.144 | 1.033 | 0.988–1.079 |
| Location of GCTB
(axial) | 0.488 | 0.355 | 0.019–6.623 |
| Clinical
presentation (fracture and neurological deficit)a | 0.041 | 6.107 | 1.075–34.70 |
| Campanacci stage
(III) | 0.547 | 0.562 | 0.086–3.671 |
| No. of local
recurrences (1 and >1)a | 0.047 | 6.480 | 1.027–40.87 |
Discussion
Metastasis of GCTBs is uncommon, as the rate of
metastasis varies from 1–9% across previous studies and 3% of GCTBs
metastasize to the lung (33–38).
In the present study, the prevalence of metastasis was 18%, which
differed from that in previously published reports (39–44).
In the present study, all nine patients had pulmonary metastases.
Among nine pulmonary metastases, recurrent patients showed higher
incidences of lung metastasis (66.66%; 2/3) than non-recurrent
patients (14.89%; 7/47). Several risk factors are associated with
the onset of metastasis, such as local tumor recurrence, a delay in
seeking treatment and pathological fractures being the most
statistically significant (45,46).
Performing tissue biopsies on every patient with a lung mass
suspected of being pulmonary metastasis from GCTB is not feasible.
It is important to balance the benefits of obtaining a definitive
diagnosis with the potential risks, resource allocation and the
well-being of the patient. Wang et al (47) defined the pulmonary metastasis from
GCTB as follows: i) The development of abnormal lesions, either
single or multiple pulmonary nodules, rounded and well-defined
opacities on chest CT; and ii) there should be evidence of growth
during the follow-up period, either in the number or size of
lesions.
Pathological fracture is significantly associated
with pulmonary metastasis. Previous studies have reported varying
incidences (5.3–11%) of pathological fractures among patients
diagnosed with pulmonary metastasis (47,48).
The rate of pulmonary metastasis from GCTB is relatively high (18%)
in the present study due to a delay in patients seeking treatment,
leading to pathological fracture. The present study demonstrated
that up to 20% of patients presented with pathological fractures
and in 55.5% of the cases of pulmonary metastasis fractures were
identified as independent risk factors for developing pulmonary
metastasis at a rate 6.107 times higher compared with patients who
did not experience fractures. The pathological fracture was a
critical risk factor for developing metastasis. Faisham et
al (38) reported that
Campanacci grade III is a risk factor for pulmonary metastasis. In
the present study, 76% of primary cases had Campanacci stage grade
III tumors, which later resulted in metastasis in 7 patients (77.7%
of malignant cases).
Local tumor recurrence and metastasis have a
positive association (45,49). Similar to findings reported in
previous studies, the present study demonstrated that local tumor
recurrence significantly increased the risk of pulmonary
metastasis. Of the 9 patients with pulmonary metastasis, 5 patients
(55.5%) experienced local recurrence at least once and this
association was confirmed in both univariate and multivariable
analysis. Local curettage and local adjuvant therapy can help
prevent local recurrence (50).
Wide resection effectively reduces tumor burden in recurrent and
pathological fracture cases (51,52).
In addition to local treatment, new drugs have been
developed for the treatment of GCTBs. Denosumab was previously
introduced for the treatment of advanced and metastatic GCTB and it
binds to the RANK ligand-receptor activator (53). Denosumab inhibits the recruitment of
osteoclast-like giant cells and prevents osteolysis (53–55).
The bisphosphate zoledronic acid, also targets the neoplastic
stromal cells in GCTBs (56). In
the present study, denosumab was administered to 3 of the patients
with pulmonary metastatic cancer and the remaining 6 patients
underwent observation only, with no radiation or resection. The
present study demonstrated that none of the patients died as a
result of pulmonary metastasis from GCTB. Resection of metastatic
lesions or administration of denosumab is considered in cases where
there is an increase in the size of the metastatic lesion(s) and
the metastatic lesion(s) are causing symptoms (57).
Although no mortality was reported in the present
study, the reported mortality rate for metastatic GCTB cases ranges
from 0–23% (58), which is a major
concern. The prognosis is good after timely and appropriate
surgical resection for patients with pulmonary metastasis, with a
71–100% survival rate at the last follow-up (59). Denosumab, a monoclonal antibody that
inhibits bone breakdown both in normal and tumor-related contexts,
by preventing the formation and activation of multinuclear
osteoclasts or giant cells mediated by receptor activator of
nuclear factor κβ, is being considered as a potential treatment
option for pulmonary metastasis in unresectable GCTB (60).
