Introduction
Renal cell carcinoma (RCC) associated with Xp11.2
translocation/transcription factor E3 (TFE3) gene fusion is a rare
subtype of RCC that was accepted as a distinct entity in the 2004
World Health Organization renal tumor classification (1). This type of RCC mainly occurs in young
adults and children, accounting for 20–40% of cases of RCC;
however, it is rarely seen in adults, with the proportion in adults
only 1–1.6% global scale (2–4). The
clinical manifestations of Xp11.2 translocation RCC are
non-specific. Patients often seek medical attention due to symptoms
such as hematuria, abdominal pain, or abdominal mass. However,
asymptomatic patients who incidentally discover the tumors during
physical examination. The present study describes the case of a
patient with Xp11.2 translocation RCC, who was admitted to the
Department of Urology (The Affiliated Hospital of Zunyi Medical
University; Zunyi, China). No tumor recurrence or metastasis was
found in the 1-year follow-up and the present study retrospectively
analyzed the diagnosis and treatment of the patient, and reviewed
the relevant literature, aiming to improve the understanding of the
symptoms, diagnosis, treatment and prognosis of RCC.
Case report
A 31-year-old man was diagnosed with a solid mass in
the left kidney during a routine health examination at the
Department of Urology (The Affiliated Hospital of Zunyi Medical
University) in October 2022. Upon tracing their medical history,
the patient had reported experiencing lower abdominal pain and
lower back pain for several months. However, they denied
experiencing symptoms such as fever, stomachache, hematuria,
frequent urination, urinary hesitancy and weight loss. The patient
had no previous history of hypertension or diabetes, no surgical
history, no family history of cancer and did not have a history of
smoking or drinking alcohol. Before the operation, the patient did
not present any significant abnormalities in their physical signs,
