Introduction
Breast cancer is the most commonly diagnosed cancer
and the second leading cause of cancer-related deaths in the United
States (1). Human epithelial growth
factor receptor 2 (HER2) is overexpressed in ~15% of breast cancer
cases in the United States (2), and
HER2-positive breast cancer is more common in metastatic settings
(3). In breast cancer treatment,
the development of targeted therapies has improved the efficacy,
reducing damage to normal tissues; however, increased drug
resistance against targeted agents has led researchers to develop
an antibody-drug conjugate comprising cytotoxic agents and
monoclonal drugs (4). Trastuzumab
emtansine (T-DM1) is the first antibody-drug conjugate to be
approved in a solid tumor and consists of trastuzumab, humanized
monoclonal antibodies against HER2 and mertansine, a microtubule
inhibitor (4). Based on the results
of the EMILIA (5), TH3RESA
(6) and MARIANNE (7) trials using various patient groups,
T-DM1 has been approved for patients with HER2-positive breast
cancer (HPBC) that have previously been treated with trastuzumab
and taxane. Although T-DM1 can be used for any line of treatment,
it is accepted as one of the standard second-line regimens for the
treatment of metastatic HPBC (8,9).
Thrombocytopenia is one of the adverse events
observed during T-DM1 treatment and is the most common reason for
dose reduction and treatment discontinuation (10–13).
Although previous reports assert the opposite (14), recent studies have reported that a
number of systemic toxicities, including thrombocytopenia, may
predict T-DM1 efficacy (15,16).
As it has been reported that the incidence of thrombocytopenia
increases with prolonged T-DM1 treatment duration (17), the predictive value for survival
outcome is debatable. Thus, the present study aimed to investigate
whether early thrombocytopenia during T-DM1 treatment could predict
survival rates.
Materials and methods
Study population and data
collection
The present retrospective, multicenter study
included patients at six oncology centers (Uludag University,
Bursa; Dokuz Eylul University, İzmir; Ataturk City Hospital,
Balıkesir; Inonu University, Malatya; Usak University, Usak; Bursa
Medicana Hospital, Bursa) in Turkey from January 2016 to December
2021. The inclusion criteria required patients to: i) Have received
at least two cycles of T-DM1 due to histopathologically confirmed
advanced-stage HPBC; ii) be female; and iii) be ≥18 years old. To
provide sufficient periods for the efficacy of the drug, the study
excluded patients who did not receive any local treatment (surgery
or radiotherapy) for symptomatic brain metastasis. Patients with a
history of hematological disease or incomplete laboratory data
during T-DM1 treatment were also excluded. T-DM1 was administered
intravenously at a dose of 3.6 mg/kg on the first day of the
treatment cycle, every 3 weeks. The dose reduction scheme provided
by the Food and Drug Administration was followed in cases of
toxicity (18). No endocrine
therapy was administered concurrently with T-DM1.
After The Clinical Research Ethics Committee of
Uludag University Faculty of Medicine (Bursa, Turkey) approved the
study, the following variables of the patients were extracted from
all electronic records in hospital databases: i) Age; ii)
menopausal status; iii) expression of estrogen receptor; iv)
expression of progesterone receptor; v) expression of HER2; vi)
sites of metastasis; vii) previous treatment regimens; viii)
treatment lines for metastatic disease; ix) Eastern Cooperative
Oncology Group (ECOG) performance status scores (19); and x) pre- and post-treatment
laboratory findings.
Definitions and outcomes
HER2 overexpression was defined as an
immunohistochemistry (IHC) staining of 3+ or from a positive
identification using in situ hybridization (ISH), and
hormone receptor status was accepted as positive in patients with
≥1% expression for estrogen and/or progesterone receptor following
the American Society of Clinical Oncology/College of American
Pathologists guidelines (20,21).
Response assessment was conducted according to the Response
Evaluation Criteria for Solid Tumors (version 1.1) (22). The overall response rate (ORR) was
defined as the proportion of patients that achieved a complete
response (CR) or a partial response (PR). The disease control rate
(DCR) was expressed as the percentage of patients with CR, PR and
stable disease.
Response assessment was performed every 3–4 cycles
of T-DM1 and more frequently in cases of clinical deterioration
attributed to treatment failure. Time-to-treatment discontinuation
(TTD) was defined as the interval from the date of initiating T-DM1
treatment to the date of treatment discontinuation or mortality.
T-DM1 treatment was continued beyond radiological disease
progression if a clinical benefit persisted according to the
evaluation of the physician with local ablative therapies
permitted. Overall survival (OS) was defined as the interval from
the beginning of T-DM1 until mortality from any cause. Adverse
events were graded using the National Cancer Institute Common
Terminology Criteria for Adverse Events (version 5.0) (23). Thrombocytopenia attributed to
infection or sepsis was not evaluated as an adverse event. Early
thrombocytopenia was defined as the occurrence of thrombocytopenia
in the first four cycles of T-DM1 treatment.
Statistical analysis
Statistical analyses were performed using SPSS
(version 22; IBM Corp.). Continuous variables were expressed as
median (minimum-maximum) values, and categorical variables were
expressed as frequency and corresponding percentage values.
Kaplan-Meier survival estimates were calculated, and comparisons
were performed using the log-rank test. The possible factors
affecting TTD were examined by Cox regression analysis. The enter
model was used for parameters with P<0.20 in univariate
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
The present study enrolled 138 patients and Table I presents the characteristics of the
patients and tumors. The median age was 50 years (range, 26–83
years). Of the 138 patients, 58% were hormone receptor-positive,
83% had a HER2 score of 3+ in the IHC evaluation and 50% were
post-menopausal. The majority of the patients (77%) presented with
visceral metastasis and 41% had de novo metastatic disease.
