Studies have reported that human hepatocyte cell
lines and primary human blood mononuclear cells produce endogenous
IL-32 and increase its levels in response to IL-1β and TNF-α
stimulation (7–9). In addition, IL-32 is secreted and
produced by primary human keratinocytes, macrophages, T lymphocytes
and NK and mast cells (7,10). Cytokines, such as IL-2, IL-12,
IL-18, IL-1β and IFN-γ, induce the expression of IL-3, whilst
recombinant IL-32 notably induces the expression of TNF-α in the
Raw 264.7 macrophage cell line (11). However, IL-32 can also induce the
opposite effects. For example, it induces release of
anti-inflammatory cytokines in immune cells, such as IL-10, and
immunosuppressive molecules, such as indoleamine 2,3-dioxygenase
(12,13).
In addition to inflammatory disease such as
ulcerative colitis and chronic obstructive pulmonary disease
(COPD), IL-32 is also involved in cancer disease progression. IL-32
is expressed in several types of cancer, such as gastric,
hepatocellular, lung and pancreatic (14,15).
However, the specific functions of each subtype of IL-32 and their
receptors are still unclear. The present review summarizes the
IL-32 receptor, the function of the different IL-32 isoforms,
mechanism of action of IL-32 and its role in four major cancer
types: Colorectal cancer (CRC) and gastric, breast and lung.
Furthermore, the corresponding mechanism of action of IL-32 in
these cancers is summarized.
Experimental analysis has identified proteinase 3
(PR3) with a molecular weight of 30 kDa, which binds specifically
with high affinity to IL-32α (16).
PR3 is a serine protease released in membrane-bound and soluble
forms. It is present in neutrophils and monocytes and acts
independently of enzymatic activity. The primary function of PR3 is
to affect cell proliferation, differentiation and apoptosis. It
also cleaves cytokines to enhance cytokine activity. Furthermore,
the specific binding of IL-32β to PR3 has been elucidated using
surface plasmon resonance. Secreted IL-32 is neutralized or
attenuated by inhibiting the activity of PR3 or by using inactive
PR3. IL-32 is also blocked by inactivating PR3. It may be possible
to exploit the specific binding activity of these molecules for the
clinical treatment of related diseases (17–19).
The aforementioned effects may be due to PR3 exposure resulting in
cleavage of p21 between Thr80 and Gly81, loss of nuclear p21 by
cytoplasmic sequestration and depletion of p21 from
cyclin/cyclin-dependent kinase (CDK) complexes, which attenuates
function of intracellular caspases at the site of inflammation
(20).
Studies have reported that expression of IL-32 is
associated with disease activity and with glomerulonephritis
(21–23). This suggests that IL-32 may be
associated with cancer prognosis. To the best of our knowledge,
however, the receptor for IL-32 has not been elucidated (16). If receptors corresponding to each
subtype of IL-32 are found, the functional role of each subtype may
be elucidated and the targeting effect exploited more efficiently
for clinical applications.
