Introduction
Lung cancer is the most common type of cancer
worldwide with an incidence rate of 11.4% and has a high mortality
rate (number of deaths per year, 1,796,144) (1). Lung adenocarcinoma, a type of
non-small cell lung cancer (NSCLC), is a common type of lung cancer
(40 to 55% of total lung cancer cases) (2). The risk factors for the disease
include long-term smoking, cooking fumes, air pollution and genetic
factors.
Targeted therapy is based on the application of
selective inhibitors and biomolecules, which can impair the growth
of lung cancer cells via interference with specific targeted
receptors or other downstream proteins, including epidermal growth
factor receptor (EGFR), anaplastic lymphoma kinase (ALK), ROS
proto-oncogene 1 (ROS1), kirsten rat sarcoma viral oncogene (KRAS),
v-raf murine sarcoma viral oncogene homolog B (BRAF), and human
epidermal growth factor receptor 2 (HER-2) (3). Targeted therapy, including tyrosine
kinase inhibitor (TKI)-based therapy, has been reported to improve
the overall survival (OS) and quality of life of patients with
NSCLC who have mutations (4). A
meta-analysis of existing data reported that the EGFR single
nucleotide polymorphisms rs712829 (−216g>T) and rs11568315 (CA
repeat) significantly affected OS and progression-free survival
(PFS) in patients with NSCLC who were treated with the TKIs
gefitinib or erlotinib (4).
However, another study reported that patients who were carriers of
the long CA repeat (SL+LL) were more likely to experience skin
toxicity associated with TKIs (5).
Targeted immunotherapy promotes the activation of
the immune system against lung cancer cells (3). In contrast to non-immune checkpoint
inhibitor (ICI) agents, such as pemetrexed and carboplatin, ICI
agents have the potential to improve long-term survival,
represented by a plateau at the tail of the survival curve in small
patient populations for several cancer types, including melanoma
(6). Programmed cell death 1 ligand
1 (PD-L1)-positive cases (≥1–49%) are recommended for the
treatment, including carboplatin or cisplatin (7) combined with pemetrexed and
pembrolizumab, or with carboplatin combined with paclitaxel,
bevacizumab and atezolizumab according to the National
Comprehensive Cancer Network guidelines (8); pembrolizumab (9) is also recommended by the European
Society of Medical Oncology (2) and
American Society of Clinical Oncology guidelines (10).
Platinum chemotherapy is the recommended first-line
treatment for patients with advanced NSCLC without targeted gene
mutations; however, the systemic toxic side effects (such as
gastrointestinal reaction, hematological toxicity and impairment of
liver and kidney function) and overall drug resistance to
chemotherapy remain significant challenges (11). Moreover, when both drug resistance
and progressive disease occur, the effectiveness of a number of
chemotherapy drugs decreases. For example, the third-generation
EGFR TKI, osetinib, was developed for the treatment of patients
with NSCLC with T790M mutations. However, although it has
demonstrated superiority over first- and second-generation
EGFR-TKIs in high selection for EGFR-activating mutations as well
as the T790M mutation, the development of drug resistance is still
a limitation (12).
Targeted therapy combined with immunotherapy has
become the primary treatment for several types of solid cancers,
such as melanoma and lung and colorectal cancer, in the last 5
years (13). Programmed cell death
protein 1 (PD-1) and PD-L1 inhibitors are types of ICI agents.
Antibody blocking of PD-1 prevents its interaction with PD-L1 and
PD-L2 by obstructing their downstream pathways and restoring the
antitumor response of T cells (14). These are the most successful amongst
all clinically applied checkpoint inhibitors. Anti-PD-1
immunotherapy provides a novel means of treating advanced lung
cancer (3).
The present study reports the case of a patient with
advanced NSCLC treated with targeted therapy and anti-PD-1
immunotherapy after multiple lines of failed therapies, providing
further evidence for adopting targeted therapy and anti-PD-1
immunotherapy in the clinical treatment of advanced NSCLC.
Case report
A 60-year-old woman was admitted to the Affiliated
Hospital of Nanjing University of Chinese Medicine (Nanjing, China)
for a routine physical examination in September 2017. The patient
was 168 cm tall and weighed 69 kg, with no medical history of
chronic illnesses. The patient did not smoke, consume alcohol or
recreational drugs, and had no notable family history of cancer.
The patient provided written consent for the publication of their
data.
A chest radiograph demonstrated a shadow in the left
upper lung. The patient was subsequently admitted to the Department
of Oncology, and the chest and abdomen were examined by enhanced
computed tomography (CT) scan, which demonstrated peripheral lung
cancer in the left upper lobe (Fig.
1). Bone emission CT (ECT) revealed that the radioactivity
distribution of the spine (both systemic and local) was not
uniform, and the radioactivity in local areas was slightly
concentrated, with possible degeneration.
