Introduction
Lung cancer is the leading cause of
cancer-associated death, with high rates of morbidity and mortality
(1,2). At present, surgery is the standard
treatment for resectable cancer (3), however, it is associated with numerous
complications and limitations, such as tissue damage and extensive
thoracic adhesion. Thus, innovative treatments are required to
eliminate tumors more effectively (4). Microwave ablation (MWA) is used in the
treatment of inoperable early stage and metastatic lung cancer. MWA
causes water molecules to vibrate and collide in microwave
electromagnetic fields within tumor tissue, resulting in high
temperatures and a coagulative necrotic zone that kills the local
tumor (5). For local treatment, MWA
is precise with minimal invasiveness and few complications
(6,7). Notably, the clinical efficacy of MWA
is comparable with that of surgery (8).
Unlike conventional surgery, MWA not only kills
local tumors but produces abscopal effects in certain cases,
resulting in reduction or removal of distant tumor lesions
(9). Previous studies have reported
that thermal ablation may promote the release of tumor-associated
antigens (TAAs) (10,11). These may induce tumor-specific
antitumor immune responses, which induce abscopal effects (12,13).
Shao et al (14) reported
that MWA induces abscopal effects and resistance to immunotherapy
in a patient with advanced squamous non-small cell lung cancer
(NSCLC). Xu et al (15)
reported spontaneous regression of distant tumor lesions after MWA
in patients with lung metastasis of endometrial carcinoma. To the
best of our knowledge, however, few cases of MWA-induced abscopal
effects have been reported (16),
and the specific mechanisms underlying the abscopal effects remain
to be elucidated.
In addition to MWA, other local therapies have
demonstrated systemic antitumor immune responses, including
abscopal effects. Radiofrequency ablation (RFA) reshapes the tumor
microenvironment (TME) by promoting the recruitment of
CD8+ T/natural killer (NK) cells and suppressing the
accumulation of myeloid-derived suppressor and regulatory T cells
(17). Notably, cryoablation
induces a strong tumor-specific tumor-infiltrating lymphocyte
response, increases CD8+ T cell and granzyme B levels
and induces abscopal effects (18).
Irreversible electroporation induces abscopal effects by increasing
the infiltration of specific T cells and enhancing specific immune
memory (19). In addition, both
radiotherapy (20) and heavy ion
therapy (21) may modulate
occurrence of immune-induced abscopal effects.
Research into MWA-induced immune responses is
primarily conducted in hepatocellular carcinoma (22) and breast cancer (23). To the best of our knowledge,
MWA-induced immune responses in patients with lung cancer have
rarely been reported in clinical studies (24,25).
Thus, the present study assessed potential changes in immune
response after MWA in patients with lung cancer. The present study
aimed to provide a novel theoretical basis to study the mechanisms
of MWA and occurrence of abscopal effects.
Materials and methods
Study design
Patients admitted to Putuo Hospital, Shanghai
University of Traditional Chinese Medicine (Shanghai, China) were
recruited. In total, 45 patients with lung cancer who successfully
received MWA were involved in the present study between January
2021 and December 2022 (Table I).
The inclusion criteria were as follows: i) Lung cancer (either
primary or metastatic) diagnosed via histopathology or artificial
intelligence-based lung computed tomography (CT) diagnostic system;
ii) MWA guided by CT; iii) ≤5 lesions and iv) a maximum tumor
diameter ≤3 cm. Patients were excluded according to the following
criteria: i) Immunotherapy, chemotherapy, radiotherapy,
glucocorticoid or immunosuppressive therapy <3 months before
enrollment; ii) serious complications after MWA, such as infection
or high levels of bleeding and iii) refusal to participate in the
study. Patients with lung cancer were divided into those with early
(stage I–II) or advanced stage (stage III–IV) disease (26). The present study was approved by the
Ethics Committee of Putuo Hospital, Shanghai University of
Traditional Chinese Medicine [Shanghai, China; approval no.
PTEC-A-2022-2(S)-1] and all patients provided written informed
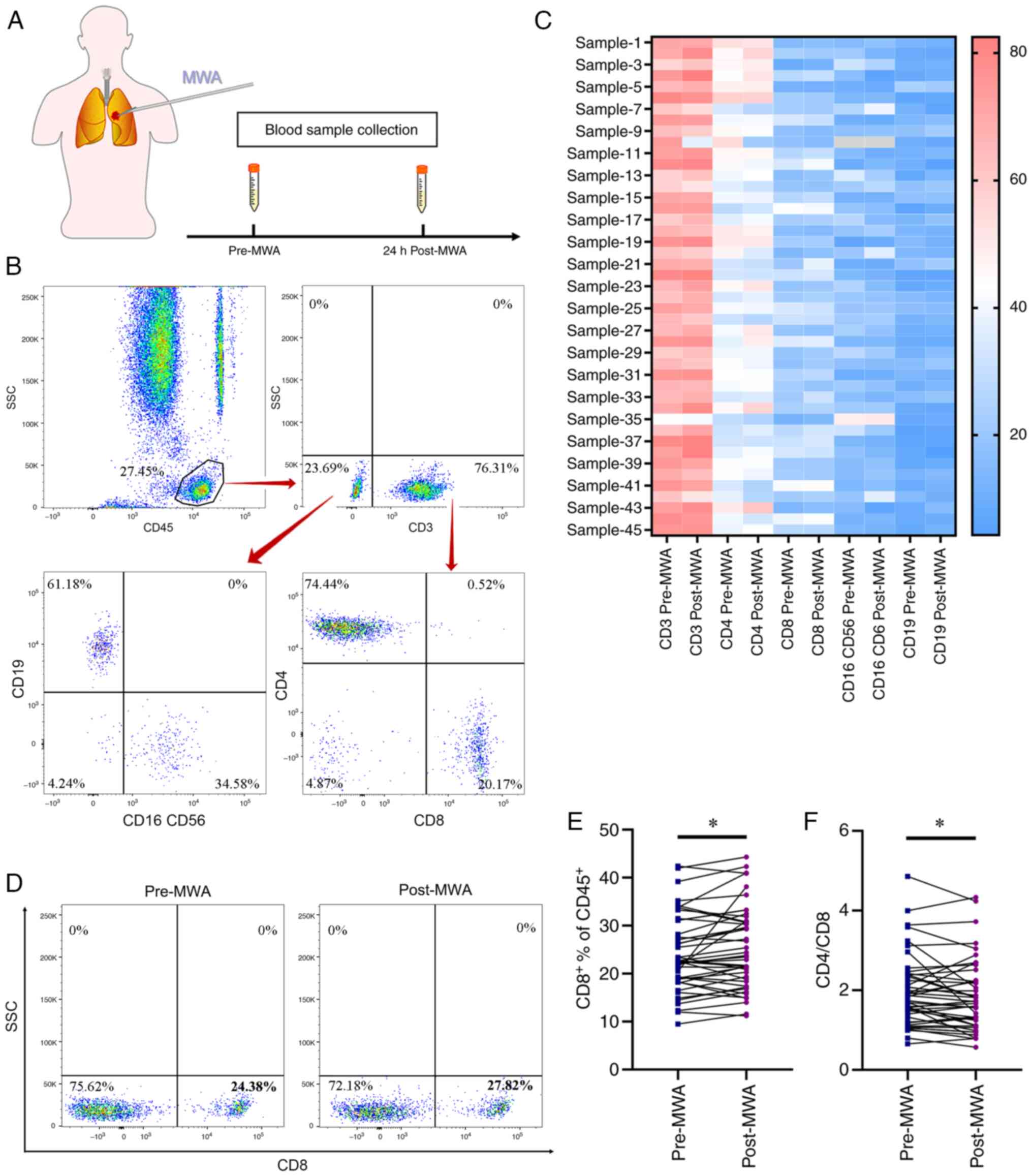
consent to participate. Peripheral blood samples were collected
(prior to intervention and 24 h after MWA) to assess levels of
immune cell subsets and cytokines (Fig.
1A).
 | Table I.Baseline characteristics of the study
population. |
Table I.