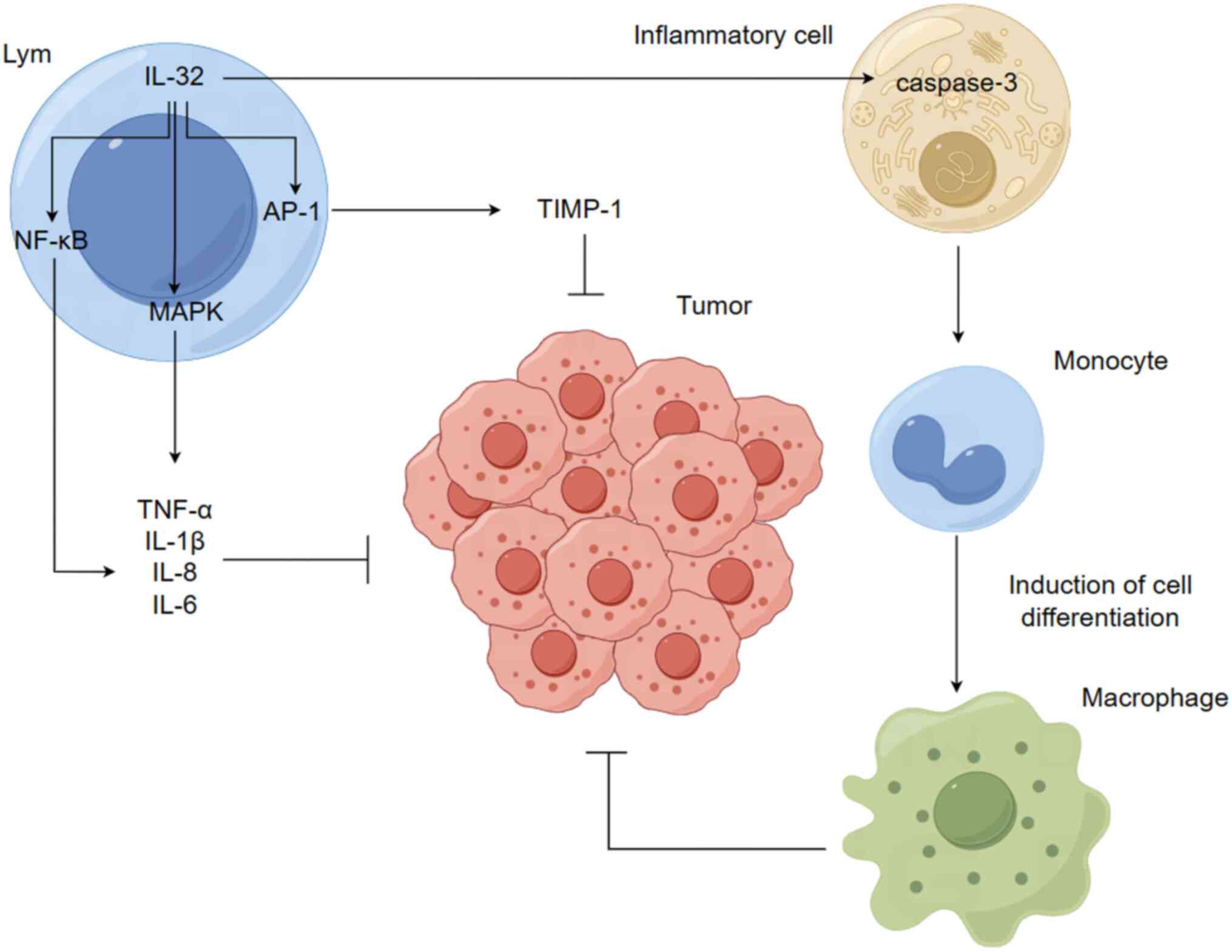
The roles of IL-32 include induction of the
secretion of several cytokines and chemokines and T cell apoptosis
and the enhancement of host defense (24,25).
IL-32 acts primarily by stimulating pro-inflammatory cytokines
through the activation of NF-κB and MAPK p38-mediated production of
TNF-α, IL-1β, IL-8 and IL-6 (2),
increasing tissue inhibitor of metalloprotease (TIMP)-1 promoter
activity and inducing TIMP-1 expression via activation of the
activator protein 1 signaling pathway (26) and serving a key role in macrophage
differentiation via activation of cysteinyl aspartate specific
proteinase-3 (caspase-3; Fig. 1)
(27); studies have reported that
IL-32 induces human monocyte secretion as well as THP-1 cell
differentiation into macrophage-like cells with phagocytic activity
against bacteria (28,29). Muramyl dipeptide, a ligand for
nucleolar oligomerization structural domain 2 receptor, exhibits no
effect on differentiation alone, whereas it enhances
monocyte-to-macrophage differentiation via IL-32 (30–36).
IL-32 also exhibits the opposite effect; in a previous study it was
reported that the granulocyte-macrophage colony-stimulating factor
IL-4-induced differentiation of dendritic cells into
macrophage-like cells is reversed by IL-32 (32,36).
Moreover, IL-32-induced differentiation of monocytes into
macrophages is mediated by a caspase-3-dependent mechanism
(26).
Isoforms of IL-32 do not serve the same roles and
IL-32 may serve completely opposite roles in different types of
cancer. IL-15 could effectively induce IL-32α expression in
dendritic cells (DCs) and additional studies of IL-32α indicate
that IL-32α could act on NK cells to inhibit IL-15-mediated
phosphorylation of STAT5 (37–39).
Moreover, it could inhibit IL-15-induced expression of effector
molecules and cytolytic activity. The biological role of IL-32 as a
cytokine has been demonstrated by inhibition of IL-15α during
co-culture of DCs with NK cells, which is reported to result in the
enhancement of NK cell effector molecule expression and
augmentation of their cytolytic activity. This suggests the
existence of a feedback mechanism of IL-32α in IL-15-mediated NK
cell activation (37–40). IL-32α can also act on DCs by
downregulating IL-15-induced IL-18 production (37). Park et al (40) reported that IL-32θ inhibits
phosphorylation of MAPK and NF-κB in vivo. In addition,
IL-32θ attenuates TNF-α promoter activity and inhibits binding of
NF-κB with the TNF-α promoter (40). Previous studies have also reported
that IL-32γ notably downregulates the expression levels of
important cancer progression proteins, including antiapoptotic,
cell proliferation and tumor progression-promoting genes, while it
markedly induces upregulation of the expression of apoptotic genes
(41–43). Furthermore, IL-32γ decreases levels
of cytokines that promote tumor growth, such as TNF-α, IL-1β and
IL-6, while increasing levels of IL-10 cytokines. IL-32γ inhibits
progression of certain cancer types, such as melanoma, colon
cancer, prostate cancer, liver cancer, and lung cancer (41,44).
