Introduction
Lung cancer is the most common malignancy
responsible for cancer-related death worldwide, accounts for 18% of
all diagnosed cancers (1). Notably,
85% of patients with lung cancer are diagnosed with non-small cell
lung cancer (NSCLC) (2). The 5-year
survival rate for NSCLC varies; while patients with stage IA have a
good prognosis (85%), only 6% of patients with stage IV cancer
survive for 5 years (3). Surgery is
currently the main treatment for early-stage NSCLC; however, for
locally advanced NSCLC, preoperative platinum-based adjuvant
chemotherapy improves the 5-year survival rate by only 5% compared
to surgery alone (4). Immune
checkpoint inhibitors (ICIs) improve survival in patients with
advanced NSCLC compared with chemotherapy alone, and patients with
NSCLC who received immunotherapy combined with chemotherapy were
reported to have higher pathological complete response (pCR) rates
and longer event-free survival (5,6). It
has been reported that the rapid development of individualized
treatment for lung cancer has brought hope to patients with lung
cancer. To improve the prognosis of NSCLC, it is necessary to
better understand the benefit of preoperative neoadjuvant therapy
in specific patient populations.
NSCLC is primarily divided into lung adenocarcinoma
(LUAD) and lung squamous cell carcinoma (LUSC). LUAD originates
from cells that secrete surfactant components, and LUSC originates
from cells lining the lung airways, the differentially expressed
genes between LUAD and LUSC cause differences in the regulatory
networks of DNA replication and repair and RNA splicing, and
further cause differences in cell structure, which causes them to
serve different roles in tumor cell proliferation and tissue
invasion, so LUAD and LUSC are not only histologically distinct
tumors, but also have unique biological characteristics and
clinical features (7,8). Although often treated similarly, LUAD
and LUSC have different prognoses and this is likely because the
unique clinical characteristics and behavior of LUAD and LUSC
remain largely unknown (9). There
are currently no clear guidelines on the efficacy of neoadjuvant
therapy for different subtypes of NSCLC (10).
The majority of previous clinical evaluations of
post-neoadjuvant therapy have focused only on tumors, and there are
few reports assessing pathological responses in the lymph node
after neoadjuvant therapy (11).
The CheckMate 816 trial was a rare exception to this practice, but
it is clear that evidence for the efficacy of neoadjuvant
immunotherapy in lymph nodes is insufficient (12). The main purpose of the present study
was to review and analyze the clinical data from patients who had
received neoadjuvant therapy for LUAD and LUSC, to preliminarily
evaluate the efficacy of neoadjuvant therapy in both tumors and
lymph node remission of patients with two distinct pathological
subtypes, and to provide clinical treatment guidelines.
Materials and methods
Patients and methods
The clinical data of patients who underwent surgery
after neoadjuvant chemotherapy or chemotherapy combined with
immunotherapy at The First Affiliated Hospital, Sun Yat-sen
University (Guangzhou, China) between January 2016 and August 2022
were retrospectively analyzed. Patients who had received
chemotherapy combined with immunotherapy were recruited from
January 2020 to August 2022. The inclusion criteria were as
follows: i) Aged ≥18 years; ii) all patients with biopsy-proven
NSCLC with a defined histological subtype; iii) patients with lymph
node metastasis, no distant metastasis, potentially operable but
not suitable for immediate resection (based on imaging
information), classified according to the tumor-lymph
node-metastasis (TNM) staging system of the 8th edition of the
American Joint Committee on Cancer (13); iv) received chemotherapy or
chemotherapy combined with immunotherapy before surgery; v)
unspecified driver gene mutations, including anaplastic lymphoma
kinase and epidermal growth factor receptor; vi) no history of
other malignancies prior to treatment and no prior antitumor
therapy; and vii) Eastern Cooperative Oncology Group (ECOG)
performance status scores of 0 or 1 (14). The general information and treatment
data of the patients, such as age, sex, smoking history,
histopathological type, clinical TNM stage, chemotherapy regimen
and frequency, imaging changes before and after treatment, and
final pathological TNM stage, were recorded in detail.
