Introduction
Acute promyelocytic leukemia (APL) represents ~10%
of all childhood acute myeloid leukemia (AML) cases (1,2).
Cytomorphologically, APL is classified as hypergranular (or typical
M3) or as hypogranular (or variant M3V) (3). APL presents a balanced reciprocal
translocation t(15;17)(q24.1;q21.2), involving the promyelocytic
leukemia (PML) gene on chromosome 15 and the retinoic acid
receptor α (RARA) gene on chromosome 17 (4,5). There
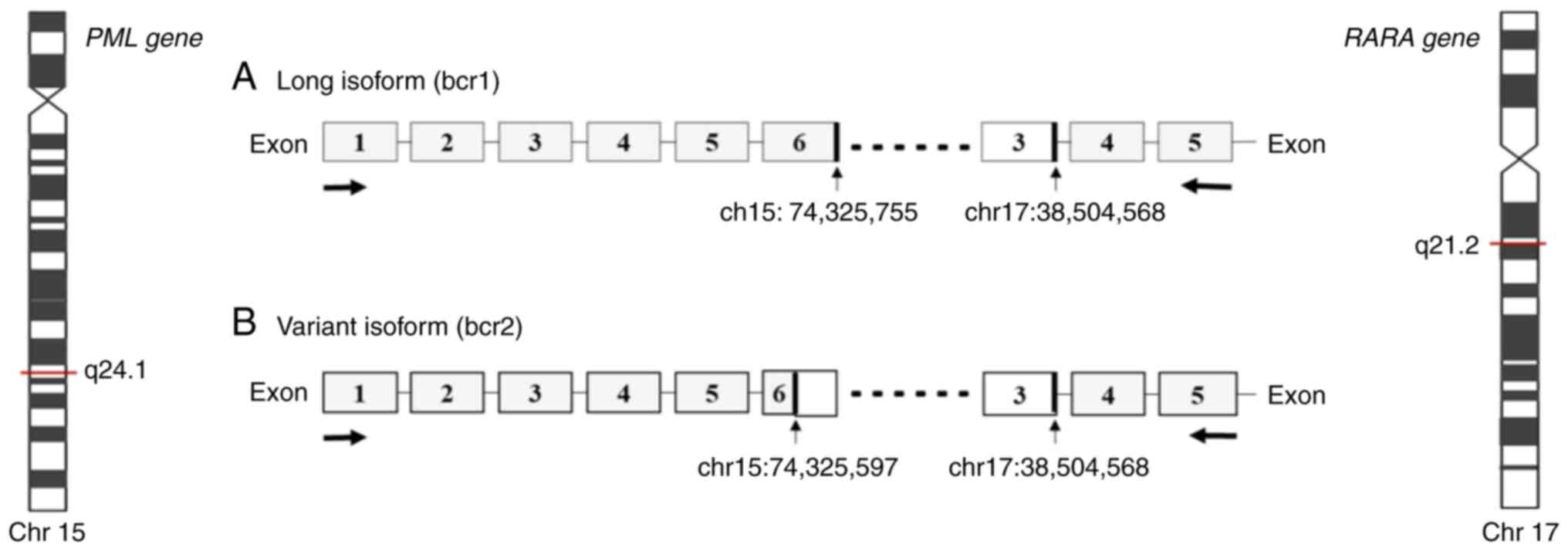
are three distinct PML breakpoint cluster regions, namely
bcr1, bcr2 and bcr3, that generate a PML/RARA fusion gene of
varying mRNA lengths. The bcr1 transcript results from a break in
intron 6 of PML with exon 3 of RARA; transcript bcr2
results from a break in exon 6 of PML with exon 3 of
RARA; transcript bcr3 results from a break in intron 3 of
PML with exon 3 of RARA (6). The frequency of the different
breakpoints is dependent principally on ethnicity and geographical
location as the bcr1 transcript is more frequent among individuals
of Latin origin (3). The
aforementioned transcripts have been detected using reverse
transcription-quantitative PCR (RT-qPCR) (7,8). The
PML/RARA fusion gene generates a PML/RARA oncoprotein that
blocks myeloid precursor differentiation, leading to the
accumulation of abnormal promyelocytes in the bone marrow (9,10). The
PML domain of the PML/RARA oncoprotein can affect the senescence
pathway, facilitating the acquisition of mutations that drive the
development of leukemia (9–11). Investigations involving children and
adults have provided information on the typical and atypical
transcripts of PML/RARA. Typical transcripts are the most
common (identified in 90–95% of cases) and have three breakpoints
in intron 6, exon 6 and intron 3 of the PML gene and only
one in intron 2 of the RARA gene. Atypical transcripts have
breakpoints in intron 4, exon 6 or exon 7 of the PML gene,
and in intron 3 of the RARA gene. However, the biological
function of atypical transcripts in the initiation and evolution of
APL have yet to be defined (11).
The present study aimed to describe a novel pediatric case that
presents atypical bcr1, bcr2 and bcr3 transcripts, as well as the
clinical and molecular aspects present in this APL case. To the
best of our knowledge, the present study is the first to identify
the three aforementioned atypical PML/RARA transcripts.
