Introduction
Lung cancer, particularly lung adenocarcinoma
(LUAD), is a major contributor to cancer-associated mortality
worldwide, accounting for approximately 11.4% of all global cancer
cases and 18.0% of cancer-related deaths. This prominence is
potentially linked to its unique patterns of invasion. (1). Aside from infiltration of
myofibroblast stroma, lymphovascular and pleural invasion, spread
through air spaces (STAS) has emerged as an invasion pattern in
LUAD (2). It was identified
initially by Kadota et al (3) and recognized as a distinct form of
tumor spread in the 2015 World Health Organization classification
(3). STAS is characterized by
presence of micropapillary clusters, solid nests or individual
cells in lung parenchyma air spaces beyond the tumor margin
(4). The current diagnostic
methodology for STAS is analysis of pathological specimens obtained
from lung tissues excised during surgical procedures in patients
(5). It is found in 14.8–56.4% of
LUAD cases and is associated with lower survival rates and a worse
prognosis compared with STAS-negative tumors. Therefore,
identification of STAS can provide key information for the clinical
treatment of patients with LUAD (6,7).
Reports indicate a significant risk of local and distant recurrence
in STAS-positive cases treated with sublobar resection (3,8),
whereas patients who undergo lobectomy have no increased recurrence
risk. Thus, early detection of STAS is of clinical importance.
Radiomics, the conversion of radiographic images
into quantifiable information, offers the potential to improve
diagnosis, prognosis and the development of predictive models
(9–11). Previous advancements in predicting
STAS status in LUAD using radiomics methods reported promising
results (12,13). However, bridging the gap between
radiomics as a research tool and its clinical implementation
presents challenges, including technical reproducibility, clinical
validity, quantification and cost-effectiveness. There is also
notable heterogeneity in previous studies, with lack of
comprehensive evaluation of the performance of radiomics in
predicting STAS in LUAD (14).
Identifying factors affecting the predictive performance of
radiomics is key for its clinical use. Several radiomics models
employing computed tomography (CT), magnetic resonance imaging and
positron emission tomography (PET)/CT have been developed for
predicting STAS, showing diverse performance and indicating
methodological variability (15,16).
However, to date, there are no relevant network meta-analyses to
evaluate the predictive value of these models, to the best of our
knowledge. Therefore, the present study aimed to assess the risk of
bias and methodological quality and to perform a network
meta-analysis (NMA) to evaluate the effectiveness of radiomics
models in predicting preoperative STAS in LUAD. This may be
valuable for clinicians, radiologists and researchers in the field
of LUAD diagnosis and treatment.
Materials and methods
Protocol and registration
The present review was performed in accordance with
AMSTAR 2 (17). The methods and
protocol for the present study were pre-registered, in accordance
with standard procedures, in the International Platform of
Registered Systematic Review and Meta-analysis Protocols
(registration no. 202390105; DOI: 10.37766/inplasy2023.9.0105).
Retrieval strategy
A comprehensive literature search was performed
using the following key terms: ‘Risk factor’, ‘predictive’, ‘spread
through air spaces’, ‘lung adenocarcinoma’ and ‘nomograms’. This
search used the PubMed (pubmed.ncbi.nlm.nih.gov/), Embase
(embase.com/),Scopus(https://www.scopus.com/),Wiley(https://onlinelibrary.wiley.com/) and Web of
Science(https://www.webofscience.com/wos/) databases, with a
cut-off date of May 1, 2023. The references of included studies
were also systematically reviewed to obtain potentially relevant
publications (Table I).
 | Table I.Search strategy. |
Table I.
Search strategy.
| Database | Search query | Number of
results |
|---|
| PubMed | (spread through air
spaces [Mesh] OR STAS [Mesh] OR spread through air spaces
[Title/Abstract] OR STAS [Title/Abstract]) AND (Lung cancer [Mesh]
OR Lung adenocarcinoma [Mesh] OR Adenocarcinoma of Lung [Mesh] OR
Lung cancer [Title/Abstract] OR Lung adenocarcinoma
[Title/Abstract] OR Adenocarcinoma of Lung [Title/Abstract]) AND
(Risk factor [Mesh] OR Prediction [Mesh] OR Nomograms [Mesh] OR
Risk factor [Title/Abstract] OR Prediction [Title/Abstract] OR
Nomograms [Title/Abstract]) | 64 |
| Embase | ((STAS)/br OR
((‘spread through air spaces’):ti)) AND ((Adenocarcinoma of
Lung)/br OR ((Lung adenocarcinoma)/br) OR ((Lung cancer)/br)) AND
((prediction)/br OR ((Risk factor)/br) OR ((Nomograms)/br)) | 120 |
| Scopus | (TITLE-ABS-KEY
(stas) OR TITLE-ABS-KEY (spread AND through AND air AND spaces) AND
(TITLE-ABS-KEY (lung AND cancer) OR TITLE-ABS-KEY (Adenocarcinoma
AND of AND Lung) OR TITLE-ABS-KEY (Lung AND adenocarcinoma)) AND
(TITLE-ABS-KEY (risk AND factor) OR TITLE-ABS-KEY (Prediction) OR
TITLE-ABS-KEY (Nomograms)) | 81 |
| Wiley | ‘STAS OR spread
through air spaces’ anywhere and ‘Lung cancer OR Lung
adenocarcinoma OR Adenocarcinoma of Lung’ anywhere and ‘prediction
OR Risk factor OR Nomograms’ anywhere | 177 |
| Web of science | ((TS=(spread
through air spaces)) OR TS=(STAS) OR TI=(STAS) OR AB=(STAS)) AND
(TS=(Lung cancer) OR TS=(Adenocarcinoma of Lung) OR TS=(Lung
adenocarcinoma) OR TI=(Lung adenocarcinoma)) AND (TS=(Prediction)
OR TS=(Risk factor) OR TS=(Nomograms)) | 112 |
Inclusion and exclusion criteria
The inclusion criteria included the following: i)
Study focuses on patients who have been diagnosed with LUAD and who
exhibit STAS; ii) objective of the study is to develop a predictive
model to accurately identify the presence of STAS in patients with
LUAD. Tumor STAS was defined as tumor cells (micropapillary
structures, solid nests, or single cells-spreading within air
spaces in the lung parenchyma beyond the edge of the main tumor
(3). The present study selected
studies that included patients who underwent segmental or lobar
resection.
The exclusion criteria were as follows: i)
Predictive models that were not constructed based on radiological
features; ii) no clear inclusion and exclusion criteria; iii)
reviews and lecture-type literature; iv) literature for which the
full text could not be obtained and v) literature for which data
could not be extracted.
Literature screening
The initial screening of titles and abstracts was
independently conducted by two researchers (CL and PW) using
Cochrane handbook's guidelines for systematic reviews of
interventions (18), adhering to
the predefined inclusion and exclusion criteria. Discrepancies or
uncertainty about article inclusion were resolved through
discussion or consultation with a third reviewer (XL).
Quality evaluation of literature
The quality of articles was assessed by two
independent reviewers using Quality Assessment of Diagnostic
Accuracy Studies-2 (QUADAS-2) (19), which is a tool for assessing quality
of diagnostic studies, focusing on ‘risk-of-bias’ and
‘applicability concerns’. The risk-of-bias was assessed across four
domains: Patient selection, index test, reference standard and flow
and timing. The applicability was evaluated for the first three
domains and rated as ‘yes’, ‘no’ or ‘unclear’, with ‘yes’ denoting
low risk, ‘no’ indicating high risk and ‘unclear’ suggesting
insufficient information. In cases of disagreement, a third
reviewer was used for resolution. A Measurement Tool to Assess
systematic Reviews was used for a stringent quality assessment
(14).
Additionally, the methodological quality of studies
was appraised using the Cochrane Handbook's risk-of-bias assessment
tool (RevMan v.5.3.5, The Cochrane Collaboration) (20), covering six aspects (selection,
performance bias, detection, attrition, reporting bias and other
bias), which were categorized as ‘yes’, ‘no’ or ‘unclear’ to
indicate the level of bias.