Treatment
All patients received platinum-based chemotherapy or
ICIs combined with chemotherapy, and were evaluated using the same
procedures before and after neoadjuvant therapy. Tumor changes were
assessed based on Response Evaluation Criteria in Solid Tumors
(RECIST; version 1.1) as complete remission (CR), partial remission
(PR), stable disease (SD) or progressive disease (PD) (15). Pathological response to treatment
was graded; pCR was defined as the complete absence of residual
tumor cells. and major pathological response (MPR) was defined as
the presence of residual viable tumor cells ≤10%. Pathological
stable disease (SD) is defined as the presence of >50% viable
tumor cells in the resected cancer specimen; incomplete
pathological response is defined as the presence of >50% viable
tumor cells in the resected cancer specimen >10% viable tumor
cells present (16).
Statistical analysis
Statistical analysis was performed using SPSS 23.0
(IBM Corp.). Continuous variables are presented as mean ± standard
deviation. Student's t-test was used to compare independent samples
of normally distributed continuous variables, and the Mann-Whitney
U test was used to compare non-normally distributed continuous
variables. Categorical data are presented as frequencies
(percentages). Comparisons of categorical variables between groups
were made using Fisher's exact test or Pearson's χ2
test. Univariate logistic regressive analysis was used to evaluate
the influence of certain clinical variables on pathological
remission. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
In the present study, a total of 67 cases were
identified, including 36 patients with LUSC and 31 patients with
LUAD. The basic information and clinical characteristics of the
patients are shown in Table I. All
patients underwent surgery after neoadjuvant therapy. Patients were
predominantly male (79.10%), and most patients were diagnosed with
stage III NSCLC (82.19%) and ipsilateral mediastinal and/or
subcarinal lymph node metastasis (N2) (55.22%). No significant
differences were demonstrated in patient characteristics between
LUSC and LUAD except for age. However, age was not a risk factor
for clinical efficacy when data were analyzed using univariate
logistic regression.
 | Table I.Demographic and tumor characteristics
of patients. |
Table I.
Demographic and tumor characteristics
of patients.
| Characteristic | Total | LUSC | LUAD | P-value |
|---|
| n | 67 | 36 | 31 |
|
| Mean age ± SD,
years | 59±10.21 | 62±8.54 | 55±10.71 | 0.01 |
| Sex, n (%) |
|
|
|
|
| Male | 53 (79.10) | 32 (88.89) | 21 (67.74) | 0.41 |
|
Female | 14 (20.90) | 4 (11.11) | 10 (32.26) |
|
| Smoking status, n
(%) |
|
|
| 0.89 |
| Former or
current | 31 (46.27) | 13 (36.11) | 18 (58.06) |
|
|
Never | 36 (53.73) | 23 (63.89) | 13 (41.94) |
|
| cT, n (%) |
|
|
| 0.23 |
| 1 | 9 (13.43) | 4 (11.11) | 5 (16.13) |
|
| 2 | 24 (35.82) | 10 (27.78) | 14 (45.14) |
|
| 3 | 21 (31.34) | 15 (41.67) | 6 (19.35) |
|
| 4 | 13 (19.41) | 7 (19.44) | 6 (19.35) |
|
| cN, n (%) |
|
|
| 0.08 |
| 1 | 24 (35.82) | 16 (44.44) | 8 (25.81) |
|
| 2 | 37 (55.22) | 19 (52.78) | 18 (58.06) |
|
| 3 | 6 (8.96) | 1 (2.78) | 5 (16.13) |
|
| cStage, n (%) |
|
|
| 0.09 |
| 2 | 12 (17.91) | 9 (25.00) | 3 (9.67) |
|
| 3 | 55 (82.19) | 27 (75.00) | 28 (90.33) |
|
| Previous treatments,
n (%) |
|
|
| >0.99 |
|
Chemotherapy | 36 (53.73) | 19 (52.78) | 17 (54.83) |
|
|
Chemotherapy combined with
immunotherapy | 31 (46.27) | 17 (47.22) | 14 (45.17) |
|
| Number of previous
treatments, n (%) |
|
|
| 0.63 |
| ≤2 | 34 (50.75) | 17 (47.22) | 17 (54.84) |
|
|
>2 | 33 (49.25) | 19 (52.78) | 14 (45.16) |
|
Efficacy
According to RECIST 1.1 criteria, 9 (13.43%)
patients achieved CR, 38 (56.72%) patients achieved PR, 18 (26.87%)
patients achieved SD and 2 (2.98%) patients achieved PD. There was
no significant difference in radiological response after
neoadjuvant therapy between patients with LUAD or LUSC (Table II).
 | Table II.Comparison of radiological and
pathological responses in patients after neoadjuvant therapy. |
Table II.
