Introduction
According to the World Health Organization (WHO),
H3K27M-mutant diffuse midline glioma (DMG) is a grade IV malignancy
that occurs at any age with no sex predilection, but commonly
occurs in children aged 5–11 years old, rarely occurs in
middle-aged and elderly people (1).
Although epidemiological data remain scant for H3K27M-mutant DMG,
the incidence of pediatric diffuse intrinsic pontine glioma (DIPG)
is estimated to be 0.54 cases per 1million person-years (2). H3K27M-mutant DMG represents 10–15% of
pediatric brain tumors and occurs at a prevalence of ~75% in DIPG
(3). Furthermore, pediatric DMG is
mainly located in the brainstem, thalamus, or spinal cord, and is
often identified in a relatively advanced stage, this indicate a
low degree of resection and the patient often has some serious
symptoms, such as headache, vomiting, nerve palsy, ataxia and even
respiratory and cardiac arrest. The prognosis of patients with this
disease is poor, with a median survival time of 1 year from
diagnosis (1). Glioma is
characterized by local recurrence and invasion; nevertheless,
extraneural metastases (ENM) are rare, occurring in <2% of
patients with glioma (4,5). Thus far, only five cases of ENM in
patients with H3K27M-mutant DMG have been reported in the
literature (6–10). In the present study, a pediatric
case of brainstem H3K27M-mutant DMG is reported, with an unusual
presentation involving multiple vertebral metastases and extensive
craniospinal leptomeningeal dissemination. Furthermore, the
relevant literature is discussed.
Case report
A 9-year-old male patient presented with a headache,
nausea and vomiting that had persisted for two weeks. He initially
attended at Beijing Tiantan Hospital (Beijing, China) in August
2019 and then was admitted to the Second Hospital of Hebei Medical
University in Shijiazhuang on September 2019. Magnetic resonance
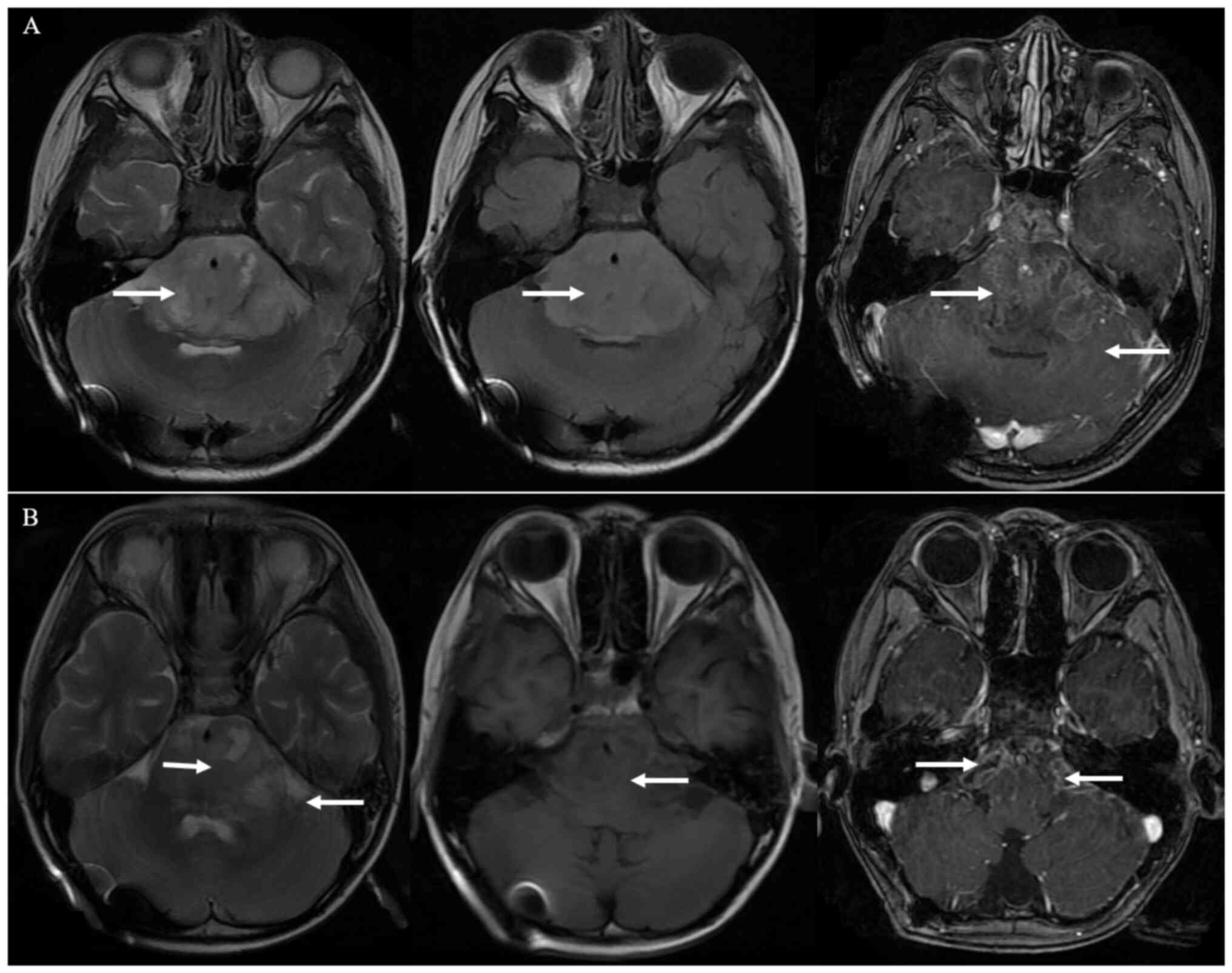
imaging (MRI) of the brain revealed the presence of a
6.0×5.4×5.9-cm3 irregular, solid and cystic mass in the
brainstem, with a slightly dilated supratentorial ventricle
(Fig. 1A). Additionally, the tumor
appeared hyperintense on T2, hypointense on T1, and T1-weighted
with heterogeneous internal enhancement. MRI of the spine did not
detect metastases. The patient had headaches and vomiting for
supratentorial ventricular dilation caused by tumor compression. so
subsequently, both a stereotactic biopsy and right
ventriculoperitoneal shunt were performed; postoperative the
patient vomiting disappeared and headache was alleviated.
