Introduction
Liver cancer is the sixth most common disease
(906,000 cases), accounting for 830,000 cancer deaths globally
(1). The prevalence and mortality
of this malignancy have increased globally and are particularly
high in North African and Asian nations (1). Currently, treatments include surgery,
transcatheter arterial chemoembolization, liver transplant,
radiotherapy, and biotherapy (2,3).
Unlike other malignancies, liver cancer is frequently discovered
only when it has progressed to the point that liver transplant,
surgical treatments, and resection are no longer feasible (4). As a result of the genetic, metabolic,
and inflammatory heterogeneity of liver cancer, the development of
therapies is difficult; chemotherapy (such as cisplatin,
gemcitabine, or doxorubicin) or treatment with multikinase
inhibitors (such as first-line sorafenib, second-line regorafenib,
Lenvatinib, or third-line cabozantinib) only slightly prolongs
overall survival (5). Thus, there
is an urgent need for new molecular therapeutic and diagnostic
targets to raise the standard of care and survival for people with
liver cancer.
Acute stress and chronic stress are two different
types of stress (6). Acute stress
can help the body adapt to harsh environments and generally have a
beneficial effect on the physiological status of a body. Yet,
growing epidemiological research indicates that long-term stress
exposure may cause various physiological issues, including the
emergence and growth of tumors (7,8).
Recent work suggests that stress hormone receptors, specifically
the Catecholamine hormone receptor and β-adrenergic receptor
(β-AR), play an essential role in tumor genesis and development,
and are considered to be an important target for cancer therapy
(9,10). Specifically, epinephrine and
norepinephrine (NE) via β-adrenergic receptors modulate the immune
response, angiogenesis, invasion, and inflammation to promote tumor
development (11). In fact,
numerous studies have shown that the AR pathway plays a role in
promoting a variety of tumor types, including melanoma, leukemia,
breast, cervical, liver, lung, gastric, oral, and pancreatic cancer
(12,13). Although it has been noted that liver
cancer cells produce more β-ARs, the exact molecular mechanism by
which β-AR regulates the development, invasion, and metastasis of
liver cancer is yet unknown (14).
β-AR belongs to the G protein-coupled receptor
(GPCR) family, which can regulate multiple malignant biological
processes, including tumor cell proliferation, angiogenesis,
epithelial-mesenchymal transition (EMT), invasion, metastasis, and
anti-apoptotic mechanisms (13).
Previous studies have shown that β-AR can activate adenylate
cyclase (AC), which in turn activates the cAMP/PKA signaling
pathways and cAMP/EPAC/Rap1/MEK1/2/extracellular signal-regulated
kinase 1/2 (ERK1/2), and promotes downstream transcription factor
expression, including the NF-κB, CREB, AP1, and Ets family of
proteins (15,16). For example, it has been reported
that NE enhances the invasion and proliferation of oral squamous
cell carcinoma (OSCC) through activating β2-AR and
inducing activation of ERK and cAMP response element binding
protein (CREB). Simultaneously, NE can promote a cancer stem
cell-like phenotype and increase the expression of stem cell
markers (13). It has been shown
that psychological stress can activate EMT, promote tumor growth,
and enhance radiation resistance via β-AR (17). By downregulating PPAR expression,
chronic stress, and hormone-induced β2-AR activation can
enhance breast cancer development and VEGF/FGF2-mediated
angiogenesis (18). Isoproterenol,
a β-AR agonist, has been shown to play critical roles in regulating
VEGF production via β-AR receptors, enhancing vascular distribution
in mouse femurs, and the release of the proinflammatory cytokines
interleukin-1 (IL-1) and interleukin-6 (IL-6), altering endothelial
cell adhesion and promoting cancer cell bone metastasis (19). By stimulating the Notch 1 pathway,
NE promotes tumor cell activity and invasion while inhibiting tumor
cell death in pancreatic ductal adenocarcinoma (20). Chronic stress may also enhance
gastric cancer (GC) cell proliferation and metastasis by
stimulating the production of NE and its binding to AR, as well as
upregulating NF-κB, CREB, and STAT3 expression (12). Furthermore, chronic stress-induced
activation of the miR-337-3P/STAT3 axis may increase breast cancer
metastasis (21). Notably, there is
limited research on unraveling the role of β-AR signaling in liver
cancer. For example, current research has revealed that the
sympathetic nervous system (SNS)/β-ARs/CCL2 alleviates
immunosuppression in liver cancer cells and overcomes PD-L1
immunotherapy resistance (22).
Additionally, β-AR promotes liver cancer growth by increasing YB-1
phosphorylation at S102 via β-arrestin-1-dependent activation of
the PI3K/AKT pathway (23).
However, the molecular mechanism by which β-AR is activated in
liver cancer cells and its downstream signaling pathways governing
the occurrence and progression of liver cancer cells are not fully
understood.
The aim of the present study was to explore the
capacity of β-AR in increasing HepG2 hepatoma cell proliferation,
migration, and epithelial cell transformation, as well as the
underlying molecular processes. The results showed that β-AR was
abundantly expressed in HepG2 cells, and that NE can boost HepG2
cell proliferation via activation of β-AR and its downstream
ERK1/2/CREB signaling pathways.
Materials and methods
Antibodies and reagents
Tocris Bioscience supplied the NE and propranolol
(PRO). Western blotting antibodies, including phospho-ERK1/2
(Thr202/Tyr204)) (cat. no. 4370; 1:2,000), ERK1/2 (cat. no. 4695;
1:2,000), phospho-CREB (Ser133) (cat. no. 9198; 1:2,000), CREB
(cat. no. 9197; 1:2,000), β-actin (cat. no. 4970; 1:5,000), and
anti-rabbit IgG HRP-linked antibody (cat. no. 7074, goat
anti-rabbit, 1:5,000) were purchased from Cell Signaling
Technology, Inc. ADRB1 (cat. no. 28323-1-AP; 1:2,000) and ADRB2
(cat. no. 29864-1-AP; 1:2,000) antibodies were purchased from
ProteinTech Group, Inc. All cell culture reagents were purchased
from Gibco (Thermo Fisher Scientific, Inc.). SYBR®
Premix Ex Taq™ II was purchased from Takara Bio, Inc. MTT was
purchased from MilliporeSigma.
Cell culture and transfection
HepG2 (hepatoblastoma), Huh7 (hepatoma), and LO2
(normal liver) cells were purchased from iCell Bioscience Inc. Both
cell lines were cultured in DMEM supplemented with 4.5 g/l glucose,
10% FBS, 50 µg/ml streptomycin, and 50 IU/ml penicillin. The cells
were regularly tested for mycoplasma and authenticated using Short
Tandem Repeat (STR) analysis to confirm their identity. The STR
profiles of both cell lines matched the reference profiles provided
by the cell bank. Additionally, their growth characteristics and
morphology were consistent with the reported characteristics of
HepG2 and LO2 cells. Cells were maintained at 37°C in an incubator
supplied with 5% CO2 air. Small interfering (si)RNAs
were used to knock down CREB expression in HepG2 cells. The cells
were transfected for 48 h using Lipofectamine® 3000
(Thermo Fisher Scientific, Inc.) with siRNAs against CREB (Santa
Cruz Biotechnology, Inc.) according to the manufacturer's
instructions.
