Introduction
Adenocarcinomas account for 96% of cases of
colorectal cancer (CRC), which is defined as cancer of the colon or
rectum (1,2). At present, therapeutic advances and
improved early detection screening are available; however, CRC
continues to rank among the leading causes of cancer-related
mortality worldwide (1,3). The primary causes of this are
post-operative cancer recurrence or metastasis, as well as cancer
cell drug resistance (4), which is
related to CRC stem cells (CCSCs), a subpopulation of CRC cells
with the capacity to self-renew, differentiate into multiple
lineages, resist therapy and develop metastasis (5). Therefore, studies are currently
underway to create novel CCSC-targeted therapeutics that will
enhance the isolation and differentiation of CCSCs from other types
of CSCs (6,7).
The most frequently employed markers in CCSC
research are cell surface markers (7). For example, targeting CD133, CD166,
CD44, aldehyde dehydrogenase 1 (ALDH1), leucine rich repeat
containing G protein-coupled receptor 5 (Lgr5) and epithelial cell
adhesion molecule, cell surface markers present on CCSCs, with
monoclonal antibodies has the potential to shrink tumors and lessen
metastasis (8). The lack of a
consistent and reliable marker for CCSCs restricts their use in
clinical practice (7), and they are
also present in varying degrees in stem cells from normal tissues
or other cancer types (9,10).
To initiate pro-inflammatory reactions to invading
pathogens, lipopolysaccharide (LPS) and CD14, a particular surface
marker of monocytes, macrophages and neutrophils, interact via the
Toll-like receptor 4 (TLR4) signaling pathway (8,11,12).
Notably, LPS promotes CRC development and metastasis via the TLR4
signaling pathway (13–15). Additionally, CD14 has been linked to
tumor recurrence, growth, metastasis and therapy resistance, which
is consistent with CSC traits (such as recurrence, growth,
metastasis and resistance to therapy), suggesting also that CD14
may be linked to CCSCs (16–18).
Furthermore, a previous study by the authors revealed that
esophageal CSCs expressed CD14, a novel surface marker (19).
In the present study, CD133 and CD14 were examined
by immunofluorescence double labeling to qualitatively assess CD14
expression in CCSCs in paraffin-embedded slices of CRC tissues and
tissues adjacent to the tumor. Subsequently, CD14+ cells
were extracted from CRC tissues to examine the stemness
characteristics by analyzing proliferation, tumorigenicity and
treatment resistance to corroborate the phenotypic identification.
Furthermore, the in vitro detection of migration enabled the
examination of CD14 function.
Materials and methods
Patients and specimens
CRC tissues were obtained from 60 patients (median
age, 60.3 years; range, 45–78 years) who underwent surgical
resection without radiotherapy from January, 2017 to January, 2022
at Hongqi Hospital, Mudanjiang Medical University (Heilongjiang,
China). Paraffin-embedded sections of colon cancer tumor tissues
[i) seven highly differentiated squamous carcinomas; ii) nine
moderately differentiated squamous carcinomas; iii) six lowly
differentiated squamous carcinomas; iv) 14 highly differentiated
adenocarcinomas; v) 12 moderately differentiated adenocarcinomas;
vi) seven lowly differentiated adenocarcinomas; and vii) seven
paracarcinomatous tissues] were obtained from Mudanjiang Tumor
Hospital, and five post-operative colon cancer tumor tissues of
patients who have not been treated with chemotherapy and
radiotherapy (two highly differentiated and three moderately
differentiated) were obtained from the Hongqi Hospital of
Mudanjiang Medical University (Heilongjiang, China). The clinical
and pathological data of the patients are presented in Table I. All patients signed an informed
consent form. The present study was approved by the Ethics
Committee of Mudanjiang Medical College (approval no.
2022-MYGZR06). The study used 12 specific pathogen-free grade nu/nu
immunodeficient mice (male; age, 4–5 weeks; median body weight, 20
g) were purchased from Beijing Vital River Laboratory Animal
Technology Co., Ltd. The animal experiments in the present study
were approved by the Laboratory Animal Welfare and Ethics Committee
of Mudanjiang Medical College (approval no. 20220228-26).
 | Table I.Clinicopathological characteristics
of the 60 patients with colon cancer. |
Table I.
Clinicopathological characteristics
of the 60 patients with colon cancer.
| Characteristic | Value |
|---|
| Sex, n (%) |
|
|
Male | 32 (53.3) |
|
Female | 28 (46.7) |
| Age, years, n
(%) |
|
|
≥60 | 36 (60.0) |
|
<60 | 24 (40.0) |
| Median age (range),
years | 60.3 (45–78) |
| Differentiation, n
(%) |
|
|
Squamous carcinoma | 22 (36.7) |
|
Well | 7 (11.7) |
|
Moderate | 9 (15.0) |
|
Poor | 6 (10.0) |
|
Adenocarcinoma | 38 (63.3) |
|
Well | 16 (26.7) |
|
Moderate | 15 (25.0) |
|
Poor | 7 (11.7) |
Reagents
The following reagents were used in the present
study: Collagenase I (Coolaber), DMEM/F12 powder (Gibco; Thermo
Fisher Scientific, Inc.); basic fibroblast growth factor (bFGF),
epidermal growth factor (EGF), leukemia inhibitory factor (LIF)
(all from PeproTech, Inc.); CD133 polyclonal antibodies (cat. no.
18495-1-AP, Proteintech Group, Inc.); CD14 polyclonal antibodies
(cat. no. CL647-65056; 1:1,000, Proteintech Group, Inc.); Protein
RIPA Lysis Solution (cat. no. abs9229, Absin); SDS-PAGE Gel Rapid
Preparation Kit (cat. no. abs9367-1Kit, Absin); ECL luminescent
solution (cat. no. abs920-2, Absin); TLR4 primary antibody (cat.
no. 132000; 1:1,000, Absin); myeloid differentiation factor 88
(MyD88) primary antibody (cat. no. abs135682; 1:1,000, Absin);
human CD14 antibody (MAB3832; 1:1,000); LPS (cat. no. abs47014848,
Absin); Cell Counting Kit-8 (CCK-8; cat. no. abs50003, Absin) and
5-ethynyl-2′-deoxyuridine (EdU) Assay/EdU Staining Proliferation
Kit (Abcam); penicillin and streptomycin (Millipore, Sigma);
β-actin antibody (AB0035, 1:1,000, Shanghai Abways Biotechnology
Co., Ltd.); horseradish enzyme labeled goat anti-rabbit IgG
(ZB-2301, 1:10,000, Beijing Zhongsui Jinqiao Biotechnology Co.);
the EasySep™ Human CD14 Positive Selection Kit II (EasySep™;
Stemcell Technologies, Inc.); Cellular Rapid RNA Extraction Kit
(abs60027, Absin); SYBR Premix Ex Taq (Takara Bio, Inc.); Prime
ScripTM RT kit (RR047A, Takara Bio, Inc.).
