Introduction
Renal cell carcinoma (RCC) is the most common solid
lesion within the kidney and accounts for ~90% of all kidney
malignancies. There is a 1.5:1 predominance for men over women and
the peak incidence of RCC is at 60–70 years of age (1). RCC comprises different subtypes with
specific histopathological and genetic characteristics, of which
clear cell RCC (ccRCC) is the predominant pathological type,
accounting for nearly 80% of all RCC cases (2). ccRCC is the most aggressive RCC
subtype with high metastasis, chemotherapy and radiotherapy
resistance, and poor prognosis (3,4). The
most common metastasis site of ccRCC is the lung, followed by
regional lymph nodes, bone, liver and brain (5). Metastasis to the head and neck is less
frequent; furthermore, thyroid metastasis is rare (6). ccRCC has previously been reported to
metastasize to normal thyroid tissue (7) or benign thyroid tumors (8). However, thyroid metastasis of ccRCC
combined with papillary thyroid carcinoma (PTC) is rarely reported.
Because of the occult nature of thyroid metastasis in ccRCC, it is
easy to be misdiagnosed or missed. Therefore, preoperative
examination is important, and its exact diagnosis depends on the
intraoperative frozen section and pathological examination. The
present study, reported a case of PTC with ccRCC metastasized to
the thyroid gland.
Case report
The patient was a 55-year-old male who was admitted
to the Thyroid Surgery Department of The Affiliated Hospital of
Southwest Medical University (Luzhou, China) in February 2022 due
to the presence of thyroid nodules. In April 2018, the patient
underwent radical resection of the left kidney for ccRCC at Xinqiao
Hospital, Third Military Medical University (Chongqing, China).
After the operation, the pathological examination results suggested
the following: Renal clear cell carcinoma, pT1aN0M0, the tumor
