Introduction
In recent decades, lung cancer has been the leading
cause of cancer death worldwide (1,2).
According to the GLOBOCAN report in 2020, the number of new cases
of lung cancer was >2.2 million, accounting for 11.4% of all
cancer cases and ranking second worldwide; the number of deaths
from lung cancer was nearly 1.8 million, accounting for 18% of all
cancer deaths and ranking first worldwide (3). Identification of modifiable risk
factors for lung cancer can help prevent this deadly malignancy.
Current epidemiological data suggest that tobacco use is the most
probable cause which leads to lung cancer (4). On the other hand, high consumption of
fruit and vegetables (5) rich in
vitamins and various antioxidants decreases the risk of lung cancer
(6–11).
Vitamin C has been reported to have a protective
effect against the risk of lung cancer (12,13).
More specifically, vitamin C is an important antioxidant regulator
(14) that traps free radicals and
reactive oxygen molecules (15),
which in turn protects cells from oxidative DNA damage, thereby
preventing cancer initiation (16).
Previous observational epidemiological studies have
reported inconsistent findings regarding the association between
vitamin C intake and the risk of lung cancer. Several observational
studies have reported that high consumption of dietary vitamin C is
associated with a decreased risk of the lung cancer (15,17,18).
For example, studies by Yong et al (6) in 1997, Voorrips et al in 2000
(19) and Yuan et al
(20) in 2003 reported that vitamin
C intake reduced the risk of lung cancer by 34, 23 and 19%,
respectively. Additionally, it has been reported that supplementary
vitamin C intake reduces the risk of lung cancer (10,21,22).
However, other observational studies have reported that there is no
association between vitamin C (dietary and/or supplementary) intake
and the risk of lung cancer (19,20,23–25).
Previous studies reported that vitamin C intake even increased the
risk of lung cancer (26–29).
Previous meta-analyses of cohort studies have also
reported inconsistent findings based on the source of vitamin C:
dietary or supplementation. Meta-analyses conducted by Cho et
al (12) in 2006, Luo et
al (13) in 2014 and Fu et
al (30) in 2021 showed that
dietary vitamin C intake significantly reduced the risk of lung
cancer by 20, 17 and 16%, respectively. On the contrary, the study
of Cho et al (12) showed
that no association between total vitamin C intake (dietary plus
supplementary) and the risk of lung cancer. Fu et al
(30) also showed no association
between supplementary vitamin C intake and the risk of lung
cancer.
The aim of the present study was to investigate the
association between dietary or supplementary vitamin C intake and
the risk of lung cancer using a comprehensive meta-analysis of
cohort studies and subgroup meta-analyses by various factors,
specifically the source of vitamin C (dietary or
supplementary).
Materials and methods
Literature search strategy
An extensive research was conducted for eligible
studies published from inception to April 15, 2022, in two core
databases: PubMed (https://pubmed.ncbi.nlm.nih.gov/) and EMBASE
(https://www.embase.com/landing?status=grey). Both the
National Library of Medicine and Medical Subject Heading (MeSH)
terms were used and a wide range of free-text search terms to
identify as numerous relevant articles as possible. A PICO
framework was used to determine the search terms associated with
the topic as follows: ‘P’ stands for population, which, in the
present study, was ‘general population’; ‘I’ for intervention
(exposure in this study), which was ‘dietary or supplementary
intake of vitamin C’; ‘C’ for comparison, which was ‘little or no
intake of vitamin C’; and ‘O’ for outcome, which was ‘incidence of
lung cancer.’ Using Boolean operators along with all selected MeSH
and free-text terms, the following search term was created:
(vitamin C or ascorbic acid) and (lung cancer or lung neoplasms).
The language of the publications was limited to English.
Selection criteria
The cohort studies that were selected (i)
investigated the association between vitamin C intake (dietary or
supplementary) and the risk of lung cancer, and (ii) reported
outcome measures using adjusted relative risks (RRs) or hazard
ratios (HRs) with 95% confidence intervals (CIs). When multiple
studies used data from the same study, the most comprehensive
analysis was included.
Selection of relevant studies and data
extraction
Based on the selection criteria, two authors (Dung V
Dung and Xuan Quy Luu) independently evaluated the eligibility of
the studies that could potentially be included in the
meta-analysis. Discrepancies between evaluators were resolved by a
third author (Seung-Kwon Myung). The following data was extracted
on the general characteristics of the included studies: Year of
publication, name of first author, region (period of enrollment),
characteristics of the population (number of participants, sex and
age), follow-up period, type of vitamin C intake, outcomes, number
of lung cancer cases, RR/HR with 95% CI and adjusted variables.
Quality assessment
The methodological quality of the individual cohort
studies using the Newcastle-Ottawa Scale (31) was evaluated (n=13). The evaluated
items were as follows: i) Selection (representativeness of the
exposed cohort, selection of the non-exposed cohort, ascertainment
of exposure, and outcome of interest that was not present at the
start of the study); ii) comparability (control for important and
additional factors); and iii) outcome (assessment of outcome,
follow-up long enough for outcomes to occur, and adequacy of
follow-up of cohorts). Each cohort study could be awarded a maximum
of one point for each numbered item within the selection and
outcome categories, whereas a maximum of two points could be given
for the comparability category. Individual studies were classified
as low (average score or lower) or high quality (higher than the
average score).
Main analysis and subgroup
analyses
In the main analysis, the association between
vitamin C intake (dietary or supplementary) and the risk of lung
cancer using adjusted RRs or HRs with 95% CIs was investigated. A
subgroup meta-analyses by region was also performed (Europe, United
States and Asia), source of vitamin C (dietary, supplementary, or
both), follow-up period (≤10 years or >10 years), sex (only
male, only female, or both) and number of participants (<30,000
participants, 30,000-60,000 participants, or >60,000
participants), and quality of study (high or low).