In addition, it induces the activation of cytotoxic T and NK cells
to the tumor site to amplify the effect of cancer therapy (30,31).
Studies have reported that IL-32β may promote antitumor effects by
downregulating key cancer progression proteins, including
antiapoptotic, proliferative and cell proliferation regulatory
proteins, via the NF-κB and STAT3 proteins (45–47).
In addition, IL-32β induces expression of proapoptotic proteins and
regulates release of cytokines from colon and prostate cancer cells
(45). However, high expression of
IL-32α activates the NF-κB and STAT3 pathways and induces IL-6
production, thereby promoting cancer progression in patients with
multiple myeloma (48). In summary,
each IL-32 isoform serves a complex role. It is possible that
interactions are present between the isoforms or that their roles
differ due to cancer type and the tumor microenvironment.
Numerous mediators of the inflammatory response,
such as cytokines, free radicals, prostaglandins and growth
factors, induce genetic and epigenetic changes, including point
mutations in tumor suppressor genes, DNA methylation and
post-translational modifications lead to alterations in key
pathways that maintain normal cellular homeostasis, thereby
promoting cancer development and progression (49). In summary, IL-32 is a cytokine that
may serve a key role in the progression of cancer. Table I outlines the major roles and
mechanisms of IL-32 in the progression of four types of
cancers.
Cytokines, such as IL-10, IL-17, IL-22, IL-23 and
IL-35, may have clinical significance in the development of CRC
(50–52). IL-32 has been reported to induce the
release of numerous cytokines and chemokines (24,26),
leading to increased expression of cytokines in the stroma. In a
previous study, immunohistochemical (IHC) and statistical analysis
of cancer tissue from patients with CRC and normal tissue
demonstrated that the lymph node metastatic rate of IL-32(+)-CRC is
60%, which is considerably higher than for the cases without lymph
node metastasis (53). This
indicated that the levels of IL-32 expression may affect the grade
of CRC, in which the overexpression of IL-32 stimulates organic and
lymph node metastasis of CRC. IL-32 may be a biological marker of
CRC metastasis. However, IL-32α expression can inhibit colon cancer
cell proliferation and suppress CRC progression via reactive oxygen
species, c-Jun N-terminal kinases and cysteine signaling. Increased
IL-32α expression can also increase expression of tumor necrosis
factor receptor 1 (TNFR1) and TNFR1-associated death domain protein
(54), which promote cell death and
reduce inflammatory damage (55).
This suggests that elevated IL-32α expression may inhibit CRC
progression.
Studies have established breast cancer tumor
xenograft models and used specific experimental assays, such as MTT
assay and TUNEL staining, to assess the effect of IL-32 on tumor
cell proliferation and apoptosis (59–61).
IL-32 notably increases the rate of cancer cell proliferation and
decreases the rate of cancer cell apoptosis. IL-32 markedly
enhances growth of tumor xenografts in vivo, indicating that
it exerts an inductive effect on the progression of breast cancer.
However, studies have reported the inhibitory effect of IL-32θ on
breast cancer progression (59,62).
The mRNA expression levels of IL-32θ and chemokine ligand 18
(CCL18) have been analyzed in breast cancer tissue by reverse
transcription (RT)-quantitative (q)PCR. To assess the effect of
IL-32θ on cancer metastasis and cancer cell molecular signaling,
in vitro cellular experiments have been performed using
MDA-MB-231 cells expressing IL-32θ. In vivo xenograft, IHC
and optical imaging models have been generated to further evaluate
the in vitro and clinical findings. Clinical data
demonstrate that IL-32θ overexpression notably attenuates the
migration, invasion and release of pro-tumorigenic factors in
breast cancer cells. IL-32θ serves as an intracellular regulator
that inhibits macrophages by targeting CCL18-dependent signaling to
promote breast cancer progression (63). The effects on cancer may be
bidirectional due to the different actions of IL-32 isoforms.
Due to the association between cancer and
inflammation, studies have assessed whether pro-inflammatory
cytokine IL-32 may be involved in lung carcinogenesis and therefore
may be a novel therapeutic target (64,65).
These studies analyzed association between IL-32 expression in
patients with lung cancer (precancerous and malignant lesions) and
clinicopathological and survival data. Confocal microscopy,
microdissection and RT-qPCR have been used to identify the cellular
origin and expression levels of IL-32 and the results indicated
that IL-32 expression is markedly absent in the majority of
squamous cell carcinoma (SCC; 76%) and precursor lesions but was
strongly upregulated in adenocarcinoma (73%) and their precursors,
64% of large and 77% of small cell lung cancers. This suggests the
possible association of IL-32 expression with pathogenesis of the
majority of lung cancer histotypes. By contrast, IL-32 expression
is not associated with the pathogenesis of SCC (66,67).
