Introduction
Cancer of unknown primary (CUP) is a heterogeneous
syndrome of metastatic cancers in which the primary site cannot be
determined after a standard and comprehensive search, and accounts
for 1–5% of all newly diagnosed malignancies (1,2).
CUP falls into either the clinicopathologically
‘favorable’ (~15% of cases) or ‘unfavorable’ (~85% of cases)
subgroups (3–5). The former includes adenocarcinoma of
the axillary lymph nodes, papillary serous carcinoma of the
peritoneum (redefined as peritoneal cancer), squamous cell
carcinoma of the cervical lymph nodes and extragonadal germ cell
tumors in young men, which are derived from the middle of the body
(4). For these patients, specific
therapeutic management can be provided, and satisfactory local
control and prolonged survival can be expected, with a median
survival time of >24 months (6).
On the other hand, the unfavorable subset (majority of patients)
includes patients with adenocarcinoma metastasis to the liver or
other organs, poorly differentiated carcinoma and squamous cell
carcinoma of the abdominal cavity (1). These patients are usually treated with
empiric chemotherapies based on platinum and taxanes (7) but are generally not chemosensitive and
have a poor prognosis, with a median survival time of 5–8 months
(4,8).
Delayed initiation of treatment and a tendency to
choose empiric chemotherapy are associated with a poor prognosis
(4). Therefore, a thorough workup
at the time of diagnosis is important in CUP. The distribution and
histology of the cancer are important for estimating the primary
site. In addition to image-based and immunohistochemical
examinations, the cancer genomic profiling (CGP) test has been used
to estimate primary sites and identify potential targets for
personalized therapies (9,10). Several studies using next-generation
sequencing have demonstrated that molecular profiling for CUP is
useful for predicting the tissue of origin (9,11–14).
However, to the best of our knowledge, it has not been determined
whether genomic profiling in CUP can help in providing
tissue-specific therapies, including targeted therapies, to improve
survival. A prospective randomized phase II trial (CUPISCO trial;
NCT03498521) is currently underway to investigate the efficacy of
CGP in CUPs; however, there are issues, such as insufficient sample
volume and cases of misdiagnosis (15,16).
In the present study, a case of CUP is reported in
which genomic profiling and lesion distribution were used to infer
the primary organs of cancer and to determine a suitable
therapeutic strategy.
Case report
A 68-year-old woman, gravida 1 para 1, who had a
history of allergic dermatitis for 2 years and had been taking
prednisone irregularly, presented to their primary care physician
(Yokohama, Japan) in August 2020 with dyspnea on exertion. Right
pleural effusion was observed, and 1,600 ml pleural fluid was
aspirated by puncture. Papanicolaou staining was performed on the
pleural fluid as previously described (17), and the cytological examination
revealed Class V adenocarcinoma (Fig.
1A) according to the Papanicolaou classification (18). Upper and lower gastrointestinal
endoscopy and gynecological examination were performed but the
primary tumor was not identified. In October 2020, the patient was
referred to the Department of Respiratory Medicine, Graduate School
of Medicine, The University of Tokyo (Tokyo, Japan). By then, the
pleural effusion had resolved spontaneously. Positron emission
tomography-computed tomography (PET-CT) revealed mild
fluorodeoxyglucose accumulation in the left predominant para-aortic
and left internal iliac lymph nodes; however, this was considered
to be a reactive change. At 11 months after the initial visit, the
Krebs von den Lungen-6 level measured using chemiluminescent enzyme
immunoassay (CLEIA) was elevated (1,243 U/ml; normal range, 0–464
U/ml), and the patient was referred back to the hospital for a
close examination of the primary site. PET-CT revealed abnormal
accumulation in the right axillary lymph node with a maximum
standardized uptake value (SUV) of 3.8, and further accumulation in
the peripancreatic, para-aortic, mesenteric, sacral and bilateral
external iliac lymph nodes with a maximum SUV of 7.2. All lymph
node accumulations were considered to indicate metastatic lymph
nodes (Fig. 1B).