Histological sections of PXAs (4 µm thick) were prepared from 10%
formalin-fixed paraffin-embedded tissue blocks for 48 h at room
temperature as follows: Tissue chips were placed in a 60°C oven for
20 min, then soaked in xylene for 3 times with 10 min per time,
soaked in absolute ethanol for 3 times with 5 min per time and
soaked successively in 90, 80, 70% ethanol for 3 times with 5 min
per time, soaked successively in tap water and distilled water for
5 min), HE stain (Hematoxylin staining for 5 min and eosin staining
for 10 s at room temperature), etc. Images were obtained using a
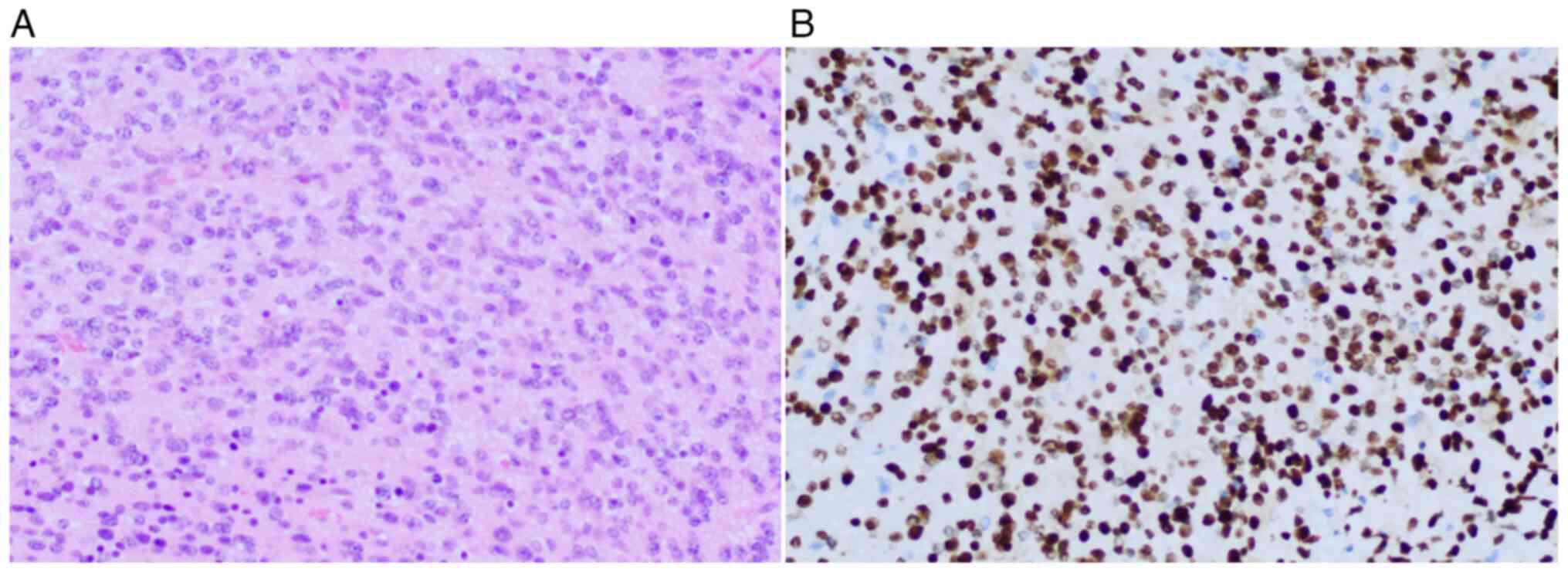
Leica light camera. Histological assessment revealed an anaplastic
astrocytic glioma with areas of variable cellularity, atypia and
focal mitotic activity (Fig. 2A).
Immunohistochemical testing of the tissue demonstrated that
H3K27M-mutant protein was expressed in tumor cells (cat. no.