such as coughing, fever, and respiratory distress. Routine blood
routine tests, blood biochemistry tests, and a coagulation test,
also did not detect any notable abnormalities.
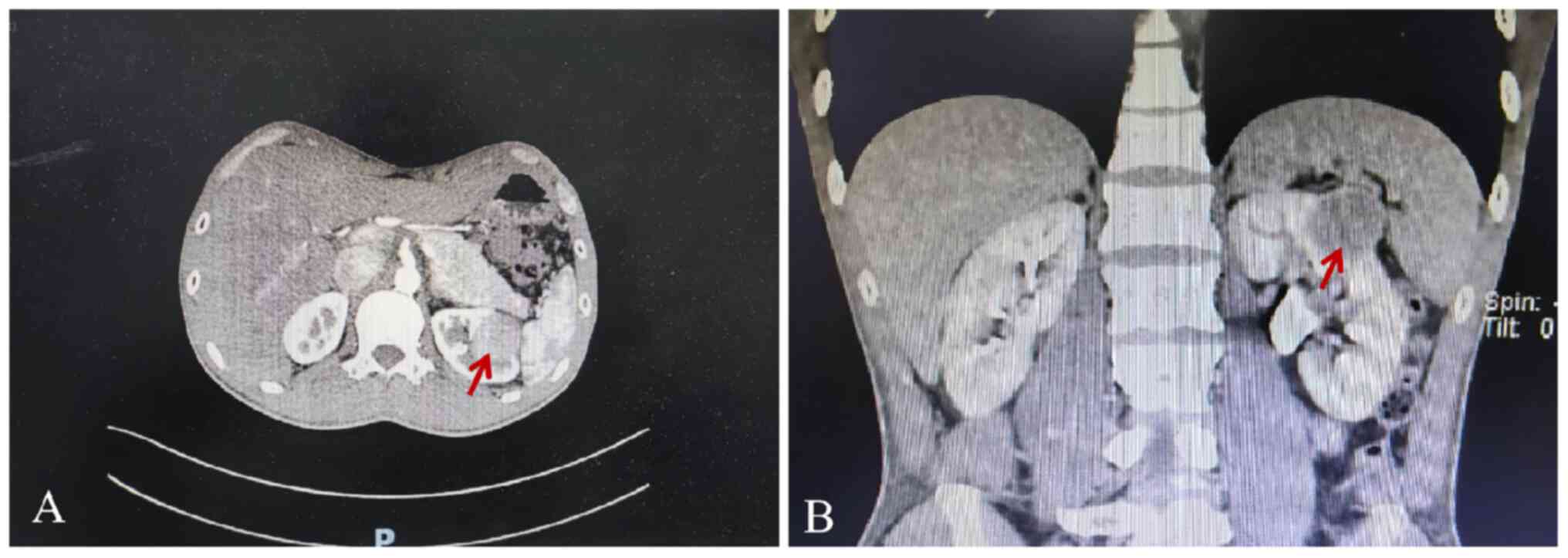
An abdominal CT scan revealed a well-defined left
renal tumor measuring 2.6×3.3×2.8 cm, slightly enhanced at the
arterial phases. No evidence of metastases or abnormalities in the
right kidney was found (Fig. 1A and
B). Based on the clinical diagnosis, the left kidney tumor was
considered to be clear cell RCC (ccRCC). After ruling out surgical
contraindications, the patient underwent a laparoscopic left
partial nephrectomy under general anesthesia 8 days after the
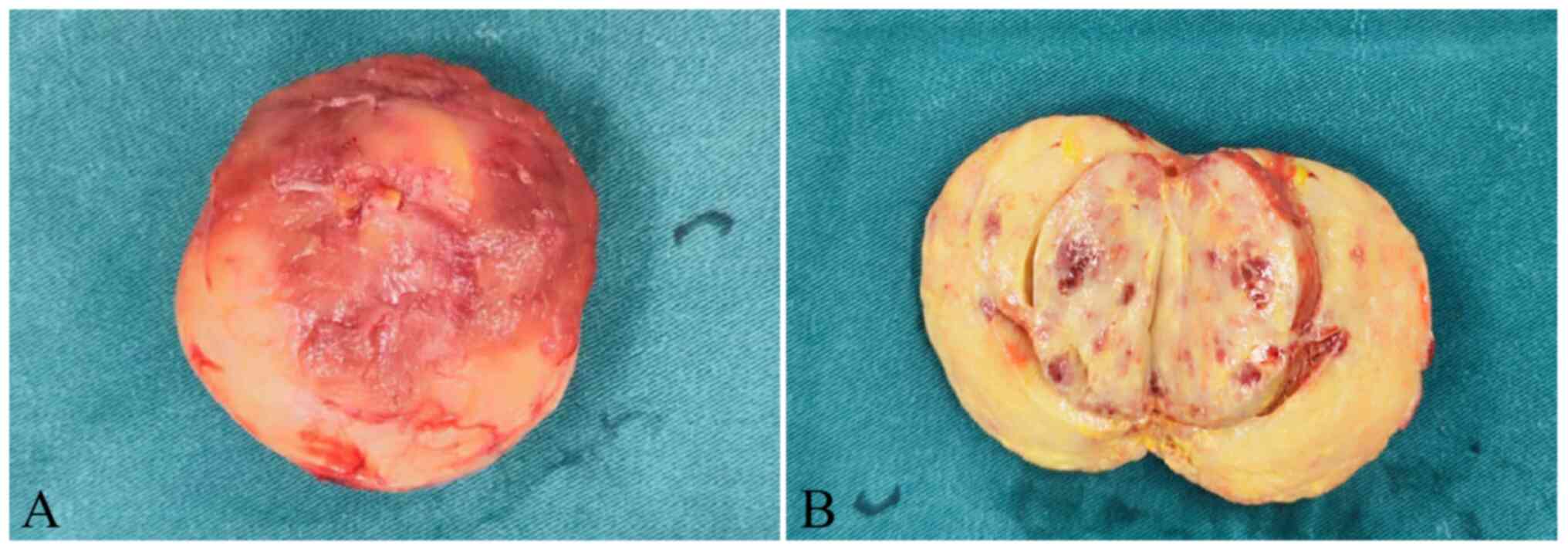
routine health examination. The operation was successful, and the
tumor was completely removed, measuring 3×2×2 cm in size and
presenting as solid, with a cut surface that was tan-yellow,
segmentally demonstrating hemorrhagic areas (Fig. 2A and B). After resection, the tumor
was sent to the Department of Pathology (Affiliated Hospital of
Zunyi Medical University) for H&E and immunohistochemical
staining. Several common kidney tumor markers were assessed,
including CD10, cytokeratin7 (CK7), vimentin, Succinate
Dehydrogenase Iron-Sulfur Subunit, P504S, carbonic anhydrase 9 and
TFE3. Ultimately, the four typical indicators, CD10, CK7, vimentin
and TFE3, were selected to support the diagnosis of Xp11.2
translocation RCC. Microscopic examination indicated that the tumor
cells had a papillary or nested architecture and consisted of cells
with voluminous, clear or eosinophilic cytoplasm, and it was
observed that these structures contained fibrovascular cores and
the focal presence of psammoma bodies (Fig. 3A-D). Immunohistochemistry (IHC)
showed clear and diffuse expression of TFE3 and CD10 (Fig. 3E and F), while vimentin and CK7 were
negative (Fig. 3G and H). The
results of the immunohistochemical staining supported the diagnosis
of Xp11.2 translocation RCC.
