Introduction
Cholangiocarcinoma is a malignant tumor located in
the bile duct epithelium and is the second most common primary
hepatobiliary malignancy after hepatocellular carcinoma (1). According to the American Cancer
Society, ~12,000 people in the United States are diagnosed with
cholangiocarcinoma each year (2).
Cholangiocarcinoma is more common in East and Southeast Asia,
potentially because eating raw, fermented or undercooked fish leads
to parasitic (liver fluke) infection, which in turn triggers
chronic bile duct inflammation and increases cancer risk (3,4). Since
cholangiocarcinoma lacks diagnostic markers and has limited
diagnostic methods, the five-year survival rate of patients with
cholangiocarcinoma is <10% (5).
Treatment guidelines for cholangiocarcinoma are primarily surgery,
radiation therapy and chemotherapy (CT), depending on the disease
stage (6). However, most patients
with cholangiocarcinoma are asymptomatic at the early stage and are
typically only diagnosed when the cholangiocarcinoma has spread to
other tissue beyond the bile duct, which limits the treatment
options (7). Accordingly,
comprehensive identification of potential cholangiocarcinoma
diagnostic biomarkers may facilitate design of more effective and
targeted therapeutic strategies.
Laminin subunit γ2 (LAMC2) is a member of the
extracellular matrix (ECM) glycoprotein family (8). It has been reported that LAMC2 is
implicated in various biological processes, including cell
adhesion, differentiation, migration, signaling and cancer
metastasis (9). For example,
previous report have shown that LAMC2 increases cell migration,
invasion and metastasis in lung adenocarcinoma by regulating
epithelial-mesenchymal transition (EMT) (10). Additionally, expression of LAMC2
enhances cell migration and invasion via directly targeting EMT
regulator zinc finger E-box binding homeobox 1 in colorectal cells
(11). Conversely, the inhibition
of LAMC2 expression promotes gemcitabine sensitivity and decreases
cancer progression via EMT signaling and ATP-binding cassette
transporters in pancreatic ductal adenocarcinoma (12). Moreover, clinical data have
demonstrated that LAMC2 is upregulated in patients with pancreatic
(13), bladder (14), lung (10), colorectal (11) and cervical cancer (15). Furthermore, high expression of LAMC2
is associated with worse clinical outcome for different cancer
types, such as pancreas, stomach, tongue, bladder, colorectal,
lung, squamous cell carcinoma of vulva, cervix andesophagus
(squamous) as well as melanoma and anaplasticthyroid carcinom
(9). However, the association
between LAMC2 expression, clinical significance and survival
outcomes in patients with cholangiocarcinoma is unknown.
The present study aimed to investigate the
expression of LAMC2 in cholangiocarcinoma and how it can impact
prognosis. By uncovering the potential of LAMC2 as a prognostic
indicator, the present study aim to provide valuable insights that
can improve the care and treatment outcomes for individuals with
cholangiocarcinoma.
Materials and methods
Analysis of expression profiles from
publicly available cholangiocarcinoma transcriptomic datasets
The cholangiocarcinoma gene expression dataset
(accession no. GSE26566) includes information on 59 non-cancerous
liver and 104 cholangiocarcinoma tumor tissue samples; data were
downloaded from Gene Expression Omnibus (GEO) (https://www.ncbi.nlm.nih.gov/geo/) and analyzed
using GeneChip™ Human Genome U133 Plus 2.0 Array (Thermo
Fisher Scientific). The comparative analysis was conducted to
generate the heatmap of significantly differently expressed genes
associated with heparin binding (GO:0008201; geneontology.org/).
The expression of the genes was then calculated by probes
combinations without preselection or filtering. Genes with
significant differential expression (log2 ratio >2;
P<0.01) were used for further study.
Patients and tumor specimens
Paraffin-embedded tissue blocks were retrieved from
182 patients with intrahepatic cholangiocarcinoma who had no lymph
node or distant metastasis and had received curative surgery. Only
individuals with T1-3N0M0 disease were included. No patients
received adjuvant CT or radiotherapy. The initial diagnosis was
made from January 1990 to December 2010 at The Chi Mei Medical
Center (Tainan, Taiwan). The present study was conducted in
accordance with the Declaration of Helsinki and approved by The
Institutional Review Board of Chi-Mei Medical Center (approval no.
09912003). Informed consent was signed and obtained from all
subjects.
In addition, histological subtypes were reevaluated
by two pathologists. The tumor stage was assessed by the 7th
edition of the American Joint Committee on Cancer (AJCC) staging
system (16).
Immunohistochemistry (IHC)
staining
The tissue blocks of cholangiocarcinoma were fixed
in 4% paraformaldehyde in PBS (4 °C), made transparent,
paraffin-embedded, and sliced into 4-µm thick serial sections using
a microtome. For antigen retrieval, slides were pressure-cooked in
10 mmol/l citrate buffer at pH 6 for 7 min and washed using TBS
buffer with 0.1% Tween-80. The tissues were dewaxed, rehydrated in
a graded ethanol submerged in 0.3% H2O2 and
in 95% ethanol for 5 min and placed in citrate buffer (pH 6). For
H&E staining, tissue section was stained in Mayers Hematoxylin
for 1 mi followed by staining blue nuclei in 1X PBS for 1 min and
counterstaining in Alcoholic-Eosin for 1 min. Then the tissue
sections were dehydrated through 100% EtOH. For
immunohistochemistry staining, the sections were stained overnight
at 4°C with anti-LAMC2 primary antibody (cat. no. ab125679; Abcam;
1:100) followed by incubation with secondary antibody HRP polymer
(car. no. ab214880; Abcam; 1:2,000) for 30 min at room temperature.
A total of two pathologists calculated H-score as follows:
H-score=π(i +1), where π is the percentage of stained tumor cells
and i is the degree of staining (0–3). The i values are indicated
as 0 (no evidence of staining), 1 (weak staining), 2 (moderate
staining), and 3 (strong staining). Based on the median H-score,
the immunostaining was categorized as low or high expression of
LAMC2.
Gene function prediction and
classification
To determine the function of LAMC2 in intrahepatic
cholangiocarcinoma, the association between the mRNA expression
levels of LAMC2 and its co-expressed genes from the
cholangiocarcinoma dataset containing 51 samples in The Cancer
Genome Atlas (TCGA) database (dbGaP Study Accession no.phs000178,
cancer.gov/ccg/research/genome-sequencing/tcga) were assessed. The
top 200 differentially expressed transcripts exhibiting positive or
negative associations with LAMC2 were downloaded. These genes were
undergoing functional annotation by the GO classification system
(geneontology.org/) and rated by fold enrichment. Fisher's exact
test was performed to identify GO terms that were over-represented
amongst differentially expressed genes. In this test, the P-value
denotes the likelihood of observing ≥x genes from the entire set of
n genes associated with a specific GO term. Subsequently, to
minimize false positives (type I errors), the original P-value was
adjusted for multiple hypothesis testing, resulting in false
discovery rate (FDR). P-value and FDR <0.05 were considered to
indicate a statistically significant difference.
