Introduction
Reactive oxygen species (ROS) are short-lived,
highly electrophilic molecules. They are produced from secondary
metabolites generated by a partial reduction of oxygen (1). When intracellular antioxidants are
reduced or ROS accumulate excessively, an imbalance in the redox
state occurs, which is referred to as oxidative stress. Under such
conditions, excessive intracellular accumulation of ROS modifies
redox-sensitive amino acid residues in regulatory proteins and
alters the actions of proteins and enzymes. Protein kinases,
transcription factors and the ubiquitin-proteasome system are
vulnerable to excessive accumulation of ROS (2). Of note, when this occurs in tumor
cells, it promotes tumor development. Stimulation of signaling
pathways by ROS promotes the proliferation, migration and invasion
of tumor cells, including in human breast, skin and liver cancers
(3). Furthermore, oxidative stress
caused by excessive accumulation of ROS can lead to genetic
instability. Cell death modalities, such as apoptosis, autophagy,
ferroptosis and pyroptosis, act as protective mechanisms to prevent
the proliferation of damaged cells (4) and this is also seen in tumor cells.
Thus, ROS effects are complex in tumor cells (5,6).
Specifically, tumor cells maintain moderate-to-high ROS levels,
namely, above the low cytostatic level and below the cytotoxic
level, by enhancing their own antioxidant capacity. Therefore, the
ROS level in tumor cells is subtoxic, which facilitates tumor cell
progression, and ROS act as signaling molecules to increase the
proliferation of tumor cells (7–10).
However, under further oxidative stress, cancer cells are equally
susceptible to excessive ROS (11),
and increased ROS unbalance the redox response of cancer cells,
ultimately leading to cellular senescence or death (12).
Curcumin is a plant polyphenol in the rhizome of
turmeric and was classified as a third-generation cancer
chemopreventive agent by the National Cancer Institute (13). Several studies have reported
anticancer mechanisms mediated by curcumin through the induction of
elevated ROS (14,15). This contradicts the antioxidant
properties of curcumin, the potential reasons for which are
discussed in the present review. Unfortunately, properties such as
poor water solubility and low bioavailability limit the clinical
application of curcumin (13).
However, the clinical efficacy of curcumin has been enhanced by
combining it with drugs, the introduction of nanocarriers and the
development of curcumin derivatives, which have brought the
clinical application of curcumin closer to reality (16–18).
Therefore, the present study reviewed articles in which the effect
of curcumin on tumors was evaluated, with a particular focus on its
relationship with oxidative stress, to provide a reference for
future studies.
Introduction to curcumin
Curcumin, also known as diferuloylmethane, has the
chemical formula C21H20O6, a
molecular weight of 368.38 g/mol and a symmetrical molecular
structure (19). Curcumin has been
commonly used as an aromatic and natural food coloring agent;
however, its biological effects are gradually being elucidated.
Curcumin has anti-inflammatory, antibacterial, hepatoprotective and
anticancer properties (20–22), and its anticancer effects have been
reported in several tumor types (Table
I). In melanoma, curcumin has been reported to increase the ROS
level and activate oxidative stress in the cysteine asparaginase
pathway, which causes tumor cell death (23). Lysosome-associated membrane protein
3 belongs to the fourth transmembrane protein superfamily, which
promotes tumor cell invasion and metastasis. It interacts with
ubiquitin-specific peptidase 4 and is positively regulated by the
latter. A recent study reported that curcumin downregulated
ubiquitin-specific peptidase 4 to modulate lysosome-associated
membrane protein 3, and thus inhibited the malignant progression of
colorectal cancer cells (24).
Furthermore, curcumin-induced accumulation of ROS in tumors to kill
tumor cells has been noted in several studies (25,26),
as discussed in the present review.
 | Table I.Cancer inhibition mechanisms of
curcumin in certain tumors. |
Table I.
Cancer inhibition mechanisms of
curcumin in certain tumors.
| First author/s,
year | Type of cell
death | Tumor type/tumor
cell | Mechanism of
carcinogenesis | (Refs.) |
|---|
| Gabr et al,
2022 | Apoptosis | Drug-resistant
tumor cells, such as M7/A549 | Curcumin promoted
ERK/JNK phosphorylation, causing elevated ROS levels and triggering
mitochondria-dependent apoptosis | (25) |
| Liczbiński et
al, 2020 |
| Human papillary
thyroid cancer | Curcumin triggered
disturbances in Ca2+ homeostasis, leading to endoplasmic
reticulum stress, mitochondrial damage and apoptosis | (41) |
| Agarwal et
al, 2018 |
| Colon cancer | Curcumin induced
mutations in the P53 gene and altered the mitochondrial membrane
potential to induce apoptosis | (45) |
| Liu et al,
2019 | Autophagy | Ovarian cancer | Curcumin inhibited
the AKT/mTOR/p70S6K signaling pathway and induced autophagy | (49) |
| Wang et al,
2020 |
| Carcinoma of the
cervix | Curcumin induced
ROS accumulation, promoted LC3 transformation and induced
autophagy | (53) |
| Li et al,
2020 | Ferroptosis | Breast cancer | Curcumin-induced
HO-1 overexpression led to a disturbed intracellular iron
distribution and triggered the Fenton reaction | (60) |
| Cao et al,
2022 |
|
| Curcumin induced
elevated ROS levels, MDA and iron accumulation, promoted SLC1A5
expression and induced ferroptosis | (64) |
| Tang et al,
2021 |
| Non-small cell lung
cancer | Curcumin induced a
decrease in GSH and an increase in ROS levels and iron
accumulation | (65) |
| Liang et al,
2021 | Pyroptosis | Liver cancer | Curcumin induced
elevated ROS levels and increased expression of GSDME-N, triggering
tumor cell pyroptosis | (68) |
Curcumin is well tolerated by humans. For example, a
study that evaluated the toxicity of curcumin in humans reported
that subjects administered 8 mg/day curcumin (99.3% purity) did not
develop toxicity (27). However,
certain physical and chemical properties of curcumin limit its
clinical application. For example, whilst its high lipophilicity
assists in penetrating lipid structures, it is insoluble in water
and has poor stability, resulting in low bioavailability (13). In a previous study, 440–2,200 mg
curcumin extract was administered to patients with advanced
colorectal cancer for 29 consecutive days, and curcumin was not
detected in patient blood or urine. In several clinical trials, ≤12
g/day curcumin was administered orally, but the amount of curcumin
detected in serum was <1% (27,28).
To overcome these drawbacks, the use of curcumin in combination
with other drugs to enhance its anticancer activity has been
reported (29). In addition, the
development of curcumin derivatives, the use of carriers or
coverings such as chitosan, and nanosystems have been suggested to
improve the bioavailability of curcumin (30). Thus, the potential for the clinical
application of curcumin is increasing.
Curcumin causes oxidative stress in tumor
cells and leads to cell death in different ways
Mechanism of action of curcumin
against cancer via oxidative stress
ROS serve an essential role in the development of
cancers as the microenvironment of cancer cells is involved in ROS
homeostasis. High ROS levels have a cytotoxic effect on cancer
cells, leading to malignant cell death and thus limiting cancer
progression. Curcumin treatment of tumor cells induces high levels
of ROS production and accumulation, resulting in a redox imbalance
in tumor cells (31). As shown in
Fig. 1, high ROS levels can cause
mitochondrial damage, thereby triggering different types of cell
death, such as apoptosis, autophagy, ferroptosis and
pyroptosis.