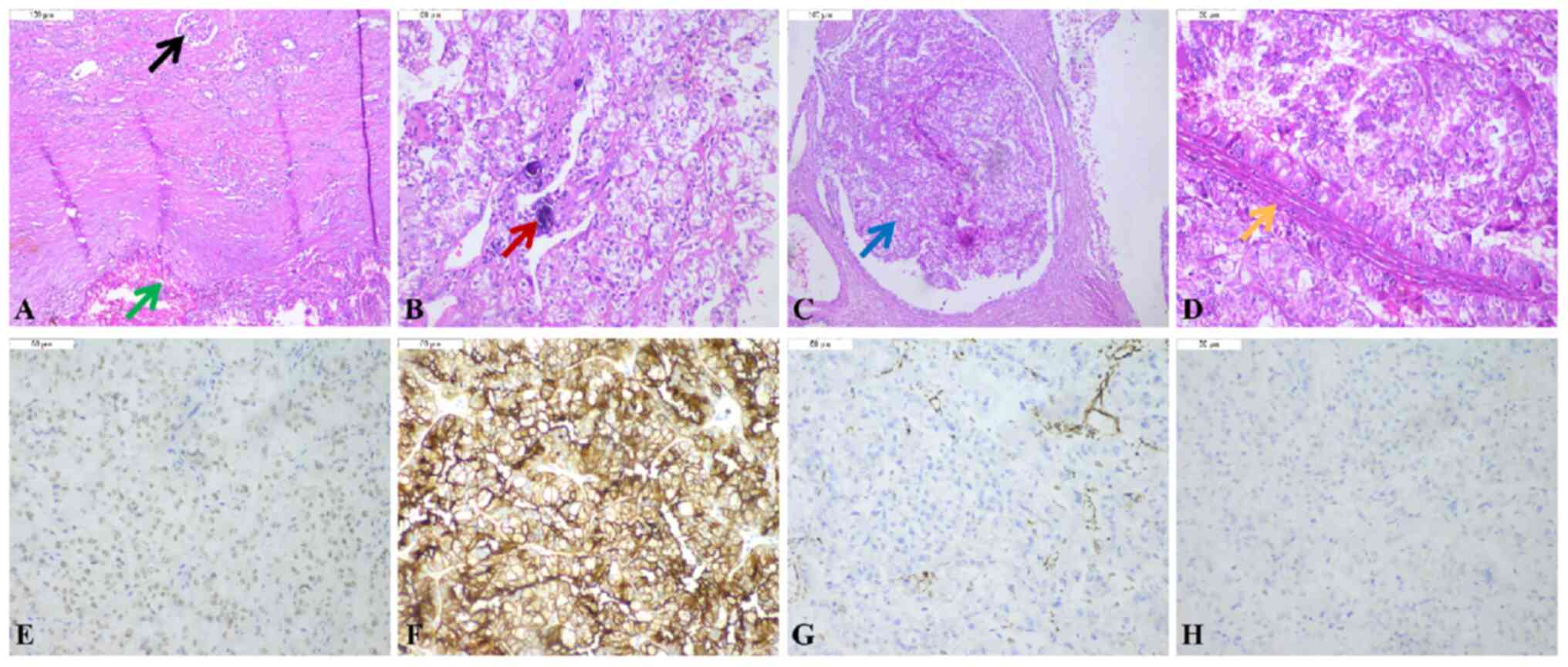 | Figure 3.Pathological features of the mass. (A)
Presence of normal kidney tissue and tumor tissue (black arrow
indicates the glomerulus; green arrow indicates the tumor tissue;
H&E staining; magnification, ×100). (B) The tumor was composed
of a single population of small, round to oval-shaped cells with
voluminous, clear or eosinophilic cytoplasm. Psammoma bodies of the
tumor were also observed (red arrow indicates the psammoma bodies;
magnification, ×200). (C) Tumor cells were arranged in nested
architecture (blue arrow; magnification, ×100). (D) Fibrovascular
cores of papillary structures were present (orange arrow;
magnification, ×200). (E) Positive immunostaining of the tumor
cells for TFE3 expression (magnification, ×200). (F) Positive
immunostaining of the tumor cells for CD10 expression
(magnification, ×200). (G) Negative immunostaining for vimentin in
the tumor cells (magnification, ×200). (H) Negative immunostaining
for CK7 in the tumor cells (magnification, ×200). CK7, cytokeratin
7; TFE3, transcription factor E3. |
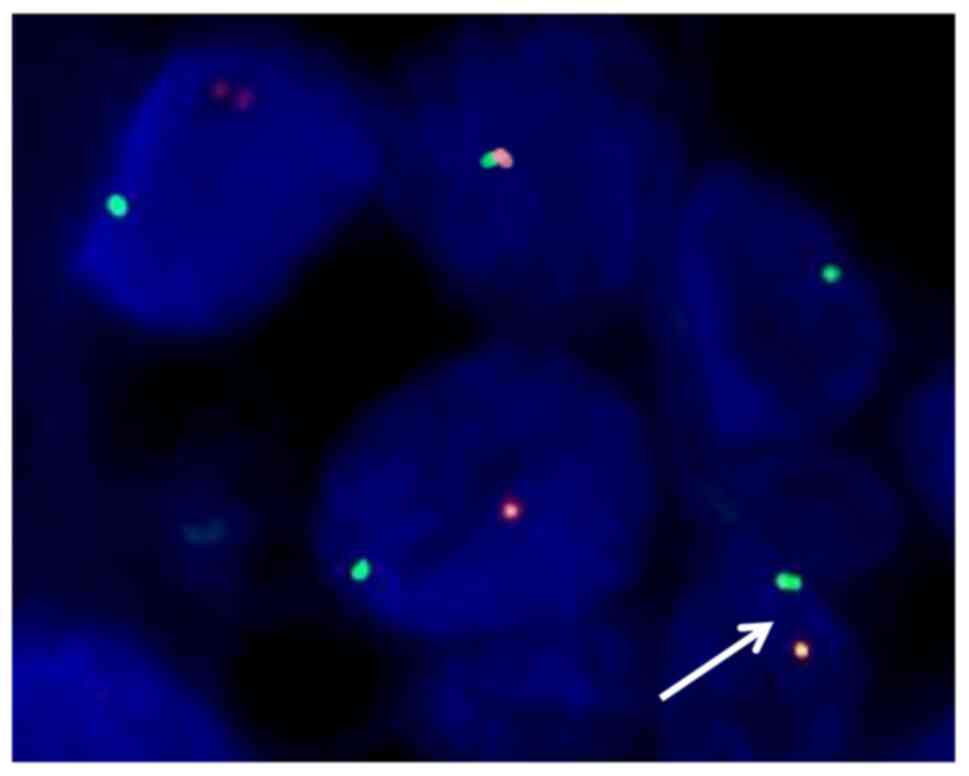
To confirm the diagnosis, the pathologist
recommended a fluorescence in situ hybridization (FISH)
assay targeting the TFE3 gene. After obtaining the consent of the
patient, the samples were sent to ShengTingGroup for the FISH
assay. The FISH assay showing the split orange (centromeric side)
and green (telomeric side) signals (white arrow) and quantitative
analysis demonstrated that the number of cells isolated by orange
and green signal was 80% (>15%) indicating TFE3 gene
