Introduction
Since the first approval of proton pump inhibitors
(PPIs) in 1989, they have proven to be an effective first-line
treatment for gastrointestinal disorders including symptomatic
peptic ulcer disease, gastresophageal reflux disease, and
Zollinger-Ellison syndrome, as well as for the prevention of
gastrointestinal bleeding in patients receiving antiplatelet
therapy (1–7). PPIs are also one of the standard
treatments for Helicobacter pylori infection, along with
antibiotics (1). Their popularity
has steadily grown, and they are now one of the most prescribed
drug classes worldwide, both in the outpatient and inpatient
clinical settings (8). In the
United States, the consumption of PPIs in non-hospitalized patients
doubled from 1999 to 2012 (9). In
England, over 50 million prescriptions that contained PPIs were
prescribed in 2015 (10). Due to
the effectiveness of PPIs in prevention and treatment of
gastrointestinal disorders, physicians tend to prescribe PPIs in
the long term for specific conditions such as Barrett's esophagus,
chronic use of non-steroidal anti-inflammatory drugs (NSAIDs) with
high to moderate bleeding risk, severe oesophagitis, and
Zollinger-Ellison syndrome, and patients usually take these
medications for longer than needed (11–14).
In England, amongst new users of PPIs in a cohort study from 1990
to 2014, 26.7% of them continued taking PPIs for more than one
year.15 Sixty percent of the long-term PPI users did not make an
attempt to step down or discontinue PPI therapy (15), and approximately 30% of the PPI
users were not appropriately prescribed for the long-term treatment
(16,17).
The long-term use of PPIs has raised concerns about
infection (18,19), dementia (20), osteoporosis, fracture (21), and cancer (22). Specifically, the risk of
gastrointestinal cancers has been a major concern to both patients
and physicians. Previous laboratory and animal studies have
reported that PPIs can suppress gastric acid secretion and
interfere with bacterial growth and nitrosamine formation (23,24).
Furthermore, PPIs have been linked to hypergastrinemia, which has
been identified as a possible risk factor for cancer progression
(25,26).
Meanwhile, observational epidemiological studies
have reported inconsistent findings on whether PPIs increase the
risk of gastrointestinal cancers (27–49).
Fourteen cohort studies reported a significant increased risk of
gastrointestinal cancers by the use of PPIs (29,30,32–37,40,42–45,47),
while 10 cohort studies did no association between them (27,28,31,38,39,41,46,48,49).
Several meta-analyses of retrospective cohort
studies and case-control studies have reported the associations
between the use of PPIs and a specific type of gastrointestinal
cancers such as gastric cancer, colorectal cancer, and pancreatic
cancer (50–54). However, no comprehensive
meta-analysis of cohort studies for all types of gastrointestinal
cancers including esophageal cancer, liver cancer, and biliary
cancer has been reported up to date.
Thus, the current study aimed to investigate the
association between the use of PPIs and the risk of
gastrointestinal cancers using a comprehensive meta-analysis of
cohort studies.
Materials and methods
Literature search strategy
This meta-analysis was conducted according to the
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
statement (55). A literature
search in both PubMed and Excerpta Medica dataBASE (EMBASE)
databases was conducted up to December 2022. This search used a
combination of the National Library of Medicine (NLM) Medical
Subject Headings (MeSH) terms with a wide range of free-text terms
as search terms to identify as many relevant articles as possible.
A PICO framework was used to determine search terms related with
the topic of this study as follows: P for population is ‘general
population’; I for intervention (exposure in this study) is ‘use of
PPIs’; C for comparison is ‘no use of PPIs’; and O for outcome is
‘incidence of cancer’. Study design of included studies was
restricted to cohort study for the current meta-analysis. Thus,
using Boolean operators for all the determined MeSH and free-text
terms, a combination of search terms was created as follows:
(proton pump inhibitors or omeprazole or esomeprazole or
pantoprazole or lansoprazole or dexlansoprazole or rabeprazole) and
cancer and cohort study. Data S1
shows the final search strategy for the PubMed example.
Additionally, the reference lists of the identified articles were
examined to identify relevant studies that were not detected
through the initial search strategy.
Eligibility criteria
Observational epidemiological studies were included
in the final meta-analysis based on the following criteria: i) an
original prospective or retrospective cohort study; ii)
investigated the association between the use of PPIs and any types
of gastrointestinal cancers; iii) reported outcome measures with an
adjusted relative risk (RR), odds ratio (OR) or hazard ratios (HR)
and its 95% confidence intervals (CI); iv) publication in English.
If data were reported in multiple publications from the same study,
the study presenting the most comprehensive data was included.
Studies that were not published in peer-reviewed journals or only
presented in academic conferences were excluded.
Selection of relevant studies
Two authors (Tran HT and Trinh TKT) independently
selected all studies retrieved from the databases. Discrepancies in
study selection were resolved by reaching a consensus with a senior
author (Myung SK). The extraction process encompassed the
collection of year of publication and first author's name, type of
study, country, year of the enrollment of participants, population
(number of participants, gender, and baseline age range), type of
cancer, definition of PPI exposure and a control group, adjusted
OR/RR/HR with 95% CI, and adjusted variables.
Assessment of methodological
quality
The methodological quality of the included studies
was assessed using the Newcastle-Ottawa Scale (NOS) for assessing
the quality of cohort studies in the meta-analyses (56). The NOS score system ranges from 0 to
9 representing the three subscales of the study quality dimensions:
study selection, comparability, and exposure assessment. Given the
absence of established cutoff criteria for designating a study as
high- or low-quality, studies scoring above the average were
categorized as high-quality.
Main and subgroup analyses
The main meta-analysis investigated the association
between the use of PPIs and the risk of gastrointestinal cancers.
Subsequently, subgroup meta-analyses were conducted, categorized by
type of cancer (esophageal, gastric, pancreatic, colorectal, liver,
gallbladder, or bile duct cancer), sex (male or female), age (over
50 years old), obesity (yes or no), smoking status (yes or no),
type of PPIs (omeprazole, lansoprazole, esomeprazole, pantoprazole,
or rabeprazole), duration of PPI use (within 1 year, 1–3 years, 3–5
years, or over 5 years), concurrent medications (aspirin or
statins), geographical region, study design (retrospective or
prospective cohort study), and methodological quality of study
(high or low quality).
Statistical analysis
A pooled OR/RR/HR with its 95% CI was calculated
using the adjusted OR/RR/HR and its respective 95% CI from each
study reporting the association between the use of PPIs and the
risk of gastrointestinal cancers. Additionally, an evaluation of
heterogeneity across the studies was performed using Higgins
I2, which measures the percentage of total variation
across the studies (57). The
I2 value is calculated as follows:
I2 = 100% × (Q -
df)/Q,
where Q is Cochran's heterogeneity statistic,
and df indicates the degrees of freedom. Negative values of
the I2 were set at zero; the I2 ranges from
0% (no observed heterogeneity) to 100% (maximal heterogeneity)
(57). An I2 value
greater than 50% indicates substantial heterogeneity (57).
The pooled estimate was computed using the
DerSimonian and Laird method (58).
A random-effects model was used due to the diverse geographical
contexts and varying populations in which the identified studies
were conducted.
Publication bias was assessed utilizing the Begg's
funnel plot and Egger's test (59).
Publication bias exists when the Begg's funnel plot shows asymmetry
or when the P-value of the Egger's test is less than 0.05 (59). Further, sensitivity analyses were
conducted to explore the influence of each study on the pooled
estimate by omitting a study one by one and re-analyzing. Stata SE
version 16.1 statistical software package (StataCorp, College
Station, Texas, USA) was used for all the meta-analyses.