Statistical analysis
All the data were analyzed using SPSS version 17.0
software (SPSS, Inc.). To explore the association between LAMC2
expression and clinicopathological characteristics in patients with
cholangiocarcinoma, medical records were collected and overall,
disease-specific, local recurrence-free and metastasis-free
survival of patients with cholangiocarcinoma from treatment start
date to the event occurrence were analyzed. Using uni- and
multivariate analysis, LAMC2 expression and clinicopathological
variables were discovered as predictors of OS (measured from
curative surgery to the time of any cause mortality), DSS (measured
from curative surgery to the time of cancer mortality), LRS
(measured from curative surgery to the time of first local
recurrence) and MFS (measured from curative surgery to the first
metastasis). Survival curves were obtained by Kaplan-Meier analysis
and log-rank test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Heparin binding-associated gene LAMC2
is significantly upregulated in patients with
cholangiocarcinoma
To identify a potential target for diagnosis of
patients with cholangiocarcinoma, the public cholangiocarcinoma
transcriptome dataset (accession no. GSE26566) in the GEO database,
which contains 104 cholangiocarcinoma tumor and 59 non-cancerous
liver tissue samples. The comparative analysis was conducted to
detect significantly differently expressed genes associated with
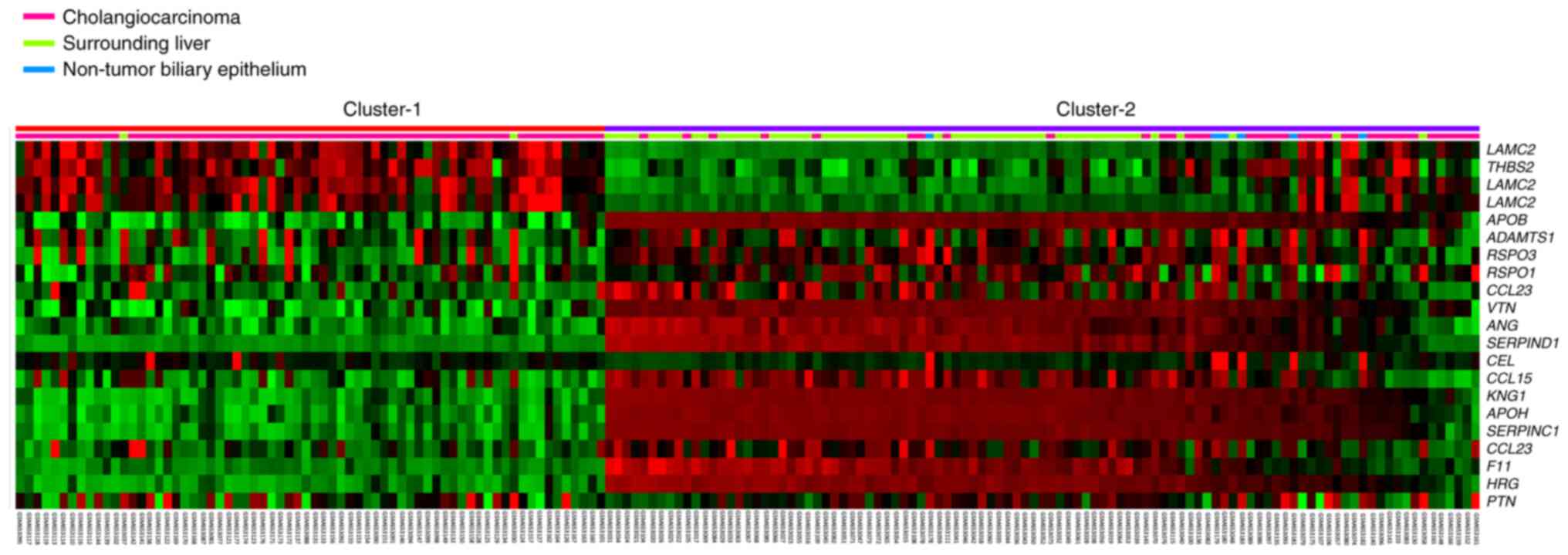
heparin binding (GO:0008201). The heatmap data revealed 19 heparin
binding-associated genes with significant differential expression
(Fig. 1). In GO Term database,
three probes for LAMC2 are used including: ILMN_1701424,
ILMN_1653824 and ILMN_1706519. All LAMC2 probes exhibited
significant expression fold-change between cholangiocarcinoma tumor
tissue and non-cancerous liver tissue. Specifically, ILMN_1701424
probe exhibited the highest expression fold change (log ratio,
2.7229; Table I). Collectively,
these findings demonstrated that LAMC2 may play an essential role
in cancer progression in cholangiocarcinoma.
 | Table I.Alteration of genes associated with
heparin binding (accession no. GO:0008201) in cholangiocarcinoma
(accession no.GSE26566). |
Table I.
Alteration of genes associated with
heparin binding (accession no. GO:0008201) in cholangiocarcinoma
(accession no.GSE26566).
|
| Cholangiocarcinoma
vs. non-tumora | Cholangiocarcinoma
vs. normal intrahepatic bile ductb |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Probe | Log ratio | P-value | Log ratio | P-value | Gene | Molecular
function | Biological
process |
|---|
| ILMN_1701424 | 2.7229 | <0.0001 | 2.3705 | <0.0001 | LAMC2 | ‘Heparin binding’,
‘protein binding’ | ‘Cell adhesion’,
‘epidermis development’ |
| ILMN_1653824 | 1.7967 | <0.0001 | 1.7476 | <0.0001 | LAMC2 | ‘Heparin binding’,
‘protein binding’ | ‘Cell adhesion’,
‘epidermis development’ |
| ILMN_1678842 | 1.2588 | 0.0008 | 1.9732 | <0.0001 | THBS2 | ‘Structural
molecule activity’, ‘heparinbinding’, ‘calcium ion
binding’,‘protein binding’ | ‘Cell
adhesion’ |
| ILMN_1706519 | 0.7668 | 0.0007 | 0.626 | <0.0001 | LAMC2 | ‘Heparin binding’,
‘protein binding’ | ‘Cell adhesion’,
‘epidermis development’ |
| ILMN_1813753 | −0.3156 | 0.0065 | −0.114 | 0.0043 | PTN | ‘Cytokine
activity’, ‘protein phosphatase inhibitor activity’, ‘heparin
binding, growth factor activity’ | Cell
proliferation’, ‘transmembrane receptor protein tyrosine
phosphatase signaling pathway’, ‘positive regulation of cell
proliferation’ |
| ILMN_1682937 | −0.3774 | 0.0003 | −0.2562 | <0.0001 | RSPO1 | ‘Electron carrier
activity’, ‘iron ion binding’, ‘heparin binding’ | ‘Wnt receptor
signaling pathway’, ‘electron transport’ |
| ILMN_1764030 | −0.6538 | 0.0005 | −0.3667 | <0.0001 | CCL23 | ‘Heparin binding’,
‘chemokine activity’ | Cell-cell
signaling’, ‘negative regulation of cell proliferation’,