The most common site of metastasis was bone (55%), followed by the
lung (50%) and non-regional lymph nodes (45%). The ECOG performance
status score was <2 in 90% of the patients. Before T-DM1
administration, all of the patients had received trastuzumab, and
95% had received taxanes. Pertuzumab was administered to only 17%
of the patients before T-DM1. The median number of lines of
treatment for T-DM1 was 2 (range, 1–8) in metastatic cases.
 | Table I.Baseline patient and disease
characteristics and prior treatments for metastatic disease
(n=138). |
Table I.
Baseline patient and disease
characteristics and prior treatments for metastatic disease
(n=138).
| Characteristic | Median (range) | n | % |
|---|
| Age, years | 50.4
(25.5–82.9) |
|
|
| Histology |
|
|
|
|
Infiltrating duct
carcinoma |
| 130 | 94.2 |
|
Othera |
| 8 | 5.8 |
| Hormone receptor
status |
|
|
|
|
Positive |
| 80 | 58.0 |
|
Negative |
| 58 | 42.0 |
| HER2 status |
|
|
|
| IHC
HER2 <3+ and |
| 24 | 17.4 |
| ISH
positive |
|
|
|
| IHC
HER2 3+ |
| 114 | 82.6 |
| Menopausal
status |
|
|
|
|
Pre-menopausal |
| 69 | 50.0 |
|
Post-menopausal |
| 69 | 50.0 |
| Disease
presentation |
|
|
|
|
Recurrent |
| 82 | 59.4 |
| De
novo metastatic |
| 56 | 40.6 |
| Disease
involvement |
|
|
|
|
Visceral |
| 106 | 76.8 |
|
Non-visceral |
| 32 | 23.2 |
| Site of
metastasis |
|
|
|
|
Bone |
| 76 | 55.1 |
|
Lung |
| 69 | 50.0 |
| Lymph
nodeb |
| 62 | 44.9 |
|
Liver |
| 45 | 32.6 |
|
Brain |
| 29 | 21.0 |
| ECOG performance
status score |
|
|
|
| 0 |
| 46 | 33.3 |
| 1 |
| 78 | 56.5 |
| 2 |
| 14 | 10.2 |
| Prior systemic
agent |
|
|
|
|
Taxanes |
| 131 | 94.9 |
|
Anthracycline |
| 101 | 73.2 |
|
Lapatinib |
| 34 | 24.6 |
|
Pertuzumab |
| 23 | 16.7 |
| Line of T-DM1
treatment in the metastatic setting | 2 (1–8) |
|
|
| 1 |
| 11 | 8.0 |
| 2 |
| 68 | 49.3 |
| 3 |
| 38 | 27.5 |
| ≥4 |
| 21 | 15.2 |
Tables II and
III present the efficacy outcomes
and laboratory toxicities of treatment with T-DM1, respectively.
The median number of cycles was 9 (range, 2–58), and 33% of the
patients received ≥15 cycles of T-DM1. Dose reduction for the