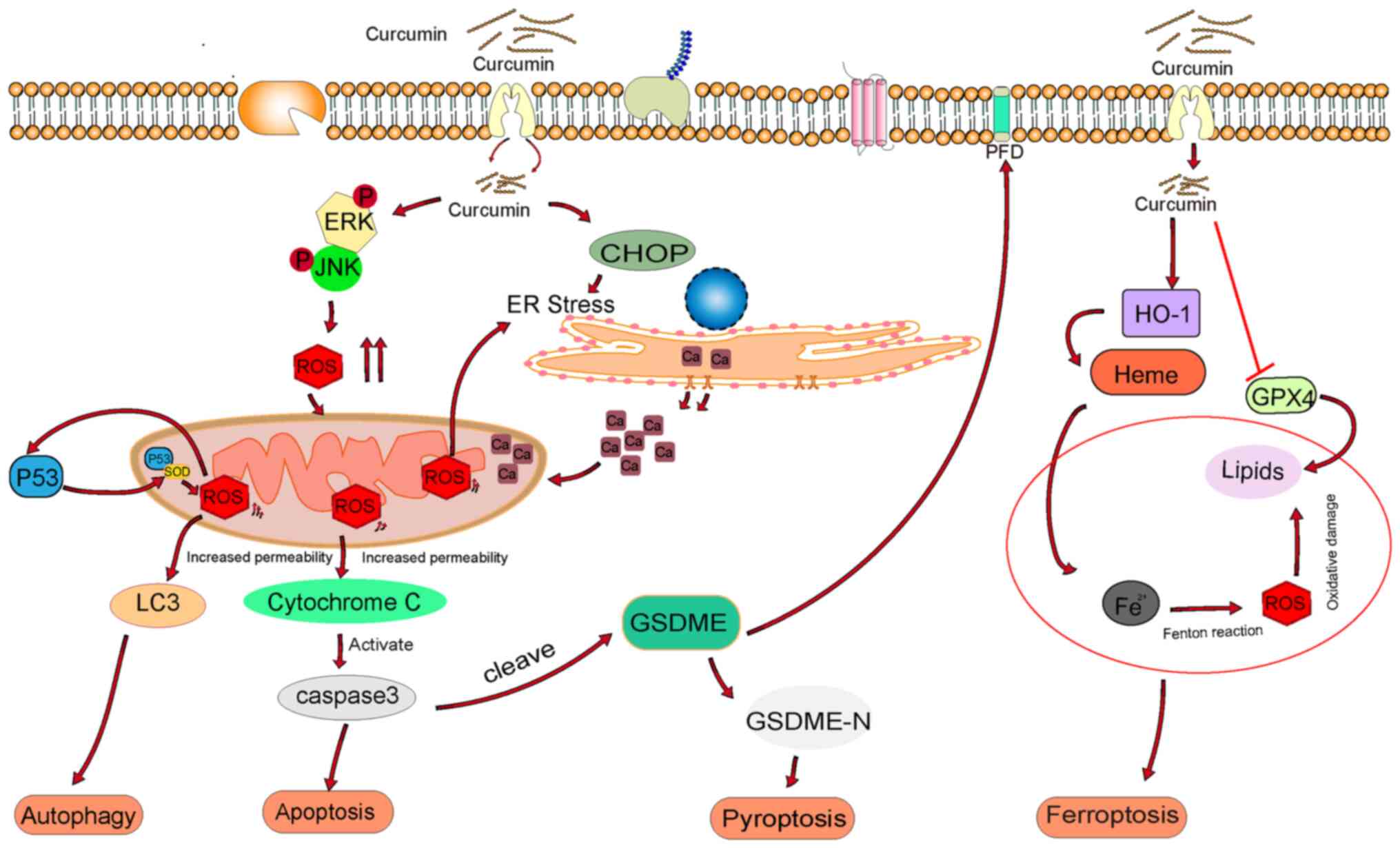 | Figure 1.Mechanism of action of curcumin in
cancer cells via oxidative stress. ROS, reactive oxygen species;
ERK, extracellular signal-regulated kinase; JNK, C-Jun N-terminal
kinase; LC3, microtubule-associated-proteinlight-chain-3; ER,
endoplasmic reticulum; CHOP, C/EBP-homologous protein; GSDME,
Gasdermin E; HO-1, heme oxygenase 1; GPX4, glutathione peroxidase
4; GSDME-N, gasdermin E N-terminal. |
Induction of mitochondrial oxidative
stress
The main source of intracellular ROS is the
respiratory chain of the inner mitochondrial membrane. ROS are
continuously produced as a by-product of aerobic metabolism and are
removed by cellular antioxidant mechanisms to maintain an
essentially non-toxic level. Under normal physiological conditions,
ROS act as specific molecular regulators of cellular signaling and
function. A typical mode of ROS regulation is the reversible
oxidation of target protein sulfhydryl groups to cystine to mediate
biological effects. In addition, ROS-induced changes in the
intracellular redox state affect cellular activities, including
signaling, metabolism, growth and apoptosis (10,32,33).
However, in tumors under inflammatory or stress conditions, the
excess ROS produced cannot be adequately neutralized. These excess
ROS propagate in the intercellular compartment and react with
proteins and nucleic acids. They also react with polyunsaturated
fatty acids to produce lipid hydroperoxides and unsaturated
aldehydes, causing oxidative stress in mitochondria (34). Such an oxidative cascade affects
several mitochondrial functions, such as biogenesis, ion
homeostasis and antioxidant defense mechanisms (34). In non-neoplastic disease studies,
curcumin is often considered a highly potent antioxidant that
reduces oxidative damage to mitochondria. In a rat model of acute
respiratory distress syndrome, curcumin treatment attenuated renal
tubular epithelial and mitochondrial damage and reduced oxidative
stress (35,36). Conversely, curcumin induced
excessive ROS production in tumor cells and caused mitochondrial
damage, which ultimately exerted its cancer-suppressive effects
through certain cell death modalities (25). In addition, a study have reported
that mitochondrial oxidative homeostasis can be disrupted by
targeting mitochondria with curcumin. Curcumin-coated micelles
effectively inhibit the progression of gastric cancer, and the
potential therapeutic mechanism may be its effect on mitochondrial
proteins to reduce the mitochondrial membrane potential and
increase ROS to disrupt oxidative homeostasis (26). In addition to targeting curcumin to
mitochondria through micelles, several other approaches may achieve
this goal. For instance, the introduction of different nanocarriers
and the development of curcumin targeting mitochondrial derivatives
(37,38). Therefore, a therapeutic approach may
be to induce oxidative stress in the mitochondria of tumor cells by
targeting curcumin to mitochondria.
Curcumin induces apoptosis via
ROS
Apoptosis is a form of programmed cell death that is
mainly induced by damage to DNA and organelles, such as the
mitochondria and endoplasmic reticulum (ER), via stimuli such as
oxidative stress, chemotherapeutic drugs and ionizing radiation
(39). The ability of curcumin to
cause apoptosis has been reported in several types of tumor. After
treatment of drug-resistant tumor cell lines, MCF7/TH, HCT116R and
A549/ADR, with curcumin, ROS levels and expression of apoptotic
markers, such as Bax and cytochrome c, were notably increased in
the high-dose group compared to the low-dose group (25).
The mitochondrion-dependent pathway is a classical
pathway that mediates apoptosis. Curcumin causes mitochondrial
damage by promoting phosphorylation of ERK and JNK, resulting in
the increased release of ROS and cytochrome c into the cytoplasm,
thereby triggering a mitochondrion-dependent pathway of apoptosis.
In addition, ER stress and mitochondrial dysfunction induce
apoptosis (40). In the treatment
of human papillary thyroid cancer with curcumin, both activating
transcription factor (ATF) 6 and the ER stress marker C/EBP
homologous protein (CHOP) were activated by curcumin and
Ca2+-ATPase activity was also affected. This led to
Ca2+ accumulation in the mitochondrial matrix, causing
mitochondrial swelling, membrane potential changes and elevated ROS
levels, which eventually resulted in mitochondrial rupture and the
release of cytochrome c and other proapoptotic proteins into the
cytoplasm (41,42).
It has been reported that 85% of patients with colon
cancer have mutations or loss of function and expression of the
Smad4 and p53 genes (43).
Therapeutic agents that induce ROS-mediated apoptosis have been
suggested to be potential agents for the treatment of Smad4- and
p53-mutant colon cancer (44).
Curcumin induced a marked increase in ROS levels in p53-mutated
colon cancer HT-29 cells and induced apoptosis in colon cancer
cells by altering the mitochondrial membrane potential (45). Another study reported the possible
involvement of p53 in curcumin-mediated apoptosis of colon cancer
cells by treating wild-type (HCT-116) and mutant (HT-29) p53 colon
cancer cell lines with curcumin, but the underlying mechanism was
not elucidated (46). Results from
the aforementioned study indicate that under stress conditions such
as tumorigenesis, p53 undergoes mitochondrial translocation and
binds to superoxide dismutase (SOD), rendering it inactive and
inducing ROS production, which triggers apoptosis. In turn,
curcumin treatment further induces ROS generation, which again
subjects p53 to oxidative stress conditions and further
mitochondrial translocation, which serves a further role in
promoting apoptosis. This may also explain why N-acetylcysteine
(NAC) inhibited the loss of the mitochondrial membrane potential in
HT-29 cells treated with both low and high concentrations of
curcumin in the aforementioned study, whereas in HCT-116 cells, it
only affected cells treated with low concentrations of
curcumin.
Curcumin induces autophagy via
ROS
Autophagy is another type of programmed cell death.
In certain cases, autophagy phagocytoses cytoplasmic proteins or
organelles and encapsulates them in vesicles for fusion with
lysosomes to form autophagic lysosomes to degrade their
encapsulated contents. During the process of degradation,
autophagic lysosomes provide essential peptides and amino acids to
cells (47,48). Inducing tumor cells to undergo
autophagy to kill the tumor cells is a potential approach for
clinical treatment and numerous studies have reported that curcumin
induces autophagy in cancer cells. For instance, in ovarian cancer,
a study demonstrated that curcumin induced autophagy via the
inhibition of the AKT/mTOR/p70S6K signaling pathway, enhanced light
chain (LC)3B-I/II expression and increased autophagy related 3 and
Beclin1 expression in a concentration-dependent manner (49). ROS also induced cell damage and
disrupted specific signaling pathways, leading to autophagic cell
death, which is also known as type II cell death (50). Furthermore, a study evaluated the
targets of curcumin in colon cancer and found that it serves a role
in ROS generation and autophagy induction (51). Similarly, another study reported
have reported that patients with colon cancer with high expression
of heat shock protein 27 are more sensitive to curcumin (52). This may be due to the high
expression of heat shock protein 27 increasing ROS levels and
activating autophagy. Treatment with curcumin enhanced this effect.
In addition, a study reported abnormal energy metabolism and
accumulation of ROS in cervical cancer cells after curcumin
treatment and further analysis of autophagy revealed that curcumin
promoted the conversion of LC3 to LC3II and degradation of
autophagosome markers. This process was blocked by the antioxidant
NAC, indicating that curcumin induced autophagy by increasing ROS
(53). Of note, curcumin
derivatives have been reported to induce ROS production and
activate autophagy. For instance, EF24 is a curcumin derivative
with promising anticancer effects, and treatment of non-small lung
cancer cells with EF24 was shown to result in a notable increase in
ROS, autophagic markers and vesicles (54).