Materials and methods
Case report
A 12-year-old female patient was admitted in March
2018 to the Civil Hospital of Guadalajara (Guadalajara, Mexico)
with gingival bleeding, hyperplasia, petechiae, ecchymosis,
paleness and traces of bleeding in the oral cavity, with no lymph
node enlargement, hepatomegaly or splenomegaly. Laboratory analyses
revealed the following: Hemoglobin levels, 10.8 g/dl (normal range,
12–16 g/dl); leucocytes, 2,670/µl (normal range, 5,000-10,000/µl);
platelets, 8,000/µl (normal range, 150,000-400,000/µl); prothrombin
time, 13.1 sec (normal range, 9–13 sec); activated partial
thromboplastin time, 25.7 sec (normal range, 26–40 sec); and
D-Dimer levels, >1,500 ng/ml (normal range, 340–729 ng/ml). Bone
marrow aspiration revealed that 98% of nucleated cells were
replaced by myeloblasts, that hypergranular promyelocytes were
densely packed, bright-pink, reddish-blue or dark-purple granules,
and that there were numerous Auer rods. Immunophenotyping revealed
a population of 91% of promyeloblasts, which was CD13+,
human leukocyte antigen (HLA)-DR−, CD38+,
CD117+ and CD45+. All the aforementioned data
were compatible with Acute Myelomonocytic Leukemia or
French-American-British (FAB) M3 classification (12). The patient was staged at
intermediate risk according to the PETHEMA APL 2012 protocol
proposed by Spanish Society of hematology and hemotherapy (13). The patient achieved remission on day
50 after receiving consolidation therapy with three chemotherapy
cycles, which was maintained for 2 years according to the protocol.
The patient completed treatment 4 years ago (2018–2022). The
patient has a good prognosis and continues to be followed up.
Ethical considerations
The present study was submitted and accepted by The
Research Committee and The Research Ethics Committee of The Civil
Hospital of Guadalajara (approval no. 00116). Bone marrow aspirates
from the patient and reference (wild-type control) were obtained
prior to treatment. Written informed consent was obtained from the
parents and the institutional review boards approved the use of
excess diagnostic material for research purposes. These studies
were conducted in accordance with the Declaration of Helsinki.
Karyotyping and fluorescence in situ
hybridization (FISH) analysis
A bone marrow sample of the patient was obtained and
G-banding karyotyping was performed. FISH analysis was then
performed for the detection of the translocation,
t(15;17)(q24.1;q21.2). Cells were dropped onto glass slides to
perform the FISH assays, which were conducted following the
manufacturer's recommendations. Images were captured using an AXIO
ImagerMI (Zeiss AG) microscope, and the images were analyzed using
ISIS software (MetaSystems). A total of 200 interphase cells were
reviewed in each slide. The PML and RARA genes were analyzed using
a Vysis LSI PML/RARA Dual Color probe, Dual Fusion
Translocation Probe (cat. no. 05J70-001; Abbott Molecular, Inc.).
For the dual-color probe, cells with 1 orange, 1 green and 2 fusion
signals were considered positive for the PML/RARA fusion.
RNA isolation and RT-PCR
RNA was isolated from lymphocytes of the bone marrow
using the method of TRIzol™ (cat. no. 15596026; Thermo Fisher
Scientific, Inc.) proposed in the study by Rio et al
(14). The Applied Biosystems™
High-Capacity cDNA RT kit (cat. no. 4368813; Applied Biosystems;
Thermo Fisher Scientific, Inc.) was used for cDNA synthesis. The
reaction included 4 µl 10× RT buffer, 4 µl 10× RT random primers,
1.8 µl 25× dNTP Mix (100 mM), 50 U/µl MultiScribe®
Reverse Transcriptase and 1 µg RNA, and was conducted using an
Applied Biosystems ProFlex PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) under the following conditions: 10 min at
25°C, 2 h at 37°C and 5 min at 85°C.
Detection of PML-RARA
rearrangements
cDNA was analyzed using the HemaVision-28N Multiplex
RT-qPCR kit in the Applied Biosystems ProFlex PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and the Qualitative
HemaVision-28Q RT-qPCR kit (cat. no. HV01-28Q; DNA Diagnostic A/S)
in the Rotor-Gene® Q system (cat. no. 1070452EN; Qiagen,
Inc.). These methodologies can identify 28 chromosomal
translocations and >145 gene breakpoints associated with
leukemia. The procedures were performed according to the
manufacturer's protocols. Duplicate analysis was considered in the
methodology using quality and negative controls from the kit.
An adapted RT-qPCR protocol from the study by Gabert
et al (15) was used to
identify the PML/RARA fusion gene. The primers used were as
follows: i) ENF903 (bcr1 forward, 5′-TCTTCCTGCCCAACAGCAA-3′; 19
bp); ii) ENF906 (bcr2 forward, 5′-ACCTGGATGGACCGCCTAG-3′; 19 bp);
iii) ENF905 (bcr3 forward, 5′-CCGATGGCTTCGACGAGTT-3′; 19 bp); iv)
ENR962 (bcr1-3 reverse, 5′-GCTTGTAGATGCGGGGTAGAG-3′, 21 bp); and v)
ENP942 (probe, 5′ FAM-AGTGCCCAGCCCTCCCTCGC-BHQ-1 3′, 20 bp). The
RT-qPCR was conducted using the following reagents: 12.5 µl TaqMan
Gene Expression Master Mix (cat. no. 4369016; Applied Biosystems;
Thermo Fisher Scientific, Inc.), 1.2 µl forward and reverse
oligonucleotides, 0.5 µl probe, 8.6 µl nuclease-free water and 1 µl
cDNA. The samples were placed in a 96-well plate and analyzed using
the 7900 HT Fast Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) with the SDS version 2.4 program (Thermo
Fisher Scientific, Inc.). The thermocycling conditions were as
follows: 50°C for 2 min, 95°C for 10 min, 95°C for 15 sec, and 60°C
for 1 min (40 cycles). To confirm the expression of PML/RARA
transcripts, the NB4 human cell line [derived from the leukemic
cells of a relapsed acute promyelocytic leukemia (M3) patient and
carrying the t(15;17) translocation; CVCL_0005; Cellosaurus,
https://www.cellosaurus.org/CVCL_0005] was donated by
St. Jude Children's Research Hospital (Memphis, USA), and validated
samples positive for bcr1, bcr2 and bcr3 variants were used as
positive controls. The NB4 cells were cultured in RPMI 1640 medium
(Gibco; Thermo Fisher Scientific, Inc.; cat. no. 11875093) with 15%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.; cat. no.