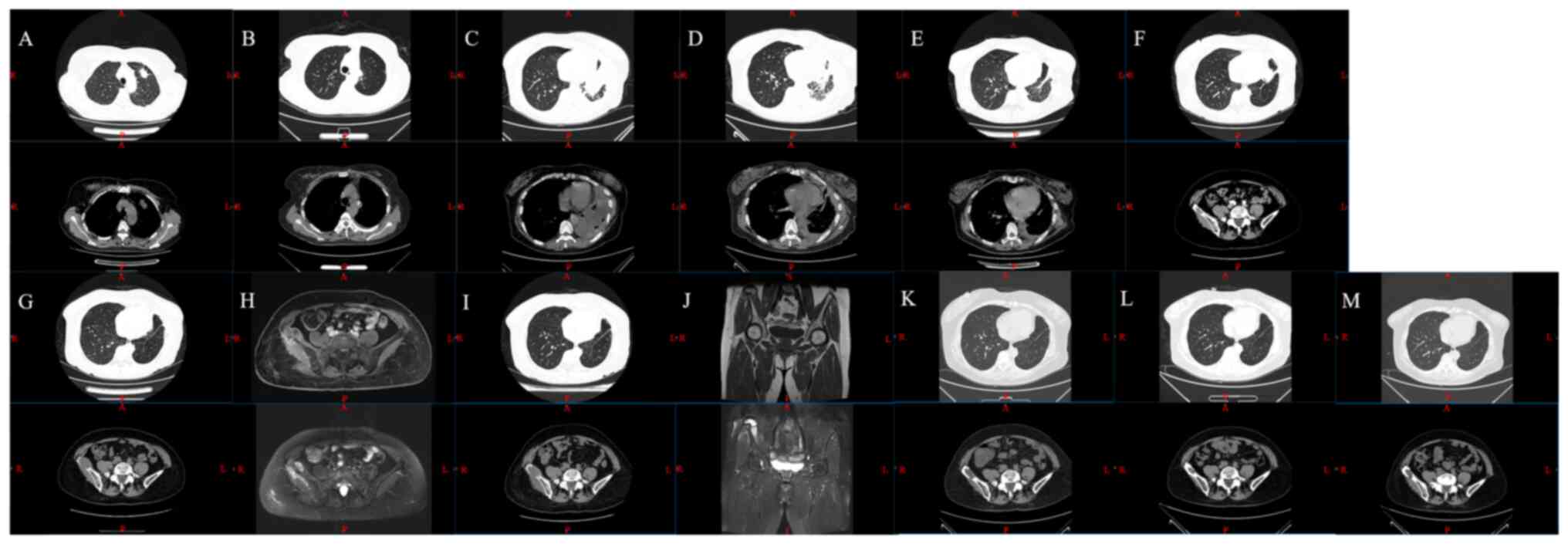 | Figure 1.CT and MRI scans images. CT scans on
(A) 2017-09, (B) 2017-10, (C) 2019-01, (D) 2019-03, (E) 2019-06,
(F) 2019-11 and (G) 2020-02. MRI scan on (H) 2020-02, CT scan on
(I) 2020-06, MRI scan on (J) 2020-08, and CT scans on (K) 2022-11,
(L) 2023-03 and (M) 2023-07. CT, computed tomography; R, right; L,
left; A, anterior; P, posterior; S, superjacent; I, inferior. |
The patient was transferred for thoracic surgery at
13 days post-admission and underwent a video-assisted left upper
lobectomy and lymphadenectomy. An interim diagnostic report of
intraoperative frozen section pathology [freezing temperature,
cryochamber temperature −23°C, object temperature −21°C; thickness
of sections, 6 µm; hematoxylin and eosin staining (according to
standard procedures); light microscope OLYMPUS BX43] showed that
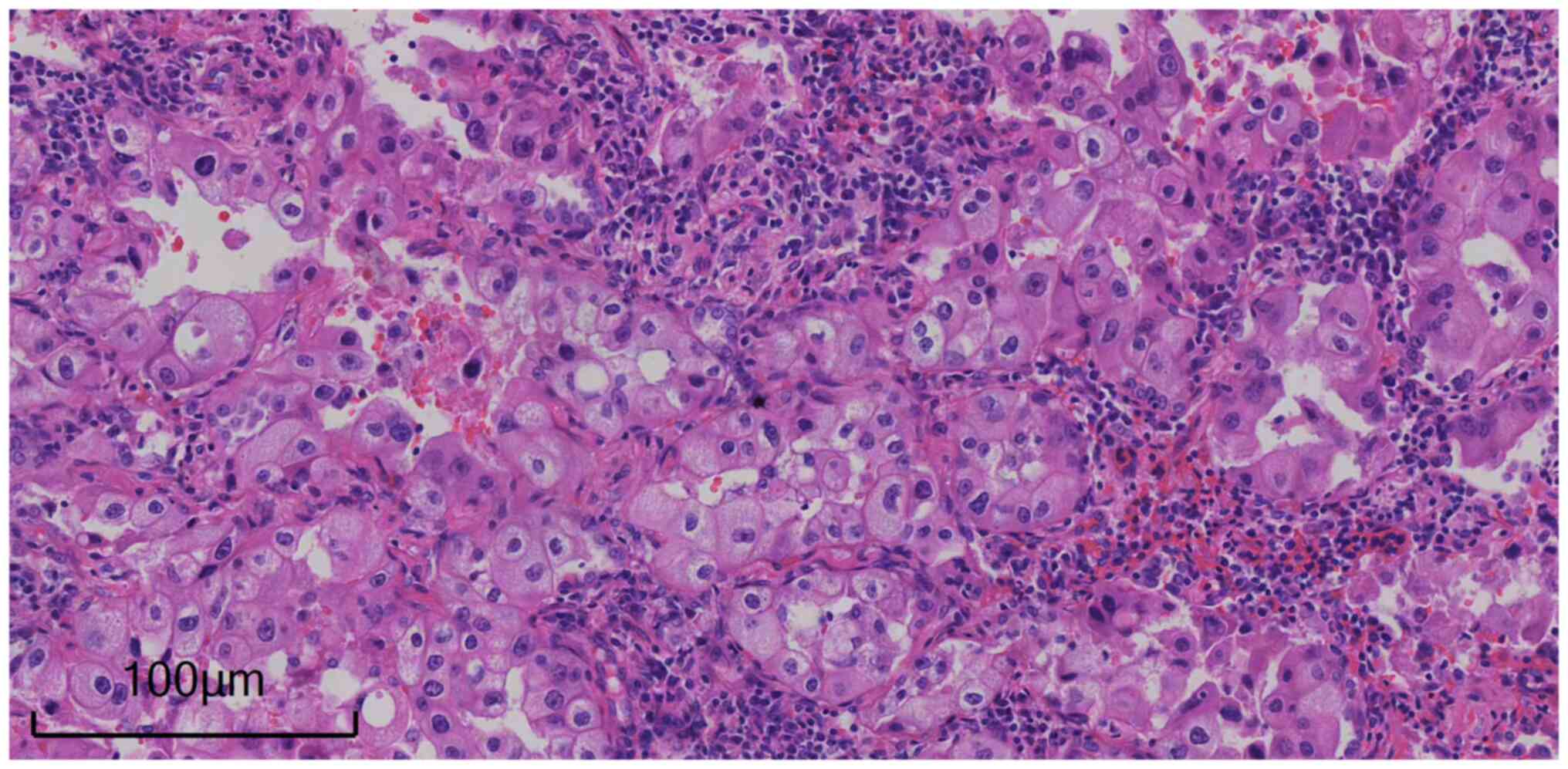
the left upper lung mass was an adenocarcinoma (characteristics of
cytology: Lung adenocarcinoma cells typically exhibit circular or
polygonal shapes, the nucleus is large and irregular, increased
nuclear chromatin with patchy appearance, more mitotic figures.