Curcumin triggers ferroptosis via
ROS
Direct induction of cytotoxicity in cancer cells is
the main goal of anticancer therapy. Unlike apoptosis, necrosis and
autophagy, ferroptosis is the iron-dependent programmed cell death
(55). It is characterized by
intracellular iron accumulation, resulting in excessive ROS
production, decreased glutathione (GSH) levels and lipid
peroxidation (56). Heme oxygenase
1 (HO-1) catalyzes the degradation of heme to carbon monoxide,
biliverdin and free iron (57). Its
upregulation alters iron homeostasis and reduces tumor cell
survival. Furthermore, oxidative stress induced by organic
oxidants, such as tert-butyl hydroperoxide, have been reported to
promote HO-1 translocation to the mitochondria, causing
mitochondrial iron overload and ferroptosis in cardiomyocytes
(58,59). Treatment of breast cancer M7 and 231
cell lines with curcumin has been reported to promote HO-1
expression, which in turn promoted heme degradation, leading to an
increase in Fe2+ and ultimately an altered cellular iron
distribution (60). Iron overload
triggers the Fenton reaction, which increases ROS levels and causes
peroxidation and oxidative damage to surrounding lipids and
proteins (61). GSH peroxidase 4
(GPX4) acts as an antioxidant and counteracts lipid peroxidation
(62). However, its expression is
downregulated in curcumin-treated breast cancer cells, which
eventually leads to ferroptosis (60). Furthermore, HO-1 is activated by
nuclear factor erythroid 2-related factor 2 (Nrf2) in the nucleus
to perform its antioxidant function (63). However, HO-1 is upregulated and
elevates ROS and induces ferroptosis, suggesting a dual role for
HO-1. In a study of breast cancer, curcumin induced elevated levels
of lipid ROS, accumulation of lipid peroxide end product
malondialdehyde and increased intracellular Fe2+ levels,
ultimately enhancing the expression of solute carrier family 1
member 5 to induce ferroptosis in vitro and in vivo
(64). Tang et al (65) assessed the specific role and
potential mechanism of ferroptosis in treating non-small cell lung
cancer with curcumin. Curcumin significantly triggered GSH
depletion, lipid peroxidation and accumulation of ROS and iron in
mice as well as in A549 and H1299 cells. In addition, pretreatment
with the hemostatic inhibitor ferrostatin-1 or iron responsive
element binding protein 2 knockdown notably reduced
curcumin-induced siderosis in A549 and H1299 cells. Notably, in
these cells, pretreatment with the autophagy inhibitor chloroquine
or knockdown of the autophagy-related gene Beclin1 reduced
curcumin-induced autophagy and subsequent ferroptosis, suggesting
that activation of the autophagy pathway may also trigger
ferroptosis in tumor cells.
Curcumin causes pyroptosis through
ROS
Pyroptosis is a recently discovered form of
programmed cell death that relies on the activation of
inflammation-associated caspase-1, −4, −5 and −11, and
apoptosis-associated caspase-3 (66). Activated caspase-3 cleaves gasdermin
E (GSDME) proteins, releasing the active GSDME N-terminal and
pore-forming structural domains, leading to the formation of
non-selective pores in the cell membrane (67). A study has reported that cell
swelling, membrane lysis, a large number of scorched vesicles and
increased expression of GSDME-N appeared after curcumin treatment
of hepatocellular carcinoma. In addition, ROS levels notably
increased. Treatment with NAC inhibited pyroptosis induced by
curcumin. These results suggest that curcumin induces pyroptosis by
regulating ROS levels, but the exact mechanism has not been
elucidated (68). Furthermore, a
study reported on the design of a dicarbonyl curcumin analog (B2)
of the curcumin β-dione structure (69). B2 treatment of lung cancer H146
cells increased the level of intracellular ROS and expression of
caspase-3 in a concentration-dependent decrease, resulting in
GSDME-N production. Thus, B2 exhibited antitumor activity through
transition from apoptosis to pyroptosis. Combined with the
possibility that high concentrations of ROS activate the ER
stress-mediated cell death pathway, Wei et al (69) further assessed the transcription
factors associated with ER stress pathways and reported that B2
increased the mRNA expression of binding immunoglobulin protein in
H460 cells and thus the mRNA expression of ATF-4 and X-box binding
protein (XBP-1). ATF-4 and XBP-1 entered the nucleus as
transcription factors to regulate ER stress, resulting in increased
protein expression of CHOP. The aforementioned studies indicate
that the inhibitory effect of B2 on tumor cells may be achieved via
the ROS-activated ER stress-mediated cell death pathway.
Combination of curcumin with anticancer
drugs improves cancer inhibition
Curcumin is a pigment with a diketone structure.
Curcumin-induced ROS is crucial for cancer cell death. In addition,
curcumin mediates chemosensitization. However, its low solubility
and poor stability limit its clinical application. To increase
curcumin use, the combination of curcumin and drugs has been
reported to sensitize drug-resistant cancer cells and enhance
therapeutic effects (Table II)
(70).
 | Table II.Anticancer effects of curcumin in
combination with other drugs. |
Table II.
Anticancer effects of curcumin in
combination with other drugs.
| First author/s,
year | Drug | Tumor | Mechanism | (Refs.) |
|---|
| Du et al,
2013 | Curcumin +
resveratrol |
Hepatocarcinoma | Promoted ROS
production and induced caspase activation to cause apoptosis | (16) |
| Arena et al,
2021 |
| Breast cancer | Induced ER stress,
activated the PERK/eIF-2α/CHOP axis and promoted apoptosis | (73) |
| Cho et al,
2019 |
| Bladder cancer | Increased the
expression of PARP, the inhibition of DNA damage, promotion of
apoptosis and the alleviation of drug resistance | (74) |
| Lee et al,
2020 | Curcumin +
paclitaxel | Lung cancer Breast
cancer | Increased ROS
production, inhibition of cell proliferation and promotion of
apoptosis | (77) |
| Attia et al,
2020 |
| Breast cancer | Reduced the
activity and expression of P-gp and MDR1 and increased paclitaxel
sensitivity | (84) |
| Ebrahimifar et
al, 2017 |
| Gastric cancer | NF-κB expression
was increased, promoting apoptosis | (85) |
| Zhang et al,
2021 | Curcumin +
doxorubicin | Colon cancer | Increased ROS,
reduced P-gp transport activity and reversed drug resistance | (86) |
| Firouzi Amoodizaj
et al, 2020 |
| Adenocarcinoma of
the stomach | Bcl-2 was
down-regulated and Bax and caspase-9 was upregulated, triggering
apoptosis | (87) |
| Dhandapani et
al, 2007 |
| Glioblastoma | Reduced the
expression of DNA repair enzymes (MGMT and DNA-PK) and enhanced DNA
damage leading to cell death | (88) |
| Kocdor et
al, 2019 | Curcumin +
deguelin | Thyroid cancer | Reduced OSI,
enhanced SOD activity and induced apoptosis | (93) |
Polyphenols, such as curcumin, are a large family of
organic compounds that contain a common ternary flavonoid ring
system structure and polyphenolic units (70). These natural compounds are mainly
found in plants and are beneficial to humans. For instance, certain
polyphenols have been used to treat tumors, such as in one study
where the treatment of breast cancer cells with resveratrol
resulted in differential expression of several genes related to the
cell cycle and induced S-phase arrest (71). Curcumin has also been reported to
mediate the arrest of tumor cells in G2/M phase via p53 (17). However, polyphenols are also
associated with negative outcomes, such as drug resistance
induction. Nevertheless, studies have reported that combinations of
certain polyphenols improved therapeutic effects on tumors, and
both curcumin and resveratrol reduced cancer cell survival by
upregulating ROS. Furthermore, the combination of curcumin and
resveratrol notably increased ROS production compared with either
agent alone (16,72). Furthermore, NAC treatment
significantly reversed apoptosis induced by the combination of
curcumin and resveratrol, and the expression of caspase-3, −8, and
−9 was also reduced. These findings suggest that combination
therapy with curcumin and resveratrol may induce apoptosis by
activating caspases and ROS production. In addition, the
combination of curcumin and resveratrol was reported to have
activated ER stress, enhanced the activity of the protein kinase
RNA-like ER kinase/eukaryotic initiation factor-2α/CHOP axis and
induced apoptosis (73). The
combination of the two increased poly (ADP-ribose) polymerase
expression inhibited DNA damage, promoted apoptosis and reduced
drug resistance in bladder cancer cells (74).
Paclitaxel (PTX), which is widely used for the
treatment of lung cancer, induces cell death by disrupting the
normal microtubule dynamics required for cell division and
important interphase processes. In addition, PTX induces cell cycle
arrest (75). However, PTX also has
several toxic effects on normal cells, such as the induction of
neurotoxicity and hematotoxicity (76). By measuring the amount of ROS
produced when the lung cancer A549 and Calu-3 cell lines were
co-treated with curcumin and PTX using a fluorescent probe, a study
reported that the combination resulted in higher levels of ROS and
showed stronger anticancer effects, such as the inhibition of cell
proliferation, as well as induction of apoptosis and cell cycle
arrest, compared with curcumin or PTX treatment alone (77). Of note, in the aforementioned study,
curcumin also attenuated the toxic effects of PTX on normal cells.
