Introduction
The burden of cancer incidence and mortality is
increasing rapidly worldwide. Primary liver cancer (PLC) is ranked
sixth most common among all cancer types worldwide, with 905,677
new cases recorded in 2020, while among the leading causes of
cancer-related mortality, it is ranked third, with 830,180 cases in
2020 (1). The association between
dietary factors and the risk of developing cancer is receiving
increasing attention. The majority of dietary guidelines worldwide
recommend that individuals should consume dairy products. Some
studies have reported the protective role of the consumption of
dairy products in breast and colorectal cancer (2,3).
However, it has also been reported that the consumption of dairy
products may increase the risk of developing prostate cancer
(4). These results indicate that
the consumption of dairy products may exert differential effects on
different cancer sites.
Some studies have explored the association between
the risk of developing PLC and the consumption of dairy products in
the general population (5,6); however, the connection between the two
is not consistent. Previously, two meta-analyses conducted on dairy
product consumption and the risk of developing PLC did not reveal
any substantial connection. However, one of the meta-analyses only
included three cohort studies associated with PLC (5). Although the other meta-analysis
included 15 studies, satisfactory results were still not reported
(6).
The present study performed a comprehensive
meta-analysis to systematically evaluate the association between
the risk of developing PLC and the consumption of dairy products,
including milk, yogurt, cheese and curd. In addition, subgroup
analyses stratified by design, location, duration, size, quality
and adjustment factors were conducted.
Materials and methods
Publication search and study
selection
A search was performed in the literature for
eligible studies published until December 2022 using the PubMed
(https://pubmed.ncbi.nlm.nih.gov/),
Cochrane Library (https://www.cochranelibrary.com/) and Embase
(www.embase.com). The search terms were as
follows: ‘(dairy OR milk OR yogurt OR cheese OR curd)’ AND
‘(primary liver cancer OR primary liver carcinoma OR hepatocellular
carcinoma OR HCC)’. Studies were selected by first reviewing the
titles and abstracts, followed by screening the full text of the
studies that were not excluded. The reference lists were also
searched for additional related literature. The present
meta-analysis included studies which met the following criteria: i)
Case-control studies or cohort studies; ii) studies on dairy
products, including total dairy product, milk, yogurt, cheese and
curd; iii) an outcome of PLC mortality or incidence; and iv) data
on hazard ratio (HR), relative risk (RR) and odds ratio (OR) with
corresponding 95% confidence intervals (CIs) were available.
Studies were excluded if they met the following conditions: i)
Non-human experiments; ii) duplicate studies; iii) reviews,
editorials, comments, letters, reports, interviews or studies
published in languages other than English; or iv) studies with
incomplete data.
Data extraction
The investigators extracted data independently,
according to the Preferred Reporting Items for Systematic reviews
and Meta-Analyses statement (7).
For each study, the following information was obtained: The
author's last name, year of publication, location, follow-up
period, design, patient sex, size of study, the quantity of cases,
dietary assessment, diagnosis approach, outcome, HR or RR or OR
with 95% CI values for the connection between the consumption of
each dairy product and the risk of developing PLC, and adjusted
factors.
Quality assessment
The Newcastle-Ottawa Scale was used to estimate the
quality of studies in the present meta-analysis (8). Each satisfactory answer was worth 1
point, with 9 maximum points. Studies with scores of ≥6 points were
considered of high methodological quality, and those with scores of
<6 points were considered of low quality.
Statistical analysis
The DerSimonian and Laird random-effects models were
used to estimate pooled RR and 95% CI values of the risk of
developing PLC for the highest compared with the lowest consumption
of each type of dairy product, which included total dairy, milk,
yogurt, cheese and curd (9), in
each included study. Subgroup analyses layered by design
(cohort/case-control), location (USA/Europe/Asia), duration (≥5
years/<5 years), size (≥1,500/<1,500) and quality (low/high)
were conducted. In addition, it was examined whether the studies
had considered for key confounders such as alcohol, smoking, body
mass index (BMI), physical activity, diabetes, energy intake, liver
diseases or viruses, and education. The studies were stratified and
analyzed by whether the factors of alcohol, smoking and BMI were
all considered, if all three were considered, it is defined as
using strong adjustments, otherwise weak adjustments were used.
Heterogeneity was assessed by the I2 statistic (10). Sensitivity analyses were conducted
by excluding each dataset one at a time. The Begg's (11) and Egger's (12) tests were conducted to assess
possible publication bias. A two-sided P-value of <0.05 was
regarded to indicate a statistically significant difference.
Stata/MP 14.0 was used for the statistical analyses.
Results
Characteristics of the included
studies
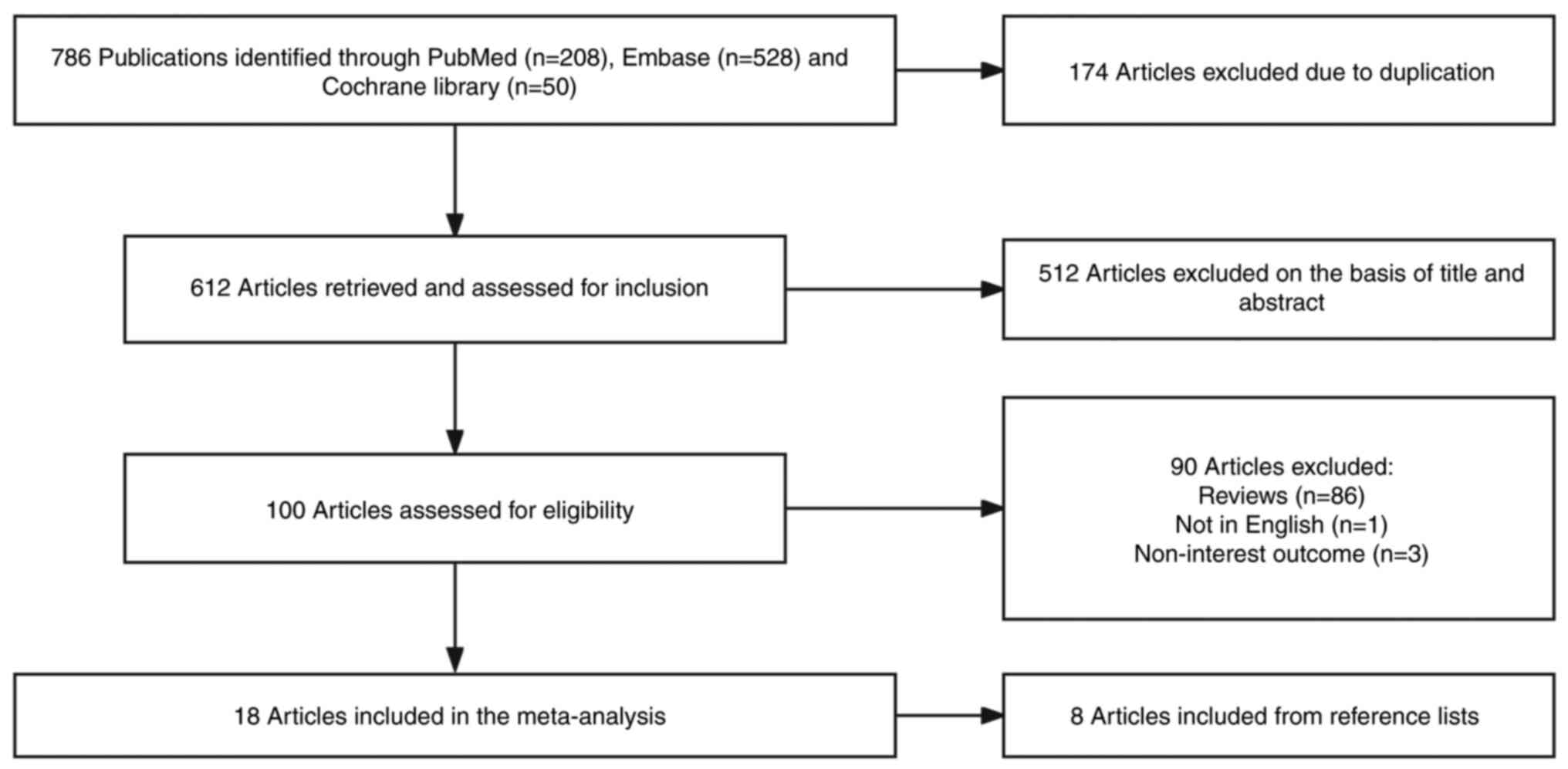
A total of 18 studies (13–30)
with 6,562,714 (ranging from 135 to 3,849,637) participants and
7,970 (ranging from 13 to 3,191) PLC cases were included in the
present study (Fig. 1). The
characteristics of the included studies are presented in Table I. In total, 10 studies were cohort
studies (13–22) and eight studies were case-control
studies (23–30). Notably, three studies were conducted
in the USA (19,21,22),
six studies in Europe (12,16,26–28,30)
and nine studies in Asia (14,15,17,18,20,23–25,29).