Results
Identification of relevant
studies
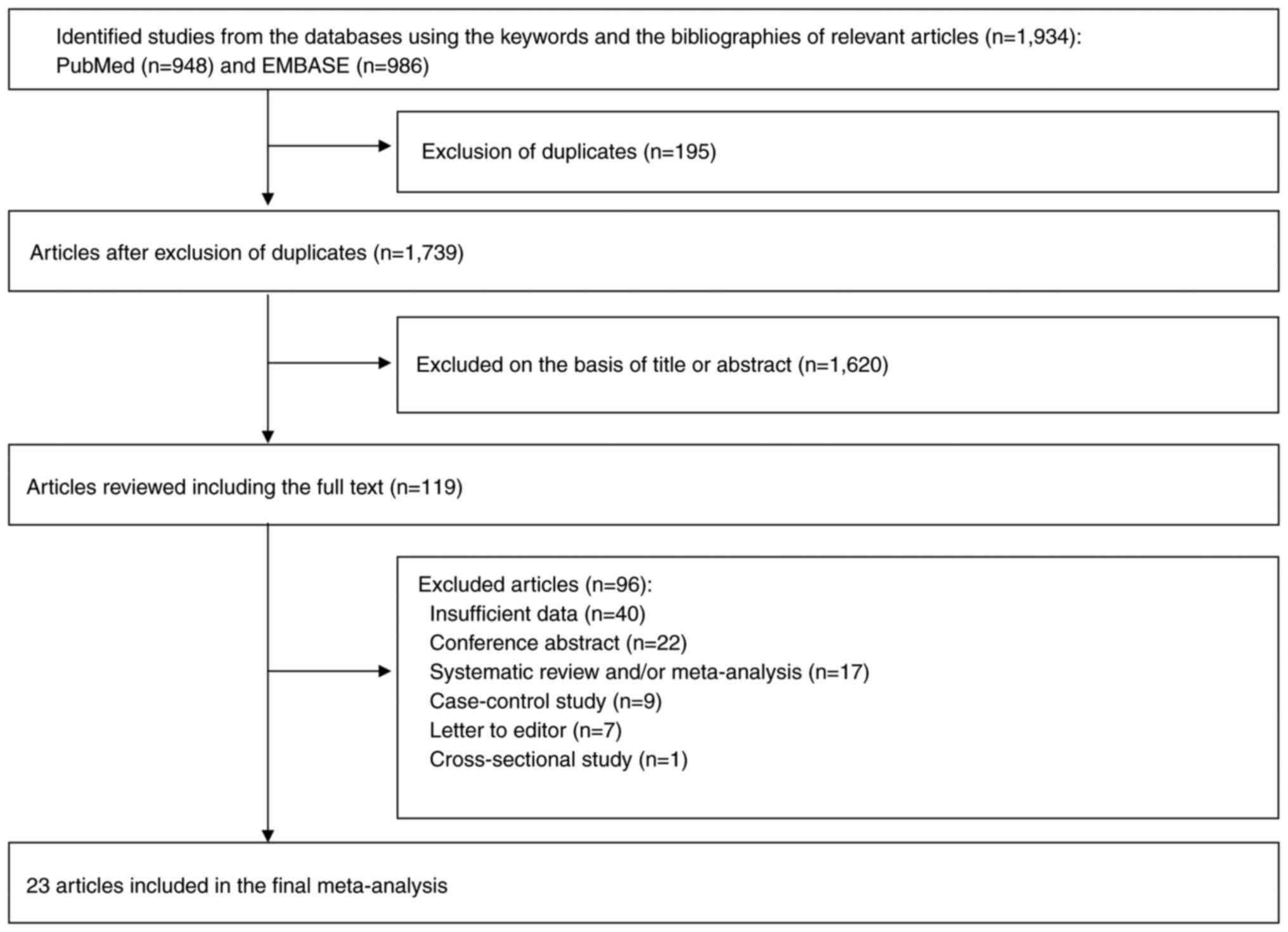
Fig. 1 shows a flow
diagram of the selection process for the current study. A total of
1,934 articles were identified by searching two electronic
databases, PubMed and EMBASE. After removing 195 duplicate
articles, an additional 1,620 articles were excluded based on the
predetermined selection criteria. A thorough review was conducted
on the remaining 119 articles. Among these, 96 articles were
excluded for the following reasons: insufficient data (n=40);
conference abstract (n=22); systematic review or/and meta-analysis
(n=17); case-control studies (n=9); letters to editor (n=7); and
cross-sectional studies (n=1). The remaining 23 articles (27–49)
were included in the final analysis. The result of the assessment
with Cohen's kappa in the selecting studies was 0.97, suggesting an
almost perfect agreement between the two authors.
Characteristics of studies included in
the final meta-analysis
This meta-analysis included 25 cohort studies from
23 articles that had a total of 10,309,227 participants. Table I shows the general characteristics
of the studies included in the final meta-analysis. Types of
cancers were as follows: esophageal, gastric, pancreatic, liver,
colorectal, gallbladder, and bile duct cancer. Of the 23 articles,
13 articles are prospective cohort studies, and 10 articles are
retrospective cohort studies. Publication dates ranged from 2009 to
2022. Eleven studies were conducted in Europe, nine studies in
Asia, and three studies in North America.
 | Table I.Characteristics of the studies
included in the final meta-analysis (n=23). |
Table I.
Characteristics of the studies
included in the final meta-analysis (n=23).
| First author/s,
year | Type of study | Country | Study period | Population (sex and
age) | Type of cancer | Definition of PPI
exposure | OR/RR/HR (95%
CI) | Adjusted
variables | (Refs.) |
|---|
| Nguyen et
al, 2009 | Retrospective
cohort study | USA | 1982-2005 | 344 individuals
(men and women; mean age, 61 years) | Esophageal
cancer | Received a
dispensed prescription for PPIs vs. non-users | 0.40
(0.16–0.97) | Sex, age, Barrett's
esophagus length and NSAIDs/COX-2/aspirin | (27) |
| Poulsen et
al, 2009 | Retrospective
cohort study | Denmark | 1990-2003 | 18,790
individuals | Gastric cancer | Patients who
received ≥2 PPI prescriptions during the study period vs.
non-users | 1.20
(0.80–2.00) | Calendar period,
sex, age, history of H. pylori eradication therapy,
gastroscopy, COPD, alcohol-related admission or therapy and ever
using NSAIDs | (28) |
| Boursi et
al, 2017 | Retrospective
cohort study | UK | 1995-2013 | 19,146
individuals | Pancreatic
cancer | PPI users vs.
non-users | 1.89
(1.52–2.36) | Age, smoking,
insulin, oral hypoglycemics, metformin, HbA1C, Hb, total
cholesterol, creatinine and alkaline phosphatase | (29) |
| Brusselaers et
al, 2017 | Prospective cohort
study | Sweden | 2005-2012 | 797,067
individuals | Gastric cancer | PPI users vs.
non-users | 3.38
(3.25–3.53) | Age, sex, calendar
and categories | (30) |
| Hwang et al,
2017 | Prospective cohort
study | South Korea | 2002-2013 | 451,284 individuals
(men and women aged ≥40 years) | Colorectal
cancer | Patients who
consumed >60 DDDs of PPIs vs. non-users | 0.98
(0.78–1.24) | Age, male sex,
obesity, current smoking, frequent drinking, low physical activity,
comorbid conditions (including type 2 diabetes), concurrent drug
use, (aspirin, metformin, statin) and low socioeconomic status | (31) |
| Wennerström et
al, 2017 | Retrospective
cohort study | Denmark | 1995-2011 | 1,563,860
individuals | Gastric cancer | PPI users vs.