Statistical analysis
From individual studies, the adjusted RRs or HRs
were used and their 95% CIs to calculate the pooled effect size
with a 95% CI. Because individual studies involved different
populations, a random-effects model was chosen with the Der
Simonian and Laird method (32). To
examine the heterogeneity in results across studies, Higgins
I2 was used, which measures the percentage of total
variation across studies. If I2 was <0, it was set to
zero. I2 ranges from 0% (no observed heterogeneity) to
100% (maximal heterogeneity); if I2 is >50%,
substantial heterogeneity exists (33).
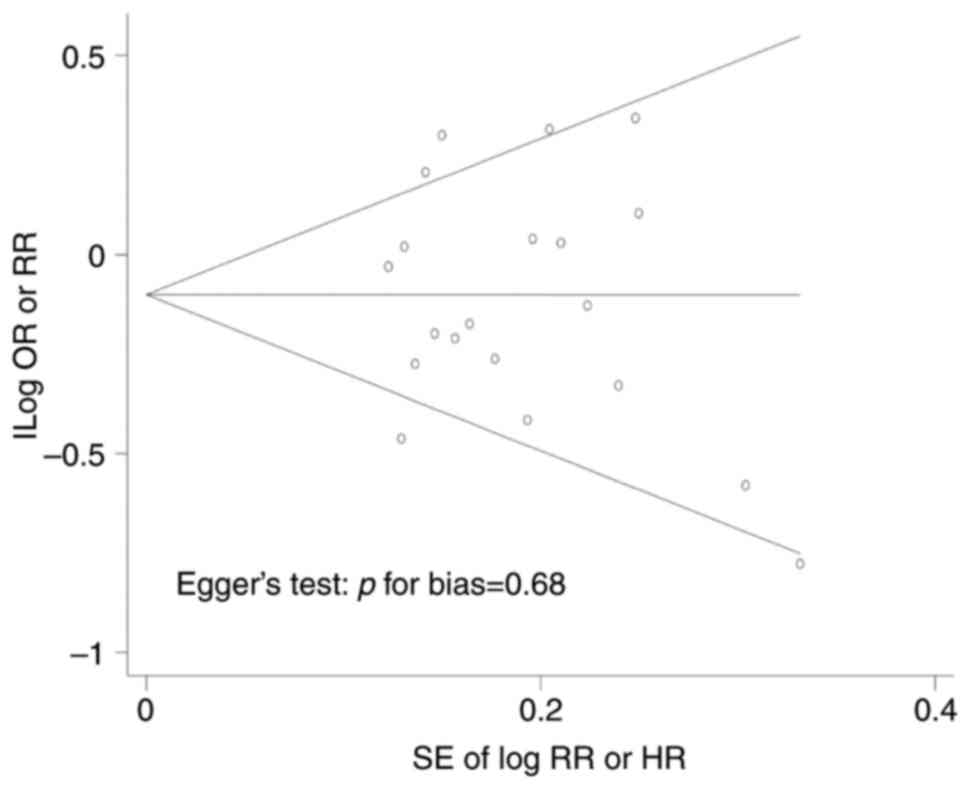
Publication bias was evaluated using Begg's funnel
plot and Egger's test. If publication bias existed, the Begg's
funnel plot was asymmetrical, or the P-value of the Egger's test
was <0.05. When the two tests showed inconsistent results, the
results from the Egger's test were adopted because the funnel plot
relies on visual inspection, which might be misleading (34). Statistical analyses were conducted
using the Stata SE software package (version 17.0; StataCorp
LP).
Results
Selection of relevant studies
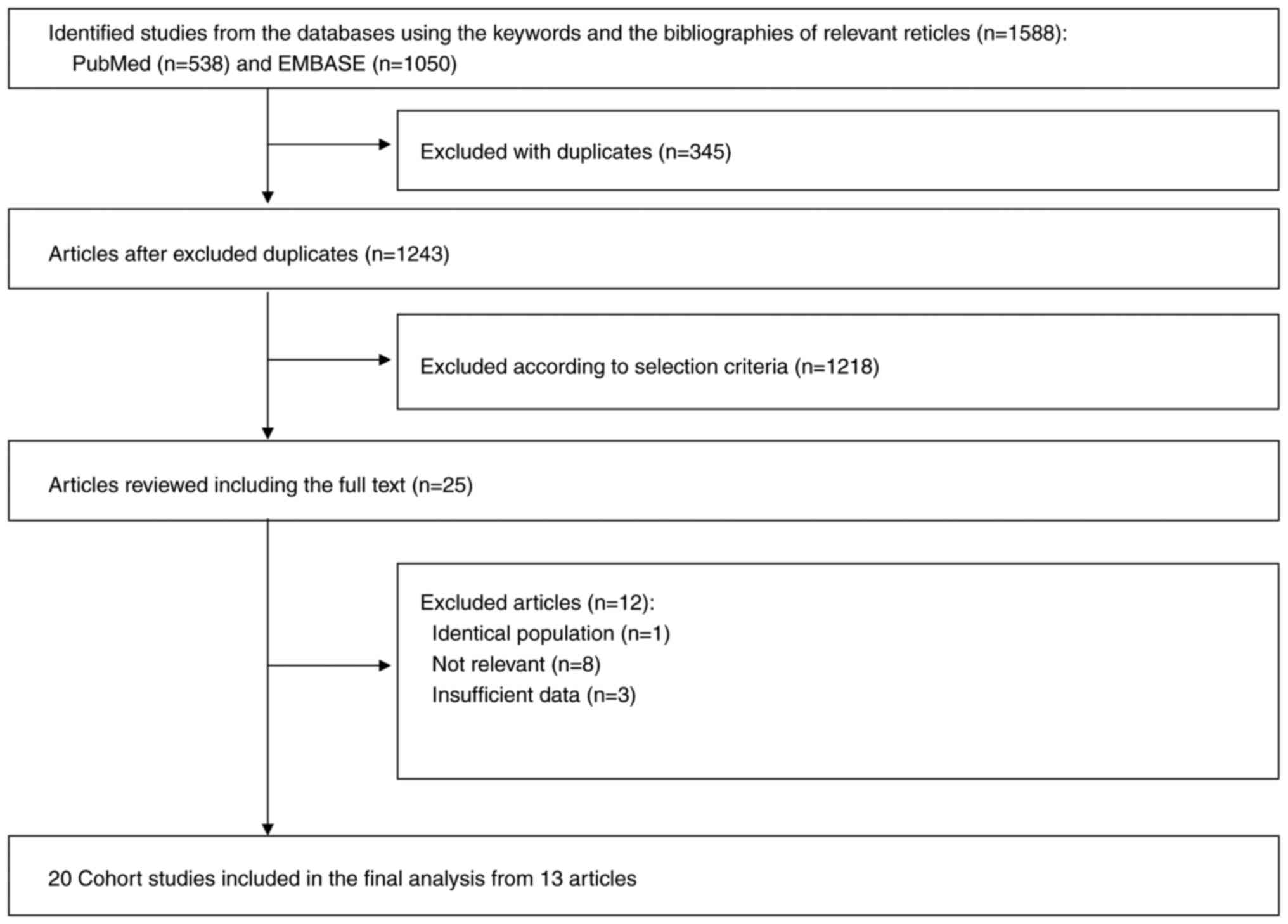
A flow diagram of the selection process of the
relevant studies is demonstrated in Fig. 1. A total of 1,588 articles were
identified by searching the databases. After removing 345 duplicate
articles, titles and abstracts were reviewed of 1,243 articles, and
subsequently 1,218 articles that did not meet the predetermined
selection criteria were excluded. After reviewing the full texts of
the remaining 25 articles, 12 articles were excluded for the
following reasons: Identical population (n=1), irrelevance (n=8),
and insufficient data (n=3). Ultimately, 20 cohort studies from the
remaining 13 articles were included in the final analysis.
General characteristics of
studies
The general characteristics of the included studies
are shown in Table I. From the 20
cohort studies, a total of 808,587 participants were identified,
including 7,227 lung cancer patients (6,8,17–20,23–29).
The individual studies were conducted in the United States (n=12),
Europe (n=4), and Asia (n=4). All participants were aged between
25–80 years. The mean follow-up period ranged from 4–20 years.
 | Table I.General characteristics of cohort
studies included in the analysis (n=13). |
Table I.
General characteristics of cohort
studies included in the analysis (n=13).
| First author,
year | Region (years
enrolled) | Population | Follow-up
Periods | Vitamin C intake
(dietary or supplementary) | Outcomes and number
of cases | RR/HR (95% CI) | Adjusted
variables | (Refs.) |
|---|
| Shibata et
al, 1992 | USA
(1981–1989) | 24,218 men of a
retirement community | 8 years | Dietary
Supplementary | Lung cancer
(n=94) | 1.11 (0.68–1.81)
1.03 (0.68–1.55) | Age and
smoking | (23) |
|
|
| Mean age: 74.9
years old 45,941 women of a retirement community |
| Dietary