Baseline characteristics of the study
population.
| Characteristic | Value |
|---|
| Median age, years
(range) | 59 (40–83) |
| Sex (%) |
|
|
Male | 25 (55.56) |
|
Female | 20 (44.44) |
| Stage (%) |
|
| I | 33 (73.33) |
| II | 0 (0.00) |
|
III | 4 (8.89) |
| IV | 8 (17.78) |
| Number of tumors
(%) |
|
| 1 | 22 (48.89) |
| 2 | 15 (33.33) |
| ≥3 | 8 (17.78) |
| Mean diameter of
index tumor, cm | 1.26±0.92 |
| Previous treatment
(%) |
|
| Local
ablative therapy | 1 (2.22) |
|
Surgical resection | 6 (13.33) |
|
None | 38 (84.44) |
CT-guided MWA
Under local anesthesia, CT-guided MWA (ECO-100E;
Yigao Microwave Electric Institute) was performed. The microwave
antenna was placed in the target lesion and MWA was performed by an
experienced respiratory doctor under CT guidance. The ablation
parameters were determined by tumor load, with a microwave
irradiation frequency of 2,450 MHz and an ablation power of 40–60 W
for 2–9 min. Ablation was performed according to the manufacturer's
recommendations to generate a safety margin of ≥0.5 cm to achieve
complete ablation.
Measurement of immune cell subset and
cytokine levels
Briefly, peripheral blood samples were centrifuged
at 1,600 × g for 20 min at 4°C to separate cells and serum. Cells
from blood samples were processed for immunophenotype analysis.
Cells were incubated with Human TruStain FcX (No. 422302,
BioLegend) for 10 min at 4°C to block the Fc receptors and fixated
and permeabilized using a fix and perm buffer for 15 min at 20°C
(No. GAS004, Thermo). Cells were stained with BD Multitest 6-Color
TBNK Reagent (No. 644611, BD Biosciences, including CD45
PerCP-Cy5.5, CD3 FITC, CD4 PE-Cy7, CD8 APC-Cy7, CD16 PE, CD56 PE,
CD19 APC). BD Multitest 6-Color TBNK dissolved in 1 ml buffered
saline, and pipette 20 µl of beforementioned antibodies into 50 µl
of blood sample according to the manufacturer's instructions.
Incubate for 30 min in the dark at 20°C. Add 450 µl of 1X BD
FACS™ Lysing Solution (No. 349202, BD Biosciences) to
the blood sample, and incubate for 30 min in the dark at 20°C.
CD3+ (CD45+CD3+), CD4+
(CD3+CD4+) and CD8+ T
(CD3+CD8+), B
(CD3−CD19+) and NK cells
(CD3−CD16+CD56+) were analyzed by
flow cytometry (BD FACSCanto™ II, BD Biosciences), and
data analysis was performed using FlowJo software (Tree Star, Inc.,
Ashland, OR, USA).
Cytokine concentrations in patients were quantified
using ELISA kits according to the manufacturer's instructions, and
these cytokines included IL-1β (No. 20212400166, Hotgen), IL-2
(cat. no. 20222400096, Hotgen), IL-4 (No. 20222400091, Hotgen),
IL-5 (No. 20222400088, Hotgen), IL-6 (No. 20172400287, Hotgen),
IL-8 (No. 20212400164, Hotgen), IL-10 (No. 20212400165, Hotgen),
IL-12p70 (No. ab213791, Abcam), IL-17A (No. ab216167, Abcam),
IL-17F (No. ab309176, Abcam), IL-22 (No. ab216170, Abcam), TNF-α
(No. 20212400161, Hotgen), TNF-β (No. ab229202, Hotgen) and IFN-γ
