Introduction
Intrahepatic cholangiocarcinoma (ICC) is a highly
lethal hepatobiliary tumor with a 140% increasing incidence in
worldwide over the past 4 decades (1–3). ICC
is characterized by aggressive progression, connective tissue
hyperplasia and vascular abnormalities (4). Complete surgical resection remains the
only cure for patients with ICC but only one third of patients have
resectable ICC (5). For patients
with unresectable or metastatic disease, combine systemic and local
therapies (such as radioembolization or hepatic artery infusion)
and targeted therapies (such as target fibroblast growth factor
receptor and isocitrate dehydrogenase) are required (3). Ferroptosis is an iron-dependent cell
death pathway involved in a number of diseases, such as acute
myeloid leukaemia, T-cell leukaemia/lymphoma, and COVID-19
infections (6). In recent years,
ferroptosis has attracted a great deal of interest in cancer
research, such as non-small-cell lung cancer, breast cancer,
gastric cancer and liver cancer (7–9).
Ferroptosis is considered to be a tumor suppressor and a novel
target for tumor therapy (6).
However, the specific mechanism of ICC and ferroptosis remains
unclear.
Circular RNAs (circRNAs) are non-coding RNAs
containing covalently closed loops that are characterized by
abundance, stability, conservation and tissue specificity (10). In cancer, circRNAs serve essential
regulatory roles, including tumor-promoting and tumor-suppressing
activity. Elevated circRNA cGGNBP2 (a circular RNA derived from
exons 4–6 of the gametogenetin binding protein 2 gene) is
associated with poor prognosis in patients with ICC and is an
independent risk factor for prognosis (11). Furthermore, circRNA SWI/SNF related,
matrix associated, actin dependent regulator of chromatin,
subfamily a, member 5 is associated with favorable clinical tumor
characteristics and prognosis and increased ICC chemosensitivity
(12). A previous study reported
that circRNA actinin alpha 4 (ACTN4), also named as
hsa_circ_0050900, is highly expressed in breast cancer and
competitively binds to the far upstream element binding protein 1
to prevent the combination of far upstream element binding protein
1 and FBP interacting repressor, thereby activating Myc
transcription and facilitating tumor progression (13). Furthermore, circACTN4
(hsa_circ_0050898), another circRNA derived from ACTN4, is
upregulated in ICC and promotes ICC cell proliferation and
metastasis by regulating the target microRNA (miR)-424-5p, as well
as transcriptional activation of Frizzled-7, by interacting with
Y-box binding protein 1 (14).
However, whether hsa_circ_0050900 is involved in ferroptosis in ICC
is unknown.
hsa-miR-605-3p is reported to play a tumor
suppressor role in different types of cancer: For example,
miR-605-3p is downregulated in colon cancer and overexpression of
miR-605-3p inactivates the Wnt/β-catenin signaling pathway induced
by overexpression of kinesin family member 3B (15). In bladder cancer, overexpression of
circRNA VANGL planar cell polarity protein 1 inhibits the
availability of miR-605-3p to promote the expression of VANGL
planar cell polarity protein 1, and the cancer cell proliferation,
migration and invasion (16).
Additionally, miR-605-3p is downregulated in glioma and high
expression of miR-605-3p decreases the expression of target
vasodilator stimulated phosphoprotein to inhibit glioma cell
proliferation, migration and invasion (17). miR-605-3p is lowly expressed in
hepatocellular carcinoma and small nucleolar RNA host gene
16/miR-605-3p/TNF receptor associated factor 6/NF-κB feedback loop
participates in development of hepatocellular carcinoma (18). Moreover, miR-605 is lowly expressed
in ICC and regulates ICC progression by controlling the downstream
target gene proteasome 26S subunit, non-ATPase 10 (19). However, whether hsa_circ_0050900
regulates hsa-miR-605-3p to participate in ferroptosis in ICC cells
is unknown.
Solute carrier family 3 member 2 (SLC3A2), also
called CD98hc or 4F2hc, is a type II membrane protein (20,21).
SLC3A2 and SLC7A11 are key targets for ferroptosis. The role of
SLC7A11 in different types of cancer has been widely explored in
recent years; to the best of our knowledge, however, there are few
studies on SLC3A2 (22,23). SLC3A2 and its light chain subunit
constitute a heterodimeric transmembrane complex that mediates
amino acid transport and regulates mTOR and
macroautophagy/autophagy (24). In
lung adenocarcinoma, YTH N6-methyladenosine RNA binding protein C2
serves as an endogenous ferroptosis inducer and inhibits SLC3A2
expression (25). In addition,
SLC3A2 negatively regulates ferroptosis in laryngeal carcinoma via
the mTOR pathway (26). SLC3A2
inhibits ferroptosis and suppresses cartilage degeneration in
osteoarthritis (27). Moreover,
liver cancer cells with hepatitis B virus infection-derived
exosomal miR-142-3p exhibit increased M1-type macrophage
ferroptosis by inhibiting expression of target SLC3A2 (28). However, it is unknown whether
hsa_circ_0050900 regulates SLC3A2 by sponging hsa-miR-605-3p and
thus affecting ferroptosis in ICC.
The present study aimed to explore
hsa_circ_0050900/hsa-miR-605-3p/SLC3A2 signaling in ICC cell
ferroptosis and the molecular mechanism underlying ICC occurrence
and development to facilitate development of novel therapeutic
targets.
Materials and methods
Cell culture and treatment
Human ICC-derived QBC-939, HUCCT-1, HCCC-9810 and
RBE cells were obtained from Shanghai Institute for Biological
Science (Shanghai, China). All cells were grown in Roswell Park
Memorial Institute-1640 (RPMI-1640) containing 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.). All cell lines were
cultured at 37°C in an incubator containing a humidified (100%)
atmosphere with 5% CO2. To investigate the role of
ferroptosis in ICC cells, 1 µM ferroptosis inhibitor ferrostatin-1
(Fer-1, cat. no. GC10380; GlpBio) was added for 24 h at 37°C, as
previously described (29).
Cell transfection
To overexpress wild-type (WT) luciferase reporter
plasmids of hsa_circ_0050900 (pmirGLO-WT, Promega Corporation),
mutant (MUT) luciferase reporter plasmids of hsa_circ_0050900,
(pmirGLO-MUT, Promega Corporation), hsa-miR-605-3p, or inhibit the
expression of hsa_circ_0050900 and hsa-miR-605-3p, Lipofectamine
2000 Transfection Reagent (Thermo Fisher Scientific, Inc.) was used
for cell transfection for 4 h. Following digestion using 0.25%
trypsin for 2 min at 37°C, 1×105 cells were inoculated
into 24-well plates and incubated overnight with 5% CO2
at 37°C. When the cell density was 80%, the medium was aspirated. A
total of 0.5 µg (in 5 µl) plasmids, siRNAs, mimics, or inhibitors
were dissolved in 250 µl OPTI-MEM (Gibco), then mixed in tube A. A
total of 1.5 µl Lipofectamine 2000 was dissolved in 250 µl
OPTI-MEM, mixed gently and stood for 5 min at room temperature in
tube B. Tube B was added to tube A, mixed well, stood for 20 min at
room temperature, then dropped into the 24-well plate well, mixed
well, and cultured at 37°C in 5% CO2. After 4 h, the
transfection medium was removed and 2 ml RPMI-1640 containing 10%
fetal bovine serum) was added to continue culture for 24 or 48 h at
37°C in 5% CO2 before further investigation. Small
interfering (si)-hsa_circ_0050900-1, hsa-miR-605-3p mimics,
hsa-miR-605-3p inhibitor and negative control (NC) si-NC, miR-NC
and NC inhibitor were synthesized by Shanghai GenePharma Co., Ltd.
Sequences were as follows: si-hsa_circ_0050900-1 forward sequences,
5′-AUGCUGGAUGCAGAGGACCUU-3′; si-hsa_circ_0050900-1 reverse,
5′-AAGGUCCUCUGCAUCCAGCAU-3′; si-hsa_circ_0050900-2 forward
sequences, 5′-AGAGGACCUUCACGGCAUGGU-3′; si-hsa_circ_0050900-2
reverse, 5′-ACCAUGCCGUGAAGGUCCUCU-3′; si-hsa_circ_0050900-3 forward
sequences, 5′-GCUGGAUGCAGAGGACCUUCA-3′; si-hsa_circ_0050900-3
reversed sequences, 5′-UGAAGGUCCUCUGCAUCCAGC-3′; si-NC forward
sequences, 5′-GAGUACCGUUUAUGGCAGGAC-3′; si-NC reversed sequences,
5′-AGAGGACCUUCACGGCAUGGU-3′; hsa-miR-605-3p mimics forward
sequences, 5′-AGAAGGCACUAUGAGAUUUAGAUU-3′; hsa-miR-605-3p mimics
reversed sequences, 5′-UCUAAAUCUCAUAGUGCCUUCUUU-3′; hsa-miR-605-3p
inhibitor, 5′-UCUAAAUCUCAUAGUGCCUUCU-3′; miR-NC forward,
5′-AAAGGUGUAGGAAAUUCACAUGUU-3′; miR-NC reverse,
5′-CAUGUGAAUUUCCUACACCUUUUU-3′ and NC inhibitor,
5′-CAGUACUUUUGUGUAGUACAA-3′.