rearrangement (Fig. 4).
Consequently, the patient was eventually diagnosed with Xp11.2
translocation RCC of the right kidney. Following the surgery, the
patient had a smooth recovery and was discharged after 2 weeks.
Subsequent follow-up examinations over a period of 6 months
revealed no tumor recurrence or metastasis.
Discussion
Xp11.2 translocation RCC with TFE3 gene fusion is a
rare subtype of RCC (5). It occurs
due to a gene fusion between the TFE3 gene on the Xp11.2 chromosome
and one of six different fusion partners (6). This fusion leads to overexpression of
the TFE3 protein, which in turn upregulates the MET tyrosine kinase
receptor, activating downstream signaling pathways that promote
cell proliferation and tumor formation (7). Xp11.2 translocation RCC differs from
other types of renal carcinoma in terms of histology,
immunophenotype and prognosis (8).
As a result, it has been classified as a rare and unique subtype of
RCC in the renal tumor classification of The World Health
Organization from the 2004 edition onwards (9). The molecular identity of five out of
the six known gene fusion partners of TFE3 are papillary renal cell
Carcinoma, Alveolar Soft Part Sarcoma, polypyrimidine tract-binding
protein-associated splicing factor, non-POU domain-containing
octamer-binding and clathrin heavy-chain genes, while the identity
of the sixth partner on chromosome 3 is still unknown (10–12).
The incidence of Xp11.2 translocation RCC is
relatively low. Previous studies have reported its incidence as
0.9% of adult RCC cases, 15% of young adult RCC cases and 54% of
child RCC cases (3,13–15).
