Immunotherapy has emerged as a rapidly advancing
treatment for tumors in recent years. Immunotherapy is based on the
tumor immune escape mechanism, which manipulates the immune system
to reactivate the antitumor immune response and overcome the
pathways that lead to tumor escape (1). Current immunotherapy methods encompass
immune checkpoint inhibitors (ICIs), adoptive cell therapy,
oncolytic viruses and cancer vaccines. Among them, ICIs, including
antibodies against programmed cell death protein 1 (PD-1), its
ligand PD-L1, cytotoxic T lymphocyte antigen-4 (CTLA-4), lymphocyte
activation gene 3 (LAG3), T cell immunoglobulin and mucin domain 3
(TIM3), and indoleamine 2, 3-dioxygenase 1 (IDO), have been widely
and rapidly developed in clinical practice, achieving satisfactory
results (2). However, there are
still several shortcomings in the treatment with ICIs. Only a small
number of tumor patients respond to ICIs, and there is still the
possibility of drug resistance. Moreover, it is unable to address
the disease progression and life-threatening nature for most cancer
patients. Additionally, ICIs rely on the activation of autoimmune
function to eliminate tumors, and these mechanisms may affect the
self-tolerance of healthy tissues, leading to immune side effects
known as immune-related adverse events (irAEs) (3). Gut microbiota plays a significant role
in the physiological and pathological processes of the human
organism. As a current research hotspot, it has made substantial
progress in various fields. The potential connection between gut
microbiota and ICIs has been extensively investigated in recent
years, encompassing the relationship between gut microbiota and its
associated metabolites, the clinical efficacy of ICIs, the
correlation between gut microbiota and adverse events related to
ICIs, the impact of antibiotic application on ICIs, and the
application and effectiveness of probiotics and fecal
transplantation in clinical practice (4). While the specific mechanism by which
gut microbiota influences the treatment of ICIs remains unclear,
the current research indicates that gut microbiota may serve as a
crucial target for regulating the efficacy of ICIs, making its
practical application in clinical settings highly promising
(5). The present review examined
the relationship between gut microbiota and ICIs, provided a
summary of the current research progress, and explored the
potential interaction mechanisms and future prospects between these
factors.
The gut microbiota is a vast microecosystem that
includes bacteria, archaea, fungi and viruses. Each person carries
up to 1014 microbial species, ~99% of which are
bacteria. The primary species are Firmicutes and
Bacteroidetes, followed by Actinobacteria and
Verrucomicrobiota (6,7). The
gut microbiota is closely linked to the activities of life and
plays a crucial role in numerous metabolic processes. For instance,
the microbiota in the colon encodes a plethora of
carbohydrate-active enzymes, allowing them to break down
non-digestible dietary residues and release short-chain fatty
acids. These microbes assist in the synthesis of micronutrients
such as vitamin K, vitamin B12, biotin, folic acid and pantothenic
acid, in addition to aiding in the absorption of calcium, magnesium
and iron. The gut microbiota can also regulate intestinal endocrine
function, nerve signals, bone mineral density, provide biogenic
energy, synthesize neurotransmitters, and metabolize bile.
Furthermore, the gut microbiota plays a crucial role in the
maturation and sustained expression of the host immune response
(8,9).
The influence of gut microbiota on the immune system
begins during early life. The immune system develops in a
relatively sterile fetal environment during early life, with its
primary exposure to antigens derived from the newly established
microbial community on the mucosal surface of the newborn. Exposure
to microbes during early life can result in lifelong changes in the
immune system (10). The infant
receives natural passive immunity from the mother through the
placental route during pregnancy. The maternal gut microbiota
profile can influence the composition of immune cells in infants.
The enrichment of Dialister, Escherichia and
Ruminococcus in the maternal gut microbiota is associated
with a lower proportion of granulocytes and a higher proportion of
central naïve CD4+ T cells
(CD4+/CD45RA+/CD31−) and naïve
regulatory T cells (Treg)
(CD4+/CD45RA+/FoxP3low) in cord blood
(11). Maternal dietary habits and
breastfeeding after birth can also impact the regulation of immune
factors in infants (12,13). The gut microbiome undergoes
significant changes before the age of 2.5 years under the influence
of various factors, but it gradually stabilizes afterward and
remains relatively constant throughout the lifetime of an
individual (14). Different age
groups exhibit distinct gut microbiota profiles, with
Bifidobacterium being more prevalent in infants and
children, while Megamosna and Peptoniphilus are
relatively enriched in the elderly (15). For instance, Akkermansia is
more abundant in the gut microbiota of frail elderly individuals.
It is positively correlated with the elevation of interleukin 6 and
can elevate serum inflammatory factor levels, as well as increase
intestinal permeability (16). In
conclusion, the influence of gut microbiota on the immune system
plays a crucial role in life development and may affect both
physiological and pathological processes.
Increasing evidence suggests that gut microbiota can
regulate the proliferation and expression of immune cells,
particularly the balance between T helper cell 17 (Th17) and Treg
cells (17). Th17 cells contribute
to autoimmunity and inflammation, while Treg cells inhibit immune
responses and maintain immune homeostasis. Both cell types
initially differentiate from naive CD4 T cells under the influence
of tumor growth factor (TGF)-β (18). A previous study demonstrated that
the balance between Th17 and Treg cells in the lamina propria of
the mouse small intestine is influenced by the presence of
Cytophaga-Flavobacter-Bacteroidetes bacteria.
Specifically, Th17 cell differentiation is associated with the
presence of these bacteria, while germ-free mice exhibit an
increase in Treg cells in the lamina propria (19). Furthermore, a previous study has
demonstrated that the presence of mixed Clostridium in mice
leads to an upregulation of Treg cell abundance and function in the
colon. This effect is attributed to the creation of a transforming
growth factor-β enriched environment (20). Thus, it is plausible that Th17/Treg
cells are regulated and proliferated by various species of gut
microbiota. Research has demonstrated that Bacteroides
fragilis (B. fragilis) can stimulate the proliferation
of Treg cells through Toll-like receptor 2 (TLR2), consequently
suppressing the activity of Th17 cells. As for the mechanism of
action, a symbiotic factor known as polysaccharide A (PSA) produced
by B. fragilis has been identified as a key player. PSA, a
representative immunomodulatory molecule of symbiotic nature, can
activate CREB-dependent transcription of anti-inflammatory genes
through the coordinated activation of TLR2 and Dectin-1. This
activation leads to the production of the immunomodulatory cytokine
IL-10 by CD4+ Treg cells. Consequently, immune tolerance
is achieved, and it may serve as a mechanism for intestinal
commensal bacteria to evade the immune system (21,22).
In conclusion, gut microbiota plays a significant role in
regulating the balance between proinflammatory responses and immune
regulation, despite the precise underlying mechanisms remaining
unclear.
Increasing evidence supports the role of gut
microbiota-derived metabolites in immune system regulation. The
majority of metabolites associated with gut microbiota have been
found to be involved in immune regulation, attenuating immune
responses, and potentially contributing to immune tolerance.
Extensive research on short-chain fatty acids (SCFAs), a
well-studied group of metabolites, has demonstrated that SCFAs
derived from mouse gut microbiota can activate STAT3 and mTOR in
Th1 cells, upregulate the transcription factor B lymphocyte-induced
maturation protein 1 (Blimp-1), and stimulate the production of
IL-10 to preserve intestinal homeostasis (23). Butyrate, a metabolite produced by
Firmicutes and Fusobacteria, can activate the
expression of TGFB1 in human intestinal epithelial cells through
the transcription factor SP1. This activation leads to the
accumulation of Treg cells in the intestine, contributing to its
immunomodulatory role (24).
Following the consumption of propionic acid by patients with
multiple sclerosis, there is a significant and sustained increase
in Treg cells. Additionally, the mitochondrial function and
morphology of Treg cells normalize, whereas the levels of Th1 and
Th17 cells markedly decrease, indicating the immunomodulatory
effects (25). Metabolites
associated with the microbiota, including taurine, histamine,
spermine and bile acids, contribute to the maintenance of
intestinal homeostasis through the regulation of NLRP6/NLRP3
inflammasomes (26,27). Probiotics such as Lactobacillus
rhamnosus GG and factors derived from LGG broth culture
supernatant can activate Akt, alleviate TNF-induced colonic
epithelial injury, suppress cytokine-induced epithelial cell
apoptosis, and foster intestinal epithelial homeostasis.