Immunofluorescence staining
CD133 and CD14 co-expression in CRC was examined
using tissue slices of the patient-derived paraffin-embedded tumor
samples. Dewaxed paraffin sections (5 µm thickness) were placed in
water, permeabilized with 0.3% Triton X-100 in PBS for 15 min at
37°C and then subjected to 3% peroxide in PBS for 10 min. In order
to label CCSCs, the sections were incubated with CD133 antibody
(1:100 dilution) at 37°C for 120 min and stained with the secondary
antibody IgG Texas Red (cat. no. ab6800; 1:100 dilution; Abcam) at
37°C for 40 min. For the analysis of CD14, the sections were
incubated again with CD14 antibody (1:100 dilution) at 37°C for 120
min and stained with another secondary antibody IgG FITC (cat. no.
abs20004; 1:100 dilution; Absin Bioscience Inc.) at 37°C for 40
min. To facilitate cell counting, the sections were counterstained
with DAPI (1:100 dilution, MilliporeSigma) at 37°C for 30 min. The
results were observed under a fluorescence microscope (Nikon
Corporation) and images were captured (scale bar, 50 µm).
Primary culture of human CRC
The surgically removed CRC tissues were transported
to the laboratory as fast as possible. The tissues were washed
three times with PBS, cut into 3-mm-thick sections and then
incubated in serum-free DMEM/F12, 37°C, humidified atmosphere with
5% CO2. This was followed by the addition of 10 ng/l
bFGF, 20 ng/l EGF, 20 ng/l LIF, 100,000 units/l penicillin and 100
mg/l streptomycin. The medium was changed every other day until no
new cells could proliferate ‘crawl’ out of the tissue block.
To mimic the process of inflammation promoting tumor
development, CD14+ cell activation was achieved using
LPS. The inhibition of CD14 by CD14-neutralizing antibodies in the
presence of LPS clarified the effect of CD14 on the migration of
colonic CSCs.
CD14+ cell activation by
LPS
A total of three groups of CD14+ cells
were randomly formed: i) CD14 group (control group; untreated); ii)
CD14 + LPS group (1 mg/l LPS for 24 h at 37°C) and CD14
neutralizing antibody + LPS group (10 µg/ml neutralizing antibody +
1 mg/l LPS for 24 h at 37°C). At the end of the treatment period,
the cell supernatant of each group was collected and cell proteins
were extracted as samples for subsequent experimental testing.
Transwell migration assay
Cell migration was detected using Nunc™
polycarbonate (cat. no. 140644; 8 µm pore size; six-well plates;
Thermo Fisher Scientific, Inc.). Cells were inoculated in the upper
chamber of the cell culture inserts in multiculture dishes at
1×104 cells/well in 500 µl DMEM/12, and 2 ml DMEM/F12
containing 10 ng/l bFGF, 20 ng/l EGF and 20 ng/l LIF as a
chemotactic incubator was added to the lower chamber. Cells were
placed in the CO2 incubator at 37°C for 24 h. The waste
solution was discarded, 0.1% crystal violet (MilliporeSigma)
staining solution was added for 20 min at 37°C and cells were
washed three times with PBS. The number of migrated cells was
observed and counted under an inverted biological microscope
(Olympus Corporation).
Wound healing assay
Cell migration was examined using a wound healing
assay. Cells were seeded into a six-well plate and cultured until
they reached 80% confluency. Following one PBS wash, the cells at
the bottom of the six-well plate were directly scraped with a
100-µl pipette tip. The cells were washed twice with PBS. After
capturing images under a microscope and measuring the scratch
width, the sample was incubated at 37°C with 5% CO2 for
24 h. An inverted biological microscope (Olympus Corporation) was
used to capture images of the affected area.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using the Cellular Rapid RNA
Extraction Kit (abs60027, Absin Bioscience Inc.) and reverse
transcribed to cDNA using the Prime ScripTM RT kit (RR047A, Japan)
and amplified using SYBR Premix Ex Taq (Takara Bio Inc.) according
to the manufacturer's instructions. The reverse transcription
products were used for RT-qPCR analysis, and the following primer
sequences were used for RT-qPCR (Shenggong Bioengineering Co.):
human GAPDH forward, 5′-CAACAGCCTCAAGATCATCAGC-3′, reverse,
5′-ATGAGTCCTTCCACGATACCAA-3′; human TLR4 forward,
5′-TGTGCAACACCTTCAGATAAGCA-3′, reverse,
5′-ACAACAGATACTACAAGCACAC-3′; and human MyD88 forward,
5′-CTGGCTGCTCTCTCAACATGCG-3′, reverse, 5′-CCAGTTGCCGGGATCTCCA-3′.
15 min at 95°C, 10 sec at 95°C, 10 sec at 60°C annealed for 40
cycles. The relative quantification of mRNA of target genes was
calculated using the 2−ΔΔCq (20) method with GAPDH as an internal
reference.
Western blotting
Total protein was extracted in the treated group
(Primary cell extracts derived from previous steps) using RIPA
(cat. no. abs9229, Absin Bioscience Inc.) lysate buffer, quantified
using a BCA Protein Assay Kit (Thermo Fisher Scientific, Inc.),
electrophoresed on an SDS-PAGE (cat. no. abs9367-1Kit) gel and
transferred to a PVDF membrane (Immobilon05317). The membrane was
blocked with 5% skimmed milk, incubated with primary antibody
(Abways AB0035, 1:1,000); TLR4 primary antibody (Absin cat. no.
132000; 1:1,000,); MyD88 primary antibody (Absin cat. no.
abs135682; 1:1,000,), overnight at 4°C, and washed three times with
TBS with Tween-20 (TBST) for 10 min each. Membranes were incubated
with Horseradish enzyme labeled goat anti-rabbit IgG
(ZB-2301,1:10,000) at room temperature for 2 h. The membrane was
washed three times with TBST for 10 min each. Quantification was
performed using Quantity-One software (Bio-Rad Laboratories, Inc.)
using the ECL chemiluminescence kit (cat. no. BL161A) to detect
protein expression. Protein band signals were semi-quantified using
ImageJ software (version 1.46r) for Windows (National Institutes of
Health).