Comparison of radiological and
pathological responses in patients after neoadjuvant therapy.
| Characteristic | Total (%) | LUSC (%) | LUAD (%) | P-value |
|---|
| n | 67 (100.00) | 36 (53.73) | 31 (46.27) |
|
| Radiological
response |
|
|
|
|
| CR | 9 (13.43) | 7 (19.44) | 2 (6.45) | 0.16 |
| PR | 38 (56.72) | 19 (52.78) | 19 (61.29) | 0.62 |
| SD | 18 (26.87) | 9 (25.00) | 9 (29.03) | 0.79 |
| PD | 2 (2.98) | 1 (2.78) | 1 (3.23) | 1.00 |
| Pathological
response |
|
|
|
|
|
pCR | 19 (28.36) | 14 (38.89) | 5 (16.13) | 0.06 |
|
MPR | 6 (8.96) | 3 (8.33) | 3 (9.68) | >0.99 |
| Downstaging of T
stage | 51 (76.12) | 27 (75.00) | 24 (77.42) | >0.99 |
| Downstaging of N
stage | 55 (82.09) | 31 (86.11) | 24 (77.42) | 0.50 |
Next, the pathological response of the two NSCLC
subtypes were compared, 38.89% of patients with LUSC achieved pCR
after surgery compared with 16.13% of patients with LUAD (Table II). Lymph node regression rates
were comparable between the two groups (86.11% vs. 77.42%; Table II). LUSC appeared to be more
responsive to neoadjuvant therapy, but there were no statistically
significant differences in tumor regression and lymph node
downstaging after neoadjuvant therapy between the LUSC and LUAD
groups.
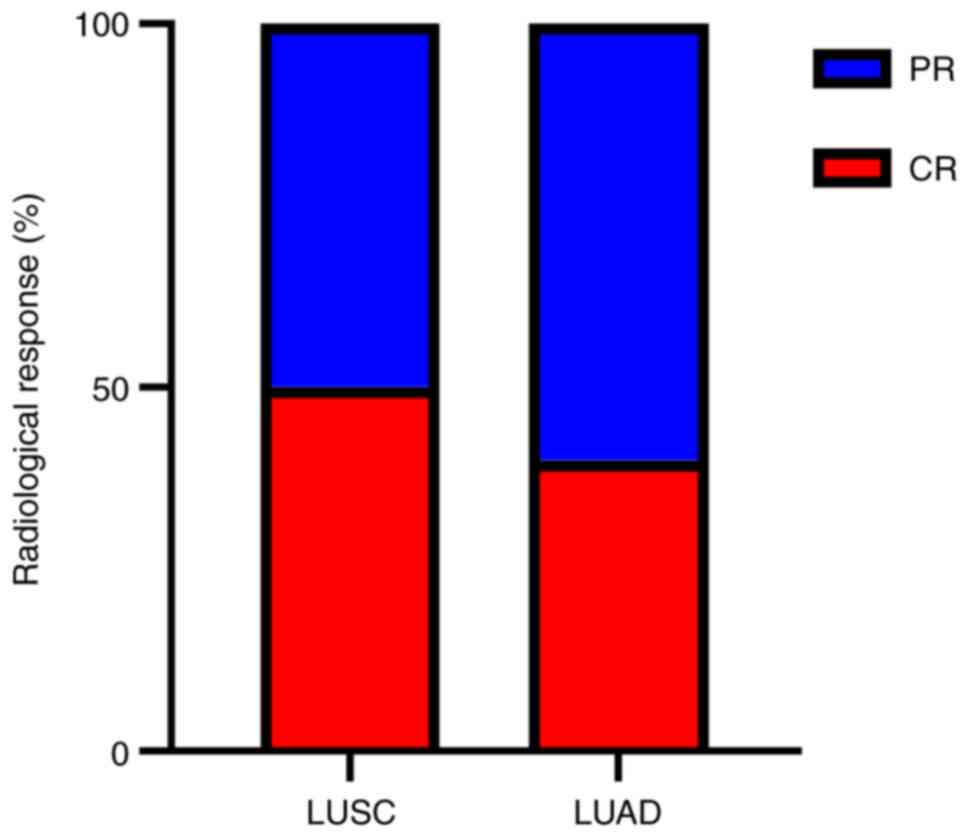
Radiological response in patients with
pCR
Analysis of the concordance between two different
subtypes of radiological responses and pCR was performed. The
results demonstrated that among all patients who achieved pCR, 50%
of patients with LUSC had radiographic CR (7/14) compared with 40%
of patients with LUAD (2/5) (Fig.