In the present study, most patient cases were
managed by close observation. Therefore, pulmonary metastasectomy
was not performed immediately post-diagnosis. Metastasectomy is
only advisable under conditions where the patient appears to have
progressing metastasis. Local tumor recurrence and pathological
fracture are independent risk factors for developing pulmonary
metastasis from GCTB. Therefore, in cases with local recurrence or
pathological fracture, more aggressive treatment, such as wide
resection, should be performed to reduce the local recurrence rate
and lower the risk of pulmonary metastasis.
The limitation of this study was the small sample
size. There were 50 cases of GCTB and only 9 patients developed
pulmonary metastasis. Therefore, a larger cohort of patients is
required to validate the risk factors associated with GCTBs that
were identified in the present study.
There are a number of unanswered questions in regard
to the effective treatment of GCTBs, especially regarding the high
recurrence rates and adverse effects observed upon systemic therapy
(61). There is a need to
investigate alternative therapeutic strategies to effectively treat
pulmonary metastasis of GCTBs.
To conclude, pulmonary metastasis from GCTB was not
uncommon in the present study. CT chest scan should be performed in
each patient with GCTB as the rate of pulmonary metastasis from
GCTB was relatively high (18%) in the present study. Local
recurrence and pathological fracture were associated with
developing pulmonary metastasis. It is unnecessary to perform
pulmonary metastasectomy immediately. Additionally, a biopsy of
metastatic lesions developed from GCT of bone is also unnecessary.
Close observation of patients with metastasis is essential and
serial imaging is recommended in every case. More studies are
required that evaluate the molecular mechanisms of GCTB.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TP and TC conceptualized the study, curated the
data, drafted, reviewed and edited the manuscript, acted as project
administrators and supervised the project. TC visualized the data.
TP and WM performed the methodology. TP, SS and KS performed data
validation, investigation and obtained resources. TP, WM and TC
performed formal data analysis. TP and TC confirm the authenticity
of all the raw data. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The current study received permission and approval
for all protocols used in the study. Due to the retrospective
nature of this study, the need for informed consent was waived by
The Institute Review Board in Human Research of Khon Kaen Hospital
(approval no. KEXP65041).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Takeuchi A, Tsuchiya H, Ishii T, Nishida
Y, Abe S, Matsumine A, Kawai A, Yoshimura K and Ueda T: Clinical
outcome of recurrent giant cell tumor of the extremity in the era
before molecular target therapy: The Japanese musculoskeletal
oncology group study. BMC Musculoskelet Disord. 17:3062016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Athanasou NA, Bansal M, Forsyth R, Reid RP
and Sapi Z: Tumours of soft tissue and bone. Pathology and
Genetics. World Health Organization Classification of Tumours.
Bridge J, Hogendoorn P and Mertens F: IARC press; 2013
|
|
3
|
Viswanathan S and Jambhekar NA: Metastatic
giant cell tumor of bone: Are there associated factors and best
treatment modalities? Clin Orthop Relat Res. 468:827–833. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Derbel O and Blay JY: Giant cell tumors of
bone. Bone Cancer. Elsevier; pp. 437–445. 2015, View Article : Google Scholar
|
|
5
|
Atkins GJ, Kostakis P, Vincent C, Farrugia
AN, Houchins JP, Findlay DM, Evdokiou A and Zannettino AC: RANK
expression as a cell surface marker of human osteoclast precursors
in peripheral blood, bone marrow, and giant cell tumors of bone. J
Bone Miner Res. 21:1339–1349. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anazawa U, Hanaoka H, Shiraishi T, Morioka
H, Morii T and Toyama Y: Similarities between giant cell tumor of
bone, giant cell tumor of tendon sheath, and pigmented villonodular
synovitis concerning ultrastructural cytochemical features of
multinucleated giant cells and mononuclear stromal cells.
Ultrastruct Pathol. 30:151–158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lindeman JH, Hanemaaijer R, Mulder A,
Dijkstra PDS, Szuhai K, Bromme D, Verheijen JH and Hogendoorn PCW:
Cathepsin K is the principal protease in giant cell tumor of bone.