non-users | 2.51
(2.26–2.79) | Age, sex and
municipality | (32) |
| Cheung et
al, 2018 | Retrospective
cohort study | Hong Kong | 2003-2012 | 63,397 individuals
(≥18-year-old male and female patients) | Gastric cancer | PPI users vs.
non-users | 2.44
(1.42–4.20) | Age of receiving
H. pylori eradication therapy, sex, smoking, alcohol use,
comorbidities and concomitant medications | (33) |
| Hwang et al,
2018 | Prospective cohort
study | South Korea | 2002-2013 | 453,655 individuals
(men and women aged ≥40 years) | Pancreatic
cancer | Patients who
consumed >60 DDDs of PPIs vs. non-users | 1.32
(1.03–1.70) | Age, male sex,
obesity, current smoking, frequent drinking, low physical activity,
comorbid conditions (type 2 diabetes, chronic pancreatitis),
Charlson Comorbidity Index score and low socioeconomic status | (34) |
| Li et al,
2018 | Prospective cohort
study | USA | 2001-2015 | 11,526 individuals
(men and women; median age, 53 years) | Liver cancer | PPI users vs.
non-users | 2.01
(1.50–2.70) | Age, sex,
ethnicity, smoking history, alcohol abuse history, body mass index,
diabetes, baseline FIB-4 score, gastroesophageal reflux disease,
HCV genotype, past completed anti-HCV treatment and attainment of
SVR | (35) |
| Tran et al,
2018 | Prospective cohort
study | UK | 1991-2004 | 475,768
individuals | Liver cancer | PPI users vs.
non-users | 1.99
(1.34–2.94) | Age, sex,
deprivation, BMI, alcohol, smoking, comorbidities (including GORD,
peptic ulcer disease, cirrhosis, hepatitis and diabetes) and other
medication use (statins, aspirin) | (36) |
| Brusselaers et
al, 2019 | Prospective cohort
study | Sweden | 2005-2012 | 796,492 individuals
(≥18-year-old male and female patients) | Gastric cancer;
esophageal cancer | ≥180 days of
accumulated use of PPIs vs. non-users | 2.97 (2.83–3.10);
3.93 (3.63–4.24) | Age and calendar
categories | (37) |
| Kao et al,
2019 | Retrospective
cohort study | Taiwan | 2003-2013 | 14,984
individuals | Liver cancer | PPI users vs.
non-users | HBV cohort, 1.25
(0.90–1.73) HCV cohort, 1.19 (0.88–1.61) | Age, sex, year of
cohort entry, comorbidities (cirrhosis, nonalcoholic liver disease,
alcoholic liver disease, hypertension, chronic kidney disease,
hyperlipidemia, diabetes) and concomitant medication
(interferon/nucleos(t)ides, nonaspirin NSAIDs, histamine 2 receptor
antagonist, aspirin, statin, fibrate, insulin, metformin) | (38) |
| Babic et al,
2020 | Prospective cohort
study | USA | 1988-2015 | 175,871 individuals
(female nurses aged 25–55 years; male health professionals aged
40–75 years) | Colorectal
cancer | PPI users vs.
non-users | 1.12
(0.78–1.59) | Age, BMI, physical
activity, family history of colorectal cancer, alcohol intake,
pack-years of smoking, history of lower endoscopy, caloric intake,
vitamin D, calcium intake, regular aspirin use, folate intake,
menopausal hormone therapy use and red meat as main dish | (39) |
| Brusselaers et
al, 2020 | Prospective cohort
study | Sweden | 2005-2012 | 796,492 individuals
(≥18-year-old male and female patients) | Pancreatic
cancer | PPI users vs.
non-users | 2.22
(2.12–2.32) | Age, sex and
calendar period | (40) |
| Liu et al,
2020 | Prospective cohort
study | UK | 2006-2014 | 471,779
individuals | Gastric cancer | PPI users vs.
non-users | 1.28
(0.86–1.90) | Age, sex,
socioeconomic status, alcohol, smoking, BMI, comorbidities
(diabetes, GORD, oesophagitis and peptic ulcer) and other
medication uses (statins and aspirin) | (41) |
| Kamal et al,
2021 | Prospective cohort
study | Sweden | 2005-2012 | 738,881
individuals | Gallbladder cancer;
extrahepatic bile ducts cancer; intrahepatic bile ducts cancer | PPI users vs.
non-users | 1.58 (1.37–1.81);
1.77 (1.56–2.00); 1.88 (1.57–2.23) | Sex, age group and
calendar period | (42) |
| Lei et al,
2021 | Retrospective
cohort study | Taiwan | 1999-2011 | 90,764 individuals
(men and women) non-users | Colorectal
cancer | Patients who used
PPIs ≥30 days vs. CVD, CAD, COPD, | 2.03
(1.56–2.63) | Age, sex,
comorbidities (hypertension, diabetes, dyslipidemia, liver
cirrhosis), and baseline medication (aspirin, NSAIDs, statin and
metformin) | (43) |
| Ng et al,
2021 | Retrospective
cohort study | Hong Kong | 2004-2017 | 13,476 individuals
(men and women) | Gastric cancer | Patients who used
PPIs for ≥30 days vs. non-users (exposed to PPIs <14 days) | 2.38
(1.20–4.76) | Age, sex,
comorbidities and baseline medication | (44) |
| Seo et al,
2021 | Retrospective
cohort study | South Korea | 2002-2013 | 23,482 individuals
(men and women; age ≥19 years) | Gastric cancer | Patients who used
PPIs for ≥30 consecutive days vs. non-users | General population
cohort, 2.44 (1.17–5.16) Post H. pylori eradication cohort,
2.22 (1.05–4.67) | Age, sex, smoking,
alcohol, comorbidities and baseline medication | (45) |
| Shin et al,
2021 | Retrospective
cohort study | South Korea | 2004-2015 | 39,799 individuals
(men and women; age ≥40 years) | Gastric cancer | PPI users vs. H2RA
users | 1.01
(0.88–1.16) | Age, sex, calendar
period of prescription, time from medication start to 180 cDDD-days
(months), socioeconomic characteristics (income, smoking and
alcohol use), indication for drug use (gastresophageal reflux
disease or peptic ulcer), Charlson Comorbidity Index,
Helicobacter pylori eradication and use of other medications
(aspirin, metformin and statin) | (46) |
| Abrahami et
al, 2022 | Prospective cohort
study | UK | 1990-2018 | 973,281 individuals
(men and women; mean age, 60.4 years) | Gastric cancer | PPI users vs. H2RA
users | 1.45
(1.06–1.98) | Age, sex,
alcohol-related disorders, smoking status, BMI, comorbidities and
baseline medication | (47) |
| Abrahami et
al, 2022 | Prospective cohort
study | UK | 1990-2018 | 1,293,749
individuals (men and women; mean age, 52.6 years) | Colorectal
cancer | PPI users vs. H2RA
users | 1.02
(0.92–1.14) | Age, sex,
alcohol-related disorders smoking status, BMI, comorbidities,
baseline medication, mammographic screening, prostate-specific
antigen testing, colorectal cancer screening and influenza
vaccination | (48) |
| Gong et al,
2022 | Prospective cohort
study | South Korea | 2002-2013 | 1,025,340
individuals (men and women; age ≥20 years) | Gastric cancer | PPI users vs. H2RA
users | 1.30
(0.75–2.27) | Age, sex,
residential area, household income and comorbidities | (49) |
Methodological quality of studies
The quality scores by the NOS for the individual
studies ranged from 6 to 9; the average score was 8.4. In this
meta-analysis, a study scored 9 was considered to possess high
level of quality. Thus thirteen studies were rated as high-quality
studies (Table II).
 | Table II.Methodological quality of studies
included in the final analysis based on the Newcastle-Ottawa
Scalea for assessing the quality of cohort studies
(n=23). |
Table II.