GT236902; Gene Tech Co., Ltd; Fig.
2B). As a result, the medical team reached a histopathological
diagnosis of H3K27M-mutant DMG (WHO grade IV). Histochemical
testing and genetic sequencing would have been beneficial to
support the understanding of the present case but were not
available.
Chemoradiotherapy was initiated 40 days after the
right ventriculoperitoneal shunt surgery. The gross tumor volume
(GTV) was defined as the area of T1-weighted and T2-flair abnormal
signals. The clinical target volume (CTV) was the expansion of the
GTV by 0.5 cm. Subsequently, the planning target volume (PTV) was
set as a 3-mm margin around the CTV. The prescribed dose to the PTV
was 50 Gy/25 fractions (2 Gy/fraction). Accordingly, the patient
received 75 mg/m2/day oral temozolomide throughout the
period of radiotherapy for 5 weeks. The tumor volume was slightly
reduced 1 month later based on the Response Assessment in
Neuro-Oncology criteria (11)
(Fig. 1B). Adjuvant chemotherapy
with temozolomide was performed intermittently for five cycles. The
dose in cycle 1 was 150 mg/m2/day, whilst in cycles 2–5
(all cycles administered on days 1–5, every 28 days) it was 200
mg/m2/day.
Drooping was observed on the right side of the
patient's mouth while laughing, and the patient reported waist and
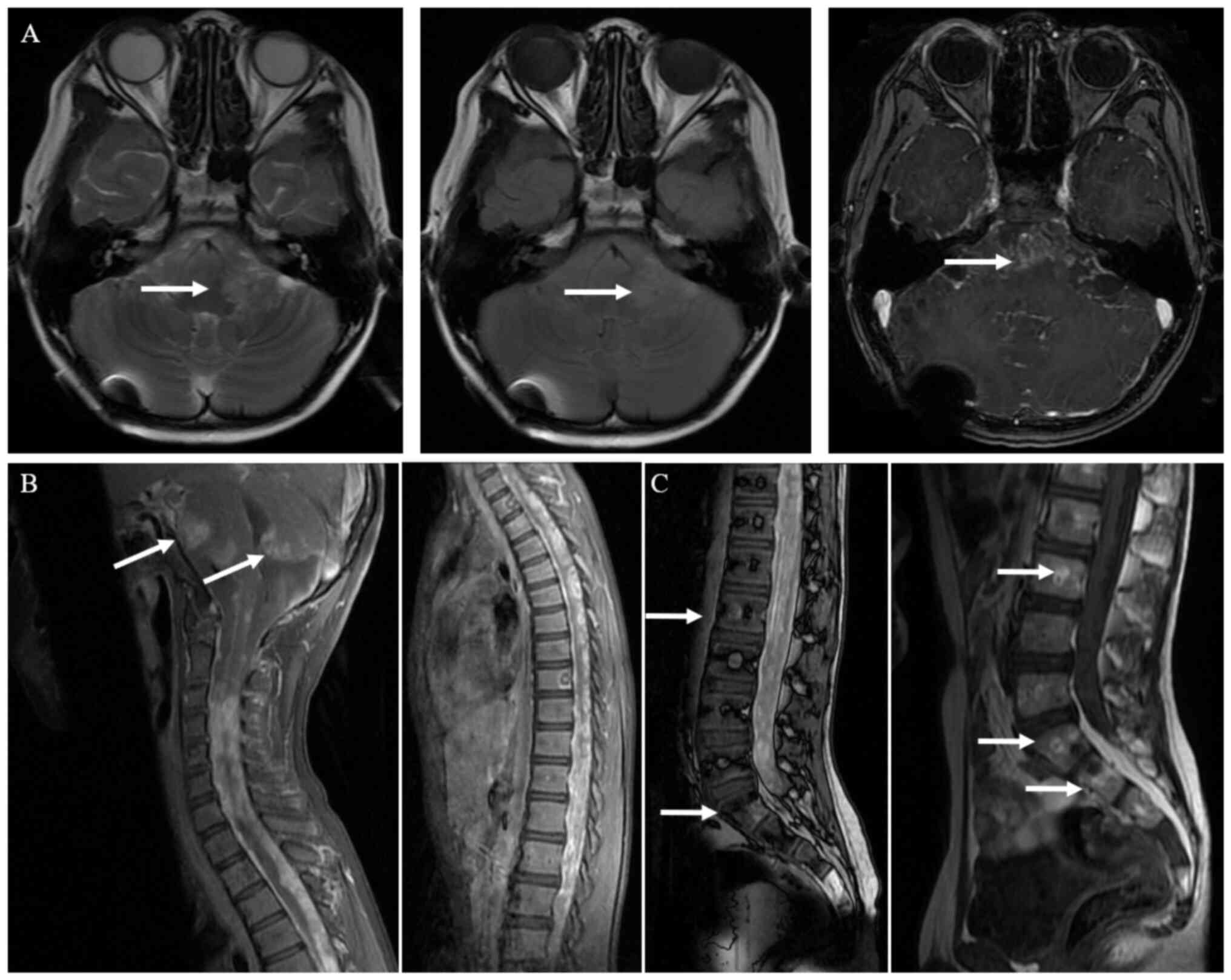
back pain 2 months after chemotherapy. MRI demonstrated nodular and
patched enhancement in the tumor located in the brainstem and
cerebellum. Around the fourth ventricle, the tumor was enlarged
compared with prior to treatment. Furthermore, enhanced MRI of the
spine showed diffuse metastases in the spinal cord and craniospinal
leptomeningeal dissemination with abnormal enhancement. Several
lumbar-sacral metastases with bone destruction and abnormal
enhancement were also noted (Fig.