‘chemotaxis’, ‘calcium ion homeostasis’, ‘G-protein coupled
receptor protein signaling pathway’, ‘signal transduction’,
‘inflammatory response’ |
| ILMN_1807101 | −1.0034 | 0.0001 | −1.7784 | <0.0001 | F11 | ‘Coagulation factor
XIa activity’, ‘peptidase activity’, ‘heparin binding’,
‘coagulation factor IXa activity’ | ‘Blood
coagulation’ |
| ILMN_1681983 | −1.0244 | <0.0001 | −0.3955 | <0.0001 | RSPO3 | ‘Electron carrier
activity’, ‘iron ion binding’, ‘heparin binding’ | ‘Wnt receptor
signaling pathway’, ‘electrontransport’ |
| ILMN_1686109 | −1.349 | 0.0001 | −0.7483 | <0.0001 | CCL23 | ‘Heparin binding’,
‘chemokine activity’ | ‘Cell-cell
signaling’, ‘negative regulation of cell proliferation’,
‘chemotaxis’, ‘calcium ion homeostasis’, ‘G-protein coupled
receptor protein signaling pathway’, ‘signal transduction,
inflammatory response’ |
| ILMN_1696974 | −1.9949 | 0.0006 | −2.6032 | <0.0001 | ANG | ‘Pancreatic
ribonuclease activity’, ‘hydrolase activity’, ‘ribonuclease
activity’, ‘DNA binding’, ‘endo-nuclease activity’, ‘receptor
binding, copper ion binding’, ‘rRNA binding’, ‘heparin binding,
actin binding’ | ‘Negative
regulation of protein biosynthesis’, ‘calcium-dependent
phospholipase A2 activation’, ‘positive regulation of endothelial
cell proliferation’, ‘homeostasis’, ‘response to hypoxia’,
‘angiogenesis’, ‘phospholipase C activation’, ‘ovarian follicle
developpment’, ‘diacylglycerol biosynthesis’, ‘ribosome
biogenesis’, ‘rRNA transcription’, ‘cell differentiation’,
‘positive regulation of protein secretion’, ‘negative regulation of
smooth muscle cell proliferation’, ‘cell communication’, ‘actin
filament polymerization’ |
| ILMN_1707975 | −2.0503 | 0.0002 | −3.0614 | <0.0001 | SER-PIND1 | ‘Serine-type
endopeptidase inhibitor activity’, ‘heparin binding’ | ‘Blood
coagulation’, ‘chemotaxis’ |
| ILMN_1691127 | −2.0625 | 0.0053 | −2.6248 | <0.0001 | VTN | ‘Heparin binding’,
‘protein binding’ | ‘Immune response’,
‘cell adhesion’ |
| ILMN_1740609 | −2.2868 | <0.0001 | −1.5285 | <0.0001 | CCL15 | ‘Chemokine
activity’, ‘chemoattractant activity’, ‘signal transducer
activity’, ‘heparin binding’ | ‘Signal
transduction’, ‘immune response’, ‘antimicrobial humoral response
(sensu Vertebrata)’, ‘cell-cell signaling’, ‘chemotaxis’, ‘calcium
ion homeostasis’ |
| ILMN_1664024 | −2.4224 | 0.0010 | −3.0416 | <0.0001 | APOB | ‘Receptor binding’,
‘lipid transporteractivity’, ‘heparin binding’ | ‘Circulation,
cholesterol metabolism’, ‘lipid trans port’, ‘lipid metabolism’,
‘steroid metabolism’, ‘signal transduction’ |
| ILMN_1807339 | −2.6598 | 0.0005 | −3.8929 | <0.0001 | HRG | ‘Heparin binding’,
‘cysteine protease inhibitor activity’ |
|
| ILMN_1761511 | −2.7374 | 0.0001 | −3.4698 | <0.0001 | APOH | ‘Lipid transporter
activity’, ‘heparin binding’ | ‘Defense
response’ |
| ILMN_1673566 | −2.8885 | <0.0001 | −0.6183 | <0.0001 | ADAMTS1 | ‘Zinc ion binding,
metal ion binding’, ‘integrin binding, heparin binding’,
‘metalloendopeptidase | ‘Negative
regulation of cell proliferation’, ‘integrinmediated signaling
pathway’ |
| ILMN_1753729 | −3.1226 | 0.0014 | −3.8105 | <0.0001 | KNG1 | activity’ ‘Receptor
binding, cysteine proteaseinhibitor activity’, ‘zinc ion binding’,
‘heparin binding’ | ‘Diuresis’,
‘negative regulation of cell adhesion’, ‘vasodilation’, ‘positive
regulation of apoptosis’, ‘blood coagulation’, ‘smooth muscle
contraction’, ‘natriuresis’,‘negative regulation of blood
coagulation’, ‘inflammatory response’ |
| ILMN_1762605 | −3.6489 | <0.0001 | −4.0868 | <0.0001 | SERPINC1 | ‘Serine-type
endopeptidase inhibitor activity’, ‘heparin binding’, ‘protein
binding’ | ‘Blood
coagulation’ |
| ILMN_1723418 | −6.3865 | <0.0001 | −0.3866 | 0.0084 | CEL | ‘Hydrolase
activity’, ‘serine esterase activity’, ‘triacylglycerol lipase
activity’, ‘sterol esterase activity’, ‘heparin binding’ | ‘Pancreatic juice
secretion’, ‘protein amino acid esterification’, ‘cholesterol