(No. ab174443, Abcam). Briefly, serum was added to microplate
strips and incubated at 20°C for 2 h. After a total of three washes
with wash buffer, primary antibodies (1:100 dilution) were added
and incubated for 2 h at 20°C. The wash step was then repeated.
Finally, after incubation with substrate solution for 30 min at
20°C, stop solution was added. Wash buffer, substrate solution,
primary antibodies and stop solution included in Elisa kit. The
levels of cytokines were measured by a fully-automatic
chemiluminescence platform (Hotgen c2000; Beijing Hotgen Biotech
Co., Ltd.) according to the manufacturer's instructions.
Clinical observation of local effects
of MWA
According to expert consensus (27), contrast-enhanced chest CT scans were
performed monthly for the first 3 months after MWA. Thereafter,
contrast-enhanced chest CT or positron emission tomography (PET)/CT
scans and tumor markers were evaluated every 3 months to assess
whether complete ablation was achieved. Complete ablation was
defined as the absence of focal enhancement signals within or at
the periphery of the tumor, confirmed via contrast-enhanced
images.
Statistical analysis
Data are presented as the mean ± standard error of
the mean of three independent experimental repeats. GraphPad Prism
8 (Dotmatics) was used to analyze the data and generate heatmaps.
Differences before and after ablation were assessed using paired
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Phenotypical characterization of
peripheral blood mononuclear cells following MWA
Changes in immune cell subsets in peripheral blood
of 45 patients with lung cancer were assessed using flow cytometry
before and 24 h after ablation (Fig.
1B). Characteristics of peripheral immune cells before and
after ablation (Fig. 1C). Notably,
the proportion of CD8+ T cells significantly increased
after ablation (23.97±1.18% vs. 25.25±1.27%; Fig. 1D and E) and the CD4/CD8 ratio
significantly decreased after ablation (1.95±0.13 vs. 1.74±0.13;
Fig. 1F). Moreover, the proportion
of CD3+ and CD4+ T, NK and B cells remained
stable, with no significant differences before and after ablation
(Fig. S1A-D). The proportion of
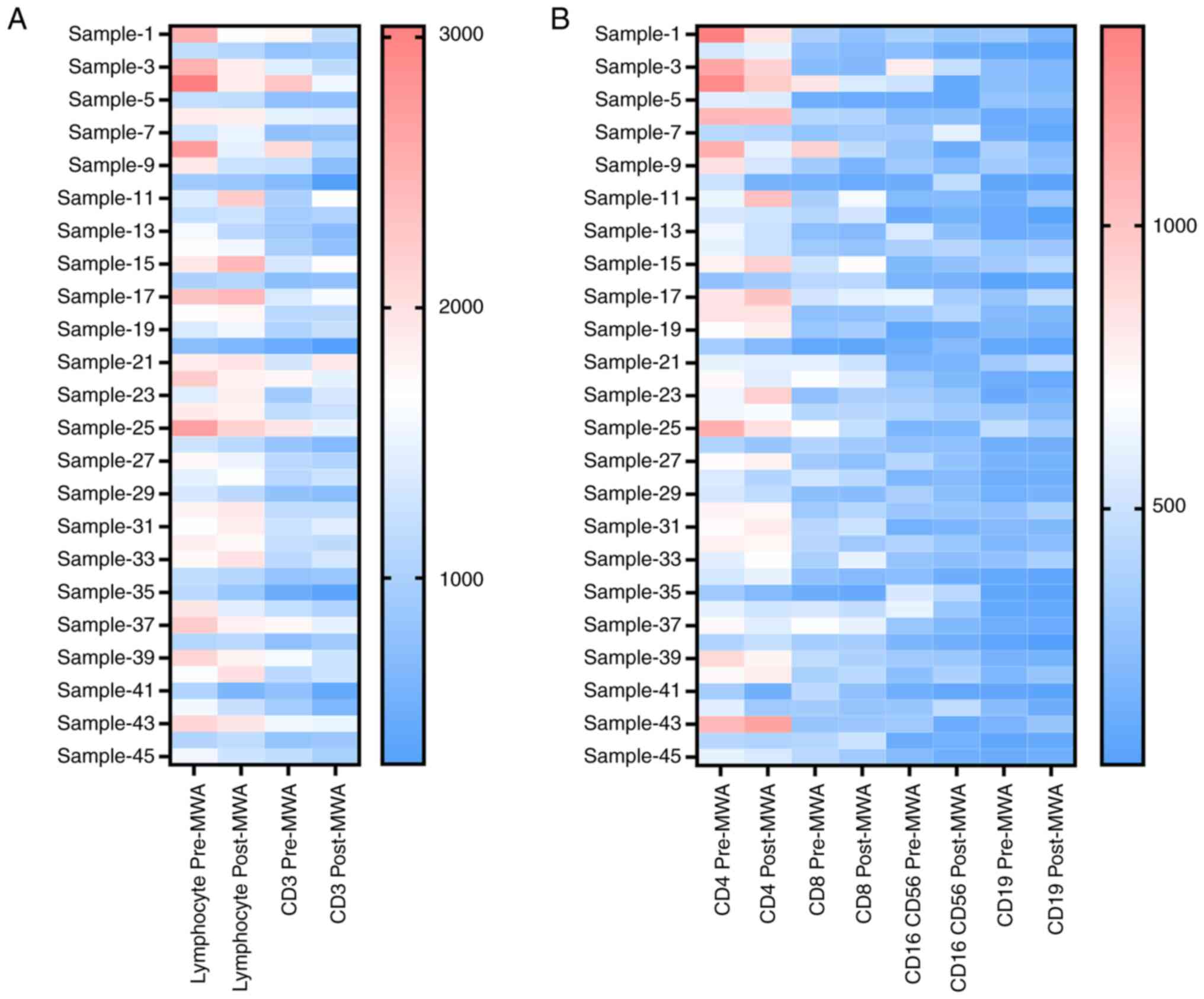
immune cell subsets in the peripheral blood was also analyzed; MWA
treatment notably reduced the proportion number of immune cells
(Figs. 2A and B and S2). In addition, the number of total
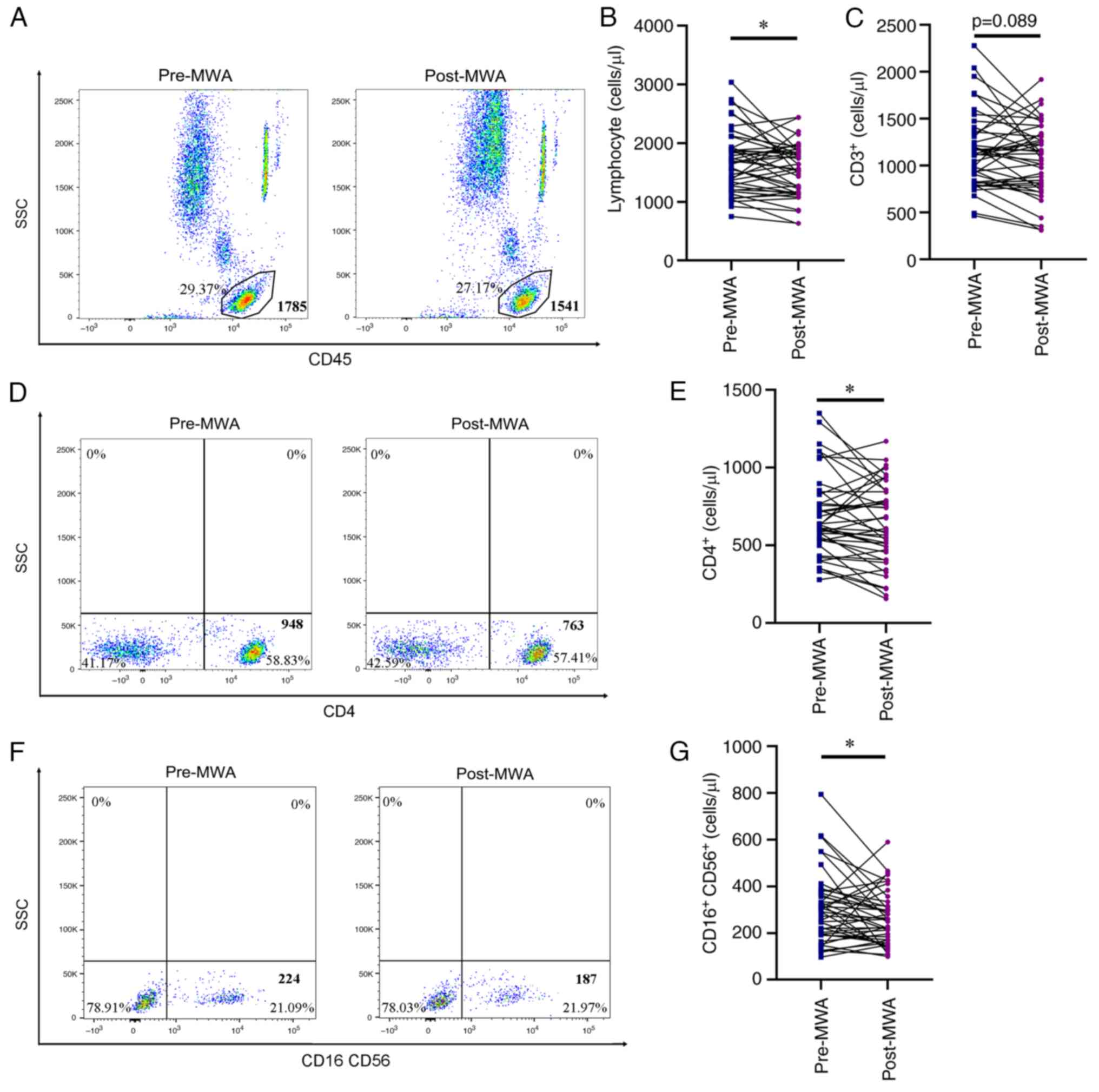
lymphocytes was not significantly increased after ablation
(1,680.27±78.33 vs. 1,543.63±66.37 cells/µl; Fig. 3A and B), as well as the number of
CD4+ T (689.41±37.83 vs. 630.95±37.37 cells/µl; Fig. 3D and E) and NK cells (304.58±22.09
vs. 260.05±16.90 cells/µl; Fig. 3F and
G). Notably, the number of CD3+ T cells was markedly
decreased, however, this was not significant (1,154.26±60.14 vs.