The follow-up period of the studies contained ranged from 2 to 32
years, with the follow-up in 10 studies being ≥5 years (13–17,19–22,25).
The majority of studies adjusted for alcohol consumption (n=13)
(13–16,19,21–24,27–30),
10 studies adjusted for smoking consumption (13–16,19,22–24,27,28),
and eight studies adjusted for BMI (13–16,19,21,22,24).
With regard to quality assessment, the studies included in the
present meta-analysis had an average score of 6.5 on a 9-point
scale; even though four studies had scores <6 (17,18,21,28),
which indicates a low quality, other studies had a scores ≥6,
indicating a high methodological quality.
 | Table I.Characteristics of studies included
in the Meta-analysis on the dairy product intake and liver cancer
risk. |
Table I.
Characteristics of studies included
in the Meta-analysis on the dairy product intake and liver cancer
risk.
| First author,
year | Location | Duration,
years | Design | Patient sex | Study size, n | No. of cases | Dietary
assessment | Diagnostic
method | Outcome | Exposures: RR (95%
CI) | Adjusted
variables | Quality score | (Refs.) |
|---|
| Yang et al,
2019 | USA | 32 | Cohort | Both | 144,845 | 164 | FFQ-131 items | Histopathology | HCC incidence | Total dairy
products: 1.85 (1.19-2.88) Milk: 1.23 (0.83-1.83) Yoghurt: 0.72
(0.49-1.05) Total cheese: 0.88 (0.59-1.31) | Age, sex,
ethnicity, physical activity, BMI, smoking, alcohol, total coffee
intake, total calorie intake, aspirin use, type 2 diabetes | 6 | (22) |
| Duarte et
al, 2014 | Europe | 20 | Cohort | Both | 477,206 | 191 | Validated
questionnaire | Histology | HCC incidence | Total dairy
products: 1.66 (1.13-2.43) Total milk: 1.51 (1.02-2.24) Cheese:
1.56 (1.02-2.38) Yoghurt: 0.94 (0.60-1.35) | Age, sex, physical
activity, BMI, smoking, self-reported diabetes status, alcohol,
energy | 7 | (16) |
| Phukan et
al, 2018 | India | 2 | Case-control | Both | 208 | 104 | Questionnaire |
Histopathology/AFP/angiogarphy/sonography/liver/tomography
scan | HCC incidence | Milk: 0.09
(0.01-0.71) Curd: 1.32 (0.22-7.83) | Age, sex, location,
alcohol, ethnicity | 8 | (29) |
| Talamini et
al, 2006 | Italy | 4 | Case-control | Both | 597 | 185 | Validated FFQ |
Histology/cytology/ultrasound/tomography/AFP | HCC incidence | Milk and yoghurt:
0.28 (0.13-0.61) Cheese: 1.31 (0.28-2.96) | Age, sex, centre,
education, place of birth, drinking habits, maximal lifetime
lifetime alcohol intake, hepatitis viruses and total energy
intake | 7 | (30) |
| Kuper et al,
2000 | Greece | 4 | Case-control | Both | 734 | 333 | Validated FFQ |
Biopsy/AFP/echotomography/other
methods | HCC incidence | Milk and dairy
products: 0.70 (0.49-1.01) | Age, sex,
schooling, infection with HBV and/or HCV, alcohol, smoking, total
energy intake, the other food groups | 8 | (27) |
| Park et al,
2010 | USA | 7 | Cohort | Both | 567,169 | 397 | FFQ-124 items | Registry | Primary liver
cancer incidence | Dairy foods: Male,
1.04 (0.72-1.48); female, 1.58 (0.78-3.20) | Ethnicity,
education, marital status, BMI, family history of cancer, physical
activity, menopausal hormone therapy use, alcohol, intake of red
meat, total energy and additional variables | 5 | (21) |
| Fukuda et
al, 1993 | Japan | 7 | Case-control | Both | 853 | 368 | Questionnaire |
Histology/angiography/ultrasonography | HCC incidence | Milk: Male, 1.88
(1.33-2.65); female, 2.63 (1.46-4.72) | Age, sex,
residence, time of hospitalization | 7 | (25) |
| La Vecchia et
al, 1988 | Italy | 4 | Case-control | Both | 1,202 | 151 | Questionnaire | Histology/AFP | HCC incidence | Milk: 0.57
(0.10-3.27) | Age, sex,
residence, | 5 education,
history of hepatitis, alcohol and smoking | (28) |
| Yu et al,
2002 | China | 3 | Case-control | Both | 496 | 248 | Questionnaire | AFP/ultrasonogra