Am J Pathol. 165:593–600. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kotake S, Sato K, Kim KJ, Takahashi N,
Udagawa N, Nakamura I, Yamaguchi A, Kishimoto T, Suda T and
Kashiwazaki S: Interleukin-6 and soluble interleukin-6 receptors in
the synovial fluids from rheumatoid arthritis patients are
responsible for osteoclast-like cell formation. J Bone Miner Res.
11:88–95. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Collier FM, Huang WH, Holloway WR, Hodge
JM, Gillespie MT, Daniels LL, Zheng MH and Nicholson GC:
Osteoclasts from human giant cell tumors of bone lack estrogen
receptors. Endocrinology. 139:1258–1267. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huvos AG: Tumors of histiocytic or
fibrohistiocytic origin: Giant cell tumor of bone. Bone Tumors:
Diagnosis, Treatment, and Prognosis. 2nd edition. Huvos AG: WB
Saunders; pp. 429–467. 1991
|
|
11
|
Kim TS and Park JS: Metastasising
recurrent giant cell tumor: A case report. J Korean Bone Joint
Tumor Soc. 7:73–79. 2001.
|
|
12
|
Mirra JM: Giant cell tumors. Bone Tumors;
Clinical Radiological, and Pathologic Correlations. Mirra JM: Lea
and Febiger; pp. 941–1020. 1989
|
|
13
|
Osaka S, Sugita H, Osaka E, Yoshida Y, Ryu
J, Hemmi A and Suzuki K: Clinical and immunohistochemical
characteristics of benign giant cell tumour of bone with pulmonary
metastases: Case series. J Orthop Surg (Hong Kong). 12:55–62. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Benevenia J, Patterson FR, Beebe KS,
Abdelshahed MM and Uglialoro AD: Comparison of phenol and argon
beam coagulation as adjuvant therapies in the treatment of stage 2
and 3 benign-aggressive bone tumors. Orthopedics. 35:e371–e378.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deventer N, Budny T, Gosheger G, Rachbauer
A, Puetzler J, Theil JC, Kovtun D, de Vaal M and Deventer N: Giant
cell tumor of bone: A single center study of 115 cases. J Bone
Oncol. 33:1004172022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Benevenia J, Rivero SM, Moore J, Ippolito
JA, Siegerman DA, Beebe KS and Patterson FR: Supplemental bone
grafting in giant cell tumor of the extremity reduces nononcologic
complications. Clin Orthop Relat Res. 475:776–783. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Campanacci M, Baldini N, Boriani S and
Sudanese A: Giant-cell tumor of bone. J Bone Joint Surg Am.
69:106–114. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Balke M, Schremper L, Gebert C, Ahrens H,
Streitbuerger A, Koehler G, Hardes J and Gosheger G: Giant cell
tumor of bone: Treatment and outcome of 214 cases. J Cancer Res
Clin Oncol. 134:969–978. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abat F, Almenara M, Peiró A, Trullols L,
Bagué S and Grácia I: Giant cell tumor of bone: A series of 97
cases with a mean follow-up of 12 years. Rev Esp Cir Ortop
Traumatol. 59:59–65. 2015.(In Spanish). PubMed/NCBI
|
|
20
|
Thomas D, Henshaw R, Skubitz K, Chawla S,
Staddon A, Blay JY, Roudier M, Smith J, Ye Z, Sohn W, et al:
Denosumab in patients with giant-cell tumour of bone: An
open-label, phase 2 study. Lancet Oncol. 11:275–280. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tian R, Su M, Tian Y, Li F, Li L, Kuang A
and Zeng J: Dual-time point PET/CT with F-18 FDG for the
differentiation of malignant and benign bone lesions. Skeletal
Radiol. 38:451–458. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cowan RW and Singh G: Giant cell tumor of
bone: A basic science perspective. Bone. 52:238–246. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Graziano V and De Laurenzi V: Role of p63
in cancer development. Biochim Biophys Acta. 1816:57–66.