Supplementary | Lung cancer
(n=70) | 0.56 (0.31–1.02)
0.72 (0.45–1.15) |
|
|
|
|
| Mean age: 73.8
years old |
|
|
|
|
|
|
| Steinmetz et
al, 1993 | USA
(1986–1990) | 41,837
postmenopausal women | 4 years | Dietary +
Supplementary | Lung cancer
(n=138) | 1.41
(0.87–2.30) | Age. energy intake,
and pack-years of smoking in multivariable logistic regression
models | (26) |
|
|
| Mean age: 61.7
years old |
|
|
|
|
|
| Bandera et
al, 1997 | USA
(1980–1987) | 27,544 men from the
New York State Cohort Age range: mostly 40–80 years old | 8 years | Dietary | Lung cancer
(n=395) | 0.63
(0.53–0.88) | Age, education,
cigarettes/ day, years smoking, and total energy intake (except
calories) based on cox proportional hazards model | (17) |
|
|
| 20,456 women from
the New York State Cohort Age range: mostly 40–80 years old |
|
| Lung cancer
(n=130) | 0.88
(0.57–1.37) |
|
|
| Ocké et al,
1997 | European
(Netherlands) (1960–1990) | 561 men from the
town of Zutphen, the Netherlands. Age range: 52–71 years old | 20 years | Dietary | Lung cancer
(n=54) | 0.46
(0.24–0.88) | Age, pack-years of
cigarettes, and energy intake | (18) |
| Yong et al,
1997 | USA
(1971–1992) | 10,068 persons
(3,968 men and 6,100 women) from the First National Health and
Nutrition Examination Survey Epidemiologic Follow up Study cohort
Age range: 25–74 years old | 19 years | Dietary | Lung cancer
(n=248) | 0.66
(0.45–0.96) | Sex, race,
educational attainment, nonrecreational activity level, BMI, family
history, smoking status, total calorie intake, and alcohol
intake | (6) |
| Speizer et
al, 1999 | USA
(1980–1992) | 118,351 women from
the Nurses' Health Study Age range: 30–69 years old | 16 years | Dietary +
Supplementary | Lung cancer
(n=593) | 1.35 (1.0–1.8) | Age, total energy
intake, smoking (past and current amount in 1980; 1–4, 5–14, 15–24,
25–34, 35–44, 45+), and age of starting to smoke (O17, 18–19,
20–21, 22+) | (27) |
| Feskanich et
al, 2000 | USA
(1976–1996) | 47,778 men from the
Health Professionals' Follow-up Study | 10 years | Dietary | Lung cancer
(n=274) | 1.04
(0.71–1.53) | n.a. | (24) |
|
|
| Age range: 40–75
years old 77,283 women from the Nurses' Health Study | 12 years |
| Lung cancer
(n=519) | 0.82
(0.62–1.1) | n.a. |
|
|
|
| Age range: 40–75
years old |
|
|
|
|
|
|
| Voorrips et
al, 2000 | European
(Netherlands) (1986–1992) | 58,279 men from the
Netherlands Cohort Study on Diet and Cancer | 6.3 years | Dietary | Lung cancer
(n=939) | 0.77
(0.54–1.08) | Age, family
history, smoking, SES, folate, and energy | (19) |
|
|
| Age range: 55–69
years old |
|
|
|
|
|
|
| Yuan et al,
2003 | Asia Singapore)
(1993–2000) | 62,392 Chinese men
and women in Singapore Age range: 45–74 years old | 8 years | Dietary | Lung cancer
(n=482) | 0.81
(0.59–1.09) | Age, sex, dialect
group, year of interview, level of education, BMI, r number of
cigarettes smoked per day, number of years of smoking, and number
of years since quitting smoking for former smokers | (20) |