Methodological quality of studies
included in the final analysis based on the Newcastle-Ottawa
Scalea for assessing the quality of cohort studies
(n=23).
|
| Selection | Comparability | Outcome |
|
|
|---|
|
|
|
|
|
|
|
|---|
| First author/s,
year (n=23) | Representativeness
of the exposed cohort | Selection of the
non-exposed cohort | Ascertainment of
exposure | Outcome of interest
was not present at start of study | Control for
important factor or additional factor | Assessment of
outcome | Follow-up long
enough for outcomes to occur | Adequacy of
follow-up of cohorts | Total | (Refs.) |
|---|
| Nguyen et
al, 2009 | 0 | 0 | 1 | 1 | 2 | 1 | 1 | 1 | 7 | (27) |
| Poulsen et
al, 2009 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (28) |
| Boursi et
al, 2017 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | (29) |
| Brusselaers et
al, 2017 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (30) |
| Hwang et al,
2017 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (31) |
| Wennerström et
al, 2017 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (32) |
| Cheung et
al, 2018 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (33) |
| Hwang et al,
2018 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (34) |
| Li et al,
2018 | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 | (35) |
| Tran et al,
2018 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (36) |
| Brusselaers et
al, 2019 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (37) |
| Kao et al,
2019 | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 | (38) |
| Babic et al,
2020 | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 | (39) |
| Brusselaers et
al, 2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (40) |
| Liu et al,
2020 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (41) |
| Kamal et al,
2021 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (42) |
| Lei et al,
2021 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (43) |
| Ng et al,
2021 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (44) |
| Seo et al,
2021 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (45) |
| Shin et al,
2021 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (46) |
| Abrahami et
al, 2022 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (47) |
| Abrahami et
al, 2022 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (48) |
| Gong et al,
2022 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (49) |
Use of PPIs and risk of
gastrointestinal cancers
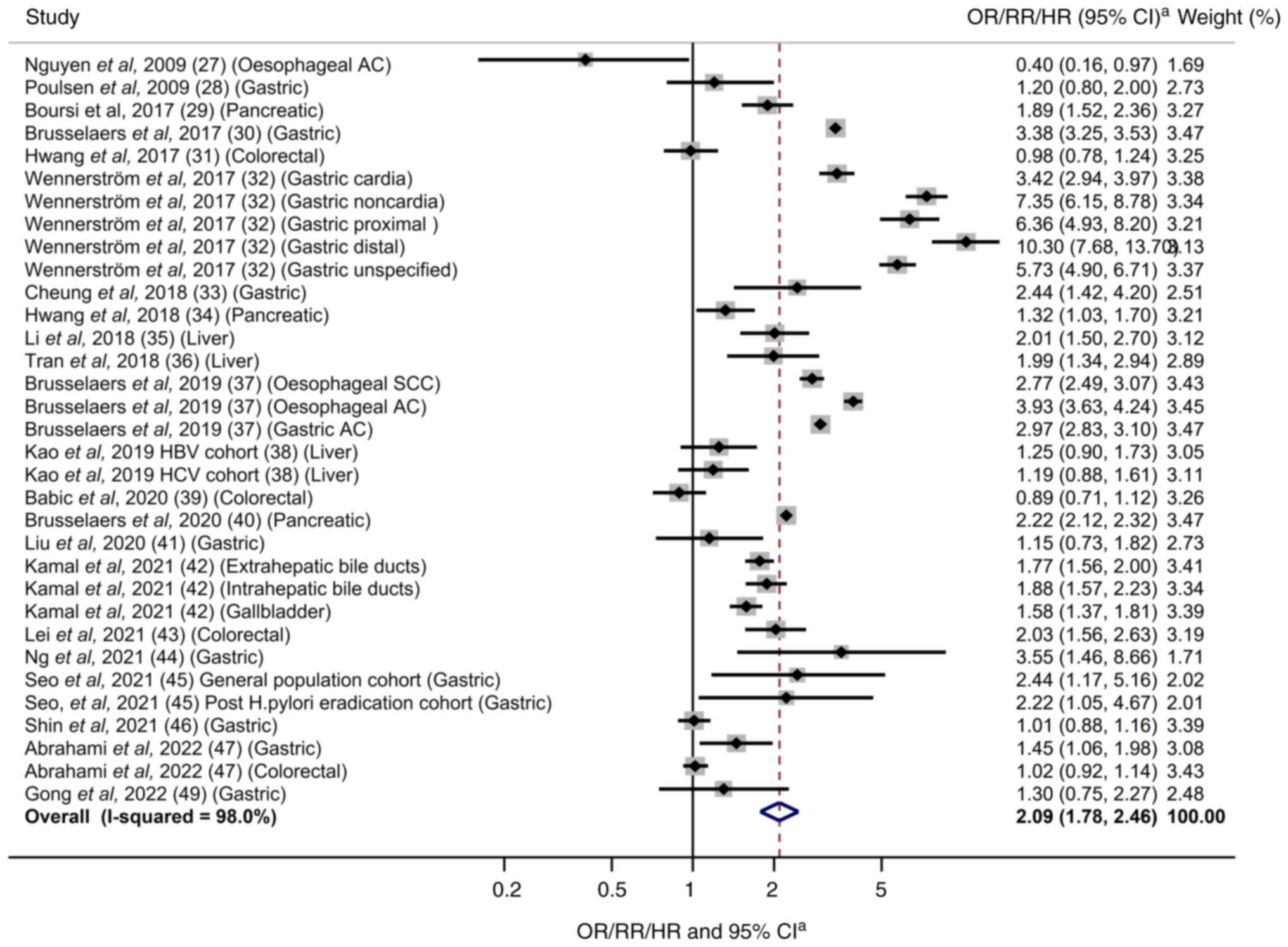
As shown in Fig. 2,
PPI use was significantly associated with a significantly increased
risk of gastrointestinal cancer (OR/RR/HR=2.09; 95% CI 1.78–2.46).
In the subgroup meta-analyses by type of cancer, the use of PPIs
was associated with a significantly increased risk of esophageal
cancer (OR/RR/HR=2.44; 95% CI 1.61–3.70; n=2), gastric cancer
(OR/RR/HR=2.88; 95% CI 2.29–3.61; n=11), pancreatic cancer
(OR/RR/HR=1.80; 95% CI 1.34–2.42; n=3), and liver cancer
(OR/RR/HR=1.55; 95% CI 1.17–2.06; n=3), while no association was
found in the risk of colorectal cancer (OR/RR/HR=1.15; 95% CI
0.85–1.54; n=4) (Table III).
 | Table III.Association between PPIs and risk of
gastrointestinal cancer in subgroup meta-analyses using a
random-effects model. |
Table III.