Although Xp11.2 translocation RCC is more common in children, the
overall number of cases may be higher in adults due to the higher
prevalence of RCC in that population (16). Additionally, sex differences in the
incidence of Xp11.2 translocation RCC may exist, given that these
translocations occur on the X chromosome (15,17). A
meta-analysis revealed a higher incidence of Xp11.2 translocation
RCC in women compared with in men, with an adult female-to-male
ratio of 1.6–3.6:1. However, this sex difference is not evident in
pediatric patients (16).
Xp11.2 translocation RCC shares similarities with
other types of RCC and lacks specific clinical manifestations.
Patients with Xp11.2 translocation RCC typically present to the
hospital with symptoms such as hematuria, abdominal pain or an
abdominal mass (known as the triad of RCC) (18). However, most patients only
experience one of these symptoms. Gross hematuria is the most
common initial symptom, while cases presenting with the complete
triad of RCC are extremely rare (19). Some patients may have distant
metastasis symptoms as their primary manifestation, including bone
pain, dull pain in the liver area or hemoptysis (19). However, numerous patients with
Xp11.2 translocation RCC are asymptomatic, and their tumors are
incidentally discovered during physical examinations (20). The tumors are typically located in
the right kidney in ~66.7% of cases and in the left kidney in ~3.3%
of cases (21).
On CT and MRI scans, Xp11.2 translocation RCC is
often indistinguishable from the more common ccRCC (22). However, there are still some
characteristic features that can help differentiate between them.
While both types can exhibit low, equal or high density on
unenhanced CT scans, Xp11.2 translocation RCC generally
demonstrates less enhancement compared with ccRCC on enhanced CT
scans (22,23). This is attributed to the presence of
fewer blood vessels within the focal area of Xp11.2 translocation
RCC. Furthermore, on MRI scans, Xp11.2 translocation RCC typically
exhibits slightly high signal intensity on T1-weighted images
before angiography, and equal-to-low signal intensity on
T2-weighted MR images (24). These
findings are in contrast to the results observed in cases of ccRCC.
Considering the age of the patient, if they are children or
adolescents, a diagnosis of Xp11.2 translocation RCC should be
highly considered. This distinction holds important guiding
significance for the development of a preoperative treatment plan
(25).
The morphological features of Xp11.2 translocation
RCC often overlap with those of other types of RCC.
Macroscopically, the tumor typically presents as solid and
occasionally cystic, with a cut surface that is mostly gray or
tan-yellow, occasionally demonstrating necrotic or hemorrhagic
areas (23). These features are
similar to what is observed in ccRCC (26). However, there are histological
characteristics that can help distinguish Xp11.2 translocation RCC
from other subtypes. The most distinct histological appearance of
Xp11.2 translocation RCC is the presence of a papillary structure
comprised of clear cells and eosinophilic cells. These structures
contain fibrovascular cores and often exhibit psammoma bodies,
which are rarely seen in conventional ccRCC (27,28).
The frequency of psammoma bodies has been reported as 50 and 62% in
two clinicopathological studies (29,30).
Although the tumor may have a relatively typical histological
appearance, an accurate diagnosis still requires supporting
immunohistochemical findings. IHC typically shows minimal
reactivity to CK7 or vimentin (31). Overexpression of the TFE3 protein is
observed due to translocation, and studies have demonstrated the
sensitivity (97.5%) and specificity (99.6%) of TFE3 protein
expression in diagnosing Xp11.2 translocation RCC (5,31).
Therefore, TFE3 protein expression detected by IHC is the most
commonly utilized auxiliary diagnostic technique in clinical
practice (32). However,
immunohistochemical results can be influenced by factors such as
immunostaining methods, incubation methods, fixation time, antigen
repair methods, and antibody sensitivity and specificity, all of
which can potentially lead to false-positive or false-negative
results (33). Qu et al
(33) reported a false-positive
rate of 6.7% and a false-negative rate of 4.3% in the diagnosis of
Xp11.2 translocation RCC using TFE3-IHC. Therefore, further tests
are essential to validate the TFE3 immunohistochemical results in
order to achieve a more accurate diagnosis (34). Common methods currently used for
validation include karyotype analysis, reverse transcription
polymerase chain reaction (RT-PCR) and FISH (34,35).