Furthermore, LGG cell-free supernatant (LGG-SN) has been observed
to enhance the sensitivity of human tumor cells to 5-fluorouracil
and irinotecan (28,29). The outer membrane protein Amuc_1100
derived from Akkermansia muciniphil stimulates the
production of IL-10 by activating TLR2 and TLR4 (30). The human gut Actinobacterium
Eggerthella lenta disrupts the inhibition of the Th17
transcription factor Rorγt by cardiac glycoside reductase 2 enzyme,
leading to Th17 activation in the intestine and the initiation of
autoimmunity (31). Overall,
certain metabolites associated with gut microbiota contribute to
the maintenance of intestinal immune balance, safeguarding the
survival of gut microbiota and protecting the intestinal tract from
immune-related harm. Consequently, the intricate mechanism through
which gut microbiota regulate the immune system via their
metabolites necessitates further investigation.
Tumor immunotherapy is initiated by the mechanisms
through which tumor cells evade the human immune system. Typically,
the immune system can identify and eliminate tumor cells in healthy
tissues based on tumor-associated antigens. Tumors, however, employ
various immune processes to evade the immune system, including
targeted modulation of Tregs function or secretion, antigen
presentation processes, modification of immunosuppressive mediator
production, development of immune tolerance, and evasion of immune
system-mediated killing (32).
Immune checkpoints play a crucial role in regulating the host's
antitumor immunity. Currently, extensively studied immune
checkpoints include PD-1, PD-L1, CTLA-4, LAG3, TIM3 and IDO. ICIs
based on these targets have significantly enhanced the efficacy of
tumor treatment and made substantial progress in recent years
(33). PD-1, a receptor in the
(immunoglobulin) Ig superfamily, negatively regulates T-cell
antigen receptor signaling through its interaction with the
specific ligand PD-L1. PD-L1, also referred to as B7-H1 or CD274,
is expressed in numerous tumors, including lung cancer, ovarian
cancer, colon cancer and melanoma. This expression reduces the
sensitivity of tumor cells to cytotoxic T cell lysis mediated by
specific T cell antigen receptors, leading to increased
tumorigenicity and aggressiveness (34–36).
CTLA-4, a member of the CD28-B7 immunoglobulin superfamily, is
expressed on activated T cell surfaces, inhibiting their activity
by competing with the costimulatory receptor CD28 for binding to
B7-1 and B7-2, thereby downregulating immune responses (37). In vivo, anti-PD-1 and
anti-CTLA-4 antibodies have varying immune effects, whether
administered alone or in combination. CTLA-4 blockade primarily
induced partial proliferation of transitional memory T cells in the
blood/tumor tissue analysis of patients undergoing immune
checkpoint blockade, whereas PD-1 blockade resulted in changes in
cytolysis and NK-cell function-related genes. Blockade of both
resulted in non-overlapping changes in gene expression patterns,
including proliferation-related and chemokine genes (38). LAG3 comprises four external
immunoglobulin superfamily domains in the cellular domain, a long
linker peptide in the transmembrane domain, and a serine
phosphorylation site in the intracellular domain. It is expressed
on the surfaces of CD4+, CD8+, natural killer
(NK), NKT and Treg cells, and inhibits T cell function. LAG3 is
expressed in various tumors and is associated with patient
prognosis. Blockade of LAG3 is also a new antitumor idea (39). TIM3 is an inhibitory checkpoint
protein expressed on Th1, Th17, Tregs, CD8+ T, NK and
dendritic cells. It is associated with antitumor immunity, and
blocking it is a promising approach to cancer therapy (40). IDO is an immunomodulatory enzyme
that metabolizes the essential amino acid tryptophan to its
downstream kynurenine, thereby inhibiting T cell immunity.
Inhibiting IDO is also a way to enhance tumor immunity (32). Furthermore, there has been an
increasing use of ICIs and targeted therapies in combination, such
as anti-PD-1/PD-L1 and anti-CTLA-4 combination therapy, as well as
anti-PD-1/PD-L1 and anti-vascular endothelial growth factor
combination therapy (41). ICIs
have achieved favorable results in clinical applications. However,
some patients initially respond to ICIs therapy but later exhibit
drug resistance, which is related to the abundant mutation function
of tumor cells, enabling them to evade T cell-mediated immune
surveillance once again (42).
Moreover, the primary focus of immunotherapy is to enhance the
immune activation mechanism. This ‘immune enhancement’ strategy
often causes frequent irAEs, although with the advancement of
immunotherapy and therapy design, related adverse events are being
gradually reduced (43). Common
adverse effects of CTLA-4 and/or PD-1 inhibition occur in the skin,
gastrointestinal tract, liver and endocrine system, such as
pruritus, rash, nausea, diarrhea and thyroid disorders (44). When irAEs occur in ICIs-treated
patients, they may need to discontinue ICIs and treat irAEs,
compromising treatment efficiency (45). The clinical studies conducted in
previous years are included in Table
I (46–61). In these clinical studies, a variety
of common tumor types were included. Their efficacy in ICIs as
monotherapy as in combination therapy is very limited. Response
rates were modest, and a substantial proportion of patients
developed grade 3–4 irAEs. Despite the progress made with ICIs,
their inefficiency and the inevitability of irAEs remain
significant challenges. Therefore, more treatment and prevention
methods need to be developed to address the deficiencies of
ICIs.
Recent studies have demonstrated that gut microbiota
plays a crucial regulatory role in ICIs therapy, offering a novel
approach to enhance the clinical effectiveness of ICIs. Assessing
the gut microbiota of patients can provide guidance and regulation
for the subsequent clinical implementation of ICIs (62–65).
Previous studies exploring the association between gut microbes and
ICIs are presented in Table II.
Generally, patients with higher levels of Firmicutes and
Verrucomicrobiota in their gut microbiota exhibited a more
favorable response to ICIs, whereas those with an abundance of
Proteobacteria showed a diminished response. The relationship
between Bacteroidetes and treatment response was found to be
varied. Regarding the occurrence of adverse reactions,
Firmicutes exhibited higher levels, whereas
Bacteroidetes displayed lower levels. Furthermore, the
administration of antibiotics is typically negatively correlated
with the clinical response to ICIs (66). A previous study encompassing
clinical and animal research demonstrated a correlation between
clinical responses to ICIs targeting the PD-1/PD-L1 axis and the
relative abundance of Akkermansia muciniphila (67). An investigation into the impact of
ICIs treatment on patients with non-small cell lung cancer (NSCLC)
revealed a higher prevalence of Akkermansiaceae in
individuals demonstrating stable disease and partial response to
immunotherapy, as opposed to those with progressive disease
(68). The study conducted by
Grenda et al (69)
demonstrated that Bacteroidaaceae, Barnesiellaceae and
Tannerellaceae were capable of extending progression-free
survival (PFS) in patients with NSCLC. Newsome et al
(70) obtained similar results in
their study involving patients with stage III/IV NSCLC who received
ICIs treatment, revealing significant enrichment of
Ruminococcus, Akkermansia and Faecalibacterium among
responders. In the context of melanoma-based ICIs therapy, response
was also linked to Bifidobacterium pseudatenulatum, Roseburia
spp. and Akkermansia muciniphila (71). The aforementioned multiple similar
studies demonstrated the more favorable effects of Akkermansia
muciniphila on ICIs. Akkermansia muciniphila, a strictly
anaerobic gut bacterium, thrives on intestinal mucin as its
exclusive carbon and nitrogen source, colonizing the intestine in a
manner intricately linked to the host's well-being. It regulates
the immune response of the organism, sustains metabolic
equilibrium, ameliorates obesity, type 2 and type 1 diabetes,
hepatic steatosis, intestinal inflammation, and augments responses
of ICIs across various cancer types (72). Concerning the mechanism underlying
the treatment of ICIs by Akkermansia muciniphila, an animal
experiment revealed that Akkermansia can modulate the
therapeutic capacity of PD-1 antibodies in mice with colorectal
cancer by influencing the metabolism of glycerophospholipid and the
expression of immune-related cytokines (IFN-γ and IL-2) within the
tumor microenvironment, thus preserving the normal effectiveness of
PD-1 antibodies (73). However, a
recent study examining the association between gastrointestinal
microbiome composition and ICIs in advanced metastatic
castration-resistant prostate cancer found a decrease in levels of
Akkermansia muciniphilia in response samples, which
contradicts previous findings in other types of tumors. The
aforementioned study observed a correlation between the abundance
of Streptococcus salivarius in fecal samples and treatment
response. It is possible that tumor type is also associated with
the mechanisms through which gut microbiota affect ICIs' therapy
(74). Additionally, the study
design and potential confounding factors may have contributed to
these findings. A study conducted with melanoma patients undergoing
anti-PD-1 treatment revealed significant disparities in the
diversity and composition of the gut microbiota between individuals
who responded to the treatment and those who did not. Responders
exhibited significantly higher alpha diversity of gut microbiota
and greater relative abundance of Ruminococcaceae compared
with non-responders. Moreover, fecal transplantation from
responders enhanced antitumor immunity in mice (75). Another analogous study, focusing on
patients undergoing ICIs treatment for melanoma, demonstrated the
abundance of certain bacterial species, such as Bifidobacterium
longum, Collinsella aerofaciens and Enterococcus
faecium, in individuals who responded to the treatment
(76). A study conducted on ICIs in
advanced NSCLC demonstrated that the α diversity of gut microbiota
was correlated with overall survival (OS), while the presence of
Ruminococcaceae UCG 13 and Agathobacter revealed a
positive association with favorable objective response rate and PFS
(77). In Chinese patients with
NSCLC who underwent anti-PD-1 treatment, the gut microbiota
exhibited enrichment of Alistipes putredinis, Bifidobacterium
longum and Prevotella copri in the responder group,
while Ruminococcus was enriched in the non-responder group.