Association between PPIs and risk of
gastrointestinal cancer in subgroup meta-analyses using a
random-effects model.
| Factors | No. of studies | Summary OR/RR/HR
(95% CI) | Heterogeneity,
I2 (%) | (Refs.) |
|---|
| All studies | 23 | 2.09
(1.78–2.46) | 98.0 | (27–49) |
| Type of cancer |
|
|
|
|
|
Esophageal cancer | 2 | 2.44
(1.61–3.70) | 49.6 | (27,37) |
| Gastric
cancer | 11 | 2.88
(2.29–3.61) | 97.4 | (28,30,32,33,37,41,44–47,49) |
|
Pancreatic cancer | 3 | 1.80
(1.34–2.42) | 88.6 | (34,40) |
|
Colorectal cancer | 4 | 1.15
(0.85–1.54) | 88.9 | (31,39,43,48) |
| Liver
cancer | 3 | 1.55
(1.17–2.06) | 67.2 | (35,36,38) |
|
Gallbladder cancer | 1 | 1.58
(1.37–1.81) | N/A | (42) |
|
Extrahepatic bile ducts
cancer | 1 | 1.17
(1.56–2.00) | N/A | (42) |
|
Intrahepatic bile ducts
cancer | 1 | 1.88
(1.57–2.33) | N/A | (42) |
| Sex |
|
|
|
|
|
Male | 11 | 1.70
(1.36–2.12) | 98.1 | (30,31,34,35,38,40,41,42,46–48) |
|
Female | 10 | 1.84
(1.55–2.19) | 96.1 | (30,31,34,38,40,41,42,46–48) |
| Age (≥50
years) | 7 | 1.76
(1.41–2.20) | 97.7 | (30,31,34,38,40,42,46) |
| Obesity | 3 | 1.14
(1.01–1.27) | 0 | (31,34,35) |
| Smoking | 4 | 1.11
(0.98–1.27) | 65.8 | (31,34,35,47) |
| Type of PPIs |
|
|
|
|
|
Omeprazole | 4 | 1.32
(0.96–1.80) | 69.4 | (41,43,47,48) |
|
Lansoprazole | 4 | 1.42
(0.99–2.06) | 81.1 | (41,43,47,48) |
|
Pantoprazole | 3 | 1.08
(0.91–1.28) | 0 | (43,47,48) |
|
Esomeprazole | 3 | 1.17
(0.70–1.96) | 72.6 | (43,47,48) |
|
Rabeprazole | 3 | 1.09
(0.77–1.56) | 51.6 | (43,47,48) |
| Duration of PPI
use |
|
|
|
|
| ≤1
year | 4 | 5.23
(2.96–9.24) | 99.6 | (30,37,40,42) |
| 1-3
years | 10 | 1.72
(1.44–2.07) | 86.8 | (30,33,37,40,42,43–45,47,48) |
| 3-5
years | 6 | 1.17
(0.96–1.43) | 79.5 | (27,30,33,37,40,43) |
| >5
years | 4 | 1.16
(0.74–1.84) | 96.6 | (30,37,40,42) |
| Concurrent
medication |
|
|
|
|
|
Aspirin | 3 | 1.09
(1.01–1.18) | 0 | (31,38,47) |
|
Statins | 4 | 0.85
(0.69–1.06) | 56.5 | (31,35,38,47) |
| Region |
|
|
|
|
|
America | 3 | 1.09
(0.55–2.17) | 86.3 | (27,35,39) |
|
Asia | 9 | 1.45
(1.17–1.80) | 79.8 | (31,33,34,38,43–46,49) |
|
Europe | 11 | 1.93
(1.51–2.45) | 98.3 | (28–30,32,36,37,40–42,47,48) |
| Study design |
|
|
|
|
|
Retrospective cohort | 10 | 2.60
(1.88–3.60) | 97.6 | (27,28,30,32,33,38,43–46) |
|
Prospective cohort | 13 | 1.71
(1.41–2.08) | 97.9 | (29,31,34–37,39–42,47–49) |
| Methodological
quality |
|
|
|
|
| High
quality | 13 | 1.43
(1.20–1.70) | 78.2 | (28,31,33,34,36,41,43–49) |
| Low
quality | 10 | 2.61
(2.20–3.09) | 98.0 | (27,29,30,32,35,37–40,42) |
Use of PPIs and risk of
gastrointestinal cancers by various factors
Table III shows
findings from the subgroup meta-analyses stratified by baseline
characteristics (sex, age over 50 years old, obesity, and smoking
status), type of PPIs, duration of PPI use, concurrent medications,
geographical region of studies, study design, and methodological
quality of study. In the subgroup meta-analyses by duration of PPI
use, a significantly increased risk was observed in people using
PPIs within 1 year and from 1 to 3 years. No significant
association was found in the subgroup meta-analyses by type of
PPIs.
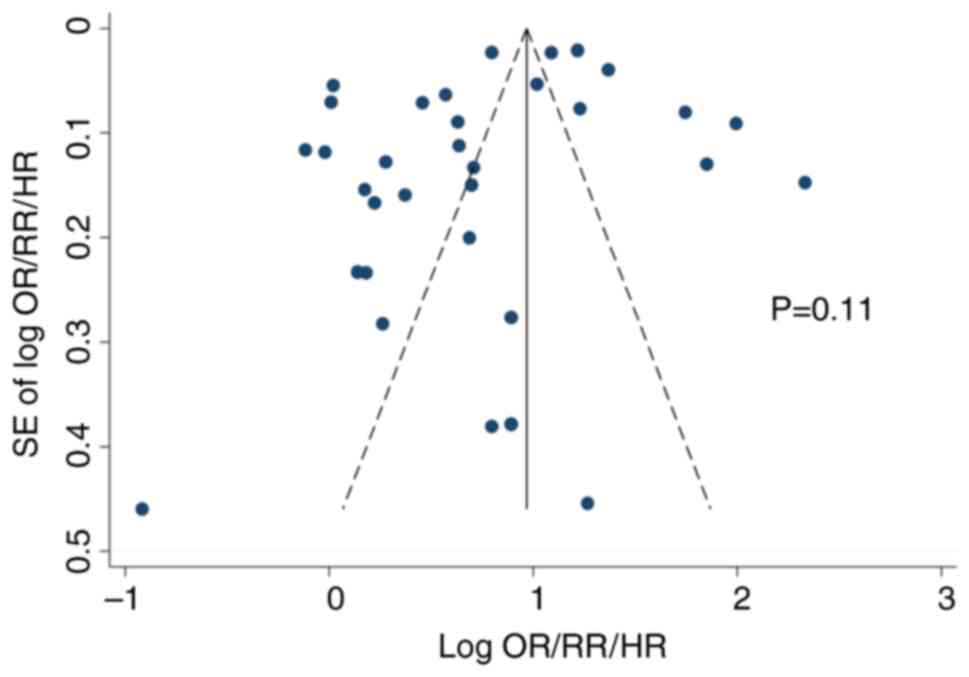
Heterogeneity, publication bias, and
sensitivity analysis
Statistical heterogeneity was observed
(I2=85.7%) in the meta-analysis of all the studies.
Publication bias was not observed in both the Begg's funnel plot
(Fig. 3) and Egger's test
(P=0.105). Sensitivity analysis to discern the influence of each
study did not show any substantial change in the pooled estimate of
the effect size and statistical significance (data not shown in
figure).
Discussion
In this meta-analysis of cohort studies, a
significant association was observed between the use of PPIs and an
increased risk of gastrointestinal cancers. In the subgroup
meta-analysis by type of gastrointestinal cancers, the use of PPIs
was significantly associated with the increased risk of gastric
cancer, liver cancer, pancreatic cancer, and esophageal cancer,
whereas there was no association for colorectal cancer. The
increased risk of gastrointestinal cancers was also observed in
people who had used PPIs within 1 year as well as up to 3
years.