sized ~4.0×4×3.5 cm3, with no lymph node cancer
metastases or distant metastasis. Therefore, the patient refused
conventional adjuvant therapy after the surgery and underwent
regular physical examination follow-up. In November 2021, abdominal
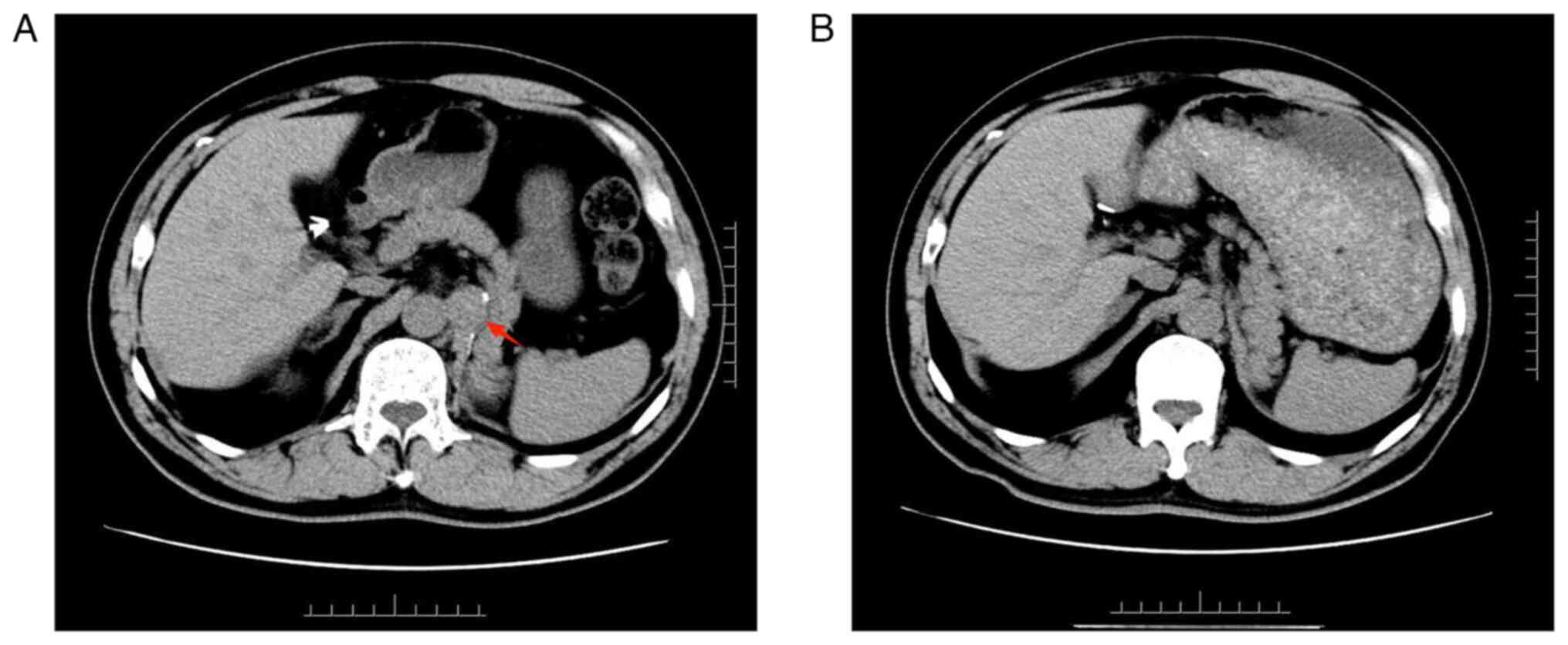
computed tomography (CT) revealed para-aortic lymph node carcinoma
metastasis (Fig. 1A), positron
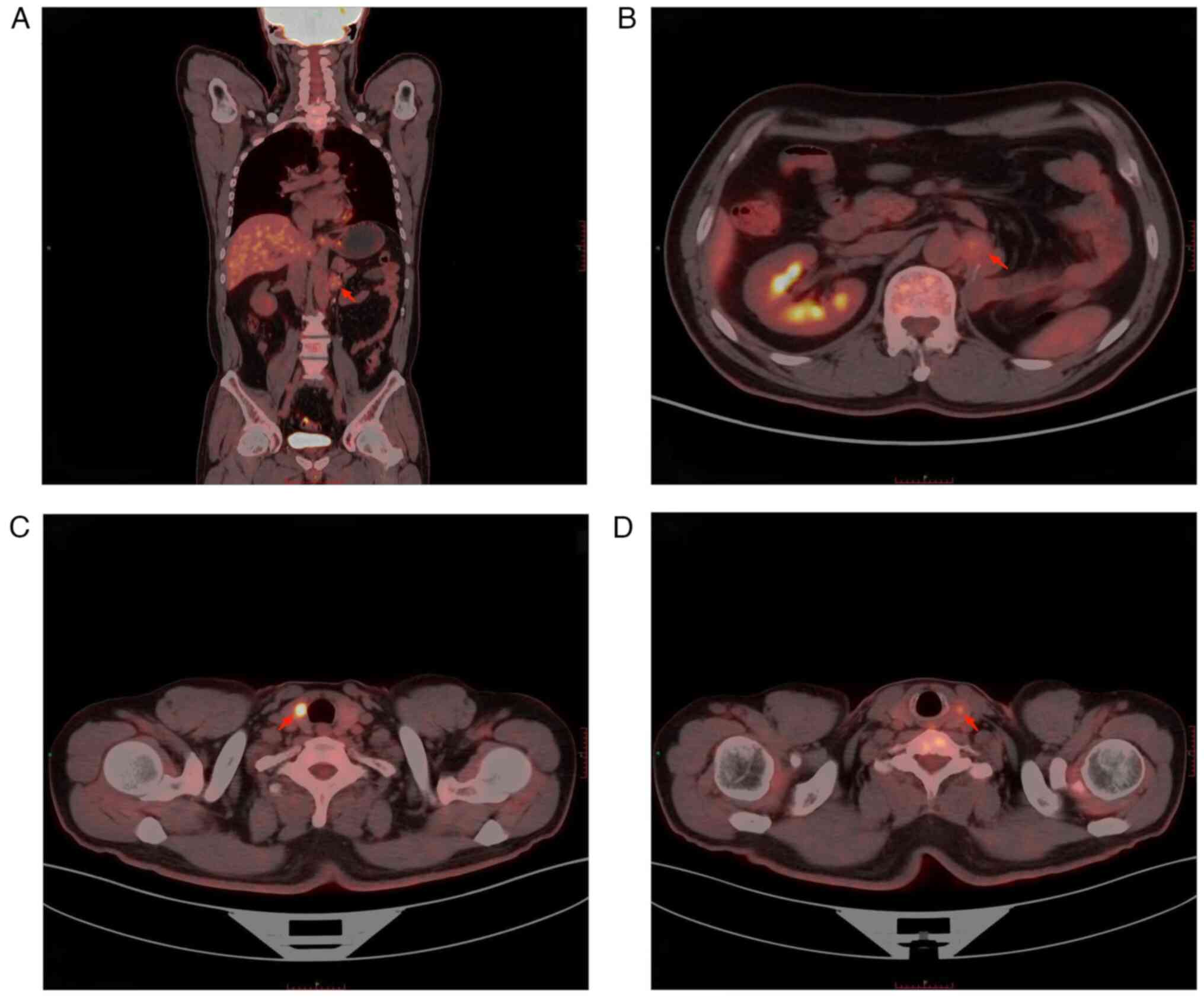
emission tomography (PET)-CT showed that the patient had a left
para-aortic nodule after left nephrectomy and furthermore, glucose
metabolism was slightly increased, and the nodule was suspected to
be lymph node metastasis (Fig. 2A and
B). Bilateral thyroid nodules and increased glucose metabolism
were considered to indicate thyroid cancer (Fig. 2C and D), and the patient underwent
laparoscopic retroperitoneal mass resection. Postoperative
pathological examination suggested lymph node metastasis of ccRCC.
Postoperative review via enhanced abdominal CT indicated complete
resection of the retroperitoneal metastases (Fig. 1B). In November 2021, thyroid
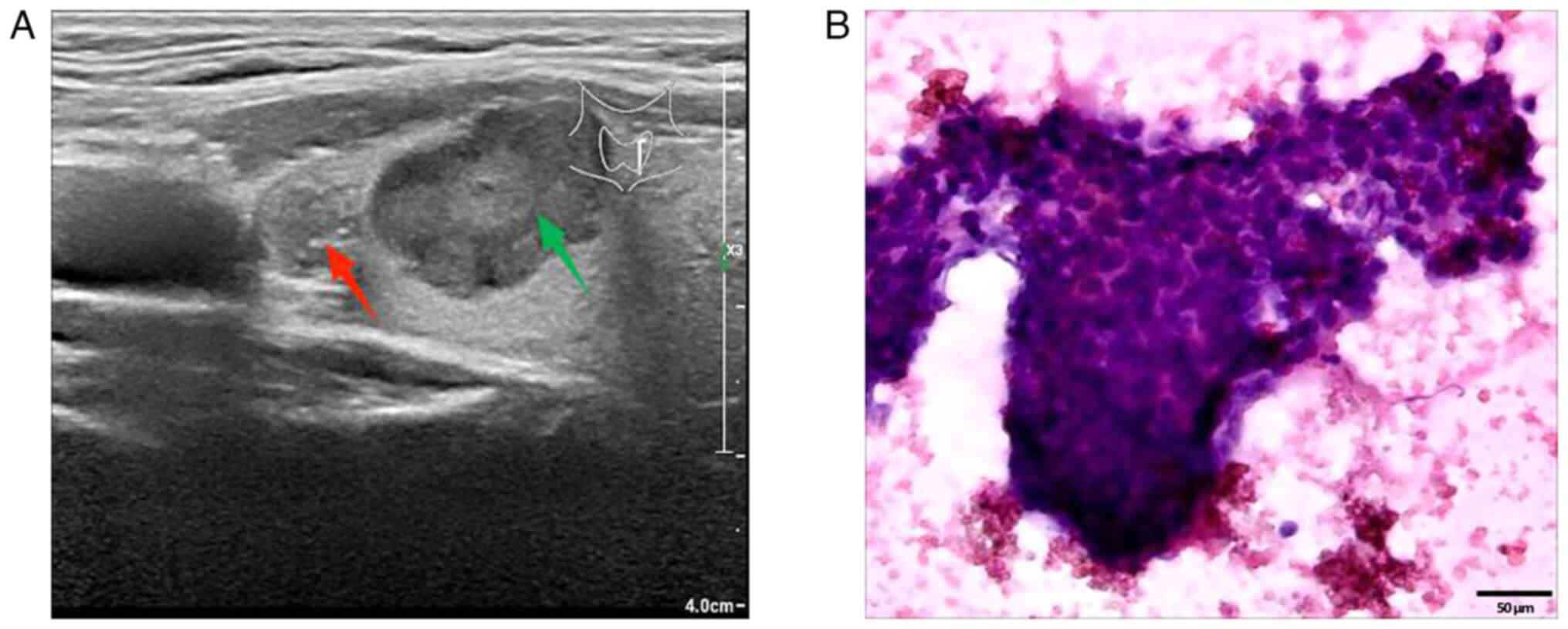
ultrasound Doppler revealed hypoechoic nodules in the left thyroid
gland, resembling Chinese-Thyroid Imaging Reporting and Data System
(C-TIRADS) 4C (nodule 1: Red arrow) and C-TIRADS 4B (nodule 2:
Green arrow) (Fig. 3A). Of note,
C-TIRADS is Chinese version of TIRADS suitable for Chinese clinical
practice modified based on thyroid ultrasound data in China. The
Chinese Medical Association Ultrasound Medical Expert Committee
modified the data system by current TIRADS and non-TIRADS risk
stratification, combined with the latest literature reported in
China and worldwide, keeping in mind the national conditions
(9). The cervical lymph node at
level VI was enlarged with an abnormal structure, with a maximum