Additionally, patients with a higher diversity of gut microbiota
demonstrate an enhanced tumor-killing effect when undergoing
anti-PD-1 treatment (78). In a
study examining the correlation between clinical response to
anti-PD-1 therapy and gut microbiota in patients with advanced
hepatobiliary cancer, individuals with a higher abundance of
Lachnospiraceae bacterium-GAM79 and Alistipes sp.
Marseille-P5997 demonstrated longer PFS and OS compared with
those with lower abundance. Furthermore, a high abundance of
Ruminococcus calidus and Erysipelotichaceae
bacterium-GAM147 was linked to extended PFS and improved
treatment response. Conversely, patients with higher abundance of
Veillonellaceae exhibited poorer PFS and OS (79). Another study investigating the gut
microbiota in patients with hepatocellular carcinoma and their
response to ICIs revealed an enrichment of Bifidobacterium,
Coprococcus and Acidaminococcus in patients with disease
control (80). However, the initial
abundance of these three taxa did not predict an OS benefit in the
aforementioned study. In a study investigating the combination of
regorafenib and toripalimab for colorectal cancer, a higher
relative abundance of Fusobacterium was linked to lack of
response and shorter PFS (81).
Additionally, a previous study provided evidence that
Helicobacter pylori infection can upregulate the expression
of PD-L1 in human gastric epithelial cells. Further investigation
into its clinical significance is warranted (82). According to Park et al
(83), the mechanism through which
gut microbiota influence ICIs involves the downregulation of PD-L2
and its binding partner, repulsive guidance molecule b, thereby
enhancing the efficacy of anti-PD-1 treatment. Ongoing studies in
this field are continuously being conducted. Generally, future
research should focus on investigating the individual species and
overall distribution of gut microbiota. The conclusions of the
previous studies are not consistent, which may be attributed to
differences in study design, tumor type, sample size and potential
confounding factors. Among the aforementioned studies, some are
animal studies, some are clinical studies, and the tumor types are
not exactly the same. In addition, the sample size was between tens
to hundreds, with large differences. Finally, confounding factors
such as sex, height, weight, diet and ethnicity can further affect
the results of experiments. Nevertheless, certain specific species
and characteristics of gut microbiota, such as a higher abundance
of Akkermansia and greater α diversity, have demonstrated a
positive effect on ICIs in multiple studies. These findings warrant
further exploration as important avenues for future research.
The role of gut microbiota in the treatment of ICIs
may be attributed to their associated metabolites. A study
conducted on patients with gastrointestinal cancers receiving
anti-PD-1/PD-L1 therapy revealed that those who exhibited a higher
Prevotella/Bacteroides ratio or higher abundance of
Prevotella, Ruminococcaceae and Lachnospiraceae
demonstrated improved responses to anti-PD-1/PD-L1 therapy. These
findings may be linked to the metabolites produced by the gut
microbiota. Specifically, gut microbiota capable of producing
SCFAs, such as Eubacterium, Lactobacillus and
Streptococcus, were found to be positively associated with
anti-PD-1/PD-L1 responses in gastrointestinal cancers (84). Another study focusing on patients
with solid cancer tumors treated with anti-PD-1 therapy
demonstrated that higher concentrations of certain SCFAs, including
fecal acetic acid, propionic acid, butyric acid, valine and plasma
isovaleric acid, were associated with longer PFS (85). The aforementioned study also
suggested that SCFAs may serve as the link between gut microbiota
and the efficacy of anti-PD-1 therapy. Furthermore, it was found
that the gut microbiota metabolite butyrate can directly enhance
the response of antitumor cytotoxic CD8+ T cells in
vitro and in vivo by promoting IL-12 signaling, thereby
improving the efficacy of antitumor therapy (86). However, another study indicated that
elevated levels of butyrate and propionate in the blood led to an
increase in the proportion of Treg cells, which resulted in a
diminished anti-CTLA-4 blockade effect and limited the activity of
anti-CTLA-4 therapy (87).
Additionally, a study focusing on ICIs for unresectable
hepatocellular carcinoma demonstrated that ursodeoxycholic acid and
ursocholic acid were significantly enriched in the feces of
patients who exhibited an objective response, and these metabolites
were correlated with the abundance of Lachnoclostridium
(88). Jiang et al (89) study revealed that Fusobacterium
nucleatum and increased succinic acid hindered the efficacy of
anti-PD-1 therapy in patients with colorectal cancer. However, it
is important to note that these studies have yielded conflicting
conclusions, emphasizing the need for further exploration into the
role of gut microbiota metabolites in ICIs treatment. Currently,
there is no further study on how gut microbiota metabolites affect
the efficiency of ICIs application by regulating the immune system.
The underlying mechanisms are likely to be highly complex,
involving interactions between different gut microbiota and various
metabolites. Additionally, investigating the intricate mechanisms
of downstream gene regulation, immune cell modulation, and
regulation of inflammatory factors presents a significant
challenge.
The use of antibiotics can affect the composition of
gut microbiota, subsequently influencing the modulating role of gut
microbiota in the effectiveness of ICIs. Generally, antibiotic
treatment is associated with poor OS (90). The utilization of antibiotics
emerged as an independent negative predictor of PFS and OS in
patients with advanced cancer undergoing ICI treatment. Patients
who underwent repetitive or prolonged antibiotic use exhibited a
poorer treatment response (91). In
a retrospective analysis of nivolumab-treated patients with NSCLC,
the median PFS was 1.2 months for patients receiving antibiotics
compared with 4.4 months for those who did not, although no
difference in OS was observed (92). Another study demonstrated that
antibiotic use diminished PFS and OS in patients with advanced
renal cell carcinoma and NSCLC, and it exacerbated disease
progression in patients with renal cell carcinoma who received
antibiotics within 30 days of commencing ICIs, in comparison with
those who did not receive antibiotics (93). The mechanism underlying the impact
of antibiotics on ICI effectiveness may lie in the disruption of
gut microbiota's ecological stability, which compromises the immune
homeostasis maintained by gut microbiota, subsequently leading to
dysregulation of intestinal immune responses. At present, no
further studies have examined how antibiotics specifically affect
gut microbiota and the immune system to alter the efficiency of
ICIs. This area requires further exploration. Nevertheless, based
on the collective results of current studies, the use of
antibiotics in patients receiving ICIs should be more strictly
regulated to ensure the efficacy of ICIs.
Gut microbiota can directly impact tumors,
regulating their occurrence and development. For instance,
Propionibacterium acidipropionici and Freudenreichii
produce cytotoxic compounds, namely SCFAs propionate and acetate,
which induce apoptosis in colorectal cancer cell lines. Similarly,
Lactobacilli stimulate immune response, and Lactobacillus
casei ATCC334 produces a killing effect on tumor cells through
its metabolite ferrichrome (94).
The probiotic LGG-SN selectively reduces cancer cell viability by
inducing mitotic arrest in the G2/M phase of the cell cycle in
tumor cells (29). On the contrary,
Fusobacterium nucleatum activates beta-catenin through
Fusobacterium adhesin A, and Peptostreptococcus
anaerobius promotes tumor cell proliferation by activating the
PI3K-Akt pathway in tumor cells and NF-κB activation in
tumor-associated macrophages (94).