Clone formation assay
After cell counting, the cells were diluted to
1×104 cell/ml, then 1×103 cell/ml was
inoculated on a 6-well plate (100 µl/well). After inoculation,
complete medium was added (2 ml/well). After 1 week of culture at
37°C in 5% CO2, the medium was removed and cells were
rinsed with PBS or normal saline twice for 10 sec each, fixed with
4% paraformaldehyde for 15 min at room temperature, washed with PBS
twice for 10 sec each and 200 µl crystal violet staining solution
was added to cover the bottom of the well. After incubation 20 min
at room temperature, the 6-well plate was rinsed under running
water for 10 sec and dried and the number of colonies (>50
cells) was calculated manually.
Cell migration assay
Transwell plate (8.0-µm pore size; Corning, Inc.)
was used to performed cell migration assay. Cells in logarithmic
growth phase were rinsed once with PBS and digested by 0.25%
trypsin (for 2 min at 37°C) into single cell suspension. Cell
suspension was centrifuged at 550 × g for 5 min at 37°C. After
supernatant was aspirated and cells were suspended with RPMI-1640,
cell concentration was adjusted to 1×106/ml with
RPMI-1640. A total of 100 µl cell suspension (in RPMI-1640) was
added to the upper chamber of Transwell chamber and 600 µl
RPMI-1640 containing 10% fetal bovine serum was added to the lower
chamber. Following incubation for 48 h at 37°C in 5%
CO2, cells in the upper chamber were removed and wiped
with cotton swabs. Cells were fixed with 4% paraformaldehyde for 15
min at 37°C, washed with PBS once for 30 sec, stained with 1%
crystal violet for 10 min at room temperature and washed with PBS
once for 30 sec. Migrated cells were observed under a light
microscope (200×).
Bioinformatics prediction and
dual-luciferase reporter gene assay
University of California-Santa Cruz (UCSC,
genome.ucsc.edu/; version: GRCh37/hg19) combined with circPrimer
2.0 (https://www.bio-inf.cn/) was utilized to
analyze the source of hsa_circ_0050900. CircAtlas 2.0 (https://ngdc.cncb.ac.cn/circatlas/) predicted the
binding sequence between hsa_circ_0050900 and hsa-miR-605-3p.
TargetScanHuman 7.1 (targetscan.org/vert_71/) predicted the binding
sites of hsa-miR-605-3p and SLC3A2 3′ untranslated region (UTR).
For dual-luciferase reporter gene assay, 293T cells were plated in
24-well plates (5×104 cells/well) and transfected with
WT or MUT luciferase reporter plasmids and hsa-miR-605-3p mimics
using Lipofectamine 2000 Transfection Reagent (Thermo Fisher
Scientific, Inc.). Following transfected for 48 h, luciferase
activity was detected using a luciferase reporter kit (cat. no.
FR201-01; TransGen Biotech Co., Ltd.). Luciferase activity was
calculated as activity of firefly luciferase activity compared with
Renilla luciferase activity. WT and MUT plasmid containing
hsa_circ_0050900 (WT1, 5′-TGGAAGGATGGTCTTGCCTTCA-3′; MUT1,
5′-TGGCGAGGGATTTTAGCATCCT-3′; WT2, 5′-ACCAACCTGAACAATGCCTTCG-3′ and
MUT2, 5′-CCGTGCCAACCCACAATGATAT-3′) and SLC3A2 3′ UTR sequence (WT,
5′-AAATAGGGTGTTTTCTGCCTTCA-3′ and MUT,
5′-GGTAACATTGGCTATATCCTGTT-3′) were inserted into pmirGLO plasmid
(Promega Corporation).
Fluorescence in situ hybridization
(FISH)
As previously described (30), subcellular localization of
hsa_circ_0050900 was detected using a FISH kit for digoxigenin
(cat. no. BIS-P0001; Guangzhou Bersin Co., Ltd.). HuCCT-1 cells
were fixed by 4% paraformaldehyde for 15 min at 25°C, washed with
PBS three times (5 min/time), and treated 0.1% TritonX-100 for 15
min at 25°C. After wash with PBS for twice (5 min/time), slides was
incubated in hybridization buffer (Wuhan Servicebio Technology CO.,
LTD., Wuhan, China) for 1 h at 37°C. Then, slides were treated with
digoxigenin-labeled hsa_circ_0050900 probe (cDNA,
5′-CCGTGAAGGTCCTCTGCATC-3′, 20 nt; Wuhan Servicebio technology CO.,
LTD.; diluted to 40 nM with hybridization buffer and denaturation
at 73°C for 5 min) hybridized at 37°C for 16 h, soaked in 2X saline
sodium citrate buffer for 10 min at 37°C, twice in 1X saline sodium
citrate buffer for 5 min at 37°C and twice in 0.5X saline sodium
citrate buffer for 5 min at 37°C. Then, slides were incubated in
normal rabbit serum (1:20 in PBS; cat. no. G1209; Wuhan Servicebio
technology CO., LTD.) for 30 min at 25°C and incubated anti-DIG-HRP
(dilution: 1:1,000; cat. no. 200-032-156; Jackson Immuno Research
Europe, Ltd.) for 50 min at 37°C, soaked in PBS thrice (5
min/time). Slides incubated with iF647-Tyramide (1:500; cat. no.
G1232; Wuhan Servicebio technology CO., LTD.) for 10 min at 25°C,
and washed using TBST (0.05% Tween-20) thrice (3 min/time) at 25°C
and stained with DAPI for 10 min at 25°C. Slides were observed
under a Zeiss LSM880 NLO confocal microscope (Leica GmbH, 1,000×)
with Zen Black software 2.0 (Carl Zeiss Microscopy GmbH).
Reverse transcription-quantitative PCR
(RT-qPCR) and Sanger sequencing
RNA was isolated from cells using TRIzol reagent
(Thermo Fisher Scientific, Inc.). Total RNA underwent RT (RT kit,
cat. no. K1622, Thermo Fisher Scientific, Inc.) and qPCR detection
was performed using SYBR-Green (cat. no. P122; Vazyme Biotech Co.,
Ltd.). For RT, 1 µl total RNA (100 ng), 1 µl oligo (dT)18 for
circRNA or mRNA expression or hsa-miR-605-3p RT primer (100 µM) for
expression of hsa-miR-605-3p and 10 µl nuclease-free water were
mixed gently, centrifuged at 250 × g for 5 sec at 25°C and
incubated at 65°C for 5 min. A total of 4 µl 5X Reaction Buffer, 1
µl RiboLock RNase Inhibitor (20 U/µl), 2 µl 10 mM dNTP Mix and 1 µl
RevertAid M-MuLV RT (200 U/µl) were mixed gently and centrifuged
250 × g for 5 sec at 25°C, followed by incubation for 60 min at
42°C. The reaction was terminated by heating at 70°C for 5 min.
Thermocycling conditions were as follows: Initial denaturation for
30 sec at 95°C, followed by 40 cycles of 30 sec at 95°C and 1 min
at 72°C. GAPDH or U6 (Gene ID 26827) was used as an internal
reference and 2−ΔΔCq was used to calculate the relative
expression (31). Primers were
designed by Primer Premier 5 and synthesized by Shanghai Sangon
Pharmaceutical Co., Ltd. In addition, qPCR products were analyzed
by Sanger sequencing to confirm the presence of hsa_circ_0050900
circularization site. The primer sequences were as follows:
hsa_circ_0050900 forward, 5′-GTCTTGCCTTCAATGCCCTG-3′ and reverse,
5′-CATGCCGTGAAGGTCCTCTG-3′; hsa-miR-605-3p RT primer,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCTAAA-3′;
hsa-miR-605-3p forward, 5′-AGAAGGCACTATGAGATTTAGA-3′ and reverse,
5′-GTGCAGGGTCCGAGGT-3′; SLC3A2 forward, 5′-ATGGAGCTACAGCCTCCTGA-3′
and reverse, 5′-CGCGCTGAGACCCTGG-3′; GAPDH forward,
5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse, 5′- GAAGATGGTGATGGGATTTC-3′
and U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′.
Western blot
Total cell protein was extracted with RIPA lysis
buffer (cat. no. P0013B; Beyotime Institute of Biotechnology) and
quantified using BCA protein concentration assay kit (cat. no.