1).
Regression analysis of risk factors
for postoperative pathological response
Univariate logistic regression models were
used to assess whether sex, age, pathological subtype, clinical
stage, neoadjuvant therapy modality and number of previous
neoadjuvant therapies could be risk factors for pCR and MPR events.
The results demonstrated that chemotherapy combined with
immunotherapy was a risk factor affecting pCR and MPR. (odds ratio
5.74; 95% confidence interval 1.93–17.01; P<0.002; Table III).
 | Table III.Univariate logistic regressive
analysis for pathological response. |
Table III.
Univariate logistic regressive
analysis for pathological response.
| Characteristic | pCR/MPR (%) | Non-pCR/MPR
(%) | OR (95% CI) | P-value |
|---|
| n | 25 (100.00) | 42 (100.00) |
|
|
| Sex |
|
| 0.38
(0.09–1.54) | 0.18 |
|
Male | 22 (88.00) | 31 (73.81) |
|
|
|
Female | 3 (12.00) | 11 (26.19) |
|
|
| Mean age ± SD,
years | 57±10.39 | 62±9.87 | 1.03
(0.98–1.09) | 0.17 |
| Smoking status |
|
|
|
|
|
Never | 11 (44.00) | 20 (47.62) | 1.16
(0.43–3.13) | 0.77 |
| Former
or current | 14 (56.00) | 22 (52.38) |
|
|
| Histological
type |
|
|
|
|
|
LUSC | 17 (68.00) | 19 (45.24) | 0.39
(0.14–1.09) | 0.07 |
|
LUAD | 8 (32.00) | 23 (54.76) |
|
|
| cT |
|
|
|
|
| 1 | 5 (20.00) | 3 (7.14) | 0.65
(0.38–1.14) | 0.14 |
| 2 | 9 (36.00) | 16 (38.10) |
|
|
| 3 | 8 (32.00) | 13 (30.95) |
|
|
| 4 | 3 (12.00) | 10 (23.81) |
|
|
| cN |
|
|
|
|
| 1 | 11 (44.00) | 13 (30.96) | 0.80
(0.36–1.81) | 0.59 |
| 2 | 11 (44.00) | 26 (61.90) |
|
|
| 3 | 3 (12.00) | 3 (7.14) |
|
|
| cStage |
|
| 0.52
(0.15–1.86) | 0.32 |
| 2 | 6 (24.00) | 6 (14.29) |
|
|
| 3 | 19 (76.00) | 36 (85.71) |
|
|
| Previous
treatment |
|
| 5.74
(1.93–17.01) | 0.002 |
|
Chemotherapy | 7 (28.00) | 29 (69.05) |
|
|
|
Chemotherapy combined
with | 18 (72.00) | 13 (30.95) |
|
|
|
immunotherapy |
|
|
|
|
| Number of previous
treatments |
|
| 2.00
(0.73–5.47) | 0.18 |
| ≤2 | 10 (40.00) | 24 (57.14) |
|
|
|
>2 | 15 (60.00) | 18 (42.86) |
|
|
To further clarify the role of ICIs in NSCLC, the
effects of neoadjuvant chemotherapy and neoadjuvant chemotherapy
combined with immunotherapy on NSCLC subtypes were compared.
Compared with chemotherapy alone, chemotherapy combined with
immunotherapy improved the pCR and MPR rates; specifically, the pCR
rate of LUAD increased from 11.76 to 21.43% (Table SIII) and that of LUSC increased
from 21.05 to 58.82% (Table IV).
Although LUSC had a higher pCR rate than LUAD after chemotherapy or
chemotherapy combined with immunotherapy, no statistically
significant difference was demonstrated between the two subtypes
(chemotherapy combined with immunotherapy, pCR P=0.07;
chemotherapy, pCR P=0.66; Tables
SI and SII). Moreover, in
LUSC, neoadjuvant chemotherapy combined with immunotherapy
significantly improved the pCR rate (P=0.01; Table IV), whereas there was no
significant difference in LUAD (P=0.64; Table SIII).
 | Table IV.Comparison of pathological remission
of neoadjuvant chemotherapy and neoadjuvant immunotherapy in
LUSC. |
Table IV.