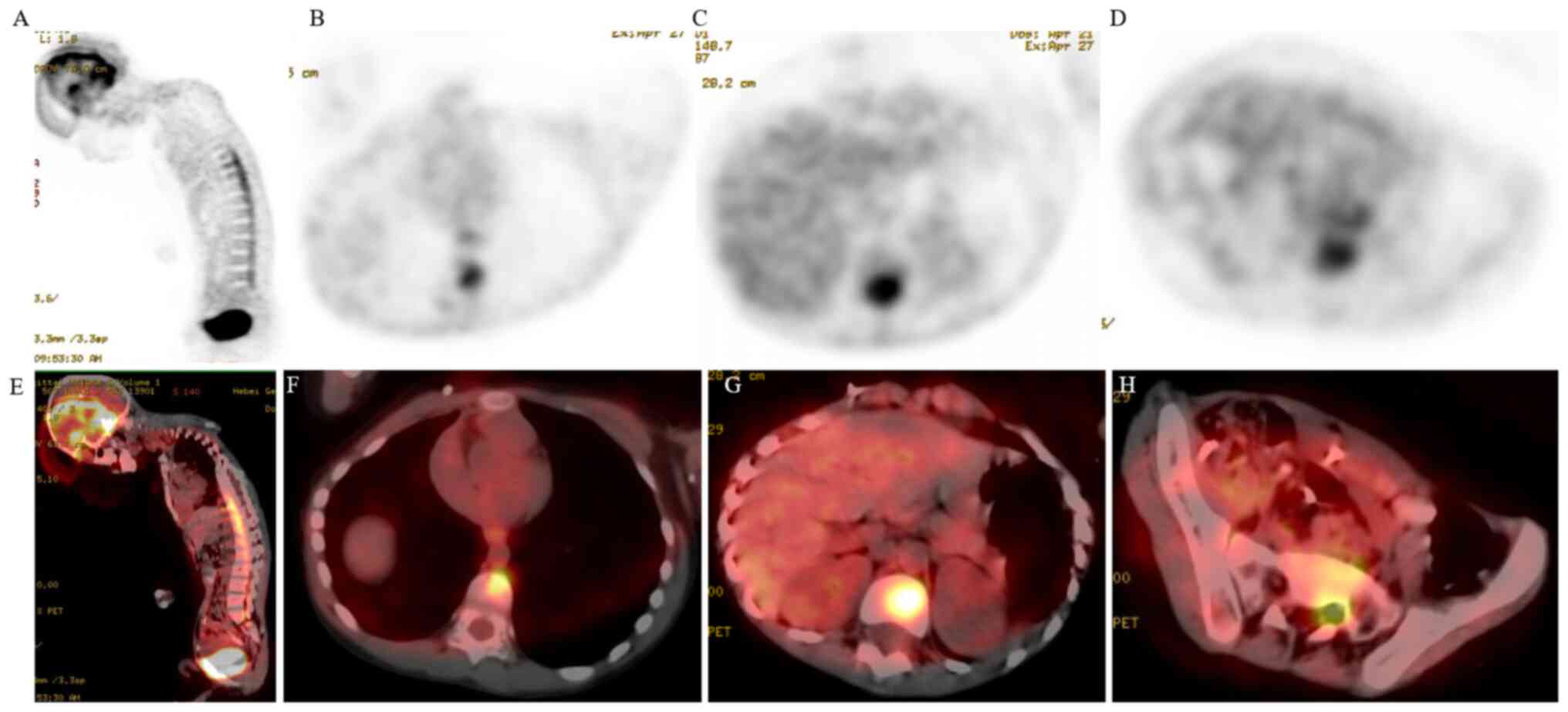
3). Examination using fluorine-18-fluorodeoxyglucose
positron-emission tomography-computed tomography (PET-CT) also
demonstrated multiple metastases in the spinal membrane and a
diffuse increase of metabolism in the cervical, thoracic and
lumbosacral vertebral areas (Fig.
4). Subsequently, the patient rapidly developed neck pain,
stiffness and urinary retention. Based on discussions between the
members of a multidisciplinary medical team, the patient underwent
one cycle of chemotherapy with cyclophosphamide 600 mg on day 1,
vincristine 1mg on day 1 and cisplatin 20 mg on day 1–3, one cycle
every 28 days. Moreover, pain relievers (Tramadol injection 50 mg
twice daily) and dehydrating drugs (Mannitol 100 ml twice daily,
Dexamethasone 3 mg once a day) were administered to relieve the
symptoms. However, the condition did not improve and the patient
died 4 weeks after diagnosis of ENM due to disease progression.