1,074.53±56.26 cells/µl; Fig. 3C).
In addition, the number of circulating B and CD8+ T
cells remained stable (Fig. S1E and
F), with no significant difference before and after
ablation.
MWA induces T helper (Th)1-type immune
response
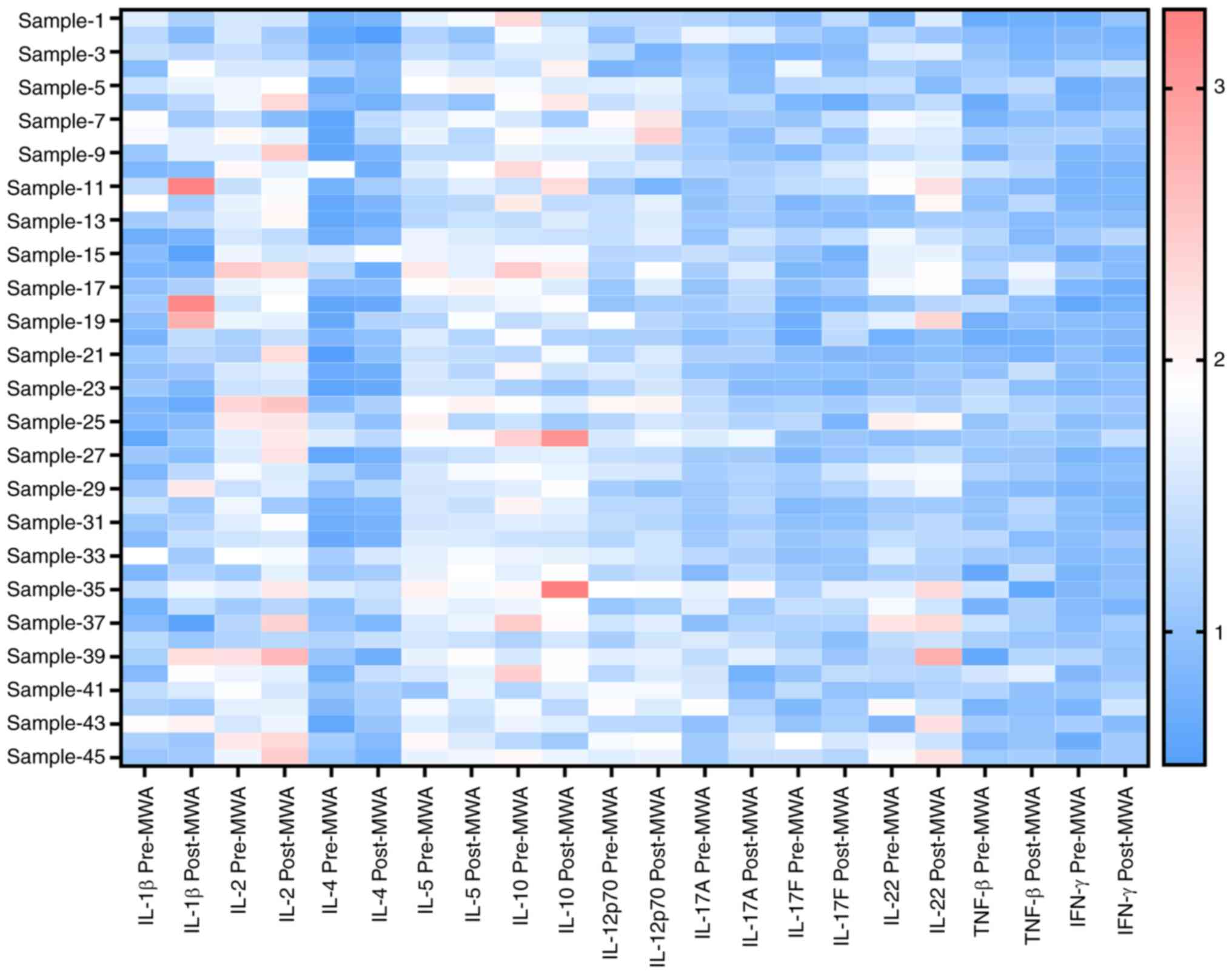
To assess serum cytokine levels after ablation in
patients with lung cancer, peripheral Th1 and Th2 cytokines were
measured, and the levels of multiple cytokines were altered
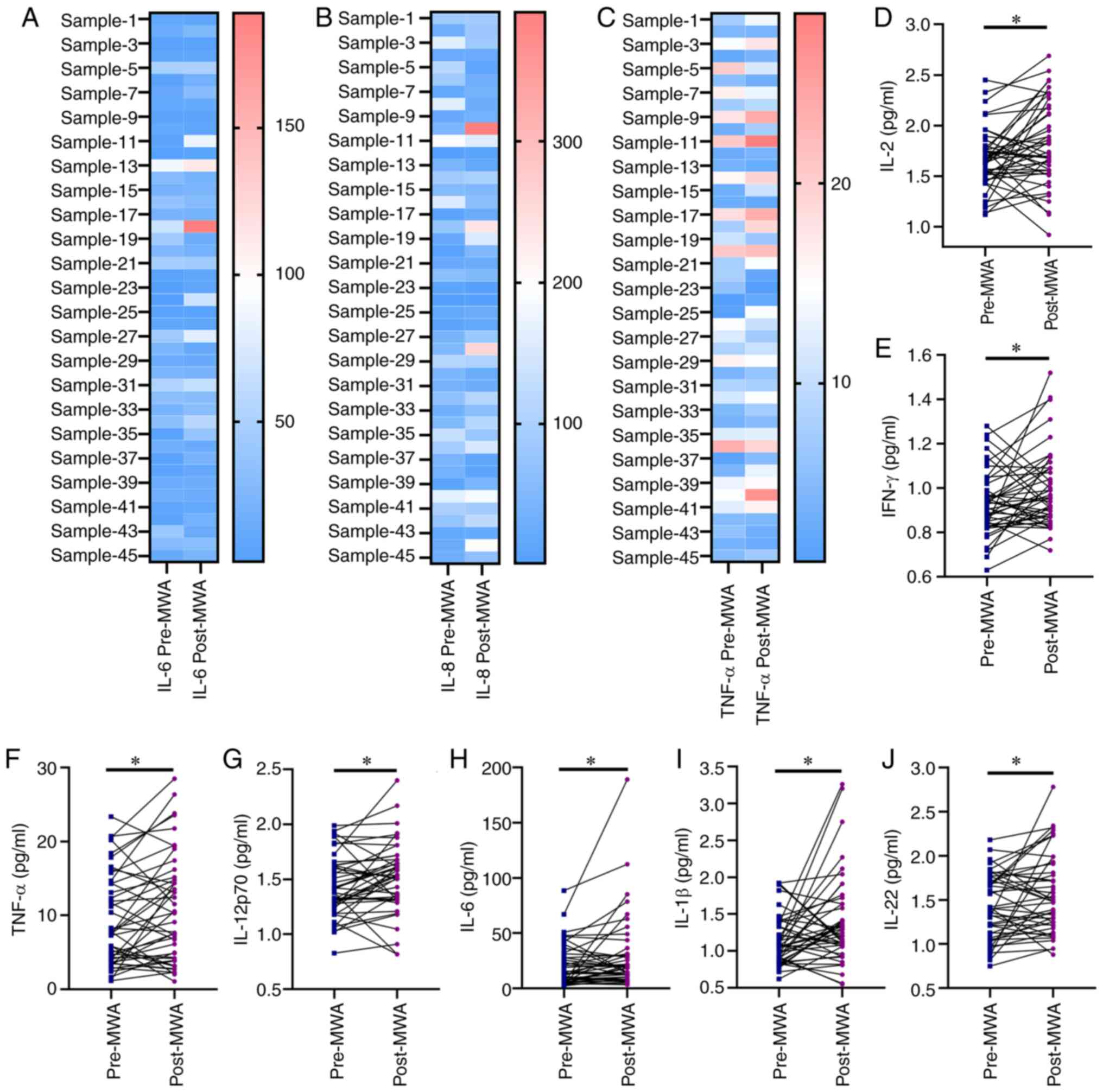
(Figs. 4 and 5A-C). Levels of Th1 cytokines IL-2
(1.66±0.04 vs. 1.81±0.06 pg/ml; Fig.
5D), IFN-γ (0.93±0.02 vs. 0.99±0.03 pg/ml; Fig. 5E), TNF-α (9.16±0.92 vs. 10.86±1.08
pg/ml; Fig. 5F) and IL-12p70
(1.44±0.04 vs. 1.52±0.05 pg/ml; Fig.
5G) were significantly elevated following ablation, indicative
of MWA-activated Th1-type immune response. By contrast, the levels
of Th2 cytokines, IL-4 and IL-10, were not significantly changed
following ablation compared with before (Fig. S3A and B). In addition,
significantly increased levels of proinflammatory cytokines IL-6
(20.73±2.81 vs. 29.54±5.06 pg/ml Fig.
5H), IL-1β (1.16±0.05 vs. 1.39±0.09 pg/ml; Fig. 5I) and IL-22 (1.43±0.06 vs. 1.56±0.07
pg/ml; Fig. 5J) were observed after
ablation, compared with before; however, no significant changes
were observed in the levels of IL-5, IL-8, IL-17A, IL-17F and TNF-β
(Fig. S3C-G).
MWA may induce immune responses in
patients with early and advanced stage disease
To assess differences in immune responses after
ablation according to tumor stage, patients with lung cancer were
divided into early and advanced stage groups. Proportion of
CD8+ T cells was significantly increased in patients
with advanced stage disease following ablation, compared with
before (26.41±2.62% vs. 27.79±2.72%; Fig. 6A), but there were no significant
changes in CD3+, CD4+ T, NK and B cells. The
overall number of lymphocytes was significantly decreased in
patients with advanced stage disease following ablation
(1,553.37±131.93 vs. 1,362.18±126.82 cells/µl; Fig. 6B), compared with before. This was
markedly decreased in comparison with the early disease stage
group, which had no significant difference between before and after
ablation (1,726.44±95.29 vs. 1,609.28±75.75 cells/µl). Moreover,
the number of CD3+ T cells was significantly reduced in
patients with advanced stage disease after ablation compared with
before (1,088.07±111.92 vs. 965.64±107.57 cells/µl; Figs. 6B and S1G). The number of CD4+ T
cells was notably decreased in patients with both early
(720.90±45.35 vs. 662.27±40.79) and advanced (602.97±63.94 vs.
544.83±79.49 cells/µl; Fig. 6B)
stage disease following ablation compared with before; however,
these results were not statistically significant. Results of the
present study also demonstrated that the number of CD8+
T, NK and B cells remained unchanged after ablation (Fig. 6B).