Comparison of pathological remission
of neoadjuvant chemotherapy and neoadjuvant immunotherapy in
LUSC.
| Characteristic | Chemotherapy
(%) | Chemotherapy
combined with immunotherapy (%) | P-value |
|---|
| n | 19 (100.00) | 17 (100.00) |
|
| pCR | 4 (21.05) | 10 (58.82) | 0.01 |
| MPR | 5 (26.31) | 12 (70.59) | 0.59 |
| Downstaging of T
stage | 11 (57.89) | 16 (94.12) | 0.02 |
| Downstaging of N
stage | 13 (68.42) | 8 (47.06) | 0.31 |
Discussion
Patients with locally advanced NSCLC can benefit
from neoadjuvant chemotherapy, and programmed cell death 1
inhibitor combined with chemotherapy has been reported to enhance
the immune response further against tumor cells (17,18).
However, NSCLC is a diverse and complex disease with distinct
differences in clinical, histopathological and molecular
characteristics between the LUAD and LUSC subtypes (19,20).
In the present study, a comprehensive analysis was performed to
evaluate the clinical efficacy of neoadjuvant therapy in these two
distinct NSCLC pathological types.
In clinical trials, MPR and pCR are currently the
most common modalities for assessing pathological responses to
neoadjuvant immunotherapy and are also used for assessing survival
(21). Several previous studies
have reported an association between pCR status and survival in
NSCLC (22,23). Neoadjuvant immunotherapy can reduce
the primary tumor volume and enable radical resection (24). It exerts its antitumor effects by
inducing antigens that trigger a durable and powerful T-cell immune
response (25,26). In the CheckMate 816 study, squamous
and non-squamous NSCLC were reported to have similar pCR rates to
immunotherapy combined with chemotherapy with 25.3% for squamous
and 22.8% for non-squamous NSCLC (11). In the present study, although there
was no significant difference in the pCR rate between LUAD and LUSC
after neoadjuvant chemotherapy combined with immunotherapy, the pCR
rate of LUSC was higher than that of LUAD.
The pCR and MPR rates in response to neoadjuvant
chemotherapy and neoadjuvant immune-combined chemotherapy in
different pathological subtypes were compared. It was demonstrated
that, compared with chemotherapy alone, the pCR and MPR of both
pathological subtypes were increased and the pCR of LUSC was
significantly improved after combined immunotherapy. Furthermore,
the radiological CR in LUSC was closer to pathological pCR. A
previous study reported that a higher primary pathological response
was observed after neoadjuvant chemotherapy in patients with
squamous cell carcinoma (26%) compared with that in patients with
adenocarcinoma (12%), possibly because of greater necrosis of the
initial tumor tissue in squamous cell carcinoma (27). Moreover, compared with
adenocarcinoma, programmed cell death 1 ligand 1 expression is more
extensive in squamous cell carcinoma and the infiltration of immune
cells such as macrophages is more obvious, thus the response to
tumors is more thorough, which may be responsible for the
difference in the responses of squamous cell carcinoma and
adenocarcinoma to immunotherapy (28).
At present, pCR and MPR only assess primary tumors
and exclude lymph nodes (29).
Although lymph node involvement is often one of the key prognostic
factors in lung cancer, there are few studies which have evaluated
lymph nodes after neoadjuvant therapy. In recent years, researchers
have emphasized the significance of lymph node changes following
adjuvant therapy (30). The aim of
the present study was to evaluate the effect of neoadjuvant therapy
on lymph nodes. However, no significant changes in lymph nodes were
identified regardless of subtype or treatment regimen.
There are certain limitations to the present study.
First, as it is a retrospective study, estimation of diagnostic
efficacy, as well as differences in scanner and image acquisition
parameters, could potentially lead to bias. Consequently, each
sample was re-evaluated for pathology and radiological response, as
part of the present study, to minimize bias. Secondly, the study
was limited by the relatively small sample size and there may be
unavoidable confounding factors; therefore, future studies with
larger sample sizes are needed to confirm these findings. Third,
the observation period was relatively short and the long-term
overall survival rate was not analyzed, as although the data
collected in this retrospective study are from January 2016 to
August 2022, the data for chemotherapy combined with immunotherapy
are from January 2021 to August 2022. Thus, the observation period
was relatively short and complete survival data were not collected;
therefore, subsequent long-term follow-up and more mature data are
necessary. Despite these limitations, the findings of the present
study demonstrated the difference in sensitivity to neoadjuvant
therapy between pathological subtypes of NSCLC.