Data extraction
The data extracted primarily encompassed the
following aspects: i) Characteristics of the included literature,
such as author information, publication date, country of origin,
predictive models and regression methods employed and predictive
factors investigated; ii) details of the study subjects, such as
sample size, sex distribution and tumor stage (according to the 8th
edition of the AJCC staging standards) (21), with all participants having
undergone surgery and iii) evaluation of effect indicators.
Statistical analysis
The effectiveness of various predictive models were
evaluated based on their accuracy, sensitivity (SEN), and
specificity (SPE). Predictive models were categorized according to
their unique features for NMA to assess performance in predicting
STAS. This NMA, conducted using Stata software (version 17.0;
StataCorp LP) within a Bayesian framework, used the Markov Chain
Monte Carlo Subset Simulation (22)
in accordance with the PRISMA NMA guidelines (23). A nodal approach for quantifying and
clarifying concordance between direct and indirect comparisons was
adopted. The consistency criterion for the NMA was P>0.05.
Network diagrams visually represented diagnostic methods, with
nodes symbolizing each method and lines representing direct
comparisons. The size of nodes and thickness of lines corresponded
to the number of studies. To detect possible publication bias in
selected studies, funnel plots were constructed for each measure of
diagnostic efficiency, employing symmetry criteria as a key
validation technique. Statistical heterogeneity was evaluated using
I2 statistic, a measure in meta-analytical methods. This
quantifies the proportion of the total variation in study estimates
due to heterogeneity rather than chance. An I2 value of
0% indicates no observed heterogeneity, whilst higher values
suggest increasing heterogeneity, with guidelines typically
considering 25, 50 and 75% as low, moderate and high heterogeneity,
respectively (18). Additionally,
to ascertain the relative superiority of one method, the level of
certainty for predictive models was quantified. This assessment was
performed using surface under the cumulative ranking curve (SUCRA),
forest plots and league tables.
Subgroup diagnostic meta-analyses were performed
using Stata to assess relative predictive efficiency of composite
models. The effectiveness of these models was evaluated using the
area under the curve (AUC) derived from the summary receiver
operating characteristics (sROC). Additionally, the Fagan plot was
utilized to quantify the overall discriminatory power of a
diagnostic test (24).
Results
Selection and characteristics of
literature
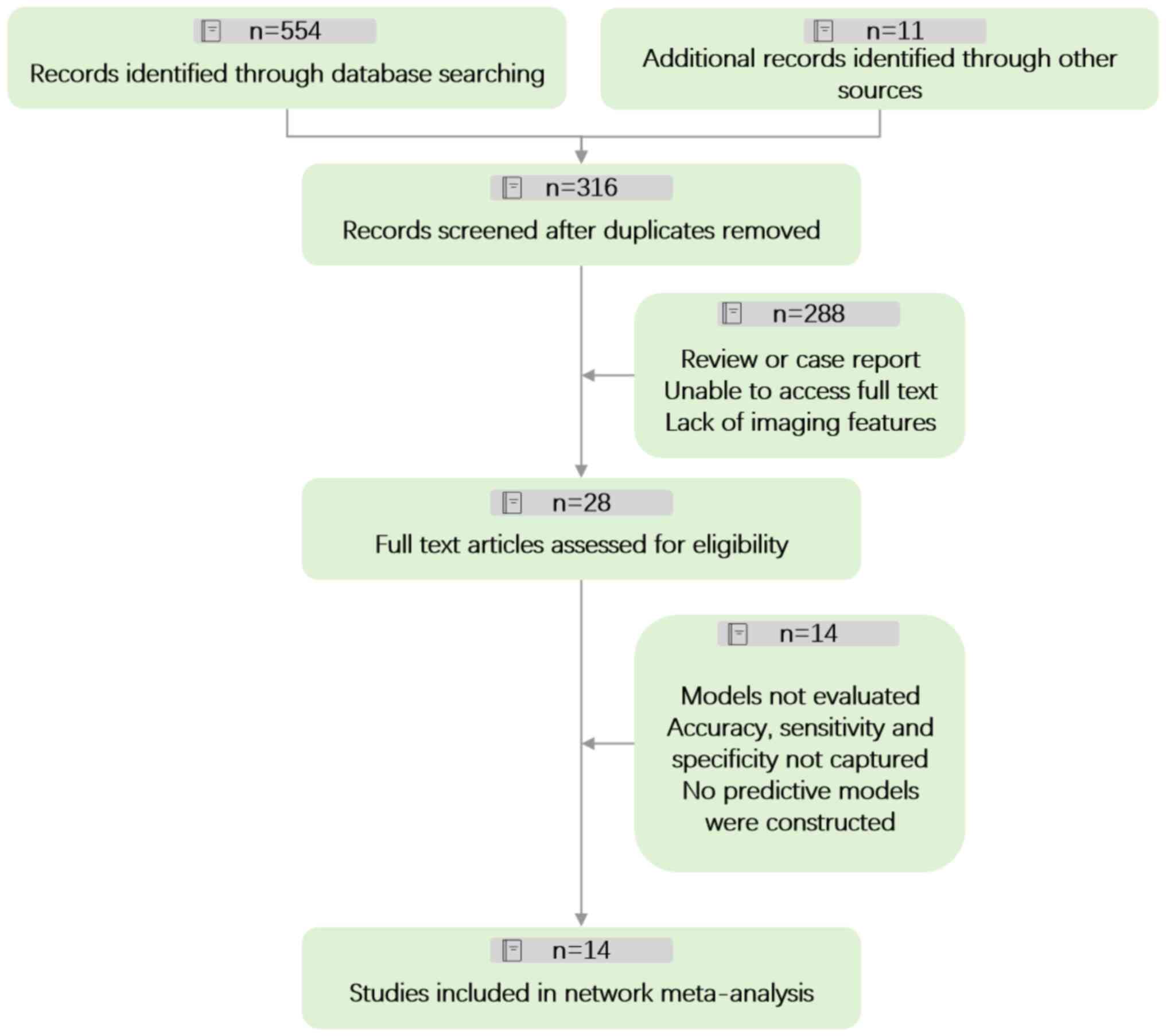
Literature review was performed using 565 articles.
Subsequent to this, a meticulous screening process was undertaken
to ensure the relevance and quality of sources. This entailed the
removal of 249 articles due to duplication. Further scrutiny,
focusing on titles and abstracts, led to the exclusion of an
additional 288 articles that were not pertinent. The remaining pool
of 28 articles was subjected to a more rigorous evaluation, which
included accessibility of full-text versions and the feasibility of
data extraction. This process led to the disqualification of 14
articles, leaving a final count of 14 articles (Fig. 1).
These 14 articles, collectively encompassing data
from 3,734 participants, were exclusively focused on patients
diagnosed with STAS in LUAD. The predictive models were categorized
into the following four distinct types based on their
methodological approaches and radiological characteristics: i)
Models developed using logistic regression analysis to screen CT
features (Features_CT); ii) models using machine learning (ML)
techniques to screen tumor radiological characteristics
(ML_Tumour); iii) models applying ML for the screening of both
tumor and peritumor radiological features (ML_Peri_tumour) and iv)
models that used logistic regression analysis for screening of
PET/CT features (pet_CT).
In the process of tumor and peritumor segmentation,
the open-source software 3D Slicer v4.8.1 (slicer.org) was used.
Moreover, all studies used pathological findings as a benchmark,
forming a control group against which predictive models were
evaluated. The data enabling direct comparative analysis in
outcomes were also assessed (Table
II) (5,15,16,25–35).
 | Table II.Characteristics of studies included
in the meta-analysis. |
Table II.