A4766801), 2 mmol/l L-glutamine (Gibco; Thermo Fisher Scientific,
Inc.; cat. no. 25030081), 1X antibiotic (Gibco; Thermo Fisher
Scientific, Inc.; cat. no. 15240062) and incubated in a humidified
incubator at 37°C with 5% CO2. The RNA isolation and
RT-qPCR protocol for the NB4 cells was the same as aforementioned.
Non-template controls (nuclease-free water) were also included in
each experiment. The quantification of relative expression of the
transcripts was calculated using the 2−ΔΔCq method, with
β-glucuronidase was as the housekeeping gene.
Genomic analysis
cDNA obtained using RT was analyzed to detect
simultaneous PML/RARA transcripts and myeloid leukemia-associated
genes using the Illumina® AmpliSeq RNA Myeloid Panel
(cat. no. 20024478; Illumina, Inc.). The procedure was performed by
Illumina Inc., and each RT reaction required 10–100 ng total
RNA.
Expression microarray
A wild-type sample (from a healthy 10-year-old
patient) and the APL case were used for the expression microarray.
RNA reconstituted in UltraPure™ DEPC-Treated Water (cat. no.
750023; Invitrogen; Thermo Fisher Scientific, Inc.) was quantified
and examined for RNA quality using a NanoDrop 2000™
spectrophotometer (cat. no. ND-2000; Thermo Fisher Scientific,
Inc.). The A260/A280 and A260/A230 ratios between 1.8 and 2.2 were
used to determine the RNA quality. RNA integrity was evaluated
using a 15% agarose gel stained with Gel Red (cat. no. 41003;
Biotium, Inc.), visualized using a FirstLight® Uniform
UV Illuminator (model, LM-20; single intensity; 302/365 nm UV;
filter size, 20×20-cm; 230 V; cat. no. 95-0449-02).
To detect transcripts, the human Clariom™ D Assay
(cat. no. 902922; Applied Biosystems; Thermo Fisher Scientific,
Inc.) was used. The Applied GeneChip System 3000Dx v.2 included the
GeneChip® Hybridization Oven 645 with
GeneChip® Fluidics Station 450 Dx v.2 and workstation.
Array images were acquired using a GeneChip® Scanner
3000Dx v.2 with AutoLoaderDx and Affymetrix Molecular Diagnostic
Software (Affymetrix; Thermo Fisher Scientific, Inc.).
Data analysis and functional
classification of differentially expressed genes
All data were captured using Applied Biosystems
Transcriptome Analysis Console (TAC) software (version 4.0.2;
Thermo Fisher Scientific, Inc.) and microarray data were deposited
in the Gene Expression Omnibus (GEO) database following the Minimum
Information about a Microarray Experiment (MIAME) and Minimum
Information about a Next-generation Sequencing Experiment (MINSEQE)
guidelines (https://www.ncbi.nlm.nih.gov/geo/). Finally, to
identify candidate differentially expressed genes, the microarray
data were analyzed using the online Database for Annotation,
Visualization and Integrated Discovery (DAVID) Bioinformatics
Resources (version 6.8; http://david-d.ncifcrf.gov/). A fold change ±1.5 and
P<0.05 were considered to indicate a statistically significant
difference in expression. However, only those genes with a fold
change > ±10.0 (the highest selection) were selected.
Results
Conventional cytogenetics
Karyotyping analysis of the patient revealed
46,XX,t(15;17)(q24;q21)[20] and the FISH result was nuc
ish(PML,RARA)x3(PML,RARA)x2[148/200](PML,RARA)x2[52/200],
with [n/n] representing the number of cells counted with the
alteration out of the total (Fig.
S1).
HemaVision RT-qPCR test
All three transcripts were identified using the
HemaVision-28N Multiplex RT-PCR kit, with a size of 353 bp for bcr1
(PMLex6-RARAex3), 97–350 bp for bcr2
(PMLδex6-RARAex3) and 325 bp for bcr3
(PMLex3-RARAex3) (Fig.
S2). Furthermore, the atypical transcripts were corroborated
using the Qualitative HemaVision-28Q RT-qPCR assay. The Cq values
were as follows: i) 29.83 (bcr1, PMLex6a-RARAex3); ii) 30.59
(bcr2, PMLex5-RARAex3); and iii) 30.12 (bcr3,
PMLex3-RARAex3). It should be noted that the breakpoints
reported are dependent on the primer design in each kit.
RT-qPCR data
The measurable residual disease was calculated at
diagnosis through the relative expression quantification of the
simultaneous transcripts using the Livak method (16). The results were 100% for bcr1 (Cq,
28.931), 38.5% for bcr2 (Cq, 30.08) and 0.83% for bcr3 (Cq, 35.86).
After the first month of treatment, expression of the three
PML/RARA transcripts decreased to 0%.
Genomic analysis of simultaneous
transcripts in the patient with APL
Genomic analysis with RNA sequencing. Genomic
analysis using the AmpliSeq RNA Myeloid Panel Illumina®
revealed two simultaneous transcripts, bcr1 and bcr2 (Fig. 1).
Data analysis of differentially
expressed genes
First, the microarray data were deposited in the GEO
database (accession no. GSE205372; http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE205372),
following the MIAME and MINSEQE guidelines. Then, the overall
pattern of gene expression in the microarray was assessed. Using
TAC Software version 4.0.2, a cut-off fold change value of ±10.00
was set to generate a reduced gene list since only 1 case was
analyzed. As a result, 613 differentially expressed genes were
identified (139 upregulated and 473 downregulated genes) compared
with the control (wild-type). The top upregulated and downregulated
genes are listed in Table SI.
Classification of differentially
expressed genes in the APL case
Using the data obtained of the 613 differentially
expressed genes, two different analyses were performed: i) First, a
search was performed for groups of genes arranged by functional
similarity and related to APL from the list using the Functional
Annotation Clustering setting in DAVID. An enrichment score >2
was deemed a significant enrichment with the highest stringency.