In conclusion, it was found that of the two major
subtypes of NSCLC, LUSC was more sensitive to immunotherapy and had
better clinical outcomes compared with LUAD.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YG and HSZ were responsible for study conception and
design; HSZ provided administrative support; YG, HSZ, ZHL, XJY, ZWT
and BZ contributed to the collection of patient data; ZHL, XJY and
ZWT collected and assembled the data; and YG and BZ performed data
analysis and interpretation. All authors read and approved the
final manuscript. YG and HSZ confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thai AA, Solomon BJ, Sequist LV, Gainor JF
and Heist RS: Lung cancer. Lancet. 398:535–554. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ahmad A and Gadgeel SM: Lung cancer and
personalized medicine: Novel therapies and clinical management. Adv
Exp Med Biol. 890:v–vi. 2016.PubMed/NCBI
|
|
4
|
NSCLC Meta-analysis Collaborative Group, .
Preoperative chemotherapy for non-small-cell lung cancer: A
systematic review and meta-analysis of individual participant data.
Lancet. 383:1561–1571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Spicer J, Wang C, Tanaka F, Saylors GB,
Chen KN, Liberman M, Vokes E, Girard N, Lu S, Provencio M, et al:
Surgical outcomes from the phase 3 CheckMate 816 trial: Nivolumab
(NIVO) + platinum-doublet chemotherapy (chemo) vs chemo alone as
neoadjuvant treatment for patients with resectable non-small cell
lung cancer (NSCLC). J Clin Oncol. 39 (Suppl 15):S85032021.
View Article : Google Scholar
|
|
6
|
Wakelee HA, Altorki NK, Zhou C, Csőszi T,
Vynnychenko IO, Goloborodko O, Goloborodko O, Luft A, Akopov A,
Martinez-Marti A, Kenmotsu H, et al: IMpower010: Primary results of
a phase III global study of atezolizumab versus best supportive
care after adjuvant chemotherapy in resected stage IB-IIIA
non-small cell lung cancer (NSCLC). J Clin Oncol. 39 (Suppl
15):S85002021. View Article : Google Scholar
|
|
7
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Relli V, Trerotola M, Guerra E and Alberti
S: Abandoning the notion of non-small cell lung cancer. Trends Mol
Med. 25:585–594. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kawase A, Yoshida J, Ishii G, Nakao M,
Aokage K, Hishida T, Nishimura M and Nagai K: Differences between
squamous cell carcinoma and adenocarcinoma of the lung: are
adenocarcinoma and squamous cell carcinoma prognostically equal?
Jpn J Clin Oncol. 42:189–195. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Detterbeck FC, Socinski MA, Gralla RJ,
Edelman MJ, Jahan TM, Loesch DM, Limentani SA, Govindan R, Zaman
MB, Ye Z, et al: Neoadjuvant chemotherapy with
gemcitabine-containing regimens in patients with early-stage
non-small cell lung cancer. J Thorac Oncol. 3:37–45. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pataer A, Weissferdt A, Vaporciyan AA,
Correa AM, Sepesi B, Wistuba II, Heymach JV, Cascone T and Swisher
SG: Evaluation of pathologic response in lymph nodes of patients
with lung cancer receiving neoadjuvant chemotherapy. J Thorac
Oncol. 16:1289–1297. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Forde PM, Spicer J, Lu S, Provencio M,
Mitsudomi T, Awad MM, Felip E, Broderick SR, Brahmer JR, Swanson
SJ, et al: Neoadjuvant nivolumab plus chemotherapy in resectable
lung cancer. N Engl J Med. 386:1973–1985. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goldstraw P, Chansky K, Crowley J,
Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P,
Mitchell A, Bolejack V, et al: The IASLC lung cancer staging
project: Proposals for revision of the TNM stage groupings in the
forthcoming (eighth) edition of the TNM classification for lung
cancer. J Thorac Oncol. 11:39–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Azam F, Latif MF, Farooq A, Tirmazy SH,
AlShahrani S, Bashir S and Bukhari N: Performance status assessment
by using ECOG (eastern cooperative oncology group) score for cancer
patients by oncology healthcare professionals. Case Rep Oncol.
12:728–736. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pataer A, Kalhor N, Correa AM, Raso MG,
Erasmus JJ, Kim ES, Behrens C, Lee JJ, Roth JA, Stewart DJ, et al:
Histopathologic response criteria predict survival of patients with
resected lung cancer after neoadjuvant chemotherapy. J Thorac
Oncol. 7:825–832. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Emens LA and Middleton G: The interplay of
immunotherapy and chemotherapy: Harnessing potential synergies.