phy/CT/liver function tests/angiography | HCC incidence | Milk: 0.69
(0.15-3.09) | Age, sex,
residence, alcohol, smoking, HBV | 7 | (23) |
| Hirayama, 1989 | Japan | 17 | Cohort | Both | 3,849,637 | 151 | Registry | AFP/liver function
test/biopsy/image | Primary liver
cancer incidence | Milk: 0.93
(0.64-1.35) | Age, sex | 4 | (17) |
| Kurozawa et
al, 2004 | Japan | 3 | Cohort | Both | 110,792 | 401 | FFQ-33 items | Death
certificates | HCC mortality | Milk: 1.79
(1.14-2.80) | Age, sex, history
of liver diseases | 5 | (18) |
| Matsumoyo et
al, 2007 | Japan | 10 | Cohort | Both | 11,606 | 13 | FFQ-30 items | Death
certificates | Primary liver
cancer mortality | Milk: 0.83
(0.27-2.54) | Age, sex | 6 | (20) |
| Chen et al,
2018 | China | 4 | Case-control | Both | 1,440 | 720 | FFQ-79 items |
Biopsy/CT/MRI/AFP | Primary liver
cancer incidence | Dairy: 0.52
(0.45-0.61) | Age, sex, BMI,
physical activity, education, household income, smoking, alcohol,
diabetes, HBV infection, total energy | 7 | (24) |
| Kanazir et
al, 2010 | Serbia | 4 | Case-control | Both | 135 | 45 | Questionnaire | Registry | HCC incidence | Milk: 2.10
(0.90-4.60) Cheese: 1.10 (0.47-3.30) Yoghurt: | Age, sex,
residence, occupation, birth history, HCV or HBV virus, family
history, occupation | 7 | (26) |
| Li et al,
2014 | USA | 12 | Cohort | Both | 494,942 | 509 | FFQ-124 items | Registry | HCC incidence | Dairy: 1.03
(0.99-1.06) | Age, sex, BMI,
ethnicity, smoking, alcohol, activity, education, diabetes | 8 | (19) |
| Kakkoura et
al, 2022 | China | 10.8 | Cohort | Both | 510,146 | 3,191 | Questionnaire | Registry | HCC incidence | Dairy: 1.18
(1.08-1.29) | Education, income,
smoking, alcohol consumption, total physical activity, family
history of cancer, fresh fruit consumption, soy consumption and
BMI | 6 | (14) |
| Wang et al,
2020 | China | 11.5 | Cohort | Both | 18,214 | 130 | Questionnaire | Registry | Primary liver
cancer mortality | Milk: 1.18
(0.72-1.93) | Sex, age, family
income, education, occupation, smoking status, alcohol use,
physical activity, body mass index, self-rated health, diabetes,
hypertension and hyperlipidemia, daily dietary energy intake and
dietary quality | 7 | (15) |
| Guo et al,
2022 | UK | 12 | Cohort | Both | 372,492 | 669 | Questionnaire | Registry | HCC incidence | Cheese: 0.95
(0.72-1.24) | Age, sex,
ethnicity, education level, Townsend Deprivation Index (quartiles),
drinking status, smoking status, exercise, BMI, diabetes | 7 | (13) |
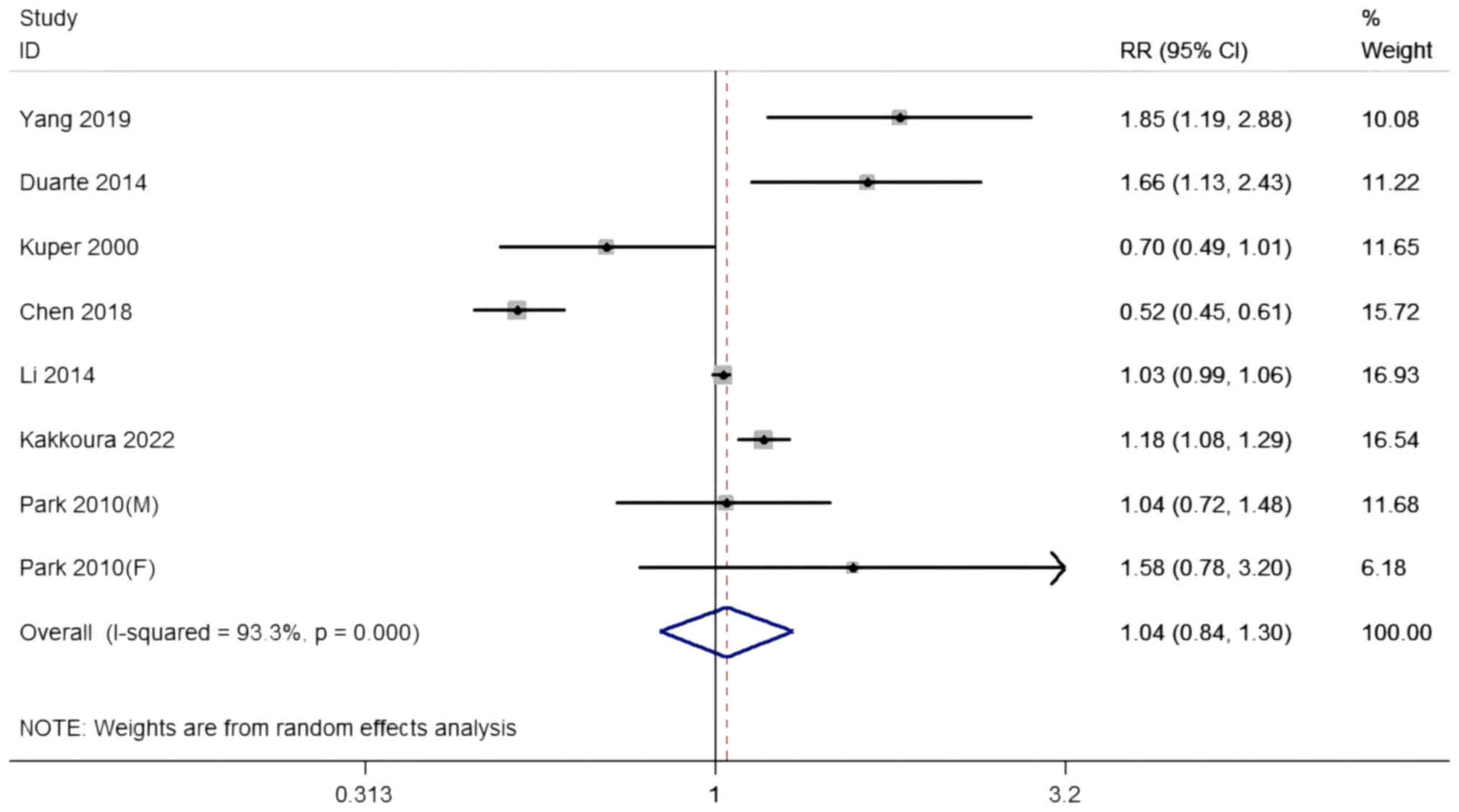
Total dairy consumption and the risk
of developing PLC
A total of five cohort studies and two case-control
studies with eight datasets, including 2,196,482 participants and
5,505 PLC cases, investigated the connection between total dairy
consumption and the risk of developing PLC. The summary RR for the
highest compared with the lowest total dairy intake was 1.04 (95%
CI, 0.84-1.30) with significant heterogeneity among the studies
(I2=93.3% P<0.001; Table
II and Fig. 2). Sensitivity
analyses revealed no visible difference, irrespective of which
dataset was excluded. No effective connections were revealed when
stratified by location, quality or adjustment for covariates, and
meta-regression analysis detected no effective connections
(P-difference ≥0.05 for all contrasts). According to the study
design, duration and study size, pooled RRs were 1.21 in the cohort
studies, ≥5 years and ≥1,500 groups (95% CI, 1.04-1.40), and 0.58
in the case-control studies, <5 years and <1,500 groups (95%
CI, 0.44-0.76). A significant contrary connection was discovered in
the cohort studies, ≥5 years and ≥1,500 groups; six studies were
contained in this analysis (P-difference=0.006).
 | Table II.Pooled RRs of PLC risk for the
highest compared with lowest dairy consumption. |
Table II.