SCFAs, which are common metabolites of gut microbiota, have
demonstrated antitumor activity in various types of tumors. They
control the proliferation and metastasis of colorectal, gastric,
lung, cervical, breast and bladder cancer, and other common tumors
through the regulation of epigenetic modifications, inhibition of
tumor cell proliferation, and regulation of antitumor immunity
(95). The impact of gut microbiota
on tumor development is closely related to the tumor
microenvironment. Gut microbiota and their metabolites modify the
tumor microenvironment by preserving the integrity of the
intestinal mucosal barrier, regulating inflammatory factors, and
controlling immune cell activation, among other aspects. These
mechanisms collectively limit the progression of tumors (96).
The relationship between irAEs and gut microbiota
has been the subject of investigation in several studies. Andrews
et al (97) demonstrated a
significant association between a higher abundance of
Bacteroides and irAEs in melanoma patients receiving ICIs.
Another clinical trial evaluating ipilimumab for the treatment of
metastatic melanoma found an inverse association between the
increase of specific bacteria in the Bacteroidetes phylum
and colitis after immunotherapy (98). Similarly, a study focusing on
immune-related diarrhea in lung cancer patients treated with
anti-PD-1 antibodies revealed that patients without diarrhea had
higher levels of Bacteroidetes and lower levels of
Firmicutes (99). At
present, there are few studies on the relationship between gut
microbiota and irAEs, no specific dominant bacteria have been
found, and no studies have further elucidated the underlying
mechanism. Further exploration of the relationship between irAEs
and gut microbiota is warranted. The underlying mechanism may be
linked to the unique properties of certain gut microbiota,
necessitating further investigation. Future studies should aim to
accurately identify and analyze the relationship between dominant
strains and specific irAEs. Nevertheless, supplementing probiotics
to modulate the microecological environment of gut microbiota may
alleviate the occurrence of irAEs, particularly intestinal-related
symptoms. Probiotic supplementation represents a clinical approach
that capitalizes on the role of gut microbiota in ICI treatment.
Sivan et al (100) animal
study demonstrated that oral administration of
Bifidobacterium alone achieved comparable melanoma control
to anti-PD-L1 treatment. Proton pump inhibitors, which facilitate
the migration of oral microbiota to the gut, generally have a
negative effect on the efficacy of ICIs in cancer patients. In a
trial involving advanced or recurrent patients with NSCLC treated
with PD-1/PD-L1, treatment with Clostridium butyricum MIYAIRI
588 (CBM588) improved the efficacy of ICIs in patients
receiving proton pump inhibitors, potentially through modulation of
specific microbiota richness (101). Another study revealed that
CBM588 supplementation increased the response rate and
prolonged PFS in the treatment of metastatic renal cell carcinoma
with nivolumab plus ipilimumab (102). Similarly, a retrospective analysis
demonstrated that CBM588 treatment significantly prolonged
PFS and OS in patients with NSCLC treated with PD-1/PD-L1 (103). Probiotic supplementation also
reduced immune-related intestinal inflammation. An animal study
indicated that Bifidobacterium attenuated intestinal
immunopathology in mice without significantly affecting
anti-melanoma immunity induced by anti-CTLA-4 treatment (104). However, the role of probiotics in
ICI treatment is not always beneficial. A clinical study involving
patients with melanoma treated with ICIs suggested that higher
dietary fiber intake was associated with significantly improved
PFS, particularly in patients who consumed adequate dietary fiber
without probiotic use. Consistent with findings in mice, low-fiber
diets or probiotics (Bifidobacterium longum- or LGG)
impaired anti-PD-1-based treatment responses (105). Gao et al (106) revealed that supplementation with
Lacticaseibacillus rhamnosus Probio-M9 enhanced the
therapeutic efficiency in colorectal cancer of anti-PD-1 treatment
through subsequent metabolism. This supplementation of probiotics
may regulate the immune balance by producing beneficial metabolites
such as SCFAs in the gut, thereby promoting the infiltration and
activation of cytotoxic T lymphocytes and inhibiting the function
of Tregs in the tumor microenvironment during ICI treatment.
However, the effectiveness of probiotics in ICIs can be influenced
by different types of probiotics used in various studies, different
tumor types, and diverse patient populations. Inappropriate
supplementation can yield contradictory outcomes. Therefore, future
research should focus on individualizing probiotic
supplementation.
Fecal transplantation is employed as a clinical
approach to assess the role of gut microbiota in ICIs treatment.
Fecal transplantation entails transferring stool from individuals
who respond to non-responders' gut. An animal study demonstrated
the superiority of combining fecal transplantation with anti-PD-1
therapy over either therapy alone (107). Experiments involving fecal
transplantation of human-germ-free mice revealed that mice
receiving response-derived fecal transplantation exhibited enhanced
antitumor responses to anti-PD-L1 treatment compared with those
receiving non-response-derived fecal transplantation (108). In clinical trials, Baruch et
al (109) reported that out of
10 patients with refractory metastatic melanoma to anti-PD-1
therapy who underwent fecal transplantation from responders, 3
patients exhibited a clinical response. Another clinical study
demonstrated that fecal transplantation from patients with melanoma
who responded to anti-PD-1 therapy provided clinical benefit to 6
out of 15 patients with anti-PD-1 resistance (110). Moreover, fecal microbiota
transplantation has been utilized to treat certain cases of
ICIs-associated colitis, resulting in clinical benefit (111,112). Koo and Morrow (113) revealed individual variation in
fecal dominant donor microbes among recipients following fecal
transplantation, which is unrelated to the response to anti-PD-1
therapy. The success of fecal transplantation demonstrates the
clinical feasibility of the gut microbiota's significant role in
ICIs treatment. Patients who underwent fecal transplantation
acquired a gut microecosystem that enhanced the efficacy of ICIs.
However, the impact of fecal transplantation is highly limited and
does not improve the non-response of the majority of patients to
ICIs. This could be attributed to variations in the ecological
environment of gut microbiota among individuals, thus suggesting
the necessity for additional experiments to explore more precise
methods in the application of fecal transplantation (114). Future research should address the
need for more accurate donor selection, more effective gut
microbiota transplantation methods, as well as the ethical
challenges and potential risks associated with fecal
transplantation.
ICIs have been extensively utilized in clinical
practice for cancer therapy, and there is a growing body of
evidence supporting the impact of gut microbiota on enhancing the
effectiveness of ICIs treatment. The immune system, serving as the
communication bridge between these entities, plays a pivotal role
in their mechanism of action. ICIs primarily eliminate tumor cells
by modulating the activation of the immune system, which is
similarly influenced by gut microbiota. In general, gut microbiota,
particularly symbiotic bacteria, primarily uphold immune tolerance
to preserve their own ecological niche, whereas the principle of
ICIs treatment operates in contrast. Conversely, the activation of
the immune system by pathogenic bacteria may inflict harm on the
body itself. Consequently, achieving a balance between gut
microbiota and ICIs treatment may prove to be a highly intricate
task. Nevertheless, this equilibrium could potentially serve as the
pivotal factor in enhancing the efficiency of ICIs, thereby
significantly impacting the prognosis of cancer patients.