Measurement of cell viability
Cells were added to 24-well plates with culture
media and incubated at 37°C for 24 h. After the cells had adhered,
the cells were serum-starved overnight and treated for 48 h with
various treatments. The culture media was then replaced with
supplemented medium containing 50 µl MTT reagent (5 mg/ml) in each
well. After a further 3 h of incubation at 37°C, the supernatant
was removed, and 500 µl dimethyl sulfoxide was added to each well
and shaken for 10 min to dissolve the precipitate. The optical
density (OD) at 490 nm was then measured. The cell viability is
represented as a percentage of the control (100%).
Western blotting
Cells were serum-starved overnight at 37°C prior to
treatment. After treatment, the cells were lysed with RIPA buffer
on ice to extract total protein. A total of 10 µg protein/lane was
loaded and resolved using 10% SDS-PAGE before transfer to a PVDF
membrane and blocked for 2 h at room temperature in 5% fat-free
milk. Subsequently, the membrane was treated with one of the
primary antibodies against ADRB1, ADRB2, phospho-ERK1/2, ERK1/2,
phospho-CREB, CREB, or β-actin overnight at 4°C, followed by
incubation with the HRP-conjugated anti-rabbit IgG secondary
antibody for 2 h at room temperature. Signals were visualized using
an enhanced chemiluminescence reagent (Thermo Fisher Scientific,
Inc.). Densitometry analysis was performed using ImageJ (version
1.47t; National Institutes of Health).
Wound-healing assays
Wound healing assays were used to assess cell
migration. Briefly, HepG2 cells were seeded into a 6-well plate
with complete growth medium containing 10% FBS and incubated until
they reached 100% confluence. The cells were then cultured in serum
starved DMEM (2% FBS) for 12 h. Subsequently, a sterile yellow
pipette tip was used to generate a wound, after which, the cells
were washed with PBS to remove any floating cells and debris. After
adding NE, the cells were cultured in DMEM containing 2% FBS at
37°C. The width of the wound was measured at 0, 12, and 24 h
post-scratch, and images of randomly selected fields from each
group were captured using a phase-contrast microscope (Olympus
Corporation).
RNA extraction and reverse
transcription (RT)-PCR
Using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). Total RNA was isolated from HepG2 and LO2
cells. The RNA purity and concentration were measured according to
the ratio of absorbance at 260 and 280 nm, using a NanoDrop
spectrophotometer (Thermo Fisher Scientific, Inc.). The RNA was
then reverse-transcribed into cDNA using the
PrimeScript® RT reagent (Takara Bio, Inc.) according to
the manufacturer's protocol. The thermocycling protocol was as
follows: Amplification step, 94°C for 5 min; followed by 35 cycles
of denaturation for 1 min at 94°C; 1 min of annealing at 55°C;
elongation at 72°C for 1 min; with a final extension step at 72°C
for 1 min. The sequences of the primers are shown in Table SI. Standard electrophoresis was
performed on a 1.2% agarose gel at 100 V for 40 min. The bands in
the gels were imaged using an UV light transilluminator (ChemiDoc
XRS+ system; Bio-Rad Laboratories, Inc.). β-actin was used as the
housekeeping gene.
Quantitative (q)PCR
The total RNA and cDNA were obtained as above and
qPCR was performed on a ViiA7 system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). A total of 20 µl qPCR reaction system
contained 6 µl nuclease-free water, 10 µl SYBR Premix Ex Taq II
(2X), 0.4 µl ROX Reference Dye II, 2 µl cDNA, 0.8 µl forward primer
(10 µM) and 0.8 µl reverse primer (10 µM). The thermocycling
conditions were: 95°C for 30 sec; followed by 40 cycles of
denaturation at 95°C for 5 sec, annealing at 60°C for 34 sec,
elongation at 95°C for 15 sec, and extension at 60°C for 1 min. The
2−ΔΔCq method was used to analyze the expression levels
of β1-AR and β2-AR (24); β-actin was used as the housekeeping
gene. Each sample was assessed in triplicate.
EMT
The HepG2 cells were seeded into 6-well plates
(1×106 cells) in supplemented media. Following adherence
of cells, NE was added, and the cells were cultured in DMEM without
FBS for 96 h. Random field images were selected and analyzed for
morphological changes using ImageJ software (version 1.47t;
National Institutes of Health).
Statistical analysis
Data are presented as the mean ± SEM of at least
three separate experiments and were analyzed using GraphPad Prism
version 6.0 (GraphPad Software, Inc.). Data were compared using a
Student's t-test (2 groups) or a one/two-way ANOVA with
Bonferroni's Multiple Comparison Corrections (>2 groups).
P<0.05 was considered to indicate a statistically significant
difference.
Results
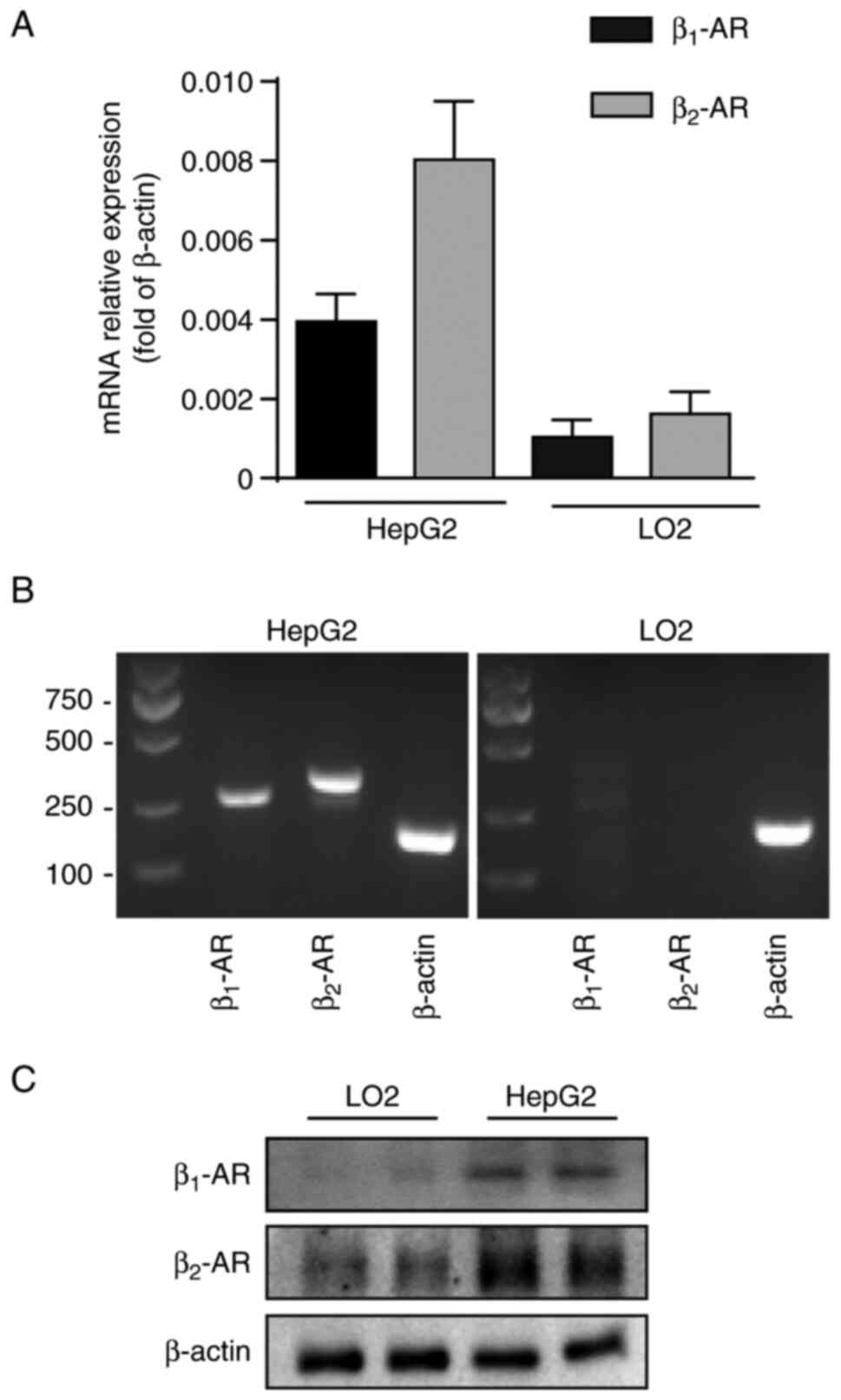
β1-AR and β2-AR
expression in HepG2 and LO2 cells
To investigate the expression of β1-AR
and β2-AR in HepG2 and LO2 cells and compare the
differences in the expression of these receptors between the cell
lines, RT-PCR and RT-qPCR assay was used. The results indicated
that β1-AR and β2-AR expression was
detectable in HepG2 and LO2 cells (Fig.