Characteristics of studies included
in the meta-analysis.
| First author/s,
year | Country of
origin | Number of
patients | Sex
(male/female) | Tumor stage | STAS (+) | Predictive
model | Regression
method | (Refs.) |
|---|
| Bassi et al,
2022 | Italy | 149 | 85/64 | I–III | 98 | CT features | Logistic | (25) |
|
|
|
|
|
|
| Tumor
radiomics | ML |
|
| Qi et al,
2021 | China | 216 | 160/56 | I–III | 56 | CT features | Logistic | (26) |
|
|
|
|
|
|
| Peritumoral and
tumoral radiomic features | ML |
|
| Liao et al,
2022 | China | 256 | 122/134 | I | 85 | Peritumoral and
tumoral radiomic features | ML | (5) |
| Chen et al,
2022 | China | 327 | 131/196 | I–III | 113 | Peritumoral and
tumoral radiomic features | ML | (27) |
| Kim et al,
2018 | Korea | 276 | 129/147 | I–III | 92 | CT features | Logistic | (28) |
| Qin et al,
2022 | China | 503 | 201/302 | I–III | 241 | CT features | Logistic | (29) |
| Chen et al,
2022 | China | 85 | Unknown | I–III | 13 | CT features | Logistic | (15) |
| Qi et al,
2020 | China | 190 | 112/78 | I | 47 | CT features | Logistic | (30) |
| Zhang et al,
2020 | China | 762 | 276/486 | I | 83 | CT features | Logistic | (31) |
| Han et al,
2022 | China | 395 | 207/188 | I | 169 | Tumor
radiomics | ML | (32) |
| Takehana et
al, 2022 | Japan | 339 | 160/179 | I | 95 | CT features | Logistic | (33) |
|
|
|
|
|
|
| Peritumoral and
tumoral radiomic features | ML |
|
| Wang et al,
2020 | China | 121 | 60/67 | I | 51 | 18F FDG-PET/CT | Logistic | (34) |
| Nishimori et
al, 2022 | Japan | 52 | 22/30 | I | 19 | 18F FDG-PET/CT | Logistic | (16) |
| Falay et al,
2021 | Turkey | 63 | 41/22 | I–III | 33 | 18F FDG-PET/CT | Logistic | (35) |
Quality assessment and publication
bias
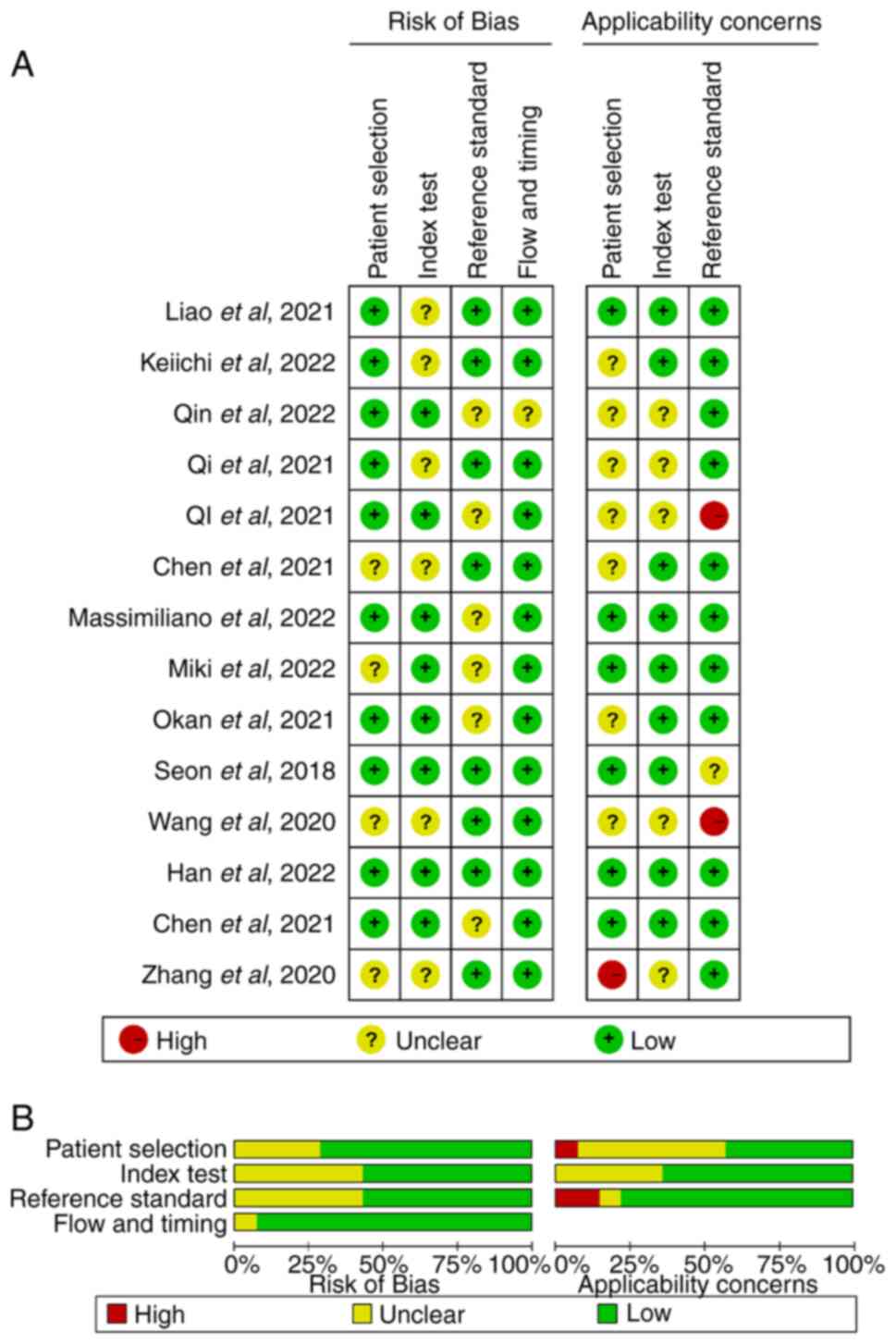
In the present study involving 14 articles and 17
predictive models, NMA was performed using Stata and the QUADAS-2
tool was used to assess quality, risk of bias and applicability of
the articles. The inter-rater reliability (κ-agreement) between the
reviewers was 0.87. A high risk of bias was detected in a few
articles (3/14) in terms of patient selection (1/14) and reference
standards (2/14); however, the overall quality of the publications
was satisfactory (Fig. 2).
NMA
NMA evaluated the relative risk (RR) values and 95%
confidence intervals (CI) across different predictive models in
terms of accuracy, SEN and SPE for STAS in LUAD.
Pairwise meta-analysis
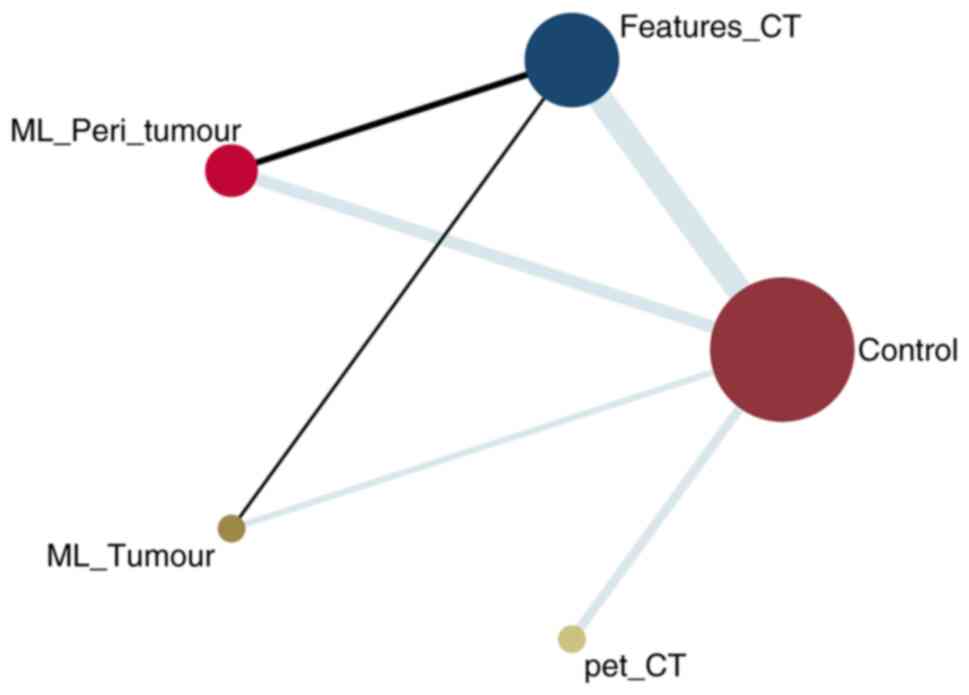
NMA graph illustrates the comparative accuracy, SEN
and SPE of predictive models (Fig.