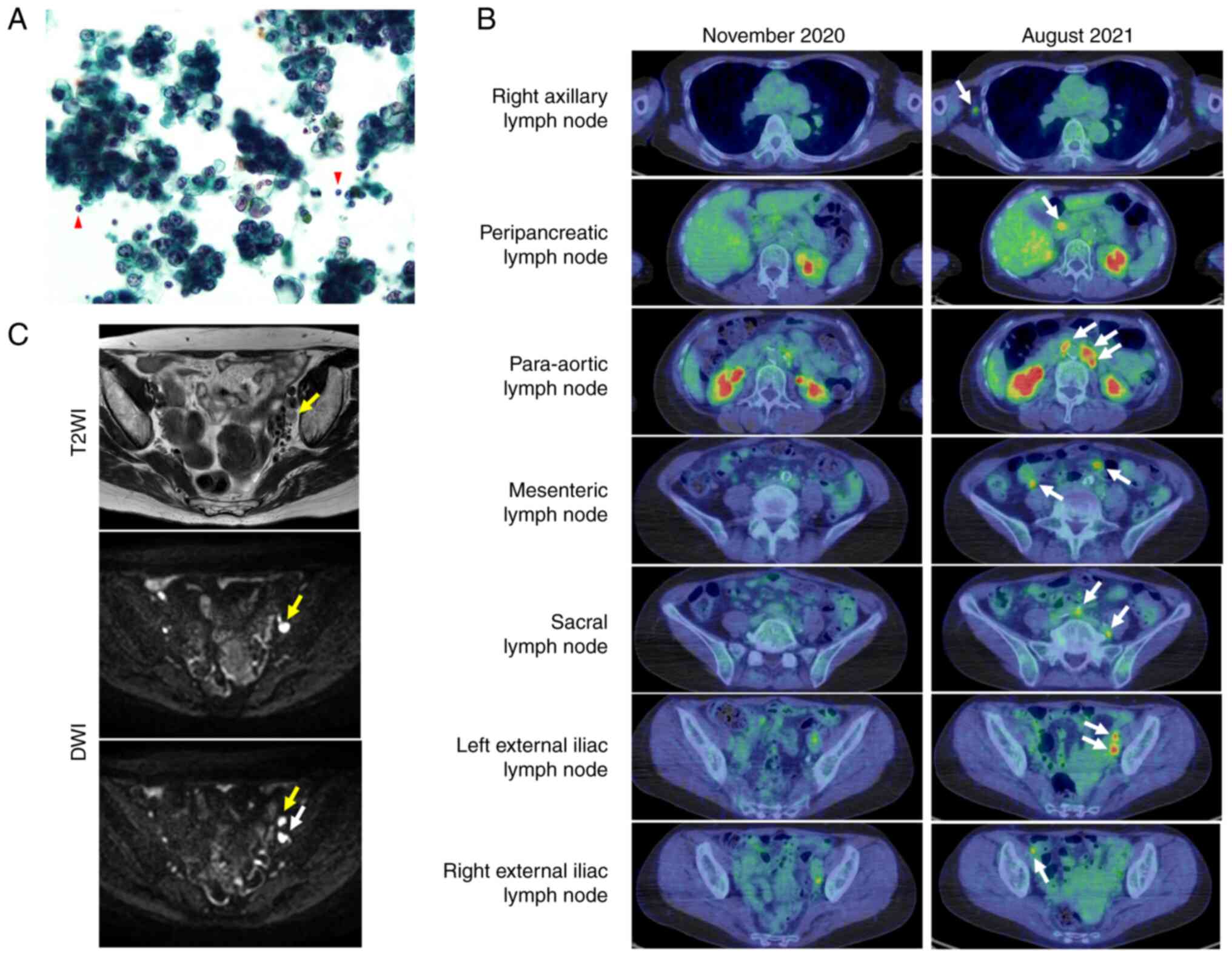 | Figure 1.Pleural fluid cytology and imaging
diagnosis. (A) Pleural fluid cytology (Papanicolaou stain;
magnification, ×400). A large number of atypical cells with
enlarged nuclei, thickened nuclear periphery and conspicuous
nucleoli were observed in pseudopapillary or spherical aggregates
or sporadically when compared with mesothelial cells, neutrophils
or lymphocytes. Cyst-like spaces were present, although mucin was
not evident. Lymphocytes indicated by the red arrows were used as
controls for comparison. (B) 18F-FDG PET-CT in November
2020 (left) and August 2021 (right). PET-CT images captured at the
primary visit indicated no conspicuous 18F-FDG
accumulation in the lymph nodes; however, 9 months later, lymph
node enlargement with 18F-FDG accumulation was observed
at the sites indicated by the white arrows, and multiple lymph node
metastasis was suspected. (C) Contrast-enhanced magnetic resonance
imaging. Arrows indicate enlarged left obturator (white) and
external iliac (yellow) lymph nodes. The upper DWI image is the
same slice as the T2WI image. DWI, diffusion-weighted imaging;
18F-FDG, 18F-fluorodeoxyglucose; PET-CT,
positron emission tomography-computed tomography; T2WI, T2-weighted
imaging. |
Due to elevated cancer antigen 125 (CA125) levels
(309 U/ml; normal range, 0–35 U/ml) measured using CLEIA and the
distribution of the metastatic lymph nodes, gynecological cancer
was suspected; however, pelvic magnetic resonance imaging showed no
obvious primary tumor and multiple uterine fibroids (Fig. 1C). The cervical and endometrial
cytologies were negative. Fine-needle aspiration cytology of the
right axillary lymph node revealed metastatic adenocarcinoma (data
not shown). For histological diagnosis, a laparoscopic para-aortic
lymph node biopsy was performed. Hematoxylin-and-eosin
(H&E)-stained slides were made from formalin-fixed
paraffin-embedded (FFPE) blocks as previously described (17). Immunohistochemical staining was
performed using the Ventana BenchMark XT automated staining system
(Roche Diagnostics) according to the manufacturer's protocol, as
previously described (19). The
primary antibodies used were as follows: Cytokeratin 7 (cat. no.
790-4462; clone SP52; Roche Diagnostics), estrogen receptor (EP1,
Envision FLEX-ER, Dako; Agilent Technologies, Inc.), paired box 8
(clone 10336-1-AP; Proteintech Group, Inc.), p53 (clone DO-7; Roche
Diagnostics), Wilm's tumor 1 (clone 6F-H2; Dako; Agilent
Technologies, Inc.), GATA binding protein 3 (clone L50-823; Biocare
Medical, LLC), cytokeratin 20 (clone SP33; Roche Diagnostics) and
D2-40 (clone D2-40; Dako; Agilent Technologies, Inc.). All the
cytopathological, histological and immunohistochemical images were
examined and captured using a light microscope (BX51; Olympus
Corporation). The tumor was diffusely positive for cytokeratin 7,
estrogen receptor, paired box 8, p53 and Wilms tumor 1, whereas it
was negative for GATA binding protein 3 and cytokeratin 20
(Fig. 2A). The histological and
immunohistochemical diagnosis was of a high-grade serous carcinoma
(HGSC) possibly derived from the gynecological organs, based on the
WHO Classification (20).