size of 0.5×0.3 cm2. Histocytological examination
performed according to standard procedures (10) by fine-needle aspiration (FNA)
suggested PTC (nodule 1) (Fig. 3B).
As the time interval between thyroid surgery and the previous
retroperitoneal mass resection was relatively short,
contrast-enhanced CT of the head, neck, chest and abdomen was
performed prior to thyroid surgery and no obvious evidence of
metastasis was found. Therefore, PET-CT was not performed again.
However, it was recommended to the patient to repeat the PET-CT for
the systemic assessment at a later follow-up visit. Based on the
abovementioned examination results, papillary carcinoma of the left
thyroid gland was suspected before surgery and surgical resection
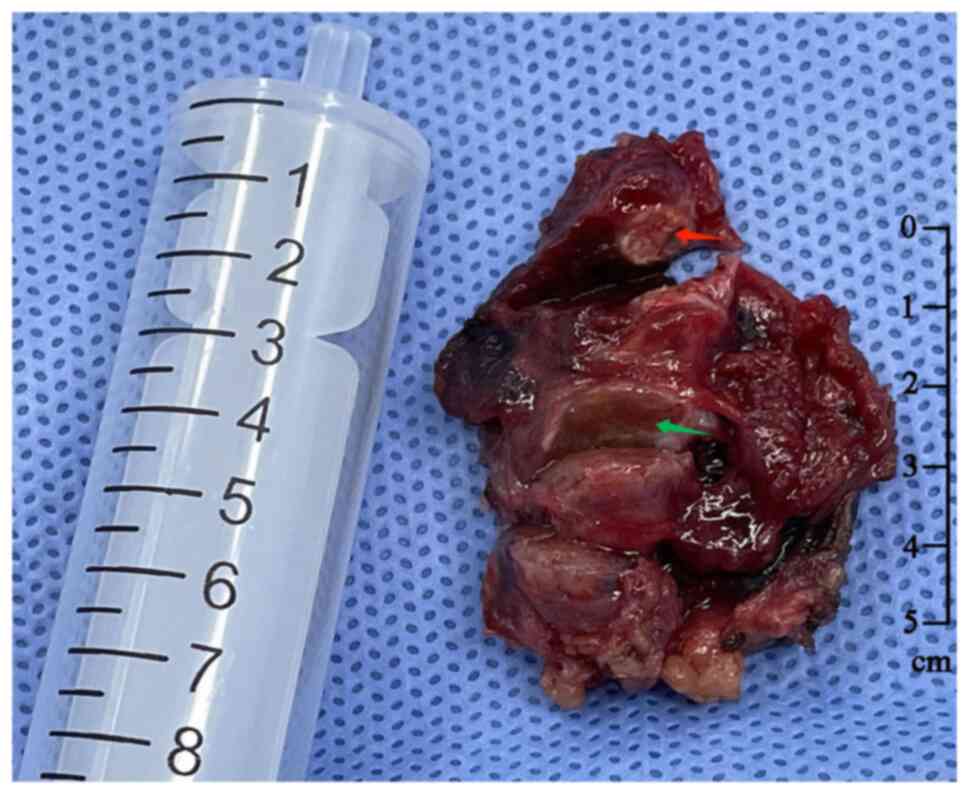
was performed in February 2022. During surgery, two gray hard
masses sized ~0.4×0.3×0.3 cm3 (nodule 1: red arrow) and
1.3×1.3×1.2 cm3 (nodule 2: Green arrow) were found in
the left thyroid gland close to the capsule, with an unclear
boundary (Fig. 4), and the distance
between the two tumors was ~1 cm. Intraoperative frozen section
analysis showed that the nodules were neoplastic, with capsule
invasion and suspected vascular invasion, and were suggestive of
follicular or medullary carcinoma. Accordingly, the nature of the
tumor could not be determined as PTC, follicular carcinoma or
medullary carcinoma based on FNA and intraoperative frozen section
analysis results of the thyroid nodule alone. According to the 2021
Chinese Society of Clinical Oncology differentiated thyroid cancer
guidelines (11) and the revised
American Thyroid Association guidelines for the diagnosis and
treatment of medullary thyroid cancer (12), the patient then underwent total
thyroidectomy and bilateral central lymph node dissection. The
