Introduction
Tyrosine kinase inhibitors (TKIs) have recently been
used to treat many solid tumors. Multi-kinase inhibitors include
sorafenib, sunitinib, and lenvatinib. Lenvatinib also has an
angiogenesis-inhibiting effect by suppressing vascular endothelial
growth factor (VEGF), fibroblast growth factor, and
platelet-derived growth factor receptors. In Japan, it is used to
treat thyroid, hepatocellular, thymic, endometrial, and renal cell
cancers (1,2).
In treatments involving angiogenesis inhibitors, a
history of radiotherapy is considered a risk factor for mucosal
damage, such as perforation and fistula formation (3–5). Fatal
colonic perforation due to sorafenib use and fatal bronchial
perforation leading to a fistula due to sunitinib use have been
reported in patients with a history of radiotherapy (6,7). To
the best of our knowledge, there are no reports of prior radiation
therapy being a risk factor for mucosal damage in the
gastrointestinal tract or other organs during lenvatinib treatment.
Herein, we report a case of rectal ulceration triggered by
lenvatinib use 15 years after definitive radiotherapy for prostate
cancer.
Case report
A 58-year-old man had a high prostate-specific
antigen level (15.6 ng/ml) during a check-up. Subsequently, he
underwent a transrectal prostate biopsy, and a diagnosis of
prostate cancer was made on pathological analysis. He then
underwent a thorough examination, which revealed a Gleason score of
7 (3+4), cT3bN0M0 cStage III, and a diagnosis of high-risk prostate
cancer according to the National Comprehensive Cancer Network
classification. A bilateral obturator lymph node biopsy was