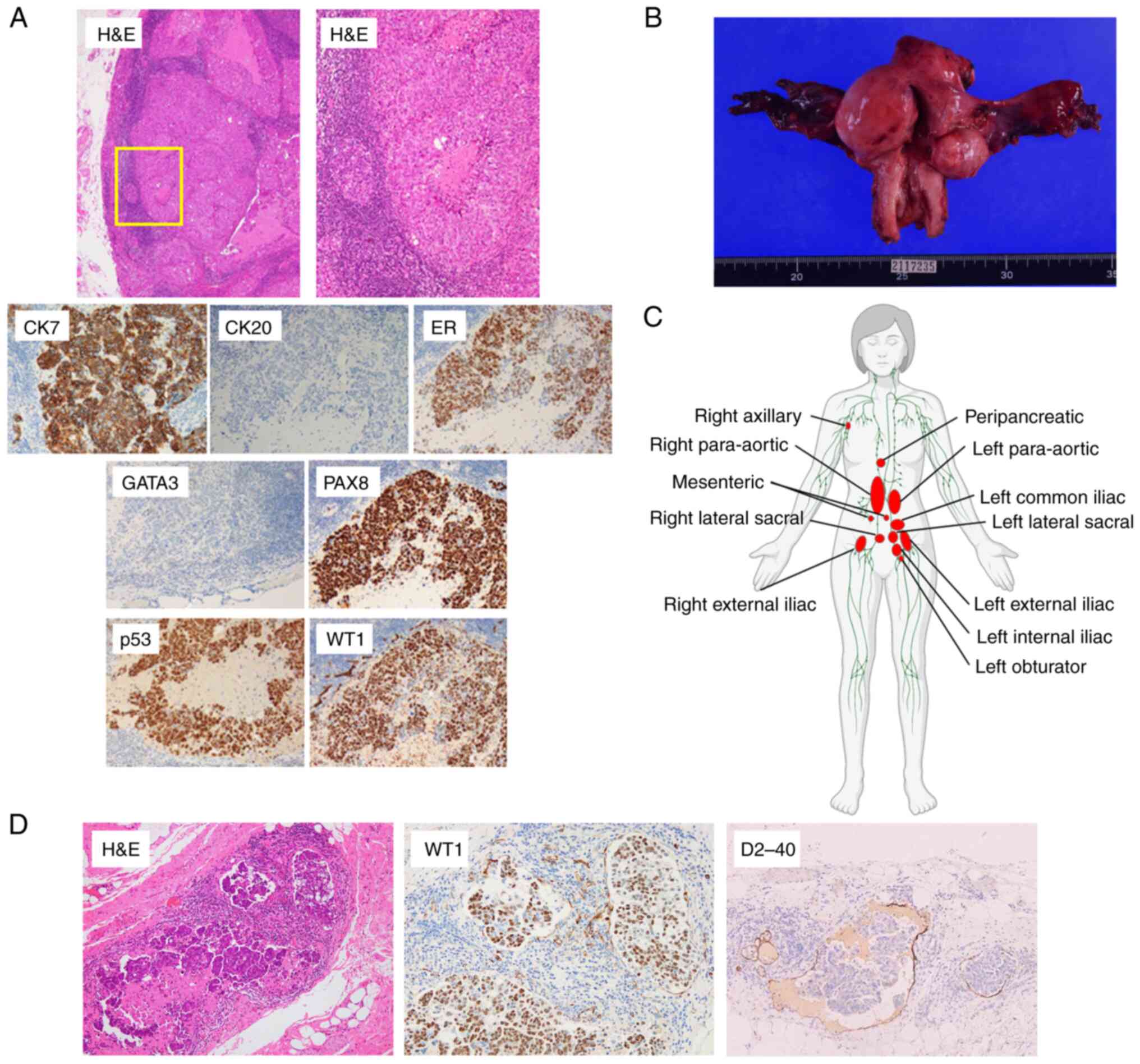 | Figure 2.Distribution and pathological
diagnosis of cancer of unknown primary. (A) Pathological
examination of para-aortic lymph node biopsy. The upper left image
shows H&E staining (magnification, ×40). The upper right image
is a magnified image of the area enclosed by the yellow square in
the upper left image (magnification, ×100). Sheets of cancer cells
with necrotic foci were observed. The cancer cells had enlarged and
irregularly shaped nuclei with distinct nucleoli. The lower images
show immunohistochemical analysis of CK7, CK20, ER, PAX8, p53 and
WT1 (all magnification, ×200). (B) Macroscopic image of surgically
removed uterus and bilateral adnexa. The unit on the scale is 1 mm.
(C) Distribution of metastatic lymph nodes determined by
pathological diagnosis of surgically removed lymph nodes and CT,
MRI and PET-CT images. Metastatic lymph nodes are shown in red.