The analysis generated several functional clusters of significantly
upregulated or downregulated genes (Table I), such as: i) Immune system-related
clusters: Immunoglobulin C1-set molecules involved in the immune
system, major histocompatibility complex (MHC) class II and the
loss of HLA-DR antigen expression; ii) C-type lectin cluster; iii)
Src-homology 2 domain (SH2) cluster; and iv) mammalian defensins
cluster. Additional clusters unrelated to APL were also found,
including graft-vs. -host disease (the patient was previously
transfused), Btk motif, AIG1, peptidase SI and Pleckstrin homology
domain. The enrichment score is a modified form of the P-value of
the exact Fisher test, and the Benjamini value is the adjusted
P-value resulting from the Benjamini and Hochberg method.
 | Table I.Functional annotation clusters
obtained by Database for Annotation, Visualization and Integrated
Discovery analysis. |
Table I.
Functional annotation clusters
obtained by Database for Annotation, Visualization and Integrated
Discovery analysis.
| Functional
annotations | No. of genes | Enrichment
scorea | Benjamini
valueb |
|---|
| Lectin C-type | 20 | 10.08 |
4.5×10−9 |
| SH2 domain | 15 | 5.36 |
4.5×10−4 |
| MHC class II, α/β
chain, N-terminal | 7 | 4.05 |
2.9×10−3 |
| Immunoglobulin
C1-set | 12 | 4.96 |
8.3×10−4 |
| AIG1 | 5 | 3.88 |
7.8×10−3 |
| Graft-vs. -host
disease | 9 | 3.79 |
8.0×10−4 |
| Peptidase SI | 12 | 2.80 |
5.5×10−2 |
| Pleckstrin homology
domain | 20 | 2.68 |
4.0×10−2 |
| Mammalian
defensins | 4 | 2.62 |
2.9×10−3 |
| Btk motif | 4 | 2.58 |
7.8×10−2 |
ii) Second, from the list generated and presented in
Table SI, only 21 genes with a
fold change >20 and 24 genes <-50 were selected that can be
associated with clinical and molecular characteristics of APL for
future research. The selected genes are part of different signaling
pathways, such as for the cell cycle, proliferation,
differentiation and adhesion, in addition to MHC and cytokine
genes.
Literature review
The present study suggested a possible signaling
pathway involving the PML/RARA oncoprotein that leads to cell
proliferation or the evasion of apoptosis, based on the microarray
analysis (613 up or downregulated genes) and a literature search.
The identified genes were selected as ‘key words’ in the literature
search in association with characteristic clinical and molecular
APL. The following databases were searched: PubMed (https://pubmed.ncbi.nlm.nih.gov/), Kyoto
Encyclopedia of Genes and Genomes (https://www.genome.jp/kegg/), Gene-Cards (https://www.genecards.org/), UniProt (https://www.uniprot.org/) and Ensembl (https://www.ensembl.org/index.html).
Discussion
APL is a subtype of AML with a unique molecular
appearance distinguished cytogenetically by balanced reciprocal
translocation t(15;17) and PML/RARA gene fusion.
Furthermore, it is often associated with a complex coagulopathy
known as disseminated intravascular coagulation (DIC), resulting in
a high hemorrhage rate and thrombosis. Patients with APL present
more frequently with severe DIC due to the increased expression of
tissue factors and annexin II, which activates fibrinolysis. During
the induction period, hemorrhaging is responsible for the majority
of deaths (17,18).
Case studies with PML/RARA transcripts are
common among adults and uncommon among children (10,19).
Generally, one transcript is usually detected in children from
common or typical breakpoints and rarely from atypical breakpoints.
The bcr1 or bcr3 transcripts are the most frequent (90–95%) and
bcr2 is infrequent (11,19–22).
Previously, a case bearing two transcripts, bcr1 and bcr2, was
detected using the HemaVision RT-qPCR kit, in a pediatric patient
with APL detected by immunophenotyping, but with normal results
from karyotyping and FISH; however, there was insufficient evidence
to define a prognostic factor (23,24).
To the best of our knowledge, there have been no cases reporting
the presence of the three different transcripts (bcr1, bcr2 and
bcr3). In the present study, alternative methods, such as genomic
analysis, were applied to identify the breakpoints between the
PML and RARA genes and only bcr1 and bcr2 transcripts
were detected. Due to the large size of the transcriptome, the RNA
sequencing methodology cannot detect fusion genes that are
expressed at low levels in the leukemic clones, which may have
affected the detection of bcr3 (25).
The effect of the PML/RARA molecular
transcripts on APL continues to be controversial as there have been
no conclusive results. The reason for the generation of the three
bcr1, bcr2 and bcr3 transcripts remains unknown, although it may
involve molecular heterogeneity or unidentified secondary
alterations. Adaptive advantages provided by such events may
contribute to a mutated phenotype during the development of APL
(26). To the best of our
knowledge, no studies to date mention differences in the
oncoprotein expressed depending on the transcript present; thus, it
would be of interest to obtain further knowledge of the molecular
function of oncoproteins in association with the prognosis of these
patients. It can be hypothesized that different PML breakpoints
that lead to protein variants could affect the prognosis or
therapeutic response; however, this issue remains unsolved
(27).
Previous studies have reported that pediatric
patients have a >25% frequency of the hypogranular morphological
subtype, and a higher frequency of the bcr2 and bcr3 transcripts,
compared with adult patients (3,17). In
addition, a higher incidence of the bcr1 transcript has been
observed in the Latin American population, and the bcr3 transcript
is associated with a worse prognosis, the M3v subtype,
hyperleukocytosis and a higher frequency of mutations in the
Fms-like tyrosine kinase 3 (FLT3) gene. According to the clinical
follow-up of the patient, it is suggested that the presence of the
three transcripts infers a good prognosis. As the expression of
bcr1 and bcr2 inhibits the effects of bcr3, we speculate that there
could be environmental influences determining the breakpoint in the
PML gene, or there is an additional secondary alteration, as
mutations in the FLT3 gene may alter cells to infer an
adaptive advantage; however, the present case did not present with
FLT3 mutations, or other clinical risk characteristics
(17,28).