Inhibition of TIMP-3 may promote tumor development based on the low
expression of TIMP-3 in the inflammatory response. Previous studies
have reported that promoter methylation results in a notable
increase in TIMP-3 expression in lung cancer cells transfected with
IL-32γ cDNA plasmid (67,68). Furthermore, mechanistic studies have
indicated that TIMP-3 overexpression decreases NF-κB activity,
leading to the inhibition of cell proliferation in
IL-32γ-transfected lung cancer cells. The aforementioned study also
indicated that IL-32γ inhibits expression of DNA
(cytosine-5-)-methyltransferase 1, which demonstrates that IL-32γ
could increase TIMP-3 expression by inactivating NF-κB activity via
hypomethylation, thereby decreasing lung tumor growth (42,68,69).
This suggests that the effect of IL-32 subgroups on lung cancer is
also bidirectional.
Increased or decreased IL-32 expression in tumor
tissue or serum may reflect the progression of certain diseases and
its abnormal expression may have several implications. Firstly,
IL-32 may serve as a marker of the presence of certain tumors.
IL-32 is upregulated in the majority of lung cancer precursor
lesions and tumor tissue compared with that of normal lung tissue
(70). The marked elevation of
IL-32α levels in peripheral blood samples of patients with
hepatocellular carcinoma may aggravate degree of liver cell damage
and a similar finding is reported in pancreatic and esophageal
cancers (71). Secondly,
upregulation of IL-32 may be associated with aggressiveness and
distant metastasis of cancer and overexpression of IL-32 in CRC
induces metastasis (72,73). Furthermore, RT-qPCR and western blot
analyses have demonstrated increased levels of IL-32 expression in
highly invasive pancreatic cancer cells at the RNA and protein
levels (74). Thirdly, IL-32 serves
as an independent prognostic assessment factor for certain types of
cancer. IL-32 expression is markedly upregulated in gastric cancer
and positively associated with cancer aggressiveness and poor
prognosis. Ectopic expression of IL-32 induces expression of IL-2,
VEGF, MMP-2, MMP-9 and MMP-3, as determined using
fluorescent-AKT/phospho-glycogen synthase kinase 8β/active β-linked
protein, to increase gastric cancer cell migration and invasion
(75). Therefore, the association
between increased IL-32 expression and poor prognosis of gastric
cancer indicates that IL-32 may be an independent prognostic
assessment factor for this cancer type (57).
In the present study the primary functions of
isoforms of IL-32 and their roles were described in different types
of cancer, indicating the complex functions of each isoform of
IL-32 and the possible presence of different isoforms in different
tumors and microenvironments. To the best of our knowledge, the
receptors of IL-32 and functions and mechanisms of action of each
isoform are not well-studied (24)
and further studies are required to determine functional
characteristics of IL-32 under different disease conditions to use
these functions for the purpose of disease treatment.
Not applicable.
The present study was supported by Natural Science Foundation of
Heilongjiang Province (grant no. LH2021H106) and the Basic Research
Fund of Universities of Heilongjiang Province (grant no.
2021-KYYWF-0598).
Not applicable.
DM performed the literature review and wrote the
paper. HD and JW was responsible for reviewing the literature and
revising the final paper. CW and RZ were involved in the literature
search and the insertion of these and were involved in summarizing
and amending the abstract. All authors have read and approved the
final manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Dahl CA, Schall RP, He HL and Cairns JS:
Identification of a novel gene expressed in activated natural
killer cells and T cells. J Immunol. 148:597–603. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim SH, Han SY, Azam T, Yoon DY and
Dinarello CA: Interleukin-32: A cytokine and inducer of TNFalpha.
Immunity. 22:131–142. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ko NY, Mun SH, Lee SH, Kim JW, Kim DK, Kim
HS, Her E, Kim SH, Won HS, Shin HS, et al: Interleukin-32α
production is regulated by MyD88-dependent and independent pathways
in IL-1β-stimulated human alveolar epithelial cells. Immunobiology.
216:32–40. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kold-Petry CA, Rudloff I, Baumer Y, Ruvo
M, Marasco D, Botti P, Farkas L, Cho SX, Zepp JA, Azam T, et al:
IL-32 promotes angiogenesis. J Immunol. 192:589–602. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goda C, Kanaji T, Kanaji S, Tanaka G,
Arima K, Ohno S and Izuhara K: Involvement of IL-32 in
activation-induced cell death in T cells. Int Immunol. 18:233–240.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hong JT, Son DJ, Lee CK, Yoon DY, Lee DH
and Park MH: Interleukin 32, inflammation and cancer. Pharmacol
Ther. 174:127–137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moschen AR, Fritz T, Clouston AD, Rebhan
I, Bauhofer O, Barrie HD, Powell EE, Kim SH, Dinarello CA,
Bartenschlager R, et al: Interleukin-32: A new proinflammatory
cytokine involved in hepatitis C virus-related liver inflammation
and fibrosis. Hepatology. 53:1819–1829. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Albuquerque R, Komsi E, Starskaia I,
Ullah U and Lahesmaa R: The role of interleukin-32 in autoimmunity.