This figure was created by the author using BioRender with
permission to reproduce images from BioRender.com. Reprinted from
‘Circulatory system (female, lymphatic)’, by BioRender.com (2023). Retrieved from https://app.biorender.com/biorender-templates. (D)
Pathological examination of the left ovary. Adenocarcinoma was
present in lymphatic vessels. The morphology of tumor clusters was
similar to the clusters observed in the pleural fluid cytology
specimens. H&E staining (left) and D2-40 (right) magnification,
×100; WT1 (center) magnification, ×200. CK7, cytokeratin 7; CK20,
cytokeratin 20; D2-40, podoplanin; ER, estrogen receptor; PAX8,
paired box 8; WT1, Wilms tumor 1; PET-CT, positron emission
tomography-computed tomography. |
Based on the distribution of the tumors, high CA125
levels, histology and immunostaining results, HGSC of the ovary or
fallopian tubes was suspected to be the primary carcinoma. For the
purpose of diagnosis and tumor debulking, a total abdominal
hysterectomy, bilateral salpingo-oophorectomy, pelvic and
para-aortic lymphadenectomy, and partial omentectomy were performed
(Fig. 2B). Pathological examination
of the surgical specimen, including histological assessment of
H&E-stained slides of FFPE tissues, revealed that there were no
primary tumors in the parenchyma of the ovaries or fallopian tubes,
and also no peritoneal dissemination, with negative peritoneal
washing cytology, or serous tubal intraepithelial carcinoma;
however, there were multiple metastatic adenocarcinomas in
para-aortic and pelvic lymph nodes. Although some lymph node
metastases were present on both sides, including the para-aortic,
sacral and external iliac lymph node metastases, some were only on
the left, including the common iliac, internal iliac and obturator
lymph node metastases. Combining preoperative imaging findings and
pathological diagnosis, the distribution of multiple lymph node
metastases was recorded as predominantly left-sided (Fig. 2C). Although no apparent primary site
was identified, lymphatic invasion of HGSC was microscopically
detected (Fig. 2D) within the left
ovarian parenchyma, and given the distribution of the lymph node
metastases, it was suggested that the tumor was left ovary-derived
(Fig. 2D). Reevaluation of the
pleural fluid cytology image at this point revealed a high
nucleus-to-cytoplasmic ratio, which was consistent with an
HGSC-like appearance (21). As no
primary tumor had been identified, the tumor was diagnosed as a
CUP. A CGP test was conducted to diagnose CUP. FoundationOne® CDx
(Foundation Medicine, Inc.) is a qualitative next-generation
sequencing-based in vitro diagnostic test performed by
Foundation Medicine, Inc. (22)
that showed 14 somatic variants (Table
I), including three likely pathogenic variants [TP53
(p.G266R), CIC (p.E1263Gfs*78) and PBRM1
(p.I223Yfs*36)], and nine gene amplifications of CCND2, CSF3R,
FGF23, FGF6, KDM5A, MYC, PIK3C2G, RAD52 and RICTOR
(Table II). The tumor was
microsatellite-stable with a tumor mutational burden (TMB) of
10.1/Mb (high) and a loss of heterozygosity (LOH) score of 23%.
Immune checkpoint inhibitors (ICIs) were recommended based on the
tumor being TMB-high (TMB-H) and poly ADP-ribose polymerase (PARP)
inhibitors were recommended based on the high LOH score. The tumor
distribution, pathological and immunohistochemical examinations,
and genomic examinations suggested gynecological organ-derived HGSC
(left ovary-derived being suspected). Myriad MyChoice CDx® (Myriad
Genetics, Inc.; protocol details not available), a next-generation
sequencing-based in vitro diagnostic test performed by
Myriad Genetics, Inc., revealed that the tumor was homologous
recombination-proficient (HRP) with a homologous
recombination-deficiency (HRD) score of 41 and no tumor
BRCA1/2 mutation.
 | Table I.Somatic variants. |
Table I.
Somatic variants.
| Gene | Site | cDNA variation | Amino acid
substitution | Clinical
significance |
|---|
| TP53 | 17p13.1 | c.796G>A | p.G266R | Likely
pathogenic |
| CIC | 19q13.2 | c.3786_3793del | p.E1263Gfs*78 | Likely
pathogenic |
| EP300 | 22q13.2 | c.2831C>T | p.A944V | - |
| ERBB2 | 17q12 | c.3149C>T | p.S1050L | - |
| FOXL2 | 3q22.3 | c.914C>A | p.P305Q | - |
| GNAS | 20q13.32 |
c.*42+13068G>C | - | - |
| JAK1 | 1p31.3 | c.1059_1082del | p.D353_R360del | - |
| MAF | 16q23.2 |
c.543_544insTAC |
p.Y181_H182insY | - |
| MPA3K13 | 3q27.2 | c.1567A>G | p.I523V | - |
| MED12 | Xq13.1 | c.2704G>T | p.V902L | - |
| NFKBIA | 14q13.2 | c.797_805del | p.Q266_Q268del | - |
| NOTCH1 | 9q34.3 | c.5422G>A | p.D1808N | - |
| NTRK2 | 9q21.33 | c.1752G>C | p.L584F | - |
| PBRM1 | 3p21.1 | c.666_679del | p.I223Yfs*36 | Likely
pathogenic |
 | Table II.Copy number alterations. |
Table II.