Pooled RRs of PLC risk for the
highest compared with lowest dairy consumption.
| A, Total dairy |
|---|
|
|---|
|
|
|
| Heterogeneity |
|
|---|
|
|
|
|
|
|
|---|
| Characteristic | Studies, n | RR (95% CI) | I2,
% | P-value | P-difference |
|---|
| All studies | 8 | 1.04
(0.84-1.30) | 93.3 | <0.001 |
|
| Design |
|
|
|
|
|
|
Cohort | 6 | 1.21
(1.04-1.40) | 76.0 | 0.001 |
|
|
Case-control | 2 | 0.58
(0.44-0.76) | 54.7 | 0.138 | 0.006 |
| Location |
|
|
|
|
|
|
USA | 4 | 1.22
(0.93-1.61) | 62.9 | 0.044 | 0.519 |
|
Asia | 2 | 0.79
(0.35-1.75) | 98.8 | <0.001 |
|
|
Europe | 2 | 1.08
(0.46-2.51) | 90.3 | 0.001 | 0.838 |
| Duration,
years |
|
|
|
|
|
| ≥5 | 6 | 1.21
(1.04-1.40) | 76.0 | 0.001 | 0.006 |
|
<5 | 2 | 0.58
(0.44-0.76) | 54.7 | 0.138 |
|
| Size, n |
|
|
|
|
|
|
≥1,500 | 6 | 1.21
(1.04-1.40) | 76.0 | 0.001 |
|
|
<1,500 | 2 | 0.58
(0.44-0.76) | 54.7 | 0.138 | 0.006 |
| Quality |
|
|
|
|
|
|
Low | 2 | 1.14
(0.81-1.61) | 6.5 | 0.301 |
|
|
High | 6 | 1.01
(0.79-1.29) | 95.2 | <0.001 | 0.671 |
| Adjustment for
covariates |
|
|
|
|
|
| Strong
adjustment | 5 | 1.07
(0.82-1.41) | 96.0 | <0.001 | 0.786 |
| Weak
adjustment | 3 | 0.97
(0.65-1.45) | 59.1 | 0.087 |
|
|
| B, Milk |
|
|
|
|
|
Heterogeneity |
|
|
|
|
|
|
|
|
Characteristic | Studies,
n | RR (95%
CI) | I2,
% | P-value |
P-difference |
|
| All studies | 13 | 1.19
(0.88-1.62) | 70.2 | <0.001 |
|
| Design |
|
|
|
|
|
|
Cohort | 6 | 1.26
(1.02-1.56) | 20.9 | 0.276 |
|
|
Case-control | 7 | 0.93
(0.44-1.96) | 81.7 | <0.001 | 0.682 |
| Location |
|
|
|
|
|
|
USA | 1 | 1.23
(0.83-1.83) | - | - | 0.735 |
|
Asia | 8 | 1.32
(0.90-1.92) | 67.4 | 0.003 | 0.584 |
|
Europe | 4 | 0.90
(0.35-2.32) | 82.7 | 0.001 |
|
| Duration,
years |
|
|
|
|
|
| ≥5 | 7 | 1.40
(1.07-1.82) | 56.2 | 0.033 | 0.216 |
|
<5 | 6 | 0.70
(0.28-1.74) | 80.1 | <0.001 |
|
| Size, n |
|
|
|
|
|
|
≥1,500 | 6 | 1.26
(1.02-1.56) | 20.9 | 0.276 | 0.682 |
|
<1,500 | 7 | 0.93
(0.44-1.96) | 81.7 | <0.001 |
|
| Quality |
|
|
|
|
|
|
Low | 3 | 1.18
(0.67-2.10) | 63.9 | 0.063 |
|
|
High | 10 | 1.17
(0.80-1.73) | 73.5 | <0.001 | 0.997 |
| Adjustment for
covariates |
|
|
|
|
|
| Strong
adjustment | 3 | 1.32
(1.03-1.68) | 0.0 | 0.681 | 0.693 |
| Weak
adjustment | 10 | 1.07