Statistical analysis
Origin 2021b SR1 v9.8.5.204 (OriginLab) and SPSS
14.0 (SPSS, Inc.) were used to conduct the statistical analysis.
The data are presented as the mean and standard deviation. One-way
ANOVA and the Least Significant Difference post hoc test were used
to compare the means of several groups, and the Student's t-test
(paired or unpaired, where appropriate) was performed to compare
the means of only two groups. P<0.05 was considered to indicate
a statistically significant difference.
Results
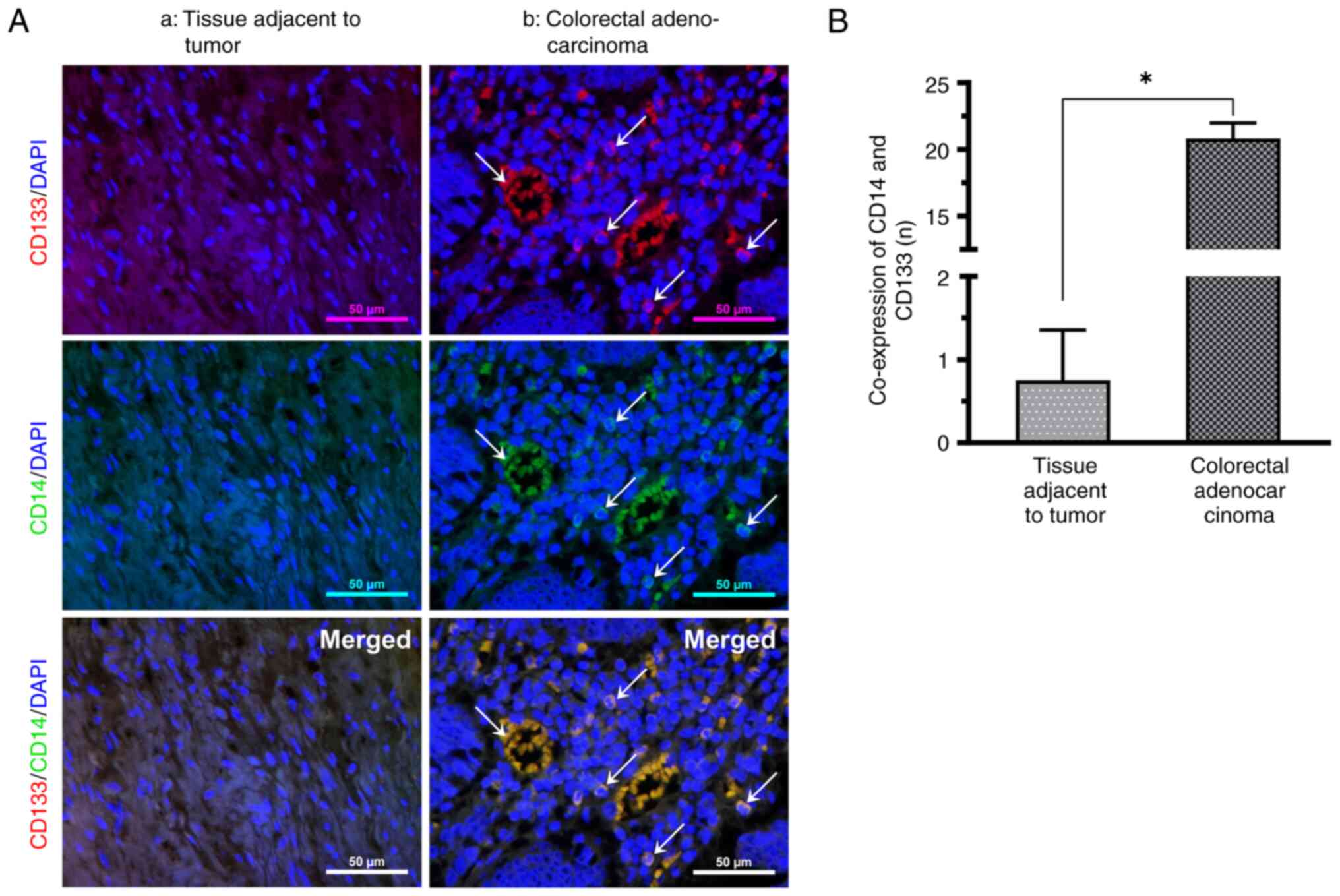
CD133-labeled CCSCs express CD14
The presence of CD14 in CRC tissues was detected
using immunofluorescence double staining. Under an orthogonal
fluorescence microscope, CD14+ cells exhibiting green
fluorescence in immunofluorescence staining, CCSC surface marker
CD133+ cells exhibiting red fluorescence, non-specific
DAPI-stained nuclei exhibiting blue fluorescence, and cells
co-expressing CD14 and CD133 exhibiting yellow fluorescence were
observed in CRC. CD133-labeled CSCs exhibited CD14 expression.
Relative to the nuclei of cancer nests, CD14 is small and the
nuclei may present a split image, located between the relatively
sparsely structured and vascularized cancer nests. The positive
rate of CD14 and CD133 co-expression was significantly increased in
all CRC tissues compared with paraneoplastic tissues (Fig. 1).
Primary cell culture
During the initial extraction, cells slowly
proliferate and ‘crawl’ out of the tissue, and several cells with
odd shapes and numerous colonies were observed. Cells gradually
adhered to the plate wall and manifested into a spindle shape. When
the cell fragments were removed from the culture after 14 days, it
was observed that the cells varied in size and shape (Fig. 2).