BL521A; Biosharp Life Sciences). Protein (20 µg/lane) was separated
by 10% SDS-PAGE, transferred onto PVDF membrane, blocked using 5%
skimmed milk for 2 h at 25°C and incubated with primary antibodies
as follows: GPX4 (cat. no. 14432-1-AP; Proteintech Group, Inc.;
1:6,000), SLC3A2 (cat. no. 66883-1-Ig; Proteintech Group, Inc.;
1:100,000) and GAPDH (cat. no. 10494-1-AP; Proteintech Group, Inc.;
1:20,000) at 4°C overnight. HRP-conjugated Affinipure Goat
Anti-Mouse IgG (H+L; cat. no. SA00001-1; Proteintech Group, Inc.;
1:10,000) and Anti-Rabbit IgG (H+L; cat. no. SA00001-2; Proteintech
Group, Inc.; 1:10,000) secondary antibodies were incubated at room
temperature for 2 h. GAPDH was used as an internal reference and
exposure was performed using an ultra-sensitive ECL
chemiluminescent substrate (cat. no. BL520A; Biosharp Life
Sciences). Image J 1.48v (National Institutes of Health, Bethesda,
MD, USA) was used for densitometry.
Detection of Fe2+ and
reactive oxygen species (ROS) levels
Iron (cat. no. #MAK025; MilliporeSigma) and ROS
assay kit (cat. no. #S0033S; Beyotime Institute of Biotechnology)
were used to measure Fe2+ and ROS levels in HuCCT-1
cells, respectively, according to the manufacturer's
instructions.
Subcellular fractionation assay
The subcellular localization of hsa_circ_0050900 was
verified using a cytoplasmic and nuclear RNA purification kit (cat.
no. #21000; Norgen Biotek Corp.). ICC cells (5×106) were
treated with 200 µl freezing cell isolation buffer for 5 min to
separate the cytoplasm and nuclei. RT-qPCR was used to measure
hsa_circ_0050900 expression in the cytoplasm and nucleus as
aforementioned.
RNA immunoprecipitation (RIP)
assay
EZ-Magna RIP RNA Binding Protein Immunoprecipitation
kit (cat. no. #17-701; EMD Millipore) was used according to the
manufacturer's instructions. After cells were lysed in complete RIP
lysis buffer, whole cell lysate was collected and treated with
conjugated anti-AGO2 (cat. no. #67934-1-Ig; Proteintech Group,
Inc.; 5 µg) or normal mouse IgG overnight at 4°C. Next, 10 µl whole
cell lysate was used as input (positive control) and IgG acted as
negative control. The immunoprecipitated RNA was extracted and
relative enrichment of hsa_circ_0050900 and SLC3A2 in
immunoprecipitated RNA was detected by RT-qPCR as
aforementioned.
Statistical analysis
SPSS 16.0 software (SPSS, Inc.) was used for
statistical analysis. All experiments were repeated three times.
Data are expressed as the mean ± standard deviation. Unpaired t
test or one-way ANOVA followed by Tukey's post hoc test was used to
analyze data. P<0.05 was considered to indicate a statistically
significant difference.
Results
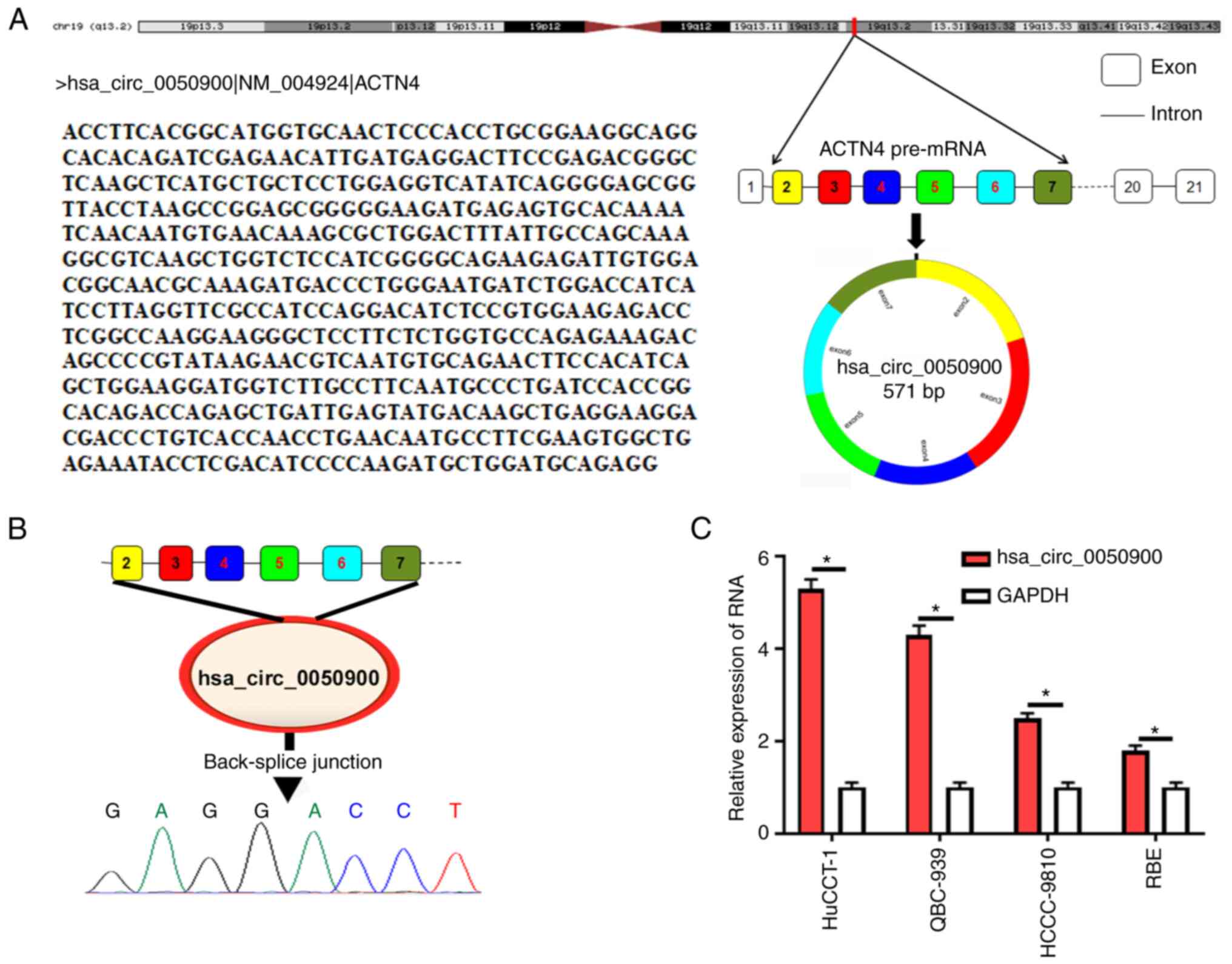
hsa_circ_0050900 is derived from ACTN4
gene and is highly expressed in ICC cells
UCSC combined with circPrimer was used to analyze
the source of hsa_circ_0050900. hsa_circ_0050900 was derived from
ACTN4 gene (Fig. 1A). Sanger
sequencing showed that there was a back-splice junction for
hsa_circ_0050900 (Fig. 1B), which
confirmed the circular structure of hsa_circ_0050900. Next,
hsa_circ_0050900 expression in ICC cells was measured relative to
GAPDH. hsa_circ_0050900 was elevated in ICC cells and highest in
HuCCT-1 cells (Fig. 1C). Therefore,
HuCCT-1 cells were selected for subsequent experiments.
Knockdown of hsa_circ_0050900
suppresses proliferation and migration by promoting ICC cell
ferroptosis
To explore the function of hsa_circ_0050900,
specific interference fragments were designed for hsa_circ_0050900.
qPCR results showed si-hsa_circ_0050900-1, si-hsa_circ_0050900-2
and si-hsa_circ_0050900-3 interfered with expression of
hsa_circ_0050900 and si-hsa_circ_0050900-3 had highest interference
efficiency (Fig. 2A). Therefore,
si-hsa_circ_0050900-3 was selected for subsequent experiments. To
investigate whether hsa_circ_0050900 was involved in ferroptosis,
HuCCT-1 cells were treated with ferroptosis inhibitor Fer-1
following transfection of si-NC or si-hsa_circ_0050900-3.
Fe2+ and ROS levels were promoted after knocking down
hsa_circ_0050900, while Fe2+ and ROS levels were
suppressed by Fer-1 (Fig. 2B and
C). In addition, ferroptosis-associated factor GPX4 expression
was suppressed after hsa_circ_0050900 was knocked down; this effect
on GPX4 expression was reversed by Fer-1 treatment (Fig. 2D). Cell proliferation and migration
were suppressed after knocking down hsa_circ_0050900 expression,
while these phenomenon were reversed by Fer-1 treatment (Fig. 2E and F). Collectively, inhibition of
hsa_circ_0050900 inhibited proliferation and migration by promoting
ICC cell ferroptosis.