For instance, when the curcumin dose was higher, the combination
was less cytotoxic to the normal lung Beas-2B cell line compared
with the higher PTX dose group. This may indicate that curcumin
cannot be internalized by normal cells (78,79)
and therefore cannot induce the production of superoxide compounds
or be toxic to normal cells. However, in tumor cells, curcumin has
been reported to be internalized and induced the production of
superoxide compounds (80), which,
in conjunction with PTX, enhanced oxidative stress in tumor cells.
In addition, as an antioxidant, curcumin in turn attenuated the
oxidative stress caused by PTX in normal cells via several
mechanisms, such as increasing the expression of glutathione and
antioxidant proteins (81,82). Therefore, the combination of
curcumin and PTX may enhance anticancer activity in cancer cells
whilst protecting healthy cells from irreversible damage.
P-glycoprotein (P-gp) is the pump responsible for
drug efflux and its high expression is associated with drug
resistance in tumors (83). Attia
et al (84) reported that
combining curcumin and PTX reduced the activity and expression of
P-gp and multidrug resistance 1 and increased PTX sensitivity in
breast cancer. Furthermore, curcumin downregulated NF-κB, which
increased the sensitivity of gastric cancer cells to PTX (85). Doxorubicin (Dox) is commonly used to
treat malignant tumors (86–88).
However, developing P-gp-mediated multidrug resistance during tumor
chemotherapy severely reduces the therapeutic efficacy of Dox.
Curcumin has been reported to have inhibited D-glutamine metabolism
by decreasing the expression of ornithine decarboxylase. It also
markedly inhibited the biosynthesis of spermine and spermidine,
thereby reducing the oxidative stress capacity of SW620/AD300 cells
and the ATP-dependent transport activity of P-gp. This increased
the intracellular accumulation of Dox in drug-resistant cells and
ultimately reversed the multidrug resistance of tumors (89). Finally, deguelin is a fish-like
pigment in an African rhizome plant, which is used as an
insecticide. Deguelin inhibits sperm function via the PI3K/AKT
signaling pathway (90) and serves
a role in cancer inhibition (91).
For instance, deguelin has been reported to kill prostate cancer
cells (92). Kocdor et al
(93) reported that curcumin and
deguelin were able to reduce the oxidative stress index and enhance
the activity of superoxide dismutase.
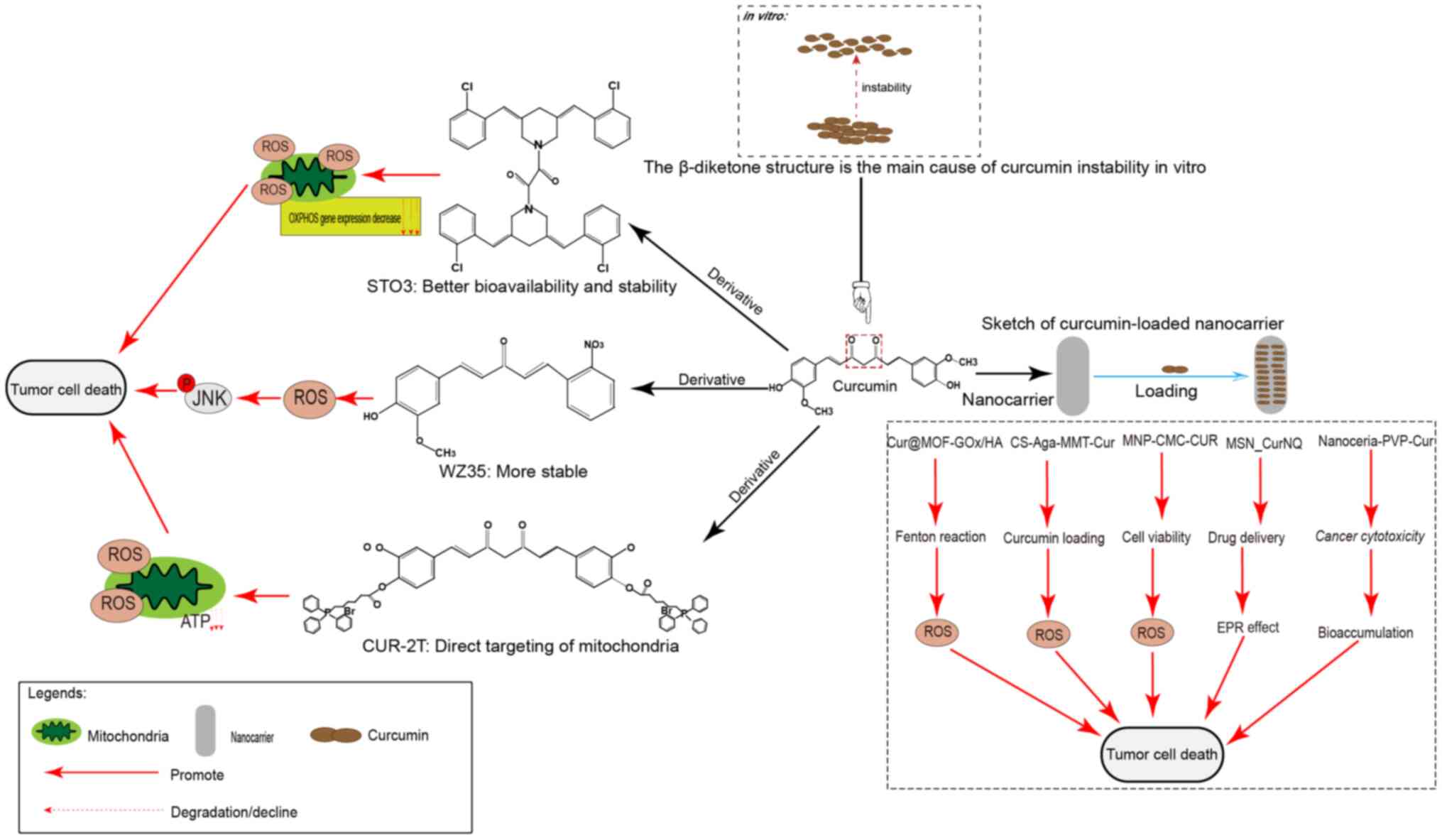
Anticancer effects of curcumin derivatives
are mediated via oxidative stress
In addition to its use in combination with drugs,
derivatives of curcumin are being developed to improve its
pharmacological effects (Fig. 2). A
study synthesized niacin (an essential vitamin necessary for normal
cell function) with curcumin to produce a new curcumin derivative
called nicotinic acid (17), which
has greater selectivity for cancer cells than curcumin itself. As
the curcumin derivative had an improved pharmacokinetic profile
in vivo, it more strongly stimulated oxidative stress.
Furthermore, β-diketones may be responsible for the instability and
weak pharmacokinetic profile of curcumin in vitro. In a
study, its β-diketone structure was deleted and a new curcumin
derivative, WZ35, was constructed, which exhibited markedly
improved chemical stability in vitro (94). In a follow-up study, WZ35 induced
apoptosis in gastric cancer cells by increasing the ROS level,
thereby causing ER stress and activating the JNK signaling pathway
(95). Furthermore, WZ35 caused
cell death in hepatocellular carcinoma and breast cancer cells via
a similar mechanism (96,97). ST03, a recently synthesized curcumin
derivative (98,99), has been reported to have improved
bioavailability and stability, and to be detectable in plasma for
up to 12 h. In a previous study, ST03 induced a peak in ROS within
1 h and a reduction in the transcription of mitochondrion
DNA-encoded respiratory chain-associated protein genes after 48 h,
which ultimately led to ovarian cancer cell death (99). A recent study synthesized novel
mitochondrion-targeted curcumin derivatives by unilateral coupling
of the curcumin phenolic hydroxyl group to triphenylphosphine
through an ester bond or bilateral coupling (CUR-2T) (38). CUR-2T demonstrated a clear
preferential selectivity for cancer cells and directly targeted
mitochondria, resulting in disruption of the redox balance
accompanied by increased ROS levels, decreased ATP levels and tumor
cell cycle arrest.
The introduction of nanocarriers and loaders has
greatly enhanced the loading and targeting ability of curcumin. A
study constructed metal-organic frameworks (MOFs) modified with
glucose oxidase (GOx). After loading with curcumin, it is further
modified with tumor-targeting hyaluronic acid (HA) to obtain
Cur@MOF-GOx/HA nano-enzymes.
Exposure to GOx triggered tumor starvation and produced
H2O2 to provide reactants for the
MOF-mediated Fenton reaction to produce large amounts of ROS,
whilst releasing curcumin to induce the autophagic death of tumor
cells (18). In another study, a
nanocomposite hydrogel platform for montmorillonite nanoparticles
added to chitosan-agarose was constructed, increasing the loading
capacity of curcumin from 63 to 76% and increasing the apoptotic
rate. The delivery platform in the present study enhanced the
curcumin load, sustained its release and increased its anticancer
effects (100). In another study,
magnetic nanoparticles containing curcumin with carboxymethyl
chitosan (MNP-CMC-CUR) were designed and used to treat breast
cancer MCF-7 and MDA-MB-231 cell lines and human fibroblasts, and
their effect was compared to that of curcumin alone in MTT assays
(101). It was observed that the
IC50 of MCF-7 cells treated with MNP-CMC-CUR was notably
reduced compared with that of curcumin itself without affecting the
metabolic activity of normal cells. Furthermore, p53 and caspase-3
gene expression was markedly increased in MCF-7 cells treated with
MNP-CMC-CUR. A study reported the cessation of G1-stage cancer cell
growth after hyperthermia, as well as an increase in caspase-3
expression and ROS production. Furthermore, MNP-CMC-CUR improved
drug effectiveness, and when combined with hyperthermia, the
therapeutic effect was enhanced (102).