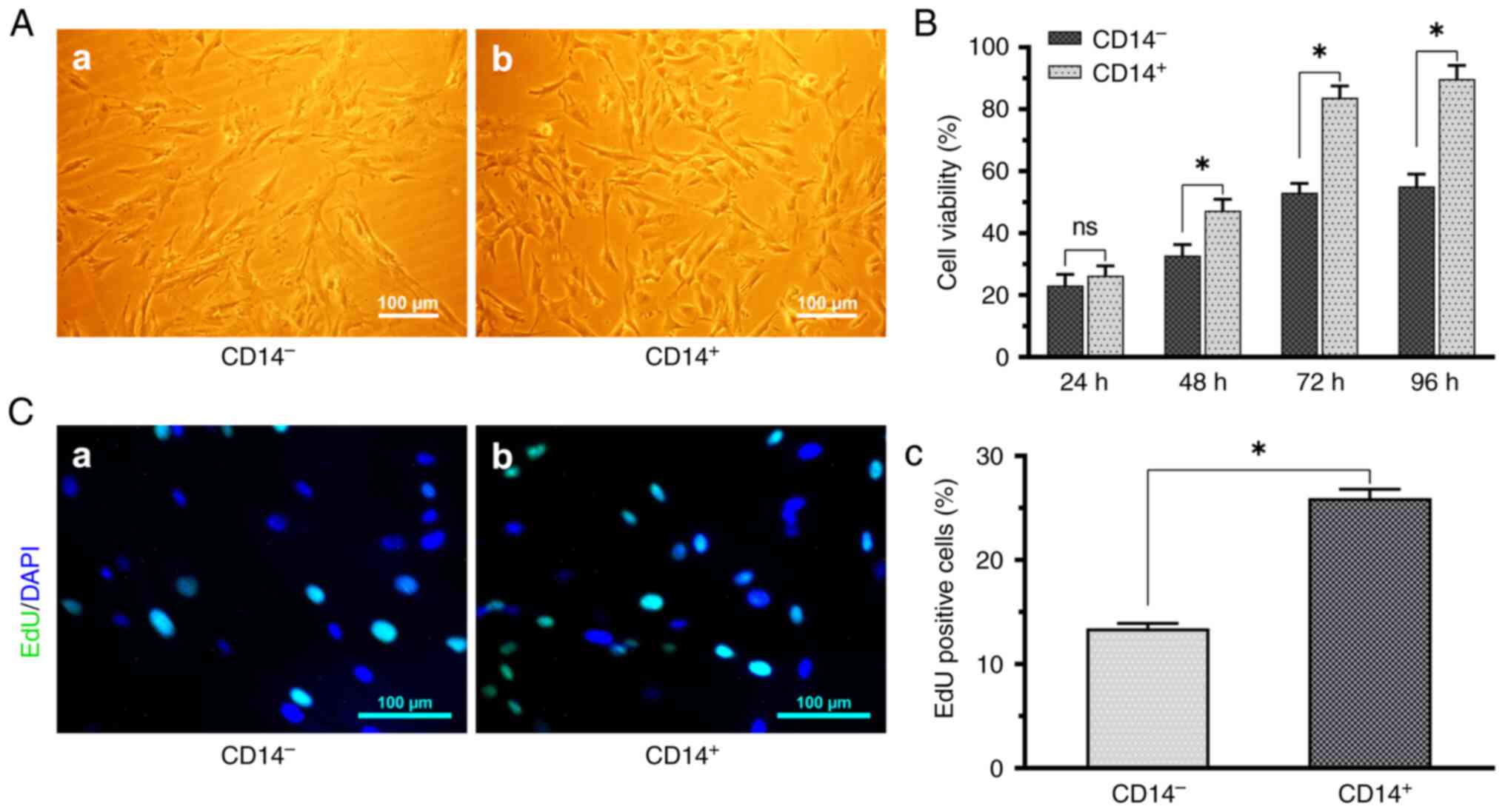
Magnetic bead sorting analysis
Following immunomagnetic bead sorting,
CD14+ (Fig. 3A-b) cells
exhibited spindle cell characteristics and decreased nuclear
division, whereas the CD14− (Fig. 3A-a) cells primarily presented with
polyhedral or irregularly formed spindles. Both CD14+
and CD14− cells could grow in multilayer cultures or
form cell clusters in monolayer cultures after confluence. The
growth time was ~5 days of CD14+ and ~7 days for the
CD14− cells (Fig.
3A).
Proliferation analysis
The EdU assay results revealed that CD14+
(Fig. 3C-b) cell growth was faster
and presenting with a higher proliferation capability in comparison
with CD14− (Fig. 3C-a)
cells (Fig. 3C). This was
consistent with the results of CCK-8 assay (Fig. 3B). This further demonstrated that
CD14+ cells exhibited an increased cell proliferative
potential.
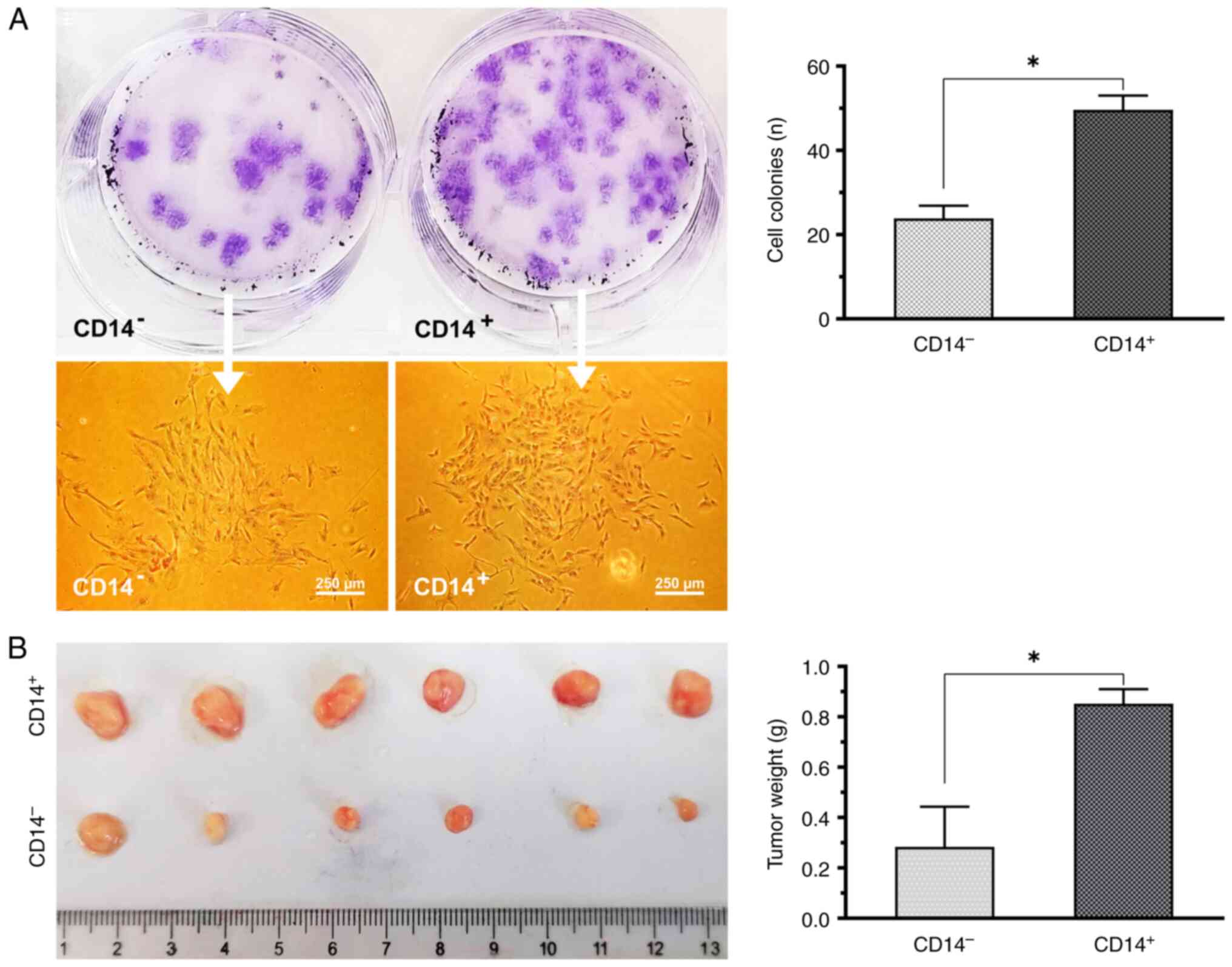
Clonality analysis
Cell cloning experiments demonstrated that the
CD14+ cells were larger, demonstrated increased nuclear
division and aggregated into clusters, as compared with the
CD14− cells (Fig. 4A).
