Endometrial carcinoma (EC) is one of the most common
gynecological malignancies and the sixth most common malignant
disease worldwide. Its incidence is increasing year on year, and
the age of onset is decreasing (1–3). The
incidence of EC in developing countries (5.1/100,000 females) is
lower than that in developed countries (13.8/100,000 females), but
there is little difference in mortality rates between developed
(2.63/100,000 females) and developing countries (1.52/100,000
females) (2,4). In addition, the incidence of EC is
increasing in numerous developing countries (5). Data on the global burden of cancer in
2020, released by the International Agency for Research on Cancer,
indicate that there were 417,000 new cases of EC and 97,000 deaths
associated with EC in 2020 (2).
Based on its pathogenesis and biological behavior,
EC can be divided into two types: Estrogen/17-β-estradiol
(E2)-dependent EC (type I) and non-E2-dependent EC (type II)
(3). The majority of type I ECs are
endometrioid carcinomas, which are well differentiated, and a few
are mucinous adenocarcinomas (6).
Type II includes serous carcinoma and clear cell carcinoma
(7). The majority of type II ECs do
not express estrogen receptors (ERs) and may develop in a
hormone-independent manner (7). The
etiology of type I EC includes age, obesity, diabetes,
hypertension, polycystic ovary syndrome, anovulation, infertility,
nonpregnancy, early age at menarche, late age at menopause, ovarian
neoplasms, use of exogenous estrogens and genetic factors (8,9).
However, the main mechanism of its pathogenesis is that atypical
hyperplasia of the endometrium occurs followed by carcinogenesis
under the long-term stimulatory effect of E2 (10). For example, an early age at
menarche, a late age at menopause and ovarian tumors all increase
the cumulative exposure of the endometrium to E2, thereby
increasing the risk of EC (11). In
addition, tamoxifen, which is commonly used in the treatment of
breast cancer, can act as an ER agonist and cause endometrial
hyperplasia, polyps, cancer or sarcoma in the long term after
surgery (12). Regarding type II,
there is as yet no consensus on the etiology of precancerous
lesions, but p53 mutations and the abnormal amplification of HER-2
are the main causes that are currently known (13). Therefore, from the classification
criteria of EC, it may be noted that the occurrence and development
of EC, particularly that of type I, are closely associated with
E2.
E2 binds specifically with ERs to form a
hormone-receptor complex, thus exerting its biological functions
(14). There are two groups of ERs.
One group includes the classical nuclear receptors ERα and ERβ,
which are located in the nucleus and exert their functions by
regulating the transcription of specific target genes (15). The other group comprises membranous
receptors, including the membrane ER and G protein-coupled ER
(GPER) family, which mainly play an indirect transcriptional
regulatory function through the second messenger system, and in
some cases appear to only have local effects in the brain (15,16).
These two types of ER have a tissue/cell-specific distribution in
the body and are involved in the regulation of various functions
such as reproduction, learning, memory and cognition (15,17).
Of all ERs, ERα was the first to be identified, has
been most comprehensively researched and is the most well
understood (15). ERα is encoded by
the ESR1 gene mapping to 6q25, and its main function is to
stimulate and maintain the development of female reproductive
organs and the emergence of secondary sexual characteristics
(10,18,19).
ERα exists not only in the reproductive tract and breast, but also
in the liver, bone, cardiovascular system and brain (18,20).
Combining immunohistochemistry with fluorescence in situ
hybridization, Lebeau et al (21) detected the expression of ERα in 100%
of 43 cases of endometrial hyperplasia, which is a precursor of EC,
and 88.5% of 368 cases of EC. Although ERα is oncogenic in EC,
patients with ERα-positive EC have an improved prognosis due to the
rapid development of hormone therapy (22,23).
In sporadic EC, it has been observed that the expression of ERα is
strongly associated with a lower histological grade, and more
effective response to hormone therapy in ~80% of EC cases (23). Notably, a search of The Cancer
Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov/genes/ENSG00000091831)
reveals that ESR1 has the highest mutation frequency in EC, at
4.47%. Among these mutations, those of Y537 are the most numerous,
suggesting that ESR1 Y537 mutation may be one of the driving
factors for the occurrence and development of EC (Table I).
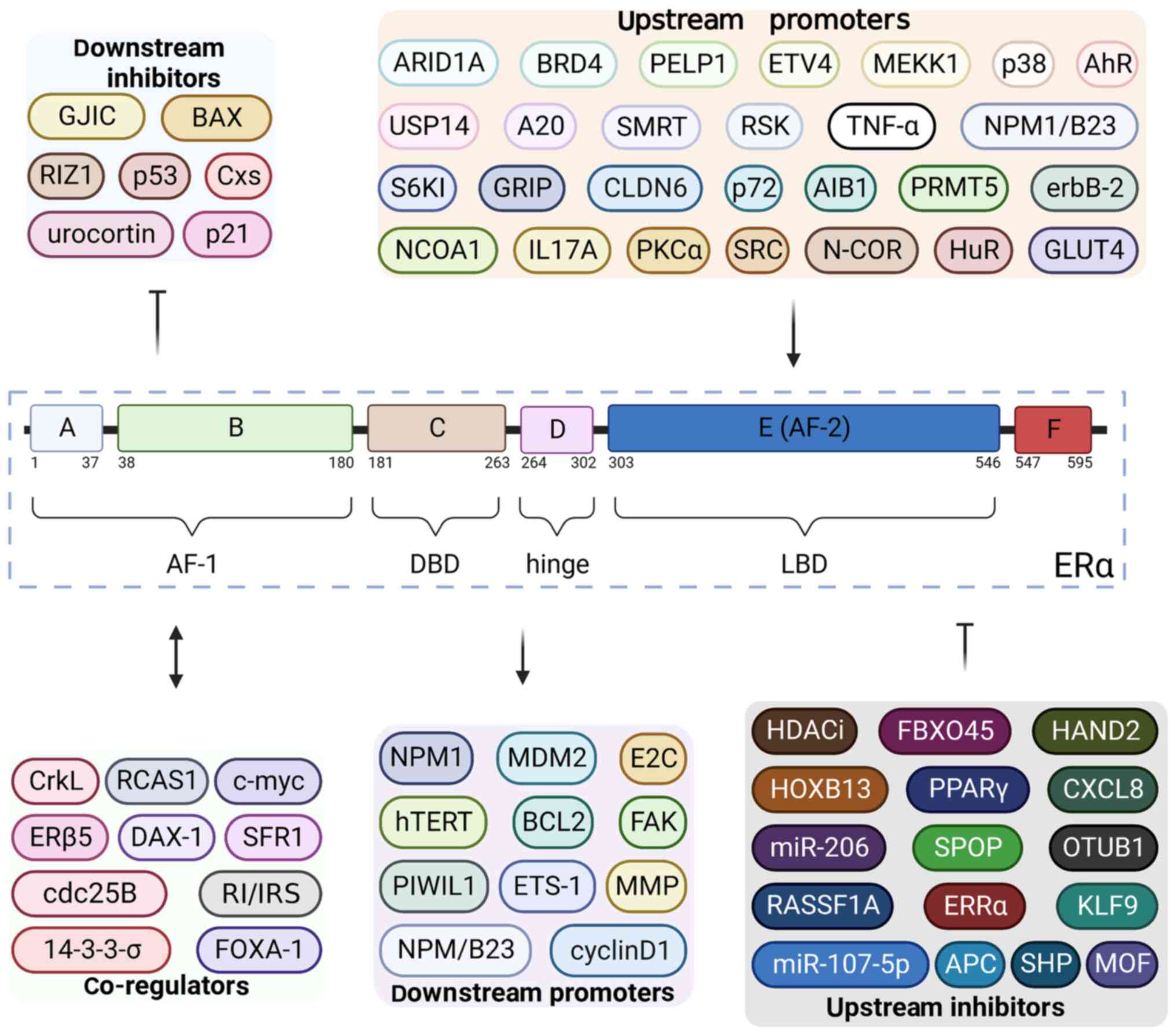
ERα is a transcriptional factor composed of 595
amino acids and six different domains: A, B, C, D, E and F
(Fig. 1) (24). The A domain (amino acids 1–37) and B
domain (amino acids 38–180) constitute the ligand-independent
activation function (AF)-1, which is independent of E2 activation
(25). However, this functional
region may regulate the transcription of E2-responsive genes by
participating in the process of E2-ERα binding (25). In general, the primary function of
AF-1 is the recruitment of co-regulatory proteins (26). The C domain (amino acids 181–263),
which is also known as the DNA binding domain (DBD), contains a
double zinc finger structure that contains four cysteines (27). The ERα homodimer binds to the
palindromic GGTCA-nnn-TGACC sequence of target genes via the DBD to
promote their transcription (28).
Additionally, the binding of DBD and DNA is stabilized due to the
action of the D domain (amino acids 264–302) (25). The D domain contributes to the
recruitment of nuclear localization signal and co-regulatory
proteins by coordinating the function of AF-1 and ligand-dependent
AF-2 in ERα (29). Moreover, the D
domain has been shown to acts as a hinge between the C domain and E
domain (amino acids 303–546) (29).
The E domain has several functions, such as binding to E2, receptor
dimerization and binding to co-activators or co-inhibitors
(28). In addition, the E domain
constitutes the ligand-binding domain (LBD), containing AF-2
(28). When AF-2 encounters
different types of estrogen, it adopts different conformations and
determines which co-activators and/or co-suppressors are required
for binding during the transcription of target genes (28). AF-1 and AF-2 coordinate with each
other to maximize the transcriptional activity of ERα (25). The function of the F domain (amino
acids 547–595) is relatively obscure. However, it has been reported
that the F domain may be necessary for transcriptional activation
and the functioning of anti-E2 drugs such as 4-hydroxytamoxifen
(28,30).
ERβ is similar to ERα in protein structure, and also
contains A, B, C, D, E and F domains (20). However, the major difference between
ERβ and ERα is in AF-1. The activity of AF-1 of ERβ is relatively
low, while that of AF-2 is similar to that of ERα, revealing that
they have different effects on various E2-responsive genes at the
transcriptional level (31,32). Specifically, when AF-1 and AF-2 are
both required for gene transcription, the effect of ERβ is weaker
than that of ERα, and they are equivalent if only AF-2 is required
(32).
The regulation of ERα can be divided into three
different aspects: Transcription of ESR1, translation of ERα
mRNA and post-translational modification of ERα protein (33–35).
The different types of regulation of ERα can produce divergent
effects, and sometimes even opposite results, particularly in the
occurrence and development of EC (36,37).
Given that the present review focuses on the roles of ERα in EC,
the following sections mainly summarize the regulation of ERα in
relation to EC (Table II).
Compared with the translational regulation of ERα
mRNA and the post-translational modification of ERα protein, the
research into the transcriptional regulation of ESR1 is
relatively unclear. However, by the analysis of ESR1 gene
amplification and ERα protein expression in 368 EC tissue
microarrays, Lebeau et al (21) found that the strong expression of
ERα protein was significantly associated with ESR1
amplification in EC, suggesting that ESR1 amplification may
be a mechanism by which ERα is overexpressed in EC, and could play
an important role in the development of a significant proportion of
EC cases. Kershah et al (38) found that the nuclear receptor
co-regulators steroid receptor coactivator (SRC)-1, SRC-2, SRC-3,
nuclear receptor corepressor and silencing mediator of retinoic
acid and thyroid hormone receptor significantly increased mRNA
expression in EC and were highly correlated with ERα mRNA,
indicating that these regulatory factors may be associated with EC.
Conversely, it has been suggested that ER-related receptor (ERR)α
may regulate ERα-mediated pathways by interfering with ERα
transcription (39). Also, in EC,
methylation of the CpG island of the ESR1 gene has been
found to be negatively associated with ERα expression (40). In addition, histone deacetylase
(HDAC) inhibitors directly inhibit the transcription of ESR1
promoters and thus regulate the E2/ERα signaling pathway (33).
Studies on translational regulation have mainly
focused on the regulation of ERα mRNA by microRNAs (miRNAs or
miRs). Bao et al (36)
showed that miR-107-5p directly targets ERα mRNA to downregulate
the expression of ERα mRNA and protein, thereby promoting tumor
proliferation and EC invasion. Similarly, other studies have shown
that miR-222-3p downregulates the expression of ERα, thereby
promoting the proliferation and invasion of EC and increasing
raloxifene resistance (41,42). Furthermore, miR-206 has been
reported to inhibit ERα-dependent proliferation, impair the
invasion ability of ERα-positive EC cells, and induce cell cycle
arrest, indicating that abnormal miR-206 expression may be
associated with the occurrence of EC (34). In addition to miRNA, a study by
Zhang et al (43) confirmed
that the stimulation of peroxisome proliferator-activated receptor
γ (PPARγ) expression inhibited ERα expression at the mRNA and
protein levels, and impaired the ability of Ishikawa cells to
migrate and invade. Therefore, activation of PPARγ may enhance the
effects of anti-E2 therapy in ERα-positive EC through ERα-mediated
ER transactivation (43).