Discussion
H3K27M-mutant DMG refers to a tumor type classified
as a central nervous system (CNS) tumor by the WHO, based on the
molecular signature, with the 2016 WHO classification of CNS tumors
recognizing H3K27M-mutant DMG as a clinical pathological entity
(1). This malignancy mostly
develops in children and seldomly in adults (1). DMG is accompanied by a mutation of
histone H3K27M and its growth is diffuse and invasive (12). Karremann et al (13) reported that H3K27M mutation was the
sole independent prognostic factor, indicating a poor prognosis,
meaning that the prognosis of H3K27M-mutant DMG was not associated
with the extent, location and grade of the tumor.
High-grade glioma (HGG) accounts for 8–15% of
pediatric CNS tumors (14,15) and ~50% of the cases occur in the
midline location, namely DMG (16).
Notably, diffuse intrinsic pontine glioma refers to the intrinsic
pontine type of DMG. In these cases, the H3K27M heterozygous
somatic mutation, which occurs in pediatric diffuse intrinsic
pontine glioma, has a prevalence rate of 78%. The rate of this
mutation in other DMGs, such as thalamic and spinal gliomas, is
~22% (17). The biological
manifestation is highly malignant and corresponds to that of a
grade IV lesion. The prognosis of H3K27M-mutant DMG is generally
poor, with a 2-year overall survival rate of <10% (18–20).
HGG is highly invasive locally, occasionally
spreading along the neuraxis. Autopsy studies have reported that
metastases in the neuraxis occur in ~20% of patients with HGG
(4,5,7).
However, the estimated incidence of ENM from intracranial malignant
gliomas is 0.4–2% of all cases (4,5). The
most commonly reported sites of HGG-ENM are the lung/pleural cavity
(60%), lymph nodes (51%), bones (31%) and liver (22%) (21–23).
The low incidence rate may be related to the barrier of the CNS and
the short life span of the patients; malignant glioma cells rarely
have the necessary time to breach the protective intrinsic
biological obstacles and thus, develop into ENM (24,25).
It has been reported that iatrogenic factors, encompassing vascular
invasion, and cranial nerve perineural and lymphatic spread may be
responsible for ENM in a number of cases (4,26). In
addition, the most recurrent site of bony metastasis is the axial
skeleton; the vertebrae are the most common site (73%), followed by
the ribs and sternum (26). This
indicates that venous invasion may reflux into the Batson plexus of
the spine. Metastatic glioma cells disseminate into the spinal
fluid and enter the Batson plexus, which supplies the vertebrae
with blood, thus facilitating the metastasis to the vertebrae
(4,8).
ENM in H3K27M-mutant DMG is extremely rare. Only
five cases of H3K27M-mutant DMG with ENM have been reported
(6–10) since it was acknowledged as a
clinical pathological entity (1)
and was classified as pediatric-type diffuse HGG in 2021 (27). Of the aforementioned five cases, two
cases were of peritoneal cavity metastases and three were bony
metastases. All five cases involved children. The characteristics
and treatment results of the five published cases of H3K27M-mutant
DMG with ENM are summarized in Table
I.
 | Table I.Cases of H3K27M-mutant diffuse midline
glioma with multiple extraneural metastases. |
Table I.
Cases of H3K27M-mutant diffuse midline
glioma with multiple extraneural metastases.
| First author,
year | Age of patient,
years | Presentation | Location of
tumor | Intervention | H3K27M status | Adjuvant
treatment | Overall survival,
months | (Refs.) |
|---|
| Stephens et
al, 2019 | 4 | Headache, left facial
droop, reduced vision and partial ptosis | Suprasellar cistern
lesion, spinal cord metastasis, and intra-abdominal metastases | Surgical debulking,
bilateral ventriculoperitoneal shunts and ascitic drainage | + | Radiotherapy and
chemotherapy | 4 | (6) |
| Bhatt et al,
2020 | 15 | Headache, neck
stiffness, paraparesis and back pain | Fourth ventricle,
craniospinal pial seeding, vertebral, rib and pelvis
metastases | Open spinal biopsy
and bone marrow aspiration from iliac rest | + | None | 0.5 | (7) |
| Handis et
al, 2021 | 16 | Blindness, back
pain, paraplegia, and urinary and fecal incontinence | Spinal
intramedullary, craniospinal pial seeding and multiple vertebral
metastases | Open spinal biopsy
and concurrent bone marrow aspiration from vertebral body | + | Radiotherapy and
chemotherapy | 5 | (8) |
| Lazow et al,
2022 | 12 | Bilateral lower
weakness, back pain, bowel/bladder incontinence and diplopia | Osseous (vertebrae,
pelvis, sternum, bilateral femurs and humeri) and pulmonary
nodules | Open spinal biopsy
and left iliac osseous disease | + | Radiotherapy and
molecularly targeted therapy-cabozantinib | 9 | (9) |
| Mohiuddin et
al, 2021 | 17 | Headaches,
diplopia, paresthesia, dizziness and short-term memory loss | Left hippocampus,
midbrain, spinal cord, chest abdomen, pelvis lymph nodes, liver and
omental fat stranding | Stereotactic biopsy
and ventriculoperitoneal shunt | + | Radiotherapy and
chemotherapy | 5 | (10) |
| Present study | 9 | Headache, nausea,
vomiting, mouth skew upon laughing, back pain, neck stiffness and
pain, and urinary retention | Brain stem,
craniospinal pial seeding, spinal intramedullary, craniospinal pial
seeding and multiple vertebral metastases | Stereotactic biopsy
and right ventriculoperitoneal shunt | + | Chemoradiotherapy
and chemotherapy | 10 | - |
Stephens et al (6) reported a case of H3K27M-mutant glioma
with peritoneal cavity seeding in a 4-year-old male patient. The
patient presented with a large, solid and cystic lesion centered on
the suprasellar cistern, and received radiochemotherapy for the
primary lesion. Moreover, the patient underwent bilateral
ventriculoperitoneal shunting due to acute hydrocephalus. Massive
ascites developed due to histologically confirmed intra-abdominal
glioma metastasis at 14 months after the initial diagnosis. The
patient died 1 month later. Another case of H3K27M-mutant DMG with
peritoneal metastases, accompanied by spinal cord metastases,
involved a 17-year-old female patient. The patient successively
underwent chemoradiotherapy and ventriculoperitoneal shunting.