Formation of acini: Lung adenocarcinoma often forms acinar
structures in the tissue, mucus secretion in the acini.
Infiltrating growth: Lung adenocarcinoma can invade surrounding
lung tissue and blood vessels and form clear boundaries of cancer
tissue). The postoperative pathological examination (according to
standard procedures) of the left upper lung mass resection specimen
involving conventional histopathological and immunohistochemical
staining (according to standard procedures) supported a diagnosis
of lung adenocarcinoma that was moderately differentiated (acinar
type, ~60%; adherent type, ~40%) with a size of 2×1.4×1 cm, with no
definite vascular tumor thrombus and no nerve invasion. No cancer
tissue was found at the cut ends of the hilum and bronchus, and 1
of the 2 lymph nodes demonstrated cancer metastasis (Fig. 2). Immunohistochemistry staining
using an Optiview DAB IHC Detection Kit (Agilent Technologies,
Inc.) was performed for thyroid transcription factor-1 (TTF-1;
clone, 8G7G3/1), NapsinA (clone, IP64), ALK (clone, D5F3),
cytokeratin (CK) 7 (clone, OV-TL 12/30), CK20 (clone, Ks 20.8) and
Villin (clone, 1D2 C3). Ready-to-use antibodies, which were not
diluted, TTF-1 and NapsinA were purchased from Beijing Zhongshan
Golden Bridge Biological Technology Co., Ltd.; ALK was purchased
from Ventana Medical Systems, Inc.; CK7, CK20 and Villin were
purchased from Agilent Technologies, Inc. Standard procedures: i)
Fixed solution, 10% neutral buffered formalin-fixed solution, fixed
at room temperature for 6 to 24 h: Using immunohistochemical tissue
anti-detachment tablets with a cross-sectional thickness of 3 µM;
ii) sealing reagent, 3% hydrogen peroxide, room temperature, 10
min; iii) the following primary antibodies were used: TTF1 (ready
to use; clone 8G7G3/1; cat. no. ZM-0250; OriGene Technologies,
Inc.) and NapsinA (ready to use; clone IP64; cat. no. ZM-0473;
OriGene Technologies, Inc.) incubated at 37°C for 32 min; ALK
(ready to use; clone D5F3; cat. no. 790-4794; Ventana Medical
Systems, Inc.) incubated at 37°C for 20 min; CK7 (ready to use;
clone OV-TL 12/30; cat. no. M7018; Agilent Technologies, Inc.),
CK20 (ready to use; clone Ks 20.8; cat. no. M7019; Agilent
Technologies, Inc.) and Villin (ready to use; clone 1D2 C3; cat.
no. IR076; Agilent Technologies, Inc.) incubated at room
temperature for 40 min; iv) the following secondary antibodies were
used: Optiview HRP Multimer (ready to use; cat. no. 760-700,
Ventana Medical Systems, Inc., room temperature, 12 min), EnVision
FLEX, EnVision FLEX/HRP (ready to use; cat. no. GV800, Agilent
Technologies, Inc., room temperature, 20 min), with 25 ml OptiView
Peroxidase Inhibitor (3.0% H2O2), 25 ml
OptiView HQ Universal Linker (~50 µg/ml), 25 ml OptiView HRP
Multimer (~40 µg/ml), 25 ml OptiView DAB (0.2% w/v DAB), 25 ml
OptiView H2O2 (0.04%
H2O2) and 25 ml OptiView Copper (5.0 g/l
CuSO4); v) DAB shows a positive result with a brownish
yellow marker, while hematoxylin is used to contrast non-positive
stained nuclei; and a vi) light microscope OLYMPUS BX43.
Immunohistochemistry of the tumor cells demonstrated the following
expression results: TTF-1(+), NapsinA(+++), ALK(−), CK7(+++),
CK20(−) and Villin local(++). Paraffin tumor tissue detection gene
testing was performed using next-generation sequencing (NGS)
according to standard protocols (15,16),
demonstrated that the G12D mutation in codon 12 of exon 2 of the
KRAS gene (c.G35A, p.G12D) had an abundance of 67.5%, while no
mutations were found in genes such as EGFR, ALK, ROS1, BRAF, MET,
HER-2, AKT1, MAP2K1 (MEK1), NRAS, NF1, PIK3CA, PTEN and RET. The
clinical stage of the cancer was determined to be IIB according to
AJCC Cancer Staging Manual (17).
Body surface area (m2) of the patient was calculated to
determine correct dose by using the following formula: 0.0061 ×
height (cm) + 0.0128 × weight (kg) −0.1529. The patient received 6
cycles of a pemetrexed + carboplatin regimen as adjuvant
chemotherapy (intravenous drip; 0.8 g pemetrexed on day 1+0.5 g
carboplatin on day 2) from October 2017, to February 2018. The
treatments received by the patient and the subsequent outcomes are
listed in Table I.
 | Table I.Timeline of treatments received by the
patient and the subsequent outcome. |
Table I.
Timeline of treatments received by the
patient and the subsequent outcome.