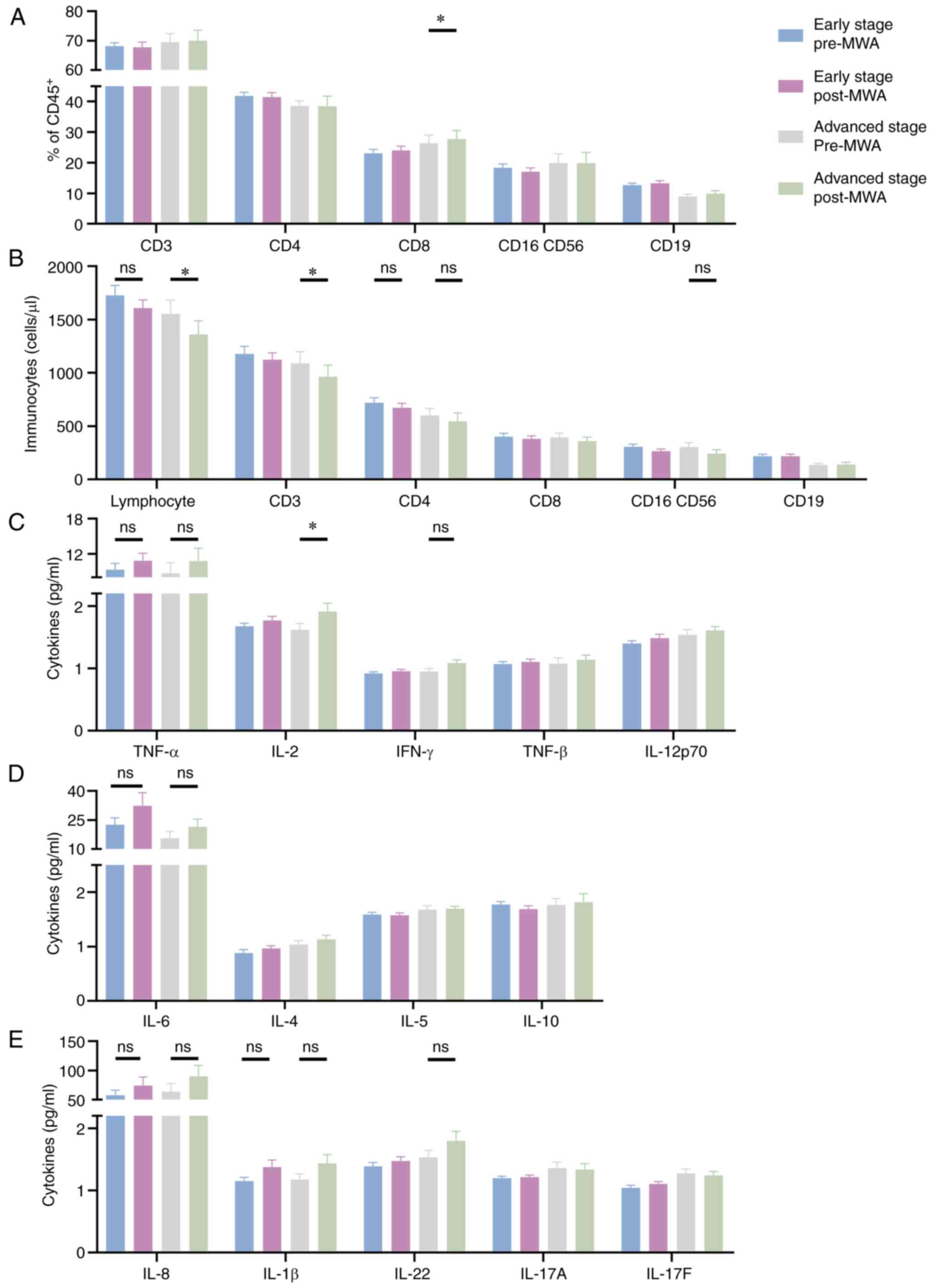 | Figure 6.Phenotypical characterization of
peripheral lymphocytes and cytokine levels before and after
ablation in patients with early or advanced stage disease. Changes
in (A) proportion and (B) levels of immunocytes after ablation in
patients with early or advanced stage disease. Changes in (C)
TNF-α, IL-2, IFN-γ, TNF-β and IL-12p70, (D) IL-6, IL-4, IL-5 and
IL-10, and (E) IL-8, IL-1β, IL-22, IL-17A and IL-17F peripheral
cytokine levels following ablation in patients with early or
advanced stages of disease. *P<0.05. MWA, microwave
ablation. |
In addition, differences in cytokine levels between
groups were assessed. IL-2 levels were significantly higher in
patients with advanced stage disease after ablation, compared with
before (1.62±0.11 vs. 1.91±0.13; Fig.
6C), while levels remained stable in patients with early stage
disease (1.68±0.05 vs. 1.77±0.07 pg/ml; Fig. 6C), with no significant differences
demonstrated. There were no significant changes in the levels of
IL-4, IL-5, IL-6, TNF-α, IL-1β, IL-8, IL-10, IL-12p70, IL-17A,
IL-17F, IL-22 TNF-β or IFN-γ in patients with early or advanced
stage disease after ablation, compared with before (Fig. 6C-E).
Discussion
As a minimally invasive therapy, MWA is increasingly
used in the treatment of lung cancer (28,29).
In addition to elimination of local tumors by MWA, the resulting
tumor fragments may serve as in situ vaccines that trigger a
series of immune responses (30–32). A
detailed study of MWA-mediated immunity is essential for combining
MWA with immunotherapy in future (33,34).
In the present study, MWA induced Th1-type immune response and
significantly increased the proportion of the peripheral
CD8+ T cell subset. However, the number of peripheral
total lymphocytes and CD4+ T and NK cells in patients
with lung cancer decreased 24 h after ablation. To the best of our
knowledge, these results have not been reported (24). Patients with advanced stage disease
had increased levels of CD8+ T cells and IL-2 and
decreased levels of total lymphocytes and CD3+ T cells,
compared with patients with early stage disease; however, the
majority of parameters were comparable between groups. These
results suggested that the stage of disease may not impact the
MWA-induced immune response.
CD8+ T cells are immune cells that
directly kill tumors. Notably, these may provide an important
immune basis for abscopal effects or for the combination of MWA
with immune checkpoint inhibitors in the treatment of cancer
(31,35). Levels of CD8+ T cells
increased and CD4/CD8 ratio decreased 24 h after ablation. By
contrast, the proportions of CD3+ and CD4+ T,
NK and B cells remained unchanged and these results were comparable
with those reported in a previous study (24). Moreover, results of the present
study demonstrated that MWA decreased the number of peripheral
immune cells, including total lymphocytes and CD4+ T and
NK cells; however, there were insignificant changes in number of
CD8+ T cells. Notably, these data were not consistent
with other reports: Zhou et al (23) reported that CD8+ T and NK
cells are notably activated in patients with breast cancer 1 week
after ablation. In addition, Wu et al (36) reported that the proportions of
CD3+ and CD4+ T cell subsets and the CD4/CD8
ratio in the peripheral blood of patients with thyroid cancer, is
significantly increased at 1 day and 2 weeks after ablation.
Leuchte et al (22) reported
that the proportion of CD3+ T cells in patients with
hepatocellular carcinoma is markedly increased 1 week after
ablation. Thus, MWA may lead to different immune cell responses in
patients with different tumors. In addition, the time after
ablation is an important factor affecting the immune response
(36). Further investigation with
increased sample sizes is required to assess the response of immune
cells.
The underlying mechanism of the MWA-induced systemic
immune response is unclear. In the present study, the levels of
Th1-type cytokines, such as IL-2, IFN-γ and TNF-α, were
significantly elevated 24 h after ablation, while the levels of
Th2-type-related cytokines, such as IL-4, IL-5 and IL-10, remained
unchanged, Notably, these results were consistent with those of
previous studies (25,37,38).
IL-2 stimulates T cell proliferation and differentiation and
enhances the function of cytotoxic T lymphocytes (39). Zhang et al (24) reported that levels of IL-2 notably
decreased and the levels of TNF-α do not change in the peripheral
blood 1 month after ablation in patients with lung cancer. In
addition, Xu et al (25)
reported that the levels of IL-2 and IFN-γ in the peripheral blood
of patients with lung cancer markedly decrease 48 h after MWA and
increase after 1 month, while TNF-α levels were not significantly
altered. The present study demonstrated that the levels of the
Th1-type cytokine IL-12p70 were markedly elevated following
ablation. IL-12p70 has been reported to independently induce the
differentiation and proliferation of Th1 cells and promote
proliferation and killing effects of T and NK cells, especially
production of IFN-γ and the formation of cytotoxic T cells
(40). Zhao et al (41) reported that IL-12p70 and IL-12p40
levels increase significantly 24 h following MWA in patients with
hepatocellular carcinoma. In summary, MWA may induce Th1-type
immune responses with antitumor effects (42), and these responses may be
time-dependent.
In the present study, levels of cytokines were
assessed after ablation; levels of proinflammatory cytokines IL-1β,
IL-6 and IL-22 were increased in the peripheral blood after
ablation. These increases may be associated with tissue repair and
the acute phase response (43–45).
Notably, levels of IL-8, IL-17A, IL-17F and TNF-β were not
significantly changed. IL-1β is considered to serve a key role in
initiation of adaptive antitumor responses, promoting Th1-type
immune responses and activating dendritic cells and cytotoxic T
lymphocytes (46). IL-6 serves a
key role in T cell proliferation and survival (47) and is also a target of NF-κB. The
simultaneous activation of NF-κB and STAT3 in non-immune cells
triggers activation of the IL-6/STAT3 axis, which is closely
associated with tumor development. IL-6 possesses both pro- and
anti-inflammatory properties; thus, the ultimate impact of the
immune response generated by IL-6 remains unclear (43). IL-22, expressed in activated T cells
(48), exhibits dual functions in
tumorigenesis. Notably, IL-22 has protective effects in the short
term, whilst uncontrolled IL-22 activity promotes cancer growth
(49). The cytokines IL-1β, IL-6
and IL-22 can be produced by macrophages, an important type of
antigen-presenting cell (50–52).