The post-translational modification of ERα includes
phosphorylation, ubiquitination, acetylation, sumoylation,
methylation and glycosylation, among which phosphorylation,
ubiquitination and acetylation are associated with EC development
(37,44–48).
Phosphorylation of ERα generally regulates the transcriptional
activity of ERα by regulating the interaction between the AF domain
and transcription co-activators (Fig.
1) (49). Kato et al
(50) showed that MAPK-mediated
phosphorylation of ERα S118 is necessary for the activity of AF-1,
in vivo and in vitro. Furthermore, another study
demonstrated that the phosphorylation of ERα S118 mediated by MAPK
signaling pathway promotes uterine leiomyoma cell growth (35). Vilgelm et al (51) found that the deletion of Pten
activates AKT in mouse endometrium, which leads to an increase in
the phosphorylation of ERα S167, thereby increasing the ability of
ERα to activate the transcription of several target genes.
Similarly, Kato et al (52)
found that the mTOR/p70 S6 kinase 1 and MAPK/p90 ribosomal S6
kinase signaling pathways co-regulate the phosphorylation of ERα at
S167, and the levels of such phosphorylation are elevated in
advanced EC. In the normal endometrium during the menstrual cycle,
phosphorylation of ERα at S104, S118 and S167 synergizes with the
phosphorylation of AKT at S473, while the phosphorylation of AKT at
T308 regulates apoptosis in endometrial cells and arterioles
(44). It has also been shown that
the p38-MAKP-mediated signaling pathway induces the phosphorylation
of ERα T311, which blocks ERα nuclear export and promotes the
interaction between ERα and steroid receptor co-activator p160
(53).
Ubiquitination of ERα is mainly mediated by
speckle-type POZ protein (SPOP), F-box protein 45 (FBXO45) and
arylhydrocarbon receptor (AhR) (45,54,55).
SPOP specifically recognizes the AF-2 domain of ERα and triggers
ERα degradation through the ubiquitin-proteasome system, thereby
inhibiting the development of EC (45). Similarly, the E3 ligase FBXO45
inhibits the progression of EC by mediating the ubiquitination and
degradation of ERα (55). AhR has
been shown to promote the ubiquitination and degradation of ERα via
the assembly of a complex with cullin 4B (CUL4B) (54). In the CUL4B-AhR complex, AhR acts as
a substrate recognition subunit that recruits ERα for degradation
(54). By contrast,
de-ubiquitination meditated by de-ubiquitinating enzymes, including
ubiquitin-specific protease 14 and ubiquitin-editing enzyme A20,
promotes the transcriptional activity of ERα by inhibiting its
degradation, thereby leading to the development of EC (56,57).
Regarding the acetylation of ERα, Wu et al (37) demonstrated that males absent on the
first (MOF), also known as lysine acetyltransferase 8, mediates the
acetylation of ERα, maintains the stability of ERα, and regulates
the activity of ERα and its target genes. However, the study also
indicated that MOF inhibits the proliferation of EC cells (37).
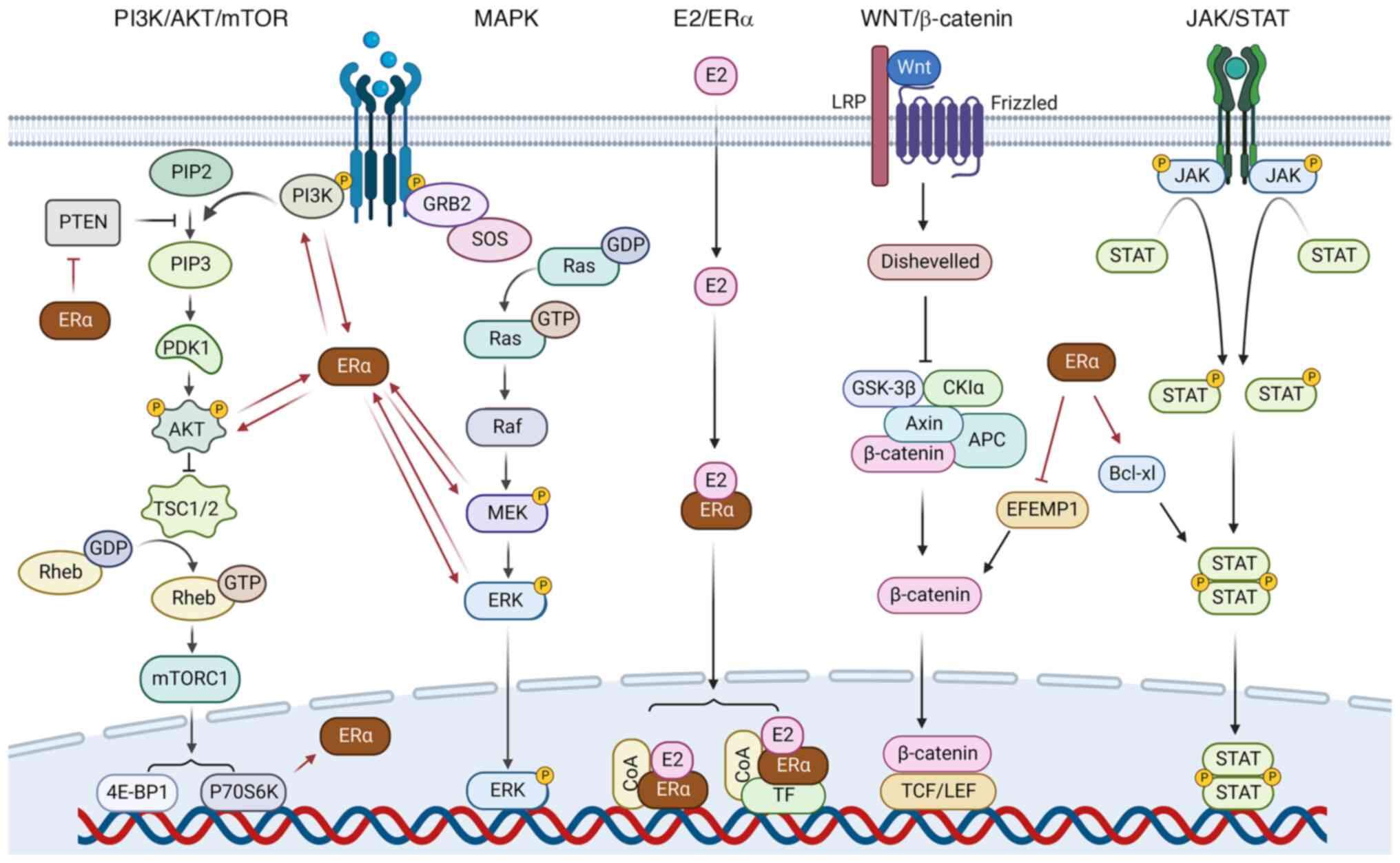
The classical E2/ERα signaling pathway regulates the
transcription of target genes through two different approaches,
namely the classical and non-classical approaches, both of which
can be divided into three steps, which differ most markedly in the
third step (29). First, E2 either
diffuses into the cell or is synthesized in situ inside the
cell (60). Second, E2 enters the
nucleus where it binds to and activates ERα to form a homologous or
heterodimer of ERα (60). In the
classic approach, the third step is that the activated ERα binds to
E2 response elements (EREs), which comprise two AGGTCA motifs in a
palindromic structure (39). The
ERα-ERE complex promotes the formation of transcription initiation
complexes and induces the transcription of target genes (39). In addition, in vivo pioneer
factors initiate chromatin remodeling by opening up the chromatin
structure to facilitate the binding of activated ERα with EREs, and
co-regulators act synergistically with ERα to enhance or reduce the
expression of specific genes, which play an important role in the
occurrence and development of EC (61). However, in the third step of the
non-classical approach, activated ERα does not directly bind to the
promoter region of the target genes (29). Instead, ligand-bound receptor dimers
first interact with other transcriptional factors, such as Fos or
Jun, for transcriptional activation (29). ERα then binds to enhancer elements
such as activating protein 1 and specific protein 1 in the promoter
region of target genes to indirectly regulate the transcription of
target genes (29).
The classical E2/ERα signaling pathway plays a key
role in the occurrence and development of EC. For instance, ERα
mediates E2-stimulated IL-6 production, which induces aromatase
expression in stromal cells, thereby producing E2 in situ,
which forms a positive feedback loop by which E2 promotes cancer
progression (62). In addition, ERα
also binds EREs and co-activators with ERRα (63). However, E2 downregulates the
expression of ERRα at the mRNA and protein levels in Ishikawa cells
by a mechanism involving ERα (64).
ERRα competes with ERα for the same target gene loci and
co-regulators, which interferes with the E2/ERα signaling pathway
and thereby potentially suppresses the development of EC (39).
ERα is generally considered to play a driving role
in endometrial malignant transformation, which has three main
aspects (Fig. 1) (65). First, upstream regulators of ERα
regulate the transcriptional activity of ERα and thus influence the
development of EC, especially cell proliferation (66). Second, ERα promotes the occurrence
of EC together with other co-regulators (67). Third, ERα mediates EC proliferation,
metastasis and apoptosis through its downstream proteins or target
genes (68).
Several upstream proteins of ERα participate in the
occurrence and development of EC by affecting the transcriptional
activity and expression of ERα (Table
III). Since the proteins that mediate the post-translational
modifications of ERα have been summarized previously in the present
review, they are not covered again in this section.
Most of the upstream regulators promote the
development of EC by enhancing the transcriptional activity of ERα.
For example, the increased expression of co-activators p72 and
amplified in breast cancer 1 (AIB1), as well as the interaction
between erbB-2 and p72, have been suggested to enhance the
interaction between E2 and ERα, thereby inducing the
transactivation of ERα in EC; this suggests that transactivation of
ERα induced by the overexpression of p72, AIB1 and erbB-2 may be
involved in the development of EC stimulated by tamoxifen (69). Similarly, interaction between
p21-activated kinase 4 (Pak4) and the E2/ERα signaling pathway has
been shown to trigger the proliferation of EC cells (66). Specifically, E2 increases the
expression and activation of Pak4 in ERα-positive EC cells via the
PI3K/AKT/mTOR signaling pathway, and the accumulation of Pak4 and
phosphorylated Pak4 in the nucleus promotes ERα transactivation,
which enhances the transcriptional activity of ERα and
ERα-dependent gene expression, leading to EC cell proliferation
(66). Although these upstream
regulators of ERα mainly promote the proliferation of EC, they have
also been shown to have some effects on migration (70,71).
In addition, protein kinase C α (PKCα) expression stimulates the
ligand-independent activation of ER-dependent promoters to enhance
the transcriptional capacity of ERα, thereby inducing endometrial
proliferation (72). Furthermore,
Frigo et al (73) showed
that p38 promotes the proliferation of EC cells by stimulating
ERα-mediated transcription via phosphorylation of the co-activator
glucocorticoid receptor-interacting protein 1. With regard to
migration, Kojima et al (74) demonstrated that claudin 6/Src-family
kinase/PI3K-dependent AKT and serum- and glucocorticoid-regulated
kinase signaling in EC cells targets ERα Ser518 in a
ligand-independent manner to activate the transcriptional activity
of ERα, thereby promoting tumor migration. In addition,
mitogen-activated protein kinase kinase kinase 1 also induces the
transcriptional activity of ERα through the N-terminal kinase of
Jun and p38/Hog1, thereby stimulating the excitatory activity of
4-hydroxytamoxifen in the endometrium (75). There are also negative upstream
regulators that inhibit the transcription of ERα and thus inhibit
EC development. For example, the activation of Pten and subsequent
inhibition of AKT have an inhibitory effect on several
ERα-dependent pathways, which suppresses the development of EC
(76). Moreover, Velard et
al (77) demonstrated that
Krüppel-like factor 9 inhibited the transcriptional activity of ERα
in endometrial epithelial cells, and suggested that it acts at a
node of the ERα genomic pathway to negatively regulate the
proliferation of EC.
Another means by which upstream regulators regulate
ERα is by affecting the expression of ERα. For example, Cheng et
al (78) found that interleukin
17A induced the proliferation and metastasis of EC cells by
promoting the expression of ERα. Likewise, human antigen R has been
reported to increase the expression of ERα protein in Ishikawa
cells, thereby promoting proliferation and inhibiting apoptosis
(79). In addition,
hyperglycemia-induced glucose transport protein 4 expression has
been shown to increase the secretion of vascular endothelial growth
factor (VEGF) and the expression of its receptor VEGFR via the
upregulation of ERα, leading to accelerated epithelial-mesenchymal
transition (EMT) in EC (80).