2011.PubMed/NCBI
|
|
24
|
Babeto E, Conceição AL, Valsechi MC, Peitl
Junior P, de Campos Zuccari DA, de Lima LG, Bonilha JL, de Freitas
Calmon M, Cordeiro JA and Rahal P: Differentially expressed genes
in giant cell tumor of bone. Virchows Arch. 458:467–476. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsukamoto S, Ciani G, Mavrogenis AF,
Ferrari C, Akahane M, Tanaka Y, Rocca M, Longhi A and Errani C:
Outcome of lung metastases due to bone giant cell tumor initially
managed with observation. J Orthop Surg Res. 15:5102020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dominkus M, Ruggieri P, Bertoni F,
Briccoli A, Picci P, Rocca M and Mercuri M: Histologically verified
lung metastases in benign giant cell tumours-14 cases from a single
institution. Int Orthop. 30:499–504. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jacopin S, Viehweger E, Glard Y, Launay F,
Jouve JL, Bouvier C and Bollini G: Fatal lung metastasis secondary
to index finger giant cell tumor in an 8-year-old child. Orthop
Traumatol Surg Res. 96:310–313. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nemes S, Jonasson JM, Genell A and
Steineck G: Bias in odds ratios by logistic regression modelling
and sample size. BMC Med Res Methodol. 9:562009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
van Smeden M, Moons KGM, de Groot JAH,
Collins GS, Altman DG, Eijkemans MJC and Reitsma JB: Sample size
for binary logistic prediction models: Beyond events per variable
criteria. Stat Methods Med Res. 28:2455–2474. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pavlou M, Ambler G, Seaman S, De Iorio M
and Omar RZ: Review and evaluation of penalised regression methods
for risk prediction in low-dimensional data with few events. Stat
Med. 35:1159–1177. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
King G and Zeng L: Logistic regression in
rare events data. Polit Anal. 9:137–163. 2001. View Article : Google Scholar
|
|
32
|
Altman DG, Gore SM, Gardner MJ and Pocock
SJ: Statistical guidelines for contributors to medical journals. Br
Med J (Clin Res Ed). 286:1489–1493. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Errani C, Ruggieri P, Asenzio MA, Toscano
A, Colangeli S, Rimondi E, Rossi G, Longhi A and Mercuri M: Giant
cell tumor of the extremity: A review of 349 cases from a single
institution. Cancer Treat Rev. 36:1–7. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kremen TJ Jr, Bernthal NM, Eckardt MA and
Eckardt JJ: Giant cell tumor of bone: Are we stratifying results
appropriately? Clin Orthop Relat Res. 470:677–683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Takeuchi A, Tsuchiya H, Niu X, Ueda T,
Jeon DG, Wang EH, Asavamongkolkul A, Kusuzaki K, Sakayama K and
Kang YK: The prognostic factors of recurrent GCT: A cooperative
study by the Eastern Asian musculoskeletal oncology group. J Orthop
Sci. 16:196–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Klenke FM, Wenger DE, Inwards CY, Rose PS
and Sim FH: Recurrent giant cell tumor of long bones: Analysis of
surgical management. Clin Orthop Relat Res. 469:1181–1187. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Donthineni R, Boriani L, Ofluoglu O and
Bandiera S: Metastatic behaviour of giant cell tumour of the spine.
Int Orthop. 33:497–501. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Faisham WI, Zulmi W, Halim AS, Biswal BM,
Mutum SS and Ezane AM: Aggressive giant cell tumour of bone.
Singapore Med J. 47:679–683. 2006.PubMed/NCBI
|
|
39
|
Prosser GH, Baloch KG, Tillman RM, Carter
SR and Grimer RJ: Does curettage without adjuvant therapy provide
low recurrence rates in giant-cell tumors of bone? Clin Orthop
Relat Res. 211–218. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Su YP, Chen WM and Chen TH: Giant-cell
tumors of bone: An analysis of 87 cases. Int Orthop. 28:239–243.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Trieb K, Bitzan P, Lang S, Dominkus M and
Kotz R: Recurrence of curetted and bone-grafted giant-cell tumours
with and without adjuvant phenol therapy. Eur J Surg Oncol.
27:200–202. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Blackley HR, Wunder JS, Davis AM, White
LM, Kandel R and Bell RS: Treatment of giant-cell tumors of long
bones with curettage and bone-grafting. J Bone Joint Surg Am.