| Slatore et
al, 2008 | USA
(2000–2006) | 77,126 men and
women from Washington State in the VITAL study | 10 years | Supplementary | Lung cancer
(n=521) | 0.97
(0.76–1.23) | Age, sex, years
smoked, pack-years, and pack-years squared | (25) |
|
|
| Age range: 50–76
years old |
|
|
|
|
|
|
| Roswall et
al, 2010 | European (Denmark)
(1993–1997) | 55,557 Danes from
the prospective Diet, Cancer and Health study | 10.6 years | Supplementary
Dietary | Lung cancer (n
=721) | 1.23
(0.93–1.62) | Not given | (28) |
|
|
| Age range: 50–64
years old |
|
|
| 0.76
(0.58–0.99) |
|
|
| Takata et
al, 2013 | Asia (China)
(2002–2009) | 61,491 adult
Chinese men from the Shanghai Men's Health study | 5.5 years | Dietary | Lung cancer
(n=359) | 0.84
(0.61–1.16) | Age, years of
smoking, the number of cigarettes smoked per day, current smoking
status, total caloric intake, education, BMI category, ever
consumption of tea, history of chronic bronchitis, and family
history of lung cancer among first-degree relatives | (8) |
|
|
| Age range: 40–74
years old |
|
|
|
|
|
| Narita et
al, 2018 | Asia (Japan)
(1990–2013) | 38,207 men from the
Japan Public Health Center-based prospective study | 18 years | Dietary | Lung cancer men
(n=1,237) | 1.02
(0.79–1.32) | Age, study area,
smoking status, alcohol consumption, vitamin supplements use, and
energy-adjusted intakes of fish, isoflavone, vegetables, and
fruits. | (29) |
|
|
| Age range: 40–69
years old |
|
|
|
|
|
|
|
| 41,498 women from
the Japan Public Health Center-based prospective study |
|
| Lung cancer women
(n=453) | 1.37
(0.92–2.05) |
|
|
|
|
| Age range: 40–69
years old |
|
|
|
|
|
|
Quality assessment
As shown in Table
II, 14 cohort studies from eight articles were considered to be
of low quality, (6,17,19,23–25,27,28)
and six studies from the five remaining articles were considered to
be of high quality (8,18,20,26,29).
 | Table II.Table
II: Methodological quality of the included studies in the final
analysis based on the Newcastle-Ottawa Scale for assessing the
quality of cohort studies (n=13). |
Table II.
Table
II: Methodological quality of the included studies in the final
analysis based on the Newcastle-Ottawa Scale for assessing the
quality of cohort studies (n=13).
|
| Selection |
| Comparability | Outcome |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Cohort studies | Representativeness
of the exposed cohort | Selection of the
non-exposed cohort | Ascertainment of
exposure | Outcome of interest
was not present at start of study | Control for
important factor or additional factor | Assessment of
outcome | Follow-up long
enough for outcomes to occur | Adequacy of
follow-up of cohorts | Total | (Refs.) |
|---|
| Shibata et
al, 1992 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | (23) |
| Steinmetz et
al, 1993 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 2 | 8 | (26) |
| Bandera et