Conversely, the inhibition of ERα expression delays the development
of EC. For example, the downregulation of ERα by RAS association
domain family 1 subtype A induces EC cell apoptosis and inhibits EC
growth (81). However, some
upstream regulators have been suggested to promote the development
of EC by inhibiting ERα expression. For example, Tanwar et
al (82) showed that reduced
adenomatous polyposis coli activity in mouse uterine stromal cells
led to transformation of the cells to a myofibroblast phenotype,
which reduced ERα expression and induced EC. In addition, another
study demonstrated that chemokine C-X-C motif chemokine ligand 8
(CXCL8) promoted the development of EC via the inhibition of ERα
expression (83). Specifically,
CXCL8 secreted by tumor-associated macrophages was shown to
downregulate the expression of ERα in EC cells via homeobox 13,
which was associated with the invasive ability of the cells
(83).
ERα contributes to the occurrence and development of
EC via interactions with several proteins, such as receptor-binding
cancer antigen expressed on SiSo cells (RCAS1), and by crosstalk
with signaling pathways including the MAPK signaling pathway and
insulin/insulin receptor signaling pathway (Table III) (84–87).
For example, ERα and ERβ5 have been found to be co-expressed in the
nuclei of endometrial adenocarcinoma cells, and to form
heterodimers that enhance the hormone sensitivity of Ishikawa
cells, thereby promoting the development of EC (88). Moreover, Bircan et al
(89) demonstrated by
immunohistochemical analysis that ERα expression was positively
correlated with c-myc expression, suggesting that c-myc expression
may contribute to the development of EC through ERα. With regard to
specific effects, ERα has been suggested to influence the
proliferation, invasion and metastasis of EC cells through
interaction with various proteins or signaling pathways (86,90).
Nakayama et al (90)
observed an inverse correlation between ERα and 14-3-3σ, and
speculated that these proteins have a synergistic effect that
promotes EC proliferation and prevents apoptotic signal
transduction in high-grade and middle-advanced endometrial
adenocarcinoma. Furthermore, Zhou et al (84) demonstrated that the co-expression of
RCAS1 and ERα may be involved in the development and metastasis of
EC. Crosstalk between ERα and the MAPK signaling pathway has been
suggested to be associated with the phenotypic plasticity of EC
cells triggered by chronic 2,2′,4,4′-tetrabromodiphenyl ether
exposure, which promoted EC tumor growth and attenuated the
resistance of EC cells to chemotherapy (86). Similarly, crosstalk between the
E2/ERα signaling pathway and the insulin/insulin receptor signaling
pathway has been demonstrated to activate downstream PI3K/AKT/mTOR
and MAPK signaling pathways, thereby contributing to occurrence and
development of EC (85).
However, interactions between ERα and certain other
proteins may inhibit EC cell proliferation (87,91).
For example, Saito et al (87) suggested that an orphan nuclear
receptor known as dosage-sensitive sex reversal adrenal hypoplasia
congenita critical region on the X chromosome gene 1 inhibits the
proliferation and progression of EC by interacting with ERα in EC
cells. Additionally, ERα and Forkhead-box A1, which is a tumor
suppressor, have been demonstrated to interact in EC cells, and to
inhibit the proliferation of EC cells (91).
In addition to the upstream and co-regulators of
ERα, downstream proteins or target genes are also involved in the
promotion of EC development by ERα. These mainly contribute to
three aspects: Proliferation, metastasis and invasion, and
anti-apoptosis (Table III)
(92–94).
ERα has been shown to induce the proliferation of EC
cells via the promotion or inhibition of downstream substrates. For
example, Chen et al (92)
found that in EC cells, ERα binds to a half-ERE on the promoter of
the gene encoding the stem cell protein Piwi-like RNA-mediated gene
silencing 1 (PIWIL1), thereby upregulating the expression of PIWIL1
(92). The authors also found that
upregulated PIWIL1 promoted the proliferation of EC cells, and that
this effect was closely associated with hypomethylation of the
PIWIL1 promoter (92).
Similarly, ERα activates the promoter of the B-cell
lymphoma/leukemia-2 (BCL2) gene to increase the
transcription of BCL2, and also downregulates the expression of
BCL2-associated X protein gene (BAX) via several miRNAs, thus
leading to an imbalance of the BCL2/BAX ratio that promotes the
proliferation of EC (95). In
addition, in primary cultured human endometrial adenocarcinoma
cells, E2 has been demonstrated to upregulate the expression of
nucleophosmin 1 (NPM1) in a dose-dependent manner through
ERα-mediated signaling rather than via ERβ, with the upregulation
of NPM1 promoting the growth and proliferation of the cells and
inhibiting their differentiation and apoptosis (3). It has also been shown that the E2/ERα
signaling pathway inhibits the formation of an NPM-alternate
reading frame complex, resulting in increased levels of NPM
protein, which promote the proliferation of endometrial tissues and
tumors (96). Additionally, ERα up-
and downregulates the expression levels of cyclin D1 and p21,
respectively, which induces dysregulation of the cell cycle and
triggers the proliferation of EC cells (97). Furthermore, ERα has also been
indicated to downregulate gap junctional intercellular
communication (GJIC) mediated by the formation of gap junctions by
connexins (Cxs), which is important in cell growth,
differentiation, homeostasis and morphogenesis (98). Saito et al (98) showed that the activation of ERα by
E2 stimulated cell growth and inhibited GJIC by inhibiting the
expression of Cxs, leading to the proliferation of EC cells.
ERα can also promote the proliferation of EC cells
via the activation of certain downstream signaling pathways
(Fig. 2). For example, Hou et
al (68) found that ERα
overexpression promotes the phosphorylation of p85α, the regulatory
subunit of PI3K, which activates the PI3K/AKT/mTOR signaling
pathway, thereby increasing the proliferation, migration and
invasion of EC cells. Moreover, another study demonstrated that the
activation of ERα by E2 induces the nuclear localization and
accumulation of fat mass and obesity-associated protein (FTO)
through the PI3K/AKT/mTOR signaling pathway, which increases the
proliferation of EC cells (99).
Furthermore, the E2/ERα signaling pathway has been shown to
increase the expression of miR-200c and decrease the expression of
Pten, leading to activation of the PI3K/AKT/mTOR signaling pathway,
thus promoting the proliferation of EC cells and inhibiting their
apoptosis (100). Moreover, when
stimulated by E2, cytoplasmic ERα forms a complex with protein
kinase 2-α, which mediates the phosphorylation of Pten and promotes
EC cell proliferation (101).
Invasion and metastasis are also affected by ERα
through its downstream substrates, either directly or indirectly.
In a Transwell experiment, Mizumoto et al (102) found that stimulation with E2
increased the invasive ability of Ishikawa cells, while the
expression levels of matrix metalloproteinase (MMP)-1, −7 and −9
and the transcriptional factor ETS-1 were also enhanced. These
results indicate that the activation of ERα stimulates EC cell
invasiveness and tumor progression by promoting the expression of
MMPs (102). In endometrial
stromal cells and Ishikawa cells, E2 has been shown to promote
cytoskeletal and membrane remodeling by the activation of focal
adhesion kinase, thus increasing the motility and invasion of the
cells (93). Furthermore, E2
upregulates the expression of ubiquitin-binding enzyme E2C via ERα
in EC, which downregulates the expression of p53 and its downstream
effector p21, thus promoting EC metastasis and invasion (103). In addition to playing a role in
proliferation, the activation of FTO via E2/ERα also stimulates the
invasion of EC cells through the PI3K/AKT/mTOR and MAPK signaling
pathways (104). However, Wik
et al (105) found that
ERα-negative tumors are also associated with EMT, which is linked
to the PI3K/AKT/mTOR signaling pathway. In addition to the
aforementioned substrates, ERα also inhibits epidermal growth
factor-containing fibulin-like extracellular matrix protein 1
(EFEMP1), retinoblastoma protein-interacting zinc finger gene 1
(RIZ1) and urocortin to promote EC cell mobility (106–108). Using chromatin immunoprecipitation
and dual-luciferase reporter assays, Yang et al (106) demonstrated that the E2/ERα
signaling pathway downregulated EFEMP1 expression in EC cells by
the direct binding of ERα to the EFEMP1 promoter. Given that EFEMP1
was also shown to inhibit EMT and the migration of EC cells via
inhibition of the WNT/β-catenin signaling pathway, it was suggested
that EFEMP1 may be an excellent candidate for EC therapy (106). The activation of ERα reduces the
expression of urocortin, a protein that inhibits EC cell migration;
therefore, the E2/ERα pathway may promote EC cell invasion and
metastasis via this mechanism (108). Furthermore, RIZ1 has been shown to
inhibit the migration and invasion of EC cells in vivo and
in vitro (107). Yang et
al (107) showed that E2
downregulated the expression of RIZ1 in EC cells, which promoted
the development of EC. They also found that the selective ERα
antagonist ICI182780 reversed this effect, suggesting that a
potential mechanism by which RIZ1 promotes EC involves the E2/ERα
signaling pathway (107).
There have been only a few studies on ERα in terms
of anti-apoptosis and drug resistance. It has been shown that by
directly binding to p53, ERα inhibits the transcriptional
activation of p53, which downregulates the inhibitory effect
of p53 on survivin (94). Survivin
inhibits apoptosis through a variety of mechanisms, including
directly binding to and inhibiting caspase-3 and caspase-9
(109). In a study of Ishikawa and
HEC-265 cells, Chuwa et al (110) found that E2 significantly induced
the co-expression of ERα and survivin in EC cells, which reduced
the apoptosis of these cells. In addition, during the G1 phase of
EC, the E2/ERα signaling pathway has been shown to promote the
translocation of phosphorylated AKT into the nucleus and thereby
inhibit the apoptosis of EC cells (111). Moreover, it has been suggested
that ERα may enhance the interaction between STAT3 and the
apoptosis regulator BCL-extra large, which is crucial for the
development of endometrioid adenocarcinoma (112). Furthermore, ERα expression has
been indicated to influence the pro-apoptotic or anti-apoptotic
effects of abnormally expressed Cx43 and Cx26 in EC (113). Regarding drug resistance, Abe
et al (114) found that ERα
upregulates the expression of BCL2-associated athanogene 3 (BAG3)
in EC cells, inhibits the expression of miR-29b, and increases the
expression of Mcl-1, which is a downstream mediator of BAG3. In
addition, the authors also found that ERα overexpression improves
the survival of EC cells in the presence of cisplatin, suggesting
that ERα may enhance the resistance of EC cells to anticancer drugs
via the overexpression of BAG3 (114).
ERα is used as a therapeutic target for EC, and
several drugs targeting ERα are currently being applied for the
treatment of EC. In addition, ERα has a role as a good prognostic
indicator for EC (Table IV)
(12,115).
Selective ER modulators including tamoxifen and
raloxifene are the most intensively studied anti-ERα agents in EC.
Tamoxifen affects the interaction of ERα with co-regulatory factors
and alters the DNA binding characteristics of ERα in EC tissue
(116). Tamoxifen contributes to
the proliferation and carcinogenesis of EC via the promotion of ERα
transcriptional activity through the constitutional activation of
MAP kinase signaling (117).
Moreover, SRC kinase promotes the role of tamoxifen in EC through
the AKT kinase-induced phosphorylation of ER S167, thereby
stabilizing ER promoter interactions and increasing ERα signaling
(118). However, despite
increasing the risk of EC, tamoxifen is also an effective
low-toxicity drug for the treatment of advanced or relapsing EC
(119). Tamoxifen exerts
excitatory or antagonistic effects on ERα through the
tissue-specific expression of co-activators and -inhibitors of
receptors (119). The development
of EC associated with tamoxifen has been suggested to be due to the
MAPK signaling pathway increasing the transcriptional activity of
ERα through AF-1 (117). It was
these negative effects of tamoxifen that drove the development of
raloxifene (120). Raloxifene not
only has the same mechanism as tamoxifen to inhibit ERα and inhibit
the proliferation of EC, but also induces mitochondria-mediated
apoptosis of EC (120). The
selective ER downregulator ICI-182780 and genistein significantly
reduce the expression level of ERα induced by E2 (121). Boisen et al (122) found that ICI-182780 binds to ERα
to inhibit E2, and also competently binds to the LBD of ERα and
induces ERα degradation through the ubiquitin-proteasome system.