Mesoporous silica nanoparticles (MSNs) are a class
of nanoparticles extensively studied for drug delivery
applications. Curcumin and naphthoquinone (NQ) are novel
therapeutic diagnostic molecules for cancer targeting, detection
and treatment (103). A novel
nanosystem has been developed using MSN_CurNQ that increases drug
delivery of CurNQ by increasing the enhanced permeability and
retention effect and sustained release (104). A study demonstrated that MSN_CurNQ
treatment did not elicit any cytotoxicity in the fibroblast 3T3
cell line, but reduced the viability of cancer cells to <50%,
indicating tumor-specific toxicity (103). Water- and alcohol-soluble cerium
oxide-curcumin conjugates were obtained by co-evaporation with
polyN-vinylpyrrolidone (PVP) (105), which induced oxidative stress
under ultraviolet (UV) irradiation or hydrogen peroxide conditions.
Nanoceramic PVP-curcumin (NPC) conjugates exhibited selective
cytotoxicity. Unlike curcumin itself, NPC conjugates demonstrated
photosensitivity in tumor cell cultures, whilst protecting
untransformed cultures from the damaging effects of UV radiation
and oxidative stress.
Curcumin combined with MOFs, nanocomposite hydrogel
platforms, nanoparticles and nanosystems may enhance the sustained
release of curcumin via several mechanisms of action to improve its
targeting and release curve, increase the solubility of curcumin
and toxicity in tumor cells, improve bioavailability and increase
its anticancer effects.
Discussion
Cancer is a significant health issue, and although
early diagnosis and targeted therapy have markedly reduced
mortality, cancer drug resistance and drug side effects are still
clinical problems that need to be resolved (106). Curcumin is a natural compound that
has been used for the treatment of numerous types of diseases, such
as Alzheimer's disease, fatty liver and cancer (53,107,108). Of note, curcumin has a dual role
in oncological and non-oncologic diseases. Specifically, in
non-neoplastic diseases, curcumin is a potent antioxidant that
attenuates oxidative stress and mitochondrial damage (35). Conversely, in tumors, curcumin binds
to several enzymes and increases ROS levels (23,24).
These different effects may be the result of differences in dosage.
For instance, in a previous study on curcumin treatment of
drug-resistant tumor cells, a low dose of curcumin showed no effect
on antioxidant proteins, whereas a high dose resulted in the
inhibition of antioxidant proteins, thereby increasing ROS levels
(25,109–112). Furthermore, mitochondria may be a
potential target for high-dose curcumin. During tumorigenesis,
mitochondria are often functionally and morphologically impaired,
leading to aberrant changes in ROS levels, and high-dose curcumin
treatment has been reported to exacerbate the effects on damaged
mitochondria (42,113). In addition, compared with normal
cells, proteins abnormally expressed in tumor cells, such as GSH
and HO-1, may be targeted by curcumin to cause oxidative stress in
tumor cells. The reasons for the dual action of curcumin need to be
further explored.
Curcumin induces high levels of ROS accumulation,
leading to an imbalance in the redox response. This results in
mitochondrial and DNA damage and subsequent activation of the cell
death pathway, providing possible approaches for cancer therapy.
The MAPK signaling pathway consists of three distinct cascades,
ERK, JNK and p38, which serve a role in cell growth, proliferation,
motility and death (114);
therefore, it may be an important pathway to target using curcumin
to induce apoptosis in cancer cells. Curcumin promotes
phosphorylation of ERK and JNK and induces ROS production, leading
to mitochondrial damage and cytochrome C release, which activates
caspase-related signaling pathways to cause apoptosis (115). In addition, it disrupts
intracellular calcium homeostasis and activates ER stress, leading
to apoptosis (42). Of note,
changes in caspase-3 expression have also been reported to induce
the production of GSDME-N and the formation of large amounts of
pore, which ultimately lead to cell membrane rupture and pyroptosis
(66). In terms of autophagy,
curcumin has been reported to induce elevated ROS levels and the
appearance of autophagy markers and autophagosomes, causing tumor
cells to undergo autophagy. Nrf2 is a classical transcription
factor that regulates the cellular oxidative stress response, which
controls several cellular behaviors, such as cellular
detoxification and oxidative stress, and induces the downstream
production of a series of cytoprotective proteins to maintain the
balance of the intracellular environment and prevent diseases
(116). Curcumin achieves
anticancer effects by regulating the expression of Nrf2 and its
downstream target HO-1, inhibiting the expression of GPX4 and
altering the accumulation of intracellular iron and inducing the
Fenton reaction (60). In addition,
the combination of curcumin with drugs, the introduction of
curcumin derivatives and nanocarriers have markedly improved the
pharmacokinetics of curcumin and enhanced its effects, including
oxidative stress to kill tumor cells.
Over the past two decades, the mechanisms by which
curcumin inhibits several types of tumor have been gradually
elucidated. In addition, research on curcumin derivatives and
nanocarriers has been performed and its clinical therapeutic
potential has been evaluated. However, research on curcumin needs
to be further deepened in terms of the following aspects: i)
Studies have reported that the cytotoxicity of curcumin
nanoparticles is notably increased and water-soluble curcumin
components demonstrate greater toxicity compared with curcumin
itself (117,118). Therefore, the toxicity of
nanoparticles and water-soluble curcumin needs to be reduced in the
development of these substances; ii) clinical trials of
nano-curcumin in tumor patients should be performed; iii) several
formulations of curcumin have produced markedly different results
in clinical trials. In a study by Sharma et al (119), patients experienced adverse
reactions such as diarrhea at a dosage of 3.6 g curcumin, whereas
no relevant therapeutic toxicity was observed at 8 g curcumin with
99.3% purity as described above. The difference between the two
studies may be due to the different formulations of curcumin.
Therefore, several formulations of curcumin should be assessed to
reduce its clinical toxicity and enhance its pharmacokinetic
effects; iv) the mechanism of action of curcumin in several tumor
types should continue to be evaluated, with oxidative stress being
the potential predominant mechanism.
In conclusion, curcumin may have the potential to
become a cutting-edge drug for the treatment of tumors and other
diseases. In-depth research on curcumin should be performed, as
well as more clinical trials related to curcumin, to evaluate its
anticancer activity.
Acknowledgements
Not applicable.
Funding
The present review was supported by the National Science
Foundation of China (grant no. 31560312).
Availability of data and materials
Not applicable.
Authors' contributions
YH and LC designed and organized this manuscript. YH
contributed the first draft of the manuscript, and LC refined and
revised the contents, tables and figures. SD wrote the basic
sections of the manuscript. KW and SL revised the manuscript. All
authors read and approved the final manuscript. Data authentication
does not apply.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dizdaroglu M: Oxidatively induced DNA
damage and its repair in cancer. Mutat Res Rev Mutat Res.
763:212–245. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moldogazieva NT, Lutsenko SV and Terentiev
AA: Reactive oxygen and nitrogen species-induced protein
modifications: Implication in carcinogenesis and anticancer
therapy. Cancer Res. 78:6040–6047. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moloney JN and Cotter TG: ROS signalling
in the biology of cancer. Semin Cell Dev Biol. 80:50–64. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Galadari S, Rahman A, Pallichankandy S and
Thayyullathil F: Reactive oxygen species and cancer paradox: To
promote or to suppress? Free Radic Biol Med. 104:144–164. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Frijhoff J, Winyard PG, Zarkovic N, Davies
SS, Stocker R, Cheng D, Knight AR, Taylor EL, Oettrich J, Ruskovska
T, et al: Clinical relevance of biomarkers of oxidative stress.
Antioxid Redox Signal. 23:1144–1170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saikolappan S, Kumar B, Shishodia G, Koul
S and Koul HK: Reactive oxygen species and cancer: A complex
interaction. Cancer Lett. 452:132–143. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saha SK, Lee SB, Won J, Choi HY, Kim K,
Yang GM, Dayem AA and Cho SG: Correlation between oxidative stress,
nutrition, and cancer initiation. Int J Mol Sci. 18:15442017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feno S, Butera G, Vecellio Reane D,
Rizzuto R and Raffaello A: Crosstalk between calcium and ROS in
pathophysiological conditions. Oxid Med Cell Longev.
2019:93240182019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mittler R: ROS Are Good. Trends Plant Sci.
22:11–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoneyama M, Kawada K, Gotoh Y, Shiba T and
Ogita K: Endogenous reactive oxygen species are essential for
proliferation of neural stem/progenitor cells. Neurochem Int.