al, 1997 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | (17) |
| Ocké et al,
1997 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 2 | 8 | (18) |
| Yong et al,
1997 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | (6) |
| Speizer et
al, 1999 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | (27) |
| Feskanich et
al, 2000 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 6 | (24) |
| Voorrips et
al, 2000 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 2 | 7 | (19) |
| Yuan et al,
2003 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (20) |
| Slatore et
al, 2008 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | (25) |
| Roswall et
al, 2010 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 6 | (28) |
| Takata et
al, 2013 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 2 | 8 | (8) |
| Narita et
al, 2018 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 2 | 8 | (29) |
|
|
|
|
|
|
|
| Average
score=7.2 |
|
|
|
Main analysis and subgroup
meta-analyses
The findings from the main analysis and subgroup
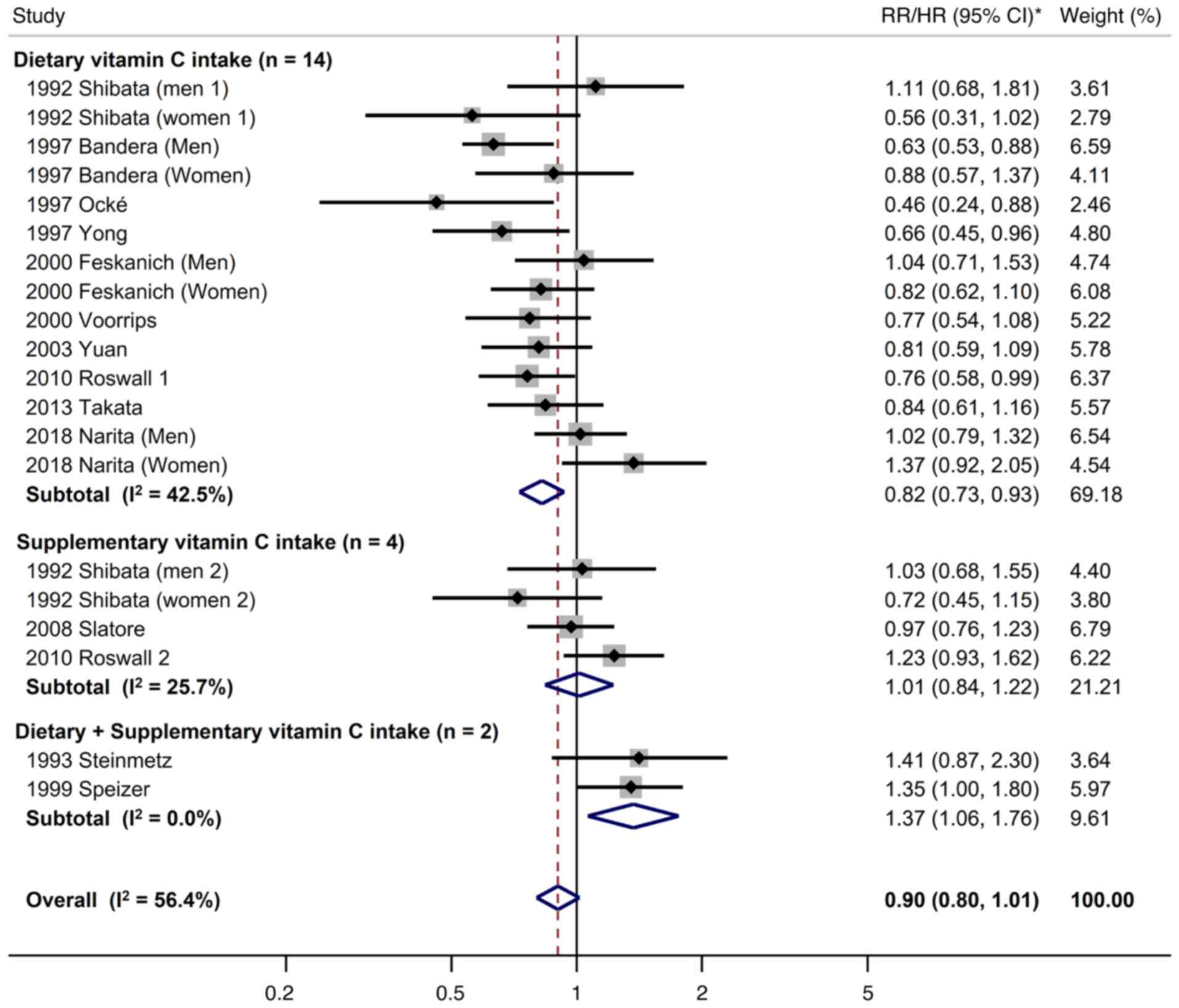
meta-analyses by the source of vitamin C are shown in Fig. 2. Overall, there was no significant
association between dietary or supplementary vitamin C intake and
the risk of lung cancer in the meta-analysis of all 20 cohort
studies (RR/HR, 0.90; 95% CI, 0.80 to 1.01; I2=56.4%).
In the subgroup meta-analysis by the source of vitamin C, dietary
vitamin C intake was associated with a decreased risk of lung
cancer (RR, 0.82; 95% CI, 0.73–0.92; I2=42.5%; n=14),
whereas there was no association between supplementary vitamin C
intake and the risk of lung cancer (RR, 1.01; 95% CI, 0.84–1.22;
I2=25.7%; n=4). In the subgroup meta-analysis of two
cohort studies, the combination of dietary and supplementary
vitamin C intake was associated with an increased risk of lung
cancer (RR, 1.37; 95% CI, 1.06–1.76; I2=0%).
Table III presents
the findings of the subgroup meta-analyses based on various
factors. In the subgroup meta-analysis by region, there was no
significant association between vitamin C intake and lung cancer
risk across regions (United States, Europe and Asia). In the
subgroup meta-analysis by follow-up period, dietary or
supplementary vitamin C intake decreased lung cancer risk during
the follow-up period of 5 to 10 years (RR, 0.83; 95% CI, 0.74–0.94;
I2=0%; n=11). However, this beneficial effect was not
observed when the follow-up period was longer than 10 years (RR,
0.94; 95% CI, 0.76–1.17; I2=0%; n=8). As demonstrated in
Fig. 3, there was no publication
bias in this meta-analysis (symmetrical Begg's funnel plot; Egger's
test, P=0.68).
 | Table III.Association between dietary or
supplementary intake of vitamin C and risk of lung cancer in
subgroup meta-analyses by various factors. |
Table III.
Association between dietary or
supplementary intake of vitamin C and risk of lung cancer in
subgroup meta-analyses by various factors.