subsequent cycle was performed in 12 patients (9%), and half of
those were due to thrombocytopenia. At the time of data cut-off,
86% of the patients had experienced TTD events. The ORR and DCR
were 50.0 and 69.6%, respectively. The most common all-grade
adverse events were increased levels of hepatic enzymes (43 and 38%
for AST and ALT, respectively), thrombocytopenia (39%) and anemia
(38%). Among grade 3 and 4 toxicities, thrombocytopenia was the
most common adverse event (10%). The median number of treatment
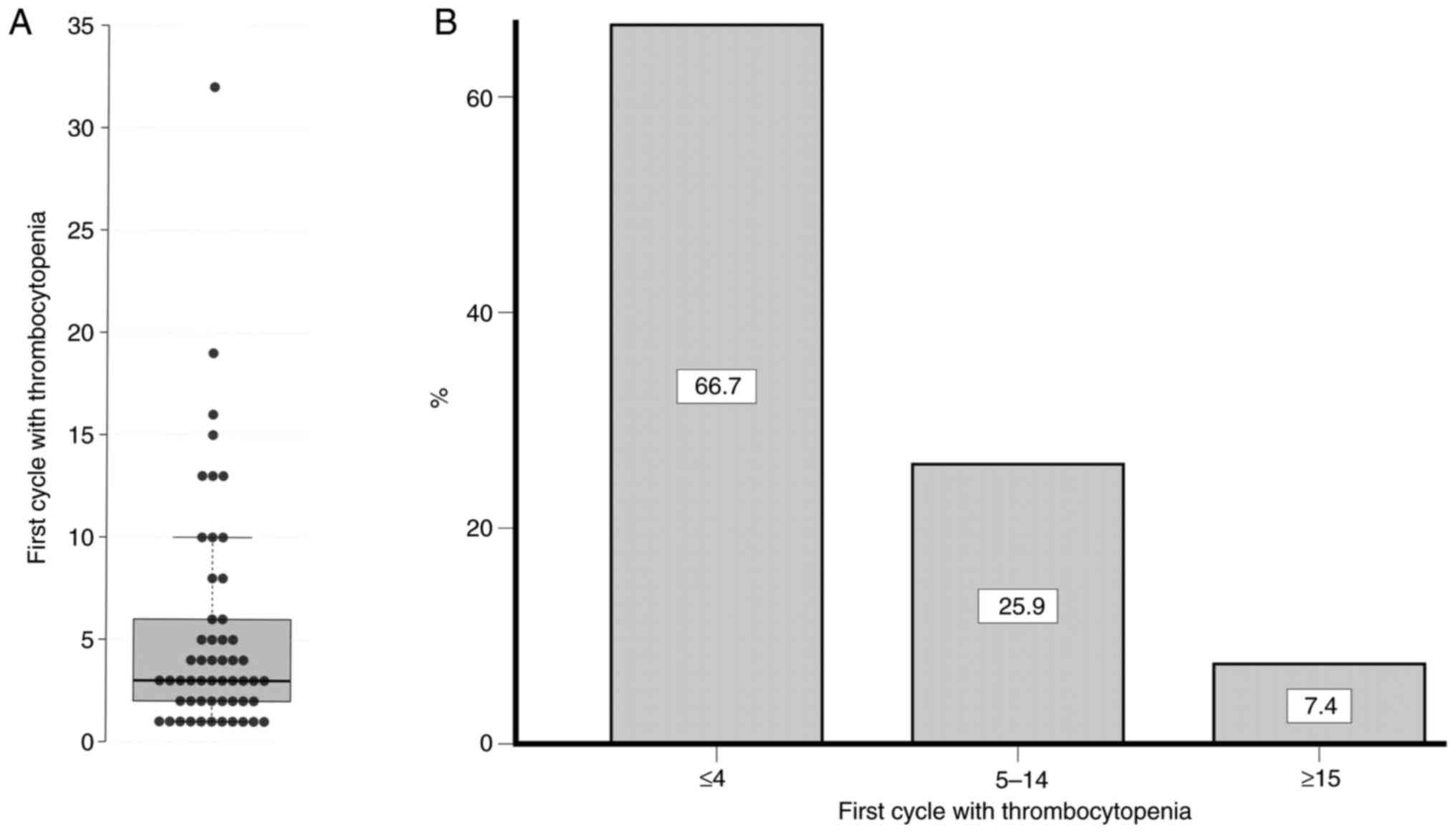
cycles in which thrombocytopenia first appeared was 3 (range, 1–32)
(Fig. 1A), and two-thirds of
occurrences of thrombocytopenia (66.7%) were observed in the first
four cycles (Fig. 1B).
 | Table II.Efficacy outcomes of treatment with
trastuzumab emtansine. |
Table II.
Efficacy outcomes of treatment with
trastuzumab emtansine.
|
Characteristics | Median
(minimum-maximum) | n, n=138 | % |
|---|
| Cycles | 9 (2–58) |
|
|
| Dose reduction |
| 12 | 8.7 |
| TTD event |
| 119 | 86.2 |
| Mortality |
| 88 | 63.8 |
| Response |
|
|
|
|
Complete response |
| 12 | 8.7 |
| Partial
response |
| 57 | 41.3 |
| Overall
response rate |
| - | 50.0 |
| Stable
disease |
| 27 | 19.6 |
| Disease
control rate |
| - | 69.6 |
|
Progressive disease |
| 42 | 30.4 |
 | Table III.Adverse effects of treatment with
trastuzumab emtansine. |
Table III.
Adverse effects of treatment with
trastuzumab emtansine.
| Adverse event | Any grade, n
(%) | Grade 3 and 4, n
(%) |
|---|
| AST increased | 59 (42.8) | 4 (2.9) |
|
Thrombocytopenia | 54 (39.1) | 14 (10.2) |
| Anemia | 53 (38.4) | 2 (1.5) |
| ALT increased | 52 (37.7) | 6 (4.4) |
| Neutropenia | 20 (14.5) | 5 (3.6) |
|
Hyperbilirubinemia | 14 (10.2) | 3 (2.2) |
| Hypokalemia | 13 (9.4) | 3 (2.2) |
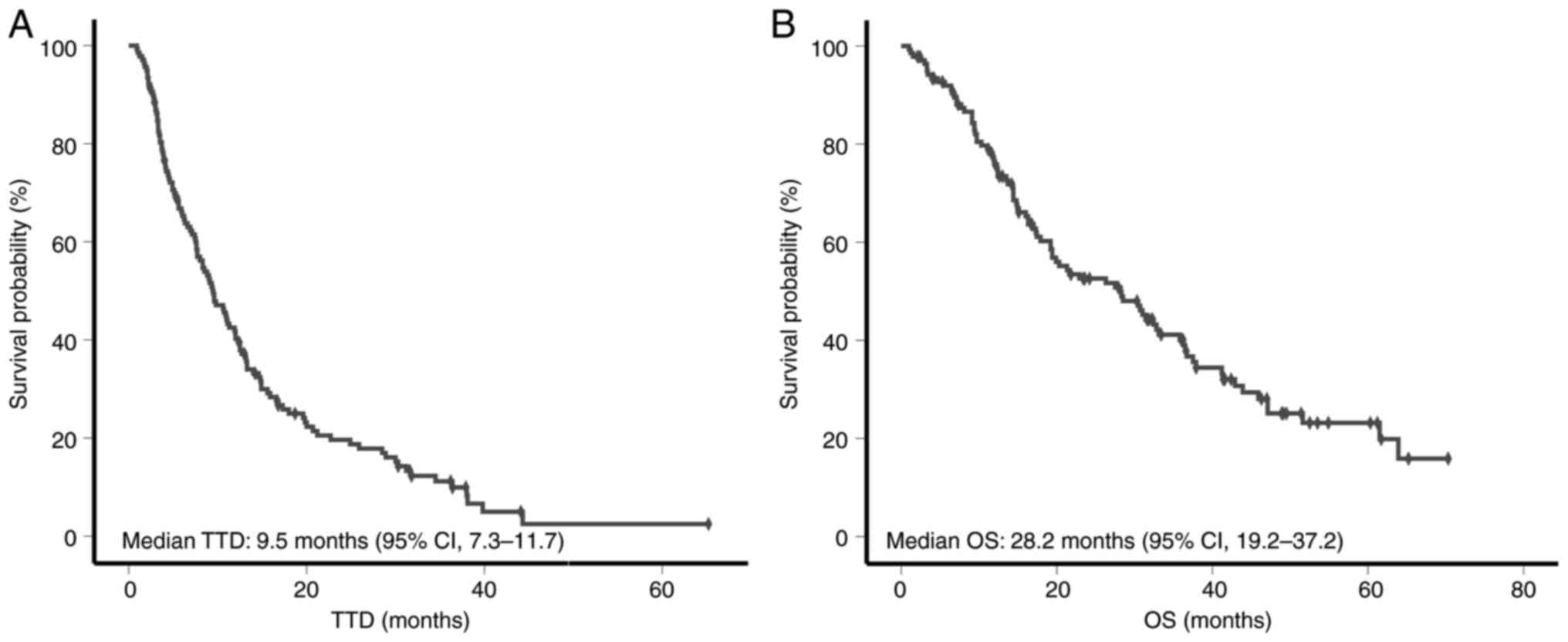
The median follow-up time was 19.3 months (range,
1–70 months). Based on the Kaplan-Meier analysis, the median TTD
was 9.5 months [95% confidence interval (CI), 7.3–11.7] (Fig. 2A), and the median OS was 28.2 months
(95% CI, 19.2–37.2) (Fig. 2B).