Scand J Immunol. 93:e130122021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nam SY, Jeong HJ and Kim HM: Kaempferol
impedes IL-32-induced monocyte-macrophage differentiation. Chem
Biol Interact. 274:107–115. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kudo M, Ogawa E, Kinose D, Haruna A,
Takahashi T, Tanabe N, Marumo S, Hoshino Y, Hirai T, Sakai H, et
al: Oxidative stress induced interleukin-32 mRNA expression in
human bronchial epithelial cells. Respir Res. 13:192012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cagnard N, Letourneur F, Essabbani A,
Devauchelle V, Mistou S, Rapinat A, Decraene C, Fournier C and
Chiocchia G: Interleukin-32, CCL2, PF4F1 and GFD10 are the only
cytokine/chemokine genes differentially expressed by in vitro
cultured rheumatoid and osteoarthritis fibroblast-like
synoviocytes. Eur Cytokine Netw. 16:289–292. 2005.PubMed/NCBI
|
|
12
|
Barksby HE, Nile CJ, Jaedicke KM, Taylor
JJ and Preshaw PM: Differential expression of immunoregulatory
genes in monocytes in response to Porphyromonas gingivalis and
Escherichia coli lipopolysaccharide. Clin Exp Immunol. 156:479–487.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jeong HJ, Shin SY, Oh HA, Kim MH, Cho JS
and Kim HM: IL-32 up-regulation is associated with inflammatory
cytokine production in allergic rhinitis. J Pathol. 224:553–563.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kang YH, Park MY, Yoon DY, Han SR, Lee CI,
Ji NY, Myung PK, Lee HG, Kim JW, Yeom YI, et al: Dysregulation of
overexpressed IL-32α in hepatocellular carcinoma suppresses cell
growth and induces apoptosis through inactivation of NF-κB and
Bcl-2. Cancer Lett. 318:226–233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kang JW, Choi SC, Cho MC, Kim HJ, Kim JH,
Lim JS, Kim SH, Han JY and Yoon DY: A proinflammatory cytokine
interleukin-32beta promotes the production of an anti-inflammatory
cytokine interleukin-10. Immunology. 128 (1 Suppl):e532–e540. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dinarello CA and Kim SH: IL-32, a novel
cytokine with a possible role in disease. Ann Rheum Dis. 65 (Suppl
3):iii61–iii64. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen J, Wang S, Su J, Chu G, You H, Chen
Z, Sun H, Chen B and Zhou M: Interleukin-32α inactivates JAK2/STAT3
signaling and reverses interleukin-6-induced epithelial-mesenchymal
transition, invasion, and metastasis in pancreatic cancer cells.
Onco Targets Ther. 9:4225–4237. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Novick D, Rubinstein M, Azam T, Rabinkov
A, Dinarello CA and Kim SH: Proteinase 3 is an IL-32 binding
protein. Proc Natl Acad Sci USA. 103:3316–3321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Heinhuis B, Netea MG, van den Berg WB,
Dinarello CA and Joosten LAB: Interleukin-32: A predominantly
intracellular proinflammatory mediator that controls cell
activation and cell death. Cytokine. 60:321–327. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pendergraft WF III, Rudolph EH, Falk RJ,
Jahn JE, Grimmler M, Hengst L, Jennette JC and Preston GA:
Proteinase 3 sidesteps caspases and cleaves p21(Waf1/Cip1/Sdi1) to
induce endothelial cell apoptosis. Kidney Int. 65:75–84. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang JJ, Pendergraft WF, Alcorta DA,
Nachman PH, Hogan SL, Thomas RP, Sullivan P, Jennette JC, Falk RJ
and Preston GA: Circumvention of normal constraints on granule
protein gene expression in peripheral blood neutrophils and
monocytes of patients with antineutrophil cytoplasmic
autoantibody-associated glomerulonephritis. J Am Soc Nephrol.