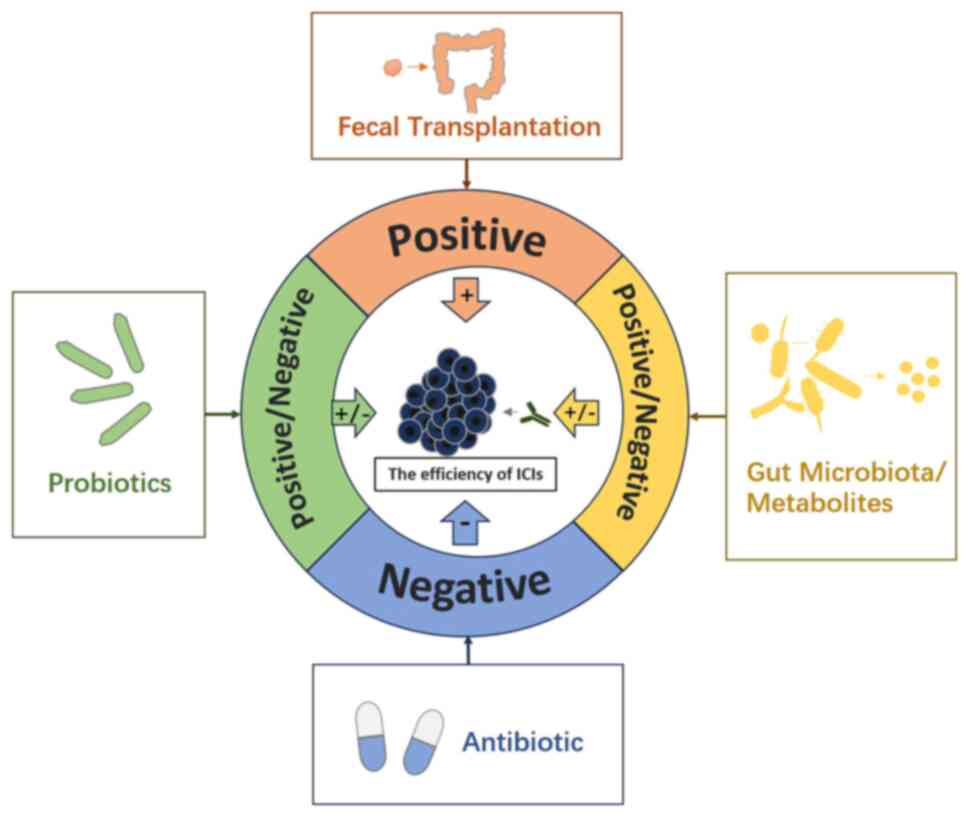
Currently, despite the ongoing nature of these investigations and
the absence of definitive conclusions, the clinical utilization of
probiotics and the exploration of fecal transplantation have
provided additional perspectives supporting the viability of this
approach (Fig. 1). Moving forward,
future research can delve into the molecular intricacies of how gut
microbiota and their downstream metabolites influence the efficacy
of ICIs. Endeavoring to elucidate the precise mechanism underlying
the maintenance of balance between gut microbiota and ICIs, as well
as identify pivotal species. Ultimately, in clinical practice,
precise and individualized implementation of specific probiotic
supplementation and fecal transplantation is warranted to enhance
the effectiveness of ICIs and optimize patient prognosis.
Not applicable.
Funding: No funding was received.
Not applicable.
HJ proposed the overall idea. HJ and QZ participated
in the collection and collation of relevant data and the writing
and revision of the manuscript. Data authentication is not
applicable. All authors read and approved the final version of the
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Christofi T, Baritaki S, Falzone L, Libra
M and Zaravinos A: Current perspectives in cancer immunotherapy.
Cancers (Basel). 11:14722019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gupta SL, Basu S, Soni V and Jaiswal RK:
Immunotherapy: An alternative promising therapeutic approach
against cancers. Mol Biol Rep. 49:9903–9913. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kennedy LB and Salama AKS: A review of
cancer immunotherapy toxicity. CA Cancer J Clin. 70:86–104. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shi Z, Li H, Song W, Zhou Z, Li Z and
Zhang M: Emerging roles of the gut microbiota in cancer
immunotherapy. Front Immunol. 14:11398212023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vivarelli S, Salemi R, Candido S, Falzone
L, Santagati M, Stefani S, Torino F, Banna GL, Tonini G and Libra
M: Gut microbiota and cancer: From pathogenesis to therapy. Cancers
(Basel). 11:382019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kumagai T, Rahman F and Smith AM: The
microbiome and radiation induced-bowel injury: Evidence for
potential mechanistic role in disease pathogenesis. Nutrients.
10:14052018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qin J, Li R, Raes J, Arumugam M, Burgdorf
KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al: A
human gut microbial gene catalogue established by metagenomic
sequencing. Nature. 464:59–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jandhyala SM, Talukdar R, Subramanyam C,
Vuyyuru H, Sasikala M and Nageshwar Reddy D: Role of the normal gut
microbiota. World J Gastroenterol. 21:8787–8803. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tuddenham S and Sears CL: The intestinal
microbiome and health. Curr Opin Infect Dis. 28:464–470. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jain N: The early life education of the
immune system: Moms, microbes and (missed) opportunities. Gut
Microbes. 12:18245642020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao Y, O'Hely M, Quinn TP, Ponsonby AL,
Harrison LC, Frøkiær H, Tang MLK, Brix S, Kristiansen K, Burgner D,
et al: Maternal gut microbiota during pregnancy and the composition
of immune cells in infancy. Front Immunol. 13:9863402022.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rio-Aige K, Azagra-Boronat I,
Massot-Cladera M, Selma-Royo M, Parra-Llorca A, González S,
García-Mantrana I, Castell M, Rodríguez-Lagunas MJ, Collado MC and
Pérez Cano FJ: Association of maternal microbiota and diet in cord
blood cytokine and immunoglobulin profiles. Int J Mol Sci.
22:17782021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Henrick BM, Rodriguez L, Lakshmikanth T,
Pou C, Henckel E, Arzoomand A, Olin A, Wang J, Mikes J, Tan Z, et
al: Bifidobacteria-mediated immune system imprinting early in life.
Cell. 184:3884–3898.e11. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koenig JE, Spor A, Scalfone N, Fricker AD,
Stombaugh J, Knight R, Angenent LT and Ley RE: Succession of
microbial consortia in the developing infant gut microbiome. Proc
Natl Acad Sci USA. 108 (Suppl 1):S4578–S4585. 2011. View Article : Google Scholar
|
|
15
|
Odamaki T, Kato K, Sugahara H, Hashikura
N, Takahashi S, Xiao JZ, Abe F and Osawa R: Age-related changes in
gut microbiota composition from newborn to centenarian: A
cross-sectional study. BMC Microbiol. 16:902016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu Y, Wang Y, Li H, Dai Y, Chen D, Wang M,
Jiang X, Huang Z, Yu H, Huang J and Xiong Z: Altered fecal
microbiota composition in older adults with frailty. Front Cell
Infect Microbiol. 11:6961862021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng H, Guan X, Chen D and Ma W: The
Th17/Treg cell balance: A gut microbiota-modulated story.
Microorganisms. 7:5832019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee GR: The balance of Th17 versus Treg
cells in autoimmunity. Int J Mol Sci. 19:7302018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ivanov II, Frutos Rde L, Manel N,
Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB and Littman DR:
Specific microbiota direct the differentiation of IL-17-producing
T-helper cells in the mucosa of the small intestine. Cell Host
Microbe. 4:337–349. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Atarashi K, Tanoue T, Shima T, Imaoka A,
Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al:
Induction of colonic regulatory T cells by indigenous
Clostridium species. Science. 331:337–341. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Round JL, Lee SM, Li J, Tran G, Jabri B,
Chatila TA and Mazmanian SK: The Toll-like receptor 2 pathway
establishes colonization by a commensal of the human microbiota.
Science. 332:974–977. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Erturk-Hasdemir D, Oh SF, Okan NA,
Stefanetti G, Gazzaniga FS, Seeberger PH, Plevy SE and Kasper DL:
Symbionts exploit complex signaling to educate the immune system.
Proc Natl Acad Sci USA. 116:26157–26166. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun M, Wu W, Chen L, Yang W, Huang X, Ma
C, Chen F, Xiao Y, Zhao Y, Ma C, et al: Microbiota-derived
short-chain fatty acids promote Th1 cell IL-10 production to
maintain intestinal homeostasis. Nat Commun. 9:35552018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Martin-Gallausiaux C, Béguet-Crespel F,
Marinelli L, Jamet A, Ledue F, Blottière HM and Lapaque N: Butyrate
produced by gut commensal bacteria activates TGF-beta1 expression
through the transcription factor SP1 in human intestinal epithelial
cells. Sci Rep. 8:97422018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Duscha A, Gisevius B, Hirschberg S,
Yissachar N, Stangl GI, Dawin E, Bader V, Haase S, Kaisler J, David
C, et al: Propionic acid shapes the multiple sclerosis disease
course by an immunomodulatory mechanism. Cell. 180:1067–1080.e16.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Levy M, Thaiss CA, Zeevi D, Dohnalová L,
Zilberman-Schapira G, Mahdi JA, David E, Savidor A, Korem T, Herzig
Y, et al: Microbiota-modulated metabolites shape the intestinal
microenvironment by regulating NLRP6 inflammasome signaling. Cell.
163:1428–1443. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo C, Xie S, Chi Z, Zhang J, Liu Y, Zhang
L, Zheng M, Zhang X, Xia D, Ke Y, et al: Bile acids control
inflammation and metabolic disorder through inhibition of NLRP3
inflammasome. Immunity. 45:802–816. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yan F, Cao H, Cover TL, Whitehead R,
Washington MK and Polk DB: Soluble proteins produced by probiotic
bacteria regulate intestinal epithelial cell survival and growth.