(0.68-1.71) | 77.2 | <0.001 |
|
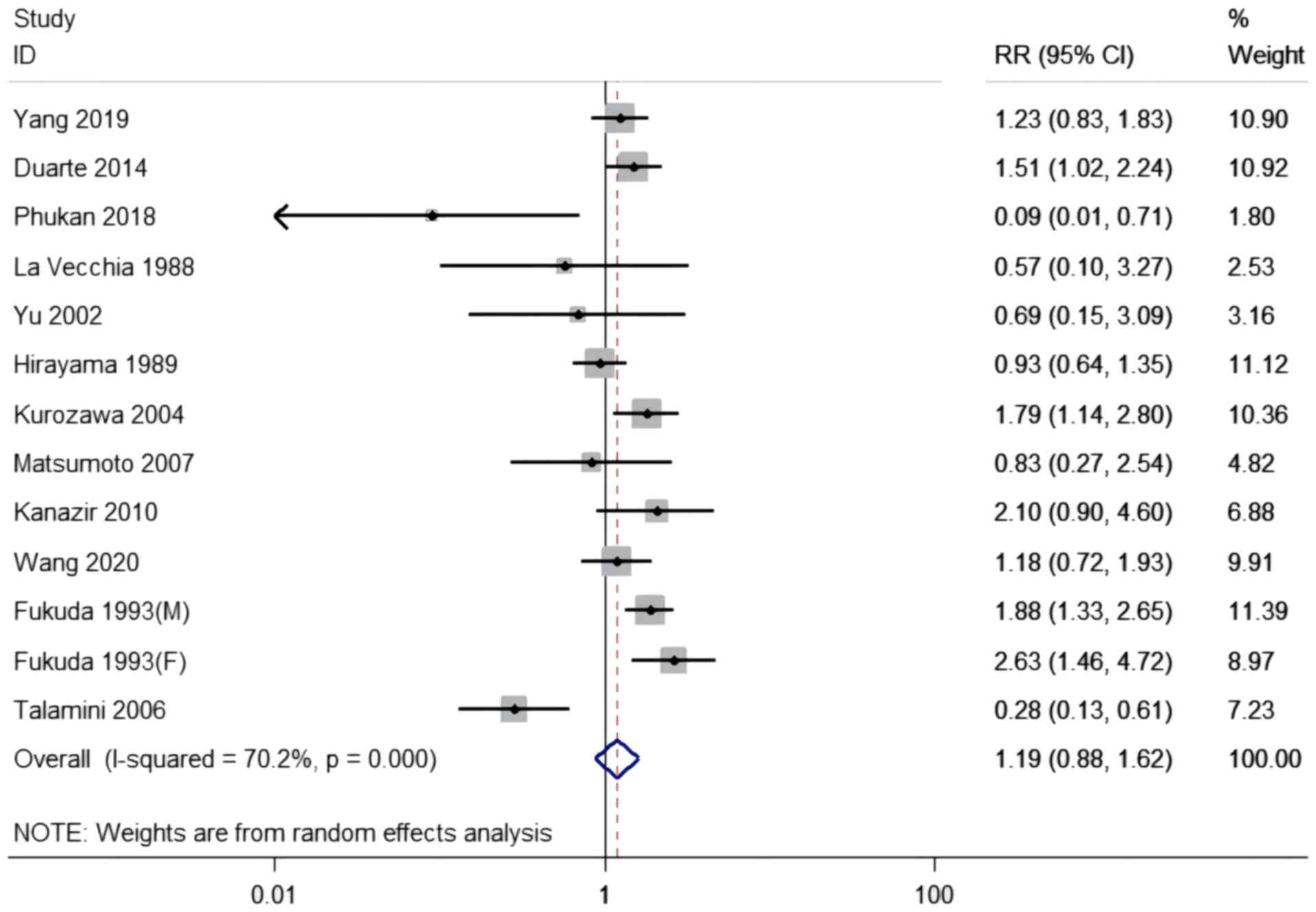
Milk consumption and risk of
developing PLC
The connection between milk consumption and the risk
of developing PLC was evaluated in six case-control studies and six
cohort studies with 13 datasets, including 4,615,791 participants
and 2,151 cases. The pooled RR for the highest consumption compared
with the lowest was 1.19 (95% CI, 0.88-1.62), with moderate
heterogeneity among the studies (I2=70.2, P<0.001;
Table II and Fig. 3). An increased risk of developing
PLC was observed in the cohort studies (RR, 1.26; 95% CI,
1.02-1.56), but not in the case-control studies (RR, 0.93; 95% CI,
0.44-1.96). With regard to duration, a significant association was
observed for ≥5 years (RR, 1.40; 95% CI, 1.07-1.82), but not for
<5 years. With regard to the size of the studies, a size ≥1,500
exhibited a significant association (RR, 1.26; 95% CI, 1.02-1.56).
Studies that used strong adjustments were associated with an
increased risk (RR, 1.32; 95% CI, 1.03-1.68), but not those that
used weak adjustments. There were no effective connections when the
analysis was stratified by location or quality, and meta-regression
analysis revealed no significant discrepancy (P-difference ≥0.05
for all comparisons). In the sensitivity analysis, a significant
association was found between the highest compared with the lowest
consumption when the study by Talamini in 2006 (30) was removed (RR, 1.38; 95% CI,
1.07-1.77) (I2=54.2%, P=0.013); however, no significant
associations were found with the removal of any of the other
studies.
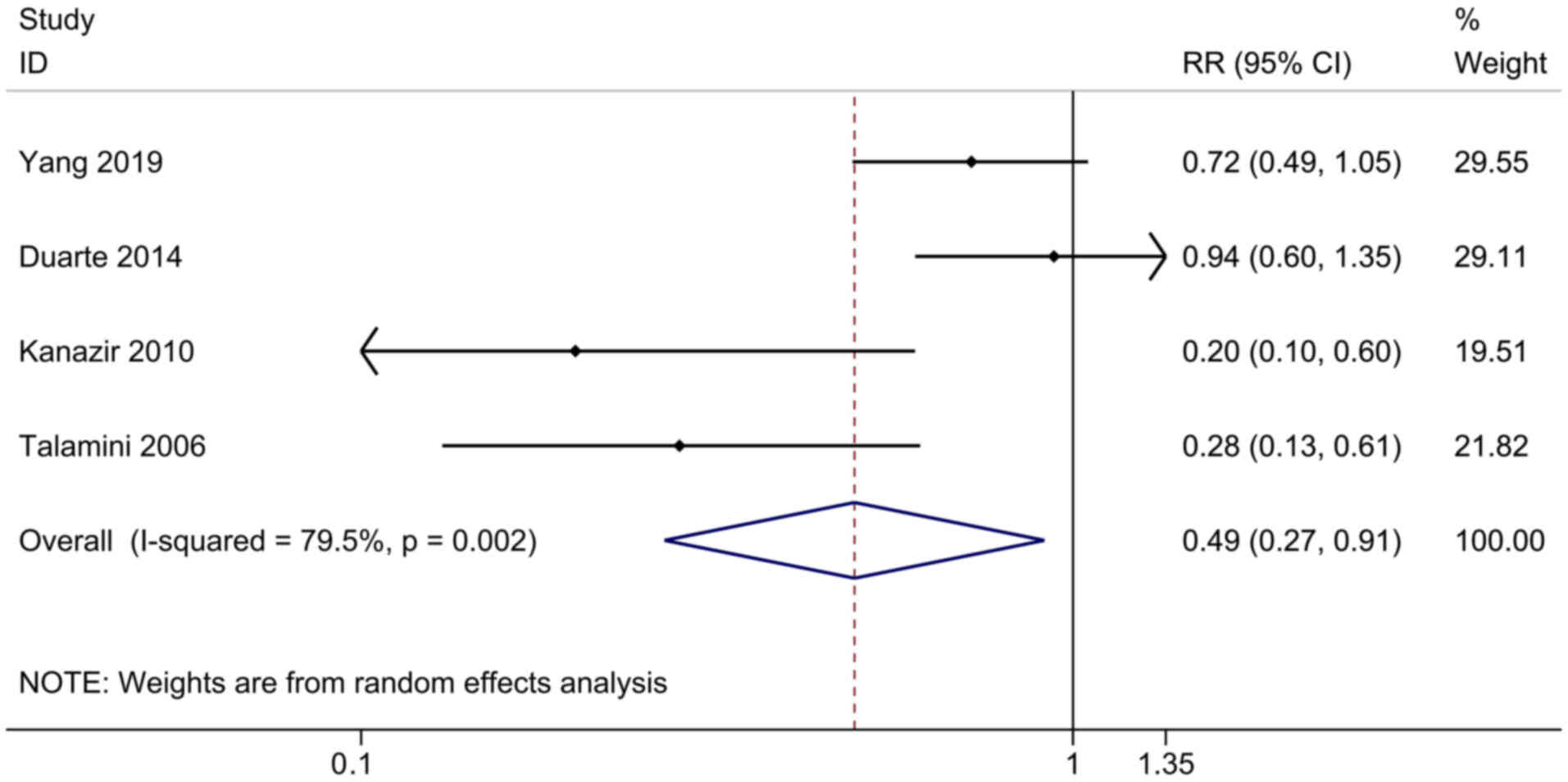
Yogurt consumption and the risk of
developing PLC
The connection between yogurt consumption and the
risk of developing PLC was evaluated in two cohort studies and two
case-control studies, including 622,783 participants and 585 cases.
The pooled RR for the highest consumption compared with the lowest
was 0.49 (95% CI, 0.27-0.91), and exhibited a high heterogeneity
among the studies (I2=79.5%, P=0.002; Fig. 4). The results of sensitivity
analysis were stable. Further subgroup and meta-regression analyses
were not executed, as only four studies were included in this
sector.