| Time period | Treatment and
dose | Result |
|---|
| October 2017-February
2018 | 6 cycles; 0.8 g
pemetrexed on day 1+0.5 g carboplatin on day 2; every 3 weeks | Progressive
disease |
| March 2019-June
2019 | 6 cycles; 120 mg
paclitaxel liposome on days 1 and 7+40 mg cisplatin on days 1–3+100
mg pembrolizumab on day 4; every 3 weeks | Progressive
disease |
| July 2019-October
2019 | 5 cycles; 100 mg
pembrolizumab on day 1; every 3 weeks | Progressive
disease |
| November 2019-January
2020 | 4 cycles; 210 mg
paclitaxel liposome on day 1+100 mg pabolizumab on day 3; every 3
weeks | Progressive
disease |
| February 2020 | 1 cycle; 210 mg
paclitaxel liposome injection on day 1+400 mg carboplatin on day
1+100 mg pembrolizumab on day 3; every 3 weeks | Progressive
disease |
| February 2020-May
2020 | 4 cycles; 200 mg
albumin-bound paclitaxel on day 1, 100 mg on day 8+240 mg
toripalimab on day 2; every 3 weeks | Progressive
disease |
| May 2020-July
2020 | 3 cycles; 240 mg
toripalimab on day 1; every 3 weeks | Progressive
disease |
| July 2020-August
2020 | 2 cycles; 200 mg
albumin-bound paclitaxel on day 1+240 mg toripalimab on day 2;
every 3 weeks | Progressive
disease |
| September
2020-September 2023 | 50 cycles; 12 mg
anlotinib on days 1–14+240 mg toripalimab on day 1; every 3
weeks | Stable disease |
In January 2019, the patient experienced notable
chest pains with chest tightness and asthma. Left thoracic puncture
drainage was performed, draining dark red liquid blood, which
indicated disease progression. In February 2019, the patient
experienced paroxysmal left lower chest pain after tiredness, and
the underarm surgical wound exhibited a notable and needle-like
pain. Chest CT demonstrated abundant pleural effusion on the left
side, partial compression of the left lung showing consolidation
and a left subpleural nodule shadow. This appeared to have
progressed compared to the CT scan performed in September 2018, as
left axillary and mediastinal lymph node metastases were observed.
Exfoliated cells in the pleural effusion were found to be
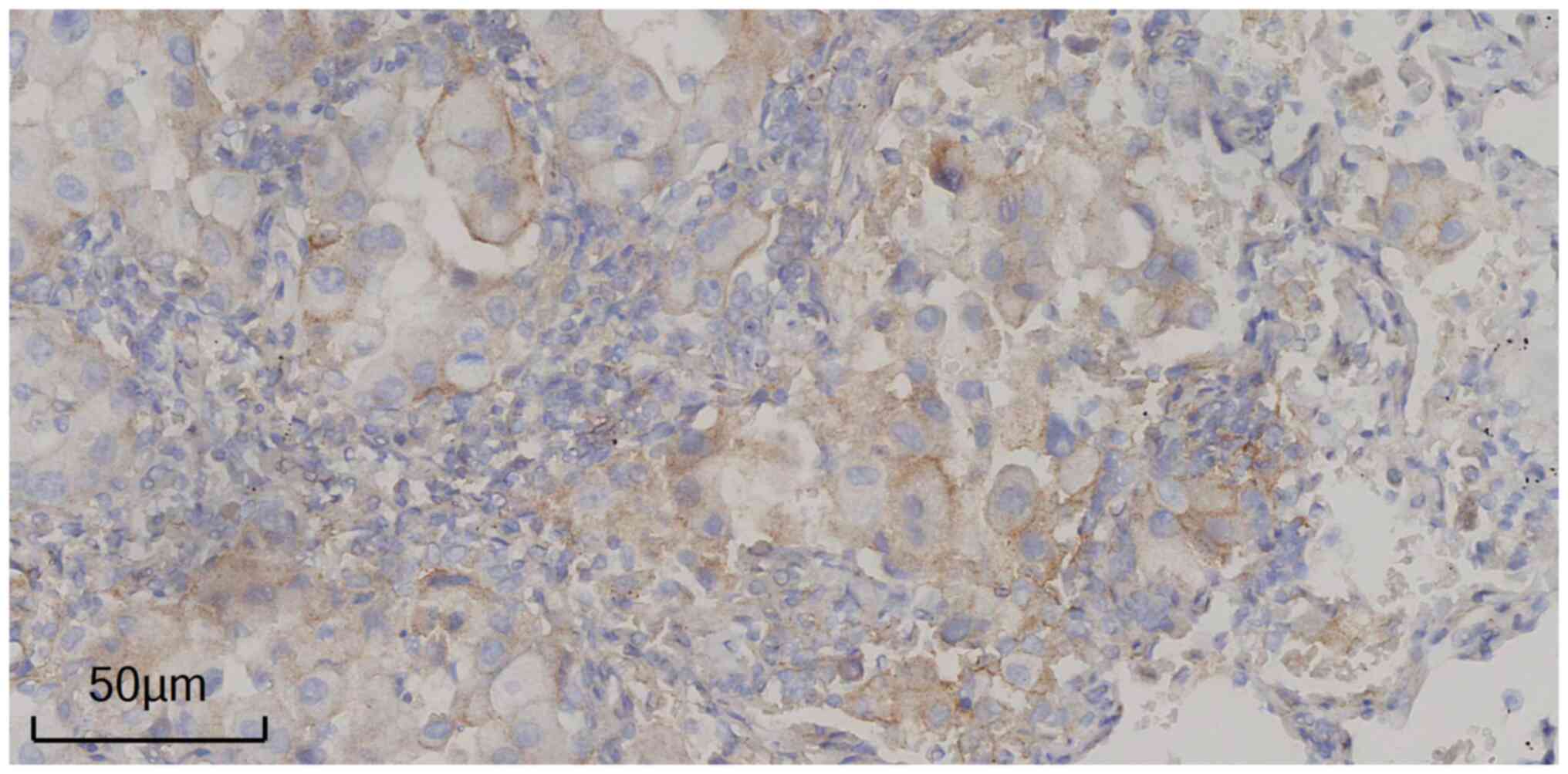
adenocarcinoma cells. In March 2019, immunohistochemistry according
to standard procedures [PD-L1 IHC 22C3 pharmDx (ready to use; clone
22C3; cat. no. SK00621; Agilent Technologies, Inc.) incubated at