Gastroenterology. 132:562–575. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Salemi R, Vivarelli S, Ricci D, Scillato
M, Santagati M, Gattuso G, Falzone L and Libra M: Lactobacillus
rhamnosus GG cell-free supernatant as a novel anti-cancer
adjuvant. J Transl Med. 21:1952023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ottman N, Reunanen J, Meijerink M, Pietilä
TE, Kainulainen V, Klievink J, Huuskonen L, Aalvink S, Skurnik M,
Boeren S, et al: Pili-like proteins of Akkermansia
muciniphila modulate host immune responses and gut barrier
function. PLoS One. 12:e01730042017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Alexander M, Ang QY, Nayak RR, Bustion AE,
Sandy M, Zhang B, Upadhyay V, Pollard KS, Lynch SV and Turnbaugh
PJ: Human gut bacterial metabolism drives Th17 activation and
colitis. Cell Host Microbe. 30:17–30.e9. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vinay DS, Ryan EP, Pawelec G, Talib WH,
Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara HMCS, et
al: Immune evasion in cancer: Mechanistic basis and therapeutic
strategies. Semin Cancer Biol. 35 (Suppl):S185–S198. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wei G, Zhang H, Zhao H, Wang J, Wu N, Li
L, Wu J and Zhang D: Emerging immune checkpoints in the tumor
microenvironment: Implications for cancer immunotherapy. Cancer
Lett. 511:68–76. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Iwai Y, Ishida M, Tanaka Y, Okazaki T,
Honjo T and Minato N: Involvement of PD-L1 on tumor cells in the
escape from host immune system and tumor immunotherapy by PD-L1
blockade. Proc Natl Acad Sci USA. 99:12293–12297. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Herbst RS, Soria JC, Kowanetz M, Fine GD,
Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger
SN, et al: Predictive correlates of response to the anti-PD-L1
antibody MPDL3280A in cancer patients. Nature. 515:563–567. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Park JJ, Thi EP, Carpio VH, Bi Y, Cole AG,
Dorsey BD, Fan K, Harasym T, Iott CL, Kadhim S, et al: Checkpoint
inhibition through small molecule-induced internalization of
programmed death-ligand 1. Nat Commun. 12:12222021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee HT, Lee SH and Heo YS: Molecular
interactions of antibody drugs targeting PD-1, PD-L1, and CTLA-4 in
immuno-oncology. Molecules. 24:11902019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Das R, Verma R, Sznol M, Boddupalli CS,
Gettinger SN, Kluger H, Callahan M, Wolchok JD, Halaban R,
Dhodapkar MV and Dhodapkar KM: Combination therapy with anti-CTLA-4
and anti-PD-1 leads to distinct immunologic changes in vivo. J
Immunol. 194:950–959. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shi AP, Tang XY, Xiong YL, Zheng KF, Liu
YJ, Shi XG, Lv Y, Jiang T, Ma N and Zhao JB: Immune checkpoint LAG3
and Its Ligand FGL1 in cancer. Front Immunol. 12:7850912022.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kandel S, Adhikary P, Li G and Cheng K:
The TIM3/Gal9 signaling pathway: An emerging target for cancer
immunotherapy. Cancer Lett. 510:67–78. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tan AC, Bagley SJ, Wen PY, Lim M, Platten
M, Colman H, Ashley DM, Wick W, Chang SM, Galanis E, et al:
Systematic review of combinations of targeted or immunotherapy in
advanced solid tumors. J Immunother Cancer. 9:e0024592021.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Syn NL, Teng MWL, Mok TSK and Soo RA:
De-novo and acquired resistance to immune checkpoint targeting.
Lancet Oncol. 18:e731–e741. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sanmamed MF and Chen L: A paradigm shift
in cancer immunotherapy: From enhancement to normalization. Cell.
175:313–326. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Boutros C, Tarhini A, Routier E, Lambotte
O, Ladurie FL, Carbonnel F, Izzeddine H, Marabelle A, Champiat S,
Berdelou A, et al: Safety profiles of anti-CTLA-4 and anti-PD-1
antibodies alone and in combination. Nat Rev Clin Oncol.
13:473–486. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Brahmer JR, Lacchetti C, Schneider BJ,
Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner
JM, Ginex P, et al: Management of immune-related adverse events in
patients treated with immune checkpoint inhibitor therapy: American
society of clinical oncology clinical practice guideline. J Clin
Oncol. 36:1714–1768. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ott PA, Bang YJ, Berton-Rigaud D, Elez E,
Pishvaian MJ, Rugo HS, Puzanov I, Mehnert JM, Aung KL, Lopez J, et
al: Safety and antitumor activity of pembrolizumab in advanced
programmed death ligand 1-positive endometrial cancer: Results from
the KEYNOTE-028 study. J Clin Oncol. 35:2535–2541. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Antonia SJ, Balmanoukian A, Brahmer J, Ou
SI, Hellmann MD, Kim SW, Ahn MJ, Kim DW, Gutierrez M, Liu SV, et
al: Clinical activity, tolerability, and long-term follow-up of
durvalumab in patients with advanced NSCLC. J Thorac Oncol.
14:1794–1806. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Schöffski P, Tan DSW, Martín M,
Ochoa-de-Olza M, Sarantopoulos J, Carvajal RD, Kyi C, Esaki T,
Prawira A, Akerley W, et al: Phase I/II study of the LAG-3
inhibitor ieramilimab (LAG525) ± anti-PD-1 spartalizumab (PDR001)
in patients with advanced malignancies. J Immunother Cancer.
10:e0037762022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Curigliano G, Gelderblom H, Mach N, Doi T,
Tai D, Forde PM, Sarantopoulos J, Bedard PL, Lin CC, Hodi FS, et
al: Phase I/Ib clinical trial of sabatolimab, an anti-TIM-3
antibody, alone and in combination with spartalizumab, an anti-PD-1
antibody, in advanced solid tumors. Clin Cancer Res. 27:3620–3629.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kelly CM, Qin LX, Whiting KA, Richards AL,
Avutu V, Chan JE, Chi P, Dickson MA, Gounder MM, Keohan ML, et al:
A phase II study of epacadostat and pembrolizumab in patients with
advanced sarcoma. Clin Cancer Res. 29:2043–2051. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zakharia Y, McWilliams RR, Rixe O, Drabick
J, Shaheen MF, Grossmann KF, Kolhe R, Pacholczyk R, Sadek R,
Tennant LL, et al: Phase II trial of the IDO pathway inhibitor
indoximod plus pembrolizumab for the treatment of patients with
advanced melanoma. J Immunother Cancer. 9:e0020572021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lynch TJ, Bondarenko I, Luft A,
Serwatowski P, Barlesi F, Chacko R, Sebastian M, Neal J, Lu H,
Cuillerot JM and Reck M: Ipilimumab in combination with paclitaxel
and carboplatin as first-line treatment in stage IIIB/IV
non-small-cell lung cancer: Results from a randomized,
double-blind, multicenter phase II study. J Clin Oncol.
30:2046–2054. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wan MT and Ming ME: Nivolumab versus
ipilimumab in the treatment of advanced melanoma: A critical
appraisal: ORIGINAL ARTICLE. Wolchok JD, Chiarion-Sileni V,
Gonzalez R, et al: Overall survival with combined nivolumab and
ipilimumab in advanced melanoma. N Engl J Med 2017; 377: 1345-1356.
Br J Dermatol. 179:296–300. 2018.PubMed/NCBI
|
|
55
|
Hellmann MD, Ciuleanu TE, Pluzanski A, Lee
JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers
S, Salman P, et al: Nivolumab plus ipilimumab in lung cancer with a
high tumor mutational burden. N Engl J Med. 378:2093–2104. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tannir NM, Signoretti S, Choueiri TK,
McDermott DF, Motzer RJ, Flaifel A, Pignon JC, Ficial M, Frontera
OA, George S, et al: Efficacy and safety of nivolumab plus
ipilimumab versus sunitinib in first-line treatment of patients
with advanced sarcomatoid renal cell carcinoma. Clin Cancer Res.