1A). Western blotting was further performed to confirm the
results (Fig. 1B and C). Notably,
the expression of β2-AR was higher in HepG2 cells than
that in the LO2 cells, and the expression of β2-AR was
evidently higher than that of β1-AR in HepG2. These
findings showed that β-AR, particularly β2-AR, may play
an important role in the tumorigenesis and development of liver
cancer.
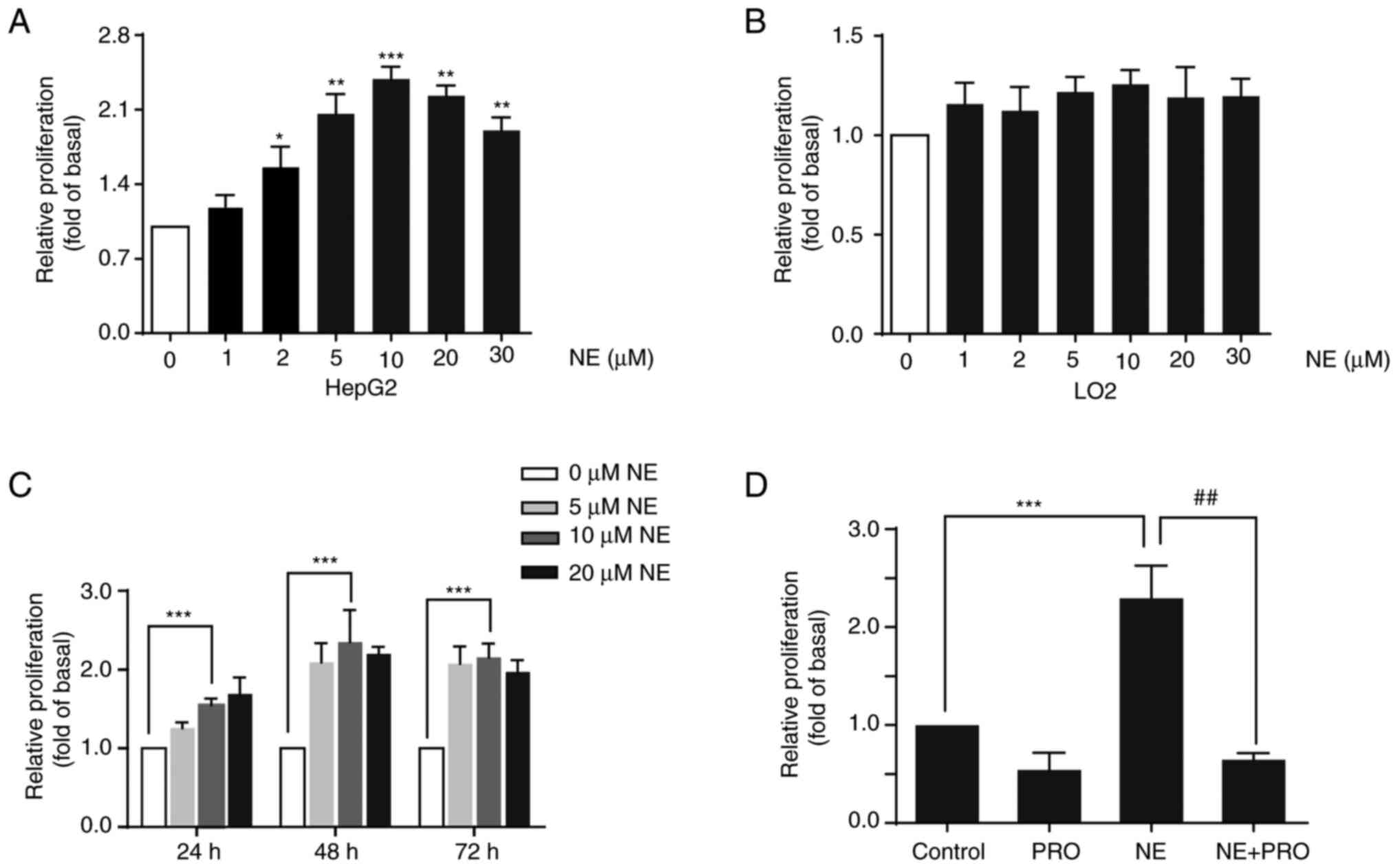
Effect of NE on the proliferation of
HepG2 and LO2 cells
To further investigate the activity of the β-ARs in
HepG2 and LO2 cells, cells were treated with the β-AR agonist NE.
Cells were treated with different doses of NE for 48 h, and cell
viability was assessed using an MTT assay. The results demonstrated
that NE significantly promoted the proliferation of HepG2 cells in
a dose-dependent manner, with 10 µM concentration showing the most
pronounced effect. However, NE did not notably affect the
proliferation of LO2 cells (Fig. 2A and
B). To confirm the effect of NE on liver cancer, Huh7 liver
cancer cells were treated with different doses of NE, and a
significant increase in the proliferation of these cells was
observed (Fig. S1). Next, the
effect of NE on cell viability after treatment of HepG2 cells with
NE for 24, 48, or 72 h was assessed. NE increased HepG2 cell growth
in a dose and time-dependent manner (Fig. 2C). Additionally, HepG2 cells were
co-treated with the non-selective β-AR blocker PRO and NE for 48 h.
The results indicated that PRO blocked NE-induced cell growth,
suggesting that the proliferative effect of NE may be mediated
through the activation of β-AR (Fig.
2D).