| Factors | No. of study | RR/HR (95% CI) | Heterogeneity,
I2 (%) |
|---|
| All | 20 | 0.90
(0.80–1.01) | 56.4 |
| Region |
|
|
|
|
Europe | 4 | 0.81
(0.58–1.14) | 73.1 |
| United
States | 12 | 0.90
(0.76–1.06) | 58.5 |
|
Asia | 4 | 0.97
(0.79–1.19) | 41% |
| Source of vitamin C
intake |
|
|
|
|
Supplementary | 4 | 1.01
(0.84–1.22) | 25.7 |
|
Dietary | 14 | 0.82
(0.73–0.92)a | 42.5 |
| Dietary
+ Supplementary | 2 | 1.37
(1.06–1.76)a | 0 |
| Follow up
period |
|
|
|
| <5
years | 1 | 1.41
(0.87–2.29) | 100 |
| 5-10
years | 11 | 0.83
(0.74–0.94) | 18.0 |
| >10
years | 8 | 0.94
(0.76–1.17) | 71.1 |
| Sex |
|
|
|
|
Men | 8 | 0.85
(0.71–1.02) | 50.2 |
|
Women | 5 | 0.88
(0.72–1.08) | 59.2 |
|
Both | 7 | 0.99
(0.77–1.27) | 62.4 |
| Number of
participants |
|
|
|
|
<30,000 | 6 | 0.76
(0.61–0.97) | 48.3 |
|
30,000-60,000 | 9 | 0.96
(0.80–1.15) | 55.4 |
|
>60,000 | 5 | 0.94
(0.78–1.13) | 50.8 |
| Quality of
study |
|
|
|
| High
quality (>7 score) | 6 | 0.95
(0.75–1.21) | 59.7 |
| Low
quality (≤7 scores) | 14 | 0.88
(0.76–1.01) | 57.1 |
Discussion
In the present meta-analysis of cohort studies,
there was no significant association between dietary or
supplementary vitamin C intake and lung cancer risk. However, in
the subgroup meta-analysis by the source of vitamin C, dietary
intake of vitamin C was found to be associated with a decreased
risk of lung cancer, whereas no association was found between
supplementary vitamin C intake and high risk of lung cancer.
Notably, the combination of dietary and supplementary vitamin C
intake was associated with an increased risk of lung cancer. In the
subgroup meta-analysis by follow-up period, dietary or
supplementary vitamin C intake was associated with a decreased risk
of lung cancer among studies with 10 years or shorter follow-up
periods. However, this beneficial effect was not observed among
studies with follow-up periods longer than 10 years.
Although the effects of vitamin C on lung cancer
development remain unclear, several potential biological mechanisms
have been proposed. Vitamin C has antioxidant properties at low
serum levels, prooxidant properties at high serum levels, and is
involved in the epigenetic regulation of genomic stability
(35–37). High levels of reactive oxygen
species (ROS), which are a subset of free radicals, are well known
to damage cellular DNA and promote oncogenesis (38). Vitamin C, as a potent antioxidant,
may scavenge these ROS, preventing DNA damage and oncogenesis
(37). Also, at high concentrations
(5–20 mM), vitamin C (ascorbate) could act as a prooxidant through
the reduction of metal ions, such as Fe3+ (2
Fe3+ + Ascorbate → 2 Fe2+ +
Dehydroascorbate), leading to the formation of ROS, such as
H2O2. Through the Fenton reaction,
H2O2 can produce free hydroxyl radicals (2
Fe2+ + 2 H2O2 → 2 Fe3+
+ 2 OH· + 2 OH-) which are selectively toxic to cancer cells
(36). Additionally, ten-eleven
translocation (TET) promotes DNA demethylation as one of the
epigenetic modifications, and loss of function in TET proteins and
changes of DNA methylation can lead to genomic instability and
carcinogenesis (39). Vitamin C, a
cofactor of TET enzymes, is also known to directly promote DNA
demethylation and alter the expression of tumor suppressors and DNA
repair enzymes (39).
The findings of the present study, are consistent
with those of previously published meta-analyses. A meta-analysis
by Cho et al (12) of eight
prospective studies in 2006 reported that dietary vitamin C intake
significantly decreased the risk of lung cancer (RR, 0.80; 95% CI,
0.71–0.91), whereas there was no significant association between
total vitamin C intake (dietary plus supplementary) and the risk of
lung cancer (RR, 1.00; 95% CI, 0.80–1.25). Additionally, Luo et
al (13) conducted a
meta-analysis of 14 prospective cohort studies in 2014 and
concluded that high dietary vitamin C intake, by consumption of
fruits and vegetables, had a protective effect against lung cancer
(RR, 0.83; 95% CI, 0.73–0.94). However, a subgroup meta-analysis of
supplementary vitamin C alone was not conducted, and additional
prospective studies have been published since this publication.
Recently, in 2021, a meta-analysis of cohort studies
by Fu et al (30) reported
findings similar to those of Cho et al's meta-analysis.
However, their analysis included only six cohort studies on dietary
vitamin C intake and three on supplementary vitamin C intake. The
present meta-analysis involved 20 cohort studies and subgroup
meta-analyses according to the source of vitamin C (dietary or
supplementary). Unlike previous meta-analyses, the present study's
subgroup meta-analysis showed that the combination of dietary and
supplementary vitamin C intake was significantly associated with a
37% increased risk of lung cancer.
There are several possible explanations for the
discrepancies regarding the findings on the effects of dietary and
supplementary vitamin C intake on the risk of lung cancer. The
majority of commercial vitamin C supplements are synthetic.
Although synthetic vitamin C supplements and food-derived dietary
vitamin C are chemically identical, the effects of fruits and
vegetables rich in vitamin C may differ from those of vitamin C
supplements in that the combination of natural vitamin C and
numerous nutrients in fruits and vegetables might have beneficial
effects of reducing the risk of lung cancer (40,41).
Additionally, it was found that the combination of dietary and
supplementary vitamin C intake significantly increased the risk of
lung cancer. A possible explanation is that when provided in
addition to dietary vitamin C, vitamin C supplements may act as
pro-oxidants under conditions of oxidative stress, such as smoking,
thereby inducing DNA damage and eventually leading to the
development of lung cancer (41,42).
The present meta-analysis revealed a difference in
the risk of lung cancer between food-derived, dietary vitamin C and
synthetic vitamin C supplements. This implies that the diverse
dietary nutrients from foods as well as vitamin C should not be
directly equated to synthetic nutrient supplements in terms of
beneficial effects. Although the dietary intake of nutrients from
foods, such as fruits and vegetables, has shown beneficial effects
in the prevention of cancer, the effects of synthetic nutrient
supplements should be investigated in further randomized controlled
trials (RCTs) as well as epidemiological observational studies.