performed, and the pathological findings revealed no lymph node
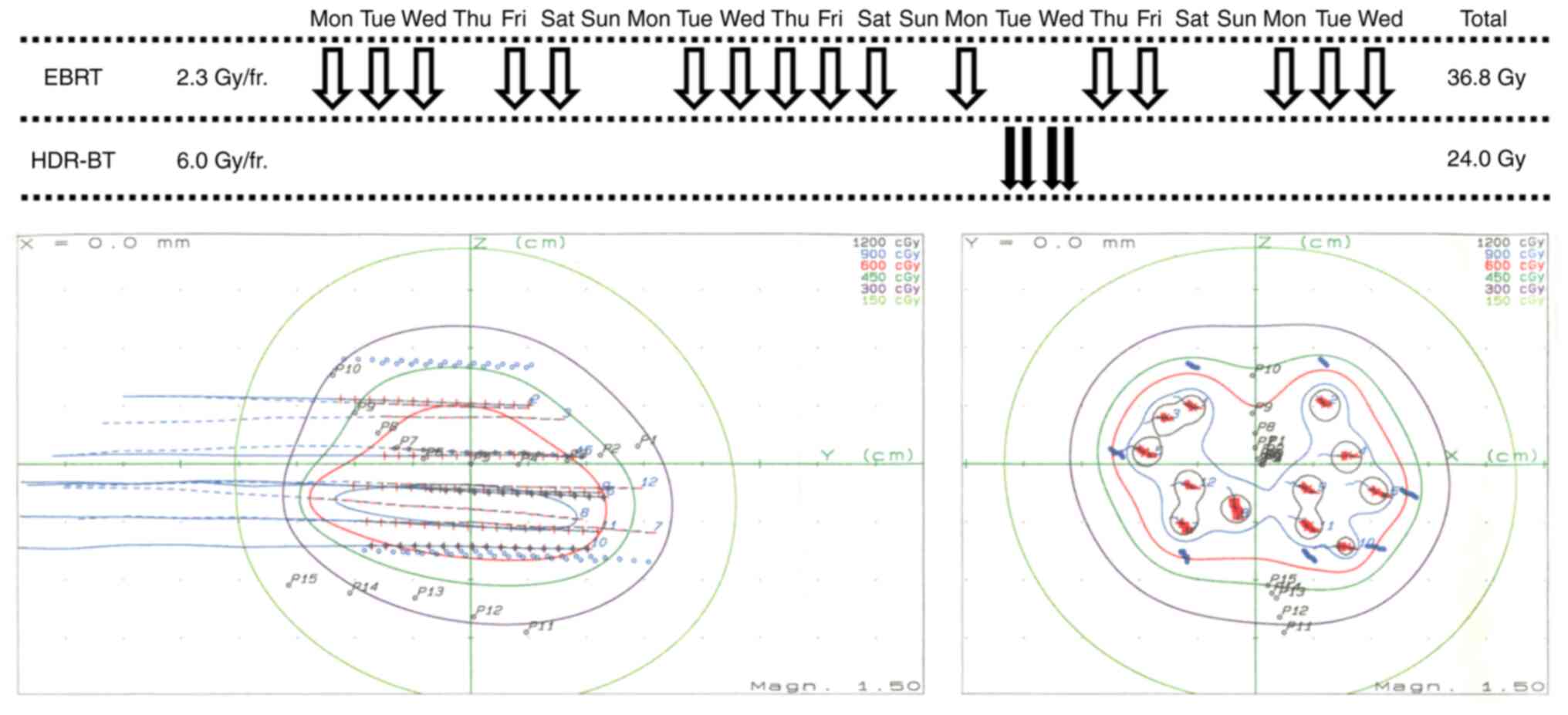
metastasis. The patient underwent external beam radiotherapy (EBRT)
in 16 fractions of 2.3 Gy to a total dose of 36.8 Gy, and
high-dose-rate brachytherapy (HDR-BT) in four fractions of 6.0 Gy
within 30 h to a total dose of 24.0 Gy for the prostate cancer
(Fig. 1). The EBRT included
four-portal irradiation with 10 MV X-rays targeting the prostate
and seminal vesicles, and HDR-BT was prescribed for the prostate
and seminal vesicle periphery with 12 applicator needles (8). The biologically effective dose (BED)
with the α/β set to 3 was 95.6 Gy for the rectum and 178.6 Gy for
the urethra. Two weeks after the completion of radiotherapy, anal
pain, odynuria, and dysuria appeared; these symptoms improved after
the administration of prednisolone 20 mg/day for 3 days. Three
weeks after prednisolone administration, a rectal endoscopic biopsy
was performed, which revealed no fibroblast or fibrinoid
degeneration of the small arteries typical of radiation colitis.
There was no biochemical recurrence of the prostate cancer.
Although grossly bloody stools were observed once a month, fecal
occult blood tests were negative once every 6 months. Ten years
after the completion of radiotherapy, a lower gastrointestinal
endoscopy was performed due to a positive stool occult blood test.
Only vasodilatation consistent with an irradiated area in the
rectoanal region was observed, with no active bleeding or ulcers
(Fig. 2).
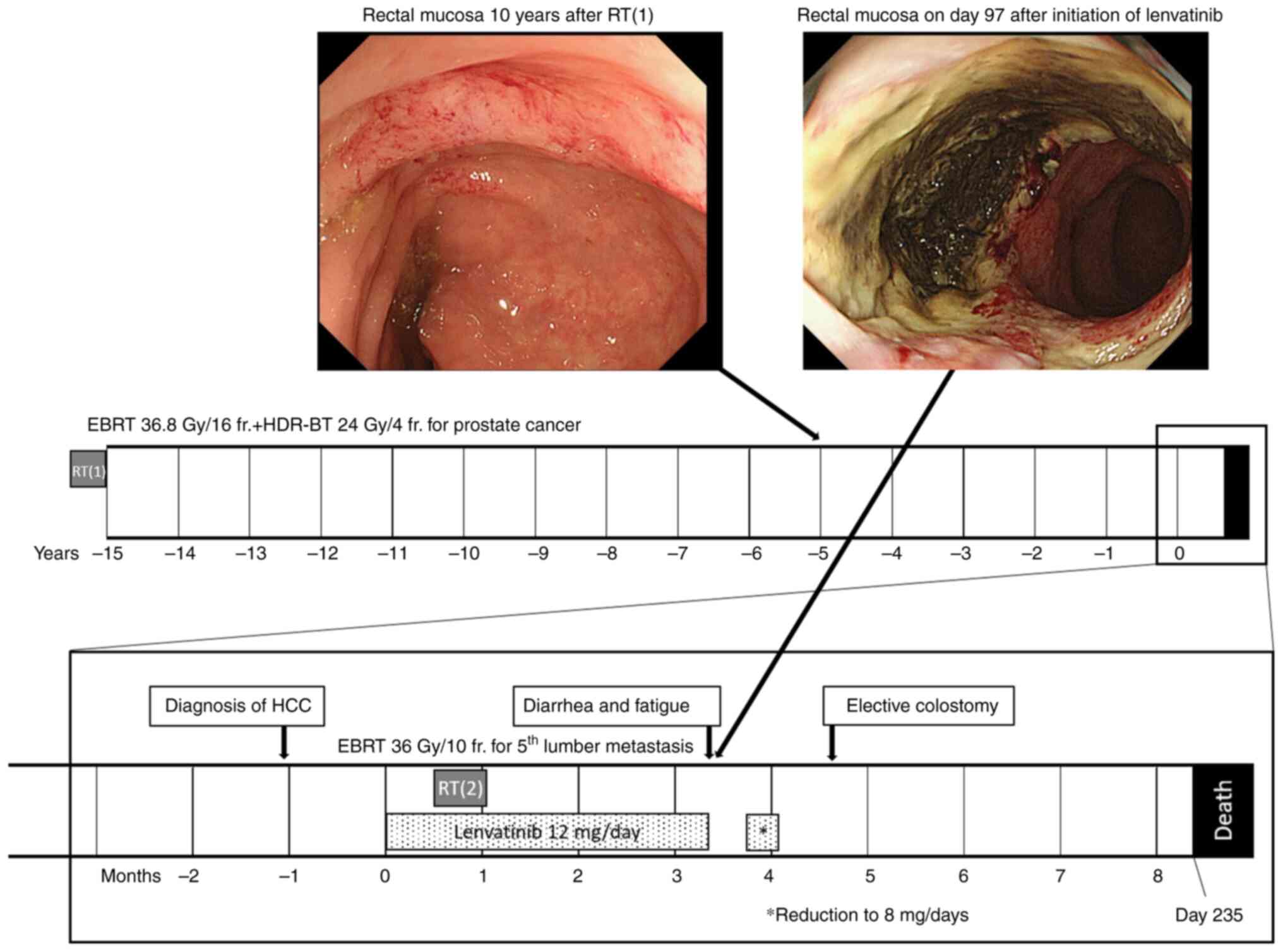
Fifteen years after radiotherapy for prostate
cancer, the patient visited a hospital for fatigue, and a blood