27:78–86. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Rini BI, Powles T, Atkins MB, Escudier B,
McDermott DF, Suarez C, Bracarda S, Stadler WM, Donskov F, Lee JL,
et al: Atezolizumab plus bevacizumab versus sunitinib in patients
with previously untreated metastatic renal cell carcinoma
(IMmotion151): A multicentre, open-label, phase 3, randomised
controlled trial. Lancet. 393:2404–2415. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Garon EB, Hellmann MD, Rizvi NA, Carcereny
E, Leighl NB, Ahn MJ, Eder JP, Balmanoukian AS, Aggarwal C, Horn L,
et al: Five-year overall survival for patients with advanced
non-small-cell lung cancer treated with pembrolizumab: Results from
the phase I KEYNOTE-001 study. J Clin Oncol. 37:2518–2527. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yuan L, Jia GD, Lv XF, Xie SY, Guo SS, Lin
DF, Liu LT, Luo DH, Li YF, Deng SW, et al: Camrelizumab combined
with apatinib in patients with first-line platinum-resistant or
PD-1 inhibitor resistant recurrent/metastatic nasopharyngeal
carcinoma: A single-arm, phase 2 trial. Nat Commun. 14:48932023.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu Y, Song Y, Zuo S, Zhang X, Liu H, Wang
J, Wang J, Tang Y, Zheng W, Ying Z, et al: Antitumor activity and
safety of camrelizumab combined with apatinib in patients with
relapsed or refractory peripheral T-cell lymphoma: An open-label,
multicenter, phase II study. Front Immunol. 14:11281722023.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhao Y, Ma Y, Fan Y, Zhou J, Yang N, Yu Q,
Zhuang W, Song W, Wang ZM, Li B, et al: A multicenter, open-label
phase Ib/II study of cadonilimab (anti PD-1 and CTLA-4 bispecific
antibody) monotherapy in previously treated advanced non-small-cell
lung cancer (AK104-202 study). Lung Cancer. 184:1073552023.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shui L, Yang X, Li J, Yi C, Sun Q and Zhu
H: Gut microbiome as a potential factor for modulating resistance
to cancer immunotherapy. Front Immunol. 10:29892020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Park EM, Chelvanambi M, Bhutiani N,
Kroemer G, Zitvogel L and Wargo JA: Targeting the gut and tumor
microbiota in cancer. Nat Med. 28:690–703. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhou Y, Liu Z and Chen T: Gut microbiota:
A promising milestone in enhancing the efficacy of PD1/PD-L1
blockade therapy. Front Oncol. 12:8473502022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Vétizou M, Pitt JM, Daillère R, Lepage P,
Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong
CP, et al: Anticancer immunotherapy by CTLA-4 blockade relies on
the gut microbiota. Science. 350:1079–1084. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Huang C, Li M, Liu B, Zhu H, Dai Q, Fan X,
Mehta K, Huang C, Neupane P, Wang F, et al: Relating gut microbiome
and its modulating factors to immunotherapy in solid tumors: A
systematic review. Front Oncol. 11:6421102021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Routy B, Le Chatelier E, Derosa L, Duong
CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C,
Roberti MP, et al: Gut microbiome influences efficacy of PD-1-based
immunotherapy against epithelial tumors. Science. 359:91–97. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Grenda A, Iwan E, Chmielewska I, Krawczyk
P, Giza A, Bomba A, Frąk M, Rolska A, Szczyrek M, Kieszko R, et al:
Presence of Akkermansiaceae in gut microbiome and
immunotherapy effectiveness in patients with advanced non-small
cell lung cancer. AMB Express. 12:862022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Grenda A, Iwan E, Krawczyk P, Frąk M,
Chmielewska I, Bomba A, Giza A, Rolska-Kopińska A, Szczyrek M,
Kieszko R, et al: Attempting to identify bacterial allies in
immunotherapy of NSCLC patients. Cancers (Basel). 14:62502022.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Newsome RC, Gharaibeh RZ, Pierce CM, da
Silva WV, Paul S, Hogue SR, Yu Q, Antonia S, Conejo-Garcia JR,
Robinson LA and Jobin C: Interaction of bacterial genera associated
with therapeutic response to immune checkpoint PD-1 blockade in a
United States cohort. Genome Med. 14:352022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lee KA, Thomas AM, Bolte LA, Björk JR, de
Ruijter LK, Armanini F, Asnicar F, Blanco-Miguez A, Board R,
Calbet-Llopart N, et al: Cross-cohort gut microbiome associations
with immune checkpoint inhibitor response in advanced melanoma. Nat
Med. 28:535–544. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Cani PD, Depommier C, Derrien M, Everard A
and de Vos WM: Akkermansia muciniphila: paradigm for
next-generation beneficial microorganisms. Nat Rev Gastroenterol
Hepatol. 19:625–637. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Xu X, Lv J, Guo F, Li J, Jia Y, Jiang D,
Wang N, Zhang C, Kong L, Liu Y, et al: Gut microbiome influences
the efficacy of PD-1 antibody immunotherapy on MSS-type colorectal
cancer via metabolic pathway. Front Microbiol. 11:8142020.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Peiffer LB, White JR, Jones CB, Slottke
RE, Ernst SE, Moran AE, Graff JN and Sfanos KS: Composition of
gastrointestinal microbiota in association with treatment response
in individuals with metastatic castrate resistant prostate cancer
progressing on enzalutamide and initiating treatment with anti-PD-1
(pembrolizumab). Neoplasia. 32:1008222022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Gopalakrishnan V, Spencer CN, Nezi L,
Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman
K, Wei SC, et al: Gut microbiome modulates response to anti-PD-1
immunotherapy in melanoma patients. Science. 359:97–103. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Matson V, Fessler J, Bao R, Chongsuwat T,
Zha Y, Alegre ML, Luke JJ and Gajewski TF: The commensal microbiome
is associated with anti-PD-1 efficacy in metastatic melanoma
patients. Science. 359:104–108. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Hakozaki T, Richard C, Elkrief A, Hosomi
Y, Benlaïfaoui M, Mimpen I, Terrisse S, Derosa L, Zitvogel L, Routy
B and Okuma Y: The gut microbiome associates with immune checkpoint
inhibition outcomes in patients with advanced non-small cell lung
cancer. Cancer Immunol Res. 8:1243–1250. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Jin Y, Dong H, Xia L, Yang Y, Zhu Y, Shen
Y, Zheng H, Yao C, Wang Y and Lu S: The diversity of gut microbiome
is associated with favorable responses to anti-programmed death 1
immunotherapy in Chinese patients with NSCLC. J Thorac Oncol.
14:1378–1389. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Mao J, Wang D, Long J, Yang X, Lin J, Song
Y, Xie F, Xun Z, Wang Y, Wang Y, et al: Gut microbiome is
associated with the clinical response to anti-PD-1 based
immunotherapy in hepatobiliary cancers. J Immunother Cancer.
9:e0033342021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Shen YC, Lee PC, Kuo YL, Wu WK, Chen CC,
Lei CH, Yeh CP, Hsu C, Hsu CH, Lin ZZ, et al: An exploratory study
for the association of gut microbiome with efficacy of immune
checkpoint inhibitor in patients with hepatocellular carcinoma. J
Hepatocell Carcinoma. 8:809–822. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wang F, He MM, Yao YC, Zhao X, Wang ZQ,
Jin Y, Luo HY, Li JB, Wang FH, Qiu MZ, et al: Regorafenib plus
toripalimab in patients with metastatic colorectal cancer: A phase
Ib/II clinical trial and gut microbiome analysis. Cell Rep Med.
2:1003832021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wu YY, Lin CW, Cheng KS, Lin C, Wang YM,
Lin IT, Chou YH and Hsu PN: Increased programmed death-ligand-1
expression in human gastric epithelial cells in Helicobacter
pylori infection. Clin Exp Immunol. 161:551–559. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Park JS, Gazzaniga FS, Wu M, Luthens AK,
Gillis J, Zheng W, LaFleur MW, Johnson SB, Morad G, Park EM, et al:
Targeting PD-L2-RGMb overcomes microbiome-related immunotherapy
resistance. Nature. 617:377–385. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Peng Z, Cheng S, Kou Y, Wang Z, Jin R, Hu
H, Zhang X, Gong JF, Li J, Lu M, et al: The gut microbiome is
associated with clinical response to anti-PD-1/PD-L1 immunotherapy
in gastrointestinal cancer. Cancer Immunol Res. 8:1251–1261. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Nomura M, Nagatomo R, Doi K, Shimizu J,
Baba K, Saito T, Matsumoto S, Inoue K and Muto M: Association of
short-chain fatty acids in the gut microbiome with clinical
response to treatment with nivolumab or pembrolizumab in patients
with solid cancer tumors. JAMA Netw Open. 3:e2028952020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
He Y, Fu L, Li Y, Wang W, Gong M, Zhang J,
Dong X, Huang J, Wang Q, Mackay CR, et al: Gut microbial
metabolites facilitate anticancer therapy efficacy by modulating
cytotoxic CD8+ T cell immunity. Cell Metab.