Nevertheless, chest, abdominal and liver metastases, and pleural
and multifocal soft tissue metastases rapidly developed, and the
patient died 5 months after the diagnosis (10). Both of the aforementioned patients
underwent bilateral ventriculoperitoneal shunting and the
subsequent presentations implied that the dissemination to the
peritoneum may have occurred via the ventriculoperitoneal
shunts.
Bhatt et al (7) was the first to report a pediatric case
of H3K27M-mutant DMG with diffuse bony metastases. A 15-year-old
female patient presented with a lesion in the fourth ventricle,
innumerable intradural lesions and leptomeningeal seeding
throughout the neuraxis, as well as several osteoblastic lesions
involving the spine, ribs, sternum, pelvis, humerus and femurs. The
pathological analysis demonstrated the presence of H3K27M-mutant
DMG with metastasis. The patient died 2 weeks after the initial
presentation. The second reported case of pediatric H3K27M-mutant
DMG with this type of presentation involved a 16-year-old female
patient who had multiple vertebral metastases within bony
structures and craniospinal pial seedings (8). The patient died 5 months after the
diagnosis. The third case involved a 12-year-old with H3K27M-mutant
DMG and vertebral and lung metastases, who received radiotherapy
and molecularly targeted therapy with cabozantinib (9). The patient expired 9 months after the
initial diagnosis.
The present case was the fourth report of a
pediatric patient with H3K27M-mutant DMG and multiple vertebral
metastases. A pathological biopsy of the vertebrae was not
performed in this case. Both MRI and PET-CT demonstrated multiple
vertebral lesions and the patient presented with multiple vertebral
metastases and extensive craniospinal leptomeningeal
dissemination.
The most common cause of bone metastasis may be
venous invasion to the Batson plexus of the spine (28). Recent study have reported that
malignant tumor cells may migrate through nerve roots (29). All the aforementioned cases reported
multiple vertebral metastases with extensive craniospinal pial
seeding. This may help glioma cells to metastasize along the nerve
roots. Therefore, it is hypothesized that the ENM in these patients
may migrate via the spinal nerve roots. However, additional
clinical cases are required to assess this hypothesis.
Currently, there is no consensus regarding the
optimal treatment for H3K27M-mutant DMG. Due to the location of
lesions, surgical treatment continues to be difficult. Therefore,
traditional radiotherapy can be used to prolong overall survival.
Radiotherapy is an independent clinical parameter influencing
overall survival (30), whereas the
effects of adjuvant chemotherapy are limited. Drugs targeting
vascular endothelial growth factor and epidermal growth factor
receptor, as well as anti-angiogenic and several multi-kinase
inhibitors, have not exhibited satisfactory efficacy (31–33).
However, programmed cell death-ligand 1/programmed cell death-1
inhibitors may be a beneficial treatment option for such patients
(34).
Further research is warranted to develop innovative
and effective treatment strategies for patients with H3K27M-mutant
DMG. H3K27M mutations alter methylation level and there are
numerous molecular pathological mechanisms that remain unknown
(16). An enhanced understanding of
this mutation and the emergence of targeted drugs may lead to the
development of more rational therapeutic methods, which may improve
overall survival.
In conclusion, the occurrence of ENM in patients
with H3K27M-mutant DMG is exceedingly rare, and the mechanisms
underlying the development of such metastases remain unclear.
Therefore, the present case report provides insight into the
clinical characteristics and metastasis mechanisms of this
aggressive disease and may help to elucidate new pathways for the
management of ENM. However, additional studies are needed in this
field.
Acknowledgements
Not applicable.