Copy number alterations.
| Gene | Site | Copy number
alteration | Copy number | Clinical
significance |
|---|
| CCND2 | 12p13.32 | Amplification | 10 | - |
| CSF3R | 1p34.3 | Amplification | 7 | - |
| FGF23 | 12p13.32 | Amplification | 10 | - |
| FGF6 | 12p13.32 | Amplification | 10 | - |
| KDM5A | 12p13.33 | Amplification | 10 | Likely
pathogenic |
| MYC | 8q24.21 | Amplification | 7 | Pathogenic |
| PIK3C2G | 12p12.3 | Amplification | 7 | - |
| RAD52 | 12p13.33 | Amplification | 10 | - |
| RICTOR | 5p13.1 | Amplification | 10 | Pathogenic |
The tumor had a high TMB, and ICIs should therefore
have been considered; however, the HRD score was 41 (relatively
high among HRP tumors) and the LOH score was also high, close to
that of the HRD tumor (HRD score ≥42). In addition, HGSC generally
has a high response rate to platinum agents and PARP inhibitors are
effective only while tumors are platinum-sensitive (23–25).
Therefore, it was decided to start a combination therapy of
paclitaxel and carboplatin (TC therapy; paclitaxel 175
mg/m2 and carboplatin AUC 6 mg/ml/min every 3–4 weeks)
and use niraparib (200 mg/body) as maintenance therapy. After eight
courses of TC therapy, the CA125 level was markedly reduced from
247 U/ml to 16 U/ml (Fig. 3), and
the patient achieved complete remission. Based on the HRD score of
41, the patient was switched to niraparib maintenance therapy
according to the treatment protocol for ovarian cancer (25). The patient has been followed up
every month since August 2022, when niraparib was started, and will
continue to be followed up at the same intervals. The patient has
been relapse-free as of July 2023.
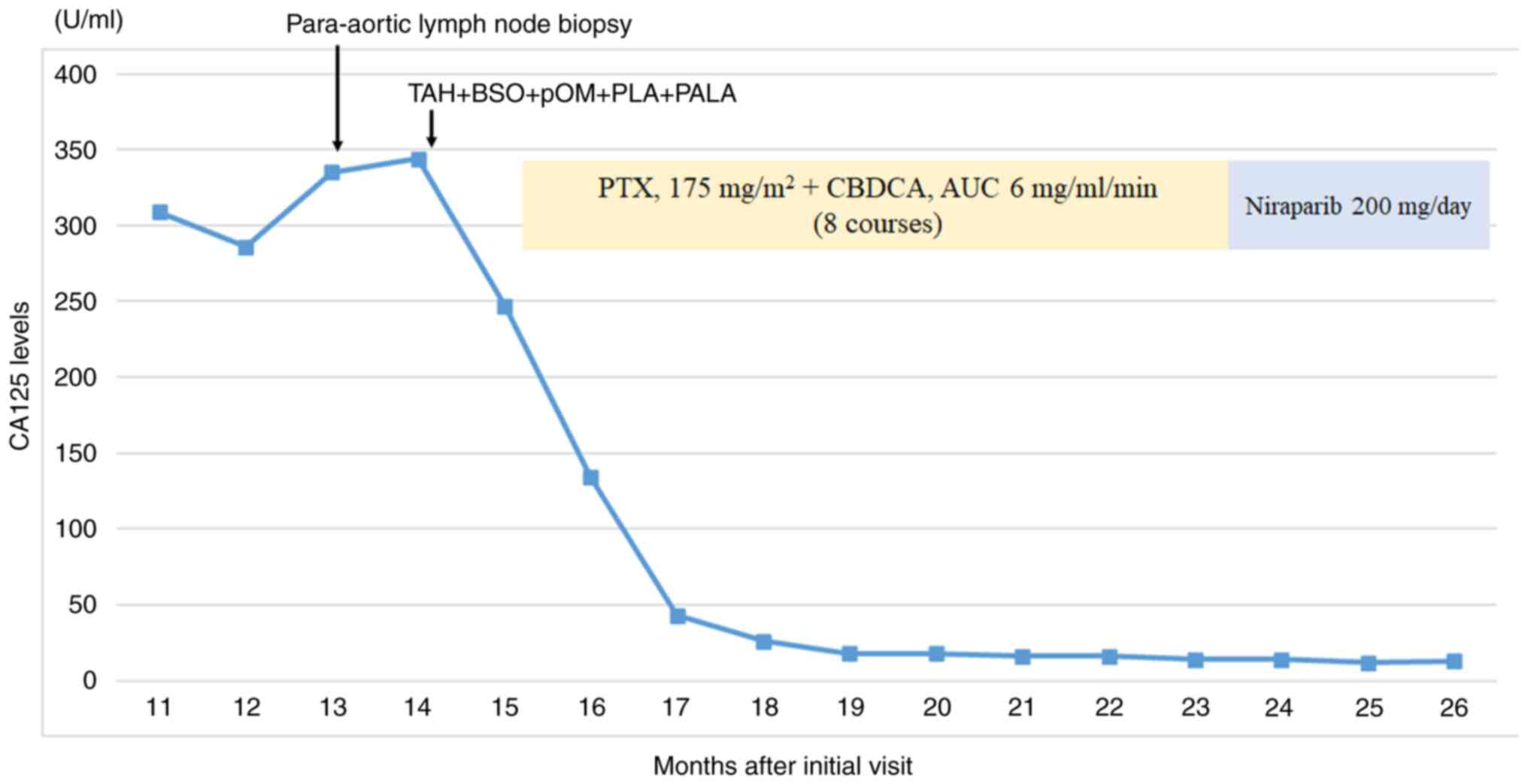 | Figure 3.Summary of clinical course and CA125
levels. No recurrence was observed during the course of the
treatment by contrast-enhanced computed tomography, and paclitaxel
and carboplatin therapy markedly reduced CA125 levels. After 8
courses of paclitaxel-carboplatin combination therapy, the patient
achieved complete remission. Since then, the patient has received
niraparib maintenance therapy and has been relapse-free. TAH, total
abdominal hysterectomy; BSO, bilateral salpingo-oophorectomy; pOM,
partial omentectomy; PLA, pelvic lymphadenectomy; PALA, paraaortic
lymphadenectomy; PTX, paclitaxel; CBDCA, carboplatin; AUC, area
under the curve; CA125, cancer antigen 125. |
Discussion
In the present case, the diagnosis was delayed by
the spontaneous resolution of the cancerous pleural effusion.