81:811–820. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
O'Donnell RJ, Springfield DS, Motwani HK,
Ready JE, Gebhardt MC and Mankin HJ: Recurrence of giant-cell
tumors of the long bones after curettage and packing with cement. J
Bone Joint Surg Am. 76:1827–1833. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kay RM, Eckardt JJ, Seeger LL, Mirra JM
and Hak DJ: Pulmonary metastasis of benign giant cell tumor of
bone. Six histologically confirmed cases, including one of
spontaneous regression. Clin Orthop Relat Res. 219–230.
1994.PubMed/NCBI
|
|
45
|
Rock MG, Pritchard DJ and Unni KK:
Metastases from histologically benign giant-cell tumor of bone. J
Bone Joint Surg Am. 66:269–274. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Goldenberg RR, Campbell CJ and Bonfiglio
M: Giant-cell tumor of bone. An analysis of two hundred and
eighteen cases. J Bone Joint Surg Am. 52:619–664. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang J, Liu X, Yang Y, Yang R, Tang X, Yan
T and Guo W: Pulmonary metastasis of giant cell tumour: A
retrospective study of three hundred and ten cases. Int Orthop.
45:769–778. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chan CM, Adler Z, Reith JD and Gibbs CP
Jr: Risk factors for pulmonary metastases from giant cell tumor of
bone. J Bone Joint Surg Am. 97:420–428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ebeid WA, Badr IT, Mesregah MK and Hasan
BZ: Risk factors and oncological outcomes of pulmonary metastasis
in patients with giant cell tumor of bone. J Clin Orthop Trauma.
20:1014992021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Errani C, Tsukamoto S, Ciani G and Donati
DM: Present day controversies and consensus in curettage for giant
cell tumor of bone. J Clin Orthop Trauma. 10:1015–1020. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kito M, Matusmoto S, Ae K, Tanizawa T,
Gokita T, Kobayashi H, Hayakawa K and Funauchi Y: Pulmonary
metastasis from giant cell tumor of bone: Clinical outcome prior to
the introduction of molecular target therapy. Jpn J Clin Oncol.
47:529–534. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen CC, Liau CT, Chang CH, Hsu YH and
Shih HN: Giant cell tumors of the bone with pulmonary metastasis.
Orthopedics. 39:e68–e73. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dufresne A, Derbel O, Cassier P, Vaz G,
Decouvelaere AV and Blay JY: Giant-cell tumor of bone, anti-RANKL
therapy. Bonekey Rep. 1:1492012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Demirsoy U, Karadogan M, Selek Ö, Anik Y,
Aksu G, Müezzinoglu B and Corapcioglu F: Golden bullet-denosumab:
Early rapid response of metastatic giant cell tumor of the bone. J
Pediatr Hematol Oncol. 36:156–158. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mak IWY, Evaniew N, Popovic S, Tozer R and
Ghert M: A translational study of the neoplastic cells of giant
cell tumor of bone following neoadjuvant denosumab. J Bone Joint
Surg Am. 96:e1272014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kumar A, Sinha S, Haider Y, Jameel J and
Kumar S: Role of zoledronic acid supplementation in reducing
post-surgical recurrence of giant cell tumor of bone: A
meta-analysis of comparative studies. Cureus.
13:e167422021.PubMed/NCBI
|
|
57
|
Li H, Gao J, Gao Y, Lin N, Zheng M and Ye
Z: Denosumab in giant cell tumor of bone: Current status and
pitfalls. Front Oncol. 10:5806052020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Siebenrock KA, Unni KK and Rock MG:
Giant-cell tumour of bone metastasising to the lungs. A long-term
follow-up. J Bone Joint Surg Br. 80:43–47. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Muheremu A and Niu X: Pulmonary metastasis
of giant cell tumor of bones. World J Surg Oncol. 12:2612014.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Borkowska AM, Szumera-Ciećkiewicz A,
Szostakowski B, Pieńkowski A and Rutkowski PL: Denosumab in giant
cell tumor of bone: Multidisciplinary medical management based on
pathophysiological mechanisms and real-world evidence. Cancers
(Basel). 14:22902022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
van der Heijden L, Dijkstra S, van de
Sande M and Gelderblom H: Current concepts in the treatment of
giant cell tumour of bone. Curr Opin Oncol. 32:332–338. 2020.
View Article : Google Scholar : PubMed/NCBI
|