Cheese and curd consumption and the
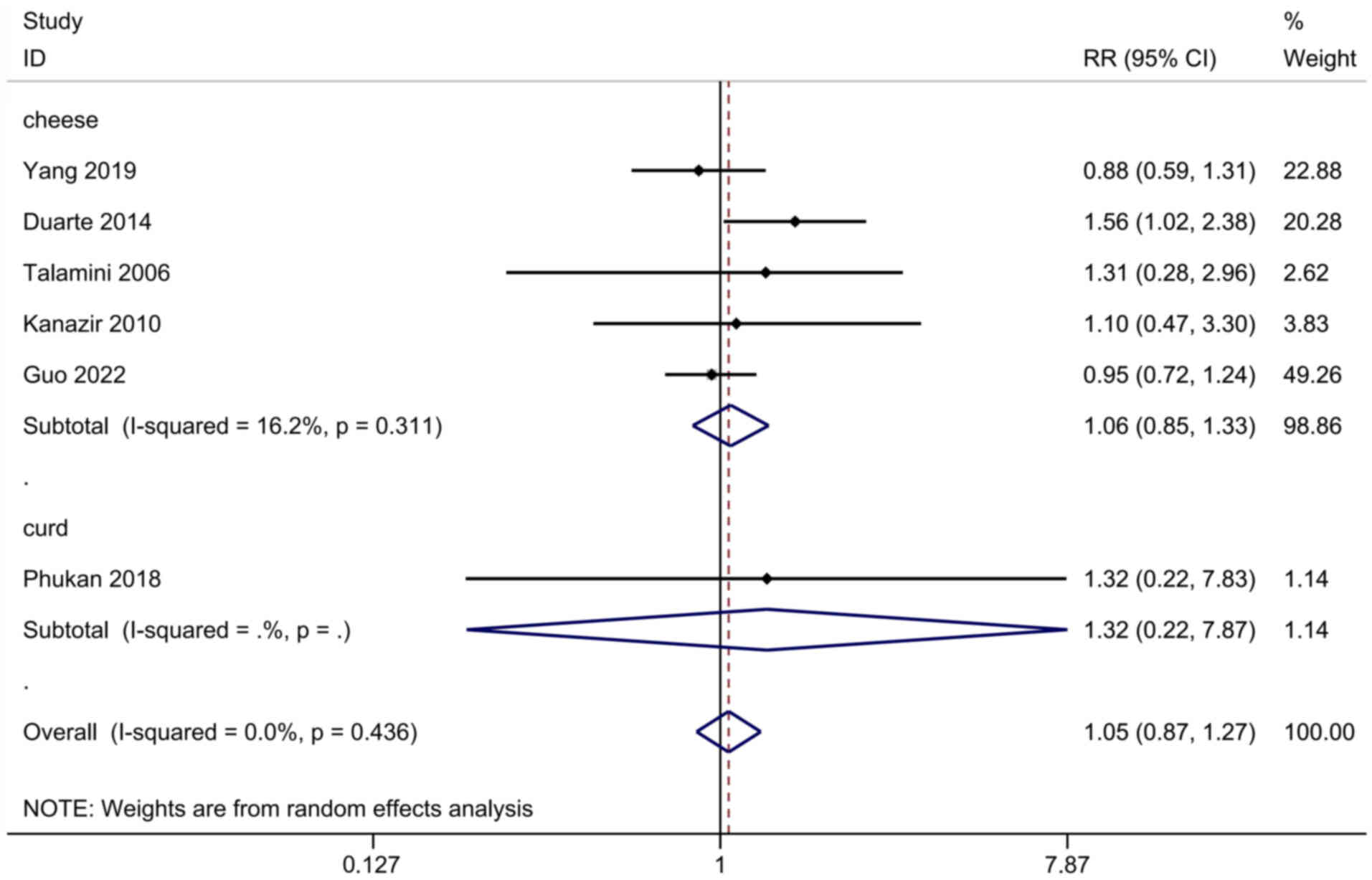
risk of developing PLC
The association between cheese or curd consumption
and the risk of developing PLC was assessed in six studies,
including 995,483 participants and 1,358 cases. The pooled RR for
all studies was 1.05 (95% CI, 0.87-1.27), and exhibited no
heterogeneity (I2=0.0%, P=0.436; Fig. 5). No subgroup or meta-regression
analyses were performed due to the insufficient numbers of
studies.
Publication bias
Begg's (P>0.07 in all analyses) and Eggers' tests
(P>0.1 in all analyses) of the risk of developing PLC for total
dairy and milk consumption revealed no evidence of publication
bias.
Discussion
In the present meta-analysis, the potential
connections between several types of dairy product consumption and
the risk of developing PLC were examined. Compared with a low level
of consumption, a high level of milk consumption was related to a
higher risk of developing PLC. By contrast, the highest type of
yogurt consumption was associated with a decreased risk of
developing PLC. There was no effective connection between the
consumption of total dairy, cheese and curd, and the risk of
developing PLC.
Previous meta-analyses have assessed the connection
between the risk of developing PLC and the highest compared with
the lowest consumption of dairy products (5,6).
However, one of the meta-analyses only included studies designed as
cohort studies. With regard to milk consumption, the meta-analysis
revealed no effective connection between milk consumption and the
risk of developing PLC (5). Another
meta-analysis reported that yogurt consumption may play a
protective role, but revealed no effective connection between the
risk of developing PLC and total dairy product, milk, cheese and
curd consumption (6). In the
present study, the analysis of Zhao et al (6) is updated by adding several recent
studies on the risk of developing PLC and the consumption of dairy
products to further validate these results. The study succeeded in
finding a link between high milk consumption and higher PLC risk.
The present analysis can therefore provide some implications for
the dietary guidelines on milk consumption, promote people to look
at milk consumption from a new angle and promote more research
related to it.
In the present analysis, it was observed that milk
consumption was connected with an increased risk of developing PLC
by conducting sensitivity analysis. Talamini et al (30) combined milk with yogurt when sorting
types of dairy products; with regard to the association of yogurt
consumption with the risk of developing PLC, it is possible that
the presence of yogurt interfered with the result. Milk, as a
health food, plays a critical role throughout the life of an
individual. Milk provides essential nutrients and is a main source
of natural bioactive ingredients (31); it is the most abundant and least
expensive provider of protein of high nutritional quality,
phosphorus, calcium and vitamin A (32). Recent research has reported that the
consumption of dairy products appears to be beneficial in building
muscle, decreasing blood pressure and low-density lipoprotein
cholesterol levels, and preventing tooth decay, cancer, diabetes
and obesity (33). With regard to
cancer prevention, it has been reported that milk consumption can
decrease the risk of developing certain cancer types, such as
colorectal, breast and bladder cancer (34), whereas it can increase the risk of
developing prostate cancer (4).