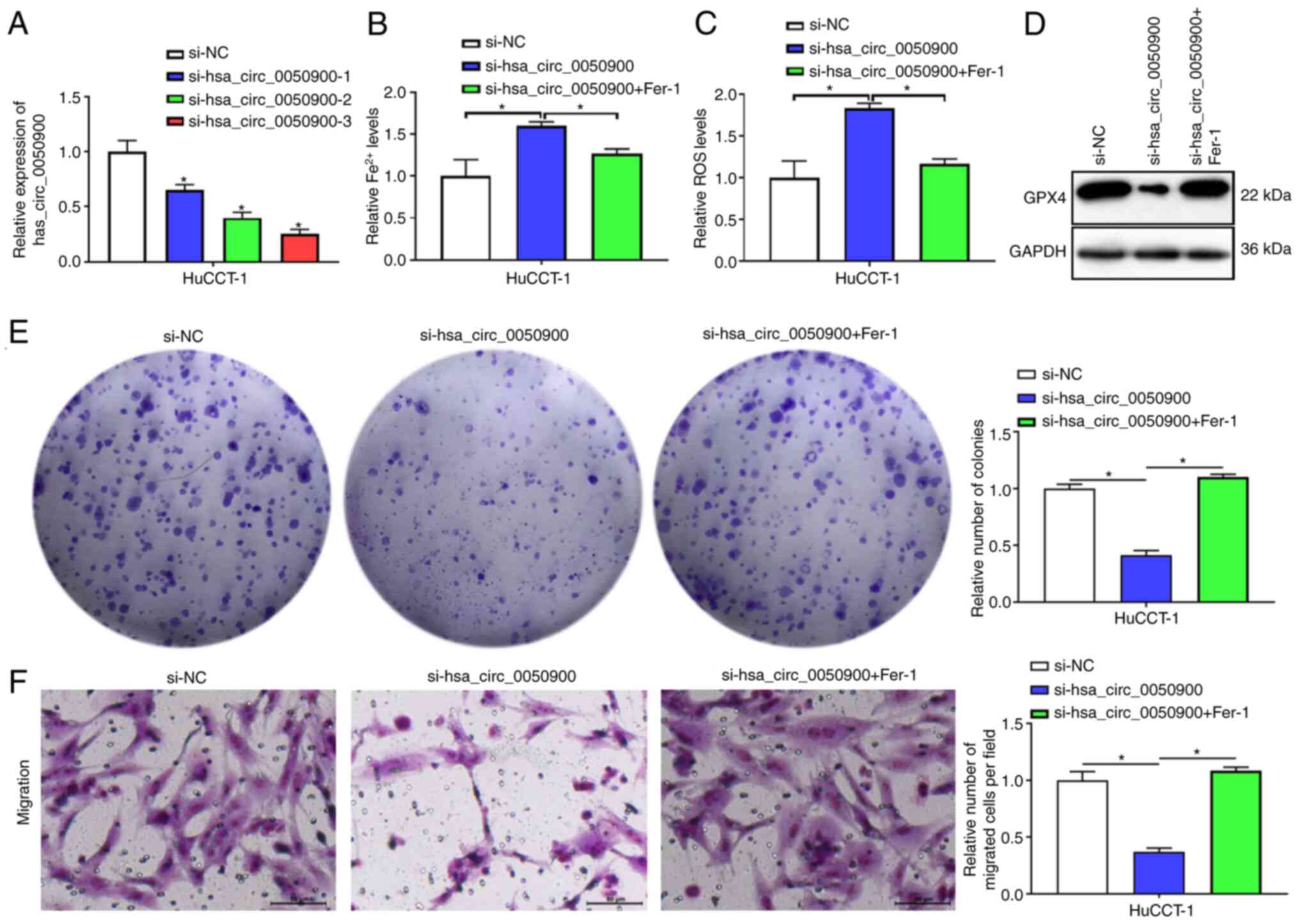 | Figure 2.Knockdown of hsa_circ_0050900
suppresses proliferation and migration by promoting ICC cell
ferroptosis. (A) si-NC, si-hsa_circ_0050900-1,
si-hsa_circ_0050900-2 and si-hsa_circ_0050900-3 were transfected
into HuCCT-1 cells for 24 h and cells were collected for reverse
transcription-quantitative PCR. si-hsa_circ_0050900-1,
si-hsa_circ_0050900-2, si-hsa_circ_0050900-3 showed knockdown
effect for hsa_circ_0050900 expression. A total of 1 µM ferroptosis
inhibitor Fer-1 was added for 24 h, then cells were collected for
further analysis. (B) Iron assay kit was used to evaluate
Fe2+ levels. (C) ROS assay kit was used to assess ROS
levels. (D) Western blot detection of GPX4 protein. (E) Clone
formation assay was used to measure proliferation. (F) Transwell
assay was used to detect migration. n=3. Magnification, 200 ×;
Scale bar, 50 µm. *P<0.05 vs. si-NC. circ, circular; ICC,
intrahepatic cholangiocarcinoma; si-NC, small interfering negative
control; Fer-1, ferrostatin-1; ROS, reactive oxygen species; GPX4,
glutathione peroxidase 4. |
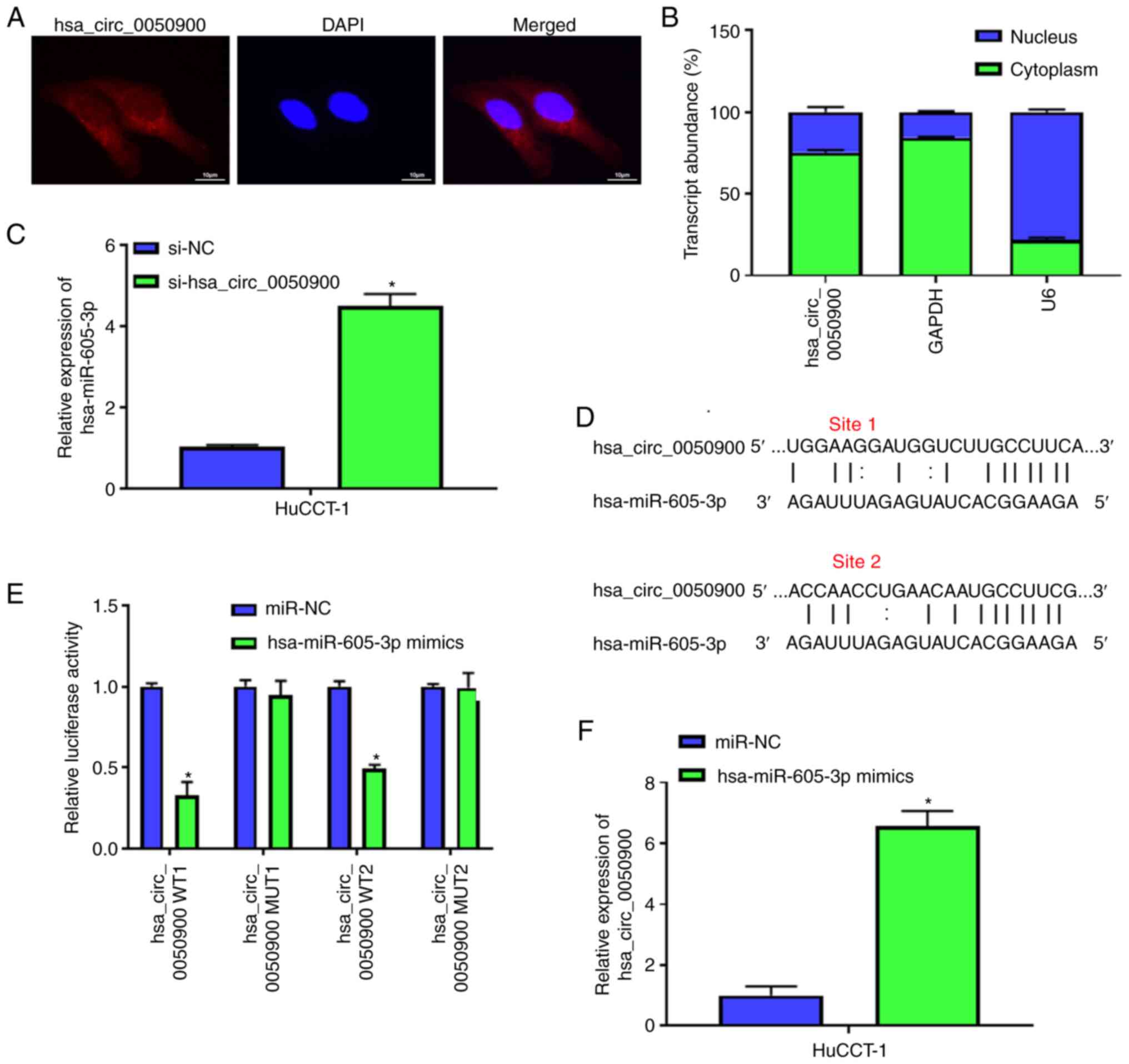
hsa_circ_0050900 negatively regulates
hsa-miR-605-3p
To explore the potential mechanism of
hsa_circ_0050900, the present study confirmed the localization of
hsa_circ_0050900. FISH showed that hsa_circ_0050900 was mainly
localized in cytoplasm in HuCCT-1 cells (Fig. 3A). RT-qPCR verified hsa_circ_0050900
was primarily located in cytoplasm (Fig. 3B). Then, the expression was detected
after knocking down expression of hsa_circ_0050900. qPCR indicated
that hsa-miR-605-3p expression was elevated after knockdown of
hsa_circ_0050900 (Fig. 3C).