Cancer Immunol Res. 3:436–443. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Provencio M, Nadal E, Insa A,
García-Campelo MR, Casal-Rubio J, Dómine M, Majem M,
Rodríguez-Abreu D, Martínez-Martí A, De Castro Carpeño J, et al:
Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell
lung cancer (NADIM): An open-label, multicentre, single-arm, phase
2 trial. Lancet Oncol. 21:1413–1422. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Z, Fillmore CM, Hammerman PS, Kim CF
and Wong KK: Non-small-cell lung cancers: A heterogeneous set of
diseases. Nat Rev Cancer. 14:535–546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang BY, Huang JY, Chen HC, Lin CH, Lin
SH, Hung WH and Cheng YF: The comparison between adenocarcinoma and
squamous cell carcinoma in lung cancer patients. J Cancer Res Clin
Oncol. 146:43–52. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Junker K, Thomas M, Schulmann K, Klinke F,
Bosse U and Müller KM: Tumour regression in non-small-cell lung
cancer following neoadjuvant therapy. Histological assessment. J
Cancer Res Clin Oncol. 123:469–477. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Waser N, Adam A, Schweikert B, Vo L,
McKenna M, Breckenridge M, Penrod JR and Goring S: 1243P Pathologic
response as early endpoint for survival following neoadjuvant
therapy (NEO-AT) in resectable non-small cell lung cancer (rNSCLC):
Systematic literature review and meta-analysis. Ann Oncol. 31
(Suppl 4):S8062020. View Article : Google Scholar
|
|
23
|
Rosner S, Liu C, Forde PM and Hu C:
Association of pathologic complete response and long-term survival
outcomes among patients treated with neoadjuvant chemotherapy or
chemoradiotherapy for NSCLC: A meta-analysis. JTO Clin Res Rep.
3:1003842022.PubMed/NCBI
|
|
24
|
Forde PM, Chaft JE and Pardoll DM:
Neoadjuvant PD-1 blockade in resectable lung cancer. N Eng J Med.
379:e142018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gandhi L, Rodríguez-Abreu D, Gadgeel S,
Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ,
Powell SF, et al: Pembrolizumab plus chemotherapy in metastatic
non-small-cell lung cancer. N Eng J Med. 378:2078–2092. 2018.
View Article : Google Scholar
|
|
26
|
Reck M, Shankar G, Lee A, Coleman S,
McCleland M, Papadimitrakopoulou VA, Socinski MA and Sandler A:
Atezolizumab in combination with bevacizumab, paclitaxel and
carboplatin for the first-line treatment of patients with
metastatic non-squamous non-small cell lung cancer, including
patients with EGFR mutations. Exp Rev Respir Med. 14:125–136. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qu Y, Emoto K, Eguchi T, Aly RG, Zheng H,
Chaft JE, Tan KS, Jones DR, Kris MG, Adusumilli PS and Travis WD:
Pathologic assessment after neoadjuvant chemotherapy for NSCLC:
Importance and implications of distinguishing adenocarcinoma from
squamous cell carcinoma. J Thorac Oncol. 14:482–493. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Q, Fu YY, Yue QN, Wu Q, Tang Y, Wang
WY, Wang YS and Jiang LL: Distribution of PD-L1 expression and its
relationship with clinicopathological variables: an audit from 1071
cases of surgically resected non-small cell lung cancer. Int J Clin
Exp Pathol. 12:774–786. 2019.PubMed/NCBI
|
|
29
|
Choi NC, Carey RW, Daly W, Mathisen D,
Wain J, Wright C, Lynch T, Grossbard M and Grillo H: Potential
impact on survival of improved tumor downstaging and resection rate
by preoperative twice-daily radiation and concurrent chemotherapy
in stage IIIA non-small-cell lung cancer. J Clin Oncol. 15:712–722.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Travis WD, Dacic S, Wistuba I, Sholl L,
Adusumilli P, Bubendorf L, Bunn P, Cascone T, Chaft J, Chen G, et
al: IASLC multidisciplinary recommendations for pathologic
assessment of lung cancer resection specimens after neoadjuvant
therapy. J Thorac Oncol. 15:709–740. 2020. View Article : Google Scholar : PubMed/NCBI
|