We hypothesized that tumor cell fragments produced by MWA may
activate macrophages, potentially inducing specific antitumor
immunity, which is consistent with a previous study (53). Further assessment of the specific
roles of IL-1β, IL-6 and IL-22 in antitumor activity and changes in
cytokine levels at different time points are required to determine
the long-term effects of MWA on immune response. IL-8 is a key
chemokine for chemotaxis of polymorphonuclear leukocytes and
monocytes/macrophages (54), and to
evaluate chemotaxis of immune cells in the short term after
ablation, IL-8 levels were measured. The levels of IL-8 were not
significantly changed after ablation, while Zhao et al
(41) reported that IL-8 levels
notably increase 24 h after MWA in patients with hepatocellular
carcinoma. The cytokines IL-17A and IL-17F are produced by
differentiated Th17 cells (55,56).
Zhou et al (57) reported
that Th17 cells in patients with hepatocellular carcinoma are
regulated 24 h after MWA, and the present study demonstrated that
IL-17A and IL-17F levels did not change significantly after
ablation. Th17 cells were not activated 24 h after MWA and these
results were consistent with those obtained by Xu et al
(25). TNF-β is an important
Th1-type cytokine that is mainly produced by activated T cells
(58). MWA did not affect levels of
TNF-β in the peripheral blood; however, a Th1-type immune response
was induced. Collectively, these results suggest that different
tumor types induce different cytokine profiles following
ablation.
It is crucial to understand the effects of MWA on
immunity in patients with early or advanced stage lung cancer.
However, to the best of our knowledge, there are no data describing
the immune response following ablation in patients with different
stages of lung cancer. In the present study, differences in immune
response were investigated after ablation in patients with
different stages of disease. The overall effects of MWA were
comparable between patients; however, MWA decreased the number of
total lymphocytes and CD3+ T cells and increased the
proportion of CD8+ T cells, in patients with advanced
stage disease; these results were consistent with those of a
previous study (59). These results
indicated that decreased levels of total lymphocytes may be
reflected by reduced levels of CD3+, but not
CD8+, T cells in patients with advanced stage disease.
Moreover, levels of NK cells were markedly reduced in patients with
advanced stage disease; however, these results were not
significant. Thus, further investigation with increased sample
sizes is required. Furthermore, the number of CD4+ T
cells decreased in both groups after ablation; however, these
results were not significant. Thus, the effects of MWA on
CD4+ T cells was independent of disease stage.
Gabrielson et al (60)
reported that high levels of CD3+ and CD8+ T
cell infiltration are associated with prolonged relapse-free
survival, and the function of immune cells is associated with
numerous factors, such as surface costimulatory molecules (61). Therefore, further preclinical
studies are required to determine the association between
circulating and infiltrating immune cells and the differences in
immune cell function after MWA.
In the present study, analysis of cytokines in the
peripheral blood demonstrated that patients with different stages
of disease responded differently to MWA. IL-2 levels were higher in
patients with advanced stage disease, indicating that these
patients were more susceptible to T cell activation by MWA
(39), which is beneficial in
advanced tumor therapy (62).
Cancer immune evasion is a major stumbling block to effective
anticancer therapeutic strategies as tumors progress, and T cell
activation contributes to antitumor effects in these patients
(63). IL-1β and IL-6 levels were
increased in both groups; however, these results were not
significant. The levels of IL-22 were increased following ablation
in patients with advanced stage compared with patients with early
stage disease; however, these results were not significant. Thus,
MWA may primarily impact IL-22 levels in patients with advanced
stage disease and may impact the TME (64). IL-10 levels remained stable after
ablation in both groups of patients; however, Leuchte et al
(22) demonstrated that IL-10 is
notably modulated after ablation. The role of IL-10 in tumors is
bidirectional, demonstrating protumor effects as a Th2-type
cytokine (65). Qiao et al
(66) reported that IL-10 notably
exhibits antitumor effects by preventing dendritic cell-mediated
apoptosis of CD8+ T cells; however, further clarification of the
antitumor role of IL-10 is required. By contrast, levels of
cytokines such as IL-4, IL-5, IL-8, IL-12p70, IL-17A, IL-17F,
TNF-α, TNF-β and IFN-γ were not significantly different between
groups after ablation and these results are consistent with those
of a previous study (41). In
summary, the immune response did not differ between patients with
early or advanced stage disease and MWA may induce more T cell
activation in patients with advanced stage disease, which may be
beneficial in treatment of advanced stage disease.
Hyperthermia generated by thermal ablation releases
TAAs, which are absorbed by antigen-presenting cells for
presentation to T cells to stimulate specific antitumor immune
responses (67). Faraoni et
al (68) reported that RFA
notably increases the number of dendritic cells in pancreatic
ductal adenocarcinoma and induces CD4+ and
CD8+ T cell-mediated abscopal effects. Kanegasaki and
Tsuchiya (69) reported that RFA
modulates an antitumor immune response in a CC-chemokine receptor
1-dependent manner. However, the immune response induced by thermal
ablation alone remains low. Immune checkpoint inhibitors (ICIs) may
enhance ablation (70) and
combination of thermal ablation and ICIs may amplify the antitumor
immune response. Yu et al (71) reported that MWA combined with
immunotherapy markedly improves the long-term prognosis in patients
with NSCLC. Huang et al (72) reported that MWA combined with
cisplatin notably prolongs local progression-free survival in
patients with large NSCLC. In addition, Feng and Lu (73) reported that systemic administration
combined with MWA under CT and fiberoptic bronchoscopy is markedly
more effective than systemic administration alone in treatment of
lung cancer. Notably, incomplete thermal ablation may promote tumor
progression and impede immunotherapy (74). The present study provides a novel
theoretical basis for the study of abscopal effects and combination
of MWA with immunotherapy in lung cancer; however, further
investigations are required to improve the feasibility of
combination therapy for long-term local efficacy and the systemic
immune response.
Several limitations exist in the present study. The
study used peripheral blood collected before and 24 h after MWA and
changes in immunity in the peripheral blood may only indicate the
short-term effects of MWA on the human immune response.
Investigations at additional time points, for example at days 1 and
7 and months 1 and 3 following MWA, are required to study the
effects of MWA on immunity. Moreover, the sample size was small and
further investigations using larger sample sizes are required.
Thus, a potential association between the immune response and
ablation of healthy lung tissue cannot be excluded.
In conclusion, MWA induced significant systemic
immune responses in patients with lung cancer, particularly
Th1-type immune responses. However, MWA may be more likely to
induce an immune response in patients with advanced stage disease
than those with early stage disease. A combination strategy using
additional therapeutic approaches that promotes MWA-induced immune
responses may exhibit potential in treatment of lung cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by The Clinical Research Special
Project of Shanghai Municipal Health Commission (grant no.
20214Y0495), One Hundred Talents Project of Putuo Hospital,
Shanghai University of Traditional Chinese Medicine (grant no.
2022-RCCY-01), Shanghai Science and Technology Committee (grant no.
20ZR1450400) and the Plan of Clinical Specialty Construction in
Shanghai Putuo District Health System (grant no. 2020tszk02).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FM and YL performed the literature review, analyzed
the data and wrote the manuscript. XM and XL designed the study and
revised the manuscript. ZN designed the study. SW, MZ, XW and ZZ
collected blood samples. FM, YL and XM confirm the authenticity of
all raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was performed according to the
guidelines of the Declaration of Helsinki and approved by the
Ethics Committee of Putuo Hospital, Shanghai University of
Traditional Chinese Medicine [Shanghai, China; approval. no.
PTEC-A-2022-2(S)-1]. Informed written consent was obtained from all
subjects involved in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wu F, Wang L and Zhou C: Lung cancer in
China: Current and prospect. Curr Opin Oncol. 33:40–46. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gao S, Li N, Wang S, Zhang F, Wei W, Li N,
Bi N, Wang Z and He J: Lung cancer in People's Republic of China. J
Thorac Oncol. 15:1567–1576. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brody H: Lung cancer. Nature. 587:S72020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aokage K, Yoshida J, Hishida T, Tsuboi M,
Saji H, Okada M, Suzuki K, Watanabe S and Asamura H: Limited
resection for early-stage non-small cell lung cancer as
function-preserving radical surgery: A review. Jpn J Clin Oncol.
47:7–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ni Y, Xu H and Ye X: Image-guided
percutaneous microwave ablation of early-stage non-small cell lung
cancer. Asia Pac J Clin Oncol. 16:320–325. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ryan MJ, Willatt J, Majdalany BS, Kielar
AZ, Chong S, Ruma JA and Pandya A: Ablation techniques for primary
and metastatic liver tumors. World J Hepatol. 8:191–199. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vogl TJ, Nour-Eldin NA, Albrecht MH,
Kaltenbach B, Hohenforst-Schmidt W, Lin H, Panahi B, Eichler K,
Gruber-Rouh T and Roman A: Thermal ablation of lung tumors: Focus
on microwave ablation. Rofo. 189:828–843. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chan MV, Huo YR, Cao C and Ridley L:
Survival outcomes for surgical resection versus CT-guided
percutaneous ablation for stage I non-small cell lung cancer
(NSCLC): A systematic review and meta-analysis. Eur Radiol.