15:2103–2114. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kwon OC, Ghang B, Lee EJ, Hong S, Lee CK,
Yoo B, Kim S and Kim YG: Interleukin-32γ: Possible association with
the activity and development of nephritis in patients with systemic
lupus erythematosus. Int J Rheum Dis. 22:1305–1311. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Inoue M, Shoda H, Seri Y, Kubo K, Kanda H,
Fujio K and Yamamoto K: Three cases of lupus nephritis patients
with serum interleukin-32γ detection. Lupus. 23:1187–1191. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Caramori G, Adcock IM, Di Stefano A and
Chung KF: Cytokine inhibition in the treatment of COPD. nt J Chron
Obstruct Pulmon Dis. 9:397–412. 2014.PubMed/NCBI
|
|
25
|
Heinhuis B, Koenders MI, van den Berg WB,
Netea MG, Dinarello CA and Joosten LAB: Interleukin 32 (IL-32)
contains a typical α-helix bundle structure that resembles focal
adhesion targeting region of focal adhesion kinase-1. J Biol Chem.
287:5733–5743. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park MH, Yoon DY, Ban JO, Kim DH, Lee DH,
Song S, Kim Y, Han SB, Lee HP and Hong JT: Decreased severity of
collagen antibody and lipopolysaccharide-induced arthritis in human
IL-32β overexpressed transgenic mice. Oncotarget. 6:38566–38577.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dos Santos JC, Heinhuis B, Gomes RS, Damen
MS, Real F, Mortara RA, Keating ST, Dinarello CA, Joosten LA and
Ribeiro-Dias F: Cytokines and microbicidal molecules regulated by
IL-32 in THP-1-derived human macrophages infected with New World
Leishmania species. PLoS Negl Trop Dis. 11:e00054132017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu H, Zhang S, Pan X, Cao H, Huang X, Xu
Q, Zhong H and Peng X: TIMP-1 expression induced by IL-32 is
mediated through activation of AP-1 signal pathway. Int
Immunopharmacol. 38:233–237. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Netea MG, Lewis EC, Azam T, Joosten LA,
Jaekal J, Bae SY, Dinarello CA and Kim SH: Interleukin-32 induces
the differentiation of monocytes into macrophage-like cells. Proc
Natl Acad Sci USA. 105:3515–3520. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Netea MG, Azam T, Ferwerda G, Girardin SE,
Walsh M, Park JS, Abraham E, Kim JM, Yoon DY, Dinarello CA and Kim
SH: IL-32 synergizes with nucleotide oligomerization domain (NOD) 1
and NOD2 ligands for IL-1beta and IL-6 production through a caspase
1-dependent mechanism. Proc Natl Acad Sci USA. 102:16309–16314.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Becker S, Warren MK and Haskill S:
Colony-stimulating factor-induced monocyte survival and
differentiation into macrophages in serum-free cultures. J Immunol.
139:3703–3709. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Delneste Y, Charbonnier P, Herbault N,
Magistrelli G, Caron G, Bonnefoy JY and Jeannin P: Interferon-gamma
switches monocyte differentiation from dendritic cells to
macrophages. Blood. 101:143–150. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lo AS, Gorak-Stolinska P, Bachy V, Ibrahim
MA, Kemeny DM and Maher J: Modulation of dendritic cell
differentiation by colony-stimulating factor-1: Role of
phosphatidylinositol 3′-kinase and delayed caspase activation. J
Leukoc Biol. 82:1446–1454. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Romani N, Gruner S, Brang D, Kämpgen E,
Lenz A, Trockenbacher B, Konwalinka G, Fritsch PO, Steinman RM and
Schuler G: Proliferating dendritic cell progenitors in human blood.
J Exp Med. 180:83–93. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sallusto F and Lanzavecchia A: Efficient
presentation of soluble antigen by cultured human dendritic cells
is maintained by granulocyte/macrophage colony-stimulating factor
plus interleukin 4 and downregulated by tumor necrosis factor
alpha. J Exp Med. 179:1109–1118. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chomarat P, Banchereau J, Davoust J and
Palucka AK: IL-6 switches the differentiation of monocytes from
dendritic cells to macrophages. Nat Immunol. 1:510–514. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gorvel L, Korenfeld D, Tung T and
Klechevsky E: Dendritic cell-derived IL-32α: A novel inhibitory
cytokine of NK cell function. J Immunol. 199:1290–1300. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Borzouei S, Gholamian-Hamadan M and Behzad
M: Impact of interleukin-32α on T helper cell-related cytokines,
transcription factors, and proliferation in patients with type 2
diabetes mellitus. Immunopharmacol Immunotoxicol. 45:268–276. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Di Sabatino A, Giuffrida P, Fornasa G,
Salvatore C, Vanoli A, Naviglio S, De Leo L, Pasini A, De Amici M,
Alvisi C, et al: Innate and adaptive immunity in self-reported
nonceliac gluten sensitivity versus celiac disease. Dig Liver Dis.
48:745–752. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Park YS, Kang JW, Lee DH, Kim MS, Bak Y,
Yang Y, Lee HG, Hong J and Yoon DY: Interleukin-32α downregulates
the activity of the B-cell CLL/lymphoma 6 protein by inhibiting
protein kinase Cε-dependent SUMO-2 modification. Oncotarget.