However, in primary EC, splicing variants and point mutations
present in the LBD are associated with hormone-independent ERα
activity, which can produce ligand-independent or anti-E2 therapy
resistance (123). Similar to
ICI-182780, clomiphene citrate has been shown to reduce the ERα
protein level via induction of ubiquitin-proteasome system without
affecting the ERα mRNA level in Ishikawa cells (124). Arsenic trioxide, however, inhibits
both ERα mRNA and protein expression in a dose-dependent manner by
promoting the rapid phosphorylation of p42/p44 in the MAPK
signaling pathway, thereby exhibiting an anti-EC effect (125). The natural dietary flavonoid
kaempferol effectively targets ERα-mediated oncogenic signaling
pathways to induce the death of EC cells, not only via the
inhibition of ERα and survivin proteins, but also by the induction
of p53 (110). Metformin exhibits
an inhibitory effect on the E2-induced enhanced proliferation of
Ishikawa cells that is weakened or partially reversed in
ESR1 knockout cells, indicating that ERα mediates the
inhibitory effect of metformin on the proliferation of EC cells
(97). It has been suggested that
this effect may be attributed metformin reducing the expression of
ERα at the protein and mRNA levels, resulting in a reduction in the
expression of the ERα-target genes keratin-19 and WNT-1 (126). Compared with anti-ERα treatment
alone, the dual targeting of ERα and ERRα in the treatment of EC
has an improved therapeutic effect, because this maximizes the
growth inhibitory and pro-apoptotic effect on EC cells (127). DY131, a selective ERRγ agonist,
inhibits the growth of ERα-positive EC cells but promotes the
growth of ERα-negative EC cells (128). In addition, melatonin has been
shown to enhance the anti-EC effect of chemotherapy, particularly
paclitaxel, by the inhibition of ERα expression in Ishikawa cells
(129). Furthermore, a combination
of S-farnesylthiosalicylic acid and medroxyprogesterone acetate was
demonstrated to inhibit growth and increase cell death in type II
EC cells by decreasing the mRNA expression of the ERα-mediated
progesterone receptor (PR), c-fos and ps2/trefoil
factor 1 (130).
A number of very promising targets and drugs for
the treatment of EC have been identified. Miki et al
(131) showed that heterogeneous
nuclear ribonucleic protein K (hnRNPK) immunoreactivity in normal
endometrium in the proliferative phase was higher than that in the
secretory phase, and the expression levels of both ERα and hnRNPK
were higher in benign endometrial tissue than in EC. In both normal
and cancerous tissues, the median hnRNPK immunoreactivity was
significantly increased in cases with high ERα, which was
significantly associated with improved disease-free survival (DFS)
and overall survival (131). Based
on these results, it was proposed that hnRNPK interacts with ERα to
regulate changes in the endometrium during the menstrual cycle,
thus having the ability to inhibit the malignant behavior of EC
(131). Krakstad et al
(132) found that the GPER protein
is significantly associated with ERα in GC, and a loss of GPER in
patients with ERα-positive GC is associated with a poor prognosis.
Additionally, using bioinformatics they found that HDAC inhibitors
may be promising drugs for the treatment of ERα-positive EC with
GPER deletion (132). Although E2
activates NPM via ERα, increased NPM expression inhibits ERα
(133). Since strategies to
promote ERα re-expression may allow patients with relapsed EC to
resume endocrine therapy, inhibition of NPM may represent a
strategy to promote ERα re-expression and ultimately restore the
sensitivity of EC to hormone therapy (133). Moreover, the expression of ERα in
EC has been found to negatively correlate with human
phosphatidylethanolamine-binding protein 4 (hPEBP4), PKCα and
antisense oligodeoxyribonucleotides against ERα, suggesting that
hPEBP4, PKCα and nucleic acid therapeutics may counter the ERα and
serve as potential agents against the proliferation of EC cells
(134–136).
Given the widespread clinical application of
endocrine therapy specific to ERα, ERα can be used as a good
prognostic indicator in EC (22).
Among patients with EC, those with ERα-positive tumors have
relatively good survival and the high expression of ERα is
associated with an improved DFS in both type I and II EC (137,138). Through the analysis of 214
patients with endometrial adenocarcinoma, Mylonas (139) found that the loss of ERα was
associated with poor survival. Furthermore, in another study ERα
mRNA upregulation was shown to be an indicator of good prognosis in
patients with EC (115). The
expression of ERα is associated with the stage, histological grade
and survival of EC (140), and ERα
upregulation is considered to provide prognostic information
independent of tumor grade and stage in women with EC (141). Although Mylonas did not find ERα
to be an independent factor affecting survival in patients with
endometrial adenocarcinoma, it was suggested that the combined
analysis of ERα and ERb may be used to identify high-risk patients
with endometrioid adenocarcinoma (139). The uptake of
16α-[18F]fluoro-17β-estradiol (FES) is closely
correlated with ERα expression, and the
2-[18F]fluoro-2-deoxy-D-glucose (FDG)/FES ratio is
negatively correlated with ERα expression, both of which can
reflect the differentiation degree of EC (142). Given the high expression of ERα in
low-grade EC, it was suggested that FES positron emission
tomography in combination with FDG can be used to noninvasively
assess ERα distribution and function, and has potential in the
prognosis of EC and determination of its treatment (142). As in EC, it has also been proposed
that ERα could be used as a prognostic indicator in serous uterine
carcinoma. The expression of ERα in serous uterine carcinoma is
associated with advanced stage, and a prognosis that is
significantly worse than that of serous uterine carcinoma without
ERα expression (143).
The role of ERα in EC is becoming increasingly
clear. In general, ERα, as a transcriptional factor, is an
oncogenic factor in EC. ERα regulates transcriptional activity with
modulation by upstream co-regulatory factors, and then promotes
transcription of its downstream target genes via the E2/ERα
signaling pathway, thus promoting the occurrence and development of
EC, with the induction of proliferation, invasion, metastasis and
anti-apoptosis effects.
However, two important aspects of ERα in EC merit
further investigation. One is that the progressive loss of ERα
seems to be associated with the progressive malignancy of EC
(89). That is, highly
differentiated EC typically retains ERα expression in the early
stages, while in advanced stages, poorly differentiated EC tends to
lack this receptor (144).
Pathirage et al (145)
found that ERα expression was significantly elevated in grade 1 EC
compared with normal tissues and higher grade EC, and observed a
significant negative correlation between ERα expression and the
grade of EC. Using immunohistochemistry, Hu et al (97) observed that the positive expression
rate of ERα was higher in patients with moderately and highly
differentiated EC than in those with poorly differentiated EC, and
showed that ERα expression was higher in the early stage of EC
development compared with the late stage of EC. Therefore, they
hypothesized that ERα promotes endometrial dysplasia and the early
progression of EC through interaction with E2 (97). However, they observed that ERα
sensitivity to E2 changed and more ERα-negative EC cells appeared
during EC progression, resulting in a lower expression of ERα in
advanced EC (97).
The other key aspect of ERα in EC is that it may
also act as a tumor suppressor. It has been shown that ERα
localized in the cytoplasm promotes cardiovascular protection in
mice but does not promote the occurrence and development of EC
(146). Furthermore, it has been
demonstrated that the ERα-mediated signaling pathway regulates the
expression of olfactomedin 4 (OLFM4), and that the expression of
OLFM4 and ERα are positively correlated (147). While the increased expression of
OLFM4 during the development of EC is associated with the
differentiation of endometrioid adenocarcinoma, the downregulation
of OLFM4 promotes the proliferation, migration and invasion of EC
cells, and is associated with a reduced survival rate in patients
with endometrioid adenocarcinoma (147). In addition, ERα blocks the
formation of tumor blood vessels (148). The high levels of ERα in EC have
been indicated to inhibit tumor growth via the regulation of
angiogenic factors such as integrin αvβ3, thereby reducing the
blood supply (148,149). In addition, ERα interacts with the
Sp3 protein, which inhibits VEGF expression and thus blood supply
in EC (150). Moreover, Joshi
et al (151) observed
endometrial hyperplasia/carcinoma in 88.9% of Pten+/−
ERα−/− mice. These mice also exhibit a high incidence of
carcinoma in situ and invasive carcinoma, suggesting that EC
can develop in the absence of ERα (151).
Although ERα is the most common target of targeted
therapy in breast cancer, anti-ERα therapy has shown inconsistent
results in EC, with very limited therapeutic efficacy and sometimes
even an increased risk of cancer (123). Since the study based on TCGA
database in 2013 (156), a new
molecular classification of EC has emerged, which is mainly based
on overall mutational burden, p53, polymerase-epsilon
(POLE), Pten mutations, microsatellite instability
and histology, which helps to refine the prognosis of EC (156–158). It divides EC into four molecular
subtypes: POLE ultra-mutated, microsatellite instability
hyper-mutated, copy-number low and copy-number high (157,158). Due to the high cost of the genetic
analysis of POLE, another simplified version of molecular
typing is commonly used in clinical practice, which divides EC into
POLE-mutant, mismatch repair deficient, no specific molecular
profile (NSMP) and p53-aberrant subtypes (157). Among these, only NSMP usually
comprises ERα and PR, while in the other three subtypes, hormone
receptors are usually absent (157,159). In NSMP, the level of copy number
alterations is low, the tumor mutation burden is moderate, and
mutation mainly occurs in the PI3K/AKT/mTOR and WNT/β-catenin
signaling pathways (157).
Targeted therapy for ERα or hormonal therapy, alone or in
combination with mTOR inhibitors, is indicated to further improve
outcomes in patients with NSMP (160). By contrast, a range of treatments
targeting ERα may not have much effect on the other three molecular
subtypes. Clinically, in addition to targeting ERα, a number of
drugs target other biological molecules: E2, including anastrozole
and letrozole; PR, such as medrysone; VEGF, including bevacizumab
and lenvatinib; mTOR, such as everolimus and ridaforolimus; and
programmed cell death protein, for example, pembrolizumab and
dostarlimab in the treatment of EC (161–165). Studies have shown that the
combination of tamoxifen with anastrozole, bevacizumab, everolimus
or pembrolizumab can be used to control the proliferation and
metastasis of breast cancer (166–169). However, in EC, there have been few
studies on the combination of anti-ERα drugs with other drugs, and
it is not clear whether they affect the prognosis of EC.
Furthermore, when combined with chemotherapy drugs or mTOR
inhibitors, anti-ERα drugs can have serious side effects and these
occur frequently (170).
Therefore, it is necessary to find improved ERα-targeting drugs or
combinations of drugs in future studies, so as to further improve
the prognosis of patients and reduce the occurrence of side
effects.
Not applicable.
This research was funded by the General Program-Education
Department of Zhejiang Province (grant no. Y202249882), Fundamental
Research Funds for the Provincial Universities of Zhejiang, The
Natural Science Foundation of Zhejiang Province (grant no.
LY20C070001), The National Natural Science Foundation of China
(grant no. 31801165) and The K.C. Wong Magna Fund of Ningbo
University.
Not applicable.
MY and XJ conceived the study. YG, XN and JL
collated the data. YG, XN and JL wrote the manuscript. MY and XJ
revised and edited the manuscript. Data authentication is not
applicable. All authors read and approved the final version of the
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Urick ME and Bell DW: Clinical
actionability of molecular targets in endometrial cancer. Nat Rev
Cancer. 19:510–521. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou Y, Shen J, Xia L and Wang Y: Estrogen
mediated expression of nucleophosmin 1 in human endometrial
carcinoma clinical stages through estrogen receptor-alpha
signaling. Cancer Cell International. 14:5402014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koskas M, Amant F, Mirza MR and Creutzberg
CL: Cancer of the corpus uteri: 2021 update. Int J Gynecol Obstet.
155:45–60. 2021. View Article : Google Scholar
|
|
5
|
Saito A, Yoshida H, Nishikawa T and
Yonemori K: Human epidermal growth factor receptor 2 targeted
therapy in endometrial cancer: Clinical and pathological
perspectives. World J Clin Oncol. 12:868–881. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Engelsen IB, Stefansson IM, Akslen LA and
Salvesen HB: GATA3 expression in estrogen receptor alpha-negative
endometrial carcinomas identifies aggressive tumors with high
proliferation and poor patient survival. Am J Obstet Gynecol.
199:543.e1–e7. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koshiyama M, Konishi I and Fujii S:
Pathology, hormonal aspects, and molecular genetics of the two
types of endometrial cancer. Cancer J France. 11:277–283. 1998.
|
|
8
|
Musicco C, Cormio G, Pesce V, Loizzi V,
Cicinelli E, Resta L, Ranieri G and Cormio A: Mitochondrial
dysfunctions in type I endometrial carcinoma: Exploring their role
in oncogenesis and tumor progression. Int J Mol Sci. 19:20762018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gullo G, Etrusco A, Cucinella G, Perino A,
Chiantera V, Laganà AS, Tomaiuolo R, Vitagliano A, Giampaolino P,
Noventa M, et al: Fertility-sparing approach in women affected by
stage I and low-grade endometrial carcinoma: An updated overview.