CircAtlas 2.0 software was used to predict the binding sequence
between hsa_circ_0050900 and hsa-miR-605-3p. There were two binding
sites between hsa_circ_0050900 and hsa-miR-605-3p (Fig. 3D). Dual-luciferase reporter gene
assay demonstrated that only hsa-miR-605-3p mimics decreased the
luciferase activity of hsa_circ_0050900 WT but not hsa_circ_0050900
MUT (Fig. 3E). AGO2-RIP assays
revealed hsa_circ_0050900 expression was promoted by hsa-miR-605-3p
mimics (Fig. 3F). These results
indicated that hsa_circ_0050900 regulated hsa-miR-605-3p expression
(Fig. 3E and F). hsa_circ_0050900
negatively regulated hsa-miR-605-3p expression.
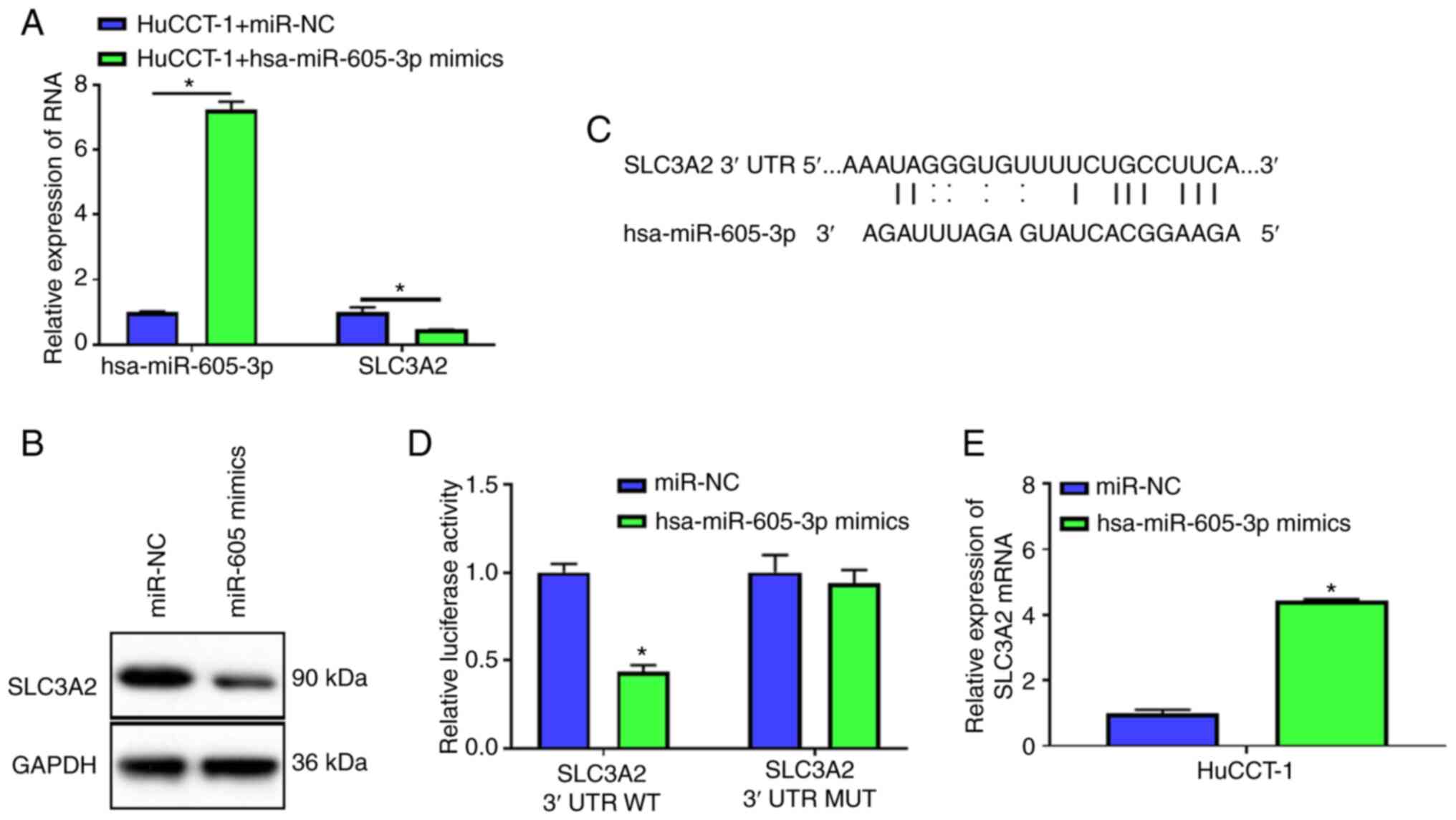
hsa-miR-605-3p targets SLC3A2
To study hsa-miR-605-3p function, hsa-miR-605-3p
mimics or miR-NC was transfected into HuCCT-1 cells. Compared with
miR-NC, hsa-miR-605-3p expression was significantly elevated after
transfection with hsa-miR-605-3p mimics, which indicated successful
overexpression of hsa-miR-605-3p (Fig.
4A). Overexpression of hsa-miR-605-3p inhibited the mRNA and
protein expression of SLC3A2 (Fig. 4A
and B). Then, TargetScanHuman 7.1 software was used to predict
the binding site between SLC3A2 3′UTR and hsa-miR-605-3p (Fig. 4C). Dual-luciferase reporter gene
assay demonstrated hsa-miR-605-3p mimics decreased the luciferase
activity of SLC3A2 3′UTR WT, but not SLC3A2 3′UTR MUT (Fig. 4D). Additionally, AGO2-RIP assay
showed hsa-miR-605-3p mimics increased expression of SLC3A2, but
not miR-NC (Fig. 4E). These results
verified that hsa-miR-605-3p could regulate SLC3A2 expression.
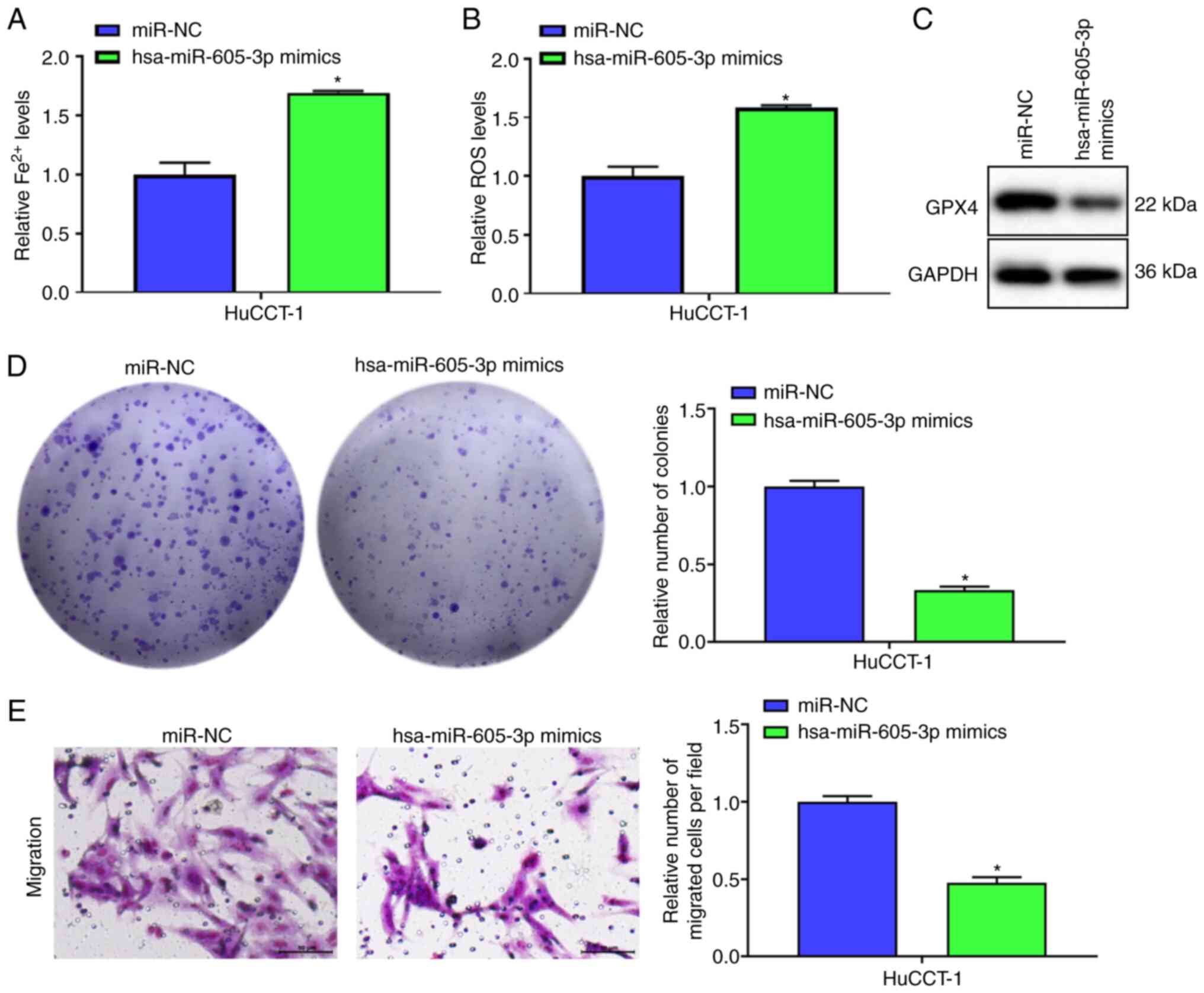
Overexpression of hsa-miR-605-3p
targets SLC3A2 to promote ICC cell ferroptosis and inhibit
proliferation and migration
Fe2+ and ROS levels were increased by
hsa-miR-605-3p mimics compared with miR-NC (Fig. 5A and B). Moreover, following
overexpression of hsa-miR-605-3p, GPX4 protein expression was
suppressed in comparison with miR-NC group (Fig. 5C). Cell function experiments
demonstrated overexpression of hsa-miR-605-3p reduced proliferation
and migration in comparison with miR-NC (Fig. 5D and E). Overexpression of
hsa-miR-605-3p targeted SLC3A2 to promote ICC cell ferroptosis and
inhibit proliferation and migration.