In the present study, to predict the possible
functional interactions of the PML/RARA oncoprotein, an expression
microarray analysis was performed. Only gene expression profiles of
genes consistently associated with APL were selected, identifying
the following clusters of genes: i) Immunoglobulin C1-set molecules
involved in the immune system, MHC class II and the loss of HLA-DR
antigen expression; ii) C-type lectin; iii) SH2; and iv) mammalian
defensins. HLA-DR is a molecule of antigen-presenting cells. The
principal function of HLA-DR is to initiate and promote the immune
response, and its expression is present in the early stages of the
APL disease (29). HLA-DR- and low
CD34 expression characterizes malignant promyelocytes. A study by
Dunn et al (30) examining
the mechanisms of immune evasion indicated an association with the
downregulation of HLA-DR antigen expression in tumor cells. The
mechanism of immune evasion is an immunoediting process that has
been described in transplanted patients with AML (30). The transplanted immune cells exert
selective pressure against AML cells that can be recognized
immunologically. Tumor clones evolve in response to selective
pressure mediated by the immune system and finally escape, leading
to resistant clones and relapse. These epigenetic alterations
suggest that therapeutic strategies to re-sensitize AML cells to
the graft-vs. -leukemia effect may be feasible (29,30).
Studies have shown that the expression levels of either CD56, CD34
or FLT3-internal tandem duplication (ITD) markers are associated
with a poor patient prognosis (31–33).
However, the prognosis of patients with APL expressing CD2, CD4,
HLA-DR and FLT3-ITD mutation remains controversial. To the best of
our knowledge, pediatric cases have not yet been reported.
The C-type lectin cluster was also found in the RNA
analysis of the present study. C-type lectin functions as a
recognition molecule in the immune system and has a variety of
roles in the defense against pathogens, immune regulation and
prevention of autoimmunity (34).
Human C-type lectin-like molecule-1 (CLL-1; CLEC12A) is a
transmembrane glycoprotein that plays a role in immune regulation
as an inhibitory receptor. CLL-1 is present in granulocytes,
monocytes and certain types of myeloid progenitors in the bone
marrow. Furthermore, CLL-1 is detected in 77.5–92% of AML blasts at
diagnosis and is also present in leukemic stem cells, causing
treatment failure and leukemia relapse (34). However, the association between the
expression of CLL-1 and other classical AML markers remains
unclear, and the predictive value of CLL-1 expression in patients
with AML has rarely been reported.
SH2 was another representative cluster found in the
present study. SH2 domain-containing phosphatase 2 (SHP2; PTPN11)
is a positive regulator of receptor tyrosine kinase-driven
signaling in response to growth factors and cytokines, including
signaling through the Ras/RAF/extracellular signal-regulated kinase
(ERK), and the JAK/STAT pathways. Hyperactive SHP2 is associated
with tumorigenesis, tumor maintenance, metastasis and therapeutic
resistance (35). Numerous somatic
gain-of-function mutations that similarly cause the constitutive
activation of SHP2 are found in leukemia (35).
In the present study, other clusters observed in the
functional analysis were mammalian defensins, which are endogenous
peptides produced by certain leukocytes and epithelial cells. In
humans, α-defensins are packaged in azurophilic granules of
neutrophils or secreted by intestinal Paneth cells. In addition,
β-defensins are constitutively expressed in various mucosa and
epithelial cells, where they are upregulated in response to
infectious and inflammatory stimuli (36). Humans produce six different
α-defensins, including four peptides (HNP-1 to HNP-4) in
neutrophils and two peptides (HD5 and HD6) in Paneth cells of the
small bowel. Several tumor types, including lung, esophageal and
skin cancer, exhibit a deregulated expression and secretion of α-
and β-defensins (36). The reasons
for this deregulated expression and the role of defensins in
oncogenesis remain poorly understood.
To the best of our knowledge, to date there have
been no studies that indicate an association of defensins with AML.
Nonetheless, α- and β-defensins have been previously reported to be
associated with chronic myeloid leukemia (37–39).
However, the present study did not establish an association between
PML/RARA transcripts and the functionally identified
clusters using gene expression microarray analysis as the
identified clusters do not participate in the PML/RARA oncoprotein
pathway. It is considered that CCL-1 may emerge as a promising
diagnostic biomarker (34). In
addition, the HLA-DR class II implication in promyelocytes was
negative. Furthermore, the present study suggested a possible
signaling pathway involving the PML/RARA oncoprotein that leads to
cell proliferation or the evasion of apoptosis, based on the
microarray analysis and literature search (Fig. 2).