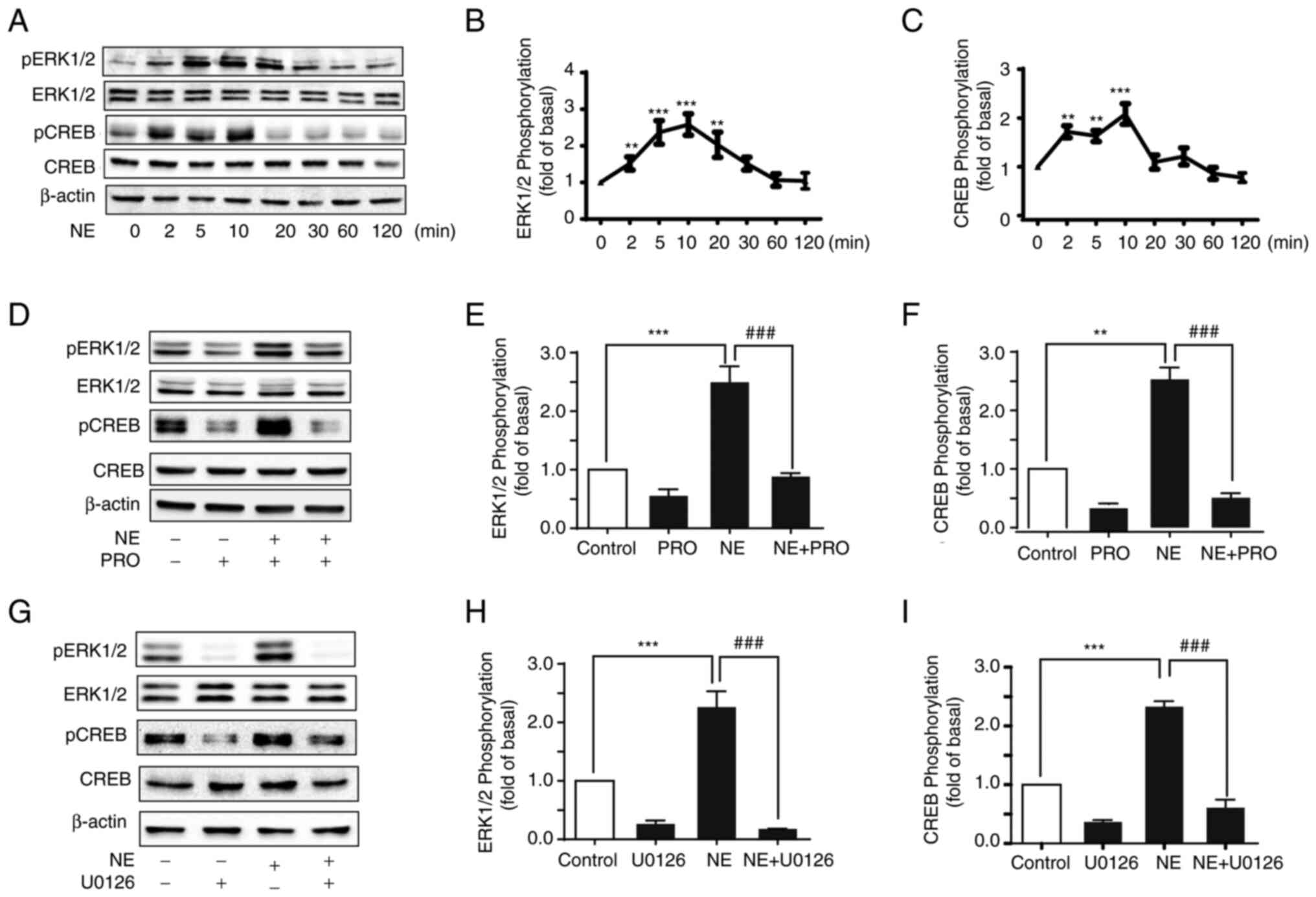
β-AR activation induces ERK1/2 and
CREB phosphorylation in HepG2 cells
As it was demonstrated that β-AR activation may have
exerted pro-proliferative effects of NE in HepG2, the specific
molecular mechanisms were next explored. It has been reported that
the MAPK/ERK1/2 pathway is crucial in regulating key processes,
such as cell proliferation, survival, and metastatic progression
(25). To determine whether ERK1/2
and CREB were implicated in β-AR activation, HepG2 cells were
treated with NE, and then, the phosphorylation levels of ERK1/2 and
CREB were measured by western blot analysis. NE induced a brief and
transitory increase in ERK1/2 phosphorylation but had no effect on
ERK1/2 expression levels (Fig. 3A and
B). The phosphorylation of ERK1/2 peaked at 10 min and then
decreased. Similarly, the transcription factor CREB, which affects
processes such as cell cycle, apoptosis, and cellular metabolism,
was also transiently phosphorylated and peaked within 10 min
(Fig. 3C).
To confirm that ERK1/2 and CREB phosphorylation was
a result of β-AR activation, HepG2 cells were pre-treated with PRO
and then stimulated with NE. PRO inhibited the phosphorylation of
ERK1/2 and CREB (Fig. 3D-F). As
CREB is a key downstream target of ERK1/2, whether NE-induced CREB
phosphorylation was mediated by ERK1/2 was assessed. The MEK1/2
inhibitor U0126 was used to treat HepG2 cells. Inhibiting the MAPK
pathway significantly inhibited NE-induced ERK1/2 and CREB
phosphorylation, suggesting that NE stimulation of β-AR promoted
ERK1/2 phosphorylation, which then activated CREB (Fig. 3G-I).
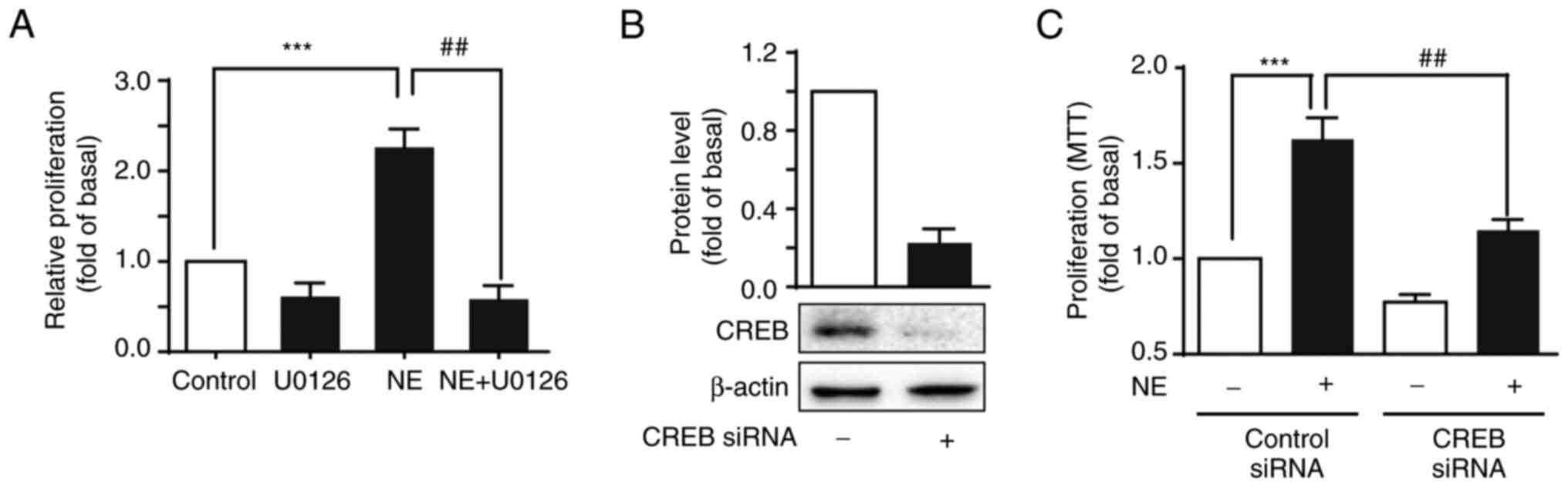
Inhibiting ERK1/2 and CREB abrogates
NE-mediated proliferation in HepG2 cells
Considering the potential of NE to significantly
increase HepG2 cell proliferation and activate ERK1/2 and CREB
phosphorylation, HepG2 cells were pre-treated with U0126 (a
selective inhibitor of ERK) and then treated with NE to further
demonstrate whether NE-mediated ERK1/2 and CREB activation were
involved in cell proliferation. U0126 inhibited the proliferative
effects of NE (Fig. 4A).