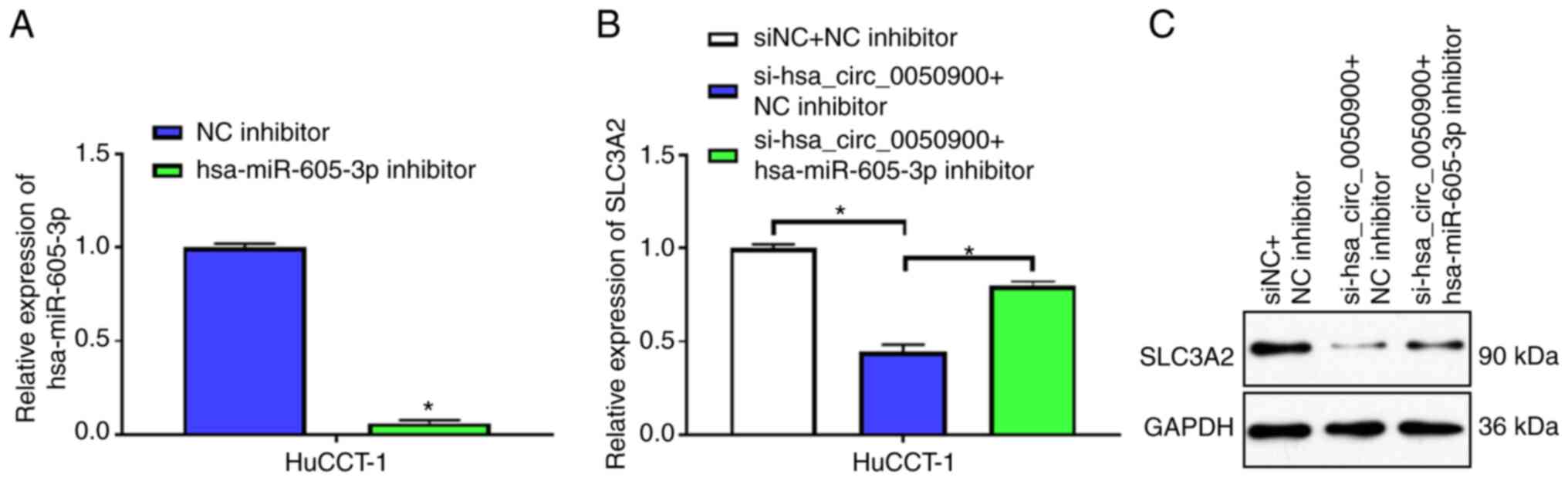
hsa_circ_0050900 regulates SLC3A2
expression via hsa-miR-605-3p
hsa-miR-605-3p expression was knocked down in
HuCCT-1 cells using hsa-miR-605-3p inhibitor. hsa-miR-605-3p
inhibitor decreased the expression of hsa-miR-605-3p relative to NC
inhibitor, indicating that hsa-miR-605-3p inhibitor had an
interfering effect (Fig. 6A). mRNA
and protein levels of the downstream target gene SLC3A2 of
hsa-miR-605-3p were suppressed after knocking down hsa_circ_0050900
(Fig. 6B and C). However, knocking
down hsa-miR-605-3p attenuated the decrease in SLC3A2 mRNA and
protein expression induced by inhibition of hsa_circ_0050900
(Fig. 6B and C). These results
indicated that hsa_circ_0050900 regulated SLC3A2 expression via
hsa-miR-605-3p.
hsa_circ_0050900 regulates SLC3A2
expression via sponging hsa-miR-605-3p to affect ICC cell
ferroptosis, proliferation and migration
Finally, the effects of hsa_circ_0050900 and
hsa-miR-605-3p on cell ferroptosis, proliferation and migration
were assessed. Knocking down hsa-miR-605-3p reduced the increases
of Fe2+ and ROS induced by inhibition of
hsa_circ_0050900 (Fig. 7A and B).
hsa-miR-605-3p inhibitor decreased the downregulation of GPX4
caused by knocking down hsa_circ_0050900 (Fig. 7C). hsa-miR-605-3p inhibitor
attenuated the decrease in cell proliferation and migration caused
by knocking down hsa_circ_0050900 (Fig.
7D and E). Taken together, hsa_circ_0050900 regulated SLC3A2
expression via sponging hsa-miR-605-3p to affect ICC cell
ferroptosis, proliferation and migration.
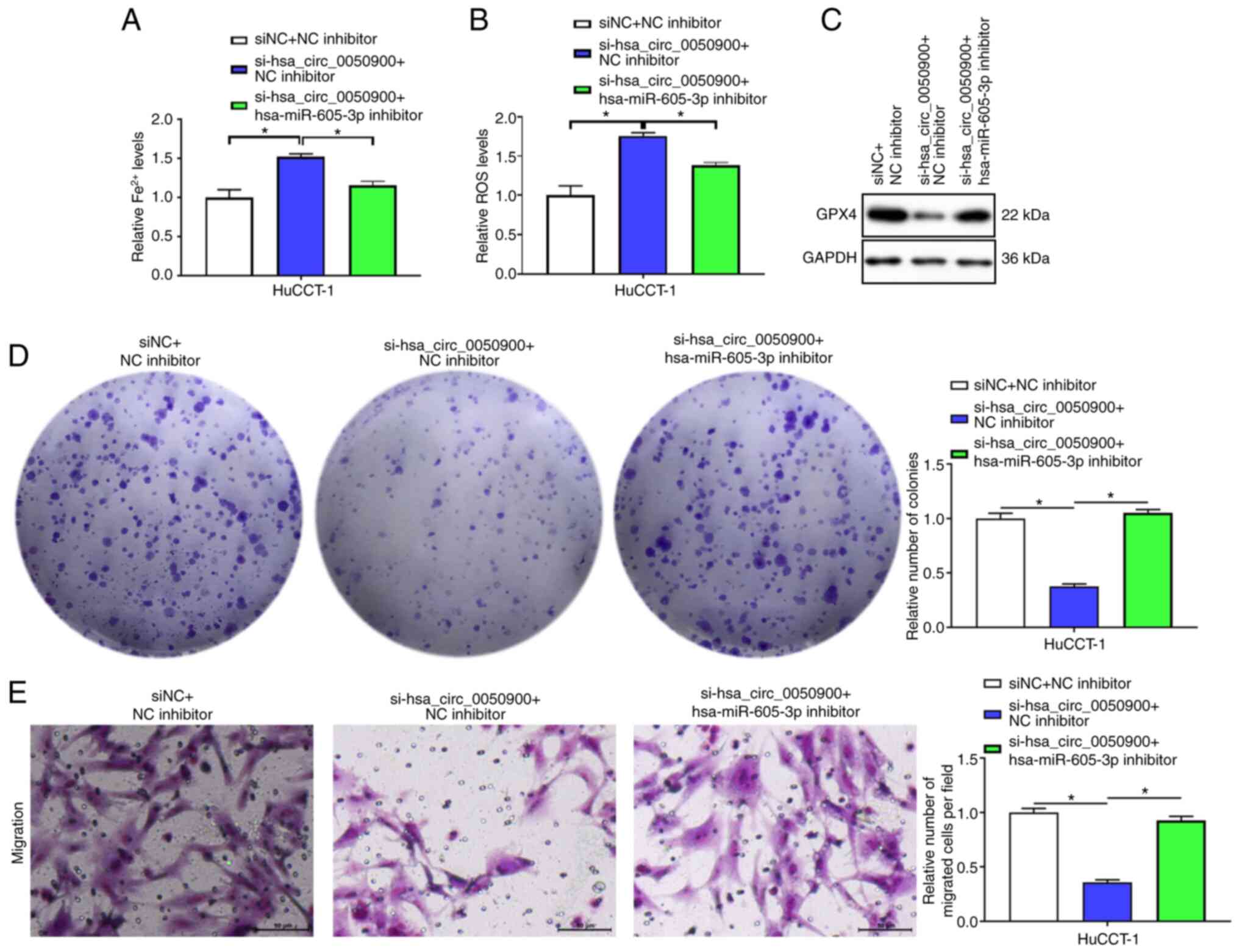 | Figure 7.hsa_circ_0050900 regulates SLC3A2
expression via sponging hsa-miR-605-3p to affect intrahepatic
cholangiocarcinoma cell ferroptosis, proliferation and migration.
si-hsa_circ_0050900-3 and hsa-miR-605-3p inhibitor or NC inhibitor
were co-transfected into HuCCT-1 cells for 48 h. (A) Iron assay kit
was used to monitor Fe2+ levels. (B) ROS assay kit was used
to examine ROS levels. (C) Western blot measurement of GPX4
protein. (D) Clone formation assay was used to test proliferation.