absorption’, ‘cholesterol catabolism’, ‘triacylglycerol
metabolism’, ‘fatty acid catabolism’, ‘lipid metabolism’, ‘lipid
catabolism’ |
LAMC2 expression is associated with
poorer clinical pathological parameters of patients with
cholangiocarcinoma
The aforementioned data confirmed that high
expression of LAMC2 may be associated with cholangiocarcinoma
progression. Therefore, the association between LAMC2 expression
and the clinicopathological features of patients with
cholangiocarcinoma was explored (Table
II). A total of 182 patients with cholangiocarcinoma were
collected including 108 male patients and 75 patients ≥65 years
old. Moreover, the clinicopathological parameters were analyzed;
LAMC2 (low vs. high expression) in the tumors of patients with
cholangiocarcinoma was significantly associated with the status of
primary tumor, histological variant and the histological grade.
However, sex, age, hepatitis, intrahepatic lithiasis and surgical
margin showed no significant difference between tumor tissue of
patients with cholangiocarcinoma with differential LAMC2
expression. LAMC2 protein expression in human cholangiocarcinoma
tumor tissue was further confirmed by IHC staining. Low-stage
cholangiocarcinoma tissue had lower LAMC2 expression (Fig. 2A-D) than high-stage
cholangiocarcinoma tissue (Fig.
2E-H). These data showed that LAMC2 expression was markedly
associated with clinicopathological characteristics and cancer
progression in patients with cholangiocarcinoma.
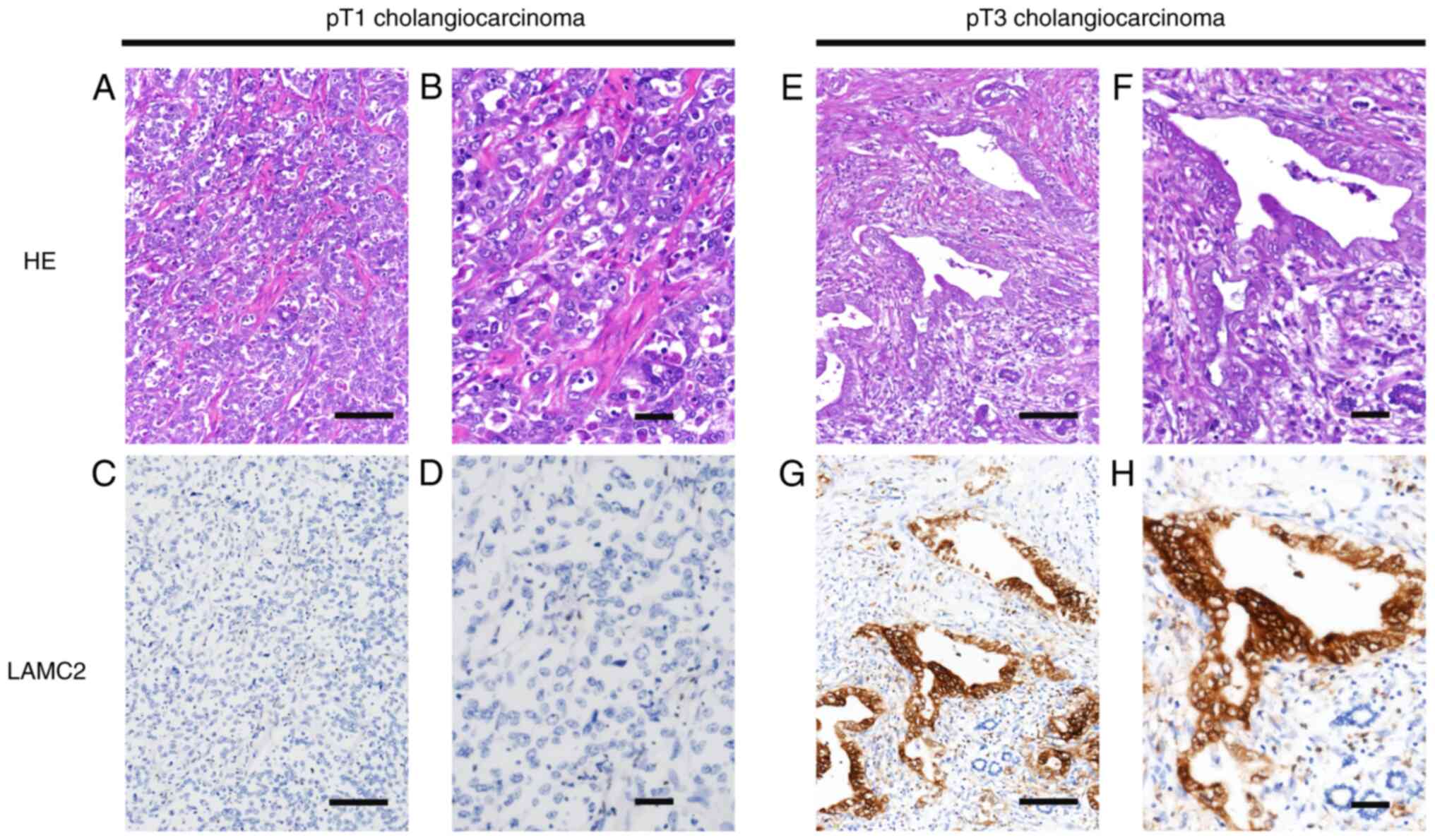 | Figure 2.Representative sections of LAMC2
immunostaning. Immunohistochemistry staining showed lower LAMC2
expression in pT1 stage cholangiocarcinoma HE staining at (A)
magnification, ×200; scale bar, 500 µm and (B) magnification, ×400;
scale bar 200 µm. LAMC2 staining at (C) magnification, ×200; scale
bar, 500 µm and (D) magnification, ×400; scale bar 200 µm compared
with pT3 stage cholangiocarcinoma HE staining at (E) magnification,
×200; scale bar, 500 µm and (F) magnification, ×400; scale bar 200
µm. LAMC2 staining at (G) magnification, ×200; scale bar, 500 µm
and (H) magnification, ×400; scale bar 200 µm. HE, hematoxylin and
eosin; LAMC2, laminin subunit γ2; pT, pathological T. |
 | Table II.Association between LAMC2 expression
and clinicopathological parameters in primary localized
cholangiocarcinoma. |
Table II.
Association between LAMC2 expression
and clinicopathological parameters in primary localized
cholangiocarcinoma.
|
|
| LAMC2
expression |
|
|---|
|
|
|
|
|
|---|
| Parameter | n | Low | High | P-value |
|---|
| Sex |
|
|
|
|
|
Male | 108 | 57 | 51 | 0.365 |
|
Female | 74 | 34 | 40 |
|
| Age, years |
|
|
|
|
|
<65 | 107 | 49 | 58 | 0.175 |
|
≥65 | 75 | 42 | 33 |
|
| Hepatitis |
|
|
|
|
| B | 72 | 38 | 34 | 0.353 |
| C | 29 | 17 | 12 |
|
| Non-B,
non-C | 81 | 36 | 45 |
|
| Intrahepatic
lithiasis |
|
|
|
|
|
Absent | 102 | 53 | 49 | 0.550 |
|
Present | 80 | 38 | 42 |
|
| Surgical
margin |
|
|
|
|
| R0 | 163 | 83 | 80 | 0.467 |
| R1 | 19 | 8 | 11 |
|
| Primary tumor
stage |
|
|
|
|
| T1 | 87 | 56 | 31 |
<0.001a |
| T2 | 61 | 27 | 34 |
|
| T3 | 34 | 8 | 26 |
|
| Histological
type |
|
|
|
|
| Large
duct | 105 | 45 | 60 | 0.024a |
| Small
duct | 77 | 46 | 31 |
|
| Histological
grade |
|
|
|
|
| Well
differentiated | 61 | 38 | 23 | 0.002a |
|
Moderately |
|
|
|
|
|
differentiated | 66 | 36 | 30 |
|
| Poorly
differentiated | 55 | 17 | 38 |
|
LAMC2 expression is associated with
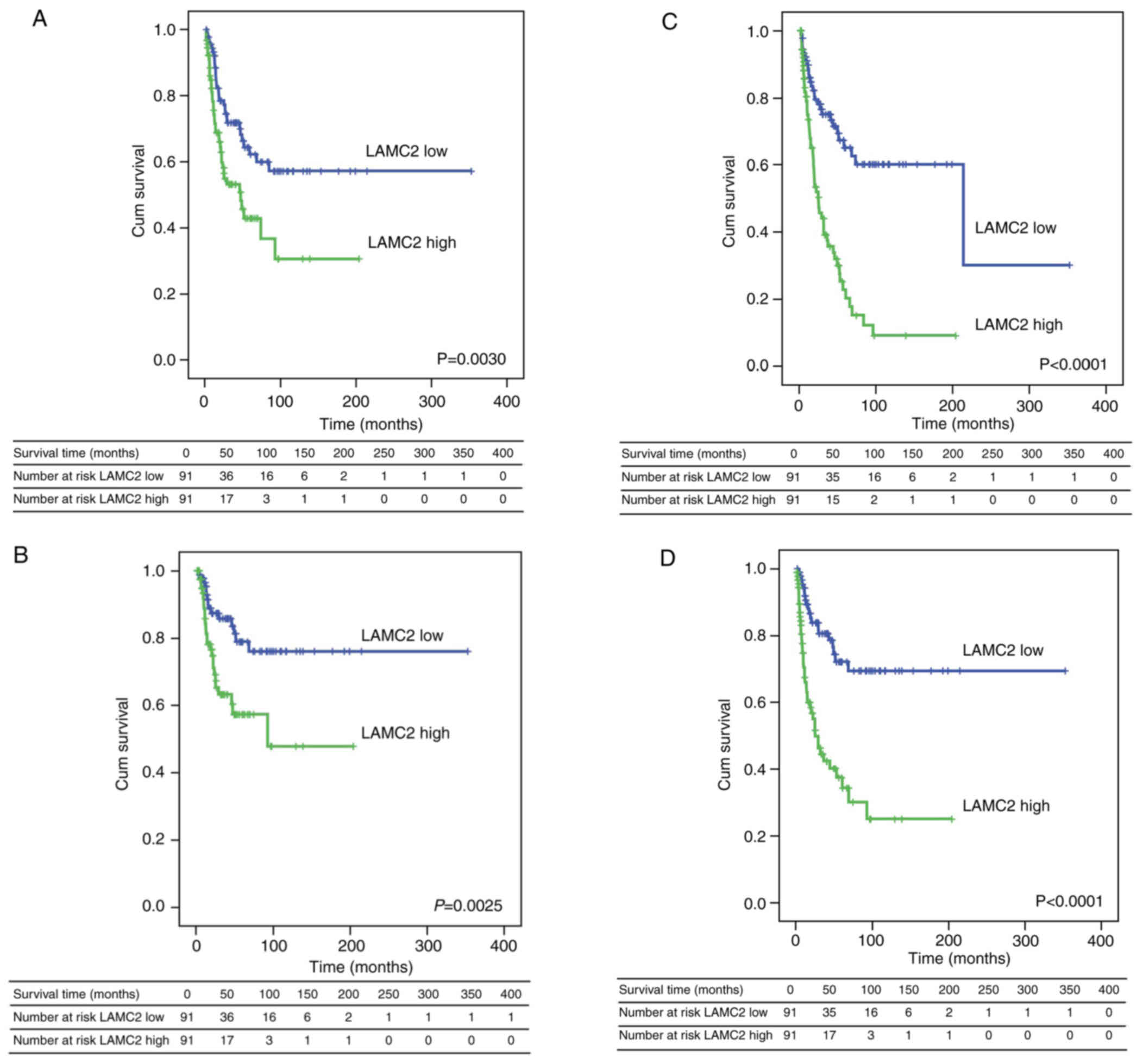
survival of patients with cholangiocarcinoma
Whether differential expression of the LAMC2 gene
affects the survival outcomes of patients with cholangiocarcinoma
was explored. Kaplan-Meier survival analysis was performed to
confirm that LAMC2 expression was associated with
clinicopathological characteristics and prognosis in patients with
cholangiocarcinoma. High LAMC2 expression was significantly
associated with lower overall (Fig.