histopathological examination results were as follows: Left lobe
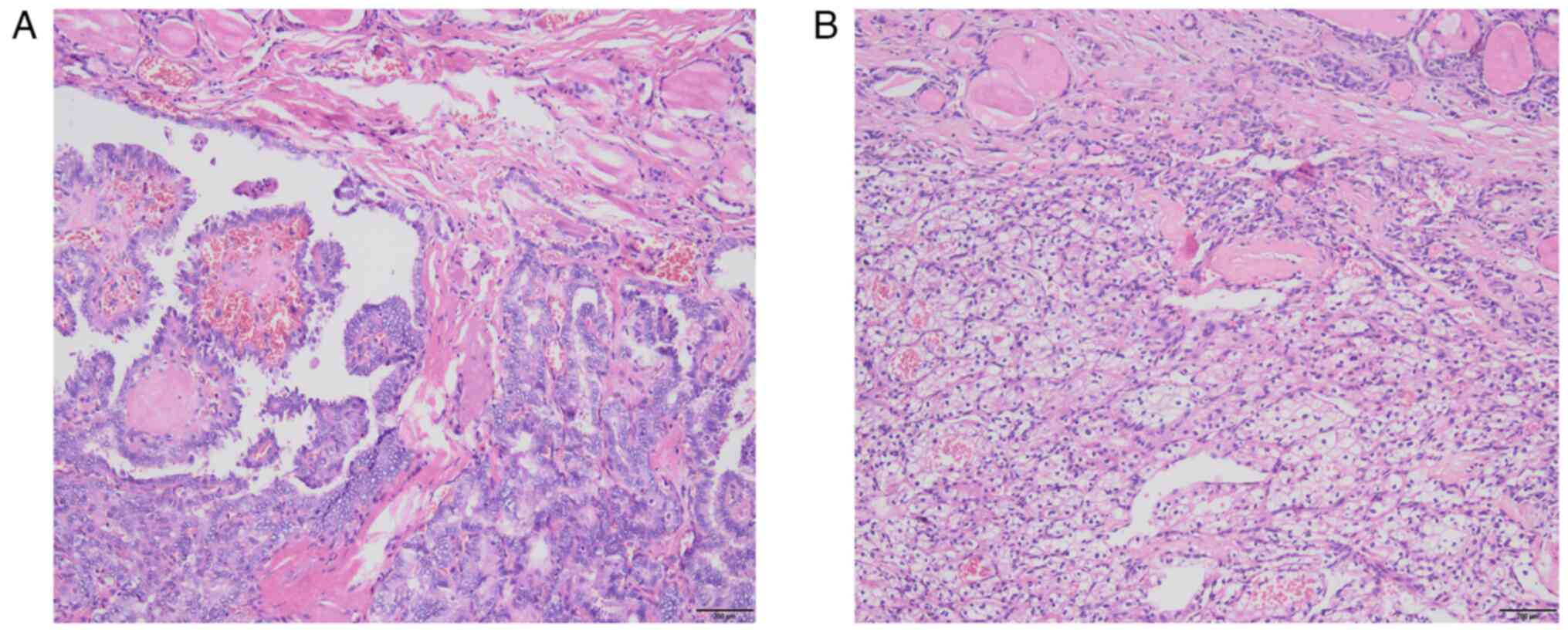
thyroid tumor (two nodules), nodule 1: The nodule composed of
partly follicles and partly papillary structures lined by tumor
cells with enlarged, crowded and overlapping nuclei; the tumor
cells showed nuclear furrows and prominent nucleoli, and certain
nuclei had a ground-glass appearance (Fig. 5A); nodule 2: The tumor cells were
arranged in nests and sheets, with a large volume, clear cytoplasm,
round and centered nuclei and no obvious nucleoli; abundant blood
vessels were seen in the background of the tumor (Fig. 5B); follicular adenoma of the right
lobe of the thyroid and reactive hyperplasia of bilateral central
lymph nodes were observed. Immunohistochemistry (dewaxing of
paraffin sections using xylene and descending ethanol 5 min → 100%
ethanol 5 min → 90% ethanol 5 min → 80% ethanol 5 min 70% ethanol 5
min → PBS buffer rinse three times, 5 min each time. 2. Antigen
repair: EDTA repair solution (PH=9.0), high-pressure repair, and
steam was added for 6 min; Citric acid buffer (PH=6.0) was used for
repair under high pressure, and steam was added for 3 min. The
sample was naturally cooled to room temperature. 3. Further, 3.3%
methanol H2O2 was soaked for 10 min to
eliminate the endogenous peroxidase activity, and PBS buffer was
used to rinse three times (3 min each time). 4. The primary
antibody was added and incubated at 37°C for 60 min; followed by
washing with PBS three times, each time for 3 min. The secondary
antibody (MaxVision™ 2/HRP) was then added and incubates at 37°C
for 30 min; followed by washing with PBS three times, each time for
3 min. DAB color development was performed at room temperature for
0.5–1 min; the process was controlled using a microscope, and the
sample was washed with tap water to stop color development. 7.