5:8765–8777. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Oh JH, Cho MC, Kim JH, Lee SY, Kim HJ,
Park ES, Ban JO, Kang JW, Lee DH, Shim JH, et al: IL-32γ inhibits
cancer cell growth through inactivation of NF-κB and STAT3 signals.
Oncogene. 30:3345–3359. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee YS, Han SB, Ham HJ, Park JH, Lee JS,
Hwang DY, Jung YS, Yoon DY and Hong JT: IL-32γ suppressed atopic
dermatitis through inhibition of miR-205 expression via
inactivation of nuclear factor-kappa B. J Allergy Clin Immunol.
146:156–168. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wallimann A and Schenk M: IL-32 as a
potential biomarker and therapeutic target in skin inflammation.
Front Immunol. 14:12642362023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Park MH, Song MJ, Cho MC, Moon DC, Yoon
DY, Han SB and Hong JT: Interleukin-32 enhances cytotoxic effect of
natural killer cells to cancer cells via activation of death
receptor 3. Mmunology. 135:63–72. 2012.
|
|
45
|
Yun HM, Oh JH, Shim JH, Ban JO, Park KR,
Kim JH, Lee DH, Kang JW, Park YH, Yu D, et al: Antitumor activity
of IL-32β through the activation of lymphocytes, and the
inactivation of NF-κB and STAT3 signals. Cell Death Dis.
4:e6402013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jung YY, Katila N, Neupane S, Shadfar S,
Ojha U, Bhurtel S, Srivastav S, Son DJ, Park PH, Yoon DY, et al:
Enhanced dopaminergic neurotoxicity mediated by MPTP in IL-32β
transgenic mice. Neurochem Int. 102:79–88. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ni X, Zhang X, Hu CH, Langridge T,
Tarapore RS, Allen JE, Oster W and Duvic M: ONC201 selectively
induces apoptosis in cutaneous T-cell lymphoma cells via activating
pro-apoptotic integrated stress response and inactivating JAK/STAT
and NF-κB pathways. Oncotarget. 8:61761–61776. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lin X, Yang L, Wang G, Zi F, Yan H, Guo X,
Chen J, Chen Q, Huang X, Li Y, et al: Interleukin-32α promotes the
proliferation of multiple myeloma cells by inducing production of
IL-6 in bone marrow stromal cells. Oncotarget. 8:92841–92854. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hussain SP and Harris CC: Inflammation and
cancer: An ancient link with novel potentials. Int J Cancer.
121:2373–2380. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ting WC, Chen LM, Huang LC, Hour MJ, Lan
YH, Lee HZ, You BJ, Chang TY and Bao BY: Impact of interleukin-10
gene polymorphisms on survival in patients with colorectal cancer.
J Korean Med Sci. 28:1302–1306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Petanidis S, Anestakis D, Argyraki M,
Hadzopoulou-Cladaras M and Salifoglou A: Differential expression of
IL-17, 22 and 23 in the progression of colorectal cancer in
patients with K-ras mutation: Ras signal inhibition and crosstalk
with GM-CSF and IFN-γ. PLoS One. 8:e736162013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zeng JC, Zhang Z, Li TY, Liang YF, Wang
HM, Bao JJ, Zhang JA, Wang WD, Xiang WY, Kong B, et al: Assessing
the role of IL-35 in colorectal cancer progression and prognosis.
Int J Clin Exp Pathol. 6:1806–1816. 2013.PubMed/NCBI
|
|
53
|
Yang Y, Wang Z, Zhou Y, Wang X, Xiang J
and Chen Z: Dysregulation of over-expressed IL-32 in colorectal
cancer induces metastasis. World J Surg Oncol. 13:1462015.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yun HM, Park KR, Kim EC, Han SB, Yoon DY
and Hong JT: IL-32α suppresses colorectal cancer development via
TNFR1-mediated death signaling. Oncotarget. 6:9061–9072. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ebach DR, Newberry R and Stenson WF:
Differential role of tumor necrosis factor receptors in TNBS
colitis. Inflamm Bowel Dis. 11:533–540. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Seo EH, Kang J, Kim KH, Cho MC, Lee S, Kim
HJ, Kim JH, Kim EJ, Park DK, Kim SH, et al: Detection of expressed
IL-32 in human stomach cancer using ELISA and immunostaining. J
Microbiol Biotechnol. 18:1606–1612. 2008.PubMed/NCBI
|
|
57
|
Tsai CY, Wang CS, Tsai MM, Chi HC, Cheng
WL, Tseng YH, Chen CY, Lin CD, Wu JI, Wang LH and Lin KH:
Interleukin-32 increases human gastric cancer cell invasion
associated with tumor progression and metastasis. Clin Cancer Res.