However, CUP was classified as cancer of ovarian origin based on
the marked lymphatic invasion around the left ovary, the
distribution of the lymph node metastases, the genomic information
and the immunohistochemical data, and the patient was able to
receive specific treatment for primary ovarian cancer.
The biological nature of CUP remains largely
unknown. The most popular hypothesis is that it is a metastatic
tumor arising from an undetectable primary tumor due to regression,
dormancy and small size (26,27).
Another hypothesis is that it is a single metastatic tumor without
a primary tumor (3). The present
case is unique in that there was no primary lesion in the uterus
and adnexa, accompanied by only lymphatic invasion within the left
ovary. These findings suggest that the primary tumor in the left
ovary or fallopian tube might either be small or regressed
spontaneously.
An important observation in this case was the
disappearance of the malignant pleural effusion. To the best of our
knowledge, the phenomenon of spontaneous resolution of malignant
pleural effusions has not yet been reported, and its precise
mechanism remains unresolved. A similar phenomenon, in which a
pleural effusion appears with ovarian malignancy and disappears
with the disappearance of the ovarian tumor, is known as
pseudo-Meigs' syndrome (28,29).
In the present case, if the malignant pleural effusion had
disappeared with the spontaneous resolution of the primary ovarian
tumor, it would be consistent with the pathogenesis of
pseudo-Meigs' syndrome.
The reason for the disappearance of the primary
tumor was subsequently considered. The patient was being
administered systemic exogenous corticosteroids around the time the
pleural effusion appeared. It is possible that the systemic
administration of steroids suppressed cancer immunity (30). Furthermore, the tumor was TMB-H.
HGSC is characterized by copy number alterations with low TMB
(31–33). TMB-H tumors are characterized by
high levels of neoantigens and immunogenicity (34). One hypothesis is that the patient
developed highly immunogenic ovarian cancer, but that cancer
immunity was suppressed during steroid administration, resulting in
pseudo-Meigs' syndrome associated with ovarian cancer. Subsequent
reactivation of immunity by steroid withdrawal may have triggered
shrinkage of the primary tumor and disappearance of the pleural
effusion.
In the present case, genomic data suggested that
PARP inhibitors could be expected to be effective as maintenance
therapy after TC therapy. In addition, the patient had a TMB-H
tumor and was expected to benefit from ICIs. Although the patient
was treated with TC therapy followed by maintenance therapy with a
PARP inhibitor based on the treatment regimen for primary ovarian
cancer, the efficacy of immunotherapy in CUP has been demonstrated
in a phase II trial (35) and is
expected to become more widespread in the future. Approximately
one-third of patients with CUP have a tumor proportion score >1%
for programmed cell death 1-ligand 1, and antitumor
immunity-related gene expression in CUP have been reported to be
comparable to those in ICI-responsive malignancies (36,37).
The pleural effusion appeared at the time of steroid administration
and the tumor was a TMB-H tumor, which is rare for ovarian HGSC
(31,32). This may suggest that the
pathophysiology of CUP in the present case report is highly
immunogenic.
The present study had some limitations. First, the
hypothesis of pseudo-Meigs' syndrome associated with ovarian cancer
was proposed as a pathogenesis of CUP; however, it is not possible
to prove this hypothesis since there was no evidence of a tumor in
the left ovary. Second, based on the high LOH score, a PARP
inhibitor was used after TC therapy; however, it was not possible
to evaluate the response to the PARP inhibitor, as the TC therapy
resulted in complete remission. Long-term observations are needed
in the future to investigate the effects of PARP inhibitors and
ICIs.
In the present case, lymphatic invasion, the
distribution of the lymph node metastases, genomic analysis and
immunohistological analysis suggested CUP of left ovarian origin,
and specific therapy for ovarian cancer was provided. This unique
course, which was characterized by the appearance and disappearance
of the CUP, may have been associated with the immune response.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The sequencing datasets generated and/or analyzed
during the current study are not publicly available as the Myriad
MyChoice CDx® and FoundationOne® CDx reports are all the raw data
that Foundation Medicine, Inc., and Myriad Genetics, Inc. can
provide. All content from the reports has been provided in the
manuscript. It has been confirmed with Chugai Pharmaceutical Co.,
and Myriad Genetics, Inc., that no other data will be provided. The
other datasets used and/or analyzed during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
HH and AT conceived and designed the study. HH, AT,
HR, AM, AN, TA, SE, YM, KS, MUM and YO obtained data and treated
this patient. HH analyzed the data and drafted the manuscript. HR
analyzed the data using pathological methods. HH and HR confirm the
authenticity of the pathological data. HH and AT confirm the
authenticity of all other raw data. AT, HR, AM, TA, AN, SE, YM, KS,
MUM and YO revised the manuscript before submission. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
This case received standard clinical treatment as a
cancer of unknown primary equivalent to ovarian cancer. For this
report, in accordance with the Act on the Protection of Personal
Information in Japan (38), this
study was approved by the Institutional Ethics Committee of The
University of Tokyo (approval no. G0683; Tokyo, Japan).