room temperature for 40 min] demonstrated that in the left upper
lung mass resection specimen, PD-L1(+) expression in the tumor
cells was 10% (Fig. 3). The patient
received 6 cycles of chemotherapy (120 mg paclitaxel liposome on
days 1 and 7+40 mg cisplatin on days 1–3) plus 100 mg pembrolizumab
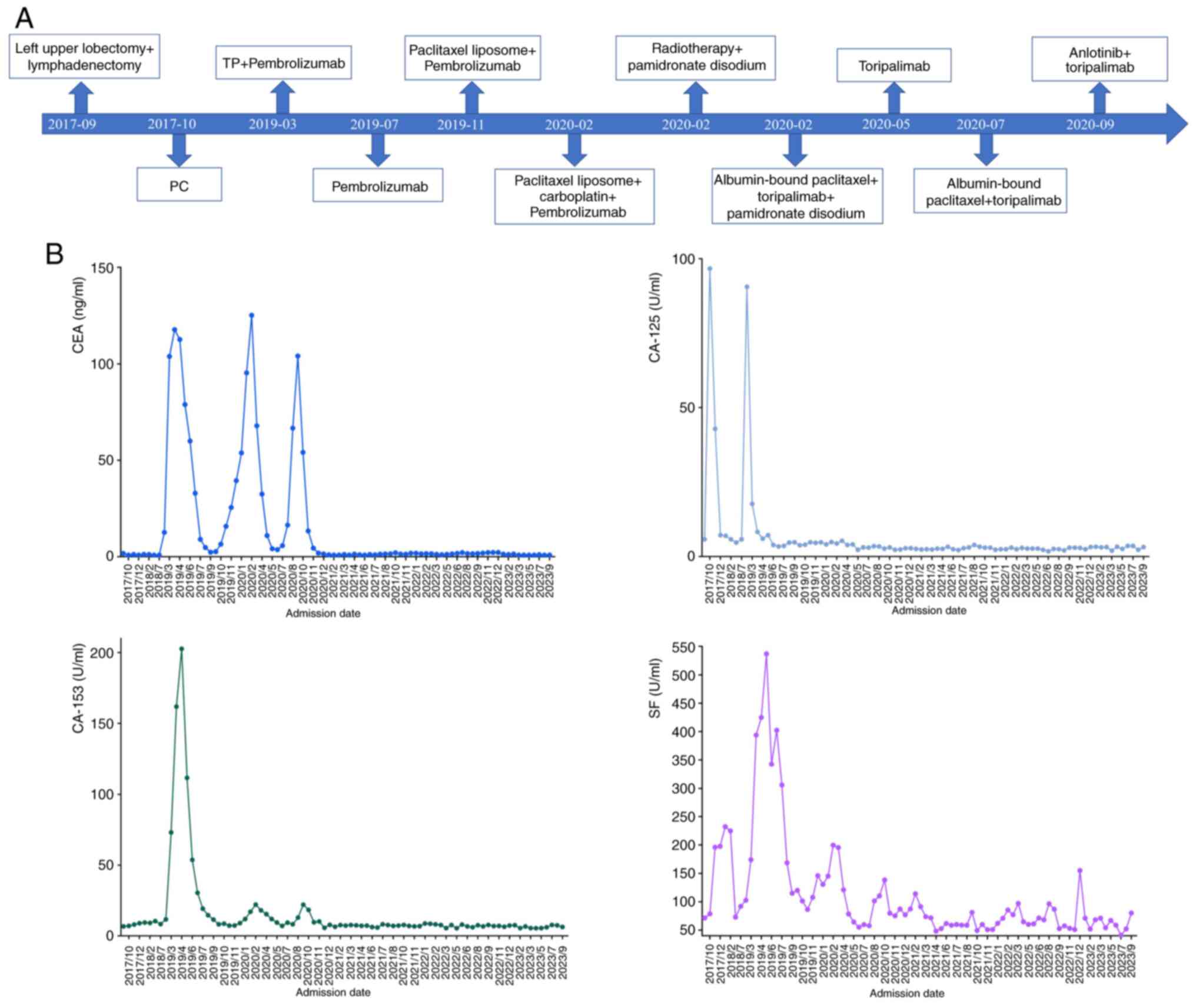
on day 4 from March 2019 to June 2019. After treatment, tumor
indicators were all reduced to their normal ranges, including the
serum carcinoembryonic antigen (CEA; normal ranges, <5.00
ng/ml), carbohydrate antigen 125 (CA-125; normal ranges, <35.00
U/ml), CA-153 (normal ranges, <31.30 U/ml), serum ferritin (SF;
normal ranges, 11.00–306.8 ng/ml) (Fig.
4). The patient received 5 cycles of 100 mg pabolizumab on day
1 from July 2019 to October 2019. However, in November 2019, the
serum carcinoembryonic antigen (CEA) level (15.78 ng/ml) was
markedly higher once more, indicating tumor progression.
The patient received 4 cycles of chemotherapy (210
mg paclitaxel liposome on day 1+100 mg pembrolizumab on day 3) from
November 2019, to January 2020, and then underwent a bone ECT scan
in an external hospital due to right hip pain. This demonstrated an
abnormal concentrated shadow at the left edge of the right ilium
and L5 vertebra, indicating active metabolism. Chest CT
demonstrated a small amount of pleural effusion on the left side
following left upper lung surgery (a video-assisted left superior
lobectomy), similar to that found on the CT scan performed in
November 2019, and focal fibrosis of the left lung with pleural
thickening and right iliac bone metastasis, with increased
progression compared with previous scans from February 2020. A
plain MRI scan of the lumbar spine and hip joint demonstrated
lumbar degeneration, lumbar 1/2, 2/3, 3/4 and 4/5, and lumbar
5/sacral 1 intervertebral disc protrusion, and metastasis of the
right iliac wing. In February 2020, the patient was administered 1
cycle of 210 mg paclitaxel liposome and 400 mg carboplatin on day
1, and 100 mg pembrolizumab on day 3. Radiotherapy was indicated in
accordance with the Chinese Society of Clinical Oncology guidelines
(18), and the patient underwent
radiotherapy (range, right iliac bone and surrounding soft tissue;
dose, 40 Gy/20 fractions combined with pamidronate disodium for
anti-bone metastasis treatment) in February 2020.