test revealed liver dysfunction. A computed tomography scan was
performed, which led to the discovery of hepatocellular carcinoma
with multiple lung metastases and bone metastasis to the fifth
lumbar vertebra. The ECOG Performance Status at the start of
lenvatinib administration was 1. The patient underwent treatment
with 12 mg/day of lenvatinib. One month after commencing
lenvatinib, the patient experienced pain due to the fifth lumbar
vertebra metastasis; therefore, EBRT at 36 Gy in 10 fractions for 2
weeks (BED 79.2 Gy at α/β=3) was concurrently administered with
lenvatinib. Three months after starting lenvatinib, diarrhea,
fatigue, and persistent anal pain developed, and a hydrocortisone
ointment was administered to the perianal area. Four months after
starting lenvatinib therapy, the patient underwent lower
gastrointestinal endoscopy due to persistent anal pain.
Circumferential vasodilatation was observed in the lower rectum,
and a deep ulceration of the anterior wall of the lower rectum on
the dorsal side of the prostate was observed with no inflammation
in other areas of the colon (Fig.
2). Ten years after radiation therapy for prostate cancer,
lower gastrointestinal endoscopy revealed blood vessel dilatation
at that exact same location. Therefore, we assumed that this ulcer
was related to the radiotherapy for the prostate cancer. An acute
hemorrhagic rectal ulcer is considered an alternative diagnosis.
However, patients presenting with acute hemorrhagic rectal ulcers
are frequently elderly individuals with an ECOG Performance Status
of 3–4. Since our case had an ECOG Performance Status of 1, it was
unlikely that this patient had an acute hemorrhagic rectal ulcer.