The CD14+ cell tumorigenic ability was observed using a
nude mouse xenograft assay. Following 45 days of implantation, the
tumors in the CD14+ cell group were considerably larger
as compared with those in the CD14− cell group (Fig. 4B).
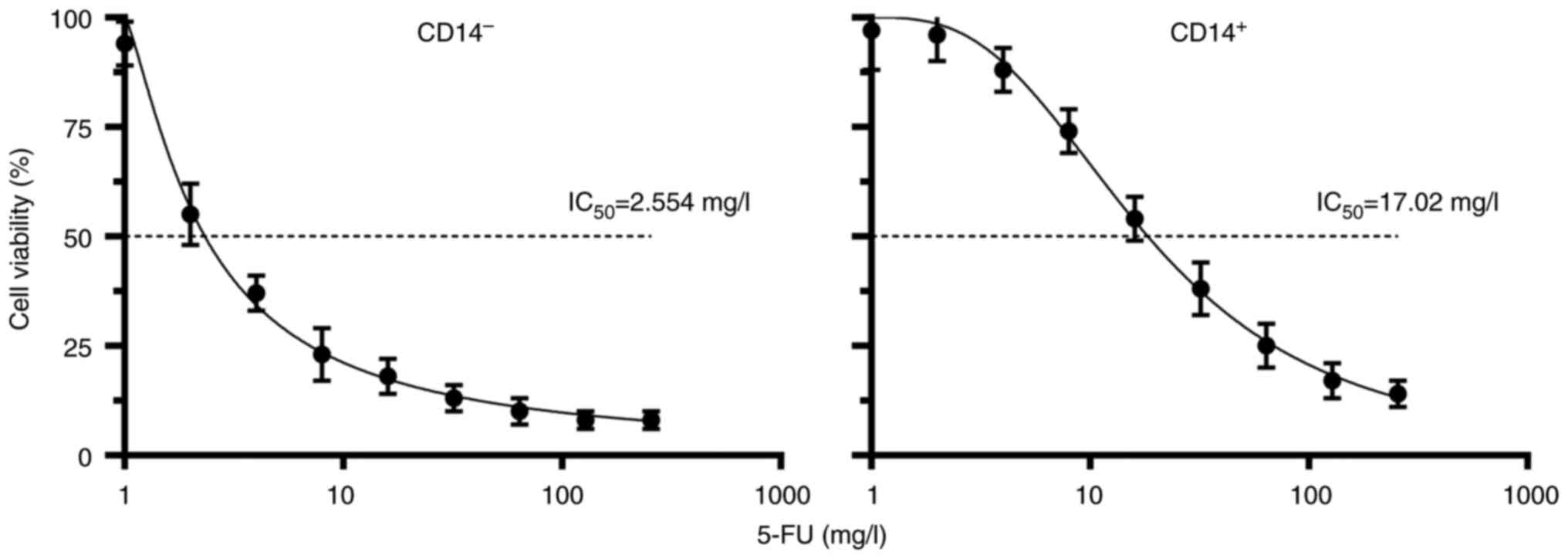
Drug resistance analysis
The IC50 of cells in the CD14+
and CD14− groups was 2.554 and 17.02 mg/l, respectively
(Fig. 5), suggesting that
resistance was increased in the positive group in comparison with
the negative group.