31:5421–5433. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Y, Dong Y, Kong L, Shi F, Zhu H and Yu
J: Abscopal effect of radiotherapy combined with immune checkpoint
inhibitors. J Hematol Oncol. 11:1042018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chu KF and Dupuy DE: Thermal ablation of
tumours: Biological mechanisms and advances in therapy. Nat Rev
Cancer. 14:199–208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van den Bijgaart RJE, Eikelenboom DC,
Hoogenboom M, Fütterer JJ, den Brok MH and Adema G J: Thermal and
mechanical high-intensity focused ultrasound: Perspectives on tumor
ablation, immune effects and combination strategies. Cancer Immunol
Immunother. 66:247–258. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vilinovszki O, Andratschke N, Huellner M,
Curioni-Fontecedro A and Kroeze SGC: True abscopal effect in a
patient with metastatic non-small cell lung cancer. Radiat Oncol.
16:1942021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pierini S, Mishra A, Perales-Linares R,
Uribe-Herranz M, Beghi S, Giglio A, Pustylnikov S, Costabile F,
Rafail S, Amici A, et al: Combination of vasculature targeting,
hypofractionated radiotherapy, and immune checkpoint inhibitor
elicits potent antitumor immune response and blocks tumor
progression. J Immunother Cancer. 9:e0016362021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shao C, Yang M, Pan Y, Xie D, Chen B, Ren
S and Zhou C: Case report: Abscopal effect of microwave ablation in
a patient with advanced squamous NSCLC and resistance to
immunotherapy. Front Immunol. 12:6967492021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu H, Sun W, Kong Y, Huang Y, Wei Z, Zhang
L, Liang J and Ye X: Immune abscopal effect of microwave ablation
for lung metastases of endometrial carcinoma. J Cancer Res Ther.
16:1718–1721. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu L, Xie H, Wang L, Cheng M, Liu J, Xu J,
Wei Z, Ye X, Xie Q and Liang J: Microwave ablation induces abscopal
effect via enhanced systemic antitumor immunity in colorectal
cancer. Front Oncol. 13:11747132023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song X, Li N, Liu Y, Wang Z, Wang T, Tan
S, Li C, Qiu C, Gao L, Asano K, et al: CD169-positive macrophages
enhance abscopal effect of radiofrequency ablation therapy in liver
cancer. Transl Oncol. 15:1013062022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khan SY, Melkus MW, Rasha F, Castro M, Chu
V, Brandi L, Khan H, Gill HS, Pruitt K and Layeequr Rahman R:
Tumor-infiltrating lymphocytes (TILs) as a biomarker of abscopal
effect of cryoablation in breast cancer: A pilot study. Ann Surg
Oncol. 29:2914–2925. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He C, Huang X, Zhang Y, Lin X and Li S:
T-cell activation and immune memory enhancement induced by
irreversible electroporation in pancreatic cancer. Clin Transl Med.
10:e392020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao Y, Zhang T, Wang Y, Lu D, Du J, Feng
X, Zhou H, Liu N, Zhu H, Qin S, et al: ICAM-1 orchestrates the
abscopal effect of tumor radiotherapy. Proc Natl Acad Sci USA.
118:e20103331182021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang YS, Zhang YH, Li XJ, Hu TC, Chen WZ,
Pan X, Chai HY and Ye YC: Bystander effect and abscopal effect in
recurrent thymic carcinoma treated with carbon-ion radiation
therapy: A case report. World J Clin Cases. 9:6538–6543. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Leuchte K, Staib E, Thelen M, Gödel P,
Lechner A, Zentis P, Garcia-Marquez M, Waldschmidt D, Datta RR,
Wahba R, et al: Microwave ablation enhances tumor-specific immune
response in patients with hepatocellular carcinoma. Cancer Immunol
Immunother. 70:893–907. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou W, Yu M, Mao X, Pan H, Tang X, Wang
J, Che N, Xie H, Ling L, Zhao Y, et al: Landscape of the peripheral
immune response induced by local microwave ablation in patients
with breast cancer. Adv Sci (Weinh). 9:e22000332022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang L, Zhang M, Wang J, Li Y, Wang T,
Xia J, Feng B and Shen J: Immunogenic change after percutaneous
microwave ablation in pulmonary malignancies: Variation in immune
cell subsets and cytokines in peripheral blood. Front Immunol.
13:10691922022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu H, Tan X, Kong Y, Huang Y, Wei Z and Ye
X: Microwave ablation of non-small cell lung cancer tumors changes
plasma levels of cytokines IL-2 and IFN-γ. J Cancer Res Ther.
18:532–544. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Puri S, Shafique M and Gray JE: Immune
checkpoint inhibitors in early-stage and locally advanced non-small
cell lung cancer. Curr Treat Options Oncol. 19:392018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ye X, Fan W, Wang H, Wang J, Wang Z, Gu S,
Feng W, Zhuang Y, Liu B, Li X, et al: Expert consensus workshop
report: Guidelines for thermal ablation of primary and metastatic
lung tumors (2018 edition). J Cancer Res Ther. 14:730–744. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Palussière J, Cazayus M, Cousin S, Cabart
M, Chomy F, Catena V and Buy X: Is there a role for percutaneous
ablation for early stage lung cancer? What Is the evidence? Curr
Oncol Rep. 23:812021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang L, Xu J, Yu J and Liang P: Review of
clinical tumor ablation advance in Asia. Int J Hyperthermia.
38:1639–1649. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Flynn MJ, Sayed AA, Sharma R, Siddique A
and Pinato DJ: Challenges and opportunities in the clinical
development of immune checkpoint inhibitors for hepatocellular
carcinoma. Hepatology. 69:2258–2270. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rangamuwa K, Leong T, Weeden C,
Asselin-Labat ML, Bozinovski S, Christie M, John T, Antippa P,
Irving L and Steinfort D: Thermal ablation in non-small cell lung
cancer: A review of treatment modalities and the evidence for
combination with immune checkpoint inhibitors. Transl Lung Cancer
Res. 10:2842–2857. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
den Brok MH, Sutmuller RP, van der Voort
R, Bennink EJ, Figdor CG, Ruers TJ and Adema GJ: In situ tumor
ablation creates an antigen source for the generation of antitumor
immunity. Cancer Res. 64:4024–4029. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang K, Wang C, Jiang H, Zhang Y, Lin W,
Mo J and Jin C: Combination of ablation and immunotherapy for
hepatocellular carcinoma: Where we are and where to go. Front
Immunol. 12:7927812021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Keisari Y: Tumor abolition and antitumor
immunostimulation by physico-chemical tumor ablation. Front Biosci
(Landmark Ed). 22:310–347. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wei Z, Zhan X, Fan L, Ye X, Yang X, Huang
G, Li W, Wang J, Han X, Meng M, et al: Programmed death-ligand 1
expression and CD8+ tumor-infiltrating lymphocytes in advanced
non-small cell lung cancer treated with microwave ablation and
chemotherapy. Int J Hyperthermia. 35:591–598. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu T, Sui GQ, Teng DK, Luo Q, Wang H and
Lin YQ: Study on changes in immune function after microwave
ablation of papillary thyroid microcarcinoma. Cancer Manag Res.
14:2861–2868. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li L, Wang W, Pan H, Ma G, Shi X, Xie H,
Liu X, Ding Q, Zhou W and Wang S: Microwave ablation combined with
OK-432 induces Th1-type response and specific antitumor immunity in
a murine model of breast cancer. J Transl Med. 15:232017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang H, Hou X, Cai H and Zhuang X:
Effects of microwave ablation on T-cell subsets and cytokines of
patients with hepatocellular carcinoma. Minim Invasive Ther Allied
Technol. 26:207–211. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Volkó J, Kenesei Á, Zhang M, Várnai P,
Mocsár G, Petrus MN, Jambrovics K, Balajthy Z, Müller G, Bodnár A,
et al: IL-2 receptors preassemble and signal in the ER/Golgi
causing resistance to antiproliferative anti-IL-2Rα therapies. Proc
Natl Acad Sci USA. 116:21120–21130. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nguyen KG, Vrabel MR, Mantooth SM, Hopkins
JJ, Wagner ES, Gabaldon TA and Zaharoff DA: Localized
interleukin-12 for cancer immunotherapy. Front Immunol.