(E) Transwell assay was used to assess migration. n=3.
magnification, 200 ×; Scale bar, 500 µm. *P<0.05. circ,
circular; SLC3A2, solute carrier family 3 member 2; miR, microRNA;
NC, negative control; si, small interfering; ROS, reactive oxygen
species; GPX4, glutathione peroxidase 4. |
Discussion
Despite technological advances and multimodal
treatment approaches, ICC prognosis remains poor due to frequent
delays in diagnosis (32).
Therefore, exploring the molecular pathogenesis of ICC is helpful
to provide targets for diagnosis and treatment. The present study
explored the mechanism of action of hsa_circ_0050900 in ferroptosis
in ICC cells in vitro. hsa_circ_0050900 affected ICC cell
ferroptosis by targeting hsa-miR-605-3p to regulate SLC3A2.
circRNAs are a class of ncRNAs that play vital roles
in cancer biology (33). circRNA
nuclear factor I B inhibits tumor growth and metastasis by
inhibiting the MEK1/ERK pathway in ICC (34). Jiang et al (35) validated in clinical tissues that
circRNA CDR1 antisense RNA may serve as a potential malignant
molecular biomarker to predict aggressive tumor progression and
poor prognosis in patients with cholangiocarcinoma. Chen et
al (14) reported elevated
circACTN4 expression is associated with enhanced tumor
proliferation and metastasis in vitro and in vivo and
poor prognosis after ICC resection. The present in vitro
experiments demonstrated that hsa_circ_0050900 was derived from
ACTN4 gene and was highly expressed in ICC cells. In addition,
inhibition of hsa_circ_0050900 suppressed proliferation and
migration by promoting ICC cell ferroptosis. The present study
showed that hsa_circ_0050900 affected the migration of ICC.
Competing endogenous RNAs (ceRNAs) have been studied
in ICC. Zhou et al (36)
revealed functional long non-coding RNAs (lncRNAs) in ICC through
comprehensive analysis of ceRNA networks, providing a novel
strategy for subsequent functional studies of lncRNAs in ICC. Kang
et al (37) predicted the
relationship between the central gene-associated ceRNA network and
overall survival of patients with ICC from transcriptome sequencing
data. There are also related studies on ceRNAs in circRNA/miR/mRNA
networks: Xu et al (38)
reported circRNA 3-hydroxy-3-methylglutaryl-CoA synthase 1–016
regulates CD73 and galectin 8 by sponging miR-1236-3p to induce ICC
cell invasion and tumor immune microenvironment remodeling. Tang
et al (39) found circRNA
reticulon 4 interacting protein 1 regulates the malignant
progression of ICC by sponging miR-541-5p to induce
hypoxia-inducible factor 1A production. These studies revealed a
network of ICC-specific ceRNAs. Here, hsa_circ_0050900 negatively
regulated hsa-miR-605-3p. In addition, hsa-miR-605-3p targeted
SLC3A2. This suggests that hsa_circ_0050900/hsa-miR-605-3p/SLC3A2
signaling functions in ICC through a ceRNA mechanism.
SLC3A2 serves a key role in amino acid and glucose
cellular nutrition, redox homeostasis and nucleotide availability,
which are all critical for cell proliferation (40). There is increasing evidence that
SLC3A2 is upregulated in a variety of cancers and is associated
with tumor growth (19,41). Decreased expression of SLC3A2
inhibits osteosarcoma cell proliferation through G2/M arrest
(42). In hepatocellular carcinoma,
overexpressed lncRNA small nucleolar RNA host gene 1 activates the
Akt pathway by regulating SLC3A2, resulting in sorafenib resistance
(43). In cholangiocarcinoma,
Janpipatkul et al (44)
reported inhibition of cell invasion and migration in L-type amino
acid transporter 1 knockdown KKU-M213 cells may be mediated in part
by upregulating miR-7 to inhibit the SLC3A2 pathway. Here,
overexpression of hsa-miR-605-3p targeted SLC3A2 to promote ICC
cell ferroptosis and inhibit proliferation and migration.
hsa_circ_0050900 affected ICC cell ferroptosis, proliferation and
migration by regulating SLC3A2 expression via sponging
hsa-miR-605-3p. To the best of our knowledge, the present study is
the first to report the targeted regulatory association between
hsa-miR-605-3p and SLC3A2 in ICC.
The present study confirmed hsa_circ_0050900 was
highly expressed in ICC cells. Furthermore, the present study
conducted a preliminary exploration of the mechanism involved in
hsa_circ_0050900. In vitro experiments found
hsa_circ_0050900 affected ICC cell ferroptosis by targeting
hsa-miR-605-3p to regulate SLC3A2. This may provide new ideas and
targets for treating ICC. However, these effects should be studied
in animal models and expression of hsa_circ_0050900, has-miR-605-3p
and SLC3A2 should be validated in ICC clinical samples and
correlation analysis should be performed.
Acknowledgements
Not applicable.
Funding
This work was funded by the National Science Foundation of China
(grant no. 82002587).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SQ and LZ conceived and designed the study. XS and
JY performed experiments and acquired the data. LX and MW analyzed
and interpreted the data. JY performed statistical analysis. XS
wrote the manuscript. LZ and XS revised the manuscript. LZ and SQ
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ICC
|
intrahepatic cholangiocarcinoma
|
|
circ
|
circular
|
|
nc
|
non-coding
|
|
SLC3A2
|
solute carrier family 3 member 2
|
|
WT
|
wild-type
|
|
MUT
|
mutant
|
|
RT-q
|
reverse transcript-quantitative
|
|
ROS
|
reactive oxygen species
|
References
|
1
|
Kelley RK, Bridgewater J, Gores GJ and Zhu
AX: Systemic therapies for intrahepatic cholangiocarcinoma. J
Hepatol. 72:353–363. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rahnemai-Azar AA, Weisbrod A, Dillhoff M,
Schmidt C and Pawlik TM: Intrahepatic cholangiocarcinoma: Molecular
markers for diagnosis and prognosis. Surg Oncol. 26:125–137. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moris D, Palta M, Kim C, Allen PJ, Morse
MA and Lidsky ME: Advances in the treatment of intrahepatic
cholangiocarcinoma: An overview of the current and future
therapeutic landscape for clinicians. CA Cancer J Clin. 73:198–222.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aoki S, Inoue K, Klein S, Halvorsen S,
Chen J, Matsui A, Nikmaneshi MR, Kitahara S, Hato T, Chen X, et al:
Placental growth factor promotes tumour desmoplasia and treatment
resistance in intrahepatic cholangiocarcinoma. Gut. 71:185–193.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hewitt DB, Brown ZJ and Pawlik TM:
Surgical management of intrahepatic cholangiocarcinoma. Expert Rev
Anticancer Ther. 22:27–38. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen Z, Jiang J, Fu N and Chen L:
Targetting ferroptosis for blood cell-related diseases. J Drug
Target. 30:244–258. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lei G, Zhuang L and Gan B: Targeting
ferroptosis as a vulnerability in cancer. Nat Rev Cancer.