Milk consumption can increase circulating IGF-I levels (35), and high IGF-I levels have been shown
to be associated with an increased risk of cancer, such as prostate
and breast cancer (36). The
translocation of IGF-I receptor to the endoplasmic reticulum
enhances the activity of sarco-endoplasmic reticulum calcium ATPase
2, thus stimulating PLC growth (37). Furthermore, a previous study
reported that IGF-1 facilitates the growth and metastasis of
hepatocellular carcinoma by inhibiting the degradation of
proteasome-mediated cathepsin B (38). Another study reported that IGF-I and
branched-chain amino acids from milk can cause PLC by
overactivating mTORC1 (39). These
are the potential mechanisms by which non-fermented dairy products
cause PLC based on the current literature, and further studies are
necessary to elucidate the implicated mechanism(s). Although
evidence of high heterogeneity among studies was indicated by a
meta-analysis of milk consumption and the risk of developing PLC in
the present study, the heterogeneity was reduced when classified by
the type of cohort, a duration of ≥5 years, a study size of ≥1,500
and strong adjustment for covariates.
Notably, there are still other dietary factors that
may affect the statistical results, such as consumption of
vegetables, fruits, coffee and tea, among others; however, it is
not possible to completely control for these dietary factors. Since
the objects included in the study were randomly selected, it was
assumed that the dietary habits were similar to those in the same
location, and a stratified analysis was conducted according to the
location to investigate whether they had an impact on the results.
The data were also stratified by location, as food safety risks
vary by populations of different ethnicities and income levels
(40). However, the results
obtained did not show any significant difference, so at this time
we consider that other dietary consumption does not have
significant effect on the conclusion of high milk consumption
linking with higher PLC risk. However, the impact of other dietary
factors still deserves some attention.
In the present meta-analysis, yogurt consumption was
found to be associated with a decreased risk of developing PLC.
Yogurt is a nutritious food, as it contains high-quality protein
and calcium, as well as other mineral substances, such as iodine,
potassium, magnesium, vitamins A and D, and several of the B
vitamins (41). Yogurt also
contains probiotics, the most common of which are
Lactobacillus and Bifidobacterium (42). Probiotics can enhance the
non-specific cellular immune response, which is characterized by
activation of macrophages, natural killer cells and
antigen-specific cytotoxic T-lymphocytes, and the release of
various cytokines, in a strain-specific and dose-dependent manner
(43). Zhang et al (42) conducted a meta-analysis that
demonstrated that yogurt consumption was associated with an overall
decreased risk of developing cancer.
The present meta-analysis has several advantages.
First, previous epidemiological studies (14,16,22)
have clarified the connection between the consumption of dairy
products and the risk of developing PLC. On this basis, the present
meta-analysis combined and analyzed the data from these studies,
thus providing firm evidence. Second, the meta-regression and
subgroup analyses were conducted using the variables of design,
location, duration, size, quality and other potential confounding
factors, in order to explore the underlying heterogeneity. Third,
Begg's and Egger's tests were used, and the results revealed that
no publication bias excited in the analysis.
Regardless of these advantages, the present
meta-analysis has certain limitations, which should be mentioned.
First, the analysis was performed on the basis of observational
studies, which cannot completely account for the unmeasured or
confounding factors. The present meta-analysis also combined cohort
and case-control studies; among these, there may be selection and
recall bias in case-control studies. However, due to the limited
amount of cohort studies, the case-control studies were not
excluded from the meta-analysis. Second, the multivariate adjusted
RR was extracted; however, only a few studies had considered key
confounding factors, such as physical activity, diabetes, liver
disease, liver viruses, energy intake or education. Third, the
stratified levels of the highest and the lowest consumption of each
dairy product across the studies differed. Fourth, although sex,
age and other factors were taken into account when the data was
extracted, most of the included literature did not distinguish
these for analysis, so the study failed to explore the relationship
between these basic characteristics of the population and PLC
caused by dairy consumption through subgroup analysis of these
potential influencing factors. Furthermore, due to the limited
amount of data, only five studies demonstrated an association with
cheese and four studies an association with yogurt.
In conclusion, in the present meta-analysis, in
comparison with low milk consumption, high milk consumption was
found to be associated with an increased risk of developing PLC.
However, high yogurt consumption was shown to be associated with a
decreased risk of developing PLC. Further well-designed studies are
warranted, however, to further analyze the connection between each
type of dairy product and the risk of developing PLC.
Acknowledgements
Not applicable.
Funding
The present study received funding from the 2023 Medical Science
Project Plan of Hebei Province (grant no. 20231865).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JD and TY designed the study and formulated the
search strategy. JD performed data collection and statistical
analysis, and wrote the original manuscript. TY inspected the
literature, and reviewed and edited the manuscript. JD, TY and LC
were responsible for data interpretation and critical revision of
important content, and have read and approved the final manuscript.
JD, TY and LC confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
He Y, Tao Q, Zhou F, Si Y, Fu R, Xu B, Xu
J, Li X and Chen B: The relationship between dairy products intake
and breast cancer incidence: A Meta-analysis of observational
studies. BMC Cancer. 21:11092021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lumsden AL, Mulugeta A and Hyppönen E:
Milk consumption and risk of twelve cancers: A large-scale
observational and Mendelian randomisation study. Clin Nutr. 42:1–8.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aune D, Navarro Rosenblatt DA, Chan DS,
Vieira AR, Vieira R, Greenwood DC, Vatten LJ and Norat T: Dairy
products, calcium, and prostate cancer risk: A systematic review
and Meta-analysis of cohort studies. Am J Clin Nutr. 101:87–117.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jin S and Je Y: Dairy consumption and
total cancer and cancer-specific mortality: A Meta-analysis of
prospective cohort studies. Adv Nutr. 13:1063–1082. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao Q, He Y, Wang K, Wang C, Wu H, Gao L,
Hu A, Yang W and Wang S: Dairy consumption and liver cancer risk: A
systematic review and dose-response meta-analysis of observational
studies. Nutr Cancer. 73:2821–2831. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wells GA, Shea B, O'Connell D, Peterson J,
Welch V, Losos M and Tugwell P: The Newcastle-Ottawa Scale (NOS)
for assessing the quality of nonrandomised studies in
meta-analyses. The Ottawa Hospital. http://www.ohri.ca/programs/clinical_
epidemiology/oxford.aspMarch 30–2021
|
|
9
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Begg CB and Mazumdar M: Operating
characteristics of a rank correlation test for publication bias.
Biometrics. 50:1088–1101. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in Meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo W, Ge X, Lu J, Xu X, Gao J, Wang Q,
Song C, Zhang Q and Yu C: Diet and risk of non-alcoholic fatty
liver disease, cirrhosis, and liver cancer: A large prospective
cohort study in UK Biobank. Nutrients. 14:53352022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kakkoura MG, Du H, Guo Y, Yu C, Yang L,
Pei P, Chen Y, Sansome S, Chan WC, Yang X, et al: Dairy consumption
and risks of total and site-specific cancers in Chinese adults: An
11-year prospective study of 0.5 million people. BMC Med.