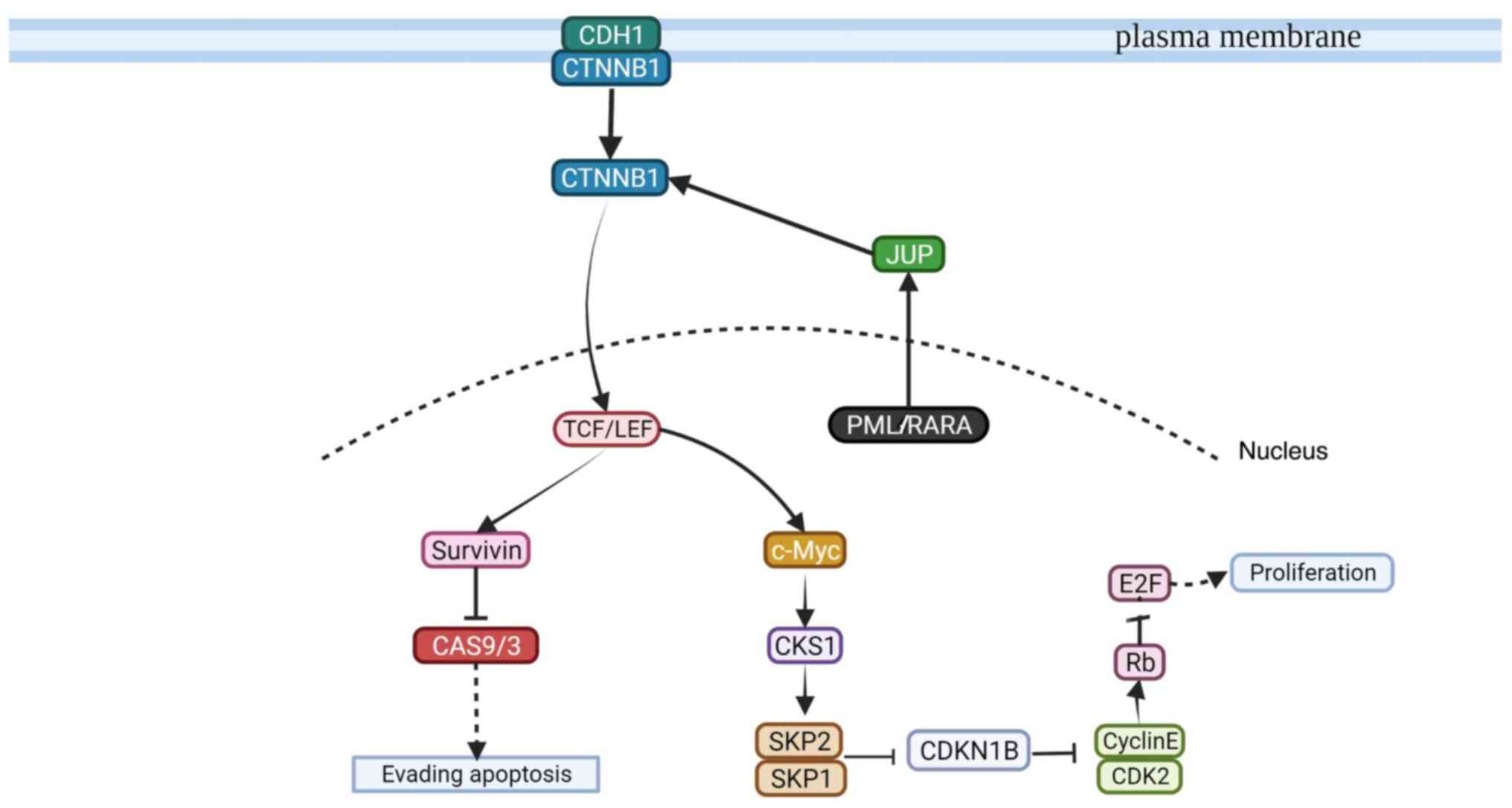 | Figure 2.Suggested PML/RARA-related signaling
pathway that leads to cell proliferation and the evasion of
apoptosis. The PML/RARA oncoprotein is active when interacting with
JUP, and in-turn interacts with CTNNB1. CTNNB1 is active in the
cellular membrane or in its free form in the cytosol. CTNNB1 is
internalized into the nucleus by TCF/LEF, leading to cell
proliferation or evasion of apoptosis. For cell proliferation, the
TCF/LEF protein activates c-Myc which then interacts with CKS1.
CKS1 then activates SKP1/2, which in-turn inhibits the function of
CDKN1B and CDK2/cyclin E. Active Rb inhibits E2F, which promotes
the constant proliferation of immature promyelocytes. For the
evasion of apoptosis, TCF/LEF activates survivin to inhibit the
function of CAS9/3. PML, promyelocytic leukemia; RARA, retinoic
acid receptor α; JUP, junction plakoglobin; CDH1, cadherin 1;
CTNNB1, catenin β 1; TCF/LEF, T-cell factor/lymphoid enhancer
factor; survivin, surviving, baculoviral IAP repeat-containing
protein 5; CAS, caspase; c-Myc, Myc proto-oncogene protein; CKS1,
cyclin-dependent kinase regulatory subunit 1; SKP, S-phase
kinase-associated protein; CDKN1B, cyclin-dependent kinase
inhibitor 1B; CDK2, cyclin-dependent kinase 2; Cyclin E,
cyclin-dependent kinase E; Rb, retinoblastoma-associated protein;
E2F, transcription factor E2F1. Created with BioRender.com (2020). |
In conclusion, to the best of our knowledge, the
present study is the first to report a pediatric patient with AML
with three simultaneous transcripts. The three transcripts may be
protective as the patient exhibited a positive response to
treatment. As it has been 4 years since the end of the treatment,
the patient is considered to be cured. Moreover, the
PML/RARA transcripts (bcr1, bcr2 and bcr3) coincide with the
good patient prognosis observed in the present case. Further
accumulation of data on similar cases may provide relevant clinical
evidence for pediatric APL. The functional clusters identified in
the patient of the present study may be related to APL biology and
suggest promising biomarkers, such as CCL-1, and α- and
β-defensins. The PML/RARA oncoprotein signaling pathway suggested
in the present study may be associated with the leukemogenic events
involved in APL.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Dr Violeta Casandra
Vera Cuevas (Molecular technical advice; Illumina, Inc.) for
support with the genomic analysis.
Funding
The present study was supported by PROSNI (support program to
improve the production conditions of research members) 2018 and The
Postgraduate Incorporation and Permanence Program from the
Postgraduate Human Genetic Department of the University of
Guadalajara.
Availability of data and materials
The gene expression datasets generated and/or
analyzed during the current study are available in the Gene
Expression Omnibus (40) repository
(https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE205372).