We believe that the EBRT for metastasis to the fifth lumbar
vertebra did not affect the rectal ulcer due to the large distance
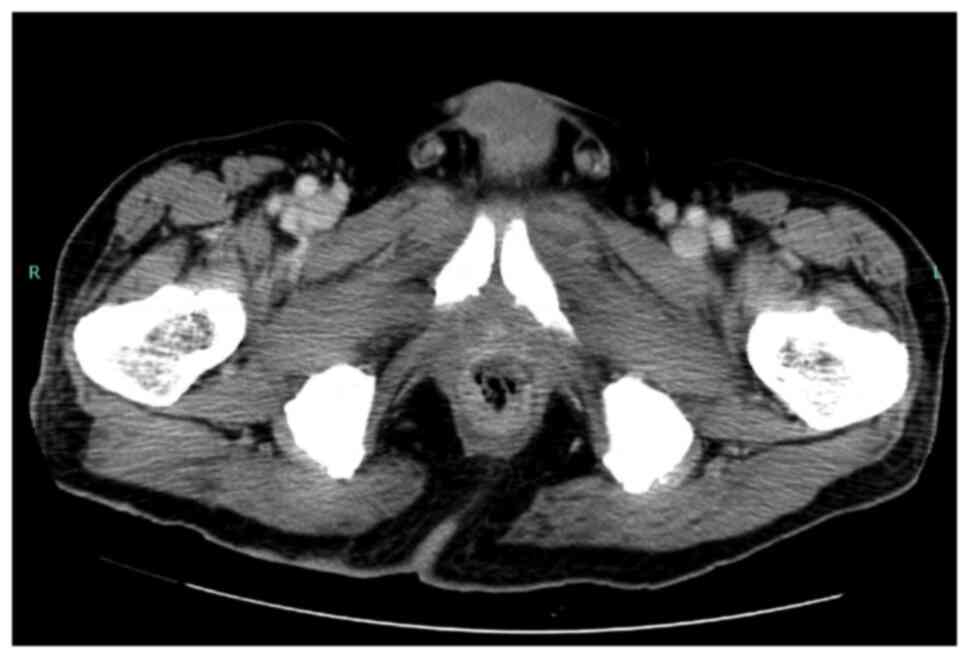
between the irradiation field and the ulcer site. The computed
tomography scan revealed rectal wall thickening, and poor contrast
effect of the rectal wall only on the prostatic side, suggesting a
deep ulcer and disruption of the rectal wall; however, there was no
evidence of air outside the gastrointestinal tract, which would
indicate perforation (Fig. 3).
Therefore conservative treatment was administered. However, the
symptoms did not improve. Therefore, the patient chose to undergo
colostomy to improve inflammation and pain at the ulcer site by
reducing the mechanical stimulation caused by defecation, and as a
result, the anal pain resolved. Based on the Common Terminology
Criteria for Adverse Events 5.0, the patient was diagnosed with a
grade 3 rectal ulcer, which could be treated by standby surgery.
Eight months after starting lenvatinib treatment, the patient died
of liver failure associated with an enlargement of the
hepatocellular carcinoma. Other than ulcers, no grade 2 or higher
adverse events were noted with the use of lenvatinib.
Discussion
To the best of our knowledge, this is the first
report of lenvatinib-induced rectal ulcers following radiotherapy.
The safety of the concurrent or heterogeneous use of radiotherapy
and molecular-targeted drugs is controversial. With respect to the
VEGF inhibitor bevacizumab, a history of radiotherapy is known to
be one of the risks for gastrointestinal perforation (5). The mechanisms of interaction between
angiogenesis inhibitors and ionizing radiation are complex and may
involve multiple interactions between the tumor stroma,
vasculature, and tumor cells (9).
Multi-kinase inhibitors inhibit angiogenesis. With
regard to the multi-kinase inhibitor sunitinib, although there are
reports of good tolerability with concurrent radiotherapy, there
are also reports of bronchobiliary fistulas and gastrointestinal
perforations in patients previously treated with radiotherapy
(10). Since lenvatinib inhibits
angiogenesis, there is a potential risk of exacerbating adverse
events with radiotherapy. A study of lenvatinib monotherapy in 261
patients with thyroid cancer showed grade 2 or less rectal bleeding
in four patients (1.5%) and grade 3 gastrointestinal fistula in 2
patients (0.8%) in terms of adverse events (11). In a Japanese nationwide survey
regarding HDR-BT for prostate cancer involving 3424 cases, there
were three cases of grade 3 rectal ulcers (0.09%) and one case of
grade 4 rectal fistula (0.03%) (12). The incidence of gastrointestinal
ulcers was low when both treatments were administered alone.