Funding
The present study was supported by Hebei Natural Science
Foundation (grant no. H2022206430) and the S&T Program of Hebei
(grant nos 22377723D and 236Z7718G).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XX designed the study. XG participated in the study
conception, performed the study, carried out the literature search
and wrote the paper. YY participated in the treatment of the
patient, analyzed aggregated data and helped write the manuscript.
WW participated in the acquisition of data and the histological
examination of the sample. LT and ZT participated in the
radiological examination of the images. GZ participated in the
acquisition of data and revision of the manuscript. ZT participated
in the critical review and substantively revised it. All authors
have read and approved the final manuscript. XG and YY confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The present case report was approved by the
Institutional Review Board of Hebei Medical University
(Shijiazhuang, China), ethics approval number:2023-R100.
Patient consent for publication
Consent for publication of the case report and
associated images was obtained from the patient's mother.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DMG
|
diffuse midline glioma
|
|
ENM
|
extraneural metastases
|
|
GTV
|
gross tumor volume
|
|
CTV
|
clinical target volume
|
|
PTV
|
planning target volume
|
|
MRI
|
magnetic resonance imaging
|
|
PET-CT
|
positron emission tomography-computed
tomography
|
References
|
1
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
Classification of Tumors of the Central Nervous System: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mackay A, Burford A, Carvalho D, Izquierdo
E, Fazal-Salom J, Taylor KR, Bjerke L, Clarke M, Vinci M,
Nandhabalan M, et al: integrated molecular meta-analysis of 1,000
pediatric high-grade and diffuse intrinsic pontine glioma. Cancer
Cell. 32:520–537.e5. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ostrom QT, Price M, Ryan K, Edelson J,
Neff C, Cioffi G, Waite KA, Kruchko C and Barnholtz-Sloan JS:
CBTRUS statistical report: Pediatric brain tumor foundation
childhood and adolescent primary brain and other central nervous
system tumors diagnosed in the United States in 2014–2018. Neuro
Oncol. 24 (Suppl 3):iii1–1iii38. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hamilton JD, Rapp M, Schneiderhan T, Sabel
M, Hayman A, Scherer A, Kröpil P, Budach W, Gerber P, Kretschmar U,
et al: Glioblastoma multiforme metastasis outside the CNS: Three
case reports and possible mechanisms of escape. J Clin Oncol.
32:e80–e84. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beauchesne P: Extra-neural metastases of
malignant gliomas: Myth or reality? Cancers (Basel). 3:461–477.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stephens S, Tollesson G, Robertson T and
Campbell R: Diffuse midline glioma metastasis to the peritoneal
cavity via ventriculo-peritoneal shunt: Case report and review of
literature. J Clin Neurosci. 67:288–293. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bhatt NS, Houser K, Belongia M, Ellison
DW, Foy A, Jarzembowski J, Kelly T, Maheshwari M, Suchi M and
Knipstein J: Diffuse midline glioma with osseous metastases at
diagnosis: A case report. J Pediatr Hematol Oncol. 42:e673–e676.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Handis C, Tanrıkulu B, Danyeli AE and Özek
MM: Spinal intramedullary H3K27M mutant glioma with vertebral
metastasis: A case report. Childs Nerv Syst. 37:3933–3937. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lazow MA, Leach JL, Trout AT, Breneman JC,
Fouladi M and Fuller C: Extraneural metastases of diffuse midline
glioma, H3 K27M-Mutant at diagnosis: Case report, review of the
literature, and identifying targetable alterations. J Pediatr
Hematol Oncol. 44:e597–e604. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mohiuddin S, Maraka S, Usman Baig M, Gupta
S, Muzzafar T, Valyi-Nagy T, Lindsay H, Moody K, Razvi S, Paulino
A, et al: Case series of diffuse extraneural metastasis in H3F3A
mutant high-grade gliomas: Clinical, molecular phenotype and
literature review. J Clin Neurosci. 89:405–411. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chukwueke UN and Wen PY: Use of the
Response Assessment in Neuro-Oncology (RANO) criteria in clinical
trials and clinical practice. CNS Oncol. 8:CNS282019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yi S, Choi S, Shin DA, Kim DS, Choi J, Ha
Y, Kim KN, Suh CO, Chang JH, Kim SH and Yoon DH: Impact of H3.3
K27M mutation on prognosis and survival of grade IV spinal cord
glioma on the basis of New 2016 World Health Organization
classification of the central nervous system. Neurosurgery.
84:1072–1081. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Karremann M, Gielen GH, Hoffmann M, Wiese
M, Colditz N, Warmuth-Metz M, Bison B, Claviez A, van Vuurden DG,
von Bueren AO, et al: Diffuse high-grade gliomas with H3 K27M
mutations carry a dismal prognosis independent of tumor location.