3A), disease-specific (Fig.
3B), local recurrence-free (Fig.
3C) and metastasis-free survival (Fig. 3D). Univariate and multivariate
analyses revealed the association between prognostic factors of
LAMC2 expression and clinicopathological factors in patients with
cholangiocarcinoma. Sex, surgical margin (R0 and R1), primary tumor
stage (T1, T2 and T3) and LAMC2 expression (high or low) were
significantly associated with overall and disease-specific survival
(Table III). However, age,
hepatitis, intrahepatic lithiasis and histological type (large and
small duct) and grade (well, moderately or poorly differentiated)
did not differ significantly in overall and disease-specific
survival (Table III). The
association between local recurrence-free and metastasis-free
survival with clinical characteristics was also evaluated by
univariate and multivariate analyses. Local recurrence-free and
metastasis-free survival were markedly associated with surgical
margins, primary tumor stage and LAMC2 expression. Local
recurrence-free survival was significantly associated with
histological type and grade by univariate, but not multivariate,
analysis (Table IV). These results
demonstrated that LAMC2 may be a potential indicator of prognosis
in patients with cholangiocarcinoma.
 | Table III.Univariate log-rank and multivariate
analysis for overall and disease-specific survival in primary
localized cholangiocarcinoma. |
Table III.
Univariate log-rank and multivariate
analysis for overall and disease-specific survival in primary
localized cholangiocarcinoma.
|
|
| Overall
survival | Disease-specific
survival |
|---|
|
|
|
|
|
|---|
|
|
| Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|
|---|
| Parameter | n | n | P-value | HR | 95% CI | P-value | n | P-value | HR | 95% CI | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
|
|
|
|
|
Male | 108 | 50 | 0.0254a | 1 | - | 0.048a | 9 | 0.0072a | 1 | - | 0.023a |
|
Female | 74 | 21 |
| 1.681 | 1.004–2.814 | - | 32 |
| 2.377 | 1.123–5.025 | - |
| Age, years |
|
|
|
|
|
|
|
|
|
|
|
|
<65 | 107 | 37 | 0.2626 | - | - | - | 28 | 0.2125 | - | - | - |
|
≥65 | 75 | 34 |
| - | - | - | 13 |
| - | - | - |
| Hepatitis |
|
|
|
|
|
|
|
|
|
|
|
| B | 72 | 32 | 0.2379 | - | - | - | 16 | 0.4561 | - | - | - |
| C | 29 | 8 |
| - | - | - | 19 |
| - | - | - |
| Non-B,
non-C | 81 | 31 |
| - | - | - | 6 |
| - | - | - |
| Intrahepatic
lithiasis |
|
|
|
|
|
|
|
|
|
|
|
|
Absent | 102 | 36 | 0.2831 | - | - | - | 19 | 0.1613 | - | - | - |
|
Present | 80 | 35 |
| - | - | - | 22 |
| - | - | - |
| Surgical
margin |
|
|
|
|
|
|
|
|
|
|
|
| R0 | 163 | 59 |
<0.0001a | 1 | - | 0.002a | 31 |
<0.0001a | 1 | - |
<0.001a |
| R1 | 19 | 12 |
| 2.978 | 1.513–5.862 |
| 10 |
| 4.446 | 2.012–9.827 |
|
| Primary tumor
stage |
|
|
|
|
|
|
|
|
|
|
|
| T1 | 87 | 25 | 0.0001a | 1 | - | 0.012a | 9 |
<0.0001a | 1 | - | 0.003a |
| T2 | 61 | 27 |
| 1.579 | 0.900–2.770 | - | 19 |
| 2.886 | 1.279–6.510 | - |
| T3 | 34 | 19 |
| 2.270 | 1.185–4.347 | - | 13 |
| 3.815 | 1.544–9.426 | - |
| Histological
type |
|
|
|
|
|
|
|
|
|
|
|
| Large
duct | 105 | 43 | 0.4281 | - | - | - | 27 | 0.1984 | - | - | - |
| Small
duct | 77 | 28 |
| - | - | - | 14 |
| - | - | - |
|
Differentiation |
|
|
|
|
|
|
|
|
|
|
|
|
Well | 61 | 20 | 0.1663 | - | - | - | 12 | 0.3881 | - | - | - |
|
Moderately | 66 | 28 |
| - | - | - | 16 |
| - | - | - |
|
Poorly | 55 | 23 |
| - | - | - | 13 |
| - | - | - |
| LAMC2
expression |
|
|
|
|
|
|
|
|
|
|
|
|
Low | 91 | 30 | 0.0030a | 1 | - | 0.034a | 15 | 0.0025a | 1 | - | 0.039a |
|
High | 91 | 41 |
| 1.713 | 1.042–2.818 | - | 26 |
| 2.011 | 1.037–3.901 | - |
 | Table IV.Univariate log-rank and multivariate
analysis for local recurrence-free and metastasis-free survival in
primary localized cholangiocarcinoma. |
Table IV.
Univariate log-rank and multivariate
analysis for local recurrence-free and metastasis-free survival in
primary localized cholangiocarcinoma.