Rinsing with running water for 5 min. 8. Re-staining with
hematoxylin for 2 min. 9. Further, differentiation was performed
using 0.1% diluted hydrochloric acid, and saturated lithium
carbonate turned blue. 10. Dehydration, transparency, and sealing:
95% ethanol (l) 1 min → 95% ethanol (l) 5 min → 100% ethanol (l) 5
min → 100% ethanol (II) 5 min → xylene 2 min → neutral gum resin
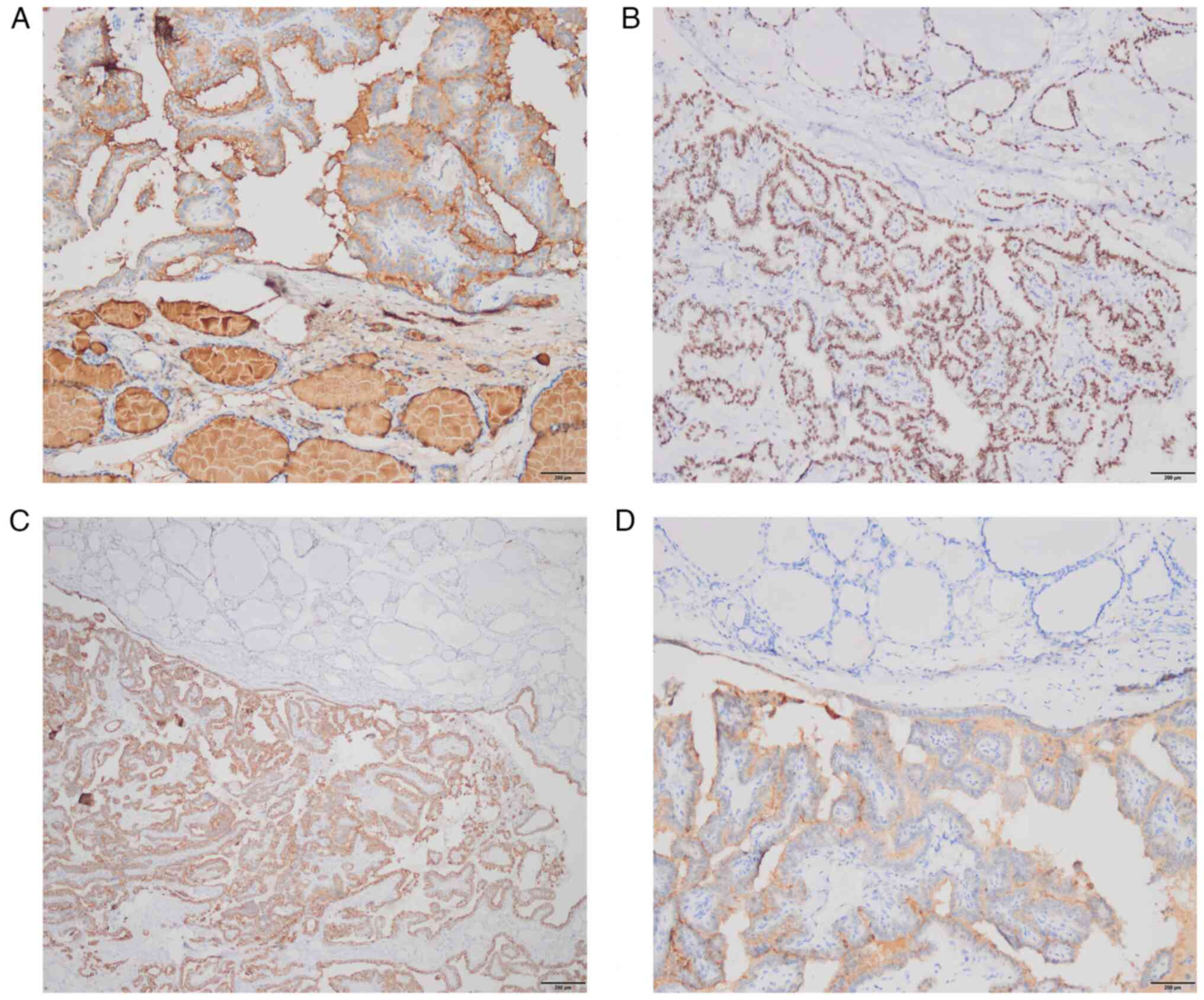
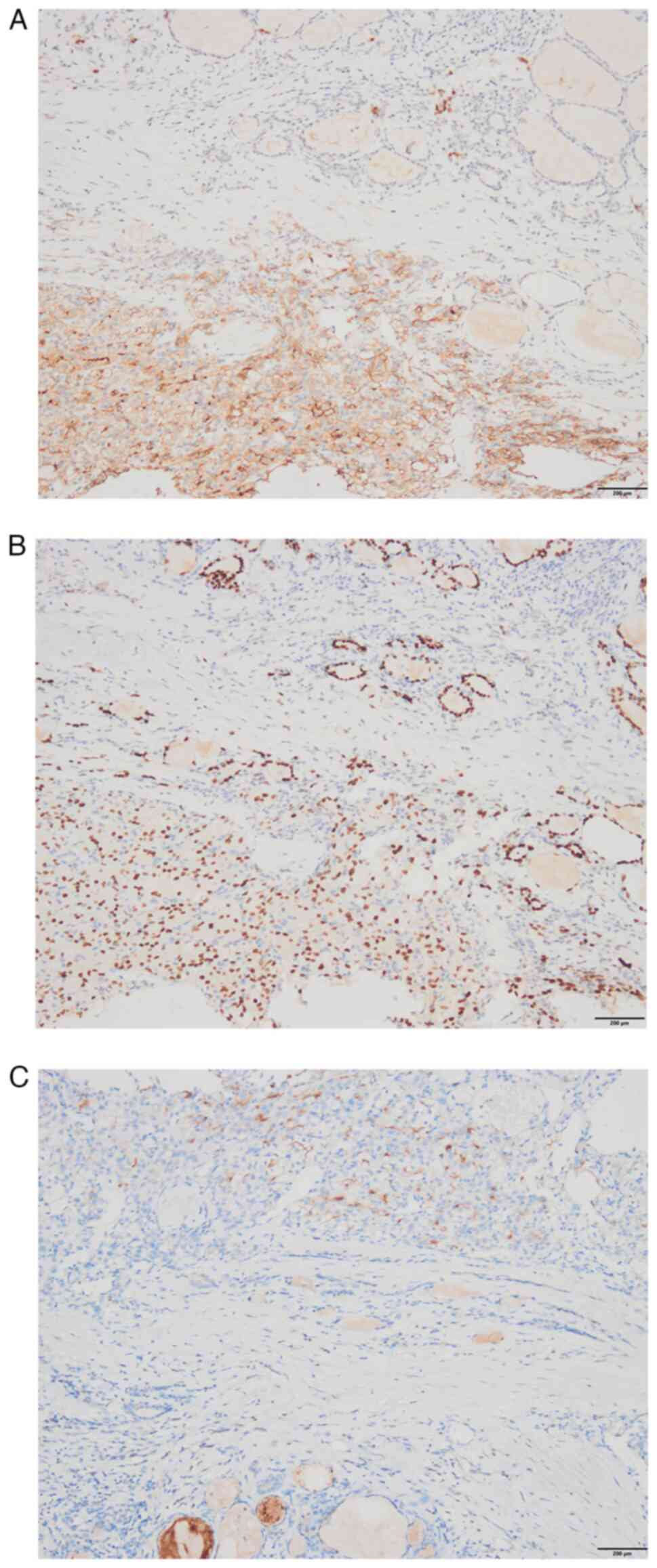
sealing.) revealed the following: Nodule 1: The tumor cell
component was immunoreactive to thyroglobulin (TG), thyroid
transcription factor-1 (TTF-1), cytokeratin 19 (CK19) and
galectin-3 (Fig. 6A-D), but common
acute lymphocyte leukemia antigen (CD10) and renal cell carcinoma
marker (RCC) staining were negative (data not shown). Nodule 2: The
clear cell component showed immunopositivity for CD10, paired box
gene 8 (PAX8) and RCC (Fig. 7A-C),
but TG and TTF-1 staining were negative (data not shown). In brief,
consecutive parallel sections were stained with the following
antibodies according to the manufacturers' recommendations: TG
(cat. no. MAB-0797), TTF-1 (mouse anti-human mAb; cat. no.
MAB-0677; Maixin Fuzhou), CK19 (mouse anti-human mAb; cat. no.
MAB-0829), galectin-3 (cat. no. MAB-0835; Maixin Fuzhou), CD10
(cat. no.MAB-0668), PAX8 (cat. no. MAB-0837; Maixin Fuzhou), RCC
(all mouse anti-human mAb; cat. no. MAB-0309; all Maixin Fuzhou).
The secondary antibody was MaxVision™ 2 plus polymer HRP
(mouse/rabbit) IHC Kit (cat. no. KIT-5930; Maixin Fuzhou). However,
the absence of quantitative results for these experiments is a
limitation of the present study. On postoperative day 1, the
parathyroid hormone level was 5.14 pg/ml (reference range, 8.7–79.6
pg/ml) and the blood calcium concentration was 1.98 mmol/l
(reference range, 2.11–2.52 mmol/l). The patient presented with
fingertip and perioral numbness, which may be caused by transient
hypocalcemia resulting from impaired parathyroid blood supply.
Treatment with calcium supplementation was provided, and the
parathyroid hormone and blood calcium concentration were reexamined
on postoperative day 3. The parathyroid hormone levels were 10.4