Possible biological mechanisms for the increased
risk of gastrointestinal cancers by the use of PPIs can be
explained by previous in vitro and in vivo studies
(60–62). First, PPIs reduce gastric acid
secretion by blocking the H+/K+ ATPase of parietal cells (63), which can induce an increase of
gastrin secretion from G-cells (64). Gastrin has long been suspected to be
a potential risk factor of gastric cancer by causing
hypergastrinemia (25).
Hypergastrinemia also could lead to the development of
gastrointestinal cancers, including esophagus, stomach, pancreatic,
and liver cancers (25). Second,
PPIs might contribute to increased bacterial colonisation and a
larger number of bacteria that are able to produce nitrosamines
(23,24). Nitrosamines and gut microbiome
alterations could lead to an increased risk of gastrointestinal
cancers (23,65,66).
Third, PPIs might increase the production of enterochromaffin-like
cells (ECL cells) by inducing hypoacidity (67). ECL cells are the key target cells of
gastrin in the oxyntic mucosa and are associated with the
expression of cholecystokinin-2 (CCK-2) receptors, which might
consequently lead to the formation of neuroendocrine tumors (NETs),
such as pancreatic cancer (67).
Some preclinical studies have reported a preventive
effect of PPIs against the development of colorectal cancer
(68,69). Pantoprazole was identified to be a
potential T-cell-originated protein kinase (TOPK) inhibitors and
blocked the anchorage-independent proliferation of colorectal
cancer cells with high TOPK levels in an in vitro cancer cell line
study and an in vivo mouse study (68). Also, a rat azoxymethane (AOM) model
study showed that omeprazole suppressed the proliferation and
carcinogenesis of colon cancer cell lines (69). On the other hand, a transgenic APC
genes (APCMin-/+) mouse model study reported that
omeprazole-induced hypergastrinemia lead to a significant increase
in the proliferation of colorectal adenomas. Thus, there are some
inconsistencies regarding the effect of PPI use on the colorectal
cancer risk.
The findings of this meta-analysis are in line with
previous meta-analyses that investigated the association between
the use of PPIs and the risk of gastric cancer (50,51,70).
Jiang et al (71) found that
long-term use of PPIs may possibly increase the risk of gastric
cancer (OR 2.50; 95% CI: 1.74–3.85). Nevertheless, they were unable
to assess publication bias due to the small number of studies, and
all studies were retrospective in design. Tran-Duy et al
(51) assessed the effects of PPI
therapy on the risk of gastric cancer by including a cohort and 3
case-control studies. They concluded that PPI therapy was
positively associated with an increased risk of gastric cancer
(51). Nonetheless, there were too
few studies included in their analysis to confirm the association.
Segna et al (50) conducted
a meta-analysis including 5 retrospective cohort and 8 case-control
studies. They found that PPI use had a 1.94-fold higher risk of
gastric cancer compared with the non-PPI group (50). They also included retrospective
studies only.
Regarding the risk of pancreatic cancer, the finding
of this meta-analysis is consistent with two previous
meta-analyses, which also included only a few studies. Laoveeravat
et al (53) and Alkhushaym
et al (54) included one and
two cohort studies, respectively. They also found that PPIs use
could significantly increase the risk of pancreatic cancer.
Regarding the risk of colorectal cancer, the finding of this
meta-analysis is consistent with that of Ma et al's
(52) meta-analysis of 3 cohort
studies, which reported that there was no statistically significant
association between PPI use and the risk of colorectal cancer.
To the best of current knowledge, this is the most
comprehensive meta-analysis of cohort studies on this topic.
Although a recent meta-analysis of observational epidemiological
studies regarding this topic was published in 2021 (50), it included only case-control and
retrospective cohort studies and revealed no clear
duration-dependent risk increase among PPI users. This
meta-analysis included a total of 23 cohort studies with 13
prospective cohort studies as well as 10 retrospective studies and
reported the evidence of the gastrointestinal cancers risk with
long-term use of PPIs. In addition, this meta-analysis provided
information regarding the types of PPI and the risk of
gastrointestinal cancers. This meta-analysis also assessed the risk
of gastrointestinal cancers by providing various subgroup analyses
that might help to minimize potential confounding factors.
This study has several limitations. This
meta-analysis only included cohort studies. In terms of
evidence-based medicine, it is important to emphasize that while
randomised controlled trials (RCTs) offer a higher level of
evidence compared to cohort studies, they pose ethical and
practical challenges when investigating the association between PPI
use and cancer risk. Nevertheless, it would be possible to conduct
a meta-analysis of RCTs using secondary outcomes from the original
trials. Second, the stratification of gastrointestinal cancer risk
based on PPI dosage was hindered by the limited availability of
relevant data from individual studies. Lastly, it was not feasible
to confirm the effect of PPI use on the risk of gallbladder and
bile duct cancers because only one study for each type of cancer
was included in the current study. Further studies are
warranted.
This meta-analysis of cohort studies suggested a
significant association between the use of PPIs and the increased
risk of gastrointestinal cancers. This finding was observed in
people using PPIs for less than 1 year as well as up to 3 years.
These findings should be confirmed by RCTs that provide a higher
level of evidence than cohort studies.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
requests.
Authors' contributions
SKM and THT conceptualized the present study,
conducted the investigation, and were involved in data curation.
THT and TTKT analyzed and interpreted data. THT wrote the original
draft. SKM, THT, and TTKT wrote, reviewed and edited the
manuscript. THT and SKM confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Agrawal NM, Campbell DR, Safdi MA, Lukasik
NL, Huang B and Haber MM: Superiority of lansoprazole vs ranitidine
in healing nonsteroidal anti-inflammatory drug-associated gastric
ulcers: Results of a double-blind, randomized, multicenter study.
NSAID-associated gastric Ulcer study group. Arch Intern Med.
160:1455–1461. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chiba N, De Gara CJ, Wilkinson JM and Hunt
RH: Speed of healing and symptom relief in grade II to IV
gastresophageal reflux disease: A meta-analysis. Gastroenterology.
112:1798–1810. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dekkers CP, Beker JA, Thjodleifsson B,
Gabryelewicz A, Bell NE and Humphries TJ: Comparison of rabeprazole
20 mg versus omeprazole 20 mg in the treatment of active duodenal
ulcer: A European multicentre study. Aliment Pharmacol Ther.
13:179–186. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Farley A, Wruble LD and Humphries TJ:
Rabeprazole versus ranitidine for the treatment of erosive
gastresophageal reflux disease: A double-blind, randomized clinical
trial. Raberprazole study group. Am J Gastroenterol. 95:1894–1899.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang JQ and Hunt RH: pH, healing rate and
symptom relief in acid-related diseases. Yale J Biol Med.
69:159–174. 1996.PubMed/NCBI
|
|
6
|
Lew EA, Pisegna JR, Starr JA, Soffer EF,
Forsmark C, Modlin IM, Walsh JH, Beg M, Bochenek W and Metz DC:
Intravenous pantoprazole rapidly controls gastric acid
hypersecretion in patients with Zollinger-Ellison syndrome.
Gastroenterology. 118:696–704. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Norton JA, Fraker DL, Alexander HR, Venzon
DJ, Doppman JL, Serrano J, Goebel SU, Peghini PL, Roy PK, Gibril F
and Jensen RT: Surgery to cure the Zollinger-Ellison syndrome. N
Engl J Med. 34:635–644. 1999. View Article : Google Scholar
|
|
8
|
Farrell B, Lass E, Moayyedi P, Ward D and
Thompson W: Reduce unnecessary use of proton pump inhibitors. BMJ.