3). Notably, CT_feature model group encompassed the largest
sample size, followed by the ML_Peri_tumour model. Specifically,
two studies directly compared the CT_feature and ML_Peri_tumour
model, and one study contrasted the CT_feature model with ML_Tumour
model (Fig. 3). Furthermore, the
comprehensive evaluation of the included studies spanned all
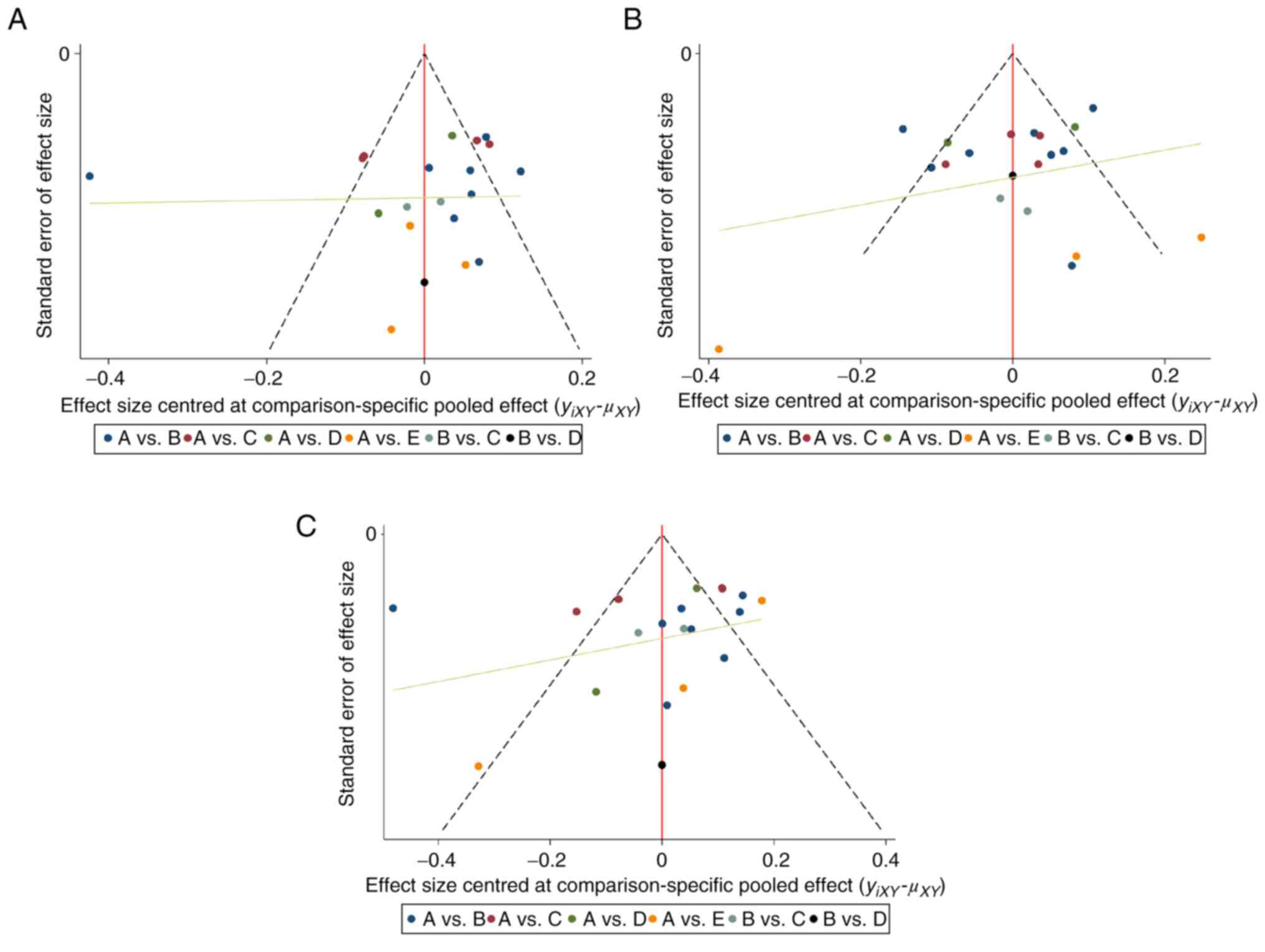
domains. Potential publication bias was assessed using funnel plots
(Fig. 4). The roughly symmetric
distribution suggested a negligible presence of publication bias or
other forms of bias within the studies. This symmetry bolstered the
reliability of findings.
Accuracy
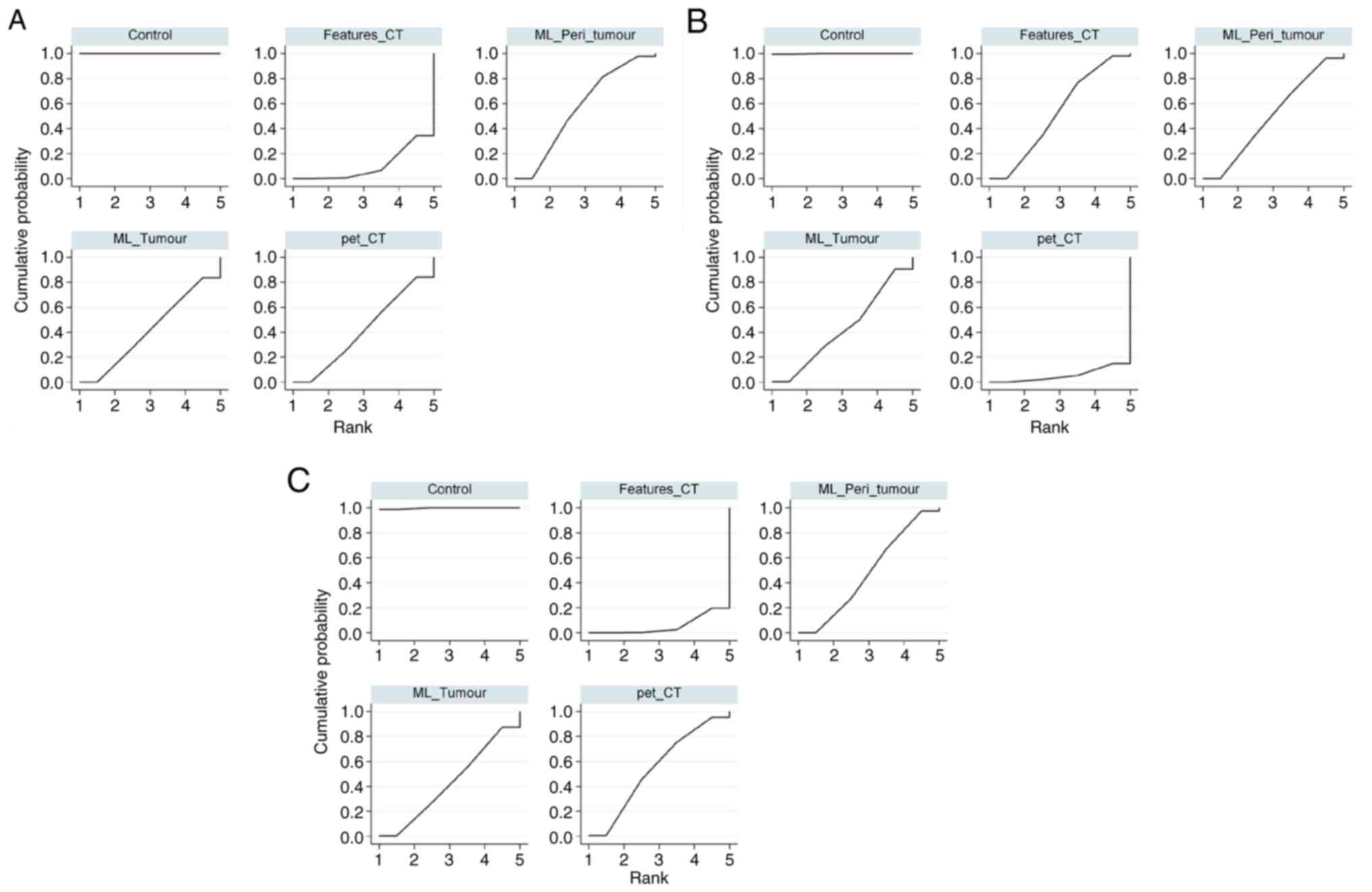
Using the SUCRA, the accuracy of several predictive
models for STAS was evaluated. Models ranked in descending order of
accuracy were as follows: Control (100.0%); ML_Peri_tumour (56.5%);
ML_Tumour (41.8%); pet_CT (41.4%) and Features_CT (10.3%; Fig. 5A). A detailed two-by-two comparative
analysis is presented in Table
IIIA, highlighting the predictive efficacy of these models.