Int J Mol Sci. 22:118252021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tan DSP, Lambros MBK, Marchio C and
Reis-Filho JS: ESR1 amplification in endometrial carcinomas: Hope
or hyperbole? J Pathol. 216:271–274. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jongen V, Sluijmer AV and Heineman MJ: The
postmenopausal ovary as an androgen-producing gland; hypothesis on
the etiology of endometrial cancer. Maturitas. 43:77–85. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
AlZaabi A, AlAmri H, ALAjmi G, Allawati M,
Muhanna F, Alabri R, AlBusaidi F, AlGhafri S, Al-Mirza AA and Al
Baimani K: Endometrial surveillance in tamoxifen and letrozole
treated breast cancer patients. Cureus. 13:e200302021.PubMed/NCBI
|
|
13
|
Travaglino A, Raffone A, Mascolo M, Guida
M, Insabato L, Zannoni GF and Zullo F: TCGA molecular subgroups in
endometrial undifferentiated/dedifferentiated carcinoma. Pathol
Oncol Res. 26:1411–1416. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barton M, Filardo EJ, Lolait SJ, Thomas P,
Maggiolini M and Prossnitz ER: Twenty years of the G
protein-coupled estrogen receptor GPER: Historical and personal
perspectives. J Steroid Biochem Mol Biol. 176:4–15. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fuentes N and Silveyra P: Estrogen
receptor signaling mechanisms. Donev R: Intracellular Signalling
Proteins; pp. pp135–170. 2019
|
|
16
|
Zhang Z, Qin P, Deng Y, Ma Z, Guo H, Guo
H, Hou Y, Wang S, Zou W, Sun Y, et al: The novel estrogenic
receptor GPR30 alleviates ischemic injury by inhibiting
TLR4-mediated microglial inflammation. J Neuroinflammation.
15:2062018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Prossnitz ER and Barton M: The
G-protein-coupled estrogen receptor GPER in health and disease. Nat
Rev Endocrinol. 7:715–726. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tecalco-Cruz AC, Zepeda-Cervantes J and
Ortega-Dominguez B: Estrogenic hormones receptors in Alzheimer's
disease. Mol Biol Rep. 48:7517–7526. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rahman MT, Nakayama K, Rahman M, Ishikawa
M, Katagiri H, Katagiri A, Ishibashi T, Sato E, Iida K, Ishikawa N,
et al: ESR1 gene amplification in endometrial carcinomas: A
clinicopathological analysis. Anticancer Res. 33:3775–3781.
2013.PubMed/NCBI
|
|
20
|
Lara-Castillo N: Estrogen signaling in
bone. Appl Sci Basel. 11:44392021. View Article : Google Scholar
|
|
21
|
Lebeau A, Grob TJ, Hoist F,
Seyedi-Fazlollahi N, Moch H, Terracciano L, Turzynski A, Choschzick
M, Sauter G and Simon R: Oestrogen receptor gene (ESR1)
amplification is frequent in endometrial carcinoma and its
precursor lesions. J Pathol. 216:151–157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo WY, Zeng SMZ, Deora GS, Li QS and Ruan
BF: Estrogen Receptor α (ERα)-targeting compounds and derivatives:
Recent advances in structural modification and bioactivity. Curr
Top Med Chem. 19:1318–1337. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Krasner C: Aromatase inhibitors in
gynecologic cancers. J Steroid Biochem Mol Biol. 106:76–80. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rao J, Jiang X, Wang Y and Chen B:
Advances in the understanding of the structure and function of
ER-alpha 36, a novel variant of human estrogen receptor-alpha. J
Steroid Biochem Mol Biol. 127:231–237. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arao Y and Korach KS: Transactivation
Function-1-mediated partial agonist activity of selective estrogen
receptor modulator requires homo-dimerization of the estrogen
receptor alpha ligand binding domain. Int J Mol Sci. 20:37182019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jia M, Dahlman-Wright K and Gustafsson J:
Estrogen receptor alpha and beta in health and disease. Best Pract
Res Clin Endocrinol Metab. 29:557–568. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bouricha EM, Hakmi M, Akachar J, Zouaidia
F and Ibrahimi A: In-silico identification of potential inhibitors
targeting the DNA binding domain of estrogen receptor alpha for the
treatment of hormone therapy-resistant breast cancer. J Biomol
Struct Dyn. 40:5203–5210. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arao Y and Korach KS: The physiological
role of estrogen receptor functional domains. Essays Biochem.
65:867–875. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tecalco-Cruz AC, Perez-Alvarado IA,
Ramirez-Jarquin JO and Rocha-Zavaleta L: Nucleo-cytoplasmic
transport of estrogen receptor alpha in breast cancer cells. Cell
Signal. 34:121–132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Skafar DF and Zhao C: The multifunctional
estrogen receptor-alpha F domain. Endocrine. 33:1–8. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zwart W, de Leeuw R, Rondaij M, Neefjes J,
Mancini MA and Michalides R: The hinge region of the human estrogen
receptor determines functional synergy between AF-1 and AF-2 in the
quantitative response to estradiol and tamoxifen. J Cell Sci.
123:1253–1261. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Božović A, Mandušić V, Todorović L and
Krajnović M: Estrogen receptor Beta: The promising biomarker and
potential target in metastases. Int J Mol Sci. 22:16562021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rocha W, Sanchez R, Deschenes J, Auger A,
Hébert E, White JH and Mader S: Opposite effects of histone
deacetylase inhibitors on glucocorticoid and estrogen signaling in
human endometrial ishikawa cells. Mol Pharmacol. 68:1852–1862.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen X, Yan Q, Li S, Zhou L, Yang H, Yang
Y, Liu X and Wan X: Expression of the tumor suppressor miR-206 is
associated with cellular proliferative inhibition and impairs
invasion in ER alpha-positive endometrioid adenocarcinoma. Cancer
Lett. 314:41–53. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hermon TL, Moore AB, Yu L, Kissling GE,
Castora FJ and Dixon D: Estrogen receptor alpha (ER alpha)
phospho-serine-118 is highly expressed in human uterine leiomyomas
compared to matched myometrium. Virchows Archiv. 453:557–569. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bao W, Zhang Y, Li S, Fan Q, Qiu M, Wang
Y, Li Y, Ji X, Yang Y, Sang Z, et al: miR-107-5p promotes tumor
proliferation and invasion by targeting estrogen receptor-alpha in
endometrial carcinoma. Oncol Rep. 41:1575–1585. 2019.PubMed/NCBI
|
|
37
|
Wu Y, Zeng K, Wang C, Wang S, Sun H, Liu
W, Wang X, Niu J, Cong SY, Zhou X and Zhao Y: Histone
acetyltransferase MOF is involved in suppression of endometrial
cancer and maintenance of ERα stability. Biochem Biophys Res
Commun. 509:541–548. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kershah SM, Desouki MM, Koterba KL and
Rowan BG: Expression of estrogen receptor coregulators in normal
and malignant human endometrium. Gynecol Oncol. 92:304–313. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gao M, Sun PM, Wang JL, Li XP, Zhao C and
Wei LH: Different biological effect of estrogen receptor-related
receptor alpha in estrogen receptor-positive and -negative
endometrial carcinoma. Mol Med Rep. 1:917–924. 2008.PubMed/NCBI
|
|
40
|
Shiozawa T, Itoh K, Horiuchi A, Konishi I,
Fujii S and Nikaido T: Down-regulation of estrogen receptor by the
methylation of the estrogen receptor gene in endometrial carcinoma.
Anticancer Res. 22:139–143. 2002.PubMed/NCBI
|
|
41
|
Liu B, Che Q, Qiu H, Bao W, Chen X, Lu W,
Li B and Wan X: Elevated MiR-222-3p promotes proliferation and
invasion of endometrial carcinoma via targeting ERα. PLoS One.
9:e875632014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Song Q, An Q, Niu B, Lu X, Zhang N and Cao
X: Role of miR-221/222 in tumor development and the underlying
mechanism. J Oncol. 2019:72520132019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang G, Hou X and Gao S: Stimulation of
peroxisome proliferator-activated receptor gamma inhibits estrogen
receptor alpha transcriptional activity in endometrial carcinoma
cells. Oncol Rep. 33:1227–1234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Uchida S, Saimi M, Li ZL, Miyaso H,
Nagahori K, Kawata S, Omotehara T, Ogawa Y and Itoh M: Effects of
phosphorylated estrogen receptor alpha on apoptosis in human
endometrial epithelial cells. Anat Sci Int. 95:240–250. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang P, Gao K, Jin X, Ma J, Peng J,
Wumaier R, Tang Y, Zhang Y, An J, Yan Q, et al: Endometrial
cancer-associated mutants of SPOP are defective in regulating
estrogen receptor-α protein turnover. Cell Death Dis. 6:e16872015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sentis S, Le Romancer M, Bianchin C,
Rostan MC and Corbo L: Sumoylation of the estrogen receptor alpha
hinge region regulates its transcriptional activity. Mol
Endocrinol. 19:2671–2684. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
O'Doherty A, Church SW, Russell SEH,
Nelson J and Hickey I: Methylation status of oestrogen
receptor-alpha gene promoter sequences in human ovarian epithelial
cell lines. Br J Cancer. 86:282–284. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li Y, Zhou Y, Mao F, Shen S, Zhao B, Xu Y,
Lin Y, Zhang X, Cao X, Xu Y, et al: miR-452 reverses abnormal
glycosylation modification of ERα and estrogen resistance in TNBC
(Triple-Negative Breast Cancer) Through Targeting UGT1A1. Front
Oncol. 10:15092020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Onate SA, Boonyaratanakornkit V, Spencer
TE, Tsai SY, Tsai MJ, Edwards DP and O'Malley BW: The steroid
receptor coactivator-1 contains multiple receptor interacting and
activation domains that cooperatively enhance the activation
function 1 (AF1) and AF2 domains of steroid receptors. J Biol Chem.
273:12101–12108. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kato S, Endoh H, Masuhiro Y, Kitamoto T,
Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H,
et al: Activation of the estrogen-receptor through phosphorylation
by mitogen-activated protein-kinase. Science. 270:1491–1494. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Vilgelm A, Lian ZL, Wang H, Beauparlant
SL, Klein-Szanto A, Ellenson LH and Di Cristofano A: Akt-mediated
phosphorylation and activation of estrogen receptor alpha is
required for endometrial neoplastic transformation in Pten(+/-)
mice. Cancer Res. 66:3375–3380. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kato E, Orisaka M, Kurokawa T, Chino Y,
Fujita Y, Shinagawa A and Yoshida Y: Relation between outcomes and
expression of estrogen receptor-alpha phosphorylated at Ser(167) in
endometrioid endometrial cancer. Cancer Sci. 105:1307–1312. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lee H and Bai W: Regulation of estrogen
receptor nuclear export by ligand-induced and p38-mediated receptor
phosphorylation. Mol Cell Biol. 22:5835–5845. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ohtake F, Fujii-Kuriyama Y and Kato S: AhR
acts as an E3 ubiquitin ligase to modulate steroid receptor
functions. Biochem Pharmacol. 77:474–484. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Han SJ, Begum K, Foulds CE, Hamilton RA,
Bailey S, Malovannaya A, Chan D, Qin J and O'Malley BW: The dual
estrogen receptor α inhibitory effects of the tissue-selective
estrogen complex for endometrial and breast safety. Mol Pharmacol.
89:14–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Su Y, Zeng K, Liu S, Wu Y, Wang C, Wang S,
Lin L, Zou R, Sun G, Luan R, et al: Ubiquitin-specific peptidase 14
maintains estrogen receptor α stability via its deubiquitination
activity in endometrial cancer. J Biol Chem. 299:1027342023.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lv Q, Xie L, Cheng Y, Shi Y, Shan W, Ning
C, Xie B, Yang B, Luo X, He Q, et al: A20-mediated deubiquitination
of ERα in the microenvironment of CD163+ macrophages
sensitizes endometrial cancer cells to estrogen. Cancer Lett.
442:137–147. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Xu ZX, Liu J, Gu LP, Huang B and Pan XJ:
Biological effects of xenoestrogens and the functional mechanisms
via genomic and nongenomic pathways. Environmental Rev. 25:306–322.
2017. View Article : Google Scholar
|
|
59
|
Stefkovich ML, Arao Y, Hamilton KJ and
Korach KS: Experimental models models for evaluating non-genomic
estrogen signaling. Steroids. 133:34–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang ZY and Yin L: Estrogen receptor
alpha-36 (ER-α36): A new player in human breast cancer. Mol Cell
Endocrinol. 418:193–206. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Manavathi B, Samanthapudi VSK and
Gajulapalli VNR: Estrogen receptor coregulators and pioneer
factors: The orchestrators of mammary gland cell fate and
development. Front Cell Dev Biol. 2:342014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Che Q, Liu BY, Liao Y, Zhang HJ, Yang TT,
He YY, Xia YH, Lu W, He XY, Chen Z, et al: Activation of a positive
feedback loop involving IL-6 and aromatase promotes intratumoral
17β-estradiol biosynthesis in endometrial carcinoma
microenvironment. Int J Cancer. 135:282–294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang ZP and Teng CT: Estrogen receptor
alpha and estrogen receptor-related receptor alpha 1 compete for
binding and coactivator. Mol Cell Endocrinol. 172:223–233. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Gao M, Sun PM, Zhao D, Wang JL, Li XP and
Wei LH: Regulatory effect of 17beta-estradiol on expression of
orphan nuclear receptor ERRalpha in endometrial carcinoma cell
lines. Ai Zheng. 25:538–542. 2006.(In Chinese). PubMed/NCBI
|
|
65
|
Mylonas I, Jeschke U, Shabani N, Kuhn C,
Kriegel S, Kupka MS and Friese K: Normal and malignant human
endometrium express immunohistochemically estrogen receptor alpha
(ER-alpha), estrogen receptor beta (ER-beta) and progesterone
receptor (PR). Anticancer Res. 25:1679–1686. 2005.PubMed/NCBI
|
|
66
|
Su T, Qu JJ, Wang K, Li BL, Zhao D, Zhu
YP, Ye L, Lu W and Wan XP: Cross-talk between p21-activated kinase
4 and ER alpha signaling triggers endometrial cancer cell
proliferation. Oncotarget. 8:68083–68094. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhou XH, Xu ST, Song WY and Teng XD:
Expression of receptor-binding cancer antigen expressed on SiSo
cells in endometrial carcinoma and the correlation thereof with the
expression of estrogen receptor subtypes. Zhonghua Yi Xue Za Zhi.