Patient consent for publication
The patient provided written informed consent for
publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CUP
|
cancer of unknown primary
|
|
CGP
|
cancer genomic profiling
|
|
PET-CT
|
positron emission tomography-computed
tomography
|
|
HGSC
|
high-grade serous carcinoma
|
|
TMB
|
tumor mutational burden
|
|
TMB-H
|
TMB-high
|
|
ICI
|
immune checkpoint inhibitor
|
|
HRP
|
homologous
recombination-proficient
|
|
HRD
|
homologous
recombination-deficiency
|
|
TC therapy
|
paclitaxel-carboplatin combination
therapy
|
|
SUV
|
standardized uptake value
|
|
CA125
|
cancer antigen 125
|
|
PARP
|
poly ADP-ribose polymerase
|
References
|
1
|
Varadhachary GR and Raber MN: Cancer of
unknown primary site. N Engl J Med. 371:757–765. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qaseem A, Usman N, Jayaraj JS, Janapala RN
and Kashif T: Cancer of unknown primary: A review on clinical
guidelines in the development and targeted management of patients
with the unknown primary site. Cureus. 11:e55522019.PubMed/NCBI
|
|
3
|
Kato S, Alsafar A, Walavalkar V,
Hainsworth J and Kurzrock R: Cancer of unknown primary in the
molecular Era. Trends Cancer. 7:465–477. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pavlidis N and Pentheroudakis G: Cancer of
unknown primary site. Lancet. 379:1428–1435. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pavlidis N, Khaled H and Gaafar R: A mini
review on cancer of unknown primary site: A clinical puzzle for the
oncologists. J Adv Res. 6:375–382. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pavlidis N, Briasoulis E, Hainsworth J and
Greco FA: Diagnostic and therapeutic management of cancer of an
unknown primary. Eur J Cancer. 39:1990–2005. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hannouf MB, Winquist E, Mahmud SM,
Brackstone M, Sarma S, Rodrigues G, Rogan PK, Hoch JS and Zaric GS:
The potential clinical and economic value of primary tumour
identification in metastatic cancer of unknown primary tumour: A
population-based retrospective matched cohort study. Pharmacoecon
Open. 2:255–270. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang H, He F, Xu W and Cao Z: Clinical
features of cancer with unknown primary site (clinical features,
treatment, prognosis of cancer with unknown primary site). BMC
Cancer. 22:13722022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hainsworth JD, Rubin MS, Spigel DR, Boccia
RV, Raby S, Quinn R and Greco FA: Molecular gene expression
profiling to predict the tissue of origin and direct site-specific
therapy in patients with carcinoma of unknown primary site: A
prospective trial of the Sarah Cannon research institute. J Clin
Oncol. 31:217–223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Greco FA, Lennington WJ, Spigel DR and
Hainsworth JD: Molecular profiling diagnosis in unknown primary
cancer: Accuracy and ability to complement standard pathology. J
Natl Cancer Inst. 105:782–790. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Varadhachary GR, Talantov D, Raber MN,
Meng C, Hess KR, Jatkoe T, Lenzi R, Spigel DR, Wang Y, Greco FA, et
al: Molecular profiling of carcinoma of unknown primary and
correlation with clinical evaluation. J Clin Oncol. 26:4442–4448.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hayashi H, Kurata T, Takiguchi Y, Arai M,
Takeda K, Akiyoshi K, Matsumoto K, Onoe T, Mukai H, Matsubara N, et
al: Randomized phase II trial comparing site-specific treatment
based on gene expression profiling with carboplatin and paclitaxel
for patients with cancer of unknown primary site. J Clin Oncol.
37:570–579. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moran S, Martínez-Cardús A, Sayols S,
Musulén E, Balañá C, Estival-Gonzalez A, Moutinho C, Heyn H,
Diaz-Lagares A, de Moura MC, et al: Epigenetic profiling to
classify cancer of unknown primary: A multicentre, retrospective
analysis. Lancet Oncol. 17:1386–1395. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Laprovitera N, Salamon I, Gelsomino F,
Porcellini E, Riefolo M, Garonzi M, Tononi P, Valente S, Sabbioni
S, Fontana F, et al: Genetic characterization of cancer of unknown
primary using liquid biopsy approaches. Front Cell Dev Biol.