379:e0692112022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Forgacs I and Loganayagam A:
Overprescribing proton pump inhibitors. BMJ. 336:2–3. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Connelly D: The development and safety of
proton pump inhibitors. The Pharm J. 296:78902016.
|
|
11
|
Lanza FL, Chan FK and Quigley EM:
Guidelines for prevention of NSAID-related ulcer complications. Am
J Gastroenterol. 104:728–738. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Targownik LE, Fisher DA and Saini SD: AGA
clinical practice update on de-prescribing of proton pump
inhibitors: Expert review. Gastroenterol. 162:1334–1342. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Malfertheiner P, Chan FK and McColl KE:
Peptic ulcer disease. Lancet. 374:1449–1461. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jankowski JAZ, de Caestecker J, Love SB,
Reilly G, Watson P, Sanders S, Ang Y, Morris D, Bhandari P, Brooks
C, et al: Esomeprazole and aspirin in Barrett's esophagus (AspECT):
A randomised factorial trial. Lancet. 392:400–408. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Othman F, Card TR and Crooks CJ: Proton
pump inhibitor prescribing patterns in the UK: A primary care
database study. Pharmacoepidemiol Drug Saf. 25:1079–1087. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Blackett JW, Faye AS, Phipps M, Li J,
Lebwohl B and Freedberg DE: Prevalence and risk factors for
inappropriate continuation of proton pump inhibitors after
discharge from the intensive care unit. Mayo Clin Proc.
96:2550–2560. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nguyen PV and Tamaz R: Inappropriate
prescription of proton pump inhibitors in a community setting. Can
J Hosp Pharm. 71:267–271. 2018.PubMed/NCBI
|
|
18
|
Cao F, Chen CX, Wang M, Liao HR, Wang MX,
Hua SZ, Huang B, Xiong Y, Zhang JY and Xu YL: Updated meta-analysis
of controlled observational studies: Proton-pump inhibitors and
risk of Clostridium difficile infection. J Hosp Infect. 98:4–13.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang CH, Li CH, Hsieh R, Fan CY, Hsu TC,
Chang WC, Hsu WT, Lin YY and Lee CC: Proton pump inhibitors therapy
and the risk of pneumonia: A systematic review and meta-analysis of
randomized controlled trials and observational studies. Expert Opin
Drug Saf. 18:163–172. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li M, Luo Z, Yu S and Tang Z: Proton pump
inhibitor use and risk of dementia: Systematic review and
meta-analysis. Medicine (Baltimore). 98:e144222019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu J, Li X, Fan L, Yang J, Wang J, Sun J
and Wang Z: Proton pump inhibitors therapy and risk of bone
diseases: An update meta-analysis. Life Sci. 218:213–223. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang ML, Fan YX, Meng R, Cai WK, Yin SJ,
Zhou T, Huang YH, Wang P, Jiang FF, Yang M and He GH: Proton pump
inhibitors and cancer risk: An umbrella review and meta-analysis of
observational studies. Am J Clin Oncol. 45:475–485. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Verdu E, Viani F, Armstrong D, Fraser R,
Siegrist HH, Pignatelli B, Idström JP, Cederberg C, Blum AL and
Fried M: Effect of omeprazole on intragastric bacterial counts,
nitrates, nitrites, and N-nitroso compounds. Gut. 35:455–460. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Imhann F, Bonder MJ, Vila AV, Fu J,
Mujagic Z, Vork L, Tigchelaar EF, Jankipersadsing SA, Cenit MC,
Harmsen HJ, et al: Proton pump inhibitors affect the gut
microbiome. Gut. 65:740–748. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lundell L, Vieth M, Gibson F, Nagy P and
Kahrilas PJ: Systematic review: The effects of long-term proton
pump inhibitor use on serum gastrin levels and gastric histology.
Aliment Pharmacol Ther. 42:649–663. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Feng J, Petersen CD, Coy DH, Jiang JK,
Thomas CJ, Pollak MR and Wank SA: Calcium-sensing receptor is a
physiologic multimodal chemosensor regulating gastric G-cell growth
and gastrin secretion. Proc Natl Acad Sci USA. 107:17791–17796.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nguyen DM, El-Serag HB, Henderson L, Stein
D, Bhattacharyya A and Sampliner RE: Medication usage and the risk
of neoplasia in patients with Barrett's esophagus. Clin
Gastroenterol Hepatol. 7:1299–1304. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Poulsen AH, Christensen S, McLaughlin JK,
Thomsen RW, Sørensen HT, Olsen JH and Friis S: Proton pump
inhibitors and risk of gastric cancer: A population-based cohort
study. Br J Cancer. 100:1503–1507. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Boursi B, Finkelman B, Giantonio BJ,
Haynes K, Rustgi AK, Rhim AD, Mamtani R and Yang YX: A clinical
prediction model to assess risk for pancreatic cancer among
patients with new-onset diabetes. Gastroenterology. 152:840–850.e3.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brusselaers N, Wahlin K, Engstrand L and
Lagergren J: Maintenance therapy with proton pump inhibitors and
risk of gastric cancer: A nationwide population-based cohort study
in Sweden. BMJ Open. 7:e0177392017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hwang IC, Chang J and Park SM: Emerging
hazard effects of proton pump inhibitor on the risk of colorectal
cancer in low-risk populations: A Korean nationwide prospective
cohort study. PLoS One. 12:e01891142017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wennerström ECM, Simonsen J, Camargo MC
and Rabkin CS: Acid-suppressing therapies and subsite-specific risk
of stomach cancer. Br J Cancer. 116:1234–1238. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cheung KS, Chan EW, Wong AYS, Chen L, Wong
ICK and Leung WK: Long-term proton pump inhibitors and risk of
gastric cancer development after treatment for Helicobacter pylori:
A population-based study. Gut. 67:28–35. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hwang IC, Chang J and Park SM: Association
between proton pump inhibitor use and the risk of pancreatic
cancer: A Korean nationwide cohort study. PLoS One.
13:e02039182018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li DK, Yan P, Abou-Samra AB, Chung RT and
Butt AA: Proton pump inhibitors are associated with accelerated
development of cirrhosis, hepatic decompensation and hepatocellular
carcinoma in noncirrhotic patients with chronic hepatitis C
infection: Results from ERCHIVES. Aliment Pharmacol Ther.
47:246–258. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tran KT, McMenamin ÚC, Hicks B, Murchie P,
Thrift AP, Coleman HG, Iversen L, Johnston BT, Lee AJ and Cardwell
CR: Proton pump inhibitor and histamine-2 receptor antagonist use
and risk of liver cancer in two population-based studies. Aliment
Pharmacol Ther. 48:55–64. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brusselaers N, Lagergren J and Engstrand
L: Duration of use of proton pump inhibitors and the risk of
gastric and esophageal cancer. Cancer Epidemiol. 62:1015852019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kao WY, Su CW, Chia-Hui Tan E, Lee PC,
Chen PH, Tang JH, Huang YH, Huo TI, Chang CC, Hou MC, et al: Proton
pump inhibitors and risk of hepatocellular carcinoma in patients
with chronic hepatitis B or C. Hepatology. 69:1151–1164. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Babic A, Zhang X, Morales-Oyarvide V, Yuan
C, Khalaf N, Khalili H, Lochhead P, Chan AT, Ogino S, Wolpin BM, et
al: Acid-suppressive medications and risk of colorectal cancer:
Results from three large prospective cohort studies. Br J Cancer.