In February 2020, CEA (125.19 ng/ml) was notably
higher compared with before in February 2020 (CEA, 95.38 ng/ml),
indicating the progression of the disease. The patient received 4
cycles of albumin-bound paclitaxel at 200 mg on day 1 and 100 mg on
day 8+240 mg toripalimab on day 2 combined with pamidronate
disodium for anti-bone metastasis treatment from February 2020, to
May 2020. The patient then received immune maintenance therapy (240
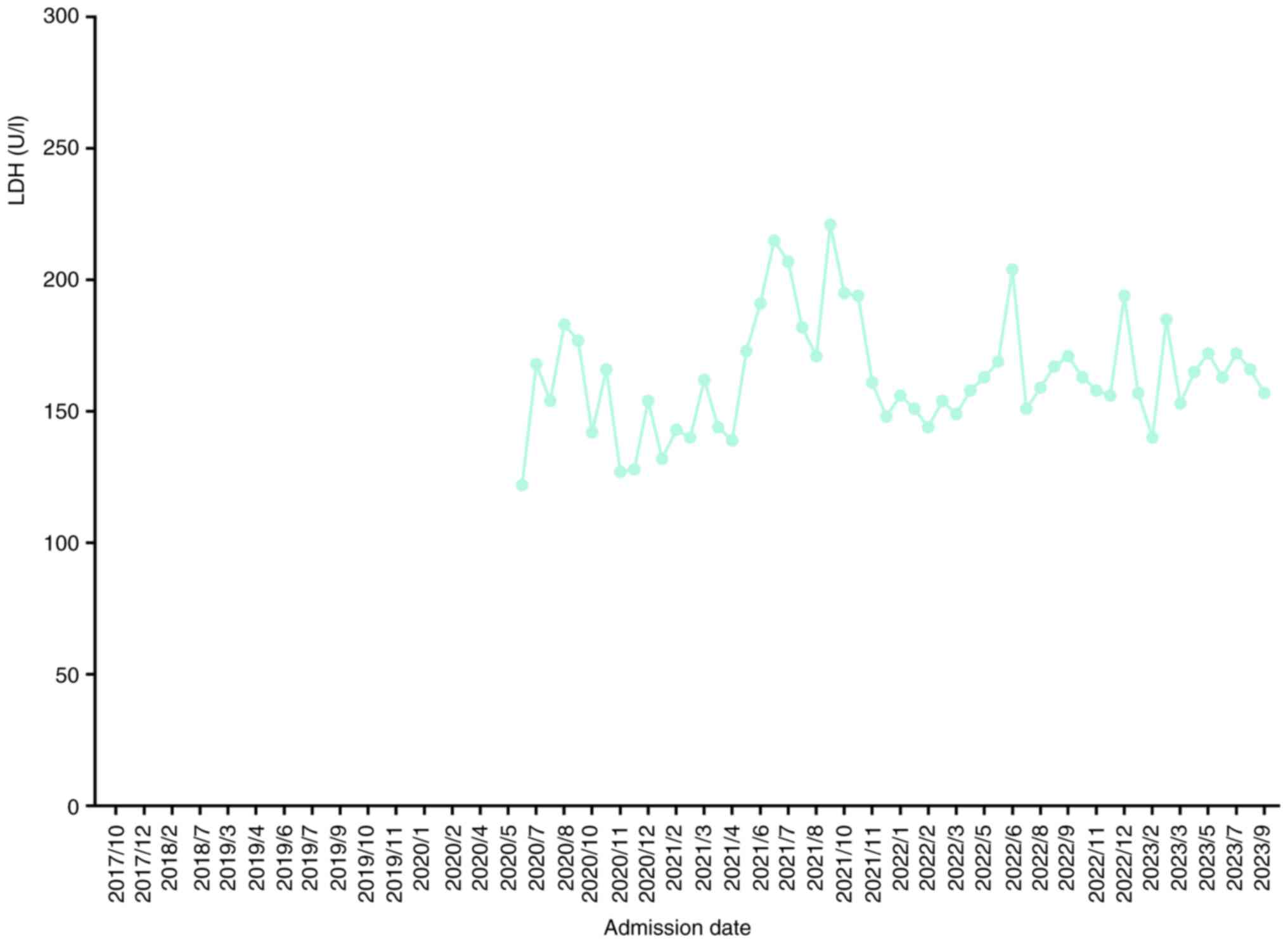
mg toripalimab) from May 2020, to July 2020. The lactate
dehydrogenase (LDH, normal ranges 135–225 U/l) levels of the
patient started to be assessed since June 2020 (Fig. 5). The CEA level (16.44 ng/ml) was
markedly higher than that in July 2020. The treatment plan was
changed to 200 mg albumin-bound paclitaxel on day 1+240 mg
toripalimab on day 2 in July 2020 and the same treatment plan was
conducted in August 2020. The patient's tumor indicators continued
to increase, suggesting drug resistance. The patient received
targeted therapy combined immunotherapy (12 mg anlotinib on days
1–14+240 mg toripalimab on day 1) from September, 2020, to
September, 2023.
In November 2022, chest and abdominal CT
demonstrated postoperative changes to the left upper lung lobe,
including scattered fibrous lesions in the left lung, localized
thickening of the left pleura, fine calcification in the right
lower lung lobe, calcification in the right lobe of the liver,
right iliac bone destruction and multiple high-density shadows of
the lumbosacral vertebral body, similar to the findings of the
previous CT scan performed in August 2022. In March 2023, and July
2023, chest and abdominal CT demonstrated the same aforementioned
features.
At the time of writing the present report (September
2023), the patient had accumulated >36 months of PFS and >72
months of OS. The patient's quality of life was good and stable,
and activities of daily living were complete without the need for
care. Moreover, the patient in the present study continued to
receive a traditional Chinese medicine (TCM) treatment in the form
of a decoction of Glehnia and Ophiopogon alongside targeted therapy
and immunotherapy, in the Department of Oncology.
Discussion
Recurrence and metastasis pose a significant threat
to the prognosis of patients with NSCLC (19). Studies have reported that anti-PD-1
immunotherapy exhibits antitumor efficacy in patients with advanced
lung cancer (20,21). With advances in Chinese health care
reforms, an increasing number of targeted therapies and
immunotherapies have been included in medical insurance programs,
to benefit an increasing number of patients and reduce the
financial burden on individuals.
In the present study, the case of a patient with
advanced lung cancer is reported. Systemic therapies, such as
chemotherapy and immunotherapy, failed and the patient subsequently
received targeted therapy (12 mg anlotinib on days 1–14) and
anti-PD-1 immunotherapy (240 mg toripalimab on day 1) when multiple
metastases were observed. Long-term survival was achieved,
determined based on tumor indicators and CT results. The patient
survived for >4 years following lung cancer progression, which
is notably longer than expected for patients with advanced lung
cancer (median PFS of 4–5 months and OS of 16–18 months) (22). This long-term survival demonstrated
the notable potential of targeted therapy and anti-PD-1
immunotherapy for the treatment of advanced lung cancer. However,
the present report focused on only one case and therefore there is
a lack of high-quality evidence (large sample and randomized);
thus, large-scale multicenter randomized controlled trials are
required.