pg/ml and the blood calcium concentration was 2.36 mmol/l. The
patient did not show any hypocalcemia again. Levothyroxine (100 mg
qd) was administered on postoperative day 2. Following thyroid
surgery, multidisciplinary consultations were acquired from the
Departments of Urology, Oncology and Thyroid Surgery, and other
disciplines. This patient had undergone surgical resection and was
treated with sunitinib (50 mg qd) for 2 cycles (taking sunitinib
for four weeks per cycle followed by a two-week interval). The
patient was followed up for 14 months and followed a good diet and
having a good sleep, and normal thyroid function and no new
metastasis was observed. Hereafter, the patient will be followed up
every 3 months and the results will be reported.
Discussion
The thyroid is an organ with the most adequate blood
supply in the body; however, the incidence of thyroid metastasis is
rare and can hardly be detected accurately during clinical and
pathological examination. Thyroid metastasis accounts for 0.36–2.1%
of all thyroid malignant tumors (13), which may be related to the occult
nature of thyroid metastasis. In the present case, based on the
results of preoperative thyroid ultrasound, FNA and intraoperative
frozen section analysis, the patient underwent bilateral thyroid
lobectomy and bilateral central lymph node dissection. Preoperative
thyroid ultrasound, FNA and intraoperative frozen section analysis
only indicated thyroid malignancy. However, postoperative
pathological examination and IHC were suggestive of PTC with ccRCC
metastasis to the thyroid gland. The possible reasons for the
abovementioned misdiagnosis and missed diagnosis are as follows.
First, metastatic thyroid cancer often lacks typical clinical
symptoms. Furthermore, doctors have an insufficient understanding
of carcinoma metastasis to the thyroid. Accordingly, if
preoperative thyroid ultrasound is indicative of typical
characteristics of PTC and FNA also suggests PTC, the doctor will
conclude the diagnosis as PTC and may not consider other tumors. In
addition, carcinoma metastasis to the thyroid is not readily
discernable before surgery.