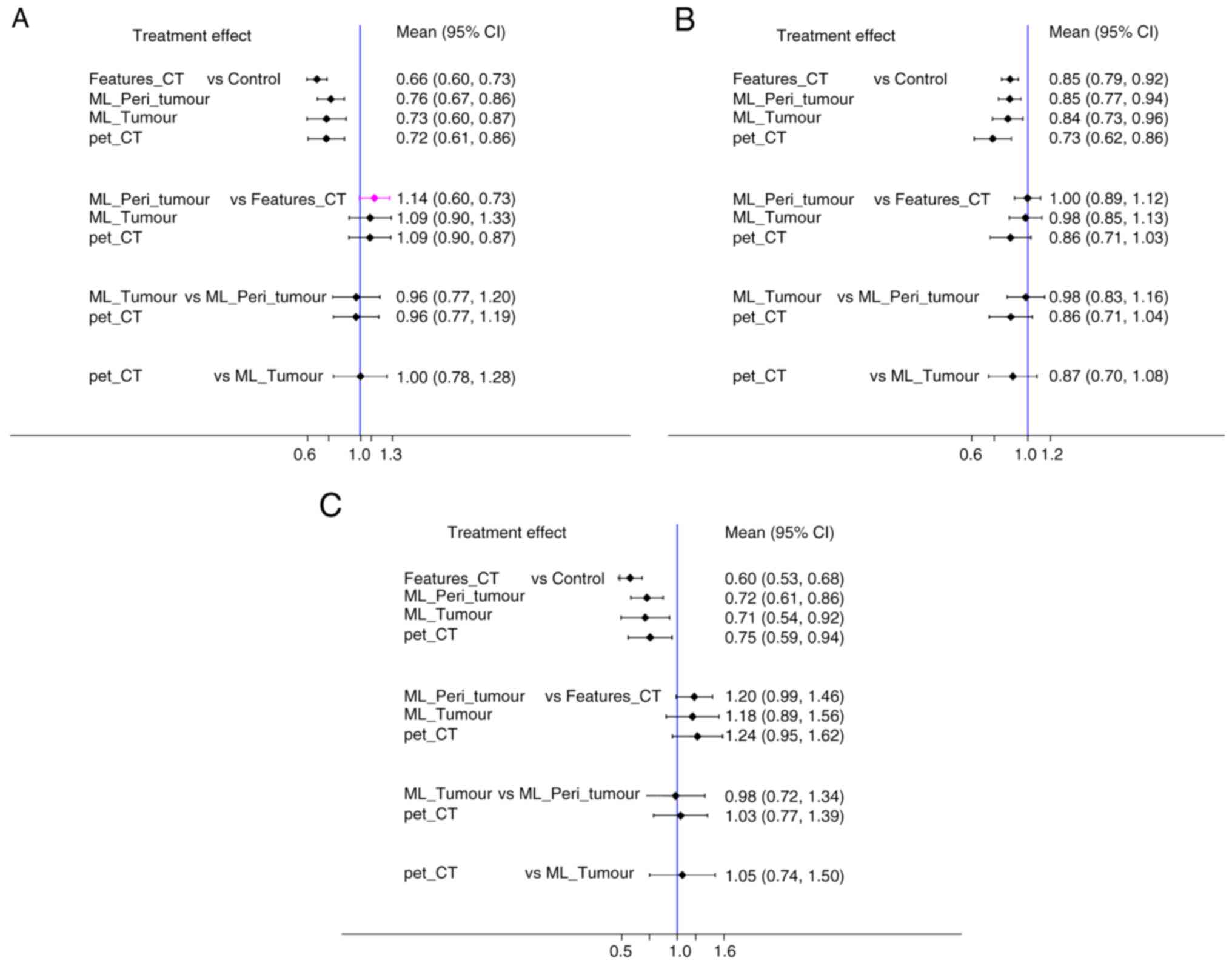
ML_Peri_tumour model demonstrated superior accuracy, particularly
compared with Features_CT (RR=1.14; 95% CI, 0.99–1.32), ML_Tumour
(RR=1.04; 95% CI, 0.83–1.30) and pet_CT (RR=1.04; 95% CI,
0.84–1.29). A heterogeneity test revealed I2 value of
20.4%. Consistently, the forest plot demonstrated the highest
predictive accuracy for ML_Peri_tumour model (Fig. 6A).
 | Table III.League tables for predictive
models. |
Table III.
League tables for predictive
models.
| A, Accuracy:
I2=20.4%; random effects results. |
|---|
| pet_CT | 1.00
(0.78,1.29) | 1.04
(0.84,1.29) | 0.92
(0.75,1.11) | 1.38
(1.16,1.64) |
| 1.00
(0.78,1.28) | ML_Tumour | 1.04
(0.83,1.30) | 0.91
(0.75,1.11) | 1.38
(1.15,1.66) |
| 0.96
(0.77,1.19) | 0.96
(0.77,1.20) | ML_Peri_tumour | 0.88
(0.76,1.01) | 1.32
(1.16,1.50) |
| 1.09
(0.90,1.33) | 1.09
(0.90,1.33) | 1.14
(0.99,1.32) | Features_CT | 1.51
(1.37,1.66) |
| 0.72
(0.61,0.86) | 0.73
(0.60,0.87) | 0.76
(0.67,0.86) | 0.66
(0.60,0.73) | Control |
|
| B, SEN:
I2=17.8%; random effects results. |
|
| pet_CT | 1.14
(0.92,1.42) | 1.16
(0.96,1.41) | 1.17
(0.97,1.40) | 1.37
(1.16,1.61) |
| 0.87
(0.70,1.08) | ML_Tumour | 1.02
(0.86,1.20) | 1.02
(0.88,1.18) | 1.20
(1.04,1.37) |
| 0.86
(0.71,1.04) | 0.98
(0.83,1.16) | ML_Peri_tumour | 1.00
(0.89,1.13) | 1.18
(1.06,1.30) |
| 0.86
(0.71,1.03) | 0.98
(0.85,1.13) | 1.00
(0.89,1.12) | Features_CT | 1.17
(1.09,1.26) |
| 0.73
(0.62,0.86) | 0.84
(0.73,0.96) | 0.85
(0.77,0.94) | 0.85
(0.79,0.92) | Control |
|
| C, SPE:
I2=9.1%; random effects results. |
|
| pet_CT | 0.95
(0.67,1.35) | 0.97
(0.72,1.29) | 0.81
(0.62,1.06) | 1.34
(1.06,1.70) |
| 1.05
(0.74,1.50) | ML_Tumour | 1.02
(0.75,1.39) | 0.85
(0.64,1.13) | 1.41
(1.09,1.84) |
| 1.03
(0.77,1.39) | 0.98
(0.72,1.34) | ML_Peri_tumour | 0.83
(0.69,1.01) | 1.39
(1.17,1.65) |
| 1.24
(0.95,1.62) | 1.18
(0.89,1.56) | 1.20
(0.99,1.46) | Features_CT | 1.66
(1.46,1.90) |
| 0.75
(0.59,0.94) | 0.71
(0.54,0.92) | 0.72
(0.61,0.86) | 0.60
(0.53,0.68) | Control |
SEN
SEN for different predictive models for STAS,
derived from the SUCRA was as follows: Control (99.9%); Features_CT
(51.9%); ML_Peri_tumour (49.9%); ML_Tumour (42.8%); and pet_CT
(5.5%; Fig. 5B). Table IIIB shows a comparative league
table for a two-by-two analysis of these models. Features_CT model
exhibited superior SEN, especially compared with ML_Peri_tumour
(RR=1.00; 95% CI, 0.89–1.13), ML_Tumour (RR=1.02; 95% CI,
0.88–1.18) and pet_CT (RR=1.17; 95% CI, 0.97–1.40). The
heterogeneity test indicated an I2 of 17.8%.