Additionally, knocking down CREB expression using specific siRNAs
significantly reduced HepG2 cell growth compared with the control
siRNA-transfected cells (Fig. 4B and
C). Together, this suggested that ERK1/2 was involved in
NE-mediated proliferation in HepG2 cells via CREB
phosphorylation.
Effects of β-AR activation on the
migration and EMT of HepG2 cells
Previous studies have reported that NE promotes
tumor metastasis in colon cancer and other types of cancer
(26,27). Therefore, the role of β-ARs in cell
migration in HepG2 cells was investigated. The wound healing assays
showed that NE had no significant effect on HepG2 cell migration
compared with the control group (Fig.
5A).
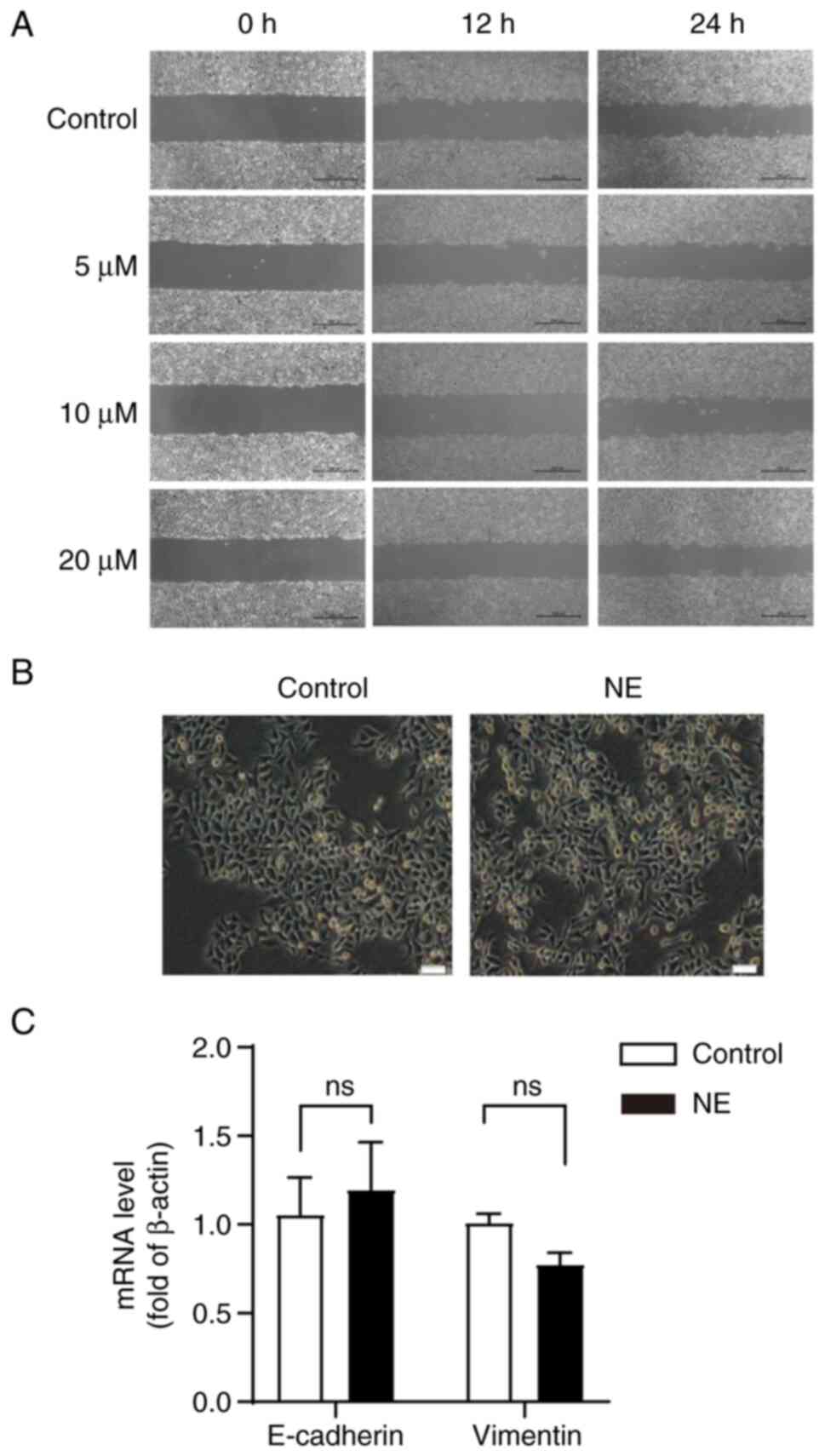 | Figure 5.Effect of β-AR activation on HepG2
cell migration and EMT. (A) Cells were treated with NE (0, 5, 10,
or 20 µM) for 24 h and the effect of NE on the migration of HepG2
cells was measured using a wound-healing assay. Scale bar, 500 µM.
(B) Effects of β-AR activation on the EMT of HepG2 cells. Cells
were cultured with NE for 96 h and the morphological changes of
NE-induced cells were observed. Scale bar, 100 µM; Magnification,
×10. (C) Effects of NE on E-cadherin and Vimentin in HepG2 cells.
EMT, epithelial-mesenchymal transition; NE, norepinephrine; β-AR,
β-Adrenergic receptor; ns, not significant. |
It has also been shown that β-AR is involved in the
EMT process in oral squamous cell carcinoma and glioma cells
(28,29). To determine whether β-ARs were
involved in the EMT process of HepG2 cells, the morphological
changes of the cell nuclei in NE-treated HepG2 cells were observed
under a phase-contrast microscope. The results showed that NE
treatment did not induce significant morphological changes in HepG2
cells (Fig. 5B). Subsequently,
cellular RNA was extracted, and RT-qPCR was used to evaluate the
expression of the EMT markers, E-cadherin, and Vimentin. The
results demonstrated that NE had no effect on the expression of
E-cadherin and Vimentin in HepG2 cells (Fig. 5C).