Thyroid metastases are usually associated with other
organs and lymph nodes. Chun et al (14) showed that 59.7% of patients with
carcinoma metastasis to the thyroid also had metastasis to other
sites. The present patient had been diagnosed ccRCC only with lymph
node metastasis, but no other organs were involved. In addition,
this patient was diagnosed with PTC during the initial diagnosis,
which was accidentally found to be accompanied with ccRCC
metastasis to the thyroid gland. The incidence of thyroid
metastasis from ccRCC is rare and the concurrent occurrence of PTC
is even rarer (6).
While the thyroid gland is a blood-rich organ, it is
a rare site of metastases (15).
RCC is one of the more common cancers to metastasize to the thyroid
gland (16). Although the mechanism
of thyroid metastasis remains elusive, most authors believe that
the abundant blood supply of the thyroid becomes a favorable factor
for thyroid metastasis (15). It
has been proposed that the thyroid gland may be more susceptible to
metastatic growth when affected by goiter, neoplasms or thyroiditis
due to metabolic changes that consist of decrements in the oxygen
and iodine content (8). Heffess
et al (17), in the largest
case series of thyroid metastasis from RCC, found pre-existing
thyroid disease in 42% of the 36 cases. Other malignant tumors
(18,19) in the abdominal cavity may also
metastasize to the thyroid gland, but the mechanism of metastasis
has remained largely elusive. In the present case, it may be
assumed that the abundant blood flow to the thyroid gland may
provide a nutritional basis for the metastasis of ccRCC. Meanwhile,
PTC may also change the metabolism of the thyroid gland and the
tumor microenvironment, thus inducing metastasis of ccRCC to the
thyroid gland. Therefore, when thyroid nodules are preliminarily
diagnosed as PTC, with ccRCC or other malignant tumors in the
abdominal cavity, it is necessary to consider whether there is a
possibility of thyroid metastatic cancer.
The diagnosis of ccRCC metastasis to the thyroid
gland is difficult, given that it is a rare metastatic disease.
Most studies (8,17,20)
report that it is difficult to make a clear diagnosis during
preoperative examination and that intraoperative freezing and
postoperative pathological examination are required, which need to
be further judged by IHC analysis. In previous reports (8,20,21),
tissue of ccRCC metastasized to the thyroid gland was found to be
positive for CD10, whereas it was negative for TG and TTF-1.
However, in the present case, the preoperative results suggested
that the nodule was PTC, and according to the IHC analysis, the PTC
tissue was positive for TG, TTF-1, CK19 and galectin-3, and the
ccRCC tissue was positive for RCC, CD10 and PAX-8. The case was
found to be ccRCC thyroid metastasis combined with PTC by IHC
analysis. Compared with previously reported cases of ccRCC
metastasized to the thyroid gland, the present case is rare;
however, previous reports (8,20,21)
have only verified part of the IHC results, and the present study
provided more comprehensive data.
The present case is different from a case of
concurrent primary ccRCC and PTC, and the perioperative management
and treatment may differ, particularly the postoperative systemic
management (15). At present, there
is no specific diagnosis and treatment plan for PTC with ccRCC
metastasis to the thyroid. However, it appears that complete
surgical resection is key in the treatment of PTC without lymph
node metastasis, and precise postoperative treatment of ccRCC
should be conducted. To date, angiogenesis inhibitors, rapamycin
(mTOR)-targeted inhibitors and immune checkpoint inhibitors, have
been approved by the Food and Drug Administration for the
first-line treatment of patients with advanced ccRCC (22). The first-line treatment options for
advanced ccRCC include: i) Targeted monotherapy, including
Sunitinib, Pezopanib and Cabozantinib; ii) Combined immunotherapy,
Immunocombination targeting (Pembrolizumab + Axitinib, Avelumab +
Axitinib, Nivolumab + Cabozantinib, pembrolizumab + Lenvatinib) and
dual immunocombination (Nivolumab + Ipilimumab). There is no clear
literature to support whether metastasis of ccRCC to the thyroid
gland affects the efficacy of iodine-131 treatment and whether
iodine-131 radiotherapy should be continued. In the present case,
the patient was subjected to standard clinical treatment. The
postoperative pathological examination of the patient indicated PTC
with ccRCC metastasis to the thyroid gland, and the cervical lymph
nodes did not suggest cancer metastasis. Given that PTC without
lymph node metastasis would be classified as low-risk for
recurrence, iodine-131 treatment was not performed (23). ccRCC with metastasis to the thyroid
gland was considered advanced ccRCC and sunitinib treatment was
conducted (24). Close follow-up
monitoring of the current patient will continue in the future.
Acknowledgements
Not applicable.
Funding
The present study was funded by The Key Laboratory of Medical
Electrophysiology (Southwest Medical University), Open Fund (grant
no. KeyME-2020-011).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FW, CX, RH, XC, ML, QG, SL and XZ made substantial
contributions to the conception, design and data acquisition of the
article. FW, CX and XZ obtained and analyzed the patient's
information and wrote the manuscript. RH, XC, SL and XZ analyzed
the patient information and reviewed the discussion part of the
clinical diagnosis and treatment. XZ critically revised the
article. ML and QG provided the pathological images and diagnosis.
SL partially revised the article and generated the figures. XZ
ensured that questions related to the integrity of any part of the
work were appropriately investigated and resolved. FW, CX, SL and
XZ confirm the authenticity of all the raw data. All authors have
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Affiliated Hospital of Southwest Medical University (Luzhou,
China; ethics approval no. KY2023140).
Patient consent for publication
Written informed consent to publish this case
information and accompanying images was obtained from the
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ljungberg B, Albiges L, Abu-Ghanem Y,
Bedke J, Capitanio U, Dabestani S, Fernández-Pello S, Giles RH,
Hofmann F, Hora M, et al: European Association of Urology
Guidelines on renal cell carcinoma: The 2022 update. Eur Urol.