This was in-line with the MIAME and MINSEQE guidelines. All other
datasets used and/or analyzed during the current study are
available from the corresponding author on reasonable request.
Author's contributions
JSH contributed to the design of the study,
performed technical procedures, drafted the article and interpreted
genomic data. LMM acquired data and performed qPCR procedures. ICQ
performed technical microarray procedures. AMM and GSS performed
cytogenetics technical procedures. SABJ and RMCO interpreted the
clinical data. UFSB contributed to the interpretation and analysis
of results. DOS contributed to the interpretation and analysis of
microarray results. FASZ coordinated the clinical management. RCR
interpreted clinical results and reviewed the manuscript. ACR
designed the study and reviewed the submitted version. LBM designed
the study and coordinated the final approval of the submitted
version. ACR and LBM confirm the authenticity of all the raw data.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was conducted following the
principles of The Declaration of Helsinki. The present study was
submitted and accepted by The Research Committee and The Research
Ethics Committee of The Civil Hospital of Guadalajara (approval no.
00116). Written informed consent and assent were obtained for
participation in this study.
Patient consent for publication
Written informed consent was obtained from the
patient and their parents to publish the present study.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
APL
|
acute promyelocytic leukemia
|
|
PML
|
promyelocytic leukemia
|
|
RARA
|
retinoic acid receptor α
|
References
|
1
|
Testi AM, D'Angiò M, Locatelli F, Pession
A and Lo Coco F: Acute promyelocytic leukemia (APL): Comparison
between children and adults. Mediterr J Hematol Infect Dis.
6:e20140322014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gregory J and Feusner J: Acute
promyelocytic leukemia in childhood. Curr Oncol Rep. 11:439–445.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jeddi R, Ghédira H, Ben Abdennebi Y, Kacem
K, Ben Amor R, Aissaoui L, Bouterâa W, Ben Lakhal R, Ben Abid H,
Menif S, et al: ATRA and anthracycline-based chemotherapy in the
treatment of childhood acute promyelocytic leukemia (APL): A
10-year experience in Tunisia. Med Oncol. 28:1618–1623. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arber DA, Orazi A, Hasserjian R, Thiele J,
Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M and Vardiman JW:
The 2016 revision to the World Health Organization classification
of myeloid neoplasms and acute leukemia. Blood. 127:2391–2405.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wiernik PH, Gallagher RE and Tallman MS:
Acute promyelocytic leukemia. Neoplastic Diseases of the Blood.
Springer International Publishing; pp. 409–463. 2018, View Article : Google Scholar
|
|
6
|
Cicconi L, Fenaux P, Kantarjian H, Tallman
M, Sanz MA and Lo-Coco F: Molecular remission as a therapeutic
objective in acute promyelocytic leukemia. Leukemia. 32:1671–1678.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim B, Lee H, Shin S, Lee ST and Choi JR:
Clinical evaluation of massively parallel RNA sequencing for
detecting recurrent gene fusions in hematologic malignancies. J Mol
Diagn. 21:163–170. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iaccarino L, Divona M, Ottone T, Cicconi
L, Lavorgna S, Ciardi C, Alfonso V, Travaglini S, Facchini L,
Cimino G, et al: Identification and monitoring of atypical PML/RARA
fusion transcripts in acute promyelocytic leukemia. Genes
Chromosomes Cancer. 58:60–65. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Korf K, Wodrich H, Haschke A, Ocampo C,
Harder L, Gieseke F, Pollmann A, Dierck K, Prall S, Staege H, et
al: The PML domain of PML-RARα blocks senescence to promote
leukemia. Proc Natl Acad Sci USA. 111:12133–12138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Conneely SE and Stevens AM: Advances in
pediatric acute promyelocytic leukemia. Children. 7:112020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liquori A, Ibañez M, Sargas C, Sanz MÁ,
Barragán E and Cervera J: Acute promyelocytic leukemia: A
constellation of molecular events around a single PML-RARA fusion
gene. Cancers (Basel). 12:6242020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Serdlow SH, Campo E, Pileri SA, Harris NL,
Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD
and Jaffe ES: The 2016 revision of the World Health Organization
classification of lymphoid neoplasms. Blood. 2375–2390. 2016.
View Article : Google Scholar
|
|
13
|
Miguel S: PETHEMA Recomendaciones LPA2012.
Hematologialafe. pp1–37. 2012.https://www.sehh.es/servicios-para-los-socios/559-servicios-para-los-socios/pethema/protocolos/lap-2012January
8–2024
|
|
14
|
Rio DC, Ares M, Hannon GJ and Nilsen TW:
Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb
Protoc. 2010.pdb.prot54392010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gabert J, Beillard E, van der Velden VHJ,
Bi W, Grimwade D, Pallisgaard N, Barbany G, Cazzaniga G, Cayuela
JM, Cavé H, et al: Standardization and quality control studies of
‘real-time’ quantitative reverse transcriptase polymerase chain
reaction of fusion gene transcripts for residual disease detection
in leukemia-a Europe against cancer program. Leukemia.