Among these, karyotype analysis has the highest accuracy, but it
requires fresh tissue samples and, clinically, tumor tissue excised
during surgery is typically preserved and fixed in formaldehyde
solution, rendering it unsuitable as a common diagnostic tool
(36). However, formalin-fixed
paraffin-embedded tissues can be used for FISH and/or RT-PCR
analysis, although the latter method is rarely employed for
diagnosis due to RNA degradation in archived materials (37). FISH has emerged as a preferred
method, offering cheapness, speed, convenience and accuracy, making
it the gold standard for clinical diagnosis of Xp11.2 translocation
RCC (38,39). Given the unclear pathological type
prior to surgery, the treatment plan for Xp11.2 translocation RCC
often follows that of ccRCC. For tumors measuring <4 cm, partial
nephrectomy is recommended; for tumors >4 cm without metastasis,
radical nephrectomy is advised (18,40);
and targeted therapy is recommended for patients with distant
metastasis (33). The overall
prognosis for Xp11.2 translocation RCC is generally worse than that
of ccRCC, with a more favorable prognosis observed in children
compared with adults (41). Zhong
et al (42) reported that
children and adolescents with lymph node-positive Xp11.2
translocation RCC in the absence of distant metastases had a
favorable prognosis, with overall survival estimates nearly triple
those of adult patients with a similar presentation. Therefore, if
postoperative pathology confirms Xp11.2 translocation RCC, more
frequent follow-ups are recommended compared with that for ccRCC.
Additionally, adults with confirmed Xp11.2 translocation RCC based
on postoperative pathology should be followed up more frequently
than children and adolescents.
In summary, Xp11.2 translocation RCC is relatively
rare, and its clinical and imaging manifestations lack specificity.
The combination of TFE3-IHC assay and FISH assay is an accurate and
effective method for separately screening and confirming the
diagnosis of Xp11.2 translocation RCC. Surgical resection without
metastasis is the preferred method, and targeted therapy is
recommended in cases of metastasis. Its prognosis is worse than
that of ccRCC, and the prognosis of adults is significantly lower
than that of children and adolescents, thus regular follow-ups are
important.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and Technology
Department of Guizhou (grant no. ZK2022665) and the Science and
Technology Department of Zunyi (grant no. HZ202111).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TW conceived and the design of the study. DY
analyzed and interpretation of the data and revised the manuscript
drafting critical revisions on the intellectual content. WT, ML, ZX
and FS acquired and analyzed the data. ZZ and XY analyzed and
interpreted the data. DY and TW confirm the authenticity of all the
original data. All authors have reviewed and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pipitone S, Vitale MG, Baldessari C,
Dominici M and Sabbatini R: Long survival of a young patient with
Xp11.2 translocation metastatic clear cell renal carcinoma: Case
report. Tumori. 107:NP131–NP135. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kmetec A and Jeruc J: Xp 11.2
translocation renal carcinoma in young adults; recently classified
distinct subtype. Radiol Oncol. 48:197–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Komai Y, Fujiwara M, Fujii Y, Mukai H,
Yonese J, Kawakami S, Yamamoto S, Migita T, Ishikawa Y, Kurata M,
et al: Adult Xp11 translocation renal cell carcinoma diagnosed by
cytogenetics and immunohistochemistry. Clin Cancer Res.
15:1170–1176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu Y, Pu X, Dong X, Ji C, Guo H, Li D,
Zhao X and Gan W: Molecular heterogeneity of Xp11.2 translocation
renal cell carcinoma: The correlation between split signal pattern
in fish and prognosis. Cancer Manag Res. 13:2419–2431. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo W, Zhu Y, Pu X, Guo H and Gan W:
Clinical and pathological heterogeneity of four common fusion
subtypes in Xp11.2 translocation renal cell carcinoma. Front Oncol.
13:11166482023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Karashima T, Kuno T, Kuroda N, Satake H,
Fukata S, Chikazawa M, Kawada C, Yamasaki I, Shuin T, Hiroi M and
Inoue K: Bilateral Xp11.2 translocation renal cell carcinoma: A
case report. BMC Urol. 18:1062018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang P, Zhang X, Shao SH, Wu F, Du FZ,
Zhang JF, Zuo ZW and Jiang R: Chemotherapy, transarterial
chemoembolization, and nephrectomy combined treated one giant renal
cell carcinoma (T3aN1M1) associated with Xp11.2/TFE3: A case
report. World J Clin Cases. 10:10180–10185. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ellis CL, Eble JN, Subhawong AP,
Martignoni G, Zhong M, Ladanyi M, Epstein JI, Netto GJ and Argani
P: Clinical heterogeneity of Xp11 translocation renal cell
carcinoma: Impact of fusion subtype, age, and stage. Mod Pathol.