|
|
| Local
recurrence-free survival | Metastasis-free
survival |
|---|
|
|
|
|
|
|---|
|
|
| Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|
|---|
| Parameter | n | n | P-value | HR | 95% CI | P-value | n | P-value | HR | 95% CI | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
|
|
|
|
|
Male | 108 | 54 | 0.2170 | - | - | - | 21 | 0.1008 | - | - | - |
|
Female | 74 | 31 |
| - | - | - | 44 |
| - | - | - |
| Age, years |
|
|
|
|
|
|
|
|
|
|
|
|
<65 | 107 | 55 | 0.2993 | - | - | - | 42 | 0.2936 | - | - | - |
|
≥65 | 75 | 30 |
| - | - | - | 23 |
| - | - | - |
| Hepatitis |
|
|
|
|
|
|
|
|
|
|
|
| B | 72 | 33 | 0.7333 | - | - | - | 26 | 0.8762 | - | - | - |
| C | 29 | 13 |
| - | - | - | 11 |
| - | - | - |
| Non-B,
non-C | 81 | 39 |
| - | - | - | 28 |
| - | - | - |
| Intrahepatic
lithiasis |
|
|
|
|
|
|
|
|
|
|
|
|
Absent | 102 | 41 | 0.0551 | - | - | - | 31 | 0.1000 | - | - | - |
|
Present | 80 | 44 |
| - | - | - | 34 |
| - | - | - |
| Surgical
margin |
|
|
|
|
|
|
|
|
|
|
|
| R0 | 163 | 71 |
<0.0001a | 1 | - |
<0.001a | 54 |
<0.0001a | 1 |
| 0.001a |
| R1 | 19 | 14 |
| 4.120 | 2.145–7.913 |
| 11 |
| 3.250 | 1.607–6.577 |
|
| Primary tumor
stage |
|
|
|
|
|
|
|
|
|
|
|
| T1 | 87 | 28 |
<0.0001a | 1 | - | 0.004a | 21 |
<0.0001a | 1 | - | 0.018a |
| T2 | 61 | 32 |
| 1.445 | 0.827–2.524 |
| 26 |
| 1.826 | 1.011–3.298 |
|
| T3 | 34 | 25 |
| 2.232 | 1.230–4.048 |
| 18 |
| 2.166 | 1.110–4.227 |
|
| Histological
type |
|
|
|
|
|
|
|
|
|
|
|
| Large
duct | 105 | 58 | 0.0085a | 1 | - | 0.373 | 43 | 0.0759 | - | - | - |
| Small
duct | 77 | 27 |
| 0.803 | 0.495–1.301 |
| 22 |
| - | - | - |
|
Differentiation |
|
|
|
|
|
|
|
|
|
|
|
|
Well | 61 | 28 | 0.0299a | 1 | - | 0.794 | 22 | 0.1794 | - | - | - |
|
Moderately | 66 | 27 |
| 0.869 | 0.498–1.516 |
| 22 |
| - | - | - |
|
Poorly | 55 | 30 |
| 1.083 | 0.616–1.903 |
| 21 |
| - | - | - |
| LAMC2
expression |
|
|
|
|
|
|
|
|
|
|
|
|
Low | 91 | 28 |
<0.0001a | 1 | - |
<0.001a | 20 |
<0.0001a | 1 | - |
<0.001a |
|
High | 91 | 57 |
| 2.721 | 1.656–4.470 |
| 45 |
| 3.117 | 1.799–5.403 |
|
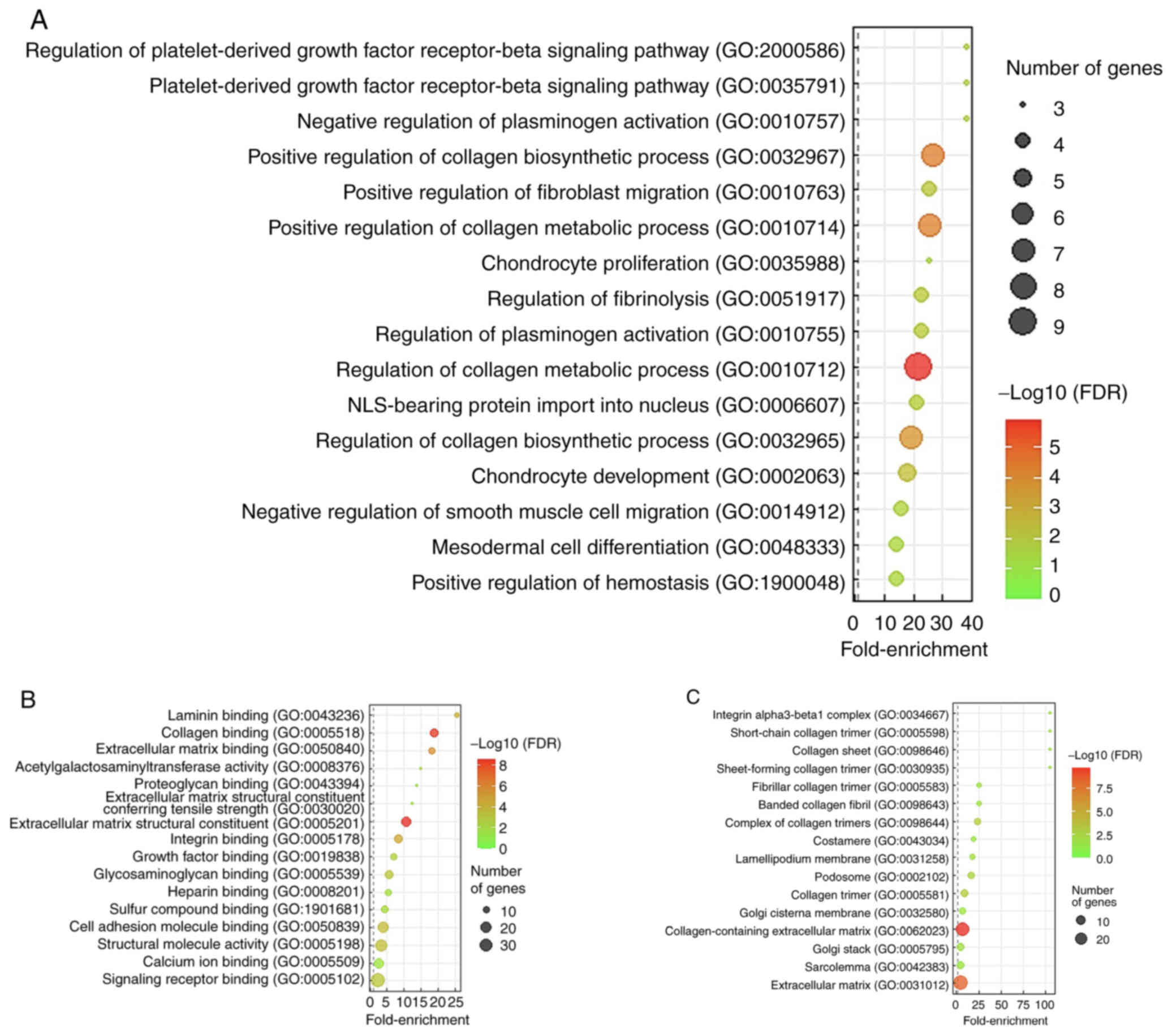
LAMC2 gene function prediction
To determine the functions of LAMC2 in
cholangiocarcinoma, the top 200 differentially expressed
transcripts exhibiting positive (Table
SI) or negative association (Table
SII) with LAMC2 were downloaded from TCGA cholangiocarcinoma
dataset (n=51). GO enrichment showed that the most significant
biological processes associated with LAMC2 upregulation were the
‘regulation of platelet-derived growth factor receptor-β signaling
pathway’ (GO: 2000586; fold-enrichment, 38.22) and
‘platelet-derived growth factor receptor-β signaling pathway’ (GO:
0035791; fold-enrichment, 38.22; Fig.
4A). Lysyl oxidase (LOX) gene was involved in both
aforementioned biological processes. The most significant molecular
function associated with LAMC2 upregulation was ‘laminin binding’
(GO: 0043236; fold-enrichment, 25.48; Fig 4B). Moreover, the most significant
cellular component associated with LAMC2 upregulation was ‘integrin
alpha3-beta1 complex’ (GO: 0034667; fold-enrichment, >100;
Fig. 4C). The integrin subunit β1
(ITGB1) and ITGA3 genes, which are implicated in both laminin
binding and integrin α3-β1 complex, were identified.
Discussion
Cholangiocarcinoma is a rare malignant tumor located
in the bile duct. However, its incidence is increasing globally and
it is a global public health problem that needs attention (1,17). To
the best of our knowledge, there is no literature identifying the
cause of cholangiocarcinoma. Certain studies have investigated risk
factors that may serve essential roles in increasing the risk of
cholangiocarcinoma, including primary sclerosing cholangitis,
chronic liver disease, smoking, diabetes and liver parasites (liver
fluke infection) (18,19). Cholangiocarcinoma is divided into
three types based on where it occurs in the bile ducts:
Intrahepatic, extrahepatic and distal cholangiocarcinoma (20). Cholangiocarcinoma is asymptomatic in
the early stages and is often diagnosed when the disease is already
at an advanced stage, which decreases affects treatment options and
leads to poor prognosis (21). The
5-year survival rate for intrahepatic cholangiocarcinoma is 9%.
However, if the cancer is diagnosed at an early stage, the 5-year
survival rate is 25%. If the tumor has spread to the regional lymph
nodes, 5-year survival rate is 8%. However, if the tumor has spread
to a distant part of the body, 5-year survival rate is 2% (22,23).
Thus, identifying potential novel biomarkers is a promising
approach to enhancing strategies to treat cholangiocarcinoma.