22:381–396. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen J, Li X, Ge C, Min J and Wang F: The
multifaceted role of ferroptosis in liver disease. Cell Death
Differ. 29:467–480. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li D, Wang Y, Dong C, Chen T, Dong A, Ren
J, Li W, Shu G, Yang J, Shen W, et al: CST1 inhibits ferroptosis
and promotes gastric cancer metastasis by regulating GPX4 protein
stability via OTUB1. Oncogene. 42:83–98. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou P, Chen X, Shi K, Qu H and Xia J: The
characteristics, tumorigenicities and therapeutics of cancer stem
cells based on circRNAs. Pathol Res Pract. 233:1538222022.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li H, Lan T, Liu H, Liu C, Dai J, Xu L,
Cai Y, Hou G, Xie K, Liao M, et al: IL-6-induced cGGNBP2 encodes a
protein to promote cell growth and metastasis in intrahepatic
cholangiocarcinoma. Hepatology. 75:1402–1419. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu Q and Fang T: Circular RNA SMARCA5
correlates with favorable clinical tumor features and prognosis,
and increases chemotherapy sensitivity in intrahepatic
cholangiocarcinoma. J Clin Lab Anal. 34:e231382020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang X, Xing L, Yang R, Chen H, Wang M,
Jiang R, Zhang L and Chen J: The circACTN4 interacts with FUBP1 to
promote tumorigenesis and progression of breast cancer by
regulating the expression of proto-oncogene MYC. Mol Cancer.
20:912021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Q, Wang H, Li Z, Li F, Liang L, Zou
Y, Shen H, Li J, Xia Y, Cheng Z, et al: Circular RNA ACTN4 promotes
intrahepatic cholangiocarcinoma progression by recruiting YBX1 to
initiate FZD7 transcription. J Hepatol. 76:135–147. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Q, Hao X, Xu G and Lv T:
Downregulated KIF3B induced by miR-605-3p inhibits the progression
of colon cancer via inactivating Wnt/β-Catenin. J Oncol.
2021:50469812021.PubMed/NCBI
|
|
16
|
Zeng Z, Zhou W, Duan L, Zhang J, Lu X, Jin
L and Yu Y: Circular RNA circ-VANGL1 as a competing endogenous RNA
contributes to bladder cancer progression by regulating
miR-605-3p/VANGL1 pathway. J Cell Physiol. 234:3887–3896. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu N, Hu G, Wang H, Wang Y and Guo Z:
LncRNA BLACAT1 regulates VASP expression via binding to miR-605-3p
and promotes giloma development. J Cell Physiol. 234:22144–22152.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu YL, Feng Y, Chen YY, Liu JZ, Su Y, Li
P, Huang H, Mao QS and Xue WJ: SNHG16/miR-605-3p/TRAF6/NF-κB
feedback loop regulates hepatocellular carcinoma metastasis. J Cell
Mol Med. 24:7637–7651. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fei F, Li X, Xu L, Li D, Zhang Z, Guo X,
Yang H, Chen Z and Xing J: CD147-CD98hc complex contributes to poor
prognosis of non-small cell lung cancer patients through promoting
cell proliferation via the PI3K/Akt signaling pathway. Ann Surg
Oncol. 21:4359–4368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu C, Li X, Li C, Zhang Z, Gao X, Jia Z,
Chen H, Jia Q, Zhao X, Liu J, et al: SLC3A2 is a novel endoplasmic
reticulum stress-related signaling protein that regulates the
unfolded protein response and apoptosis. PLoS One. 13:e02089932018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Palacín M and Kanai Y: The ancillary
proteins of HATs: SLC3 family of amino acid transporters. Pflugers
Arch. 447:490–494. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
He J, Liu D, Liu M, Tang R and Zhang D:
Characterizing the role of SLC3A2 in the molecular landscape and
immune microenvironment across human tumors. Front Mol Biosci.
9:9614102022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koppula P, Zhuang L and Gan B: Cystine
transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient
dependency, and cancer therapy. Protein Cell. 12:599–620. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Digomann D, Linge A and Dubrovska A:
SLC3A2/CD98hc, autophagy and tumor radioresistance: A link
confirmed. Autophagy. 15:1850–1851. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma L, Zhang X, Yu K, Xu X, Chen T, Shi Y,
Wang Y, Qiu S, Guo S, Cui J, et al: Targeting SLC3A2 subunit of
system XC− is essential for m6A
reader YTHDC2 to be an endogenous ferroptosis inducer in lung
adenocarcinoma. Free Radic Biol Med. 168:25–43. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu F, Xiong G, Chen Z, Lei C, Liu Q and
Bai Y: SLC3A2 inhibits ferroptosis in laryngeal carcinoma via mTOR
pathway. Hereditas. 159:62022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu H, Deng Z, Yu B, Liu H, Yang Z, Zeng A
and Fu M: Identification of SLC3A2 as a potential therapeutic
target of osteoarthritis involved in ferroptosis by integrating
bioinformatics, clinical factors and experiments. Cells.
11:34302022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu Z, Yin Y, Jiang J, Yan C, Wang Y, Wang
D and Li L: Exosomal miR-142-3p secreted by hepatitis B virus
(HBV)-hepatocellular carcinoma (HCC) cells promotes ferroptosis of
M1-type macrophages through SLC3A2 and the mechanism of HCC
progression. J Gastrointest Oncol. 13:754–767. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qin X, Zhang J, Wang B, Xu G, Yang X, Zou
Z and Yu C: Ferritinophagy is involved in the zinc oxide
nanoparticles-induced ferroptosis of vascular endothelial cells.
Autophagy. 17:4266–4285. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shang A, Gu C, Wang W, Wang X, Sun J, Zeng
B, Chen C, Chang W, Ping Y, Ji P, et al: Exosomal circPACRGL
promotes progression of colorectal cancer via the
miR-142-3p/miR-506-3p- TGF-β1 axis. Mol Cancer. 19:1172020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Krenzien F, Nevermann N, Krombholz A,
Benzing C, Haber P, Fehrenbach U, Lurje G, Pelzer U, Pratschke J,
Schmelzle M and Schöning W: Treatment of intrahepatic
cholangiocarcinoma-a multidisciplinary approach. Cancers (Basel).
14:3622022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang J, Qi M, Fei X, Wang X and Wang K:
Hsa_circRNA_0088036 acts as a ceRNA to promote bladder cancer
progression by sponging miR-140-3p. Cell Death Dis. 13:3222022.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Du J, Lan T, Liao H, Feng X, Chen X, Liao
W, Hou G, Xu L, Feng Q, Xie K, et al: CircNFIB inhibits tumor
growth and metastasis through suppressing MEK1/ERK signaling in
intrahepatic cholangiocarcinoma. Mol Cancer. 21:182022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jiang XM, Li ZL, Li JL, Xu Y, Leng KM, Cui
YF and Sun DJ: A novel prognostic biomarker for cholangiocarcinoma:
circRNA Cdr1as. Eur Rev Med Pharmacol Sci. 22:365–371.
2018.PubMed/NCBI
|
|
36
|
Zhou D, Gao B, Yang Q, Kong Y and Wang W:
Integrative analysis of ceRNA network reveals functional lncRNAs in
intrahepatic cholangiocarcinoma. Biomed Res Int. 2019:26012712019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kang Z, Guo L, Zhu Z and Qu R:
Identification of prognostic factors for intrahepatic
cholangiocarcinoma using long non-coding RNAs-associated ceRNA
network. Cancer Cell Int. 20:3152020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu YP, Dong ZN, Wang SW, Zheng YM, Zhang
C, Zhou YQ, Zhao YJ, Zhao Y, Wang F, Peng R, et al: circHMGCS1-016
reshapes immune environment by sponging miR-1236-3p to regulate
CD73 and GAL-8 expression in intrahepatic cholangiocarcinoma. J Exp
Clin Cancer Res. 40:2902021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tang J, Wang R, Tang R, Gu P, Han J and
Huang W: CircRTN4IP1 regulates the malignant progression of
intrahepatic cholangiocarcinoma by sponging miR-541-5p to induce
HIF1A production. Pathol Res Pract. 230:1537322022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cano-Crespo S, Chillarón J, Junza A,
Fernández-Miranda G, García J, Polte C, R de la Ballina L, Ignatova
Z, Yanes Ó, Zorzano A, et al: CD98hc (SLC3A2) sustains amino acid
and nucleotide availability for cell cycle progression. Sci Rep.
9:140652019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Furuya M, Horiguchi J, Nakajima H, Kanai Y
and Oyama T: Correlation of L-type amino acid transporter 1 and
CD98 expression with triple negative breast cancer prognosis.
Cancer Sci. 103:382–389. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu B, Cheng D, Hou L, Zhou S, Ying T and
Yang Q: SLC3A2 is upregulated in human osteosarcoma and promotes
tumor growth through the PI3K/Akt signaling pathway. Oncol Rep.
37:2575–2582. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li W, Dong X, He C, Tan G, Li Z, Zhai B,
Feng J, Jiang X, Liu C, Jiang H and Sun X: LncRNA SNHG1 contributes
to sorafenib resistance by activating the Akt pathway and is
positively regulated by miR-21 in hepatocellular carcinoma cells. J
Exp Clin Cancer Res. 38:1832019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Janpipatkul K, Suksen K, Borwornpinyo S,
Jearawiriyapaisarn N, Hongeng S, Piyachaturawat P and Chairoungdua
A: Downregulation of LAT1 expression suppresses cholangiocarcinoma
cell invasion and migration. Cell Signal. 26:1668–1679. 2014.
View Article : Google Scholar : PubMed/NCBI
|