In this case, the BED with the α/β set to 3 was 95.6
Gy for the rectum. Clark et al showed a low incidence of
rectal adverse events when the rectal dose was less than 125 Gy for
the BED (13), which was not a
high-risk group for severe rectal ulceration in terms of rectal
dose. In a long-term observational study of cervical cancer treated
with a combination of EBRT and HDR-BT, the incidence rates of grade
3–5 rectosigmoid colon complications were 3.8, 4.4, and 5.3% at 5,
10, and 20 years, respectively (14). Moreover, rectosigmoid colon
complications occurred most frequently during the first 2 years,
after which the incidence decreased markedly (14). Therefore, it is unlikely that a
serious radiation-related rectal adverse event occurred for the
first time 15 years after HDR-BT.
In this case, only vasodilation of the rectum,
consistent with the irradiated area, was observed 10 years after
the completion of HDR-BT. Nevertheless, because the rectal ulcer
appeared after lenvatinib administration, it is reasonable to
assume that lenvatinib administration triggered a synergistic
effect with the burden of the previous radiotherapy. Regarding late
gastrointestinal complications after radiotherapy, Pollom et
al reported complex wounds associated with fibrosis, vascular
hypodensity, and thrombosis (15).
VEGF inhibition not only inhibits the ulcer healing process but can
also result in vascular hypodensity and thrombosis similar to those
seen with radiation (15). Although
the role of VEGF in repairing chronic or late radiation injury
remains unclear, it has been reported that radiation-damaged
vessels are more sensitive to VEGF receptor inhibition in tumor
model systems (16). The same
mechanisms may have occurred in this case. In contrast, no
enteritis was observed in the intestinal tract around the lumbar
spine, where palliative irradiation was performed. Metcalfe et
al reported that the labial chorionic villus structure was
unchanged at doses of 2 Gy or less; however, it began to be
compromised at higher doses of 6 Gy or more (17). If the dose in one fraction is low,
the combination of both therapies may not cause impairment.
Evaluation of the prevalence and potential risk factors of
lenvatinib fistula and organ perforation in radioiodine-refractory
thyroid cancer suggests that EBRT should not be considered an
exclusion criterion for lenvatinib initiation (18). However, it should be noted that no
details on the number of fractions are available because they are
evaluated as greater than or less than 30 Gy. Moreover, the
histologic type and degree of tumor invasion have been shown to be
risks for fistula and organ perforation.
We believe that lenvatinib and anti-VEGF agents are
important treatment options for hepatocellular and other carcinomas
and may improve the prognosis; thus, their use should not be
avoided even if there is a history of radiation therapy. However,
it is important to keep in mind that, although rare, a history of
high-dose radiotherapy of one fraction may lead to ulceration, as
in this case. In conclusion, we present a case of rectal ulceration
induced by lenvatinib treatment 15 years after the completion of
definitive radiotherapy for prostate cancer. Further studies
regarding this type of case are warranted.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YK collected the data. YK, KW and KK drafted the
manuscript. YK, KW, RT, YM, AS and KK designed the study. YK and KW
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the wife
of the patient with respect to the publication of this case
report.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BED
|
biologically effective dose
|
|
EBRT
|
external beam radiotherapy
|
|
HDR-BT
|
high-dose-rate brachytherapy
|
|
TKIs
|
tyrosine kinase inhibitors
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Motzer RJ, Taylor MH, Evans TRJ, Okusaka
T, Glen H, Lubiniecki GM, Dutcus C, Smith AD, Okpara CE, Hussein Z,
et al: Lenvatinib dose, efficacy, and safety in the treatment of
multiple malignancies. Expert Rev Anticancer Ther. 22:383–400.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hao Z and Wang P: Lenvatinib in management
of solid tumors. Oncologist. 25:e302–e310. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gore E, Currey A and Choong N:
Tracheoesophageal fistula associated with bevacizumab 21 months
after completion of radiation therapy. J Thorac Oncol. 4:1590–1591.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lordick F, Geinitz H, Theisen J, Sendler A
and Sarbia M: Increased risk of ischemic bowel complications during
treatment with bevacizumab after pelvic irradiation: Report of
three cases. Int J Radiat Oncol Biol Phys. 64:1295–1298. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kozloff M, Yood MU, Berlin J, Flynn PJ,
Kabbinavar FF, Purdie DM, Ashby MA, Dong W, Sugrue MM, Grothey A,
et al: Clinical outcomes associated with bevacizumab-containing
treatment of metastatic colorectal cancer: The BRiTE observational
cohort study. Oncologist. 14:862–870. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Basille D, Andrejak M, Bentayeb H, Kanaan
M, Fournier C, Lecuyer E, Boutemy M, Garidi R, Douadi Y and Dayen
C: Bronchial fistula associated with sunitinib in a patient
previously treated with radiation therapy. Ann Pharmacother.