Additionally, forest plot highlighted the superior predictive SEN
of the Features_CT model (Fig.
6B).
SPE
SPE of different predictive models for STAS,
ascertained using the SUCRA, was as follows: Control (99.7%);
pet_CT (53.9%); ML_Peri_tumour (48.0%); ML_Tumour (42.7%); and
Features_CT (5.7%; Fig. 5C). A
comprehensive league table in Table
IIIC compares these models in a two-by-two format. The pet_CT
model showed enhanced SPE, particularly against ML_Peri_tumour
(RR=1.03; 95% CI, 0.77–1.39), ML_Tumour (RR=1.05; 95% CI,
0.74–1.50) and Features_CT (RR=1.24; 95% CI, 0.95–1.62). The
heterogeneity test yielded I2 of 9.1%. The forest plot
indicated the superior predictive SPE of the pet_CT model (Fig. 6C).
Subgroup diagnostic MA
Diagnostic MA scrutinized the predictive
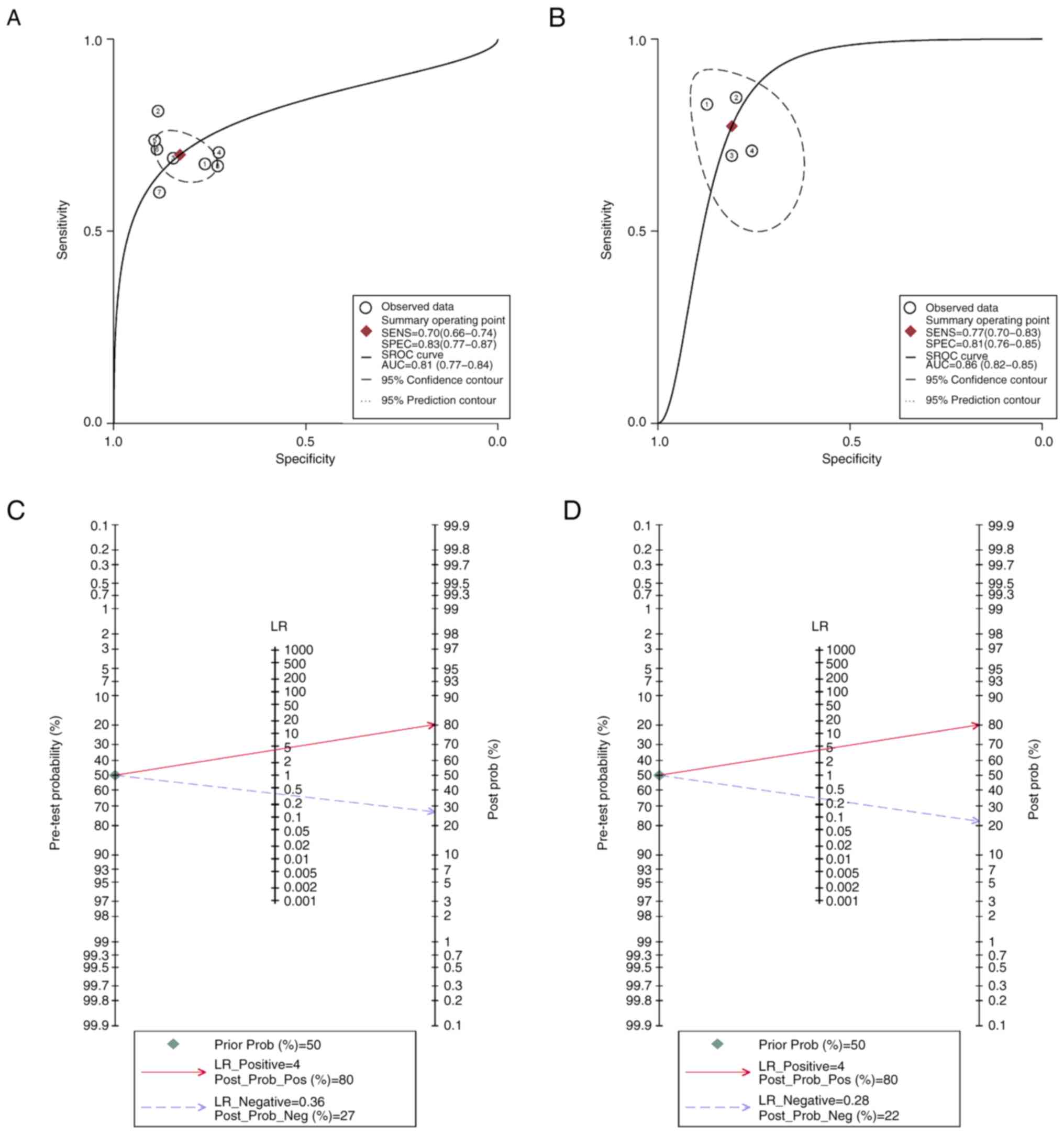
capabilities of the ML_Peri_tumour and Features_CT models. AUC of
the sROC for the ML_Peri_tumour model was 0.86 (95% CI, 0.82–0.88),
while for the Features_CT model it was 0.81 (95% CI, 0.77–0.84;
Fig. 7A and B, respectively). Fagan
plot analysis, which assessed the predictive potency of models,
demonstrated the relative superiority of the ML_Peri_tumour model
(Fig. 7C and D).
Discussion
The present MA evaluated predictive accuracy of
several models for STAS in LUAD. Analyzing 14 studies encompassing
3,734 patients, four predictive models were assessed. Among these,
the ML_Peri_tumour model, using ML to analyze tumor and peritumor
radiographic features, was the most effective. This model
demonstrated superior performance in accuracy, SEN and SPE,
evidenced by its SUCRA values of 56.5, 49.9 and 48.0, respectively.
Furthermore, a diagnostic MA supported the efficacy of the
ML_Peri_tumour model, indicating a pooled AUC of 0.86 (95% CI,
0.82–0.88).
Previous studies have substantially deepened
understanding of STAS in LUAD, especially regarding its prediction
via radiological and pathological features (36,37).
Investigations into predictive CT characteristics for STAS in
small-sized LUAD have reported that attributes such as
consolidation tumor ratio, spiculation, satellites, ground glass
ribbon sign, pleural attachment and unclear tumor-lung interface
are effective predictors of STAS (30,38).
This aligns with the present accuracy of the ML_Peri_tumour and
other ML-based models, underscoring the significance of CT features
in these models.
The evolving understanding of the association
between tumor stromal cells and STAS, along with the role of
stromal cells in STAS pathogenesis, is noteworthy. Advanced medical
information technology, including three-dimensional space
convolution and fuzzy neural networks, has demonstrated potential
in enhancing diagnostic SEN and SPE for lung cancer, suggesting
promising avenues for future STAS prediction models (39).
Another notable development is the association
between fluorodeoxyglucose (FDG) metabolic tumor burden, measured
by PET/CT and STAS. Studies using PET/CT metrics such as
standardized uptake value and total lesion glycolysis have reported
that LUAD with low FDG uptake is associated with a lower incidence
of STAS, whilst subtypes with higher FDG uptake, such as solid
predominant adenocarcinoma, show a higher incidence of STAS
(34,35). Furthermore, integration of ML
techniques for analyzing radiological data for tumor and peritumor
features has resulted in models with improved predictive accuracy
for STAS. A study by Liao et al (5) involving 256 patients, integrated tumor
radiomic signature (TRS) with peritumoral radiomic signatures (PRS)
and developed an effective gross radiomic signature model.