Table IV presents univariate and
multivariate Cox regression analyses for factors affecting the TTD
of T-DM1. The multivariate analyses revealed that hormone receptor
status [hazard ratio (HR), 1.837; 95% CI, 1.249–2.701; P=0.002],
ECOG performance status score (HR, 3.269; 95% CI, 1.788–5.976;
P<0.001) and thrombocytopenia during treatment (HR, 0.297; 95%
CI, 0.198–0.446; P<0.001) were independent factors for TTD.
Patients who were hormone receptor-positive and patients with high
ECOG performance scores had an increased risk of an TTD event. By
contrast, patients who developed thrombocytopenia during T-DM1
treatment had a reduced risk of developing a TTD event.
 | Table IV.Univariate and multivariate cox
regression analysis of the predictors for time-to-treatment
discontinuation. |
Table IV.
Univariate and multivariate cox
regression analysis of the predictors for time-to-treatment
discontinuation.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Factor | Category | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age | Years | 1.000 | 0.985–1.016 | 0.996 |
|
|
|
| Menopausal
status | Pre-men. (R) vs.
post-men. | 0.978 | 0.681–1.406 | 0.906 |
|
|
|
| Hormone receptor
status | Negative (R) vs.
positive | 1.547 | 1.065–2.247 | 0.022 | 1.837 | 1.249–2.701 | 0.002a |
| HER2 status | IHC 3+ (R) vs. ISH
positive | 1.186 | 0.744–1.892 | 0.474 |
|
|
|
| Histotype | IDC (R) vs.
other | 1.330 | 0.646–2.735 | 0.439 |
|
|
|
| Disease
presentation | Recurrent (R) vs.
de novo metastatic | 1.111 | 0.765–1.611 | 0.581 |
|
|
|
| Visceral
metastasis | Absent (R) vs.
present | 1.205 | 0.782–1.858 | 0.397 |
|
|
|
| CNS metastasis | Absent (R) vs.
present | 1.194 | 0.771–1.850 | 0.427 |
|
|
|
| ECOG performance
score | 0–1 (R) vs. 2 | 3.219 | 1.795–5.774 | <0.001 | 3.269 | 1.788–5.976 |
<0.001a |
| Pertuzumab before
T-DM1 | Absent (R) vs.
present | 1.153 | 0.656–2.026 | 0.620 |
|
|
|
| Line of T-DM1 | <3 (R) vs.
≥3 | 1.082 | 0.752–1.557 | 0.671 |
|
|
|
|
Thrombocytopenia | No (R) vs. yes | 0.300 | 0.202–0.445 | <0.001 | 0.297 | 0.198–0.446 |
<0.001a |
| AST increased | No (R) vs. yes | 0.963 | 0.667–1.390 | 0.841 |
|
|
|
| ALT increased | No (R) vs. yes | 1.149 | 0.796–1.659 | 0.458 |
|
|
|
|
Hyperbilirubinemia | No (R) vs. yes | 0.736 | 0.404–1.341 | 0.316 |
|
|
|
| Hypokalemia | No (R) vs. yes | 0.928 | 0.520–1.656 | 0.928 |
|
|
|
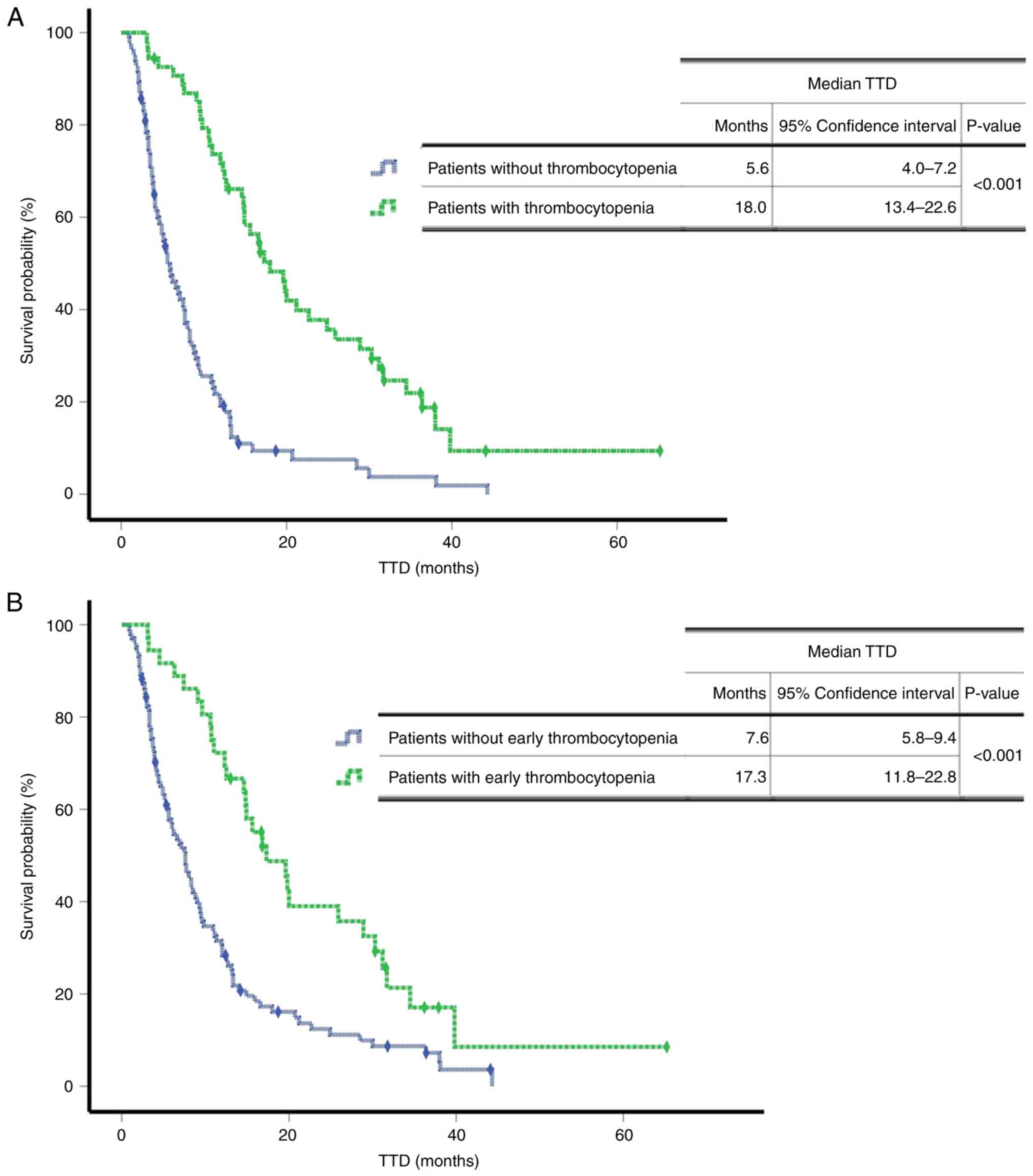
Fig. 3 presents the
TTD survival curves according to thrombocytopenia. Patients with
thrombocytopenia during treatment had a longer TTD (P<0.001)
(Fig. 3A). As a longer duration of
T-DM1 treatment was associated with a high incidence of
thrombocytopenia, a further analysis was performed to evaluate
whether thrombocytopenia was predictive of a prolonged survival
time. A comparison of the survival rates between patients with and