82:399–410. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang A, Ye J, Zhou Y, Zhu B, Lu J, Ge S,
Qu L, Xiao J, Wang L and Cai C: Copper death inducer, FDX1, as a
prognostic biomarker reshaping tumor immunity in clear cell renal
cell carcinoma. Cells. 12:3492023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feng X, Yan N, Sun W, Zheng S, Jiang S,
Wang J, Guo C, Hao L, Tian Y, Liu S and Sun MZ: miR-4521-FAM129A
axial regulation on ccRCC progression through TIMP-1/MMP2/MMP9 and
MDM2/p53/Bcl2/Bax pathways. Cell Death Discov. 5:892019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qu Y, Feng J, Wu X, Bai L, Xu W, Zhu L,
Liu Y, Xu F, Zhang X, Yang G, et al: A proteogenomic analysis of
clear cell renal cell carcinoma in a Chinese population. Nat
Commun. 13:20522022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beutner U, Leowardi C, Bork U, Lüthi C,
Tarantino I, Pahernik S, Wente MN, Büchler MW, Schmied BM and
Müller SA: Survival after renal cell carcinoma metastasis to the
thyroid: Single center experience and systematic review of the
literature. Thyroid. 25:314–324. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ramírez-Plaza CP, Domínguez-López ME and
Blanco-Reina F: Thyroid metastasis as initial presentation of clear
cell renal carcinoma. Int J Surg Case Rep. 10:101–103. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tian P, Du W, Liu X, Xu W, Rong X, Zhang Z
and Wang Y: Ultrasonographic characteristics of thyroid metastasis
from clear cell renal cell carcinoma: A case report. Medicine
(Baltimore). 99:e230702020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Medas F, Calò PG, Lai ML, Tuveri M, Pisano
G and Nicolosi A: Renal cell carcinoma metastasis to thyroid tumor:
A case report and review of the literature. J Med Case Rep.
7:2652013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou J, Yin L, Wei X, Zhang S, Song Y, Luo
B, Li J, Qian L, Cui L, Chen W, et al: 2020 Chinese guidelines for
ultrasound malignancy risk stratification of thyroid nodules: The
C-TIRADS. Endocrine. 70:256–279. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang CG, Li MZ, Wang SH, Liu Y, Zhang HL,
Haybaeck J and Yang ZH: Analysis of cytological misdiagnosis and
oversight of adenoid cystic carcinoma of salivary gland. Cancer
Control. 30:107327482211316522023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guidelines Working Committee of Chinese
Society of Clinical Oncology, . Guidelines of chinese society of
clinical oncology (CSCO) differentiated thyroid cancer. J Cancer
Control Treat. 34:1164–1201. 2021.
|
|
12
|
Wells SA Jr, Asa SL, Dralle H, Elisei R,
Evans DB, Gagel RF, Lee N, Machens A, Moley JF, Pacini F, et al:
Revised American Thyroid Association guidelines for the management
of medullary thyroid carcinoma. Thyroid. 25:567–610. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ghossein CA, Khimraj A, Dogan S and Xu B:
Metastasis to the thyroid gland: A single-institution 16-year
experience. Histopathology. 78:508–519. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chung AY, Tran TB, Brumund KT, Weisman RA
and Bouvet M: Metastases to the thyroid: A review of the literature
from the last decade. Thyroid. 22:258–268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Velez Torres JM, Briski LM, Martinez
Duarte E, Sadow PM, Kerr DA and Kryvenko ON: Metastatic clear cell
renal cell carcinoma involving the thyroid gland: A
clinicopathologic study of 17 patients. Int J Surg Pathol.
30:743–752. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tjahjono R, Phung D, Gurney H, Gupta R,
Riffat F and Palme CE: Thyroid gland metastasis from renal cell
carcinoma: A case series and literature review. ANZ J Surg.
91:708–715. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heffess CS, Wenig BM and Thompson LD:
Metastatic renal cell carcinoma to the thyroid gland: A
clinicopathologic study of 36 cases. Cancer. 95:1869–1878. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Minami S, Inoue K, Irie J, Mine T, Tada N,
Hirabaru M, Noda K, Ito S and Haraguchi M: Metastasis of colon
cancer to the thyroid and cervical lymph nodes: A case report. Surg
Case Rep. 2:1082016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Delitala AP, Vidili G, Manca A, Dial U,
Delitala G and Fanciulli G: A case of thyroid metastasis from
pancreatic cancer: case report and literature review. BMC Endocr
Disord. 14:62014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu J, Nikiforova MN, Hodak SP, Yim JH, Cai
G, Walls A, Nikiforov YE and Seethala RR: Tumor-to-tumor metastases
to follicular variant of papillary thyroid carcinoma: Histologic,
immunohistochemical, and molecular studies of two unusual cases.
Endocr Pathol. 20:235–242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kefeli M and Mete O: An unusual solitary
thyroid nodule with bloody follicles: Metastatic renal cell
carcinoma within an infiltrative follicular variant papillary
carcinoma. Endocr Pathol. 27:171–174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schiavoni V, Campagna R, Pozzi V, Cecati
M, Milanese G, Sartini D, Salvolini E, Galosi AB and Emanuelli M:
Recent advances in the management of clear cell renal cell
carcinoma: Novel biomarkers and targeted therapies. Cancers
(Basel). 15:32072023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pacini F, Fuhrer D, Elisei R,
Handkiewicz-Junak D, Leboulleux S, Luster M, Schlumberger M and
Smit JW: 2022 ETA Consensus Statement: What are the indications for
post-surgical radioiodine therapy in differentiated thyroid cancer?
Eur Thyroid J. 11:e2100462022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Goebell PJ, Ivanyi P, Bedke J, Bergmann L,
Berthold D, Boegemann M, Busch J, Doehn C, Krege S, Retz M, et al:
Consensus paper: Current state of first- and second-line therapy in
advanced clear-cell renal cell carcinoma. Future Oncol.
16:2307–2328. 2020. View Article : Google Scholar : PubMed/NCBI
|