Particularly, TRS combined with the PRS-15 mm model exhibited
substantial predictive accuracy, achieving an AUC of 0.854 in the
development and 0.870 in the validation cohort. The focus on the
peritumoral environment represents a notable advancement over prior
research (28), which predominantly
concentrated on the primary tumor alone. The success of the
ML_Peri_tumour model in the present study highlights the potential
of merging radiomic features from both tumor and peritumor regions,
providing a more comprehensive approach to STAS prediction. This is
relevant since STAS, typically found at the tumor periphery, may be
more accurately predicted using preoperative CT images of tumor
margins (26,33).
The present analysis revealed that the majority of
radiomics studies on STAS prediction were in early or intermediate
stages of research. The rigorous design of these studies is vital
for validating the feasibility of radiological approaches. The
present study identified limitations, including a lack of
reproducibility analysis, internal validation and comprehensive
performance evaluation of models. Notably, none of the included
studies performed phantom or test-retest analyses for validating
feature robustness (40,41) and only three studies addressed
calibration, which is a key metric for evaluating prediction
consistency with actual outcomes (5,15,32).
To advance clinical application and practicality of radiomics,
attention must be paid to external validation, cost-effectiveness
and availability of open data. Validation with data from other
institutions or different time periods is key for confirming model
generalizability (42). However,
only one study in the present analysis validated radiomic
signatures with external data (15). Furthermore, a lack of open data and
code availability, essential for assessing reproducibility, was a
common limitation across studies (43). In the present NMA, included studies
encompassed two different regression methods for constructing
predictive models: ML (6/14) and binary logistic regression (8/14).
ML focuses on the accuracy of the final model, while binary
logistic regression also pays attention to metrics such as the odds
ratio for each variable (44). The
present research did not reveal any heterogeneity between different
types of regression method. However, the comprehensive analysis
indicated that the ML_Peri_tumour model held greater value in
predicting STAS, potentially due to its consideration of radiomic
signatures in the peritumoral region. Nevertheless, use of models
developed using diverse regression methods is a limitation of the
present study. To enhance robustness and comprehensiveness of
results of the present study, the incorporation of additional
studies for detailed subgroup analyses is key. Additionally, 8/14
studies reviewed focused on the occurrence of STAS in patients with
stage I LUAD. However, the MA did not demonstrate inter-study
heterogeneity in this aspect. There is need for more research to
evaluate the predictive efficiency of radiomic models for STAS in
early-stage LUAD, where accurate diagnosis is of paramount
importance. Assessing STAS status prior to developing a surgical
plan is key, as it significantly influences the selection of
surgical strategies.
In conclusion, the present study is the first NMA to
integrate several predictive models, to the best of our knowledge.
The findings underscore the superior predictive efficacy of tumor
and peritumor-based radiological models. Nonetheless, research in
radiological features for STAS prediction is in its early stages,
and significant enhancements are needed, particularly in technical
reproducibility and comprehensive model evaluation. The reliability
of these studies requires experimental verification due to limited
external validation. Additionally, the scarcity of direct model
comparisons in the analyzed studies, primarily relying on indirect
comparisons, may affect quality assessment results, underscoring
the need for more direct model comparisons in future. The
ethnographic and geographic applicability of these findings,
primarily contributed to by researchers in Asia, also needs further
validation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Clinical Medicine Science
and Technology Development Foundation of Jiangsu University (grant
no. JLY2021082) and Xuzhou Science and Technology Bureau (grant no.
KC23229).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XL designed the study and revised the manuscript. CL
performed statistical analysis and wrote the manuscript. CL and PW
performed the literature review and quality assessment. PW, YW, FG
and ZS interpreted the data. CL and XL confirm the authenticity of
all the raw data. HZ and QW were involved in the study design and
critically revised the manuscript. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhuo Y, Feng M, Yang S, Zhou L, Ge D, Lu
S, Liu L, Shan F and Zhang Z: Radiomics nomograms of tumors and
peritumoral regions for the preoperative prediction of spread
through air spaces in lung adenocarcinoma. Transl Oncol.
13:1008202020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blaauwgeers H, Flieder D, Warth A, Harms
A, Monkhorst K, Witte B and Thunnissen E: A prospective study of
loose tissue fragments in non-small cell lung cancer resection
specimens: An alternative view to ‘spread through air spaces’. Am J
Surg Pathol. 41:1226–1230. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kadota K, Nitadori JI, Sima CS, Ujiie H,
Rizk NP, Jones DR, Adusumilli PS and Travis WD: Tumor spread
through air spaces is an important pattern of invasion and impacts
the frequency and location of recurrences after limited resection
for small stage I lung adenocarcinomas. J Thorac Oncol. 10:806–814.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Travis WD, Brambilla E, Nicholson AG,
Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E,
Flieder DB, et al: The 2015 world health organization
classification of lung tumors: Impact of genetic, clinical and
radiologic advances since the 2004 classification. J Thorac Oncol.
10:1243–1260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liao G, Huang L, Wu S, Zhang P, Xie D, Yao
L, Zhang Z, Yao S, Shanshan L, Wang S, et al: Preoperative CT-based
peritumoral and tumoral radiomic features prediction for tumor
spread through air spaces in clinical stage I lung adenocarcinoma.
Lung Cancer. 163:87–95. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Terada Y, Takahashi T, Morita S,
Kashiwabara K, Nagayama K, Nitadori JI, Anraku M, Sato M,
Shinozaki-Ushiku A and Nakajima J: Spread through air spaces is an
independent predictor of recurrence in stage III (N2) lung
adenocarcinoma. Interact Cardiovasc Thorac Surg. 29:442–448. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Niu Y, Han X, Zeng Y, Nanding A, Bai Q,
Guo S, Hou Y, Yu Y, Zhang Q and Li X: The significance of spread
through air spaces in the prognostic assessment model of stage I
lung adenocarcinoma and the exploration of its invasion mechanism.
J Cancer Res Clin Oncol. 149:7125–7138. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eguchi T, Kameda K, Lu S, Bott MJ, Tan KS,
Montecalvo J, Chang JC, Rekhtman N, Jones DR, Travis WD and
Adusumilli PS: Lobectomy is associated with better outcomes than
sublobar resection in spread through air spaces (STAS)-positive T1
lung adenocarcinoma: A propensity score-matched analysis. J Thorac
Oncol. 14:87–98. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ji GW, Zhang YD, Zhang H, Zhu FP, Wang K,
Xia YX, Zhang YD, Jiang WJ, Li XC and Wang XH: Biliary tract cancer
at CT: A radiomics-based model to predict lymph node metastasis and
survival outcomes. Radiology. 290:90–98. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Autorino R, Gui B, Panza G, Boldrini L,
Cusumano D, Russo L, Nardangeli A, Persiani S, Campitelli M,
Ferrandina G, et al: Radiomics-based prediction of two-year
clinical outcome in locally advanced cervical cancer patients
undergoing neoadjuvant chemoradiotherapy. Radiol Med. 127:498–506.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cong H, Peng W, Tian Z, Vallières M,
Chuanpei X, Aijun Z and Benxin Z: FDG-PET/CT radiomics models for
the early prediction of locoregional recurrence in head and neck
cancer. Curr Med Imaging. 17:374–383. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang C, Luo Y, Yuan J, You S, Chen Z, Wu
M, Wang G and Gong J: CT-based radiomics and machine learning to
predict spread through air space in lung adenocarcinoma. Eur
Radiol. 30:4050–4057. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li C, Jiang C, Gong J, Wu X, Luo Y and Sun
G: A CT-based logistic regression model to predict spread through
air space in lung adenocarcinoma. Quant Imaging Med Surg.