87:1900–1903. 2007.(In Chinese). PubMed/NCBI
|
|
68
|
Hou X, Zhao M, Wang T and Zhang G:
Upregulation of estrogen receptor mediates migration, invasion and
proliferation of endometrial carcinoma cells by regulating the
PI3K/AKT/mTOR pathway. Oncol Rep. 31:1175–1182. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhao L, Watanabe M, Yano T, Yanagisawa J,
Nakagawa S, Oishi H, Wada-Hiraike O, Oda K, Minaguchi T, Yasugi T,
et al: Analysis of the status of the novel estrogen receptor alpha
(ERα) coactivator p72 in endometrial cancer and its cross talk with
erbB-2 in the transactivation of ERα. Mol Med Rep. 1:387–390.
2008.PubMed/NCBI
|
|
70
|
Nagarajan S, Hossan T, Alawi M, Najafova
Z, Indenbirken D, Bedi U, Taipaleenmäki H, Ben-Batalla I, Scheller
M, Loges S, et al: Bromodomain protein BRD4 is required for
estrogen receptor-dependent enhancer activation and gene
transcription. Cell Rep. 8:460–469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Vadlamudi RK, Balasenthil S, Broaddus RR,
Gustafsson JA and Kumar R: Deregulation of estrogen receptor
coactivator proline-, glutamic acid-, and leucine-rich
protein-1/modulator of nongenomic activity of estrogen receptor in
human endometrial tumors. J Clin Endocrinol Metab. 89:6130–6138.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Thorne AM, Jackson TA, Willis VC and
Bradford AP: Protein Kinase C α modulates
estrogen-receptor-dependent transcription and proliferation in
endometrial cancer cells. Obstet Gynecol Int. 2013:5374792013.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Frigo DE, Basu A, Nierth-Simpson EN,
Weldon CB, Dugan CM, Elliott S, Collins-Burow BM, Salvo VA, Zhu Y,
Melnik LI, et al: p38 mitogen-activated protein kinase stimulates
estrogen-mediated transcription and proliferation through the
phosphorylation and potentiation of the p160 coactivator
glucocorticoid receptor-interacting protein 1. Mol Endocrinol.
20:971–983. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kojima M, Sugimoto K, Kobayashi M,
Ichikawa-Tomikawa N, Kashiwagi K, Watanabe T, Soeda S, Fujimori K
and Chiba H: Aberrant Claudin-6-adhesion signaling promotes
endometrial cancer progression via estrogen receptor α. Mol Cancer
Res. 19:1208–1220. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lee H, Jiang F, Wang Q, Nicosia SV, Yang
J, Su B and Bai W: MEKK1 activation of human estrogen receptor
alpha and stimulation of the agonistic activity of
4-hydroxytamoxifen in endometrial and ovarian cancer cells. Mol
Endocrinol. 14:1882–1896. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lian Z, De Luca P and Di Cristofano A:
Gene expression analysis reveals a signature of estrogen receptor
activation upon loss of Pten in a mouse model of endometrial
cancer. J Cell Physiol. 208:255–266. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Velarde MC, Zeng Z, McQuown JR, Simmen FA
and Simmen RCM: Kruppel-like factor 9 is a negative regulator of
ligand-dependent estrogen receptor alpha signaling in Ishikawa
endometrial adenocarcinoma cells. Mol Endocrinol. 21:2988–3001.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Cheng R, Xue X and Liu X: Expression of
IL17A in endometrial carcinoma and effects of IL17A on biological
behaviour in Ishikawa cells. J Int Med Res. 48:3000605209505632020.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wang D, Wang M, Hu CE, Shuang T, Zhou Y
and Yan X: Expression of the ELAV-like protein HuR in the cytoplasm
is associated with endometrial carcinoma progression. Tumor Biol.
35:11939–11947. 2014. View Article : Google Scholar
|
|
80
|
Gu CJ, Xie F, Zhang B, Yang HL, Cheng J,
He YY, Zhu XY, Li DJ and Li MQ: High glucose promotes
epithelial-mesenchymal transition of uterus endometrial cancer
cells by increasing ER/GLUT4-mediated VEGF secretion. Cell Physiol
Biochem. 50:706–720. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Nan F, Wei S, Guan D, Zhang L, Guo Q, Cao
S, Liu Y, Liu Y and Sun M: Suppressive efficiency of RASSF1A in
endometrial carcinoma via inhabiting estrogen receptor alpha
expression and ERK pathway activation. Int J Clin Exp Pathol.
11:577–585. 2018.PubMed/NCBI
|
|
82
|
Tanwar PS, Zhang L, Roberts DJ and
Teixeira JM: Stromal deletion of the APC tumor suppressor in mice
triggers development of endometrial cancer. Cancer Res.
71:1584–1596. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Tong H, Ke JQ, Jiang FZ, Wang XJ, Wang FY,
Li YR, Lu W and Wan XP: Tumor-associated macrophage-derived CXCL8
could induce ERα suppression via HOXB13 in endometrial cancer.
Cancer Lett. 376:127–136. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhou XH, Teng XD, Song WY and Wu YJ:
Expression of receptor-binding cancer antigen expressed on SiSo
cells and estrogen receptor subtypes in the normal, hyperplastic,
and carcinomatous endometrium. Int J Gynecol Cancer. 18:152–158.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Tian W, Teng F, Gao J, Gao C, Liu G, Zhang
Y, Yu S, Zhang W, Wang Y and Xue F: Estrogen and insulin
synergistically promote endometrial cancer progression via
crosstalk between their receptor signaling pathways. Cancer Biol
Med. 16:55–70. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zhang F, Peng L, Huang Y, Lin X, Zhou L
and Chen J: Chronic BDE-47 exposure aggravates malignant phenotypes
and chemoresistance by activating ERK through ERα and GPR30 in
endometrial carcinoma. Front Oncol. 9:10792019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Saito S, Ito K, Suzuki T, Utsunomiya H,
Akahira J, Sugihashi Y, Niikura H, Okamura K, Yaegashi N and Sasano
H: Orphan nuclear receptor DAX-1 in human endometrium and its
disorders. Cancer Sci. 96:645–652. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Collins F, Itani N, Esnal-Zufiaurre A,
Gibson DA, Fitzgerald C and Saunders PTK: The ERβ 5 splice variant
increases oestrogen responsiveness of ERαpos Ishikawa cells. Endocr
Relat Cancer. 27:55–66. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Bircan S, Ensari A, Ozturk S, Erdogan N,
Dundar I and Ortac F: Immunohistochemical analysis of c-myc, c-jun
and estrogen receptor in normal, hyperplastic and neoplastic
endometrium. Pathol Oncol Res. 11:32–39. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Nakayama H, Sano T, Motegi A, Oyama T and
Nakajima T: Increasing 14-3-3 sigma expression with declining
estrogen receptor alpha and estrogen-responsive finger protein
expression defines malignant progression of endometrial carcinoma.
Pathol Int. 55:707–715. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Wang J, Bao W, Qiu M, Liao Y, Che Q, Yang
T, He X, Qiu H and Wan X: Forkhead-box A1 suppresses the
progression of endometrial cancer via crosstalk with estrogen
receptor α. Oncol Rep. 31:1225–1234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Chen Z, Yang HJ, Lin Q, Zhu MJ, Yu YY, He
XY and Wan XP: Estrogen-ERα signaling and DNA hypomethylation
co-regulate expression of stem cell protein PIWIL1 in ER
alpha-positive endometrial cancer cells. Cell Commun Signal.
18:842020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Flamini MI, Sanchez AM, Genazzani AR and
Simoncini T: Estrogen regulates endometrial cell cytoskeletal
remodeling and motility via focal adhesion kinase. Fertility
Sterility. 95:722–726. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Sayeed A, Konduri SD, Liu W, Bansal S, Li
F and Das GM: Estrogen receptor alpha inhibits p53-mediated
transcriptional repression: Implications for the regulation of
apoptosis. Cancer Res. 67:7746–7755. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhang R, He Y, Zhang X, Xing B, Sheng Y,
Lu H and Wei Z: Estrogen receptor-regulated microRNAs contribute to
the BCL2/BAX imbalance in endometrial adenocarcinoma and
precancerous lesions. Cancer Lett. 314:155–165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Chao A, Lin CY, Tsai CL, Hsueh S, Lin YY,
Lin CT, Chou HH, Wang TH, Lai CH and Wang HS: Estrogen stimulates
the proliferation of human endometrial cancer cells by stabilizing
nucleophosmin/B23 (NPM/B23). J Mol Med (Berl). 91:249–259. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Hu G, Zhang J, Zhou X, Liu J, Wang Q and
Zhang B: Roles of estrogen receptor α and β in the regulation of
proliferation in endometrial carcinoma. Pathol Res Pract.
216:1531492020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Saito T, Tanaka R, Wataba K, Kudo R and
Yamasaki H: Overexpression of estrogen receptor-α gene suppresses
gap junctional intercellular communication in endometrial carcinoma
cells. Oncogene. 23:1109–1116. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhu Y, Shen J, Gao L and Feng Y: Estrogen
promotes fat mass and obesity-associated protein nuclear
localization and enhances endometrial cancer cell proliferation via
the mTOR signaling pathway. Oncol Rep. 35:2391–2397. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Chen R, Zhang M, Liu W, Chen H, Cai T,
Xiong H, Sheng X, Liu S, Peng J, Wang F, et al: Estrogen affects
the negative feedback loop of PTENP1-miR200c to inhibit PTEN
expression in the development of endometrioid endometrial
carcinoma. Cell Death Dis. 10:42018. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Scully MM, Palacios-Helgeson LK, Wah LS
and Jackson TA: Rapid estrogen signaling negatively regulates PTEN
activity through phosphorylation in endometrial cancer cells. Horm
Cancer. 5:218–231. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Mizumoto H, Saito T, Ashihara K, Nishimura
M, Tanaka R and Kudo R: Acceleration of invasive activity via
matrix metalloproteinases by transfection of the estrogen
receptor-α gene in endometrial carcinoma cells. Int J Cancer.
100:401–406. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Liu Y, Zhao R, Chi S, Zhang W, Xiao C,
Zhou X, Zhao Y and Wang H: UBE2C is upregulated by estrogen and
promotes epithelial-mesenchymal transition via p53 in endometrial
cancer. Mol Cancer Res. 18:204–215. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhang Z, Zhou D, Lai Y, Liu Y, Tao X, Wang
Q, Zhao G, Gu H, Liao H, Zhu Y, et al: Estrogen induces endometrial
cancer cell proliferation and invasion by regulating the fat mass
and obesity-associated gene via PI3K/AKT and MAPK signaling
pathways. Cancer Lett. 319:89–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Wik E, Raeder MB, Krakstad C, Trovik J,
Birkeland E, Hoivik EA, Mjos S, Werner HM, Mannelqvist M,
Stefansson IM, et al: Lack of estrogen receptor-alpha is associated
with epithelial-mesenchymal transition and PI3K alterations in
endometrial carcinoma. Clin Cancer Res. 19:1094–1105. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Yang T, Zhang H, Qiu H, Li B, Wang J, Du
G, Ren C and Wan X: EFEMP1 is repressed by estrogen and inhibits
the epithelial-mesenchymal transition via Wnt/β-catenin signaling
in endometrial carcinoma. Oncotarget. 7:25712–25725. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Yang T, Ren C, Jiang A, Yu Z, Li G, Wang G
and Zhang Q: RIZ1 is regulated by estrogen and suppresses tumor
progression in endometrial cancer. Biochem Biophys Res Commun.
489:96–102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Owens GL, Lawrence KM, Jackson TR, Crosbie
EJ, Sayan BS, Kitchener HC and Townsend PA: Urocortin suppresses
endometrial cancer cell migration via CRFR2 and its system
components are differentially modulated by estrogen. Cancer Med.
6:408–415. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Mita AC, Mita MM, Nawrocki ST and Giles
FJ: Survivin: Key regulator of mitosis and apoptosis and novel
target for cancer therapeutics. Clin Cancer Res. 14:5000–5005.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Chuwa AH, Sone K, Oda K, Tanikawa M,
Kukita A, Kojima M, Oki S, Fukuda T, Takeuchi M, Miyasaka A, et al:
Kaempferol, a natural dietary flavonoid, suppresses
17β-estradiol-induced survivin expression and causes apoptotic cell
death in endometrial cancer. Oncol Lett. 16:6195–6201.