The present study, to the best of the authors'
knowledge, was the most comprehensive meta-analysis of cohort
studies on the association between vitamin C intake and the risk of
lung cancer, including subgroup meta-analyses of various
factors.
However, the present study had some limitations.
First, most studies included in this meta-analysis did not report
the risk of lung cancer based on the specific levels of dietary
vitamin C consumed by the participants. Thus, it was impossible to
perform subgroup meta-analyses by dietary intake levels of vitamin
C; second, although the subgroup meta-analyses revealed that
dietary intake of vitamin C along with supplements increased the
risk of lung cancer, only two studies were included in this
analysis. Further prospective studies are warranted to confirm
these findings. Third, only studies published in English were
included to ensure the high quality of the included studies and
accessibility of the data. If studies published in languages other
than English had been included in the present study, the findings
may have changed. However, this would not have significantly
affected the conclusions. Fourth, this meta-analysis only included
cohort studies. Regarding the effects of vitamin C supplementation,
RCTs provide a higher level of evidence than cohort studies.
Finally, all the included cohort studies were published before
2010. Further RCTs and meta-analyses of RCTs are warranted to
confirm the association between supplementary vitamin C intake and
lung cancer risk.
In conclusion, in the present meta-analysis of
cohort studies it was found that the dietary intake of vitamin C is
beneficial for the prevention of lung cancer, whereas its
supplementary intake does not have any beneficial effect. The
findings of the present study should be confirmed by further
prospective studies and RCTs.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SKM and DVT conceptualized the present study,
conducted investigation and data curation. SKM implemented the
methodology. DVT performed formal analysis. DVT and XQL wrote the
original draft. XQL and HTTT made substantial contributions to
acquisition and interpretation of data. SKM, DVT and HTTT wrote,
reviewed and edited the manuscript. All authors have read and
approved the final version of the manuscript. DVT and SKM confirm
the authenticity of all the raw data
Ethics approval and consent to
participate
Not applicable.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Glade MJ: Food, nutrition, and the
prevention of cancer: A global perspective. American institute for
cancer research/world cancer research fund, American institute for
cancer research, 1997. Nutrition. 15:523–526. 1999.PubMed/NCBI
|
|
2
|
World Health Organization, . Cancer.
https://www.who.int/news-room/fact-sheets/detail/cancerDecember
22–2022
|
|
3
|
Sung H, Ferlay JF, Siegel RL, Laversanne
M, Soerjomataram I, Jemal A and Bray F: Global cancer statistics
2020: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim CH, Lee YC, Hung RJ, McNallan SR, Cote
ML, Lim WY, Chang SC, Kim JH, Ugolini D, Chen Y, et al: Exposure to
secondhand tobacco smoke and lung cancer by histological type: A
pooled analysis of the international lung cancer consortium
(ILCCO). Int J Cancer. 135:1918–1930. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Norat T, Aune D, Chan D and Romaguera D:
Fruits and vegetables: Updating the epidemiologic evidence for the
WCRF/AICR lifestyle recommendations for cancer prevention. Cancer
Treat Res. 159:35–50. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yong LC, Brown CC, Schatzkin A, Dresser
CM, Slesinski MJ, Cox CS and Taylor PR: Intake of vitamins E, C,
and A and risk of lung cancer. The NHANES I epidemiologic followup
study. First national health and nutrition examination survey. Am J
Epidemiol. 146:231–243. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng TY, Lacroix AZ, Beresford SA,
Goodman GE, Thornquist MD, Zheng Y, Chlebowski RT, Ho GY and
Neuhouser ML: Vitamin D intake and lung cancer risk in the Women's
health initiative. Am J Clin Nutr. 98:1002–1011. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takata Y, Xiang YB, Yang G, Li H, Gao J,
Cai H, Gao YT, Zheng W and Shu XO: Intakes of fruits, vegetables,
and related vitamins and lung cancer risk: Results from the
Shanghai Men's health study (2002–2009). Nutr Cancer. 65:51–61.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Redaniel MT, Gardner MP, Martin RM and
Jeffreys M: The association of vitamin D supplementation with the
risk of cancer in postmenopausal women. Cancer Causes Control.
25:267–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vieira AR, Abar L, Vingeliene S, Chan DS,
Aune D, Navarro-Rosenblatt D, Stevens C, Greenwood D and Norat T:
Fruits, vegetables and lung cancer risk: A systematic review and
meta-analysis. Ann Oncol. 27:81–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shareck M, Rousseau MC, Koushik A,
Siemiatycki J and Parent ME: Inverse association between dietary
intake of selected carotenoids and vitamin C and risk of lung
cancer. Front Oncol. 7:232017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cho E, Hunter DJ, Spiegelman D, Albanes D,
Beeson WL, van den Brandt PA, Colditz GA, Feskanich D, Folsom AR,
Fraser GE, et al: Intakes of vitamins A, C and E and folate and
multivitamins and lung cancer: A pooled analysis of 8 prospective
studies. Int J Cancer. 118:970–978. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo J, Shen L and Zheng D: Association
between vitamin C intake and lung cancer: A dose-response
meta-analysis. Sci Rep. 4:61612014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fairfield KM and Fletcher RH: Vitamins for
chronic disease prevention in adults: Scientific review. JAMA.
287:3116–3126. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee KW, Lee HJ, Surh YJ and Lee CY:
Vitamin C and cancer chemoprevention: Reappraisal. Am J Clin Nutr.