without early thrombocytopenia using a log-rank test revealed that
patients with early thrombocytopenia had a significantly longer TTD
of 17.3 months (95% CI, 11.8–22.8) compared with 7.6 months (95%
CI, 5.8–9.4) for patients without early thrombocytopenia
(P<0.001) (Fig. 3B).
Discussion
The present study evaluated the factors affecting
survival time during T-DM1 treatment. It was revealed that
increased survival time was associated with low ECOG performance
scores, negative hormone receptor status and thrombocytopenia.
Furthermore, thrombocytopenia that developed in the first four
T-DM1 cycles was predictive of a longer TTD in T-DM1 treatment.
Thrombocytopenia has been identified as a
characteristic of T-DM1 and as the most common grade ≥3 and
dose-limiting adverse event in both clinical trials (10–12)
and real-life studies (17,24,25).
To the best of our knowledge, only two reports in the literature
have studied the predictive value of thrombocytopenia during T-DM1
treatment. Tataroglu et al (16) reported that a multivariate Cox
regression analysis of 78 patients demonstrated that patients with
thrombocytopenia had longer progression-free survival (PFS)
compared with those without thrombocytopenia, consistent with the
results of the present study. In the second study, which evaluated
73 patients, Tang et al (15) proposed a toxicity score including
thrombocytopenia and hepatitis. It was revealed that an increased
toxicity score was associated with an improved response and
prolonged PFS. However, Yardley et al (17) observed that the incidence of
thrombocytopenia increased with T-DM1 cycles, and thrombocytopenia
was more often observed in patients receiving >18 cycles of
T-DM1 compared with in those receiving ≤18 cycles.
Considering that the incidence of thrombocytopenia
increases with the duration of T-DM1 treatment, it is a matter of
debate whether thrombocytopenia is an inevitable adverse event
secondary to long-term use or is a predictive marker for improved
survival rates. To investigate this issue, the survival rates of
patients with thrombocytopenia in the first four T-DM1 cycles were
compared with those without thrombocytopenia in the first four
cycles after thrombocytopenia was confirmed as a significant
independent factor for survival rate using a multivariate analysis.
It was revealed that patients with early thrombocytopenia exhibited
improved survival rates compared with those without early
thrombocytopenia (17.3 vs. 7.6 months, respectively; P<0.001).
Therefore, it was hypothesized that thrombocytopenia may be a
predictive marker of TTD in treatment with T-DM1, considering that,
in previous studies, the majority of instances of grade ≥3
thrombocytopenia had occurred within the first 42 days (4,26) and
that 70% of total dose reductions due to adverse events had
occurred within the first 4 months (27).