20:1342022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang XJ, Jiang CQ, Zhang WS, Zhu F, Jin
YL, Woo J, Cheng KK, Lam TH and Xu L: Milk consumption and risk of
mortality from all-cause, cardiovascular disease and cancer in
older people. Clin Nutr. 39:3442–3451. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Duarte-Salles T, Fedirko V, Stepien M,
Trichopoulou A, Bamia C, Lagiou P, Lukanova A, Trepo E, Overvad K,
Tjønneland A, et al: Dairy products and risk of hepatocellular
carcinoma: The European prospective investigation into cancer and
nutrition. Int J Cancer. 135:1662–1672. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hirayama T: A large-scale cohort study on
risk factors for primary liver cancer, with special reference to
the role of cigarette smoking. Cancer Chemother Pharmacol. 23
(Suppl):S114–S117. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kurozawa Y, Ogimoto I, Shibata A, Nose T,
Yoshimura T, Suzuki H, Sakata R, Fujita Y, Ichikawa S, Iwai N, et
al: Dietary habits and risk of death due to hepatocellular
carcinoma in a large scale cohort study in Japan. Univariate
analysis of JACC study data. Kurume Med J. 51:141–149. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li WQ, Park Y, McGlynn KA, Hollenbeck AR,
Taylor PR, Goldstein AM and Freedman ND: Index-based dietary
patterns and risk of incident hepatocellular carcinoma and
mortality from chronic liver disease in a prospective study.
Hepatology. 60:588–597. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsumoto M, Ishikawa S, Nakamura Y,
Kayaba K and Kajii E: Consumption of dairy products and cancer
risks. J Epidemiol. 17:38–44. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park Y, Leitzmann MF, Subar AF, Hollenbeck
A and Schatzkin A: Dairy food, calcium, and risk of cancer in the
NIH-AARP Diet and Health Study. Arch Intern Med. 169:391–401. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang W, Sui J, Ma Y, Simon TG, Chong D,
Meyerhardt JA, Willett WC, Giovannucci EL, Chan AT and Zhang X: A
prospective study of dairy product intake and the risk of
hepatocellular carcinoma in U.S. men and women. Int J Cancer.
146:1241–1249. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu SZ, Huang XE, Koide T, Cheng G, Chen
GC, Harada K, Ueno Y, Sueoka E, Oda H, Tashiro F, et al: Hepatitis
B and C viruses infection, lifestyle and genetic polymorphisms as
risk factors for hepatocellular carcinoma in Haimen, China. Jpn J
Cancer Res. 93:1287–1292. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen PY, Fang AP, Wang XY, Lan QY, Liao
GC, Liu ZY, Zhang DM, Zhang YY, Chen YM and Zhu HL: Adherence to
the Chinese or American dietary guidelines is associated with a
lower risk of primary liver cancer in China: A case-control study.
Nutrients. 10:11132018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fukuda K, Shibata A, Hirohata I, Tanikawa
K, Yamaguchi G and Ishii M: A hospital-based case-control study on
hepatocellular carcinoma in Fukuoka and Saga Prefectures, northern
Kyushu, Japan. Jpn J Cancer Res. 84:708–714. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kanazir M, Boricic I, Delic D, Tepavcevic
DK, Knezevic A, Jovanovic T and Pekmezovic T: Risk factors for
hepatocellular carcinoma: A case-control study in Belgrade
(Serbia). Tumori. 96:911–917. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kuper H, Tzonou A, Lagiou P, Mucci LA,
Trichopoulos D, Stuver SO and Trichopoulou A: Diet and
hepatocellular carcinoma: A case-control study in Greece. Nutr
Cancer. 38:6–12. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
La Vecchia C, Negri E, Decarli A, D'Avanzo
B and Franceschi S: Risk factors for hepatocellular carcinoma in
northern Italy. Int J Cancer. 42:872–876. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Phukan RK, Borkakoty BJ, Phukan SK,
Bhandari K, Mahanta J, Tawsik S, Bhandari S, Rai A and Narain K:
Association of processed food, synergistic effect of alcohol and
HBV with Hepatocellular Carcinoma in a high incidence region of
India. Cancer Epidemiol. 53:35–41. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Talamini R, Polesel J, Montella M, Dal
Maso L, Crispo A, Tommasi LG, Izzo F, Crovatto M, La Vecchia C and
Franceschi S: Food groups and risk of hepatocellular carcinoma: A
multicenter case-control study in Italy. Int J Cancer.
119:2916–2921. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin T, Meletharayil G, Kapoor R and
Abbaspourrad A: Bioactives in bovine milk: Chemistry, technology,
and applications. Nutr Rev. 79 (Suppl 2):S48–S69. 2021. View Article : Google Scholar
|
|
32
|
Kwon Y, Lee SW, Cho YS, Jeong SJ and Han
MY: Is high milk intake good for children's health? A National
population-based observational cohort study. Nutrients.
13:34942021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tunick MH and Van Hekken DL: Dairy
products and health: Recent insights. J Agric Food Chem.
63:9381–9388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Larsson SC, Mason AM, Kar S, Vithayathil
M, Carter P, Baron JA, Michaëlsson K and Burgess S: Genetically
proxied milk consumption and risk of colorectal, bladder, breast,
and prostate cancer: A two-sample Mendelian randomization study.
BMC Med. 18:3702020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qin LQ, He K and Xu JY: Milk consumption
and circulating insulin-like growth factor-I level: A systematic
literature review. Int J Food Sci Nutr. 60 (Suppl 7):S330–S340.
2009. View Article : Google Scholar
|
|
36
|
Renehan AG, Zwahlen M, Minder C, O'Dwyer
ST, Shalet SM and Egger M: Insulin-like growth factor (IGF)-I, IGF
binding protein-3, and cancer risk: Systematic review and
meta-regression analysis. Lancet. 363:1346–1353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Y, Li K, Pan T, Xie Q, Cheng Y, Wu X,
Xu R, Liu X, Liu L, Gao J, et al: Translocation of IGF-1R in
endoplasmic reticulum enhances SERCA2 activity to trigger
Ca2+ER perturbation in hepatocellular
carcinoma. Acta Pharm Sin B. 13:3744–3755. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lei T and Ling X: IGF-1 promotes the
growth and metastasis of hepatocellular carcinoma via the
inhibition of proteasome-mediated cathepsin B degradation. World J
Gastroenterol. 21:10137–10149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Melnik BC: Lifetime impact of Cow's milk
on overactivation of mTORC1: From fetal to childhood overgrowth,
acne, diabetes, cancers, and neurodegeneration. Biomolecules.
11:4042021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Signs RJ, Darcey VL, Carney TA, Evans AA
and Quinlan JJ: Retail food safety risks for populations of
different races, ethnicities, and income levels. J Food Prot.
74:1717–1723. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Donovan SM and Goulet O: Introduction to
the Sixth global summit on the health effects of yogurt: Yogurt,
more than the sum of its parts. Adv Nutr. 10:913S–916S. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang K, Dai H, Liang W, Zhang L and Deng
Z: Fermented dairy foods intake and risk of cancer. Int J Cancer.
144:2099–2108. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ashraf R and Shah NP: Immune system
stimulation by probiotic microorganisms. Crit Rev Food Sci Nutr.
54:938–956. 2014. View Article : Google Scholar : PubMed/NCBI
|