56:740–746. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Trachootham D, Alexandre J and Huang P:
Targeting cancer cells by ROS-mediated mechanisms: A radical
therapeutic approach? Nat Rev Drug Discov. 8:579–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zoi V, Galani V, Lianos GD, Voulgaris S,
Kyritsis AP and Alexiou GA: The role of curcumin in cancer
treatment. Biomedicines. 9:10862021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nelson KM, Dahlin JL, Bisson J, Graham J,
Pauli GF and Walters MA: The essential medicinal chemistry of
curcumin. J Med Chem. 60:1620–1637. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abd El-Hack ME, El-Saadony MT, Swelum AA,
Arif M, Abo Ghanima MM, Shukry M, Noreldin A, Taha AE and
El-Tarabily KA: Curcumin, the active substance of turmeric: Its
effects on health and ways to improve its bioavailability. J Sci
Food Agric. 101:5747–5762. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Du Q, Hu B, An HM, Shen KP, Xu L, Deng S
and Wei MM: Synergistic anticancer effects of curcumin and
resveratrol in Hepa1-6 hepatocellular carcinoma cells. Oncol Rep.
29:1851–1858. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He YC, He L, Khoshaba R, Lu FG, Cai C,
Zhou FL, Liao DF and Cao D: Curcumin nicotinate selectively induces
cancer cell apoptosis and cycle arrest through a P53-Mediated
mechanism. Molecules. 24:41792019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yao H, Gong X, Geng M, Duan S, Qiao P, Sun
F, Zhu Z and Du B: Cascade nanozymes based on the ‘butterfly
effect’ for enhanced starvation therapy through the regulation of
autophagy. Biomater Sci. 10:4008–4022. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kotha RR and Luthria DL: Curcumin:
Biological, pharmaceutical, nutraceutical, and analytical aspects.
Molecules. 24:29302019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Amalraj A, Pius A and Gopi S and Gopi S:
Biological activities of curcuminoids, other biomolecules from
turmeric and their derivatives-A review. J Tradit Complement Med.
7:205–233. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gopi S, Jacob J, Varma K, Jude S, Amalraj
A, Arundhathy CA, George R, Sreeraj TR, Divya C, Kunnumakkara AB
and Stohs SJ: Comparative oral absorption of curcumin in a natural
turmeric matrix with two other curcumin formulations: An open-label
Parallel-arm study. Phytother Res. 31:1883–1891. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kunnumakkara AB, Bordoloi D, Padmavathi G,
Monisha J, Roy NK, Prasad S and Aggarwal BB: Curcumin, the golden
nutraceutical: Multitargeting for multiple chronic diseases. Br J
Pharmacol. 174:1325–1348. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Manica D, Silva GBD, Silva APD, Marafon F,
Maciel SFVO, Bagatini MD and Moreno M: Curcumin promotes apoptosis
of human melanoma cells by caspase 3. Cell Biochem Funct. Oct
4–2023.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wei H, Li X, Liu F, Li Y, Luo B, Huang X,
Chen H, Wen B and Ma P: Curcumin inhibits the development of
colorectal cancer via regulating the USP4/LAMP3 pathway. Naunyn
Schmiedebergs Arch Pharmacol. Sep 20–2023.(Epub ahead of print).
View Article : Google Scholar
|
|
25
|
Gabr SA, Elsaed WM, Eladl MA, El-Sherbiny
M, Ebrahim HA, Asseri SM, Eltahir YAM, Elsherbiny N and Eldesoqui
M: Curcumin modulates oxidative stress, fibrosis, and apoptosis in
Drug-resistant cancer cell lines. Life (Basel).
12:14272022.PubMed/NCBI
|
|
26
|
Lin X, Wang L, Zhao L, Zhu Z, Chen T, Chen
S, Tao Y, Zeng T, Zhong Y, Sun H, et al: Curcumin micelles suppress
gastric tumor cell growth by upregulating ROS generation,
disrupting redox equilibrium and affecting mitochondrial
bioenergetics. Food Funct. 11:4146–4159. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sharma RA, McLelland HR, Hill KA, Ireson
CR, Euden SA, Manson MM, Pirmohamed M, Marnett LJ, Gescher AJ and
Steward WP: Pharmacodynamic and pharmacokinetic study of oral
Curcuma extract in patients with colorectal cancer. Clin Cancer
Res. 7:1894–1900. 2001.PubMed/NCBI
|
|
28
|
Lao CD, Ruffin MT IV, Normolle D, Heath
DD, Murray SI, Bailey JM, Boggs ME, Crowell J, Rock CL and Brenner
DE: Dose escalation of a curcuminoid formulation. BMC Complement
Altern Med. 6:102006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Faião-Flores F, Suarez JA, Pardi PC and
Maria DA: DM-1, sodium
4-[5-(4-hydroxy-3-methoxyphenyl)-3-oxo-penta-1,4-dienyl]-2-methoxy-phenolate:
A curcumin analog with a synergic effect in combination with
paclitaxel in breast cancer treatment. Tumour Biol. 33:775–785.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stohs SJ, Chen O, Ray SD, Ji J, Bucci LR
and Preuss HG: Highly bioavailable forms of curcumin and promising
avenues for curcumin-based research and application: A review.
Molecules. 25:13972020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang ZJ, Huang SY, Zhou DD, Xiong RG, Zhao
CN, Fang AP, Zhang YJ, Li HB and Zhu HL: Effects and mechanisms of
curcumin for the prevention and management of cancers: An updated
review. Antioxidants (Basel). 11:14812022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Freyre-Fonseca V, Delgado-Buenrostro NL,
Gutiérrez-Cirlos EB, Calderón-Torres CM, Cabellos-Avelar T,
Sánchez-Pérez Y, Pinzón E, Torres I, Molina-Jijón E, Zazueta CP, et
al: Titanium dioxide nanoparticles impair lung mitochondrial
function. Toxicol Lett. 202:111–119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cremers CM and Jakob U: Oxidant sensing by
reversible disulfide bond formation. J Biol Chem. 288:26489–26496.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Trujillo J, Granados-Castro LF, Zazueta C,
Andérica-Romero AC, Chirino YI and Pedraza-Chaverrí J: Mitochondria
as a target in the therapeutic properties of curcumin. Arch Pharm
(Weinheim). 347:873–884. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mortezaee K, Salehi E, Mirtavoos-Mahyari
H, Motevaseli E, Najafi M, Farhood B, Rosengren RJ and Sahebkar A:
Mechanisms of apoptosis modulation by curcumin: Implications for
cancer therapy. J Cell Physiol. 234:12537–12550. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang M, Tian H, Shen P, Xu L, Liu H, Zhu
J, Wang Q and Shi Y: Curcumin alleviates nuclear factor-κB/NOD-like
receptor protein 3 mediated renal injury caused by acute
respiratory distress syndrome through reducing mitochondrial
oxidative stress. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue.
35:393–397. 2023.(In Chinese). PubMed/NCBI
|
|
37
|
Feng L, Wang Y, Bi Z, Wei Z, Zhang H and
Zhang S: Single-Atom nanoenzyme-based autoluminescence system for
cancer cell imaging and mitochondrial-targeted therapy. ACS Appl
Bio Mater. 6:5086–5096. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lu Z, Gao Z, Song H, Zhou Y, Yuan W, Wang
X, Zhang L, Hong Y, Meng Y, Hu J, et al: Synthesis, biological
evaluation and action mechanism study of new mitochondria-targeted
curcumin derivative as potential antitumor drugs. Chem Biodivers.
20:e2023000862023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sarosiek K and Wood KC: Endogenous and
imposed determinants of apoptotic vulnerabilities in cancer. Trends
Cancer. 9:96–110. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen Y, Tao Y, Hu K and Lu J: GRP78
inhibitor HA15 increases the effect of Bortezomib on eradicating
multiple myeloma cells through triggering endoplasmic reticulum
stress. Heliyon. 9:e198062023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liczbiński P, Michałowicz J and Bukowska
B: Molecular mechanism of curcumin action in signaling pathways:
Review of the latest research. Phytother Res. 34:1992–2005. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang L, Cheng X, Xu S, Bao J and Yu HJM:
Curcumin induces endoplasmic reticulum stress-associated apoptosis
in human papillary thyroid carcinoma BCPAP cells via disruption of
intracellular calcium homeostasis. Medicine (Baltimore).
97:e110952018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Goswami RS, Patel KP, Singh RR,
Meric-Bernstam F, Kopetz ES, Subbiah V, Alvarez RH, Davies MA,
Jabbar KJ, Roy-Chowdhuri S, et al: Hotspot mutation panel testing
reveals clonal evolution in a study of 265 paired primary and
metastatic tumors. Clin Cancer Res. 21:2644–2651. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hail N Jr: Mitochondrial reactive oxygen
species affect sensitivity to curcumin-induced apoptosis. Free
Radic Biol Med. 44:1382–1393. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Agarwal A, Kasinathan A, Ganesan R,
Balasubramanian A, Bhaskaran J, Suresh S, Srinivasan R, Aravind KB
and Sivalingam N: Curcumin induces apoptosis and cell cycle arrest
via the activation of reactive oxygen species-independent
mitochondrial apoptotic pathway in Smad4 and p53 mutated colon
adenocarcinoma HT29 cells. Nutr Res. 51:67–81. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sritharan S and Sivalingam N: Curcumin
induced apoptosis is mediated through oxidative stress in mutated
p53 and wild type p53 colon adenocarcinoma cell lines. J Biochem
Mol Toxicol. 35:e226162021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Onorati AV, Dyczynski M, Ojha R and
Amaravadi RK: Targeting autophagy in cancer. Cancer. 124:3307–3318.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ashrafizadeh M, Zarrabi A, Orouei S,
Kiavash Hushmandi, Hakimi A, Amirhossein Zabolian, Daneshi S,
Samarghandian S, Baradaran B and Najafi M: MicroRNA-mediated
autophagy regulation in cancer therapy: The role in
chemoresistance/chemosensitivity. Eur J Pharmacol. 892:1736602021.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu LD, Pang YX, Zhao XR, Li R, Jin CJ,
Xue J, Dong RY and Liu PS: Curcumin induces apoptotic cell death
and protective autophagy by inhibiting AKT/mTOR/p70S6K pathway in
human ovarian cancer cells. Arch Gynecol Obstet. 299:1627–1639.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mortezaee K, Parwaie W, Motevaseli E,
Mirtavoos-Mahyari H, Musa AE, Shabeeb D, Esmaely F, Najafi M and
Farhood B: Targets for improving tumor response to radiotherapy.