Experimental studies evaluating the potential
mechanisms of thrombocytopenia have demonstrated that, rather than
exerting a direct effect on platelets, T-DM1 inhibits the
proliferation and differentiation of proplatelet precursors by
disrupting microtubules after the uptake of megakaryocytes via
micropinocytosis (28,29). Tang et al (15) hypothesized that emtansine molecules
released from lysed HER2+ tumor cells after the
administration of T-DM1 enter the systemic circulation and cause
adverse events, such as hepatitis and thrombocytopenia, explaining
the association between efficacy and adverse events. The findings
of the present study and the high incidence of adverse effects in
the first cycles of treatment, that is, during the period when the
tumor burden was highest, are in agreement with this hypothesis,
but it should be confirmed by further studies.
Treatment beyond radiological progression with
targeted anticancer therapies, including tyrosine kinase
inhibitors, anti-vascular endothelial growth factor and checkpoint
inhibitors, has been studied in various solid organ malignancies,
such as lung cancer, renal cell carcinoma and melanoma (30–37).
These studies indicate that the continuation of these agents after
progression could contribute to increased survival rates,
especially in selected patients, and the significance of predictive
markers in this regard has been observed. The unique adverse events
of numerous targeted agents that occur during treatment have been
associated with improved treatment responses and prolonged survival
times (38–41). In this context, it is hypothesized
that thrombocytopenia may be used to select patients to continue
T-DM1 treatment in a post-progression setting after the predictive
value is confirmed in large scale studies.
Other factors affecting TTD were the ECOG
performance score and hormone receptor status. A low ECOG
performance score was associated with improved survival outcomes,
consistent with previous reports (16,42,43).
It is hypothesized that the significantly shorter TTD of patients
with a hormone receptor-positive status in the present study was
associated with the preference of clinicians to discontinue T-DM1
and add endocrine therapy after radiological progression in these
patients.
The main strength of the present study was the use
of TTD instead of PFS as the endpoint. TTD was reported to inform
the clinician regarding the continuation of an anticancer agent
after objective progression, which is common in oncology practice,
particularly for targeted agents, and has a high association with
PFS, which is an objective evaluation (44). In addition, the present study
included a relatively high number of patients compared with the
aforementioned studies on the predictive value of thrombocytopenia.
However, the present study also had limitations, including its
retrospective design and the lack of toxicity data other than
laboratory findings. Furthermore, factors impacting
thrombocytopenia could not be analyzed due to a lack of associated
data, such as pretreatment platelet counts, which have been
identified as a strong predictor for thrombocytopenia during T-DM1
treatment (26). Moreover, tumor
burden, one of the aspects affecting response in cancer treatments
(45), could not be assessed due to
the retrospective and multicenter design of the present study.
In conclusion, the patients that experienced
thrombocytopenia in the first four cycles of T-DM1 treatment had a
longer TTD compared with those without thrombocytopenia. Thus,
future prospective studies and large-scale cohorts should aim to
confirm the predictive role of thrombocytopenia for improved
survival rates in order to maximize the potential benefit of T-DM1
treatment.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
EC, TE and AS designed the study. The data were
collected by AS, BC, BO, SO, AD, BH, IS, AG, MC and HO. GO and AS
performed the data analysis. AS, TE, EC, AD and IS conducted the
literature review. AS wrote the manuscript. BC, BO, BH, AG, HO and
SO contributed significantly to the writing of the manuscript. TE,
EC, AD and IS revised the manuscript critically for important
intellectual content. AS, BC, BO, SO, AD, AG, HO, BH and MC confirm
the authenticity of all the raw data. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
The study was conducted in accordance with the
ethical standards of the institutional research committee and the
1964 Declaration of Helsinki. The Clinical Research Ethics
Committee of Uludag University Faculty of Medicine approved the
study (Bursa, Turkey; approval no. 2022-18/46), waiving the need
for informed consent due to the retrospective nature of the present
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Noone AM, Cronin KA, Altekruse SF,
Howlader N, Lewis DR, Petkov VI and Penberthy L: Cancer incidence
and survival trends by subtype using data from the surveillance
epidemiology and end results program, 1992–2013. Cancer Epidemiol
Biomarkers Prev. 26:632–641. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ross JS, Slodkowska EA, Symmans WF,
Pusztai L, Ravdin PM and Hortobagyi GN: The HER-2 receptor and
breast cancer: Ten years of targeted anti-HER-2 therapy and
personalized medicine. Oncologist. 14:320–368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Verma S, Miles D, Gianni L, Krop IE,
Welslau M, Baselga J, Pegram M, Oh DY, Diéras V, Guardino E, et al:
Trastuzumab emtansine for HER2-positive advanced breast cancer. N
Engl J Med. 367:1783–1791. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fu Z, Li S, Han S, Shi C and Zhang Y:
Antibody drug conjugate: The ‘biological missile’ for targeted
cancer therapy. Signal Transduct Target Ther. 7:932022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krop IE, Kim SB, González-Martín A,
LoRusso PM, Ferrero JM, Smitt M, Yu R, Leung AC and Wildiers H;
TH3RESA study collaborators, : Trastuzumab emtansine versus
treatment of physician's choice for pretreated HER2-positive
advanced breast cancer (TH3RESA): A randomised, open-label, phase 3
trial. Lancet Oncol. 15:689–699. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Perez EA, Barrios C, Eiermann W, Toi M, Im
YH, Conte P, Martin M, Pienkowski T, Pivot XB, Burris HA III, et
al: Trastuzumab emtansine with or without pertuzumab versus
trastuzumab with taxane for human epidermal growth factor receptor
2-positive advanced breast cancer: Final results from MARIANNE.
Cancer. 125:3974–3984. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gennari A, André F, Barrios CH, Cortés J,
de Azambuja E, DeMichele A, Dent R, Fenlon D, Gligorov J, Hurvitz
SA, et al: ESMO clinical practice guideline for the diagnosis,
staging and treatment of patients with metastatic breast cancer.