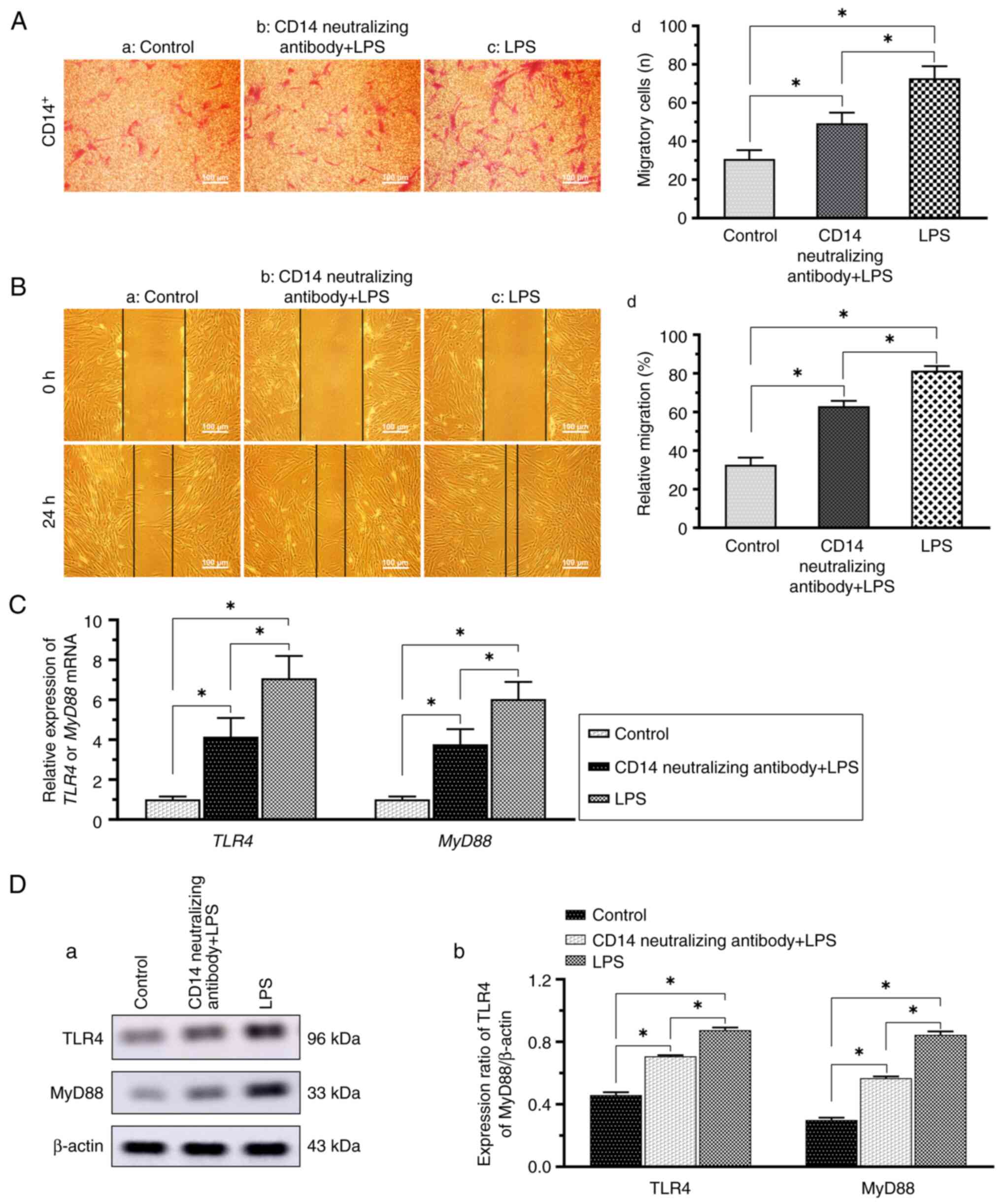
Migration via the TLR4/MyD88 pathway
following LPS stimulation
The results of Transwell assay revealed that the LPS
group (Fig. 6A-c) exhibited a
greater migratory ability as compared with the control group
(Fig. 6A-a), and the migratory
ability was greater in the LPS + neutralizing antibody group
(Fig. 6A-b) compared with the
control group (Fig. 6A-a).
According to the results of the wound healing experiments,
migration was significantly enhanced in the LPS group and slightly
enhanced in the LPS + neutralizing antibody group compared with the
control group; the cell migratory ability was higher in the LPS
group than in the LPS + neutralizing antibody group (Fig. 6B). The RT-qPCR results demonstrated
that the MyD88 mRNA levels in the treatment groups were increased
in comparison with the control group, with the highest mRNA levels
observed in the LPS group (Fig.
6C). Western blotting also revealed that TLR4 and MyD88 protein
expression was significantly elevated in both the LPS +
neutralizing antibody group and the LPS group, as compared with the
control group, with a greater increase observed in the LPS group
(Fig. 6D).
Discussion
Specific cell surface indicators have been suggested
for the identification of CCSCs (21). Excluding CD133, other cell surface
markers of CCSCs have been identified, including CD44, CD166, Lgr5
and ALDH1 (8). The present study
demonstrated that the cell surface marker CD14 was expressed in the
CD133-labeled CCSCs. Nuclear division could also be observed in
smaller-sized CCSCs as with the cancer nest cells and CCSCs were
primarily distributed in the tumor around the cancer nests, which
was consistent with the findings of a previous study on CSC
distribution in tumor tissues (22). To validate the phenotypic detection,
the assessment of the functional capacities of CCSCs by using in
vitro and in vivo assays is required (23).
To identify and confirm the stemness of tumor cells,
the infinite capacity for proliferation, the capacity for
self-renewal and the capacity for tumorigenesis are frequently
examined (24). The proliferation
and/or self-renewal capacity of cancer cells can be examined using
CCK-8 and EdU assays (25). Colony
formation and xenograft assays are two widely used methods for
determining the features of tumorigenesis (25,26).
Additionally, since CCSCs may be able to survive
chemotherapy-induced toxicity, drug resistance can also be used for
identification (21,27). The present study demonstrated that
CD14+ CRC cells possessed CSC-like stemness using the
aforementioned methods.
Numerous microorganisms in the colon may activate
similar receptors through their antigens (for example, the antigen
LPS), which promote the development of CRC (28). The present study is one of numerous
studies that have used an in vivo and in vitro
experimental setup to perform research (29–31).
LPS, a crucial part of the outer membrane of Gram-negative
bacteria, can elicit immune system activation and acute or chronic
inflammation (32). LPS has also
been linked to carcinogenesis and the emergence of colon cancer in
addition to its role in inflammatory reactions. In previous
studies, increased LPS levels were detected in the blood and CRC
tissues of patients with CRC, even including cases of early-stage
adenoma (33,34), revealing also that circulating LPS
could lead to systemic inflammation and a disordered coagulation
system, with the ensuing chronic inflammation and active
coagulation system being linked to tumorigenesis (35). By examining human CRC cell lines, it
was also revealed that LPS increases CRC metastasis (13,34).
The results of the present study demonstrated that LPS may have
induced the proliferation and migration of CD14+ CRC
cells that were CSC-like.
TLR4 is a transmembrane protein that is expressed in
various cancer cells and is largely involved in proliferation,
migration and invasion (36,37).
TLR4 stimulates two signaling pathways, the MyD88-dependent (also
known as TRIF-dependent) and the MyD88-independent (also known as
LPS-dependent) pathways, which are both regulated by CD14 and
triggered by LPS (12,28). TLR4/MyD88-dependent pathway
activation may encourage the development and metastasis of CRC
(38,39). The findings of the present study
demonstrated that following LPS stimulation, CD14 regulated the
proliferation and migration of CD14+ CRC cells through
the TLR4/MyD88 pathway. Multiple studies have demonstrated that the
TLR4/MyD88 pathway may activate cyclooxygenase-2, the EGF receptor
and -catenin-dependent pathways that promote CRC cell proliferation
(14,38,40–42).