Int Immunopharmacol. 76:1058472019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang J, Zhang J, Zhang CJ, Wong YK, Lim
TK, Hua ZC, Liu B, Tannenbaum SR, Shen HM and Lin Q: In situ
proteomic profiling of curcumin targets in HCT116 colon cancer cell
line. Sci Rep. 6:221462016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liang HH, Huang CY, Chou CW, Makondi PT,
Huang MT, Wei PL and Chang YJ: Heat shock protein 27 influences the
anti-cancer effect of curcumin in colon cancer cells through ROS
production and autophagy activation. Life Sci. 209:43–51. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang T, Wu X, Al Rudaisat M, Song Y and
Cheng H: Curcumin induces G2/M arrest and triggers autophagy, ROS
generation and cell senescence in cervical cancer cells. J Cancer.
11:6704–6715. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chang M, Shang M, Yuan F, Guo W and Wang
C: EF24 exerts cytotoxicity against NSCLC via inducing ROS
accumulation. Cancer Cell Int. 21:5312021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mou Y, Wang J, Wu J, He D, Zhang C, Duan C
and Li B: Ferroptosis, a new form of cell death: Opportunities and
challenges in cancer. J Hematol Oncol. 12:342019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang L, Li XM, Shi XH, Ye K, Fu XL, Wang
X, Guo SM, Ma JQ, Xu FF, Sun HM, et al: Sorafenib triggers
ferroptosis via inhibition of HBXIP/SCD axis in hepatocellular
carcinoma. Acta Pharmacol Sin. 44:622–634. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tang X, Li Y, Zhao J, Liang L, Zhang K,
Zhang X, Yu H and Du H: Heme oxygenase-1 increases intracellular
iron storage and suppresses inflammatory response of macrophages by
inhibiting M1 polarization. Metallomics. 15:mfad0622023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Giorgi G, Mascaró M, Gandini N, Rabassa
ME, Coló GP, Arévalo J, Curino AC, Facchinetti MM and Roque ME:
Iron cycle disruption by heme oxygenase-1 activation leads to a
reduced breast cancer cell survival. Biochim Biophys Acta Mol Basis
Dis. 1869:1666212023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chen Y, Guo X, Zeng Y, Mo X, Hong S, He H,
Li J, Fatima S and Liu Q: Oxidative stress induces mitochondrial
iron overload and ferroptotic cell death. Sci Rep. 13:155152023.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Li R, Zhang J, Zhou Y, Gao Q, Wang R, Fu
Y, Zheng L and Yu H: Transcriptome investigation and in vitro
verification of curcumin-induced HO-1 as a feature of ferroptosis
in breast cancer cells. Oxid Med Cell Longev. 2020:34698402020.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Suttner DM and Dennery PA: Reversal of
HO-1 related cytoprotection with increased expression is due to
reactive iron. FASEB J. 13:1800–1809. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
McBean GJ: The transsulfuration pathway: A
source of cysteine for glutathione in astrocytes. Amino Acids.
42:199–205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhou XL, Zhu CY, Wu ZG, Guo X and Zou W:
The oncoprotein HBXIP competitively binds KEAP1 to activate NRF2
and enhance breast cancer cell growth and metastasis. Oncogene.
38:4028–4046. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Cao X, Li Y, Wang Y, Yu T, Zhu C, Zhang X
and Guan J: Curcumin suppresses tumorigenesis by ferroptosis in
breast cancer. PLoS One. 17:e02613702022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tang X, Ding H, Liang M, Chen X, Yan Y,
Wan N, Chen Q, Zhang J and Cao J: Curcumin induces ferroptosis in
non-small-cell lung cancer via activating autophagy. Thorac Cancer.
12:1219–1230. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Shi J, Gao W and Shao F: Pyroptosis:
Gasdermin-Mediated programmed necrotic cell death. Trends Biochem
Sci. 42:245–254. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Frank D and Vince JE: Pyroptosis versus
necroptosis: Similarities, differences, and crosstalk. Cell Death
Differ. 26:99–114. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Liang WF, Gong YX, Li HF, Sun FL, Li WL,
Chen DQ, Xie DP, Ren CX, Guo XY, Wang ZY, et al: Curcumin activates
ROS signaling to promote pyroptosis in hepatocellular carcinoma
HepG2 cells. In Vivo. 35:249–257. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wei T, Zheng Z, Wei X, Liu Y, Li W, Fang
B, Yun D, Dong Z, Yi B, Li W, et al: Rational design, synthesis,
and pharmacological characterisation of dicarbonyl curcuminoid
analogues with improved stability against lung cancer via ROS and
ER stress mediated cell apoptosis and pyroptosis. J Enzyme Inhib
Med Chem. 37:2357–2369. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Li AN, Li S, Zhang YJ, Xu XR, Chen YM and
Li HB: Resources and biological activities of natural polyphenols.
Nutrients. 6:6020–6047. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wu H, Chen L, Zhu F, Han X, Sun L and Chen
K: The cytotoxicity effect of resveratrol: Cell cycle arrest and
induced apoptosis of breast cancer 4T1 cells. Toxins (Basel).
11:7312019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Tanaka T, Aoki R and Terasaki M: Potential
chemopreventive effects of dietary combination of phytochemicals
against cancer development. Pharmaceuticals (Basel). 16:15912023.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Arena A, Romeo MA, Benedetti R, Masuelli
L, Bei R, Gilardini Montani MS and Cirone M: New insights into
curcumin- and resveratrol-mediated anti-cancer effects.
Pharmaceuticals (Basel). 14:10682021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Cho CJ, Yang CW, Wu CL, Ho JY, Yu CP, Wu
ST and Yu DS: The modulation study of multiple drug resistance in
bladder cancer by curcumin and resveratrol. Oncol Lett.
18:6869–6876. 2019.PubMed/NCBI
|
|
75
|
Xia RL, Lu Y, Zhu LN, Zhang SF, Zhao FK
and Fu CY: Different regulatory pathways are involved in the
proliferative inhibition of two types of leukemia cell lines
induced by paclitaxel. Oncol Rep. 30:1853–1859. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Rosière R, Van Woensel M, Mathieu V,
Langer I, Mathivet T, Vermeersch M, Amighi K and Wauthoz N:
Development and evaluation of well-tolerated and tumor-penetrating
polymeric micelle-based dry powders for inhaled anti-cancer
chemotherapy. Int J Pharm. 501:148–159. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lee WH, Loo CY, Traini D and Young PM:
Development and evaluation of paclitaxel and curcumin dry powder
for inhalation lung cancer treatment. Pharmaceutics. 13:92020.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lee WH, Bebawy M, Loo CY, Luk F, Mason RS
and Rohanizadeh R: Fabrication of curcumin micellar nanoparticles
with enhanced anti-cancer activity. J Biomed Nanotechnol.
11:1093–1105. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lee WH, Loo CY, Ong HX, Traini D, Young PM
and Rohanizadeh R: Synthesis and characterization of inhalable
flavonoid nanoparticle for lung cancer cell targeting. J Biomed
Nanotechnol. 12:371–386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Syng-Ai C, Kumari AL and Khar A: Effect of
curcumin on normal and tumor cells: Role of glutathione and bcl-2.
Mol Cancer Ther. 3:1101–1108. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Tapia E, Zatarain-Barrón ZL,
Hernández-Pando R, Zarco-Márquez G, Molina-Jijón E,
Cristóbal-García M, Santamaría J and Pedraza-Chaverri J: Curcumin
reverses glomerular hemodynamic alterations and oxidant stress in
5/6 nephrectomized rats. Phytomedicine. 20:359–366. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Serafini MM, Catanzaro M, Fagiani F,
Simoni E, Caporaso R, Dacrema M, Romanoni I, Govoni S, Racchi M,
Daglia M, et al: Modulation of Keap1/Nrf2/ARE signaling pathway by
Curcuma- and Garlic-Derived Hybrids. Front Pharmacol. 10:15972020.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Waghray D and Zhang Q: Inhibit or evade
multidrug resistance P-Glycoprotein in cancer treatment. J Med
Chem. 61:5108–5121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Attia YM, El-Kersh DM, Ammar RA, Adel A,
Khalil A, Walid H, Eskander K, Hamdy M, Reda N, Mohsen NE, et al:
Inhibition of aldehyde dehydrogenase-1 and p-glycoprotein-mediated
multidrug resistance by curcumin and vitamin D3 increases
sensitivity to paclitaxel in breast cancer. Chem Biol Interact.