Ann Oncol. 32:1475–1495. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
National Comprehensive Cancer Network, .
Breast cancer version 4.2022, 2022. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdfAugust
31–2022
|
|
10
|
Diéras V, Miles D, Verma S, Pegram M,
Welslau M, Baselga J, Krop IE, Blackwell K, Hoersch S, Xu J, et al:
Trastuzumab emtansine versus capecitabine plus lapatinib in
patients with previously treated HER2-positive advanced breast
cancer (EMILIA): A descriptive analysis of final overall survival
results from a randomised, open-label, phase 3 trial. Lancet Oncol.
18:732–742. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krop IE, Kim SB, Martin AG, LoRusso PM,
Ferrero JM, Badovinac-Crnjevic T, Hoersch S, Smitt M and Wildiers
H: Trastuzumab emtansine versus treatment of physician's choice in
patients with previously treated HER2-positive metastatic breast
cancer (TH3RESA): Final overall survival results from a randomised
open-label phase 3 trial. Lancet Oncol. 18:743–754. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Montemurro F, Ellis P, Anton A, Wuerstlein
R, Delaloge S, Bonneterre J, Quenel-Tueux N, Linn SC, Irahara N,
Donica M, et al: Safety of trastuzumab emtansine (T-DM1) in
patients with HER2-positive advanced breast cancer: Primary results
from the KAMILLA study cohort 1. Eur J Cancer. 109:92–102. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu F, Ke J and Song Y: T-DM1-induced
thrombocytopenia in breast cancer patients: New perspectives.
Biomed Pharmacother. 129:1104072020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Girish S, Gupta M, Wang B, Lu D, Krop IE,
Vogel CL, Burris Iii HA, LoRusso PM, Yi JH, Saad O, et al: Clinical
pharmacology of trastuzumab emtansine (T-DM1): An antibody-drug
conjugate in development for the treatment of HER2-positive cancer.
Cancer Chemother Pharmacol. 69:1229–1240. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang SC, Capra CL, Ajebo GH, Meza-Junco J,
Mairs S, Craft BS, Zhu X, Maihle N and Hillegass WB: Systemic
toxicities of trastuzumab-emtansine predict tumor response in HER2+
metastatic breast cancer. Int J Cancer. 149:909–916. 2021.(Epub
ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tataroglu Ozyukseler D, Basak M, Ay S,
Koseoglu A, Arıcı S, Oyman A, Sürmeli H, Turan M, Turan N, Odabaş H
and E Yıldırım M: Prognostic factors of ado-trastuzumab emtansine
treatment in patients with metastatic HER-2 positive breast cancer.
J Oncol Pharm Pract. 27:547–554. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yardley DA, Krop IE, LoRusso PM, Mayer M,
Barnett B, Yoo B and Perez EA: Trastuzumab emtansine (T-DM1) in
patients With HER2-positive metastatic breast cancer previously
treated with chemotherapy and 2 or more HER2-targeted agents:
Results from the T-PAS expanded access study. Cancer J. 21:357–364.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
U.S. Food and Drug Administration (FDA), .
FDA approves ado-trastuzumab emtansine for early breast cancer.
FDA, Silver Spring, MD. 2019.https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ado-trastuzumab-emtansine-early-breast-cancer
|
|
19
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Allison KH, Hammond MEH, Dowsett M,
McKernin SE, Carey LA, Fitzgibbons PL, Hayes DF, Lakhani SR,
Chavez-MacGregor M, Perlmutter J, et al: Estrogen and progesterone
receptor testing in breast cancer: ASCO/CAP guideline update. J
Clin Oncol. 38:1346–1366. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wolff AC, Hammond MEH, Allison KH, Harvey
BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P,
Hanna W, et al: Human epidermal growth factor receptor 2 testing in
breast cancer: American society of clinical oncology/college of
American pathologists clinical practice guideline focused update.
Arch Pathol Lab Med. 142:1364–1382. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
NCI, . Common terminology criteria for
adverse events v5.0, 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5×11.pdfApril
19–2020
|
|
24
|
Fabi A, De Laurentiis M, Caruso M, Valle
E, Moscetti L, Santini D, Cannita K, Carbognin L, Ciccarese M,
Rossello R, et al: Efficacy and safety of T-DM1 in the
‘common-practice’ of HER2+ advanced breast cancer setting: A
multicenter study. Oncotarget. 8:64481–64489. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yeo W, Luk MY, Soong IS, Yuen TY, Ng TY,
Mo FK, Chan K, Wong SY, Tsang J, Leung C, et al: Efficacy and
tolerability of trastuzumab emtansine in advanced human epidermal
growth factor receptor 2-positive breast cancer. Hong Kong Med J.
24:56–62. 2018.PubMed/NCBI
|
|
26
|
Modi ND, Sorich MJ, Rowland A, McKinnon
RA, Koczwara B, Wiese MD and Hopkins AM: Predicting
thrombocytopenia in patients with breast cancer treated with
ado-trastuzumab emtansine. Clin Breast Cancer. 20:e220–e228. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang E, Rowland A, McKinnon RA, Sorich MJ
and Hopkins AM: Effect of early adverse events resulting in
ado-trastuzumab emtansine dose adjustments on survival outcomes of
HER2+ advanced breast cancer patients. Breast Cancer Res Treat.