Cheah et al (43) reported that bladder cancer
CD14−high cells expressed higher levels of numerous
inflammatory mediators (IL-6 and IL8/CXCL1) even in the absence of
LPS stimulation and formed larger tumors with higher
vascularization than CD14−low cells, demonstrating that
CD14 may promote tumorigenesis and development through a variety of
mechanisms, which should be investigated in further studies.
The present study revealed that CD133-labeled CCSCs
expressed the surface marker CD14. In vitro and in
vivo experiments demonstrated that primary CD14+
cells of CRC exhibited CSC stemness, and CD14 could regulate the
migration of CD14+ CRC cells through the TLR4/MyD88
pathway following LPS stimulation, suggesting that CD14 may be
regarded as a unique surface marker of CCSCs, potentially providing
a therapeutic target against CCSCs. However, the disadvantage of
the present study was the absence of CD14 expression-related rescue
and knockdown tests, and the functional mechanism of CD14 was not
explored in-depth. Therefore, additional research is necessary for
the further elucidation of the CD14 function in CCSCs.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Special Program for
Graduate Student Supervisors' Research (grant no. YJS2X202201).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JuL, YuL, JS and JiL conceived and designed the
study. ZL, YoL and AX and WS contributed to the execution of the
experiments, statistical analysis of the data and drafting of the
manuscript. YuL and JuL confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Mudanjiang Medical University (approval no.
2022-MYGZR06). All patients signed an informed consent form. The
animal experiments were approved by the Laboratory Animal Welfare
and Ethics Committee of Mudanjiang Medical University (approval no.
20220228-26).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ahmad R, Singh JK, Wunnava A, Al-Obeed O,
Abdulla M and Srivastava SK: Emerging trends in colorectal cancer:
Dysregulated signaling pathways (Review). Int J Mol Med. 47:142021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lewandowska A, Rudzki G, Lewandowski T,
Stryjkowska-Góra A and Rudzki S: Title: risk factors for the
diagnosis of colorectal cancer. Cancer Control.
29:107327482110566922022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Salibasic M, Pusina S, Bicakcic E, Pasic
A, Gavric I, Kulovic E, Rovcanin A and Beslija S: Colorectal cancer
surgical treatment, our experience. Med Arch. 73:412–414. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Y, Wu M, Xu S, Huang H, Yan L and Gu Y:
Colorectal cancer stem cell-derived exosomal long intergenic
noncoding RNA 01315 (LINC01315) promotes proliferation, migration,
and stemness of colorectal cancer cells. Bioengineered.
13:10827–10842. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Batlle E and Clevers H: Cancer stem cells
revisited. Nat Med. 23:1124–1134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hervieu C, Christou N, Battu S and
Mathonnet M: The role of cancer stem cells in colorectal cancer:
From the basics to novel clinical trials. Cancers (Basel).
13:10922021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kopenhaver J, Crutcher M, Waldman SA and
Snook AE: The shifting paradigm of colorectal cancer treatment: A
look into emerging cancer stem cell-directed therapeutics to lead
the charge toward complete remission. Expert Opin Biol Ther.
21:1335–1345. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gupta R, Bhatt LK, Johnston TP and
Prabhavalkar KS: Colon cancer stem cells: Potential target for the
treatment of colorectal cancer. Cancer Biol Ther. 20:1068–1082.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Munro MJ, Wickremesekera SK, Peng L, Tan
ST and Itinteang T: Cancer stem cells in colorectal cancer: A
review. J Clin Pathol. 71:110–116. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu X, Lin Y, Yan X, Tian Q, Li L and Lin
EH: CD133, stem cells, and cancer stem cells: Myth or Reality? Curr
Colorectal Cancer Rep. 7:253–259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Janova H, Böttcher C, Holtman IR, Regen T,
van Rossum D, Götz A, Ernst AS, Fritsche C, Gertig U, Saiepour N,
et al: CD14 is a key organizer of microglial responses to CNS
infection and injury. Glia. 64:635–649. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ciesielska A, Matyjek M and Kwiatkowska K:
TLR4 and CD14 trafficking and its influence on LPS-induced
pro-inflammatory signaling. Cell Mol Life Sci. 78:1233–1261. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hsu RY, Chan CH, Spicer JD, Rousseau MC,
Giannias B, Rousseau S and Ferri LE: LPS-induced TLR4 signaling in
human colorectal cancer cells increases beta1 integrin-mediated
cell adhesion and liver metastasis. Cancer Res. 71:1989–1998. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Santaolalla R, Sussman DA, Ruiz JR, Davies
JM, Pastorini C, España CL, Sotolongo J, Burlingame O, Bejarano PA,
Philip S, et al: TLR4 activates the β-catenin pathway to cause
intestinal neoplasia. PLoS One. 8:e632982013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song W, Tiruthani K, Wang Y, Shen L, Hu M,
Dorosheva O, Qiu K, Kinghorn KA, Liu R and Huang L: Trapping of
lipopolysaccharide to promote immunotherapy against colorectal
cancer and attenuate liver metastasis. Adv Mater. 30:e18050072018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao D, Sun T, Zhang X, Guo Y, Yu D, Yang
M, Tan W, Wang G and Lin D: Role of CD14 promoter polymorphisms in
Helicobacter pylori infection-related gastric carcinoma. Clin
Cancer Res. 13:2362–2368. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Arihara F, Mizukoshi E, Kitahara M, Takata
Y, Arai K, Yamashita T, Nakamoto Y and Kaneko S: Increase in
CD14+HLA-DR-/low myeloid-derived suppressor cells in hepatocellular
carcinoma patients and its impact on prognosis. Cancer Immunol
Immunother. 62:1421–1430. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang A, Zhang B, Wang B, Zhang F, Fan KX
and Guo YJ: Increased CD14(+)HLA-DR (−/low) myeloid-derived
suppressor cells correlate with extrathoracic metastasis and poor
response to chemotherapy in non-small cell lung cancer patients.