27:875–886. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lopez-Beltran A, Scarpelli M, Montironi R
and Kirkali Z: 2004 WHO classification of the renal tumors of the
adults. Eur Urol. 49:798–805. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Argani P, Lui MY, Couturier J, Bouvier R,
Fournet JC and Ladanyi M: A novel CLTC-TFE3 gene fusion in
pediatric renal adenocarcinoma with t(X;17)(p11.2;q23). Oncogene.
22:5374–5378. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Clark J, Lu YJ, Sidhar SK, Parker C, Gill
S, Smedley D, Hamoudi R, Linehan WM, Shipley J and Cooper CS:
Fusion of splicing factor genes PSF and NonO (p54nrb) to the TFE3
gene in papillary renal cell carcinoma. Oncogene. 15:2233–2239.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mathur M, Das S and Samuels HH: PSF-TFE3
oncoprotein in papillary renal cell carcinoma inactivates TFE3 and
p53 through cytoplasmic sequestration. Oncogene. 22:5031–5044.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Argani P and Ladanyi M: Translocation
carcinomas of the kidney. Clin Lab Med. 25:363–378. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ramphal R, Pappo A, Zielenska M, Grant R
and Ngan BY: Pediatric renal cell carcinoma: Clinical, pathologic,
and molecular abnormalities associated with the members of the mit
transcription factor family. Am J Clin Pathol. 126:349–364. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sukov WR, Hodge JC, Lohse CM, Leibovich
BC, Thompson RH, Pearce KE, Wiktor AE and Cheville JC: TFE3
rearrangements in adult renal cell carcinoma: Clinical and
pathologic features with outcome in a large series of consecutively
treated patients. Am J Surg Pathol. 36:663–670. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu L, Yang R, Gan W, Chen X, Qiu X, Fu K,
Huang J, Zhu G and Guo H: Xp11.2 translocation renal cell
carcinomas in young adults. BMC Urol. 15:572015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu Y, Chen S, Zhang M, Liu K, Jing J, Pan
K, Zhang L, Xu B, Lu X and Chen M: Factors associated with survival
from Xp11.2 translocation renal cell carcinoma diagnosis-A
systematic review and pooled analysis. Pathol Oncol Res.
27:6103602021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Wang Y, Feng M, Lian X, Lei Y and
Zhou H: Renal cell carcinoma associated with Xp11.2
translocation/transcription factor E3 gene fusion: An adult case
report and literature review. J Int Med Res.
48:3000605209420952020.PubMed/NCBI
|
|
19
|
Hung CC, Pan CC, Lin CC, Lin ATL, Chen KK
and Chang YH: XP11.2 translocation renal cell carcinoma: Clinical
experience of Taipei Veterans General Hospital. J Chin Med Assoc.
74:500–504. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Armah HB and Parwani AV: Xp11.2
translocation renal cell carcinoma. Arch Pathol Lab Med.
134:124–129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lim B, You D, Jeong IG, Kwon T, Hong S,
Song C, Cho YM, Hong B, Hong JH, Ahn H and Kim CS:
Clinicopathological features of Xp11.2 translocation renal cell
carcinoma. Korean J Urol. 56:212–217. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen X, Zhu Q, Li B, Cui W, Zhou H, Duan
N, Liu Y, Kundra V and Wang Z: Renal cell carcinoma associated with
Xp11.2 translocation/TFE gene fusion: Imaging findings in 21
patients. Eur Radiol. 27:543–552. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong H, Ni Y, Liu Z, Wang Z, Hu B, Xu H
and Cai S: Imaging findings, clinical and pathological characters
of 28 patients with Xp11.2/TFE3 translocation renal cell carcinoma.
J Cancer Res Ther. 19:132–140. 2023.PubMed/NCBI
|
|
24
|
Wang W, Ding J, Li Y, Wang C, Zhou L, Zhu
H and Peng W: Magnetic resonance imaging and computed tomography
characteristics of renal cell carcinoma associated with Xp11.2
translocation/TFE3 gene fusion. PLoS One. 9:e999902014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu K, Xie P, Peng W and Zhou Z: Renal
carcinomas associated with Xp11.2 translocations/TFE3 gene fusions:
Findings on MRI and computed tomography imaging. J Magn Reson
Imaging. 40:440–447. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ge Y, Lin X, Zhang Q, Lin D, Luo L, Wang H
and Li Z: Xp11.2 translocation renal cell carcinoma with TFE3
rearrangement: Distinct morphological features and prognosis with
different fusion partners. Front Oncol. 11:7849932021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Al-Maghrabi JA and Khabaz MN: Uncommon
localization of extrarenal Xp11.2 translocation-associated renal
cell carcinoma (RCC): Case report. Appl Immunohistochem Mol
Morphol. 28:e33–e35. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou J, Zhao L, Yang Z, Chen Y, Wu X and
Xue W: Clinicopathologic, treatment and prognosis study of 46
Xp11.2 translocation/TFE3 gene fusion renal cell carcinomas. BMC
Urol. 22:1092022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu N, Qu F, Wei K, Gan W, Wang Z, Zhuang
W, Agizamhan S, Ma W, Yang J, Chen M, et al: Incidence and
significance of psammoma bodies in Xp11.2 translocation renal cell
carcinoma and papillary renal cell carcinoma. Oncol Lett.