44:383–386. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peters NA, Richel DJ, Verhoeff JJ and
Stalpers LJ: Bowel perforation after radiotherapy in a patient
receiving sorafenib. J Clin Oncol. 26:2405–2406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jo Y, Hiratsuka J, Fujii T, Takenaka A and
Fujisawa M: High-dose-rate iridium-192 afterloading therapy
combined with external beam radiotherapy for T1c-T3bN0M0 prostate
cancer. Urology. 64:556–560. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wachsberger P, Burd R and Dicker AP: Tumor
response to ionizing radiation combined with antiangiogenesis or
vascular targeting agents: Exploring mechanisms of interaction.
Clin Cancer Res. 9:1957–1971. 2003.PubMed/NCBI
|
|
10
|
Kleibeuker EA, Ten Hooven MA, Verheul HM,
Slotman BJ and Thijssen VL: Combining radiotherapy with sunitinib:
lessons (to be) learned. Angiogenesis. 18:385–395. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schlumberger M, Tahara M, Wirth LJ,
Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff
AO, et al: Lenvatinib versus placebo in radioiodine-refractory
thyroid cancer. N Engl J Med. 372:621–630. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ishiyama H, Kamitani N, Kawamura H, Kato
S, Aoki M, Kariya S, Matsumura T, Kaidu M, Yoshida K, Hashimoto Y,
et al: Nationwide multi-institutional retrospective analysis of
high-dose-rate brachytherapy combined with external beam
radiotherapy for localized prostate cancer: An Asian Prostate
HDR-BT Consortium. Brachytherapy. 16:503–510. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Clark BG, Souhami L, Roman TN, Chappell R,
Evans MD and Fowler JF: The prediction of late rectal complications
in patients treated with high dose-rate brachytherapy for carcinoma
of the cervix. Int J Radiat Oncol Biol Phys. 38:989–993. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakano T, Kato S, Ohno T, Tsujii H, Sato
S, Fukuhisa K and Arai T: Long-term results of high-dose rate
intracavitary brachytherapy for squamous cell carcinoma of the
uterine cervix. Cancer. 103:92–101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pollom EL, Deng L, Pai RK, Brown JM,
Giaccia A, Loo BW Jr, Shultz DB, Le QT, Koong AC and Chang DT:
Gastrointestinal toxicities with combined antiangiogenic and
stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys.
92:568–576. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zips D, Eicheler W, Geyer P, Hessel F,
Dörfler A, Thames HD, Haberey M and Baumann M: Enhanced
susceptibility of irradiated tumor vessels to vascular endothelial
growth factor receptor tyrosine kinase inhibition. Cancer Res.
65:5374–5379. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Metcalfe C, Kljavin NM, Ybarra R and de
Sauvage FJ: Lgr5+ stem cells are indispensable for
radiation-induced intestinal regeneration. Cell Stem Cell.
14:149–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Valerio L, Giani C, Agate L, Molinaro E,
Viola D, Bottici V, Matrone A, Puleo L, Lorusso L, Cappagli V, et
al: Prevalence and risk factors of developing fistula or organ
perforation in patients treated with lenvatinib for
radioiodine-refractory thyroid cancer. Eur Thyroid J. 10:399–407.
2021. View Article : Google Scholar : PubMed/NCBI
|