11:5755972020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao J, Li Q, Muktiali M, Ren B, Hu Y, Li
D, Li Z, Li D, Xie Y, Tao M and Liang R: Effect of microwave
ablation treatment of hepatic malignancies on serum cytokine
levels. BMC Cancer. 20:8122020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou W, Yu M, Pan H, Qiu W, Wang H, Qian
M, Che N, Zhang K, Mao X, Li L, et al: Microwave ablation induces
Th1-type immune response with activation of ICOS pathway in
early-stage breast cancer. J Immunother Cancer. 9:e0023432021.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bent EH, Millán-Barea LR, Zhuang I, Goulet
DR, Fröse J and Hemann MT: Microenvironmental IL-6 inhibits
anti-cancer immune responses generated by cytotoxic chemotherapy.
Nat Commun. 12:62182021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu Y, Min J, Ge C, Shu J, Tian D, Yuan Y
and Zhou D: Interleukin 22 in liver injury, inflammation and
cancer. Int J Biol Sci. 16:2405–2413. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sun R, Gao DS, Shoush J and Lu B: The IL-1
family in tumorigenesis and antitumor immunity. Semin Cancer Biol.
86:280–295. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bent R, Moll L, Grabbe S and Bros M:
Interleukin-1 beta-A friend or foe in malignancies? Int J Mol Sci.
19:21552018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fisher DT, Appenheimer MM and Evans SS:
The two faces of IL-6 in the tumor microenvironment. Semin Immunol.
26:38–47. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hossein-Khannazer N, Zian Z, Bakkach J,
Kamali AN, Hosseinzadeh R, Anka AU, Yazdani R and Azizi G: Features
and roles of T helper 22 cells in immunological diseases and
malignancies. Scand J Immunol. 93:e130302021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Perez LG, Kempski J, McGee HM, Pelzcar P,
Agalioti T, Giannou A, Konczalla L, Brockmann L, Wahib R, Xu H, et
al: TGF-β signaling in Th17 cells promotes IL-22 production and
colitis-associated colon cancer. Nat Commun. 11:26082020.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rodriguez AE, Ducker GS, Billingham LK,
Martinez CA, Mainolfi N, Suri V, Friedman A, Manfredi MG, Weinberg
SE, Rabinowitz JD and Chandel NS: Serine metabolism supports
macrophage IL-1β production. Cell Metab. 29:1003–1011.e4. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hirani D, Alvira CM, Danopoulos S, Milla
C, Donato M, Tian L, Mohr J, Dinger K, Vohlen C, Selle J, et al:
Macrophage-derived IL-6 trans-signalling as a novel target in the
pathogenesis of bronchopulmonary dysplasia. Eur Respir J.
59:20022482022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hou Y, Zhu L, Tian H, Sun HX, Wang R,
Zhang L and Zhao Y: IL-23-induced macrophage polarization and its
pathological roles in mice with imiquimod-induced psoriasis.
Protein Cell. 9:1027–1038. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nishiga Y, Drainas AP, Baron M,
Bhattacharya D, Barkal AA, Ahrari Y, Mancusi R, Ross JB, Takahashi
N, Thomas A, et al: Radiotherapy in combination with CD47 blockade
elicits a macrophage-mediated abscopal effect. Nat Cancer.
3:1351–1366. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Teijeira A, Garasa S, Ochoa MDC, Cirella
A, Olivera I, Glez-Vaz J, Andueza MP, Migueliz I, Alvarez M,
Rodriguez-Ruiz ME, et al: Differential Interleukin-8 thresholds for
chemotaxis and netosis in human neutrophils. Eur J Immunol.
51:2274–2280. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yasuda K, Takeuchi Y and Hirota K: The
pathogenicity of Th17 cells in autoimmune diseases. Semin
Immunopathol. 41:283–297. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu T, Li S, Ying S, Tang S, Ding Y, Li Y,
Qiao J and Fang H: The IL-23/IL-17 pathway in inflammatory skin
diseases: From bench to bedside. Front Immunol. 11:5947352020.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhou Y, Xu X, Ding J, Jing X, Wang F, Wang
Y and Wang P: Dynamic changes of T-cell subsets and their relation
with tumor recurrence after microwave ablation in patients with
hepatocellular carcinoma. J Cancer Res Ther. 14:40–45. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Takaoka Y, Abe Y, Haraguchi R and Kito K:
Lymphotoxin (TNF-beta). Nihon Rinsho. 68 (Suppl 7):S93–S95.
2010.(In Japanese).
|
|
59
|
Chen Z, Huang Y, Hu Z, Zhao M, Li M, Bi G,
Zheng Y, Liang J, Lu T, Jiang W, et al: Landscape and dynamics of
single tumor and immune cells in early and advanced-stage lung
adenocarcinoma. Clin Transl Med. 11:e3502021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gabrielson A, Wu Y, Wang H, Jiang J,
Kallakury B, Gatalica Z, Reddy S, Kleiner D, Fishbein T, Johnson L,
et al: Intratumoral CD3 and CD8 T-cell densities associated with
relapse-free survival in HCC. Cancer Immunol Res. 4:419–430. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Huff WX, Kwon JH, Henriquez M, Fetcko K
and Dey M: The evolving role of CD8+CD28−
immunosenescent T cells in cancer immunology. Int J Mol Sci.
20:28102019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ren Z, Zhang A, Sun Z, Liang Y, Ye J, Qiao
J, Li B and Fu YX: Selective delivery of low-affinity IL-2 to PD-1+
T cells rejuvenates antitumor immunity with reduced toxicity. J
Clin Invest. 132:e1536042022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Tison A, Garaud S, Chiche L, Cornec D and
Kostine M: Immune-checkpoint inhibitor use in patients with cancer
and pre-existing autoimmune diseases. Nat Rev Rheumatol.
18:641–656. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jiang R and Sun B: IL-22 signaling in the
tumor microenvironment. Adv Exp Med Biol. 1290:81–88. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Rallis KS, Corrigan AE, Dadah H,
Stanislovas J, Zamani P, Makker S, Szabados B and Sideris M: IL-10
in cancer: An essential thermostatic regulator between homeostatic
immunity and inflammation-a comprehensive review. Future Oncol.
18:3349–3365. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Qiao J, Liu Z, Dong C, Luan Y, Zhang A,
Moore C, Fu K, Peng J, Wang Y, Ren Z, et al: Targeting tumors with
IL-10 prevents dendritic cell-mediated CD8+ T cell
apoptosis. Cancer Cell. 35:901–915.e4. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Dromi SA, Walsh MP, Herby S, Traughber B,
Xie J, Sharma KV, Sekhar KP, Luk A, Liewehr DJ, Dreher MR, et al:
Radiofrequency ablation induces antigen-presenting cell
infiltration and amplification of weak tumor-induced immunity.
Radiology. 251:58–66. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Faraoni EY, O'Brien BJ, Strickland LN,
Osborn BK, Mota V, Chaney J, Atkins CL, Cen P, Rowe J, Cardenas J,
et al: Radiofrequency ablation remodels the tumor microenvironment
and promotes neutrophil-mediated abscopal immunomodulation in
pancreatic cancer. Cancer Immunol Res. 11:4–12. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kanegasaki S and Tsuchiya T: Alarmins
released during local antitumor treatments play an essential role
in enhancing tumor growth inhibition at treated and non-treated
sites via a derivative of CCL3. Oncoimmunology. 3:e9589562014.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yin J, Dong J, Gao W and Wang Y: A case
report of remarkable response to association of radiofrequency
ablation with subsequent Atezolizumab in stage IV nonsmall cell
lung cancer. Medicine (Baltimore). 97:e131122018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yu W, Sun J, Wang T and Du Y: The effect
of microwave ablation combined with anti-PD-1 monoclonal antibody
on T cell subsets and long-term prognosis in patients suffering
from non-small-cell lung cancer. Comput Math Methods Med.
2022:70954232022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Huang G, Li W, Meng M, Ni Y, Han X, Wang
J, Zou Z, Zhang T, Dai J, Wei Z, et al: Synchronous microwave
ablation combined with cisplatin intratumoral chemotherapy for
large non-small cell lung cancer. Front Oncol. 12:9555452022.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Feng K and Lu Y: Clinical analysis of
systemic chemotherapy combined with microwave ablation in the
treatment of lung cancer. Asian J Surg. 45:1107–1112. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Shi L, Wang J, Ding N, Zhang Y, Zhu Y,
Dong S, Wang X, Peng C, Zhou C, Zhou L, et al: Inflammation induced
by incomplete radiofrequency ablation accelerates tumor progression
and hinders PD-1 immunotherapy. Nat Commun. 10:54212019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kutob L and Schneider F: Lung cancer
staging. Surg Pathol Clin. 13:57–71. 2020. View Article : Google Scholar : PubMed/NCBI
|