315:1088652020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Ebrahimifar M, Hasanzadegan Roudsari M,
Kazemi SM, Ebrahimi Shahmabadi H, Kanaani L, Alavi SA and Izadi
Vasfi M: Enhancing effects of curcumin on cytotoxicity of
paclitaxel, methotrexate and vincristine in gastric cancer cells.
Asian Pac J Cancer Prev. 18:65–68. 2017.PubMed/NCBI
|
|
86
|
Zhang N, Gao M, Wang Z, Zhang J, Cui W, Li
J, Zhu X, Zhang H, Yang DH and Xu X: Curcumin reverses doxorubicin
resistance in colon cancer cells at the metabolic level. J Pharm
Biomed Anal. 201:1141292021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Firouzi Amoodizaj F, Baghaeifar S, Taheri
E, Farhoudi Sefidan Jadid M, Safi M, Seyyed Sani N, Hajazimian S,
Isazadeh A and Shanehbandi D: Enhanced anticancer potency of
doxorubicin in combination with curcumin in gastric adenocarcinoma.
J Biochem Mol Toxicol. 34:e224862020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Dhandapani KM, Mahesh VB and Brann DW:
Curcumin suppresses growth and chemoresistance of human
glioblastoma cells via AP-1 and NFkappaB transcription factors. J
Neurochem. 102:522–538. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Abbaspour H and Afshar AS: Curcumin
inhibits the expression of ornithine decarboxylase and adenosine
deaminase genes in MCF-7 human breast cancer cells. Arch Biol Sci.
70:639–645. 2018. View Article : Google Scholar
|
|
90
|
Lee WJ, Jo JH, Jang SI, Jung EJ, Hwang JM,
Bae JW, Ha JJ, Kim DH and Kwon WS: The natural flavonoid compound
deguelin suppresses sperm (Sus Scrofa) functions through abnormal
activation of the PI3K/AKT pathway. Reprod Toxicol. 120:1084262023.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Lin ZY, Yun QZ, Wu L, Zhang TW and Yao TZ:
Pharmacological basis and new insights of deguelin concerning its
anticancer effects. Pharmacol Res. 174:1059352021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Russell DA, Bridges HR, Serreli R, Kidd
SL, Mateu N, Osberger TJ, Sore HF, Hirst J and Spring DR:
Hydroxylated rotenoids selectively inhibit the proliferation of
prostate cancer cells. J Nat Prod. 83:1829–1845. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Kocdor MA, Cengiz H, Ates H and Kocdor H:
Inhibition of cancer stem-like phenotype by curcumin and deguelin
in CAL-62 anaplastic thyroid cancer cells. Anticancer Agents Med
Chem. 19:1887–1898. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Liang G, Shao L, Wang Y, Zhao C, Chu Y,
Xiao J, Zhao Y, Li X and Yang S: Exploration and synthesis of
curcumin analogues with improved structural stability both in vitro
and in vivo as cytotoxic agents. Bioorg Med Chem. 17:2623–2631.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zou P, Zhang J, Xia Y, Kanchana K, Guo G,
Chen W, Huang Y, Wang Z, Yang S and Liang G: ROS generation
mediates the anti-cancer effects of WZ35 via activating JNK and ER
stress apoptotic pathways in gastric cancer. Oncotarget.
6:5860–5876. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wang L, Han L, Tao Z, Zhu Z, Han L, Yang
Z, Wang H, Dai D, Wu L, Yuan Z and Chen T: The curcumin derivative
WZ35 activates ROS-dependent JNK to suppress hepatocellular
carcinoma metastasis. Food Funct. 9:2970–2978. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Wang L, Wang C, Tao Z, Zhao L, Zhu Z, Wu
W, He Y, Chen H, Zheng B, Huang X, et al: Curcumin derivative WZ35
inhibits tumor cell growth via ROS-YAP-JNK signaling pathway in
breast cancer. J Exp Clin Cancer Res. 38:4602019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Koroth J, Nirgude S, Tiwari S,
Gopalakrishnan V, Mahadeva R, Kumar S, Karki SS and Choudhary B:
Investigation of anti-cancer and migrastatic properties of novel
curcumin derivatives on breast and ovarian cancer cell lines. BMC
Complement Altern Med. 19:2732019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Koroth J, Mahadeva R, Ravindran F,
Parashar TR, Teja V, Karki SS and Choudhary B: Curcumin derivative
1, 2-bis [(3E, 5E)-3, 5-bis [(2-chlorophenyl)
methylene]-4-oxo-1-piperidyl] ethane-1, 2-dione (ST03) induces
mitochondria mediated apoptosis in ovarian cancer cells and
inhibits tumor progression in EAC mouse model. Transl Oncol.
15:1012802022. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Haseli S, Pourmadadi M, Samadi A, Yazdian
F, Abdouss M, Rashedi H and Navaei-Nigjeh M: A novel pH-responsive
nanoniosomal emulsion for sustained release of curcumin from a
chitosan-based nanocarrier: Emphasis on the concurrent improvement
of loading, sustained release, and apoptosis induction. Biotechnol
Prog. 38:e32802022. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Hou CH, Lin FL, Hou SM and Liu JF:
Hyperthermia induces apoptosis through endoplasmic reticulum and
reactive oxygen species in human osteosarcoma cells. Int J Mol Sci.
15:17380–17395. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Pazouki N, Irani S, Olov N, Atyabi SM and
Bagheri-Khoulenjani S: Fe3O4 nanoparticles
coated with carboxymethyl chitosan containing curcumin in
combination with hyperthermia induced apoptosis in breast cancer
cells. Prog Biomater. 11:43–54. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Freidus LG, Kumar P, Marimuthu T, Pradeep
P and Choonara YE: Theranostic Mesoporous Silica Nanoparticles
Loaded With a Curcumin-Naphthoquinone Conjugate for Potential
Cancer Intervention. Front Mol Biosci. 8:6707922021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Freidus LG, Kumar P, Marimuthu T, Pradeep
P, Pillay V and Choonara YE: Synthesis and Properties of CurNQ for
the theranostic application in ovarian cancer intervention.
Molecules. 25:44712020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zholobak NM, Shcherbakov AB, Ivanova OS,
Reukov V, Baranchikov AE and Ivanov VK: Nanoceria-curcumin
conjugate: Synthesis and selective cytotoxicity against cancer
cells under oxidative stress conditions. J Photochem Photobiol B.
209:1119212020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Haider T, Pandey V, Banjare N, Gupta PN
and Soni V: Drug resistance in cancer: Mechanisms and tackling
strategies. Pharmacol Rep. 72:1125–1151. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Li L, Wang F, Jia X, Yao L and Liu Y:
Research mechanism and progress of the natural compound curcumin in
treating Alzheimer´s disease. Mini Rev Med Chem. Oct 30–2023.(Epub
ahead of print). View Article : Google Scholar
|
|
108
|
Molani-Gol R, Dehghani A and Rafraf M:
Effects of curcumin/turmeric supplementation on the liver enzymes,
lipid profiles, glycemic index, and anthropometric indices in
non-alcoholic fatty liver patients: An umbrella meta-analysis.
Phytother Res. Nov 2–2023.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Wu WS, Wu JR and Hu CT: Signal cross talks
for sustained MAPK activation and cell migration: The potential
role of reactive oxygen species. Cancer Metastasis Rev. 27:303–314.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Das L and Vinayak M: Long term effect of
curcumin in restoration of tumour suppressor p53 and phase-II
antioxidant enzymes via activation of Nrf2 signalling and
modulation of inflammation in prevention of cancer. PLoS One.
10:e01240002015. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Birben E, Sahiner UM, Sackesen C, Erzurum
S and Kalayci O: Oxidative stress and antioxidant defense. World
Allergy Organ J. 5:9–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Lin X, Bai D, Wei Z, Zhang Y, Huang Y,
Deng H and Huang X: Curcumin attenuates oxidative stress in
RAW264.7 cells by increasing the activity of antioxidant enzymes
and activating the Nrf2-Keap1 pathway. PLoS One. 14:e02167112019.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Ghosh P, Vidal C, Dey S and Zhang L:
Mitochondria targeting as an effective strategy for cancer therapy.
Int J Mol Sci. 21:33632020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y and
Hu LL: ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med.
19:1997–2007. 2020.PubMed/NCBI
|
|
115
|
Wu MF, Huang YH, Chiu LY, Cherng SH, Sheu
GT and Yang TY: Curcumin induces apoptosis of chemoresistant lung
cancer cells via ROS-Regulated p38 MAPK phosphorylation. Int J Mol
Sci. 23:82482022. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
He F, Antonucci L and Karin M: NRF2 as a
regulator of cell metabolism and inflammation in cancer.
Carcinogenesis. 41:405–416. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Karthikeyan A, Senthil N and Min T:
Nanocurcumin: A promising candidate for therapeutic applications.
Front Pharmacol. 11:4872020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Safavy A, Raisch KP, Mantena S, Sanford
LL, Sham SW, Krishna NR and Bonner JA: Design and development of
water-soluble curcumin conjugates as potential anticancer agents. J
Med Chem. 50:6284–6288. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Sharma RA, Euden SA, Platton SL, Cooke DN,
Shafayat A, Hewitt HR, Marczylo TH, Morgan B, Hemingway D, Plummer
SM, et al: Phase I clinical trial of oral curcumin: biomarkers of
systemic activity and compliance. Clin Cancer Res. 10:6847–6854.
2004. View Article : Google Scholar : PubMed/NCBI
|