78:1074–1078. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pathak SK, Sharma RA, Steward WP, Mellon
JK, Griffiths TR and Gescher AJ: Oxidative stress and
cyclooxygenase activity in prostate carcinogenesis: Targets for
chemopreventive strategies. Eur J Cancer. 41:61–70. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bandera EV, Freudenheim JL, Marshall JR,
Zielezny M, Priore RL, Brasure J, Baptiste M and Graham S: Diet and
alcohol consumption and lung cancer risk in the New York state
cohort (United States). Cancer Causes Control. 8:828–840. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ocké MC, Bueno-de-Mesquita HB, Feskens EJ,
van Staveren WA and Kromhout D: Repeated measurements of
vegetables, fruits, beta-carotene, and vitamins C and E in relation
to lung cancer. The Zutphen study. Am J Epidemiol. 145:358–365.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Voorrips LE, Goldbohm RA, Brants HA, van
Poppel GA, Sturmans F, Hermus RJ and van den Brandt PA: A
prospective cohort study on antioxidant and folate intake and male
lung cancer risk. Cancer Epidemiol Biomarkers Prev. 9:357–365.
2000.PubMed/NCBI
|
|
20
|
Yuan JM, Stram DO, Arakawa K, Lee HP and
Yu MC: Dietary cryptoxanthin and reduced risk of lung cancer: The
Singapore Chinese health study. Cancer Epidemiol Biomarkers Prev.
12:890–898. 2003.PubMed/NCBI
|
|
21
|
Lee DH and Jacobs DR Jr: Interaction among
heme iron, zinc, and supplemental vitamin C intake on the risk of
lung cancer: Iowa Women's health study. Nutr Cancer. 52:130–137.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin J, Cook NR, Albert C, Zaharris E,
Gaziano JM, Van Denburgh M, Buring JE and Manson JE: Vitamins C and
E and beta carotene supplementation and cancer risk: A randomized
controlled trial. J Natl Cancer Inst. 101:14–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shibata A, Paganini-Hill A, Ross RK and
Henderson BE: Intake of vegetables, fruits, beta-carotene, vitamin
C and vitamin supplements and cancer incidence among the elderly: A
prospective study. Br J Cancer. 66:673–679. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Feskanich D, Ziegler RG, Michaud DS,
Giovannucci EL, Speizer FE, Willett WC and Colditz GA: Prospective
study of fruit and vegetable consumption and risk of lung cancer
among men and women. J Natl Cancer Inst. 92:1812–1823. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Slatore CG, Littman AJ, Au DH, Satia JA
and White E: Long-term use of supplemental multivitamins, vitamin
C, vitamin E, and folate does not reduce the risk of lung cancer.
Am J Respir Crit Care Med. 177:524–530. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Steinmetz KA, Potter JD and Folsom AR:
Vegetables, fruit, and lung cancer in the Iowa Women's Health
Study. Cancer Res. 53:536–543. 1993.PubMed/NCBI
|
|
27
|
Speizer FE, Colditz GA, Hunter DJ, Rosner
B and Hennekens C: Prospective study of smoking, antioxidant
intake, and lung cancer in middle-aged women (USA). Cancer Causes
Control. 10:4754–4782. 1999. View Article : Google Scholar
|
|
28
|
Roswall N, Olsen A, Christensen J,
Dragsted LO, Overvad K and Tjønneland A: Source-specific effects of
micronutrients in lung cancer prevention. Lung Cancer. 67:275–281.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Narita S, Saito E, Sawada N, Shimazu T,
Yamaji T, Iwasaki M, Ishihara J, Takachi R, Shibuya K, Inoue M, et
al: Dietary consumption of antioxidant vitamins and subsequent lung
cancer risk: The Japan public health center-based prospective
study. Int J Cancer. 142:2441–2460. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fu Y, Xu F, Jiang L, Miao Z, Liang X, Yang
J, Larsson SC and Zheng JS: Circulating vitamin C concentration and
risk of cancers: A Mendelian randomization study. BMC Med.
19:1712021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wells GA, Shea B, O'Connell D, Peterson J,
Welch V, Losos M and Tugwell P: The newcastle-ottawa scale (NOS)
for assessing the quality of nonrandomised studies in
meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm
|
|
32
|
Borenstein M, Hedges LV, Higgins JP and
Rothstein HR: A basic introduction to fixed-effect and
random-effects models for meta-analysis. Res Synth Methods.
1:97–111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Simmonds M: Quantifying the risk of error
when interpreting funnel plots. Syst Rev. 4:242015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vissers MCM and Das AB: Potential
mechanisms of action for vitamin c in cancer: Reviewing the
evidence. Front Physiol. 9:8092018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Villagran M, Ferreira J, Martorell M and
Mardones L: The role of vitamin C in cancer prevention and therapy:
A literature review. Antioxidants (Basel). 10:18942021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pawlowska E, Szczepanska J and Blasiak J:
Pro- and antioxidant effects of vitamin c in cancer in
correspondence to its dietary and pharmacological concentrations.
Oxid Med Cell Longev. 24:72867372019.PubMed/NCBI
|
|
38
|
Singh R and Manna PP: Reactive oxygen
species in cancer progression and its role in therapeutics. Explor
Med. 3:43–57. 2022. View Article : Google Scholar
|
|
39
|
Brabson JP, Leesang T, Mohammad S and
Cimmino L: Epigenetic regulation of genomic stability by vitamin C.
Front Genet. 12:6757802021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Myung SK, Kim Y, Ju W, Choi HJ and Bae WK:
Effects of antioxidant supplements on cancer prevention:
Meta-analysis of randomized controlled trials. Ann Oncol.
21:166–179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Carr AC and Vissers MC: Synthetic or
food-derived vitamin C-are they equally bioavailable? Nutrients.
5:4284–4304. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mayne ST, Handelman GJ and Beecher G:
Beta-carotene and lung cancer promotion in heavy smokers-a
plausible relationship? J Natl Cancer Inst. 88:1513–1515. 1996.
View Article : Google Scholar : PubMed/NCBI
|