18:472–478. 2019.PubMed/NCBI
|
|
30
|
Manucha V, Sessums MT, Lewin J and Akhtar
I: Cyto-histological correlation of Xp11.2 translocation/TFE3 gene
fusion associated renal cell carcinoma: Report of a case with
review of literature. Diagn Cytopathol. 46:267–270. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Argani P, Lal P, Hutchinson B, Lui MY,
Reuter VE and Ladanyi M: Aberrant nuclear immunoreactivity for TFE3
in neoplasms with TFE3 gene fusions: A sensitive and specific
immunohistochemical assay. Am J Surg Pathol. 27:750–761. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dey B, Badhe B, Govindarajan KK and Ramesh
RA: Xp11.2 translocation renal cell carcinoma diagnosed by
immunohistochemistry and cytogenetics. J Lab Physicians. 8:123–125.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qu Y, Gu C, Wang H, Chang K, Yang X, Zhou
X, Dai B, Zhu Y, Shi G, Zhang H and Ye D: Diagnosis of adults
Xp11.2 translocation renal cell carcinoma by immunohistochemistry
and FISH assays: Clinicopathological data from ethnic Chinese
population. Sci Rep. 6:216772016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Klatte T, Streubel B, Wrba F, Remzi M,
Krammer B, de Martino M, Waldert M, Marberger M, Susani M and
Haitel A: Renal cell carcinoma associated with transcription factor
E3 expression and Xp11.2 translocation: Incidence, characteristics,
and prognosis. Am J Clin Pathol. 137:761–768. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gaillot-Durand L, Chevallier M, Colombel
M, Couturier J, Pierron G, Scoazec JY and Mege-Lechevallier F:
Diagnosis of Xp11 translocation renal cell carcinomas in adult
patients under 50 years: Interest and pitfalls of automated
immunohistochemical detection of TFE3 protein. Pathol Res Pract.
209:83–89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhuang W, Liu N, Guo H, Zhang C and Gan W:
Gender difference analysis of Xp11.2 translocation renal cell
carcinomas's attack rate: A meta-analysis and systematic review.
BMC Urol. 20:1302020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Malouf GG, Su X, Yao H, Gao J, Xiong L, He
Q, Compérat E, Couturier J, Molinié V, Escudier B, et al:
Next-generation sequencing of translocation renal cell carcinoma
reveals novel RNA splicing partners and frequent mutations of
chromatin-remodeling genes. Clin Cancer Res. 20:4129–4140. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim SH, Choi Y, Jeong HY, Lee K, Chae JY
and Moon KC: Usefulness of a break-apart FISH assay in the
diagnosis of Xp11.2 translocation renal cell carcinoma. Virchows
Arch. 459:299–306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rao Q, Williamson SR, Zhang S, Eble JN,
Grignon DJ, Wang M, Zhou XJ, Huang W, Tan PH, Maclennan GT and
Cheng L: TFE3 break-apart FISH has a higher sensitivity for Xp11.2
translocation-associated renal cell carcinoma compared with TFE3 or
cathepsin K immunohistochemical staining alone: Expanding the
morphologic spectrum. Am J Surg Pathol. 37:804–815. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Song HC, Sun N, Zhang WP, He L, Fu L and
Huang C: Biological characteristics of pediatric renal cell
carcinoma associated with Xp11.2 translocations/TFE3 gene fusions.
J Pediatr Surg. 49:539–542. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dong J, Xu W, Ji Z and Pan B: Xp11.2
translocation renal cell carcinoma: Clinical characteristics and
potential prognostic predictors. Dis Markers. 2021:56479332021.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhong M, De Angelo P, Osborne L,
Paniz-Mondolfi AE, Geller M, Yang Y, Linehan WM, Merino MJ,
Cordon-Cardo C and Cai D: Translocation renal cell carcinomas in
adults: A single-institution experience. Am J Surg Pathol.
36:654–662. 2012. View Article : Google Scholar : PubMed/NCBI
|