Activation of β-AR increases the
expression of genes related to cell proliferation and cycle
regulation
There is growing evidence that NE treatment can
regulate the expression of genes related to the cell cycle and
proliferation, thereby regulating the occurrence and development of
tumors (30). To determine the
effect of β-AR on gene expression in HepG2 cells, HepG2 cells were
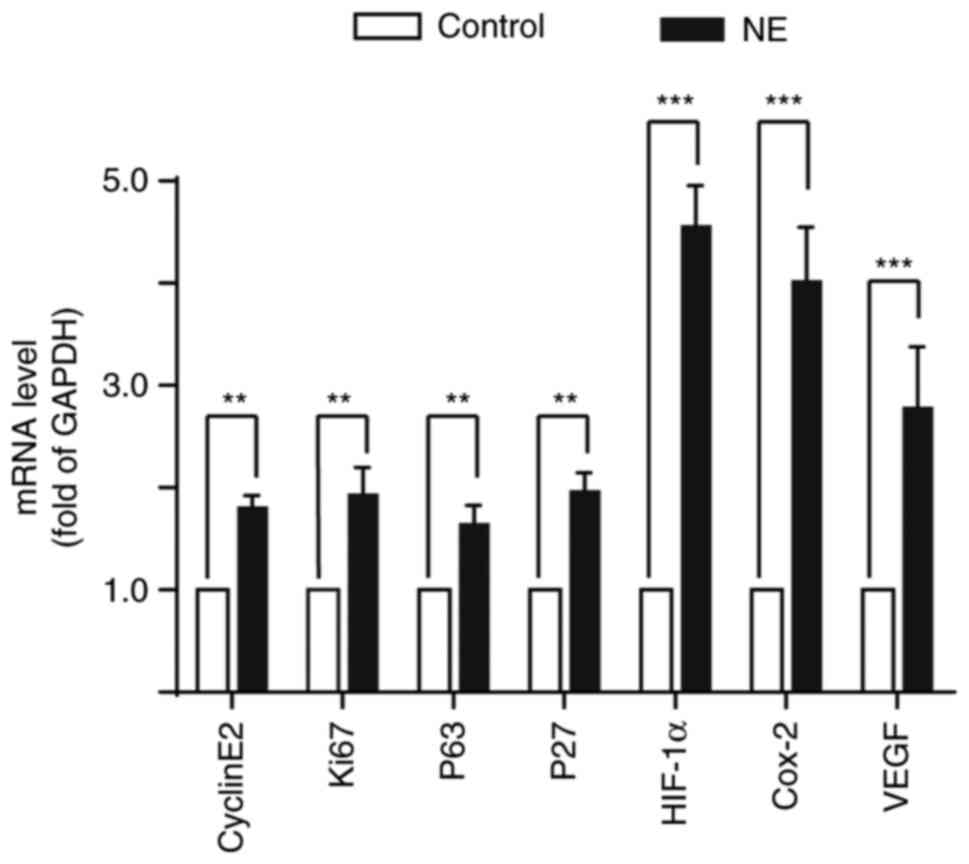
treated with NE, and the expression of cyclinE2, Ki67, P53, P27,
HIF-1α, COX-2, and VEGF in HepG2 cells was detected. The results
showed that β-AR activation significantly increased the expression
of these genes (Fig. 6).
Discussion
In the past decade, despite the continuous
improvements in understanding the etiology of liver cancer and
techniques for diagnosing liver cancer, the prognosis of patients
has remained poor (31,32). Therefore, there is an urgent need to
identify novel and effective treatment methods. Chronic stress can
activate AR through catecholamine neurotransmitters to mediate
tumorigenesis; in addition, β-AR is crucial in the link between
tumor development and psychological stress (33,34).
Although the activated β-AR can regulate the proliferation of
various types of cancer, including lung cancer, gastric cancer,
pancreatic cancer, prostate cancer, and glioma, amongst others.,
there are still relatively fewer studies on the regulation of β-AR
in the occurrence of hepatocellular carcinoma (12,16,34–36).
In the present study, the possible mechanisms by which β-AR
regulated the proliferation of hepatoma HepG2 cells were explored.
First, it was shown that both β1-AR and β2-AR
were expressed in hepatoma HepG2 cells, and the expression levels
were higher than those in normal hepatocytes. Second, NE stimulated
HepG2 cell proliferation in a dose-and time-dependent manner. The
NE-induced pro-proliferative effect could be inhibited after the
application of the β-AR blocker PRO, suggesting that the NE-induced
pro-proliferative effect was mediated through β-AR. However, NE has
no obvious pro-proliferative effect on normal hepatocytes, which is
consistent with the expression of β-AR in normal hepatocytes.
β-AR-mediated cAMP/PKA and MAPK signaling pathways
are essential signaling pathways regulating tumorigenesis and
development (15). Previous studies
have found that activation of β-AR by isoproterenol upregulates the
phosphorylation of ERK1/2 and CREB in neuroblastoma (36,37).
In the present study, NE administration significantly enhanced
ERK1/2 and CREB phosphorylation levels in HepG2 cells; the
pro-proliferative effect induced by NE was inhibited by treatment
with the β-AR blocker PRO; this suggested that NE promoted HepG2
cell proliferation through β-AR, However, the role of α-AR was not
determined, nor was the β-AR subtype involved, and subsequent
studies should specifically activate α-AR, β1-AR, or
β1-AR to address these shortcomings.
Since CREB is a key downstream target of ERK1/2,
U0126, a selective inhibitor of ERK1/2, was used to pretreat HepG2
cells to detect changes in CREB protein expression and cell
viability. The results showed that NE treatment induced CREB
phosphorylation and the increase in HepG2 cell viability was
significantly inhibited. Moreover, similar conclusions were
obtained after knocking down CREB expression. These findings
suggest that NE regulates the ERK1/2/CREB signaling pathway by
activating β-AR, which in turn promotes HepG2 cell proliferation.
PDK1 has been linked to several pathological traits, including
uncontrolled cell reproduction, apoptosis resistance, invasion,
dissemination, metastasis, metabolic reprogramming, and aberrant
angiogenesis (38). Increased PDK1
expression can increase PI3K/AKT/MTOR signaling, resulting in a
radiation-resistant and dedifferentiated phenotype of liver cancer
(31). As a consequence, the effect
of NE on the phosphorylation levels of PDK1 and AKT proteins was
assessed, and the results suggested that the NE-induced increase in
proliferation may also be mediated via the PDK1/AKT signaling
pathway (Fig. S2). These findings
highlight the critical role of β-AR in hepatocarcinogenesis.
EMT is a key step for tumor cell invasion and
migration. Tumor cells acquire mesenchymal and fibroblast-like
phenotypes during EMT, and this promotes cell migration and spread
to tissues distant from the site of origin (39,40).
Simultaneously, several studies have shown that NE may also be
involved in the metastatic process of various types of cancer
(26). However, the effect of NE on
cell migration and EMT has not been demonstrated in HepG2 cells.
Here, it was found that NE had a negligible effect on the EMT of
HepG2 cells. Furthermore, β-AR has been shown to regulate the
expression of genes involved in cell proliferation and cycle
regulation, thereby regulating the occurrence and development of
tumors (30). Consistent with these
findings, the results of the present study showed that β-AR
activation significantly enhanced the expression of CyclinE2, Ki67,
P53, P27, HIF-1α, Cox-2, and VEGF genes in HepG2 cells.
While the current study has revealed the crucial
role of NE/AR signaling in regulating the cell proliferation of
HepG2 and Huh7 cells, some important questions remain to be
addressed. For example, it is unknown whether NE has a
broad-spectrum pro-proliferative effect on hepatocellular
carcinoma. Additional types of hepatoma cell lines, with different
degrees of malignancy, such as SMMC7721 and HCCLM3, will be used to
further confirm the role of β-AR/ERK1/2/CREB signaling cascade in
hepatoma cell proliferation. Second, in vivo experiments
based on animal models of liver cancer should be performed to
determine the effect of β-AR-ERK1/2-CREB signaling on tumor growth.
Answering these questions is expected to expand our understanding
of the role of β-AR in controlling the occurrence and development
of hepatocellular carcinoma.