Neuro Oncol. 20:123–131. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bondy ML, Scheurer ME, Malmer B,
Barnholtz-Sloan JS, Davis FG, Il'yasova D, Kruchko C, McCarthy BJ,
Rajaraman P, Schwartzbaum JA, et al: Brain tumor epidemiology:
Consensus from the Brain tumor epidemiology consortium. Cancer. 113
(7 Suppl):S1953–S1968. 2008. View Article : Google Scholar
|
|
15
|
Ostrom QT, Gittleman H, Fulop J, Liu M,
Blanda R, Kromer C, Wolinsky Y, Kruchko C and Barnholtz-Sloan JS:
CBTRUS statistical report: Primary brain and central nervous system
tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 17
(Suppl 4):iv1–1iv62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jones C and Baker SJ: Unique genetic and
epigenetic mechanisms driving paediatric diffuse high-grade glioma.
Nat Rev Cancer. 14:10.1038/nrc3811. 2014. View Article : Google Scholar
|
|
17
|
Wu G, Broniscer A, McEachron TA, Lu C,
Paugh BS, Becksfort J, Qu C, Ding L, Huether R, Parker M, et al:
Somatic histone H3 alterations in pediatric diffuse intrinsic
pontine gliomas and non-brainstem glioblastomas. Nat Genet.
44:251–253. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu VM, Alvi MA, McDonald KL and Daniels
DJ: Impact of the H3K27M mutation on survival in pediatric
high-grade glioma: A systematic review and meta-analysis. J
Neurosurg Pediatr. 23:308–316. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Navarro RE, Golub D, Hill T, McQuinn MW,
William C, Zagzag D and Hidalgo ET: Pediatric midline H3K27M-mutant
tumor with disseminated leptomeningeal disease and glioneuronal
features: Case report and literature review. Childs Nerv Syst.
37:2347–2356. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Feng LL, Ji PG, Liu JH, Guo SC,
Zhai YL, Sankey EW, Wang Y, Xue YR, Wang N, et al: Clinical
features and molecular markers on diffuse midline gliomas With
H3K27M Mutations: A 43 cases retrospective cohort study. Front
Oncol. 10:6025532020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gamis AS, Egelhoff J, Roloson G, Young J,
Woods GM, Newman R and Freeman AI: Diffuse bony metastases at
presentation in a child with glioblastoma multiforme. A case
report. Cancer. 66:180–184. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Beauchesne P, Soler C and Mosnier JF:
Diffuse vertebral body metastasis from a glioblastoma multiforme: A
technetium-99m Sestamibi single-photon emission computerized
tomography study. J Neurosurg. 93:887–890. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Seoane J and De Mattos-Arruda L: Escaping
out of the brain. Cancer Discov. 4:1259–1261. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Piccirilli M, Brunetto GM, Rocchi G,
Giangaspero F and Salvati M: Extra central nervous system
metastases from cerebral glioblastoma multiforme in elderly
patients. Clinico-pathological remarks on our series of seven cases
and critical review of the literature. Tumori. 94:40–51. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bernstein JJ and Woodard CA: Glioblastoma
cells do not intravasate into blood vessels. Neurosurgery.
36:124–132; discussion 132. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goodwin CR, Liang L, Abu-Bonsrah N, Hdeib
A, Elder BD, Kosztowski T, Bettegowda C, Laterra J, Burger P and
Sciubba DM: Extraneural glioblastoma multiforme vertebral
metastasis. World Neurosurg. 89:578–582.e3. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Smith HL, Wadhwani N and Horbinski C:
Major Features of the 2021 WHO Classification of CNS Tumors.
Neurotherapeutics. 19:1691–1704. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li ZG, Zheng MY, Zhao Q, Liu K, Du JX and
Zhang SW: Solitary vertebral metastatic glioblastoma in the absence
of primary brain tumor relapse: A case report and literature
review. BMC Med Imaging. 20:892020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang W, Cai YY, Wang XL, Wang XX, Li Y,
Han GY, Chu YJ, Zhang YX and Hao FR: Bone metastases of
glioblastoma: A case report and review of the literature. Front
Oncol. 11:7054552021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang H, Yang K, Ren X, Cui Y, Li M, Lei Y
and Lin S: Diffuse midline glioma with H3 K27M mutation: A
comparison integrating the clinical, radiological, and molecular
features between adult and pediatric patients. Neuro Oncol.
22:e1–1e9. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Long W, Yi Y, Chen S, Cao Q, Zhao W and
Liu Q: Potential new therapies for pediatric diffuse intrinsic
pontine glioma. Front Pharmacol. 8:4952017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ramaswamy V, Remke M and Taylor MD: An
epigenetic therapy for diffuse intrinsic pontine gliomas. Nat Med.
20:1378–1379. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wiese M, Schill F, Sturm D, Pfister S,
Hulleman E, Johnsen SA and Kramm CM: No significant cytotoxic
effect of the EZH2 inhibitor tazemetostat (EPZ-6438) on pediatric
glioma cells with wildtype histone 3 or mutated histone 3.3. Klin
Padiatr. 228:113–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang G, Fang Y, Zhou M, Li W, Dong D, Chen
J, Da Y, Wang K, Li X, Zhang X, et al: Case report: The effective
response to pembrolizumab in combination with bevacizumab in the
treatment of a recurrent glioblastoma with multiple extracranial
metastases. Front Oncol. 12:9489332022. View Article : Google Scholar : PubMed/NCBI
|