A previous case study reported that a patient with
advanced NSCLC received multi-line therapy, including chemotherapy
combined with targeted therapy (consisting of pemetrexed,
carboplatin and bevacizumab), chemotherapy with abraxane, anlotinib
targeted therapy and subsequent single-agent treatment with
toliximab, with no progression for 13 months (23). Another study reported that a patient
with late-stage NSCLC and EGFR-sensitized mutations responded to
topolizumab combined with chemotherapy after resistance to
osetinib, with a PFS time of >8 months and an OS time of >28
months (24). A multicenter phase
II trial involving patients with advanced NSCLC and EGFR mutations
who were previously treated with an EGFR-TKI, administered treated
with tolipizumab + chemotherapy as a second-line treatment. The
study reported a median PFS of 7 months and an OS of 23.5 months
(25). A phase III study reported
that the combination of toripalimab with first-line chemotherapy
for advanced NSCLC significantly improved PFS time compared with
placebo combined with chemotherapy, with a median PFS time of 8.3
months compared with 5.6 months, respectively (26). It is important to follow up on
patients to observe the outcomes and prognosis, which can help to
improve clinical diagnoses and treatments. Tumor markers, such as
CEA, can be used to observe disease progression. However, when
assessing progression, LDH should also be assessed, as it is a good
indicator of a tumor (27). The
Affiliated Hospital of Nanjing University of Chinese Medicine only
began to routinely test patients' LDH levels in June 2020;
therefore, the patient's LDH levels in the present study were not
assessed before June 2020.
Most cancer patients with a Chinese background tend
to seek TCM treatment (28), which
has been reported to alleviate clinical symptoms, prolong survival
time and decrease the adverse effects of conventional therapy
(29). The pathogenesis theory of
cancerous toxins is a TCM-based theory on the differentiation and
treatment of tumors (30). Studies
have reported that TCM decoctions, such as the Long-Zhua-Jie-Du
decoction based on the pathogenesis theory of cancerous toxins, may
have anticancer effects on advanced lung cancer (31). Tumorigenesis is a multistep process,
and its occurrence and developmental mechanisms remain to be fully
explored. In a case where single therapy may have little or no
effect, holistic integrative medicine may be utilized (32). Furthermore, a combination of
multiple therapies, including conventional therapy, targeted
therapy, immunotherapy, and complementary and alternative
medicines, such as TCM (33), are a
relatively newer route for the treatment of tumor and metastasis in
the last 5 years. Research has showed that Qianjinweijing decoction
combined with chemotherapy improves the quality of life of
patients, and prolongs the survival time of patients with advanced
NSCLC (34).
In conclusion, the present patient survived for
>4 years following lung cancer progression, which is notably
longer than expected for patients with advanced lung cancer. The
present case demonstrated the efficacy of targeted therapy and
anti-PD-1 immunotherapy for the treatment of advanced lung cancer
following the occurrence of drug resistance and progressive
disease.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Key R&D
Program of China (grant no. 2022YFC3500200), the Innovation Team
and Talents Cultivation Program of National Administration of
Traditional Chinese Medicine (grant no. ZYYCXTD-C-202208), the
Traditional Chinese Medicine Inheritance and Innovation ‘Tens of
Millions’ Talent Project (Qihuang Project) of National
Administration of Traditional Chinese Medicine and Jiangsu
Postgraduate Practice Innovation Plan (grant no. SJCX22_0706).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
TG and HC made substantial contributions to the
conception and design of the study. TG, WZ and SZ were primarily
responsible for writing the manuscript. TG, WZ, SZ, WQ, YW, XL and
FK were responsible for collecting the patient's clinical data and
data analysis. TG and HC confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written consent for the
publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hendriks LE, Kerr KM, Menis J, Mok TS,
Nestle U, Passaro A, Peters S, Planchard D, Smit EF, Solomon BJ, et
al: Non-oncogene addicted metastatic non-small-cell lung cancer:
ESMO Clinical Practice Guideline for diagnosis, treatment and
follow-up. Ann Oncol. 34:358–376. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leone GM, Candido S, Lavoro A, Vivarelli
S, Gattuso G, Calina D, Libra M and Falzone L: Clinical relevance
of targeted therapy and immune-checkpoint inhibition in lung
cancer. Pharmaceutics. 15:12522023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jurisic V, Vukovic V, Obradovic J,
Gulyaeva LF, Kushlinskii NE and Djordjević N: EGFR polymorphism and
survival of NSCLC patients treated with TKIs: A systematic review
and meta-analysis. J Oncol. 2020:19732412020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Obradovic J, Todosijevic J and Jurisic V:
Side effects of tyrosine kinase inhibitors therapy in patients with
non-small cell lung cancer and associations with EGFR
polymorphisms: A systematic review and meta-analysis. Oncol Lett.
25:622022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Everest L, Shah M and Chan KKW: Comparison
of long-term survival benefits in trials of immune checkpoint
inhibitor vs. Non-immune checkpoint inhibitor anticancer agents
using ASCO value framework and ESMO magnitude of clinical benefit
scale. JAMA Netw Open. 2:e1968032019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Falzone L, Bordonaro R and Libra M:
SnapShot: Cancer chemotherapy. Cell. 186:1816.e12023. View Article : Google Scholar
|
|
8
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, D'Amico
TA, et al: Non-Small cell lung cancer, version 3.2022, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
20:497–530. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bironzo P and Di Maio M: A review of
guidelines for lung cancer. J Thorac Dis. 10 (Suppl
13):S1556–S1563. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hanna N, Johnson D, Temin S, Baker S Jr,
Brahmer J, Ellis PM, Giaccone G, Hesketh PJ, Jaiyesimi I, Leighl
NB, et al: Systemic therapy for stage IV Non-small-cell lung
cancer: American Society of Clinical Oncology Clinical Practice
Guideline Update. J Clin Oncol. 35:3484–3515. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li C, Qiu Y and Zhang Y: Research progress
on therapeutic targeting of cancer-associated fibroblasts to tackle
treatment-resistant NSCLC. Pharmaceuticals (Basel). 15:14112022.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leonetti A, Sharma S, Minari R, Perego P,
Giovannetti E and Tiseo M: Resistance mechanisms to osimertinib in
EGFR-mutated non-small cell lung cancer. Brit J Cancer.