33:988–1000.e7. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Coutzac C, Jouniaux JM, Paci A, Schmidt J,
Mallardo D, Seck A, Asvatourian V, Cassard L, Saulnier P, Lacroix
L, et al: Systemic short chain fatty acids limit antitumor effect
of CTLA-4 blockade in hosts with cancer. Nat Commun. 11:21682020.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Lee PC, Wu CJ, Hung YW, Lee CJ, Chi CT,
Lee IC, Yu-Lun K, Chou SH, Luo JC, Hou MC and Huang YH: Gut
microbiota and metabolites associate with outcomes of immune
checkpoint inhibitor-treated unresectable hepatocellular carcinoma.
J Immunother Cancer. 10:e0047792022. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Jiang SS, Xie YL, Xiao XY, Kang ZR, Lin
XL, Zhang L, Li CS, Qian Y, Xu PP, Leng XX, et al: Fusobacterium
nucleatum-derived succinic acid induces tumor resistance to
immunotherapy in colorectal cancer. Cell Host Microbe.
31:781–797.e9. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yang M, Wang Y, Yuan M, Tao M, Kong C, Li
H, Tong J, Zhu H and Yan X: Antibiotic administration shortly
before or after immunotherapy initiation is correlated with poor
prognosis in solid cancer patients: An up-to-date systematic review
and meta-analysis. Int Immunopharmacol. 88:1068762020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Tinsley N, Zhou C, Tan G, Rack S, Lorigan
P, Blackhall F, Krebs M, Carter L, Thistlethwaite F, Graham D and
Cook N: Cumulative antibiotic use significantly decreases efficacy
of checkpoint inhibitors in patients with advanced cancer.
Oncologist. 25:55–63. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Hakozaki T, Okuma Y, Omori M and Hosomi Y:
Impact of prior antibiotic use on the efficacy of nivolumab for
non-small cell lung cancer. Oncol Lett. 17:2946–2952.
2019.PubMed/NCBI
|
|
93
|
Derosa L, Hellmann MD, Spaziano M,
Halpenny D, Fidelle M, Rizvi H, Long N, Plodkowski AJ, Arbour KC,
Chaft JE, et al: Negative association of antibiotics on clinical
activity of immune checkpoint inhibitors in patients with advanced
renal cell and non-small-cell lung cancer. Ann Oncol. 29:1437–1444.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kim J and Lee HK: The role of gut
microbiota in modulating tumor growth and anticancer agent
efficacy. Mol Cells. 44:356–362. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Son MY and Cho HS: Anticancer effects of
gut microbiota-derived short-chain fatty acids in cancers. J
Microbiol Biotechnol. 33:849–856. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Li T, Han L, Ma S, Lin W, Ba X, Yan J,
Huang Y, Tu S and Qin K: Interaction of gut microbiota with the
tumor microenvironment: A new strategy for antitumor treatment and
traditional Chinese medicine in colorectal cancer. Front Mol
Biosci. 10:11403252023. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Andrews MC, Duong CPM, Gopalakrishnan V,
Iebba V, Chen WS, Derosa L, Khan MAW, Cogdill AP, White MG, Wong
MC, et al: Gut microbiota signatures are associated with toxicity
to combined CTLA-4 and PD-1 blockade. Nat Med. 27:1432–1441. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Dubin K, Callahan MK, Ren B, Khanin R,
Viale A, Ling L, No D, Gobourne A, Littmann E, Huttenhower C, et
al: Intestinal microbiome analyses identify melanoma patients at
risk for checkpoint-blockade-induced colitis. Nat Commun.
7:103912016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Liu T, Xiong Q, Li L and Hu Y: Intestinal
microbiota predicts lung cancer patients at risk of immune-related
diarrhea. Immunotherapy. 11:385–396. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Sivan A, Corrales L, Hubert N, Williams
JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B,
Alegre ML, et al: Commensal Bifidobacterium promotes
antitumor immunity and facilitates anti-PD-L1 efficacy. Science.
350:1084–1089. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Tomita Y, Goto Y, Sakata S, Imamura K,
Minemura A, Oka K, Hayashi A, Jodai T, Akaike K, Anai M, et al:
Clostridium butyricum therapy restores the decreased
efficacy of immune checkpoint blockade in lung cancer patients
receiving proton pump inhibitors. Oncoimmunology. 11:20810102022.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Dizman N, Meza L, Bergerot P, Alcantara M,
Dorff T, Lyou Y, Frankel P, Cui Y, Mira V, Llamas M, et al:
Nivolumab plus ipilimumab with or without live bacterial
supplementation in metastatic renal cell carcinoma: A randomized
phase 1 trial. Nat Med. 28:704–712. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Tomita Y, Ikeda T, Sakata S, Saruwatari K,
Sato R, Iyama S, Jodai T, Akaike K, Ishizuka S, Saeki S and
Sakagami T: Association of probiotic Clostridium butyricum
therapy with survival and response to immune checkpoint blockade in
patients with lung cancer. Cancer Immunol Res. 8:1236–1242. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Wang F, Yin Q, Chen L and Davis MM:
Bifidobacterium can mitigate intestinal immunopathology in
the context of CTLA-4 blockade. Proc Natl Acad Sci USA.
115:157–161. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Spencer CN, McQuade JL, Gopalakrishnan V,
McCulloch JA, Vetizou M, Cogdill AP, Khan MAW, Zhang X, White MG,
Peterson CB, et al: Dietary fiber and probiotics influence the gut
microbiome and melanoma immunotherapy response. Science.
374:1632–1640. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Gao G, Shen S, Zhang T, Zhang J, Huang S,
Sun Z and Zhang H: Lacticaseibacillus rhamnosus Probio-M9
enhanced the antitumor response to anti-PD-1 therapy by modulating
intestinal metabolites. EBioMedicine. 91:1045332023. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Huang J, Zheng X, Kang W, Hao H, Mao Y,
Zhang H, Chen Y, Tan Y, He Y, Zhao W and Yin Y: Metagenomic and
metabolomic analyses reveal synergistic effects of fecal microbiota
transplantation and anti-PD-1 therapy on treating colorectal
cancer. Front Immunol. 13:8749222022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Shaikh FY, Gills JJ, Mohammad F, White JR,
Stevens CM, Ding H, Fu J, Tam A, Blosser RL, Domingue JC, et al:
Murine fecal microbiota transfer models selectively colonize human
microbes and reveal transcriptional programs associated with
response to neoadjuvant checkpoint inhibitors. Cancer Immunol
Immunother. 71:2405–2420. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Baruch EN, Youngster I, Ben-Betzalel G,
Ortenberg R, Lahat A, Katz L, Adler K, Dick-Necula D, Raskin S,
Bloch N, et al: Fecal microbiota transplant promotes response in
immunotherapy-refractory melanoma patients. Science. 371:602–609.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Davar D, Dzutsev AK, McCulloch JA,
Rodrigues RR, Chauvin JM, Morrison RM, Deblasio RN, Menna C, Ding
Q, Pagliano O, et al: Fecal microbiota transplant overcomes
resistance to anti-PD-1 therapy in melanoma patients. Science.
371:595–602. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wang Y, Wiesnoski DH, Helmink BA,
Gopalakrishnan V, Choi K, DuPont HL, Jiang ZD, Abu-Sbeih H, Sanchez
CA, Chang CC, et al: Fecal microbiota transplantation for
refractory immune checkpoint inhibitor-associated colitis. Nat Med.
24:1804–1808. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Fasanello MK, Robillard KT, Boland PM,
Bain AJ and Kanehira K: Use of fecal microbial transplantation for
immune checkpoint inhibitor colitis. ACG Case Rep J. 7:e003602020.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Koo H and Morrow CD: Incongruence between
dominant commensal donor microbes in recipient feces post fecal
transplant and response to anti-PD-1 immunotherapy. BMC Microbiol.
21:2512021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Jamal R, Messaoudene M, de Figuieredo M
and Routy B: Future indications and clinical management for fecal
microbiota transplantation (FMT) in immuno-oncology. Semin Immunol.
67:1017542023. View Article : Google Scholar : PubMed/NCBI
|