Cancer Immunol Immunother. 62:1439–1451. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin Y, Dong J, Yu W, Li Y, Liu Z, Liu J,
Wang C, Qin J, Zhu L and Liang J: CD14, a novel surface marker of
esophageal cancer stem cells. Oncol Rep. 49:132023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zalewski A, Snook AE and Waldman SA: Stem
cells as therapeutic targets in colorectal cancer. Per Med.
18:171–183. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cui G, Xu G, Zhu L, Pang Z, Zheng W, Li Z
and Yuan A: Temporal and spatial changes of cells positive for
stem-like markers in different compartments and stages of human
colorectal adenoma-carcinoma sequence. Oncotarget. 8:45311–45322.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Akbarzadeh M, Maroufi NF, Tazehkand AP,
Akbarzadeh M, Bastani S, Safdari R, Farzane A, Fattahi A, Nejabati
HR, Nouri M and Samadi N: Current approaches in identification and
isolation of cancer stem cells. J Cell Physiol. 234:14759–14772.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han GD, Sun Y, Hui HX, Tao MY, Liu YQ and
Zhu J: MiR-1224 acts as a prognostic biomarker and inhibits the
progression of gastric cancer by targeting SATB1. Front Oncol.
11:7488962021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vargo-Gogola T and Rosen JM: Modelling
breast cancer: One size does not fit all. Nat Rev Cancer.
7:659–672. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsai RY: Balancing self-renewal against
genome preservation in stem cells: How do they manage to have the
cake and eat it too? Cell Mol Life Sci. 73:1803–1823. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chiodi I, Belgiovine C, Donà F, Scovassi
AI and Mondello C: Drug treatment of cancer cell lines: A way to
select for cancer stem cells? Cancers (Basel). 3:1111–1128. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fang Y, Yan C, Zhao Q, Zhao B, Liao Y,
Chen Y, Wang D and Tang D: The association between gut microbiota,
toll-like receptors, and colorectal cancer. Clin Med Insights.
16:117955492211305492022.PubMed/NCBI
|
|
29
|
Liu WT, Jing YY, Yan F, Han ZP, Lai FB,
Zeng JX, Yu GF, Fan QM, Li R, Zhao QD, et al: LPS-induced
CXCR4-dependent migratory properties and a mesenchymal-like
phenotype of colorectal cancer cells. Cell Adh Migr. 11:13–23.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jeong JW, Park C, Cha HJ, Hong SH, Park
SH, Kim GY, Kim WJ, Kim CH, Song KS and Choi YH: Cordycepin
inhibits lipopolysaccharide-induced cell migration and invasion in
human colorectal carcinoma HCT-116 cells through down-regulation of
prostaglandin E2 receptor EP4. BMB Rep. 51:532–537. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu G, Cheng Z, Lin C, Hoffman RM, Huang
Y, Singh SR, Zheng W, Yang S and Ye J: MyD88 Regulates LPS-induced
NF-ĸB/MAPK cytokines and promotes inflammation and malignancy in
colorectal cancer cells. Cancer Genomics Proteomics. 16:409–419.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
d'Hennezel E, Abubucker S, Murphy LO and
Cullen TW: Total Lipopolysaccharide from the human gut microbiome
silences toll-like receptor signaling. mSystems. 2:e00046–17.
2017.PubMed/NCBI
|
|
33
|
Kang M, Edmundson P, Araujo-Perez F, McCoy
AN, Galanko J and Keku TO: Association of plasma endotoxin,
inflammatory cytokines and risk of colorectal adenomas. BMC Cancer.
13:912013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu G, Huang Q, Huang Y, Zheng W, Hua J,
Yang S, Zhuang J, Wang J and Ye J: Lipopolysaccharide increases the
release of VEGF-C that enhances cell motility and promotes
lymphangiogenesis and lymphatic metastasis through the TLR4-
NF-κB/JNK pathways in colorectal cancer. Oncotarget. 7:73711–73724.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
de Waal GM, de Villiers WJS, Forgan T,
Roberts T and Pretorius E: Colorectal cancer is associated with
increased circulating lipopolysaccharide, inflammation and
hypercoagulability. Sci Rep. 10:87772020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou W, Chen X, Hu Q, Chen X, Chen Y and
Huang L: Galectin-3 activates TLR4/NF-κB signaling to promote lung
adenocarcinoma cell proliferation through activating lncRNA-NEAT1
expression. BMC Cancer. 18:5802018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang W and Wang J: Toll-Like Receptor 4
(TLR4)/Cyclooxygenase-2 (COX-2) regulates prostate cancer cell
proliferation, migration, and invasion by NF-κB activation. Med Sci
Monit. 24:5588–5597. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rakoff-Nahoum S and Medzhitov R:
Regulation of spontaneous intestinal tumorigenesis through the
adaptor protein MyD88. Science. 317:124–127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang HY, Zhang ZJ, Cao CB, Wang N, Liu
FF, Peng JQ, Ren XJ and Qian J: The TLR4/NF-κB signaling pathway
mediates the growth of colon cancer. Eur Rev Med Pharmacol Sci.
18:3834–3843. 2014.PubMed/NCBI
|
|
40
|
Fukata M, Chen A, Vamadevan AS, Cohen J,
Breglio K, Krishnareddy S, Hsu D, Xu R, Harpaz N, Dannenberg AJ, et
al: Toll-like receptor-4 promotes the development of
colitis-associated colorectal tumors. Gastroenterology.
133:1869–1881. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fukata M, Chen A, Klepper A, Krishnareddy
S, Vamadevan AS, Thomas LS, Xu R, Inoue H, Arditi M, Dannenberg AJ
and Abreu MT: Cox-2 is regulated by Toll-like receptor-4 (TLR4)
signaling: Role in proliferation and apoptosis in the intestine.
Gastroenterology. 131:862–877. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fukata M, Shang L, Santaolalla R,
Sotolongo J, Pastorini C, España C, Ungaro R, Harpaz N, Cooper HS,
Elson G, et al: Constitutive activation of epithelial TLR4 augments
inflammatory responses to mucosal injury and drives
colitis-associated tumorigenesis. Inflamm Bowel Dis. 17:1464–1473.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cheah MT, Chen JY, Sahoo D,
Contreras-Trujillo H, Volkmer AK, Scheeren FA, Volkmer JP and
Weissman IL: CD14-expressing cancer cells establish the
inflammatory and proliferative tumor microenvironment in bladder
cancer. Proc Natl Acad Sci USA. 112:4725–4730. 2015. View Article : Google Scholar : PubMed/NCBI
|