121:725–737. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee KWC, Li MSC, Gai W, Lau YM, Chan AKC,
Chan OSH, Lee CK, Yeung RMW, Fung SYH, Cheung WF, et al: Testing
for EGFR variants in pleural and pericardial effusion cell-free DNA
in patients with non-small cell lung cancer. JAMA Oncol. 9:261–265.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Patsoukis N, Wang Q, Strauss L and
Boussiotis VA: Revisiting the PD-1 pathway. Sci Adv.
6:eabd27122020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang Z, Yang N, Ou Q, Xiang Y, Jiang T, Wu
X, Bao H, Tong X, Wang X, Shao YW, et al: Investigating novel
resistance mechanisms to third-generation EGFR tyrosine kinase
inhibitor osimertinib in non-small cell lung cancer patients. Clin
Cancer Res. 24:3097–3107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shu Y, Wu X, Tong X, Wang X, Chang Z, Mao
Y, Chen X, Sun J, Wang Z, Hong Z, et al: Circulating tumor DNA
mutation profiling by targeted next generation sequencing provides
guidance for personalized treatments in multiple cancer types. Sci
Rep. 7:5832017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu S, Sagiv O, Rubin ML, Sa HS, Tetzlaff
MT, Nagarajan P, Ning J and Esmaeli B: Validation Study of the AJCC
Cancer Staging Manual, Eighth Edition, Staging System for eyelid
and periocular squamous cell carcinoma. JAMA Ophthalmol.
137:537–542. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou Q and Wu YL: Developing CSCO lung
cancer practice guidelines stratified by resource availability and
treatment value. J Glob Oncol. 3:285–288. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang CY, Yeh YC, Wang LC, Lin YY, Lin SY,
Wang SY, Chu PY, Liu ZY, Su YC, Ho HL and Chou TY: Genomic
profiling with large-scale next-generation sequencing panels
distinguishes separate primary lung adenocarcinomas from
intrapulmonary metastases. Mod Pathol. 36:1000472023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song X, Xiong A, Wu F, Li X, Wang J, Jiang
T, Chen P, Zhang X, Zhao Z, Liu H, et al: Spatial multi-omics
revealed the impact of tumor ecosystem heterogeneity on
immunotherapy efficacy in patients with advanced non-small cell
lung cancer treated with bispecific antibody. J Immunother Cancer.
11:e0062342023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Villaruz LC, Blumenschein GR Jr, Otterson
GA and Leal TA: Emerging therapeutic strategies for enhancing
sensitivity and countering resistance to programmed cell death
protein 1 or programmed death-ligand 1 inhibitors in non-small cell
lung cancer. Cancer. 129:1319–1350. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mok TSK, Kim SW, Wu YL, Nakagawa K, Yang
JJ, Ahn MJ, Wang J, Yang JC, Lu Y, Atagi S, et al: Gefitinib plus
chemotherapy versus chemotherapy in epidermal growth factor
receptor mutation-positive non-small-cell lung cancer resistant to
first-line gefitinib (IMPRESS): Overall survival and biomarker
analyses. J Clin Oncol. 35:4027–4034. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He Y and An T: Toripalimab in an advanced
non-small cell lung cancer patient with poor general condition
after multiline treatment: A case report. J Int Med Res.
49:30006052110429882021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou J, Zhou F, Xie H, Wu Y, Zhao J and Su
C: An advanced non-small cell lung cancer patient with epidermal
growth factor receptor sensitizing mutation responded to
toripalimab in combination with chemotherapy after resistance to
osimertinib: A case report. Transl Lung Cancer Res. 9:354–359.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang T, Wang P, Zhang J, Zhao Y, Zhou J,
Fan Y, Shu Y, Liu X, Zhang H, He J, et al: Toripalimab plus
chemotherapy as second-line treatment in previously EGFR-TKI
treated patients with EGFR-mutant-advanced NSCLC: A multicenter
phase-II trial. Signal Transduct Target Ther. 6:3552021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang J, Wang Z, Wu L, Li B, Cheng Y, Li X,
Wang X, Han L, Wu X, Fan Y, et al: MA13.08 CHOICE-01: A Phase 3
study of toripalimab versus placebo in combination with first-line
chemotherapy for advanced NSCLC. J Thorac Oncol. 16:S927–S928.
2021. View Article : Google Scholar
|
|
27
|
Jurisic V, Radenkovic S and Konjevic G:
The actual role of LDH as tumor marker, biochemical and clinical
aspects. Adv Exp Med Biol. 867:115–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Y, Lou Y, Wang J, Yu C and Shen W:
Research status and molecular mechanism of the traditional chinese
medicine and antitumor therapy combined strategy based on tumor
microenvironment. Front Immunol. 11:6097052021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Br Cassileth KS and Yeung: Supportive
Cancer Care with Chinese Medicine. Focus on Alternative and
Complementary Therapies. 15:261–262. 2010. View Article : Google Scholar
|
|
30
|
Sang T, Qiu W, Li W and Zhou H, Chen H and
Zhou H: The Relationship between prevention and treatment of
colorectal cancer and cancerous toxin pathogenesis theory basing on
gut microbiota. Evid Based Complement Alternat Med.
2020:71625452020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang S, Fu Jl, Hao Hf, Jiao YN, Li PP and
Han SY: Metabolic reprogramming by traditional Chinese medicine and
its role in effective cancer therapy. Pharmacol Res.
170:1057282021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fan D: Holistic integrative medicine:
Toward a new era of medical advancement. Front Med. 11:152–159.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang L, Gong Y, Zhang L, Liang B, Xu H,
Hu W, Jin Z, Wu X, Chen X, Li M, et al: Gou Qi Zi inhibits
proliferation and induces apoptosis through the PI3K/AKT1 signaling
pathway in non-small cell lung cancer. Front Oncol. 12:10347502022.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu P, Zhao Q, Xu Y, Ye J, Tan J, Hou J,
Wang Y, Li J, Cui W, Wang S and Wang X: A Chinese classical
prescription Qianjinweijing Decoction in treatment of lung cancer:
An overview. Biomed Pharmacother. 156:1139132022. View Article : Google Scholar : PubMed/NCBI
|