Here, the tumorigenesis-associated genes in the
transcriptome of cholangiocarcinoma (GSE26566) were compared with
heparin binding in GO (GO:0008201). Heparin-binding associated gene
LAMC2 showed upregulated expression in the cholangiocarcinoma
compared with non-tumor tissue. LAMC2 is a key laminin in the ECM
glycoprotein family and regulates numerous biological processes,
including cell adhesion, differentiation, migration, signaling and
metastasis (24). Moreover,
accumulating evidence indicates that LAMC2 is also involved in
regulating progression in multiple types of cancer (25–27).
For example, inhibition of LAMC2 expression decreases cell
proliferation, migration and invasion in non-small-cell lung cancer
(28). In pancreatic cancer,
upregulation of LAMC2 enhances cell migration and invasion through
the activation of Akt/sodium-hydrogen antiporter 1) signaling
(26). Furthermore, overexpression
of LAMC2 increases cell proliferation and decreases cell apoptosis
via p38/MAPK signaling activation in ovarian cancer (29). Zhou et al (27) demonstrated that silencing LAMC2
expression suppresses cell migration, invasion and cancer stemness
by inhibiting the PI3K/Akt signaling pathway in oral squamous cell
carcinoma. Clinical results have shown that LAMC2 is highly
expressed and associated with worse survival outcomes in
pancreatic, bladder, colorectal, oral and ovarian cancer (9,30,31).
To the best of our knowledge, no studies have investigated the
association between LAMC2 expression and prognostic outcomes and
survival in patients with cholangiocarcinoma. In the present study,
IHC showed that LAMC2 protein was upregulated in advanced
cholangiocarcinoma tumor tissues compared with early
cholangiocarcinoma tumor tissue. Patients with cholangiocarcinoma
with a high LAMC2 expression had worse overall, disease-specific,
local recurrence-free and metastasis-free survival than patients
with cholangiocarcinoma with low LAMC2 expression. Collectively,
these results indicated that LAMC2 may serve as a novel predictive
marker for patients with cholangiocarcinoma.
The association between LAMC2 and
clinicopathological parameters of patients with cholangiocarcinoma
was investigated. It was found that LAMC2 expression was markedly
associated with primary tumor stage and histological type and
grade. Moreover, univariate log-rank and multivariate analyses were
performed for overall, disease-specific, local recurrence-free and
metastasis-free survival in primary localized IHCC. Univariate and
multivariate analysis indicated that sex, surgical margin, primary
tumor stage and LAMC2 expression were markedly associated with
overall, disease-specific, local recurrence-free and
metastasis-free survival. Additionally, univariate, but not
multivariate, analysis showed that histological type and grade were
significantly associated with local recurrence-free survival in
patients with cholangiocarcinoma. These analyses suggested that
LAMC2 may be a potential biomarker in patients with
cholangiocarcinoma.
A characteristic of cholangiocarcinoma is dense ECM
featuring highly desmoplastic stroma comprising collagen, which
increases tumor stiffness and decreases drug penetration (32). The LOX family, composed of LOX and
LOX-like 1–4, is characterized by catalytic activity leading to
collagen crosslinking and ECM remodeling (33). Notably, LOX was a significant gene
that was positively associated with LAMC2 in the context of
biological processes. LOX also plays a crucial role in EMT and its
elevated expression is associated with poor prognosis in
hepatocellular carcinoma (34).
Nevertheless, whether LAMC2 promotes cholangiocarcioma progression
via LOX needs further exploration. ITGB1 and ITGA3 genes were
positively associated with LAMC2 in terms of molecular functions
and cellular components. Integrin α3β1, formed of ITGA3 and ITGB1,
is a receptor for ECM components including laminin, collagen and
fibronectin (35,36). Integrin α3β1 is suggested to play an
important role in tumor cell invasion of the basement membrane
(37). Additionally, the role of
laminin in cholangiocarcinoma cell migration (38) and upregulated ITGA3 and ITGB1 levels
in cholangiocarcinoma (39) have
been documented. Accordingly, the involvement of ITGA3 and ITGB1 in
cholangiocarcinoma development mediated by LAMC2 (a laminin
component) deserves further investigation.
The present study research has certain limitations.
Firstly, it was a retrospective study conducted at a single
institution and lacked experimental validation. Secondly, the exact
molecular mechanism underlying disease progression and adverse
outcomes in LAMC2-overexpressing cholangiocarcinoma remains
unclear. Thirdly, there is currently no standardized immunostaining
and scoring scheme for assessing LAMC2 expression. Due to the lack
of agreed staining standards, it is difficult to reach a consensus.
Lastly, to validate the findings, prospective multicenter studies
are required.
In conclusion, to the best of our knowledge, the
present study is the first to indicate that LAMC2 may serve as a
novel biomarker for prognosis of patients with cholangiocarcinoma.
Public transcriptome datasets were analyzed with clinical cohorts
and LAMC2 was notably upregulated in cholangiocarcinoma tumor
tissues. IHC staining was consistent with this result. The
expression of LAMC2 in patients with advanced cholangiocarcinoma
was higher than in patients with early cholangiocarcinoma.
Furthermore, the present study demonstrated that high expression of
LAMC2 was associated with poorer overall, disease-specific, local
recurrence-free and metastasis-free survival in patients with
cholangiocarcinoma. Notably, differential expression of LAMC2 was
significantly associated with the primary tumor stage and
histological type and histological grade. Therefore, LAMC2 may be a
novel biomarker to detect cholangiocarcinoma.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets generated and analyzed during the
current study are available in the Gene Expression Omnibus database
(National Center for Biotechnology Information,
ncbi.nlm.nih.gov/geo/) and in The Cancer Genome Atlas database
(National Cancer Institute and National Human Genome Research
Institute, cancer.gov/ccg/research/genome-sequencing/tcga).
Authors' contributions
YLS, CFL, KHO, YYH, HYL and YHK conceptualized the
study. SKHH and YFT performed the experiments. HCW, TCC, TJC, DPS,
CLC and HHT performed the data analysis. KHO, YYH and HYL wrote the
manuscript. CLC, CFL and YHK wrote, reviewed and edited the
manuscript. YLS, CFL, YHK, SKHH, YFT, TJC, DPS, HCW, TCC, HHT, KHO,
YYH, HYL and CLC confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki and approved by The Institutional
Review Board of Chi-Mei Medical Center (Tainan, Taiwan; approval
no. 09912003). Informed consent was signed and obtained from all
subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Banales JM, Marin JJG, Lamarca A,
Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen
JB, Braconi C, et al: Cholangiocarcinoma 2020: The next horizon in
mechanisms and management. Nat Rev Gastroenterol Hepatol.
17:557–588. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Neuzillet C, Emery C, Teissier C, Bouée S
and Lièvre A: Patient healthcare trajectories of intrahepatic
cholangiocarcinoma in France: A nationwide retrospective analysis.