20:2276–2288. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ishigami S, Arigami T, Uchikado Y,
Setoyama T, Kita Y, Sasaki K, Okumura H, Kurahara H, Kijima Y,
Harada A, et al: IL-32 expression is an independent prognostic
marker for gastric cancer. Med Oncol. 30:4722013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang S, Chen F and Tang L: IL-32 promotes
breast cancer cell growth and invasiveness. Oncol Lett. 9:305–307.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lin J, Xu R, Hu L, You J, Jiang N, Li C,
Che C, Wang Q, Xu Q and Li J: Interleukin-32 induced thymic stromal
lymphopoietin plays a critical role in the inflammatory response in
human corneal epithelium. Cell Signal. 49:39–45. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Nicholl MB, Chen X, Qin C, Bai Q, Zhu Z,
Davis MR and Fang Y: IL-32α has differential effects on
proliferation and apoptosis of human melanoma cell lines. J Surg
Oncol. 113:364–369. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Park HM, Park JY, Kim NY, Kim J, Pham TH,
Hong JT and Yoon DY: Modulatory effects of point-mutated IL-32θ
(A94V) on tumor progression in triple-negative breast cancer cells.
Biofactors. Sep 2–2023.(Epub ahead of print). View Article : Google Scholar
|
|
63
|
Pham TH, Bak Y, Kwon T, Kwon SB, Oh JW,
Park JH, Choi YK, Hong JT and Yoon DY: Interleukin-32θ inhibits
tumor-promoting effects of macrophage-secreted CCL18 in breast
cancer. Cell Commun Signal. 17:532019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lee YS, Kim KC, Mongre RK, Kim JY, Kim YR,
Choi DY, Song S, Yun J, Han SB, Yoon DY and Hong JT: IL-32γ
suppresses lung cancer stem cell growth via inhibition of
ITGAV-mediated STAT5 pathway. Cell Death Dis. 10:5062019.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Felaco P, Castellani ML, De Lutiis MA,
Felaco M, Pandolfi F, Salini V, De Amicis D, Vecchiet J, Tete S,
Ciampoli C, et al: IL-32: A newly-discovered proinflammatory
cytokine. J Biol Regul Homeost Agents. 23:141–147. 2009.PubMed/NCBI
|
|
66
|
Ma Z, Dong Z, Yu D, Mu M, Feng W, Guo J,
Cheng B, Guo J and Ma J: IL-32 promotes the radiosensitivity of
esophageal squamous cell carcinoma cell through STAT3 pathway.
Biomed Res Int. 2021:66537472021.PubMed/NCBI
|
|
67
|
Sorrentino C and Di Carlo E: Expression of
IL-32 in human lung cancer is related to the histotype and
metastatic phenotype. Am J Respir Crit Care Med. 180:769–779. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yun J, Park MH, Son DJ, Nam KT, Moon DB,
Ju JH, Hwang OK, Choi JS, Kim TH, Hwang DY, et al: IL-32 gamma
reduces lung tumor development through upregulation of TIMP-3
overexpression and hypomethylation. Cell Death Dis. 9:3062018.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Liu H, Pan X, Cao H, Shu X, Sun H, Lu J,
Liang J, Zhang K, Zhu F, Li G and Zhang Q: IL-32γ promotes integrin
αvβ6 expression through the activation of NF-κB in HSCs. Exp Ther
Med. 14:3880–3886. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wang Y, Yang Y, Zhu Y, Li L, Chen F and
Zhang L: Polymorphisms and expression of IL-32: Impact on genetic
susceptibility and clinical outcome of lung cancer. Biomarkers.
22:165–170. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zou Y, Bao J, Pan X, Lu Y, Liao S, Wang X,
Wang G and Lin D: NKP30-B7-H6 interaction aggravates hepatocyte
damage through up-regulation of interleukin-32 expression in
hepatitis b virus-related acute-on-chronic liver failure. PLoS One.
10:e01345682015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Nishida A, Andoh A, Inatomi O and Fujiyama
Y: Interleukin-32 expression in the pancreas. J Biol Chem.
284:17868–17876. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Yousif NG, Al-Amran FG, Hadi N, Lee J and
Adrienne J: Expression of IL-32 modulates NF-κB and p38 MAP kinase
pathways in human esophageal cancer. Cytokine. 61:223–227. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Takagi K, Imura J, Shimomura A, Noguchi A,
Minamisaka T, Tanaka S, Nishida T, Hatta H and Nakajima T:
Establishment of highly invasive pancreatic cancer cell lines and
the expression of IL-32. Oncol Lett. 20:2888–2896. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Cai A, Qi S, Su Z, Shen H, Ma W and Dai Y:
Tripterygium glycosides inhibit inflammatory mediators in the rat
synovial RSC-364 cell line stimulated with interleukin-1β. Biomed
Rep. 3:763–766. 2015. View Article : Google Scholar : PubMed/NCBI
|