178:473–477. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thon JN, Devine MT, Begonja AJ, Tibbitts J
and Italiano JE Jr: High-content live-cell imaging assay used to
establish mechanism of trastuzumab emtansine (T-DM1)-mediated
inhibition of platelet production. Blood. 120:1975–1984. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao H, Gulesserian S, Ganesan SK, Ou J,
Morrison K, Zeng Z, Robles V, Snyder J, Do L, Aviña H, et al:
Inhibition of megakaryocyte differentiation by antibody-drug
conjugates (ADCs) is mediated by macropinocytosis: Implications for
ADC-induced thrombocytopenia. Mol Cancer Ther. 16:1877–1886. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park K, Yu CJ, Kim SW, Lin MC, Sriuranpong
V, Tsai CM, Lee JS, Kang JH, Chan KC, Perez-Moreno P, et al:
First-line erlotinib therapy until and beyond response evaluation
criteria in solid tumors progression in asian patients with
epidermal growth factor receptor mutation-positive non-small-cell
lung cancer: The ASPIRATION study. JAMA Oncol. 2:305–312. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Haddad R, Concha-Benavente F, Blumenschein
G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Kasper S, Vokes
EE, Worden F, et al: Nivolumab treatment beyond RECIST-defined
progression in recurrent or metastatic squamous cell carcinoma of
the head and neck in CheckMate 141: A subgroup analysis of a
randomized phase 3 clinical trial. Cancer. 125:3208–3218. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Goto Y, Tanai C, Yoh K, Hosomi Y, Sakai H,
Kato T, Kaburagi T, Nishio M, Kim YH, Inoue A, et al: Continuing
EGFR-TKI beyond radiological progression in patients with advanced
or recurrent, EGFR mutation-positive non-small-cell lung cancer: An
observational study. ESMO Open. 2:e0002142017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Escudier B, Motzer RJ, Sharma P, Wagstaff
J, Plimack ER, Hammers HJ, Donskov F, Gurney H, Sosman JA, Zalewski
PG, et al: Treatment beyond progression in patients with advanced
renal cell carcinoma treated with nivolumab in CheckMate 025. Eur
Urol. 72:368–376. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ge X, Zhang Z, Zhang S, Yuan F, Zhang F,
Yan X, Han X, Ma J, Wang L, Tao H, et al: Immunotherapy beyond
progression in patients with advanced non-small cell lung cancer.
Transl Lung Cancer Res. 9:2391–2400. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Czarnecka AM, Sobczuk P, Rogala P, Świtaj
T, Placzke J, Kozak K, Mariuk-Jarema A, Spałek M, Dudzisz-Śledź M,
Teterycz P, et al: Efficacy of immunotherapy beyond RECIST
progression in advanced melanoma: A real-world evidence. Cancer
Immunol Immunother. 71:1949–1958. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bennouna J, Sastre J, Arnold D, Österlund
P, Greil R, Van Cutsem E, von Moos R, Viéitez JM, Bouché O, Borg C,
et al: Continuation of bevacizumab after first progression in
metastatic colorectal cancer (ML18147): A randomised phase 3 trial.
Lancet Oncol. 14:29–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Guven DC, Yekeduz E, Erul E, Yazgan SC,
Sahin TK, Karatas G, Aksoy S, Erman M, Yalcin S, Urun Y and
Kilickap S: The benefit of treatment beyond progression with immune
checkpoint inhibitors: A multi-center retrospective cohort study. J
Cancer Res Clin Oncol. 149:3599–3606. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu Y, Zhou L, Chen Y, Liao B, Ye D, Wang
K and Li H: Hypertension as a prognostic factor in metastatic renal
cell carcinoma treated with tyrosine kinase inhibitors: A
systematic review and meta-analysis. BMC Urol. 19:492019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ou SHI, Tong WP, Azada M, Siwak-Tapp C, Dy
J and Stiber JA: Heart rate decrease during crizotinib treatment
and potential correlation to clinical response. Cancer.
119:1969–1975. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bar-Ad V, Zhang QE, Harari PM, Axelrod R,
Rosenthal DI, Trotti A, Jones CU, Garden AS, Song G, Foote RL, et
al: Correlation between the severity of cetuximab-induced skin rash
and clinical outcome for head and neck cancer patients: The RTOG
experience. Int J Radiat Oncol Biol Phys. 95:pp1346–1354. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Holch JW, Held S, Stintzing S, Fischer von
Weikersthal L, Decker T, Kiani A, Kaiser F, Heintges T, Kahl C,
Kullmann F, et al: Relation of cetuximab-induced skin toxicity and
early tumor shrinkage in metastatic colorectal cancer patients:
Results of the randomized phase 3 trial FIRE-3 (AIO KRK0306). Ann
Oncol. 31:72–78. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Vici P, Pizzuti L, Michelotti A, Sperduti
I, Natoli C, Mentuccia L, Di Lauro L, Sergi D, Marchetti P, Santini
D, et al: A retrospective multicentric observational study of
trastuzumab emtansine in HER2 positive metastatic breast cancer: A
real-world experience. Oncotarget. 8:56921–56931. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hopkins AM, Rowland A, Logan JM and Sorich
MJ: Primary predictors of survival outcomes for HER2-positive
advanced breast cancer patients initiating ado-trastuzumab
emtansine. Breast. 46:90–94. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Blumenthal GM, Gong Y, Kehl K,
Mishra-Kalyani P, Goldberg KB, Khozin S, Kluetz PG, Oxnard GR and
Pazdur R: Analysis of time-to-treatment discontinuation of targeted
therapy, immunotherapy, and chemotherapy in clinical trials of
patients with non-small-cell lung cancer. Ann Oncol. 30:830–838.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dall'Olio FG, Marabelle A, Caramella C,
Garcia C, Aldea M, Chaput N, Robert C and Besse B: Tumour burden
and efficacy of immune-checkpoint inhibitors. Nat Rev Clin Oncol.
19:75–90. 2022. View Article : Google Scholar : PubMed/NCBI
|