9:6661562021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pauli C, Bochtler T, Mileshkin L,
Baciarello G, Losa F, Ross JS, Pentheroudakis G, Zarkavelis G,
Yalcin S, Özgüroğlu M, et al: A challenging task: Identifying
patients with cancer of unknown primary (CUP) According to ESMO
Guidelines: The CUPISCO trial experience. Oncologist. 26:e769–e779.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pisacane A, Cascardi E, Berrino E,
Polidori A, Sarotto I, Casorzo L, Panero M, Boccaccio C, Verginelli
F, Benvenuti S, et al: Real-world histopathological approach to
malignancy of undefined primary origin (MUO) to diagnose cancers of
unknown primary (CUPs). Virchows Arch. 482:463–475. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Uchikura E, Fukuda T, Imai K, Yamauchi M,
Kasai M, Ichimura T, Yasui T, Kuwae Y and Sumi T: Carcinomatous
meningitis from ovarian serous carcinoma: A case report. Oncol
Lett. 25:662022.PubMed/NCBI
|
|
18
|
Papanicolaou GN: Atlas of Exfoliative
Cytology. Cambridge, Mass: Harvard University Press; 1954
|
|
19
|
Hinata M, Kunita A, Abe H, Morishita Y,
Sakuma K, Yamashita H, Seto Y, Ushiku T and Fukayama M: Exosomes of
epstein-barr virus-associated gastric carcinoma suppress dendritic
cell maturation. Microorganisms. 8:17762020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
WHO Classification of Tumours Editorial
Board, . Female Genital Tumors, WHO Classification of Tumors. Vol
4:(5th edition). 2020.
|
|
21
|
Pereira TC, Saad RS, Liu Y and Silverman
JF: The diagnosis of malignancy in effusion cytology: A pattern
recognition approach. Adv Anat Pathol. 13:174–184. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Milbury CA, Creeden J, Yip WK, Smith DL,
Pattani V, Maxwell K, Sawchyn B, Gjoerup O, Meng W, Skoletsky J, et
al: Clinical and analytical validation of FoundationOne® CDx, a
comprehensive genomic profiling assay for solid tumors. PLoS One.
17:e02641382022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moore K, Colombo N, Scambia G, Kim BG,
Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A, Sonke
GS, et al: Maintenance olaparib in patients with newly diagnosed
advanced ovarian cancer. N Engl J Med. 379:2495–2505. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
González-Martín A, Pothuri B, Vergote I,
DePont Christensen R, Graybill W, Mirza MR, McCormick C, Lorusso D,
Hoskins P, Freyer G, et al: Niraparib in patients with newly
diagnosed advanced ovarian cancer. N Engl J Med. 381:2391–2402.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Coleman RL, Fleming GF, Brady MF, Swisher
EM, Steffensen KD, Friedlander M, Okamoto A, Moore KN, Efrat
Ben-Baruch N, Werner TL, et al: Veliparib with first-line
chemotherapy and as maintenance therapy in ovarian cancer. N Engl J
Med. 381:2403–2415. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Olivier T, Fernandez E, Labidi-Galy I,
Dietrich PY, Rodriguez-Bravo V, Baciarello G, Fizazi K and
Patrikidou A: Redefining cancer of unknown primary: Is precision
medicine really shifting the paradigm? Cancer Treat Rev.
97:1022042021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Conway AM, Mitchell C, Kilgour E, Brady G,
Dive C and Cook N: Molecular characterisation and liquid biomarkers
in Carcinoma of Unknown Primary (CUP): taking the ‘U’ out of ‘CUP’.
Br J Cancer. 120:141–153. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kazanov L, Ander DS, Enriquez E and Jaggi
FM: Pseudo-Meigs' Syndrome. Am J Emerg Med. 16:404–405. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gücer F, Oz-Puyan F, Mülayim N and Yüce
MA: Ovarian dysgerminoma associated with Pseudo-Meigs' syndrome and
functioning ovarian stroma: A case report. Gynecol Oncol.
97:681–684. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Coutinho AE and Chapman KE: The
anti-inflammatory and immunosuppressive effects of glucocorticoids,
recent developments and mechanistic insights. Mol Cell Endocrinol.
335:2–13. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cristescu R, Mogg R, Ayers M, Albright A,
Murphy E, Yearley J, Sher X, Lin XQ, Lu H, Nebozhyn M, et al:
Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based
immunotherapy. Science. 362:eaar35932018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Park J, Lee JY and Kim S: How to use
immune checkpoint inhibitor in ovarian cancer? J Gynecol Oncol.
30:e1052019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Leary A, Tan D and Ledermann J: Immune
checkpoint inhibitors in ovarian cancer: Where do we stand? Ther
Adv Med Oncol. 13:175883592110398992021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ward JP, Gubin MM and Schreiber RD: The
role of neoantigens in naturally occurring and therapeutically
induced immune responses to cancer. Adv Immunol. 130:25–74. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Raghav KP, Stephen B, Karp DD, Piha-Paul
SA, Hong DS, Jain D, Chudy Onwugaje DO, Abnofal A, Willett AF,
Overman M, et al: Efficacy of pembrolizumab in patients with
advanced cancer of unknown primary (CUP): A phase 2 non-randomized
clinical trial. J Immunother Cancer. 10:e0048222022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tanizaki J, Yonemori K, Akiyoshi K, Minami
H, Ueda H, Takiguchi Y, Miura Y, Segawa Y, Takahashi S, Iwamoto Y,
et al: Open-label phase II study of the efficacy of nivolumab for
cancer of unknown primary. Ann Oncol. 33:216–226. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Haratani K, Hayashi H, Takahama T,
Nakamura Y, Tomida S, Yoshida T, Chiba Y, Sawada T, Sakai K, Fujita
Y, et al: Clinical and immune profiling for cancer of unknown
primary site. J Immunother Cancer. 7:2512019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Japanese government, . Act on the
Protection of Personal Information: Last Version: Act No. 37 of
2021. Translated Date: November 5, 2021. https://www.japaneselawtranslation.go.jp/ja/laws/view/130July
24–2023
|

