10:1984–1993. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu A, Sun X, Xu J, Xuan Y, Zhao Y, Qiu T,
Hou F, Qin Y, Wang Y, Lu T, et al: Relevance and prognostic ability
of Twist, Slug and tumor spread through air spaces in lung
adenocarcinoma. Cancer Med. 9:1986–1998. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Y, Jiang C, Kang W, Gong J, Luo D,
You S, Cheng Z, Luo Y and Wu K: Development and validation of a
CT-based nomogram to predict spread through air space (STAS) in
peripheral stage IA lung adenocarcinoma. Jpn J Radiol. 40:586–594.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nishimori M, Iwasa H, Miyatake K, Nitta N,
Nakaji K, Matsumoto T, Yamanishi T, Yoshimatsu R, Iguchi M, Tamura
M and Yamagami T: 18F FDG-PET/CT analysis of spread through air
spaces (STAS) in clinical stage I lung adenocarcinoma. Ann Nucl
Med. 36:897–903. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shea BJ, Reeves BC, Wells G, Thuku M,
Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E and
Henry DA: AMSTAR 2: A critical appraisal tool for systematic
reviews that include randomised or non-randomised studies of
healthcare interventions, or both. BMJ. 358:j40082017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Higgins JPT, Thomas J, Chandler J,
Cumpston M, Li T, Page MJ and Welch VA: Cochrane handbook for
systematic reviews of interventions version 6.4 (updated August
2023). Cochrane; 2023
|
|
19
|
Whiting PF, Rutjes AW, Westwood ME,
Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA and Bossuyt
PM; QUADAS-2 group, : QUADAS-2: A revised tool for the quality
assessment of diagnostic accuracy studies. Ann Intern Med.
155:529–536. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang SR, Li QL, Tian F, Li J, Li WX, Chen
M, Sang T, Cao CL and Shi LN: Diagnostic value of multiple
diagnostic methods for lymph node metastases of papillary thyroid
carcinoma: A systematic review and meta-analysis. Front Oncol.
12:9906032022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kutob L and Schneider F: Lung cancer
staging. Surg Pathol Clin. 13:57–71. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Heinecke A, Tallarita M and De Iorio M:
Bayesian splines versus fractional polynomials in network
meta-analysis. BMC Med Res Methodol. 20:2612020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hutton B, Salanti G, Caldwell DM, Chaimani
A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen
JP, et al: The PRISMA extension statement for reporting of
systematic reviews incorporating network meta-analyses of health
care interventions: Checklist and explanations. Ann Intern Med.
162:777–784. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kapor S, Rankovic MJ, Khazaei Y, Crispin
A, Schüler I, Krause F, Lussi A, Neuhaus K, Eggmann F, Michou S, et
al: Systematic review and meta-analysis of diagnostic methods for
occlusal surface caries. Clin Oral Investig. 25:4801–4815. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bassi M, Russomando A, Vannucci J,
Ciardiello A, Dolciami M, Ricci P, Pernazza A, D'Amati G,
Terracciano CM, Faccini R, et al: Role of radiomics in predicting
lung cancer spread through air spaces in a heterogeneous dataset.
Transl Lung Cancer Res. 11:560–571. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qi L, Li X, He L, Cheng G, Cai Y, Xue K
and Li M: Comparison of diagnostic performance of spread through
airspaces of lung adenocarcinoma based on morphological analysis
and perinodular and intranodular radiomic features on chest CT
images. Front Oncol. 11:6544132021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen LW, Lin MW, Hsieh MS, Yang SM, Wang
HJ, Chen YC, Chen HY, Hu YH, Lee CE, Chen JS, et al: Radiomic
values from high-grade subtypes to predict spread through air
spaces in lung adenocarcinoma. Ann Thorac Surg. 114:999–1006. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim SK, Kim TJ, Chung MJ, Kim TS, Lee KS,
Zo JI and Shim YM: Lung Adenocarcinoma: CT features associated with
spread through air spaces. Radiology. 289:831–840. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qin L, Sun Y, Zhu R, Hu B and Wu J:
Clinicopathological and CT features of tumor spread through air
space in invasive lung adenocarcinoma. Front Oncol. 12:9591132022.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qi L, Xue K, Cai Y, Lu J, Li X and Li M:
Predictors of CT morphologic features to identify spread through
air spaces preoperatively in small-sized lung adenocarcinoma. Front
Oncol. 10:5484302020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Z, Liu Z, Feng H, Xiao F, Shao W,
Liang C, Sun H, Gu X and Liu D: Predictive value of radiological
features on spread through air space in stage cIA lung
adenocarcinoma. J Thorac Dis. 12:6494–6504. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Han X, Fan J, Zheng Y, Ding C, Zhang X,
Zhang K, Wang N, Jia X, Li Y, Liu J, et al: The value of CT-based
radiomics for predicting spread through air spaces in stage IA lung
adenocarcinoma. Front Oncol. 12:7573892022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takehana K, Sakamoto R, Fujimoto K, Matsuo
Y, Nakajima N, Yoshizawa A, Menju T, Nakamura M, Yamada R, Mizowaki
T and Nakamoto Y: Peritumoral radiomics features on preoperative
thin-slice CT images can predict the spread through air spaces of
lung adenocarcinoma. Sci Rep. 12:103232022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang XY, Zhao YF, Yang L, Liu Y, Yang YK
and Wu N: Correlation analysis between metabolic tumor burden
measured by positron emission tomography/computed tomography and
the 2015 World Health Organization classification of lung
adenocarcinoma, with a risk prediction model of tumor spread
through air spaces. Transl Cancer Res. 9:6412–6422. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Falay O, Selçukbiricik F, Tanju S, Erus S,
Kapdağli M, Cesur E, Yavuz Ö, Bulutay P, Firat P, Mandel NM and
Dilege Ş: The prediction of spread through air spaces with
preoperative 18F-FDG PET/CT in cases with primary lung
adenocarcinoma, its effect on the decision for an adjuvant
treatment and its prognostic role. Nucl Med Commun. 42:922–927.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang S, Shou H, Wen H, Wang X, Wang H, Lu
C, Gu J, Xu F, Zhu Q, Wang L and Ge D: An individual nomogram can
reliably predict tumor spread through air spaces in non-small-cell
lung cancer. BMC Pulm Med. 22:2092022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Toki MI, Harrington K and Syrigos KN: The
role of spread through air spaces (STAS) in lung adenocarcinoma
prognosis and therapeutic decision making. Lung Cancer.
146:127–133. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun F, Huang Y, Yang X, Zhan C, Xi J, Lin
Z, Shi Y, Jiang W and Wang Q: Solid component ratio influences
prognosis of GGO-featured IA stage invasive lung adenocarcinoma.
Cancer Imagin. 20:872020. View Article : Google Scholar
|
|
39
|
Bai S, Wang Z, Sun Z and Liu Z: Study on
the relationship between lung cancer stromal cells and air cavity
diffusion based on an image acquisition system. Contrast Media Mol
Imaging. 2022:24921242022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Y, Reyhan M, Zhang Y, Wang X, Zhou J,
Zhang Y, Yue NJ and Nie K: The impact of phantom design and
material-dependence on repeatability and reproducibility of
CT-based radiomics features. Med Phys. 49:1648–1659. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Steyerberg EW, Vickers AJ, Cook NR, Gerds
T, Gonen M, Obuchowski N, Pencina MJ and Kattan MW: Assessing the
performance of prediction models: A framework for traditional and
novel measures. Epidemiology. 21:128–138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sauerbrei W, Taube SE, McShane LM,
Cavenagh MM and Altman DG: Reporting recommendations for tumor
marker prognostic studies (REMARK): An abridged explanation and
elaboration. J Natl Cancer Inst. 110:803–811. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nagendran M, Chen Y, Lovejoy CA, Gordon
AC, Komorowski M, Harvey H, Topol EJ, Ioannidis JPA, Collins GS and
Maruthappu M: Artificial intelligence versus clinicians: systematic
review of design, reporting standards, and claims of deep learning
studies. BMJ. 368:m6892020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Christodoulou E, Ma J, Collins GS,
Steyerberg EW, Verbakel JY and Van Calster B: A systematic review
shows no performance benefit of machine learning over logistic
regression for clinical prediction models. J Clin Epidemiol.
110:12–22. 2019. View Article : Google Scholar : PubMed/NCBI
|