123:844–851. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Brusselaers N, Sadr-Azodi O and Engstrand
L: Long-term proton pump inhibitor usage and the association with
pancreatic cancer in Sweden. J Gastroenterol. 55:453–461. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu P, McMenamin ÚC, Johnston BT, Murchie
P, Iversen L, Lee AJ, Vissers PAJ and Cardwell CR: Use of proton
pump inhibitors and histamine-2 receptor antagonists and risk of
gastric cancer in two population-based studies. Br J Cancer.
123:307–315. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kamal H, Sadr-Azodi O, Engstrand L and
Brusselaers N: Association between proton pump inhibitor use and
biliary tract cancer risk: A Swedish population-based cohort study.
Hepatology. 74:2021–2031. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lei WY, Wang JH, Yi CH, Liu TT, Hung JS,
Wong MW, Bair MJ, Vaezi MF, Orr WC and Chen CL: Association between
use of proton pump inhibitors and colorectal cancer: A nationwide
population-based study. Clin Res Hepatol Gastroenterol.
45:1013972021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ng AK, Ng PY, Ip A, Cheung KS and Siu CW:
Association between proton pump inhibitors after percutaneous
coronary intervention and risk of gastric cancer. BMJ Open
Gastroenterol. 8:e0007192021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Seo SI, Park CH, You SC, Kim JY, Lee KJ,
Kim J, Kim Y, Yoo JJ, Seo WW, Lee HS and Shin WG: Association
between proton pump inhibitor use and gastric cancer: A
population-based cohort study using two different types of
nationwide databases in Korea. Gut. 70:2066–2075. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shin GY, Park JM, Hong J, Cho YK, Yim HW
and Choi MG: Use of proton pump inhibitors vs histamine 2 receptor
antagonists for the risk of gastric cancer: Population-based cohort
study. Am J Gastroenterol. 116:1211–1219. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Abrahami D, McDonald EG, Schnitzer ME,
Barkun AN, Suissa S and Azoulay L: Proton pump inhibitors and risk
of gastric cancer: population-based cohort study. Gut. 71:16–24.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Abrahami D, McDonald EG, Schnitzer ME,
Barkun AN, Suissa S and Azoulay L: Proton pump inhibitors and risk
of colorectal cancer. Gut. 71:111–118. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gong EJ, Bang CS, Kim DK, Lee JJ and Baik
GH: Use of proton pump inhibitors and the risk for the development
of gastric cancers: A nationwide population-based cohort study
using balanced operational definitions. Cancers. 14:51722022.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Segna D, Brusselaers N, Glaus D, Krupka N
and Misselwitz B: Association between proton-pump inhibitors and
the risk of gastric cancer: A systematic review with meta-analysis.
Therap Adv Gastroenterol. 14:175628482110514632021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tran-Duy A, Spaetgens B, Hoes AW, de Wit
NJ and Stehouwer CD: Use of proton pump inhibitors and risks of
fundic gland polyps and gastric cancer: Systematic review and
meta-analysis. Clin Gastroenterol Hepatol. 14:1706–1719.e5. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ma T, Wu M, Jia S and Yang L: Proton pump
inhibitors and the risk of colorectal cancer: A systematic review
and meta-analysis of observational studies. Int J Colorectal Dis.
35:2157–2169. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Laoveeravat P, Thavaraputta S,
Vutthikraivit W, Suchartlikitwong S, Mingbunjerdsuk T, Motes A,
Nugent K, Rakvit A, Islam E and Islam S: Proton pump inhibitors and
histamine-2 receptor antagonists on the risk of pancreatic cancer:
A systematic review and meta-analysis. QJM. 113:100–107.
2020.PubMed/NCBI
|
|
54
|
Alkhushaym N, Almutairi AR, Althagafi A,
Fallatah SB, Oh M, Martin JR, Babiker HM, McBride A and Abraham I:
Exposure to proton pump inhibitors and risk of pancreatic cancer: A
meta-analysis. Expert Opin Drug Saf. 19:327–334. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wells GA, Shea B, O'Connell D, et al: The
newcastle-ottawa scale (NOS) for assessing the quality of
nonrandomised studies in meta-analyses. Ottawa Hospital Research
Institute. Ottawa; ON, Canada: 2000
|
|
57
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
DerSimonian R and Kacker R: Random-effects
model for meta-analysis of clinical trials: An update. Contemp Clin
Trials. 28:105–114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Macaskill P, Walter SD and Irwig L: A
comparison of methods to detect publication bias in meta-analysis.
Stat Med. 20:641–654. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lee Y, Urbanska AM, Hayakawa Y, Wang H, Au
AS, Luna AM, Chang W, Jin G, Bhagat G, Abrams JA, et al: Gastrin
stimulates a cholecystokinin-2-receptor-expressing cardia
progenitor cell and promotes progression of Barrett's-like
esophagus. Oncotarget. 8:203–214. 2016. View Article : Google Scholar
|
|
61
|
Betton GR, Dormer CS, Wells T, Pert P,
Price CA and Buckley P: Gastric ECL-cell hyperplasia and carcinoids
in rodents following chronic administration of H2-antagonists
SK&F 93479 and oxmetidine and omeprazole. Toxicol Pathol.
16:288–298. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kidd M, Tang LH, Modlin IM, Zhang T, Chin
K, Holt PR and Moss SF: Gastrin-mediated alterations in gastric
epithelial apoptosis and proliferation in a mastomys rodent model
of gastric neoplasia. Digestion. 62:143–151. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Joo MK, Park JJ and Chun HJ: Proton pump
inhibitor: The dual role in gastric cancer. World J Gastroenterol.
25:2058–2070. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Henwood M, Clarke P, Smith AM and Watson
SA: Expression of gastrin in developing gastric adenocarcinoma. Br
J Surg. 88:564–568. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wroblewski LE, Peek RM and Coburn LA: The
role of the microbiome in gastrointestinal cancer. Gastroenterol
Clin North Am. 45:543–556. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Weitsman S, Celly S, Leite G, Mathur R,
Sedighi R, Barlow GM, Morales W, Sanchez M, Parodi G,
Villanueva-Millan MJ, et al: Effects of proton pump inhibitors on
the small bowel and stool microbiomes. Dig Dis Sci. 67:224–232.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Waldum HL, Sørdal Ø and Fossmark R: Proton
pump inhibitors (PPIs) may cause gastric cancer-clinical
consequences. Scand J Gastroenterol. 53:639–642. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zeng X, Liu L, Zheng M, Sun H, Xiao J, Lu
T, Huang G, Chen P, Zhang J, Zhu F, et al: Pantoprazole, an
FDA-approved proton-pump inhibitor, suppresses colorectal cancer
growth by targeting T-cell-originated protein kinase. Oncotarget.
7:22460–22473. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Patlolla JM, Zhang Y, Li Q, Steele VE and
Rao CV: Anti-carcinogenic properties of omeprazole against human
colon cancer cells and azoxymethane-induced colonic aberrant crypt
foci formation in rats. Int J Oncol. 40:170–175. 2012.PubMed/NCBI
|
|
70
|
Watson SA and Smith AM: Hypergastrinemia
promotes adenoma progression in the APC(Min-/+) mouse model of
familial adenomatous polyposis. Cancer Res. 61:625–631.
2001.PubMed/NCBI
|
|
71
|
Jiang K, Jiang X, Wen Y, Liao L and Liu
FB: Relationship between long-term use of proton pump inhibitors
and risk of gastric cancer: A systematic analysis. J Gastroenterol
Hepatol. 34:1898–1905. 2019. View Article : Google Scholar : PubMed/NCBI
|