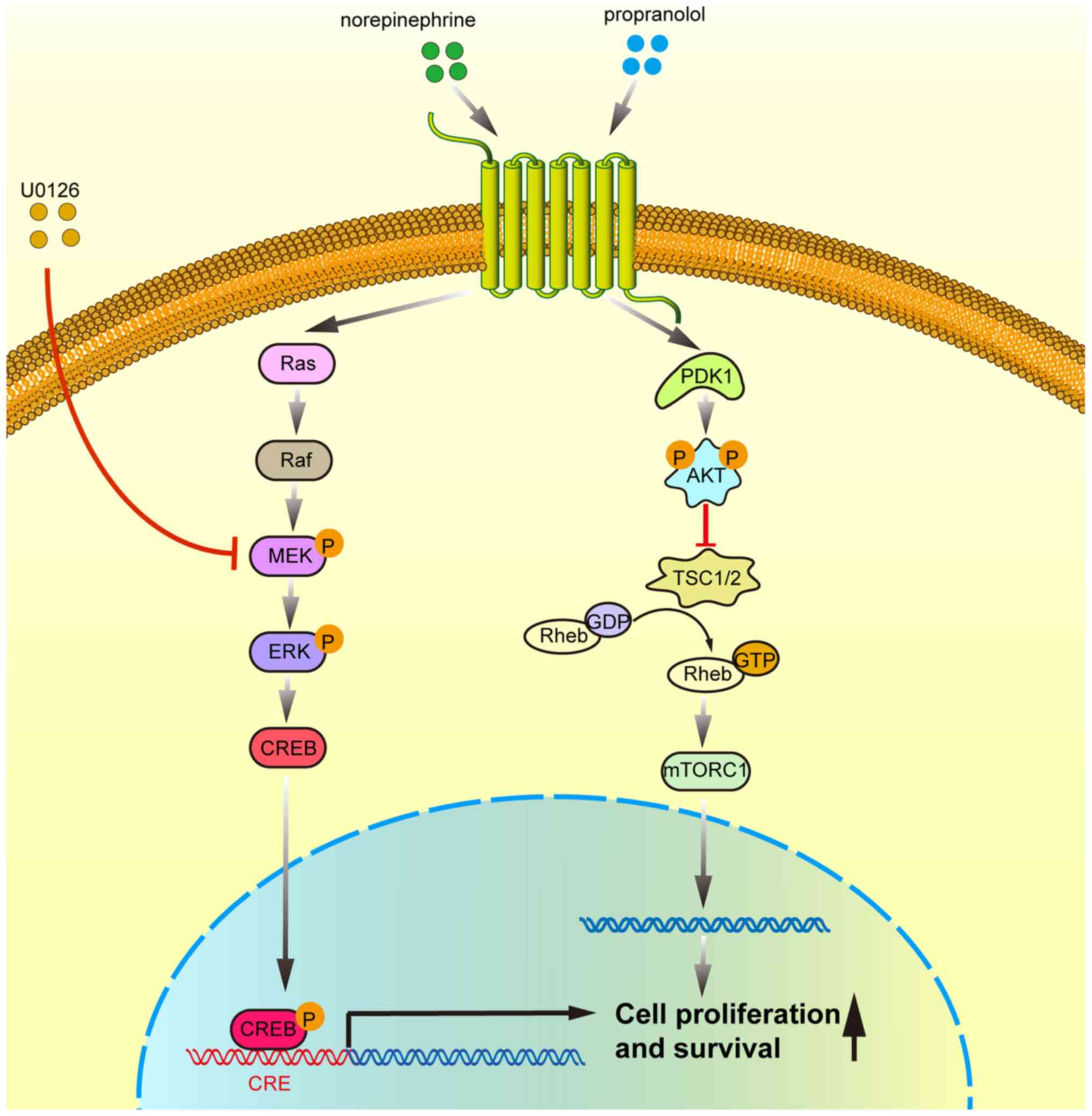
In conclusion, the results of the present study
indicate that β-adrenergic receptor activation promotes the
proliferation of HepG2 cells by activating the ERK1/2/CREB
signaling pathway (Fig. 7), which
highlights the significance of β-AR activation in
hepatocarcinogenesis and provides a theoretical basis for the
development of novel therapeutic approaches that target the
ERK1/2/CREB signaling pathway for the management of liver
cancer.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National Natural
Science Foundation of China (grant no. 32160184), the Scientific
Project of Jiangxi Province (grant nos. 20202BAB206043 and 190017),
and the Department of Public Health of Jiangxi Province (grant no.
20185226).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JH, PH, XLiu and XLin designed the study. JH and
XLin performed the experiments. FL, XLiu and LL analyzed the data.
HX, LS, LN and YZ analyzed the western blotting data. JH, XLin,
XLiu and PH wrote the manuscript. XLiu and PH were responsible for
ensuring that any issues concerning the accuracy or integrity of
the work were properly addressed and resolved. PH and XLin confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang Y, Shen J, Feng S, Liang R, Lai J, Li
D, Peng B, Wang Z, Huang C and Kuang M: Hepatic resection versus
transarterial chemoembolization in infiltrative hepatocellular
carcinoma: A multicenter study. J Gastroenterol Hepatol.
35:2220–2228. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim JJ, McFarlane T, Tully S and Wong WWL:
Lenvatinib versus sorafenib as first-line treatment of unresectable
hepatocellular carcinoma: A cost-utility analysis. Oncologist.
25:e512–e519. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li J, Zhou M, Liu F, Xiong C, Wang W, Cao
Q, Wen X, Robertson JD, Ji X, Wang YA, et al: Hepatocellular
carcinoma: Intra-arterial delivery of doxorubicin-loaded hollow
gold nanospheres for photothermal ablation-chemoembolization
therapy in rats. Radiology. 281:427–435. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li X, Ramadori P, Pfister D, Seehawer M,
Zender L and Heikenwalder M: The immunological and metabolic
landscape in primary and metastatic liver cancer. Nat Rev Cancer.
21:541–557. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dai S, Mo Y, Wang Y, Xiang B, Liao Q, Zhou
M, Li X, Li Y, Xiong W, Li G, et al: Chronic stress promotes cancer
development. Front Oncol. 10:14922020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Antoni MH and Dhabhar FS: The impact of
psychosocial stress and stress management on immune responses in
patients with cancer. Cancer. 125:1417–1431. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krizanova O, Babula P and Pacak K: Stress,
catecholaminergic system and cancer. Stress. 19:419–428. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xia Y, Wei Y, Li ZY, Cai XY, Zhang LL,
Dong XR, Zhang S, Zhang RG, Meng R, Zhu F and Wu G: Catecholamines
contribute to the neovascularization of lung cancer via
tumor-associated macrophages. Brain Behav Immun. 81:111–121. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seiler A, Sood AK, Jenewein J and Fagundes
CP: Can stress promote the pathophysiology of brain metastases? A
critical review of biobehavioral mechanisms. Brain Behav Immun.
87:860–880. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bastos DB, Sarafim-Silva BAM, Sundefeld M,
Ribeiro AA, Brandao JDP, Biasoli ER, Miyahara GI, Casarini DE and
Bernabe DG: Circulating catecholamines are associated with
biobehavioral factors and anxiety symptoms in head and neck cancer
patients. PLoS One. 13:e02025152018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang X, Zhang Y, He Z, Yin K, Li B, Zhang
L and Xu Z: Chronic stress promotes gastric cancer progression and
metastasis: An essential role for ADRB2. Cell Death Dis.