17:2318–2357. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoo ES: Recent advances in the diagnosis
and management of childhood acute promyelocytic leukemia. Korean J
Pediatr. 54:95–105. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ookura M, Hosono N, Tasaki T, Oiwa K,
Fujita K, Ito K, Lee S, Matsuda Y, Morita M, Tai K, et al:
Successful treatment of disseminated intravascular coagulation by
recombinant human soluble thrombomodulin in patients with acute
myeloid leukemia. Medicine (Baltimore). 97:e129812018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barragán E, Bolufer P, Martín G, Cervera
J, Moreno I, Capote FJ, Rosique P and Sanz MA: Identification of
two atypical PML-RAR(alpha) transcripts in two patients with acute
promyelocytic leukemia. Leuk Res. 26:439–442. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim M, Lim J, Kim Y, Han K, Lee DH, Chung
NG, Cho B, Kim HK, Eom KS, Min CK and Min WS: The genetic
characterization of acute promyelocytic leukemia with cryptic
t(15;17) including a new recurrent additional cytogenetic
abnormality i(17)(q10). Leukemia. 22:881–883. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim MJ, Cho SY, Kim MH, Lee JJ, Kang SY,
Cho EH, Huh J, Yoon HJ, Park TS, Lee WI, et al: FISH-negative
cryptic PML-RARA rearrangement detected by long-distance polymerase
chain reaction and sequencing analyses: A case study and review of
the literature. Cancer Genet Cytogenet. 203:278–283. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Manola KN, Karakosta M, Sambani C,
Terzoudi G, Pagoni M, Gatsa E and Papaioannou M: Isochromosome
der(17)(q10)t(15;17) in acute promyelocytic leukemia resulting in
an additional copy of the RARA-PML fusion gene: Report of 4 cases
and review of the literature. Acta Haematol. 123:162–170. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rasekh EO, Elsayed GM, Madney Y and El
Gammal MM: Prognostic significance of bcr-1 and bcr-3 isoforms of
PML-RARA and FLT3-ITD in patients with acute promyelocytic
leukemia. Clin Lymphoma Myeloma Leuk. 20:156–167. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rasekh EO, Elsayed GM and Fathy S: No
prognostic significance of normalized copy number of PML-RARA
transcript at diagnosis in patients with acute promyelocytic
leukemia. Hematol Oncol Stem Cell Ther. 14:119–125. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heyer EE, Deveson IW, Wooi D, Selinger CI,
Lyons RJ, Hayes VM, O'Toole SA, Ballinger ML, Gill D, Thomas DM, et
al: Diagnosis of fusion genes using targeted RNA sequencing. Nat
Commun. 10:13882019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Loeb LA: A mutator phenotype in cancer.
Cancer Res. 61:3230–3239. 2001.PubMed/NCBI
|
|
27
|
De Braekeleer E, Douet-Guilbert N and De
Braekeleer M: RARA fusion genes in acute promyelocytic leukemia: A
review. Expert Rev Hematol. 7:347–357. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Castro-Mujica Mdel C and
Sullcahuamán-Allende Y: Molecular subtypes of PML/RARα in patients
with acute promyelocytic leukemia. Rev Peru Med Exp Salud Publica.
30:37–40. 2013.(In Spanish). PubMed/NCBI
|
|
29
|
Christopher MJ, Petti AA, Rettig MP,
Miller CA, Chendamarai E, Duncavage EJ, Klco JM, Helton NM,
O'Laughlin M, Fronick CC, et al: Immune escape of relapsed AML
cells after allogeneic transplantation. N Engl J Med.
379:2330–2341. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dunn GP, Bruce AT, Ikeda H, Old LJ and
Schreiber RD: Cancer immunoediting: From immunosurveillance to
tumor escape. Nat Immunol. 3:991–998. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oelschlaegel U, Mohr B, Schaich M, Schäkel
U, Kroschinsky F, Illmer T, Ehninger G and Thiede C: HLA-DRneg
patients without acute promyelocytic leukemia show distinct
immunophenotypic, genetic, molecular, and cytomorphologic
characteristics compared to acute promyelocytic leukemia. Cytometry
B Clin Cytom. 76:321–327. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Roerden M, Märklin M, Salih HR, Bethge WA,
Klein R, Rammensee HG, Nelde A and Walz JS: Expression levels of
HLA-DR in acute myeloid leukemia: Implications for antigenicity and
clinical outcome. Leuk Lymphoma. 62:1907–1919. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nie L, Ma R, Yuan X, Jiang L, Yang S, Xu
H, Liu X, Liu Y, Zhang L and Zhu Z: The prognostic value of CD2,
CD4, and HLA-DR expression and FLT3-ITD mutation in adult acute
promyelocytic leukemia. Leuk Lymphoma. 61:2482–2487. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang J, Wang W, Chen H, Li W, Huang T,
Zhang W, Ling W, Lai P, Wang Y, Geng S, et al: C-type lectin-like
molecule-1 as a biomarker for diagnosis and prognosis in acute
myeloid leukemia: A preliminary study. Biomed Res Int.
2021:66439482021.PubMed/NCBI
|
|
35
|
Raveendra-Panickar D, Finlay D, Layng FI,
Lambert LJ, Celeridad M, Zhao M, Barbosa K, De Backer LJS, Kwong E,
Gosalia P, et al: Discovery of novel furanylbenzamide inhibitors
that target oncogenic tyrosine phosphatase SHP2 in leukemia cells.
J Biol Chem. 298:1014772022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ghosh SK, McCormick TS and Weinberg A:
Human beta defensins and cancer: Contradictions and common ground.
Front Oncol. 9:3412019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Stretch C, Khan S, Asgarian N, Eisner R,
Vaisipour S, Damaraju S, Graham K, Bathe OF, Steed H, Greiner R and
Baracos VE: Effects of sample size on differential gene expression,
rank order and prediction accuracy of a gene signature. PLoS One.
8:e653802013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Maleki F, Ovens K, McQuillan I and Kusalik
AJ: Size matters: How sample size affects the reproducibility and
specificity of gene set analysis. Hum Genomics. 13 (Suppl
1):S422019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tsai CA, Wang SJ, Chen DT and Chen JJ:
Sample size for gene expression microarray experiments.
Bioinformatics. 21:1502–1508. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Edgar R, Domrachev M and Lash AE: Gene
expression omnibus: NCBI gene expression and hybridization array
data repository. Nucleic Acids Res. 30:207–210. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kent WJ, Sugnet CW, Furey TS, Roskin KM,
Pringle TH, Zahler AM and Haussler D: The human genome browser at
UCSC. Genome Res. 12:996–1006. 2002. View Article : Google Scholar : PubMed/NCBI
|