2018.PubMed/NCBI
|
|
111
|
Abe N, Watanabe J, Tsunoda S, Kuramoto H
and Okayasu I: Significance of nuclear p-Akt in endometrial
carcinogenesis rapid translocation of p-Akt into the nucleus by
estrogen, possibly resulting in inhibition of apoptosis. Int J
Gynecological Cancer. 21:194–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Wincewicz A, Baltaziak M, Kanczuga-Koda L,
Koda M, Sulkowska U, Famulski W and Sulkowski S: STAT3 and
apoptosis regulators: Bak and Bcl-xL in endometrioid
adenocarcinomas of different estrogen receptor-alpha immunoprofile.
Gynecol Endocrinol. 27:536–540. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Sulkowska U, Wincewicz A, Kanczuga-Koda L,
Koda M and Sulkowski S: Eventual proapoptotic or anti-apoptotic
impact of aberrantly expressed Cx43 and Cx26 can depend on ER-alpha
overexpression in human endometrioid adenocarcinoma. Gynecol
Endocrinol. 31:604–608. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Abe S, Iwasaki M, Habata S, Mariya T,
Tamate M, Matsuura M, Satohisa S and Saito T: ERα increases
endometrial cancer cell resistance to cisplatin via upregulation of
BAG3. Oncol Lett. 21:202021.PubMed/NCBI
|
|
115
|
Zhang JW and Peng ZL: Association between
the expression of estrogen receptor subunit in endometrial
carcinoma and the prognostic factors of endometrial carcinoma.
Sichuan Da Xue Xue Bao Yi Xue Ban. 35:843–845. 2004.(In Chinese).
PubMed/NCBI
|
|
116
|
Droog M, Nevedomskaya E, Dackus GM, Fles
R, Kim Y, Hollema H, Mourits MJ, Nederlof PM, van Boven HH, Linn
SC, et al: Estrogen receptor alpha wields treatment-specific
enhancers between morphologically similar endometrial tumors. Proc
Natl Acad Sci USA. 114:E1316–E1325. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Sakamoto T, Eguchi H, Omoto Y, Ayabe T,
Mori H and Hayashi S: Estrogen receptor-mediated effects of
tamoxifen on human endometrial cancer cells. Mol Cell Endocrinol.
192:93–104. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Shah YM and Rowan BG: The Src kinase
pathway promotes tamoxifen agonist action in Ishikawa endometrial
cells through phosphorylation-dependent stabilization of estrogen
receptor (alpha) promoter interaction and elevated steroid receptor
coactivator 1 activity. Mol Endocrinol. 19:732–748. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Emons G, Mustea A and Tempfer C: Tamoxifen
and endometrial cancer: A Janus-Headed Drug. Cancers. 12:25352020.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Nikolic I, Andjelkovic M, Zaric M, Zelen
I, Canovic P, Milosavljevic Z and Mitrovic M: Induction of
mitochondrial apoptotic pathway by raloxifene and estrogen in human
endometrial stromal ThESC cell line. Arch Med Sci. 13:293–301.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Wu Y, Niwa K, Onogi K, Tang L, Mori H and
Tamaya T: Effects of selective estrogen receptor modulators and
genistein on the expression of ER alpha/beta and COX-1/2 in
ovarectomized mouse uteri. Eur J Gynaecol Oncol. 28:89–94.
2007.PubMed/NCBI
|
|
122
|
Boisen MM, Andersen CL, Sreekumar S, Stern
AM and Oesterreich S: Treating gynecologic malignancies with
selective estrogen receptor downregulators (SERDs): Promise and
challenges. Mol Cell Endocrinol. 418:322–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Holst F, Hoivik EA, Gibson WJ,
Taylor-Weiner A, Schumacher SE, Asmann YW, Grossmann P, Trovik J,
Necela BM, Thompson EA, et al: Recurrent hormone-binding domain
truncated ESR1 amplifications in primary endometrial cancers
suggest their implication in hormone independent growth. Sci Rep.
6:255212016. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Amita M, Takahashi T, Igarashi H and
Nagase S: Clomiphene citrate down-regulates estrogen receptor-alpha
through the ubiquitin-proteasome pathway in a human endometrial
cancer cell line. Mol Cell Endocrinol. 428:142–147. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Bae-Jump VL, Zhou C, Boggess JF and Gehrig
PA: Arsenic trioxide (As2O3) inhibits expression of estrogen
receptor-alpha through regulation of the mitogen-activated protein
kinase (MAPK) pathway in endometrial cancer cells. Reproductive
Sci. 15:1011–1017. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Collins G, Mesiano S and DiFeo A: Effects
of metformin on cellular proliferation and steroid hormone
receptors in patient-derived, low-grade endometrial cancer cell
lines. Reprod Sci. 26:609–618. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Mao X, Dong B, Gao M, Ruan G, Huang M,
Braicu EI, Sehouli J and Sun P: Dual targeting of estrogen receptor
alpha and estrogen-related receptor alpha: A novel endocrine
therapy for endometrial cancer. Onco Targets Ther. 12:6757–6767.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Yamamoto T, Mori T, Sawada M, Kuroboshi H,
Tatsumi H, Yoshioka T, Matsushima H, Iwasaku K and Kitawaki J:
Estrogen-related receptor-γ regulates estrogen receptor-α
responsiveness in uterine endometrial cancer. Int J Gynecol Cancer.
22:1509–1516. 2012.PubMed/NCBI
|
|
129
|
Watanabe M, Kobayashi Y, Takahashi N,
Kiguchi K and Ishizuka B: Expression of melatonin receptor (MT1)
and interaction between melatonin and estrogen in endometrial
cancer cell line. J Obstet Gynaecol Res. 34:567–573. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Faigenbaum R, Haklai R, Ben-Baruch G and
Kloog Y: Growth of poorly differentiated endometrial carcinoma is
inhibited by combined action of medroxyprogesterone acetate and the
Ras inhibitor Salirasib. Oncotarget. 4:316–328. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Miki Y, Iwabuchi E, Takagi K, Suzuki T,
Sasano H, Yaegashi N and Ito K: Co-expression of nuclear
heterogeneous nuclear ribonucleic protein K and estrogen receptor α
in endometrial cancer. Pathol Res Pract. 231:153795. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Krakstad C, Trovik J, Wik E, Engelsen IB,
Werner HM, Birkeland E, Raeder MB, Øyan AM, Stefansson IM, Kalland
KH, et al: Loss of GPER identifies new targets for therapy among a
subgroup of ERα-positive endometrial cancer patients with poor
outcome. Br J Cancer. 106:1682–1688. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Lin CY, Chao A, Wang TH, Lee LY, Yang LY,
Tsai CL, Wang HS and Lai CH: Nucleophosmin/B23 is a negative
regulator of estrogen receptor α expression via AP2γ in endometrial
cancer cells. Oncotarget. 7:60038–60052. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Guo T, Li B and Gu C: Expression of hPEBP4
negatively correlates with estrogen and progesterone receptors in
endometrial carcinoma. J Buon. 18:465–470. 2013.PubMed/NCBI
|
|
135
|
Taylor AH, Al-Azzawi F, Pringle JH and
Bell SC: Inhibition of endometrial carcinoma cell growth using
antisense estrogen receptor oligodeoxyribonucleotides. Anticancer
Res. 22:3993–4003. 2002.PubMed/NCBI
|
|
136
|
Fournier DB, Chisamore M, Lurain JR,
Rademaker AW, Jordan VC and Tonetti DA: Protein kinase C α
expression is inversely related to ER status in endometrial
carcinoma: Possible role in AP-1-mediated proliferation of
ER-negative endometrial cancer. Gynecol Oncol. 81:366–372. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Jongen V, Briet J, de Jong R, Ten Hoor K,
Boezen M, van der Zee A, Nijman H and Hollema H: Expression of
estrogen receptor-alpha and -beta and progesterone receptor-A and
-B in a large cohort of patients with endometrioid endometrial
cancer. Gynecol Oncol. 112:537–542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Ren S, Wu J, Yin W, Liao Q, Gong S, Xuan B
and Mu X: Researches on the correlation between estrogen and
progesterone receptors expression and disease-free survival of
endometrial cancer. Cancer Manag Res. 12:12635–12647. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Mylonas I: Prognostic significance and
clinical importance of estrogen receptor alpha and beta in human
endometrioid adenocarcinomas. Oncol Rep. 24:385–393. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Shabani N, Kuhn C, Kunze S, Schulze S,
Mayr D, Dian D, Gingelmaier A, Schindlbeck C, Willgeroth F, Sommer
H, et al: Prognostic significance of oestrogen receptor alpha
(ERalpha) and beta (ERbeta), progesterone receptor A (PR-A) and B
(PR-B) in endometrial carcinomas. Eur J Cancer. 43:2434–2444. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Creasman WT: Prognostic significance of
hormone receptors in endometrial cancer. Cancer. 71:1467–1470.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Tsujikawa T, Yoshida Y, Kiyono Y, Kurokawa
T, Kudo T, Fujibayashi Y, Kotsuji F and Okazawa H: Functional
oestrogen receptor α imaging in endometrial carcinoma using
16α-[18F]fluoro-17β-oestradiol PET. Eur J Nucl Med Mol
Imaging. 38:37–45. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Sho T, Hachisuga T, Nguyen TT, Urabe R,
Kurita T, Kagami S, Kawagoe T, Matsuura Y and Shimajiri S:
Expression of estrogen receptor-α as a prognostic factor in
patients with uterine serous carcinoma. Int J Gynecol Cancer.
24:102–106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Kreizman-Shefer H, Pricop J, Goldman S,
Elmalah I and Shalev E: Distribution of estrogen and progesterone
receptors isoforms in endometrial cancer. Diagnostic Pathology.
9:772014. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Pathirage N, Di Nezza LA, Salmonsen LA,
Jobling T, Simpson ER and Clyne CD: Expression of aromatase,
estrogen receptors, and their coactivators in patients with
endometrial cancer. Fertility Sterility. 86:469–472. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Chambliss KL, Wu Q, Oltmann S, Konaniah
ES, Umetani M, Korach KS, Thomas GD, Mineo C, Yuhanna IS, Kim SH,
et al: Non-nuclear estrogen receptor alpha signaling promotes
cardiovascular protection but not uterine or breast cancer growth
in mice. J Clin Invest. 120:2319–2330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Duan C, Liu X, Liang S, Yang Z, Xia M,
Wang L, Chen S and Yu L: Oestrogen receptor-mediated expression of
Olfactomedin 4 regulates the progression of endometrial
adenocarcinoma. J Cell Mol Med. 18:863–874. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Ali SH, O'Donnell AL, Balu D, Pohl MB,
Seyler MJ, Mohamed S, Mousa S and Dandona P: Estrogen
receptor-alpha in the inhibition of cancer growth and angiogenesis.
Cancer Res. 60:7094–7098. 2000.PubMed/NCBI
|
|
149
|
Ali SH, O'Donnell AL, Mohamed S, Mousa S
and Dandona P: Overexpression of estrogen receptor-α in the
endometrial carcinoma cell line Ishikawa: Inhibition of growth and
angiogenic factors. Gynecol Oncol. 95:637–645. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Stoner M, Wang F, Wormke M, Nguyen T,
Samudio I, Vyhlidal C, Marme D, Finkenzeller G and Safe S:
Inhibition of vascular endothelial growth factor expression in
HEC1A endometrial cancer cells through interactions of estrogen
receptor alpha and Sp3 proteins. J Biol Chem. 275:22769–22779.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Joshi A, Wang H, Jiang G, Douglas W, Chan
JS, Korach KS and Ellenson LH: Endometrial tumorigenesis in
Pten(+/-) mice is independent of coexistence of estrogen and
estrogen receptor α. Am J Pathol. 180:2536–2547. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Wedren S, Lovmar L, Humphreys K, Magnusson
C, Melhus H, Syvänen AC, Kindmark A, Landegren U, Fermér ML, Stiger
F, et al: Estrogen receptor alpha gene polymorphism and endometrial
cancer risk-a case-control study. BMC Cancer. 8:3222008. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Jazaeri O, Shupnik MA, Jazaeri AA and Rice
LW: Expression of estrogen receptor alpha mRNA and protein variants
in human endometrial carcinoma. Gynecol Oncol. 74:38–47. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Tu BB, Lin SL, Yan LY, Wang ZY, Sun QY and
Qiao J: ER-α36, a novel variant of estrogen receptor α, is involved
in EGFR-related carcinogenesis in endometrial cancer. Am J Obstet
Gynecol. 205:227.e1–e6. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Bryant W, Snowhite AE, Rice LW and Shupnik
MA: The estrogen receptor (ER)alpha variant Delta5 exhibits
dominant positive activity on ER-regulated promoters in endometrial
carcinoma cells. Endocrinology. 146:751–759. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Cancer Genome Atlas Research Network, .
Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H,
Robertson AG, Pashtan I, Shen R, et al: Integrated genomic
characterization of endometrial carcinoma. Nature. 497:67–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
McAlpine J, Leon-Castillo A and Bosse T:
The rise of a novel classification system for endometrial
carcinoma; integration of molecular subclasses. J Pathol.
244:538–549. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Travaglino A, Raffone A, Gencarelli A,
Saracinelli S, Riccardi C, Mollo A, Zullo F and Insabato L:
Clinico-pathological features associated with mismatch repair
deficiency in endometrial undifferentiated/dedifferentiated
carcinoma: A systematic review and meta-analysis. Gynecol Oncol.
160:579–585. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Stelloo E, Nout RA, Osse EM,
Jürgenliemk-Schulz IJ, Jobsen JJ, Lutgens LC, van der Steen-Banasik
EM, Nijman HW, Putter H, Bosse T, et al: Improved risk assessment
by integrating molecular and clinicopathological factors in
Early-stage endometrial cancer-combined analysis of the PORTEC
cohorts. Clin Cancer Res. 22:4215–4224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Stelloo E, Bosse T, Nout RA, MacKay HJ,
Church DN, Nijman HW, Leary A, Edmondson RJ, Powell ME, Crosbie EJ,
et al: Refining prognosis and identifying targetable pathways for
high-risk endometrial cancer; a TransPORTEC initiative. Mod Pathol.
28:836–844. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Gao C, Wang Y, Tian W, Zhu Y and Xue F:
The therapeutic significance of aromatase inhibitors in endometrial
carcinoma. Gynecol Oncol. 134:190–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Chen H, Liang M and Min J: Efficacy and
safety of Bevacizumab-combined chemotherapy for advanced and
recurrent endometrial cancer: A systematic review and
Meta-analysis. Balkan Med J. 38:7–12. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Stringer EM and Fleming GF: Hormone
therapy plus mTOR inhibitors in the treatment of endometrial
carcinoma. Eur Endocrinol. 9:18–21. 2013.PubMed/NCBI
|
|
164
|
De Jaeghere A, Tuyaerts S, van Nuffel AMT,
Lippens L, Hendrix A, Vuylsteke P, Henry S, Trinh XB, Van Dam PA,
Aspeslagh S, et al: Pembrolizumab, SBRT, and immunomodulation for
recurrent and/or refractory cervical or endometrial carcinoma. Ann
Oncol. 32 (Suppl 7):S1449–S1450. 2021. View Article : Google Scholar
|
|
165
|
Tsoref D, Welch S, Lau S, Biagi J, Tonkin
K, Martin LA, Ellard S, Ghatage P, Elit L, Mackay HJ, et al: Phase
II study of oral ridaforolimus in women with recurrent or
metastatic endometrial cancer. Gynecol Oncol. 135:184–189. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Bachelot T, Bourgier C, Cropet C,
Ray-Coquard I, Ferrero JM, Freyer G, Abadie-Lacourtoisie S, Eymard
JC, Debled M, Spaëth D, et al: Randomized phase II trial of
everolimus in combination with tamoxifen in patients with hormone
Receptor-positive, human epidermal growth factor receptor
2-negative metastatic breast cancer with prior exposure to
aromatase inhibitors: A GINECO study. J Clin Oncol. 30:2718–2724.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Smith IE, Dowsett M, Ebbs SR, Dixon JM,
Skene A, Blohmer JU, Ashley SE, Francis S, Boeddinghaus I and Walsh
G; IMPACT Trialists Group, : Neoadjuvant treatment of
postmenopausal breast cancer with anastrozole, tamoxifen, or both
in combination: The Immediate Preoperative Anastrozole, Tamoxifen,
or Combined with Tamoxifen (IMPACT) multicenter double-blind
randomized trial. J Clin Oncol. 23:5108–5116. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Roviello G, Francini E, Perrella A, Laera
L, Mazzei MA, Guerrini S, Roviello F, Marrelli D and Petrioli R:
Five years of stable disease with maintenance therapy using
bevacizumab and tamoxifen in a patient with metastatic breast
cancer. Cancer Biol Ther. 16:493–497. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Barberio MT, Thomas S, Chien AJ, Rugo HS,
Melisko ME, Angelidakis AN, Pawlowska N, Deal T and Munster PN:
Phase II trial with tamoxifen in combination with vorinostat and
pembrolizumab in estrogen receptor (+) hormone therapy resistant
metastatic breast cancer patients (NCT02395627). J Clin Oncol. 34
(Suppl 15):2016.
|
|
170
|
van Weelden WJ and Massuger LFAG; ENITEC,
; Pijnenborg JMA and Romano A: Anti-estrogen Treatment in
Endometrial Cancer: A Systematic Review. Front Oncol. 9:3592019.
View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Hirschfeld M, Ouyang YQ, Jaeger M, Erbes
T, Orlowska-Volk M, Zur Hausen A and Stickeler E: HNRNP G and
HTRA2-BETA1 regulate estrogen receptor alpha expression with
potential impact on endometrial cancer. BMC Cancer. 15:862015.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Stanišić V, Malovannaya A, Qin J, Lonard
DM and O'Malley BW: OTU Domain-containing ubiquitin
aldehyde-binding protein 1 (OTUB1) deubiquitinates estrogen
receptor (ER) alpha and affects ERalpha transcriptional activity. J
Biol Chem. 284:16135–16145. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Mei S, Ge S, Wang J, Li H, Jing X, Liang
K, Zhang X, Xue C, Zhang C and Zhang T: PRMT5 promotes progression
of endometrioid adenocarcinoma via ERα and cell cycle signaling
pathways. J Pathol Clin Res. 7:154–164. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Tong Z, Liu Y, Yu X, Martinez JD and Xu J:
The transcriptional co-activator NCOA6 promotes estrogen-induced
GREB1 transcription by recruiting ERα and enhancing
enhancer-promoter interactions. J Biol Chem. 294:19667–19682. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Gori I, Pellegrini C, Staedler D, Russell
R, Jan C and Canny GO: Tumor necrosis factor-α activates estrogen
signaling pathways in endometrial epithelial cells via estrogen
receptor α. Mol Cell Endocrinol. 345:27–37. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Hu H, Chen Z, Ji L, Wang Y, Yang M, Lai R,
Zhong Y, Zhang X and Wang L: ARID1A-dependent permissive chromatin
accessibility licenses estrogen-receptor signaling to regulate
circadian rhythms genes in endometrial cancer. Cancer Lett.
492:162–173. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Rodriguez AC, Vahrenkamp JM, Berrett KC,
Clark KA, Guillen KP, Scherer SD, Yang CH, Welm BE, Janát-Amsbury
MM, Graves BJ and Gertz J: ETV4 is necessary for estrogen signaling
and growth in endometrial cancer cells. Cancer Res. 80:1234–1245.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Ring KL, Yates MS, Schmandt R, Onstad M,
Zhang Q, Celestino J, Kwan SY and Lu KH: Endometrial cancers with
activating KRas mutations have activated estrogen signaling and
paradoxical response to MEK inhibition. Int J Gynecol Cancer.
27:854–862. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Fukuda T, Shirane A, Wada-Hiraike O, Oda
K, Tanikawa M, Sakuabashi A, Hirano M, Fu H, Morita Y, Miyamoto Y,
et al: HAND2-mediated proteolysis negatively regulates the function
of estrogen receptor α. Mol Med Rep. 12:5538–5544. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Klinge CM, Jernigan SC and Risinger KE:
The agonist activity of tamoxifen is inhibited by the short
heterodimer partner orphan nuclear receptor in human endometrial
cancer cells. Endocrinology. 143:853–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Feng Y, Singleton D, Guo C, Gardner A,
Pakala S, Kumar R, Jensen E, Zhang J and Khan S: DNA homologous
recombination factor SFR1 physically and functionally interacts
with estrogen receptor alpha. PLoS One. 8:e680752013. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Wu WG, Slomovitz BM, Celestino J, Chung L,
Thornton A and Lu KH: Coordinate expression of Cdc25B and ER-α is
frequent in low-grade endometrioid endometrial carcinoma but
uncommon in high-grade endometrioid and nonendometrioid carcinomas.
Cancer Res. 63:6195–6199. 2003.PubMed/NCBI
|
|
183
|
Padmanabhan RA, Nirmala L, Murali M and
Laloraya M: CrkL is a co-activator of estrogen receptor alpha that
enhances tumorigenic potential in cancer. Mol Endocrinol.
25:1499–1512. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Suga S, Kato K, Ohgami T, Yamayoshi A,
Adachi S, Asanoma K, Yamaguchi S, Arima T, Kinoshita K and Wake N:
An inhibitory effect on cell proliferation by blockage of the
MAPK/estrogen receptor/MDM2 signal pathway in gynecologic cancer.
Gynecol Oncol. 105:341–350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Boggess JF, Zhou C, Bae-Jump VL, Gehrig PA
and Whang YE: Estrogen-receptor-dependent regulation of telomerase
activity in human endometrial cancer cell lines. Gynecol Oncol.
103:417–424. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Jing X, Peng J, Dou Y, Sun J, Ma C, Wang
Q, Zhang L, Luo X, Kong B, Zhang Y, et al: Macrophage ERα promoted
invasion of endometrial cancer cell by mTOR/KIF5B-mediated
epithelial to mesenchymal transition. Immunol Cell Biol.
97:563–576. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Xu D, Lin TH, Yeh CR, Cheng MA, Chen LM,
Chang C and Yeh S: The wedelolactone derivative inhibits estrogen
receptor-mediated breast, endometrial, and ovarian cancer cells
growth. Biomed Res Int. 2014:7132632014. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Labrie F, Labrie C, Bélanger A, Simard J,
Giguère V, Tremblay A and Tremblay G: EM-652 (SCH57068), a pure
SERM having complete antiestrogenic activity in the mammary gland
and endometrium. J Steroid Biochem Mol Biol. 79:213–225. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Fadiel A, Song J, Tivon D, Hamza A,
Cardozo T and Naftolin F: Phenytoin is an estrogen receptor
α-selective modulator that interacts with helix 12. Reprod Sci.
22:146–155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Lian Z, Niwa K, Onogi K, Mori H, Harrigan
RC and Tamaya T: Anti-tumor effects of herbal medicines on
endometrial carcinomas via estrogen receptor-α-related mechanism.
Oncol Rep. 15:1133–1136. 2006.PubMed/NCBI
|
|
191
|
Zhang W, Chen JH, Aguilera-Barrantes I,
Shiau CW, Sheng X, Wang LS, Stoner GD and Huang YW: Urolithin A
suppresses the proliferation of endometrial cancer cells by
mediating estrogen receptor-α-dependent gene expression. Mol Nutr
Food Res. 60:2387–2395. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Karaboğa Arslan AK and Yerer MB:
α-Chaconine and α-Solanine Inhibit RL95-2 Endometrium Cancer Cell
Proliferation by Reducing Expression of Akt (Ser473) and ERα
(Ser167). Nutrients. 10:6722018. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Leong H, Firestone GL and Bjeldanes LF:
Cytostatic effects of 3,3′-diindolylmethane in human endometrial
cancer cells result from an estrogen receptor-mediated increase in
transforming growth factor-alpha expression. Carcinogenesis.
22:1809–1817. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Aoyama H, Couse JF, Hewitt SC, Haseman JK,
He H, Zheng X, Majstoravich S, Korach KS and Dixon D: Upregulation
of estrogen receptor expression in the uterus of ovariectomized
B6C3F1 mice and Ishikawa cells treated with bromoethane. Toxicol
Appl Pharmacol. 209:226–235. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Kim HI, Kim T, Kim JE, Lee J, Heo J, Lee
NR, Kim NJ and Inn KS: NJK14013, a novel synthetic estrogen
receptor-α agonist, exhibits estrogen receptor-independent, tumor
cell-specific cytotoxicity. Int J Oncol. 47:280–286. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Karaca B, Bakır E, Yerer MB, Cumaoğlu A,
Hamurcu Z and Eken A: Doxazosin and erlotinib have anticancer
effects in the endometrial cancer cell and important roles in ERα
and Wnt/β-catenin signaling pathways. J Bioch Mol Toxicol.
35:e229052021. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Wang J, Liu X, Zhang X, Liu J, Ye S, Xiao
S, Chen H and Wang H: Induction of apoptosis by c9, t11-CLA in
human endometrial cancer RL 95-2 cells via ERα-mediated pathway.
Chem Phys Lipids. 175–176. 27–32. 2013.PubMed/NCBI
|
|
198
|
Shah YM, Al-Dhaheri M, Dong Y, Ip C, Jones
FE and Rowan BG: Selenium disrupts estrogen receptor (alpha)
signaling and potentiates tamoxifen antagonism in endometrial
cancer cells and tamoxifen-resistant breast cancer cells. Mol
Cancer Ther. 4:1239–1249. 2005. View Article : Google Scholar : PubMed/NCBI
|