Lancet Reg Health Eur. 15:1003242022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khuntikeo N, Titapun A, Loilome W,
Yongvanit P, Thinkhamrop B, Chamadol N, Boonmars T, Nethanomsak T,
Andrews RH, Petney TN and Sithithaworn P: Current perspectives on
opisthorchiasis control and cholangiocarcinoma detection in
Southeast Asia. Front Med (Lausanne). 5:1172018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arunsan P, Ittiprasert W, Smout MJ,
Cochran CJ, Mann VH, Chaiyadet S, Karinshak SE, Sripa B, Young ND,
Sotillo J, et al: Programmed knockout mutation of liver fluke
granulin attenuates virulence of infection-induced hepatobiliary
morbidity. Elife. 8:e414632019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hoyos S, Navas MC, Restrepo JC and Botero
RC: Current controversies in cholangiocarcinoma. Biochim Biophys
Acta Mol Basis Dis. 1864:1461–1467. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rizvi S, Khan SA, Hallemeier CL, Kelley RK
and Gores GJ: Cholangiocarcinoma-evolving concepts and therapeutic
strategies. Nat Rev Clin Oncol. 15:95–111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brindley PJ, Bachini M, Ilyas SI, Khan SA,
Loukas A, Sirica AE, The BT, Wongkham S and Gores GJ:
Cholangiocarcinoma. Nat Rev Dis Primers. 7:652021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ljubimova JY, Fujita M, Khazenzon NM,
Ljubimov AV and Black KL: Changes in laminin isoforms associated
with brain tumor invasion and angiogenesis. Front Biosci. 11:81–88.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garg M, Braunstein G and Koeffler HP:
LAMC2 as a therapeutic target for cancers. Expert Opin Ther
Targets. 18:979–982. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moon YW, Rao G, Kim JJ, Shim HS, Park KS,
An SS, Kim B, Steeg PS, Sarfaraz S, Changwoo Lee L, et al: LAMC2
enhances the metastatic potential of lung adenocarcinoma. Cell
Death Differ. 22:1341–1352. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang D, Du C, Ji D, Xi J and Gu J:
Overexpression of LAMC2 predicts poor prognosis in colorectal
cancer patients and promotes cancer cell proliferation, migration,
and invasion. Tumour Biol. 39:10104283177058492017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okada Y, Takahashi N, Takayama T and Goel
A: LAMC2 promotes cancer progression and gemcitabine resistance
through modulation of EMT and ATP-binding cassette transporters in
pancreatic ductal adenocarcinoma. Carcinogenesis. 42:546–556. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takahashi S, Hasebe T, Oda T, Sasaki S,
Kinoshita T, Konishi M, Ochiai T and Ochiai A: Cytoplasmic
expression of laminin gamma2 chain correlates with postoperative
hepatic metastasis and poor prognosis in patients with pancreatic
ductal adenocarcinoma. Cancer. 94:1894–1901. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang JL, Wang CCN, Cai JH, Chou CY, Lin YC
and Hung CC: Identification of GSN and LAMC2 as key prognostic
genes of bladder cancer by integrated bioinformatics analysis.
Cancers (Basel). 12:18092020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guess CM and Quaranta V: Defining the role
of laminin-332 in carcinoma. Matrix Biol. 28:445–455. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khan SA, Tavolari S and Brandi G:
Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 39
(Suppl 1):S19–S31. 2019. View Article : Google Scholar
|
|
18
|
Ceci L, Zhou T, Lenci I, Meadows V,
Kennedy L, Li P, Ekser B, Milana M, Zhang W, Wu C, et al: Molecular
mechanisms linking risk factors to cholangiocarcinoma development.
Cancers (Basel). 14:14422022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baidoun F, Sarmini MT, Merjaneh Z and
Moustafa MA: Controversial risk factors for cholangiocarcinoma. Eur
J Gastroenterol Hepatol. 34:338–344. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cholangiocarcinoma Working Group, :
Italian clinical practice guidelines on cholangiocarcinoma-part I:
Classification, diagnosis and staging. Dig Liver Dis. 52:1282–1293.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chung T and Park YN: Up-to-date pathologic
classification and molecular characteristics of intrahepatic
cholangiocarcinoma. Front Med (Lausanne). 9:8571402022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Forner A, Vidili G, Rengo M, Bujanda L,
Ponz-Sarvisé M and Lamarca A: Clinical presentation, diagnosis and
staging of cholangiocarcinoma. Liver Int. 39 (Suppl 1):S98–S107.
2019. View Article : Google Scholar
|
|
23
|
Geizhals S and Lipner SR: Review of
onychocryptosis: Epidemiology, pathogenesis, risk factors,
diagnosis and treatment. Dermatol Online J. 25:13030/qt9985w2n0.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rousselle P and Scoazec JY: Laminin 332 in
cancer: When the extracellular matrix turns signals from cell
anchorage to cell movement. Semin Cancer Biol. 62:149–165. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsuruta D, Kobayashi H, Imanishi H,
Sugawara K, Ishii M and Jones JCR: Laminin-332-integrin
interaction: A target for cancer therapy? Curr Med Chem.
15:1968–1975. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang H, Cai J, Du S, Wei W and Shen X:
LAMC2 modulates the acidity of microenvironments to promote
invasion and migration of pancreatic cancer cells via regulating
AKT-dependent NHE1 activity. Exp Cell Res. 391:1119842020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou YM, Yao YL, Liu W, Shen XM, Shi LJ
and Wu L: MicroRNA-134 inhibits tumor stem cell migration and
invasion in oral squamous cell carcinomas via downregulation of
PI3K-Akt signaling pathway by inhibiting LAMC2 expression. Cancer
Biomark. 29:51–67. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu M, Cai R, Wang T, Yang X, Wang M,
Kuang Z, Xie Y, Zhang J and Zheng Y: LAMC2 promotes the
proliferation of cancer cells and induce infiltration of
macrophages in non-small cell lung cancer. Ann Transl Med.
9:13922021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang D, Guo H, Feng W and Qiu H: LAMC2
regulated by microRNA-125a-5p accelerates the progression of
ovarian cancer via activating p38 MAPK signalling. Life Sci.
232:1166482019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Islam S, Kitagawa T, Baron B, Abiko Y,
Chiba I and Kuramitsu Y: ITGA2, LAMB3, and LAMC2 may be the
potential therapeutic targets in pancreatic ductal adenocarcinoma:
an integrated bioinformatics analysis. Sci Rep. 11:105632021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Garg M, Kanojia D, Okamoto R, Jain S,
Madan V, Chien W, Sampath A, Ding LW, Xuan M, Said JW, et al:
Laminin-5γ-2 (LAMC2) is highly expressed in anaplastic thyroid
carcinoma and is associated with tumor progression, migration, and
invasion by modulating signaling of EGFR. J Clin Endocrinol Metab.
99:E62–E72. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carpino G, Overi D, Melandro F, Grimaldi
A, Cardinale V, Di Matteo S, Mennini G, Rossi M, Alvaro D, Barnaba
V, et al: Matrisome analysis of intrahepatic cholangiocarcinoma
unveils a peculiar cancer-associated extracellular matrix
structure. Clin Proteomics. 16:372019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ye M, Song Y, Pan S, Chu M, Wang ZW and
Zhu X: Evolving roles of lysyl oxidase family in tumorigenesis and
cancer therapy. Pharmacol Ther. 215:1076332020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lin HY, Li CJ, Yang YL, Huang YH, Hsiau YT
and Chu PY: Roles of lysyl oxidase family members in the tumor
microenvironment and progression of liver cancer. Int J Mol Sci.
21:97512020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Barczyk M, Carracedo S and Gullberg D:
Integrins. Cell Tissue Res. 339:269–280. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brown AC, Dysart MM, Clarke KC,
Stabenfeldt SE and Barker TH: Integrin α3β1 binding to fibronectin
is dependent on the ninth type III repeat. J Biol Chem.
290:25534–25547. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Giannelli G, Astigiano S, Antonaci S,
Morini M, Barbieri O, Noonan DM and Albini A: Role of the
alpha3beta1 and alpha6beta4 integrins in tumor invasion. Clin Exp
Metastasis. 19:217–223. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Islam K, Thummarati P, Kaewkong P, Sripa B
and Suthiphongchai T: Role of laminin and cognate receptors in
cholangiocarcinoma cell migration. Cell Adh Migr. 15:152–165. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Samaržija I, Dekanić A, Humphries JD,
Paradžik M, Stojanović N, Humphries MJ and Ambriović-Ristov A:
Integrin crosstalk contributes to the complexity of signalling and
unpredictable cancer cell fates. Cancers (Basel). 12:19102020.
View Article : Google Scholar : PubMed/NCBI
|