10:7882019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang B, Wu C, Chen W, Qiu L, Li S, Wang
T, Xie H, Li Y, Li C and Li L: The stress hormone norepinephrine
promotes tumor progression through beta2-adrenoreceptors in oral
cancer. Arch Oral Biol. 113:1047122020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li XQ, Peng WT, Shan S, Wu JJ, Li N, Du
JJ, Sun JC, Chen TT, Wei W and Sun WY: beta-arrestin2 regulating
beta2-adrenergic receptor signaling in hepatic stellate cells
contributes to hepatocellular carcinoma progression. J Cancer.
12:7287–7299. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cole SW and Sood AK: Molecular pathways:
Beta-adrenergic signaling in cancer. Clin Cancer Res. 18:1201–1206.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nilsson MB, Le X and Heymach JV:
β-adrenergic signaling in lung cancer: A potential role for
beta-blockers. J Neuroimmune Pharmacol. 15:27–36. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Y, Zanos P, Jackson IL, Zhang X, Zhu
X, Gould T and Vujaskovic Z: Psychological stress enhances tumor
growth and diminishes radiation response in preclinical model of
lung cancer. Radiother Oncol. 146:126–135. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou J, Liu Z, Zhang L, Hu X, Wang Z, Ni
H, Wang Y and Qin J: Activation of beta2-adrenergic receptor
promotes growth and angiogenesis in breast cancer by
down-regulating PPARγ. Cancer Res Treat. 52:830–847. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moraes RM, Elefteriou F and Anbinder AL:
Response of the periodontal tissues to β-adrenergic stimulation.
Life Sci. 281:1197762021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qian W, Lv S, Li J, Chen K, Jiang Z, Cheng
L, Zhou C, Yan B, Cao J, Ma Q and Duan W: Norepinephrine enhances
cell viability and invasion, and inhibits apoptosis of pancreatic
cancer cells in a Notch-1-dependent manner. Oncol Rep.
40:3015–3023. 2018.PubMed/NCBI
|
|
21
|
Du P, Zeng H, Xiao Y, Zhao Y, Zheng B,
Deng Y, Liu J, Huang B, Zhang X, Yang K, et al: Chronic stress
promotes EMT-mediated metastasis through activation of STAT3
signaling pathway by miR-337-3p in breast cancer. Cell Death Dis.
11:7612020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu C, Yang Y, Chen C, Li L, Li J, Wang X,
Chu Q, Qiu L, Ba Q, Li X and Wang H: Environmental eustress
modulates beta-ARs/CCL2 axis to induce anti-tumor immunity and
sensitize immunotherapy against liver cancer in mice. Nat Commun.
12:57252021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu J, Qu L, Wan C, Xiao M, Ni W, Jiang F,
Fan Y, Lu C and Ni R: A novel β2-AR/YB-1/β-catenin axis mediates
chronic stress-associated metastasis in hepatocellular carcinoma.
Oncogenesis. 9:842020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Braicu C, Buse M, Busuioc C, Drula R,
Gulei D, Raduly L, Rusu A, Irimie A, Atanasov AG, Slaby O, et al: A
comprehensive review on MAPK: A promising therapeutic target in
cancer. Cancers (Basel). 11:16182019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Han J, Jiang Q, Ma R, Zhang H, Tong D,
Tang K, Wang X, Ni L, Miao J, Duan B, et al:
Norepinephrine-CREB1-miR-373 axis promotes progression of colon
cancer. Mol Oncol. 14:1059–1073. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pan C, Wu J, Zheng S, Sun H, Fang Y, Huang
Z, Shi M, Liang L, Bin J, Liao Y, et al: Depression accelerates
gastric cancer invasion and metastasis by inducing a neuroendocrine
phenotype via the catecholamine/β2 -AR/MACC1 axis. Cancer Commun
(Lond). 41:1049–1070. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sakakitani S, Podyma-Inoue KA, Takayama R,
Takahashi K, Ishigami-Yuasa M, Kagechika H, Harada H and Watabe T:
Activation of beta2-adrenergic receptor signals suppresses
mesenchymal phenotypes of oral squamous cell carcinoma cells.
Cancer Sci. 112:155–167. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang X, Wang Y, Xie F, Song ZT, Zhang ZQ,
Zhao Y, Wang SD, Hu H, Zhang YS and Qian LJ: Norepinephrine
promotes glioma cell migration through up-regulating the expression
of Twist1. BMC Cancer. 22:2132022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mravec B, Horvathova L and Hunakova L:
Neurobiology of cancer: The role of beta-adrenergic receptor
signaling in various tumor environments. Int J Mol Sci.
21:79582020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bamodu OA, Chang HL, Ong JR, Lee WH, Yeh
CT and Tsai JT: Elevated PDK1 expression drives PI3K/AKT/MTOR
signaling promotes radiation-resistant and dedifferentiated
phenotype of hepatocellular carcinoma. Cells. 9:7462020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Villanueva A: Hepatocellular carcinoma. N
Engl J Med. 380:1450–1462. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pu J, Zhang X, Luo H, Xu L, Lu X and Lu J:
Adrenaline promotes epithelial-to-mesenchymal transition via
HuR-TGFbeta regulatory axis in pancreatic cancer cells and the
implication in cancer prognosis. Biochem Biophys Res Commun.
493:1273–1279. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xiao MB, Jin DD, Jiao YJ, Ni WK, Liu JX,
Qu LS, Lu CH, Ni RZ, Jiang F and Chen WC: β2-AR regulates the
expression of AKR1B1 in human pancreatic cancer cells and promotes
their proliferation via the ERK1/2 pathway. Mol Biol Rep.
45:1863–1871. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Decker AM, Jung Y, Cackowski FC, Yumoto K,
Wang J and Taichman RS: Sympathetic signaling reactivates quiescent
disseminated prostate cancer cells in the bone marrow. Mol Cancer
Res. 15:1644–1655. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
He JJ, Zhang WH, Liu SL, Chen YF, Liao CX,
Shen QQ and Hu P: Activation of β-adrenergic receptor promotes
cellular proliferation in human glioblastoma. Oncol Lett.
14:3846–3852. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Deng J, Jiang P, Yang T, Huang M, Xie J,
Luo C, Qi W, Zhou T, Yang Z, Zou Y, et al: beta2adrenergic receptor
signaling promotes neuroblastoma cell proliferation by activating
autophagy. Oncol Rep. 42:1295–1306. 2019.PubMed/NCBI
|
|
38
|
Gagliardi PA, Puliafito A and Primo L:
PDK1: At the crossroad of cancer signaling pathways. Semin Cancer
Biol. 48:27–35. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shan T, Cui X, Li W, Lin W, Li Y, Chen X
and Wu T: Novel regulatory program for norepinephrine-induced
epithelial-mesenchymal transition in gastric adenocarcinoma cell
lines. Cancer Sci. 105:847–856. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bhat AA, Syed N, Therachiyil L, Nisar S,
Hashem S, Macha MA, Yadav SK, Krishnankutty R, Muralitharan S,
Al-Naemi H, et al: Claudin-1, a double-edged sword in cancer. Int J
Mol Sci. 21:5692020. View Article : Google Scholar : PubMed/NCBI
|