Introduction
Testicular cancer is the most common type of cancer
in men aged 14–44 years; its incidence has increased over the past
two decades in Western countries (1). Approximately 50% of patients with
testicular cancer are diagnosed with seminoma, whereas the
remaining are diagnosed with various types of non-seminoma or mixed
testicular germ cell tumors (1).
The implementation of cisplatin-based chemotherapy regimens and
refinement of surgical procedures have improved the long-term
survival. The cure rate in all patients with testicular cancer and
those with metastatic disease is >95 and 90%, respectively
(2). Undescended testis,
contralateral testicular tumor, and familial testis cancer are
established risk factors for testicular cancer (3). Moreover, there is a proven correlation
between infertility and testicular cancer, and infertile men with
semen abnormalities are 20 times more likely to develop testicular
cancer (4). Future fertility is a
concern for young patients undergoing cancer treatment (5). Oligozoospermia is present in >50%
of patients with testicular tumors before treatment (6) and testicular tumors are sometimes
identified during infertility examinations (7). Declining semen quality in testicular
cancer could be due to mechanical loss of physical testicular
volume in the affected testis, paracrine and endocrine effects on
the ipsilateral and contralateral testis from the tumor, and
congenital factors (5).
The present report describes a case in which a
patient with infertility and history of cryptorchidism surgery was
diagnosed with two types of testicular tumor in one testis; semen
parameters and hormonal status improved following high
orchiectomy.
Case study
A 38-year-old male patient was referred to Dokkyo
Medical University Saitama Medical Center, Saitama, Japan) in April
2021 with oligozoospermia, detected during investigation of
infertility. The patient had a history of surgery for left
cryptorchidism during infancy and no medication history. A physical
examination revealed no abnormalities in the testes. Scrotal color
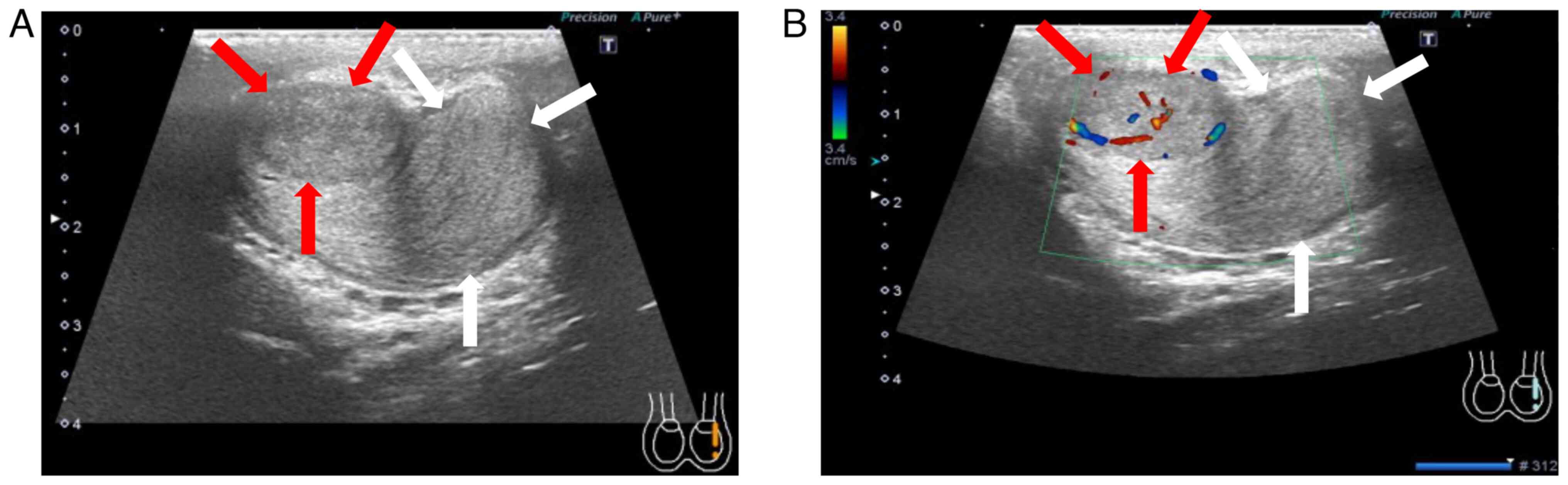
Doppler ultrasonography showed that the right testis was normal;
however, the left testis had a mass with clear margins and abundant
blood flow in the cranial part and a mass with clear margins but
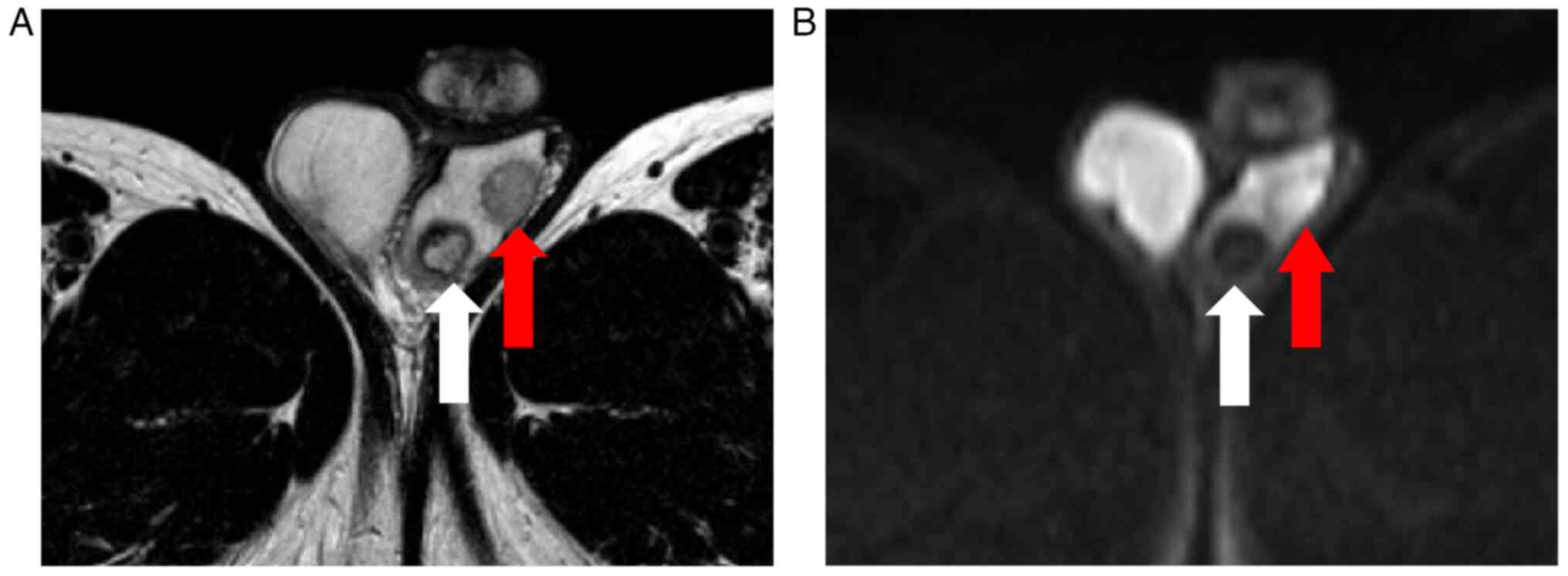
poor blood flow in the caudal part (Fig. 1). Magnetic resonance imaging showed
diffusion limitation in the cranial, but not the caudal part, of
the left testis (Fig. 2).
Chest-abdomen-pelvis computed tomography (CT; TSX-301C/3A, Canon
Medical Systems) did not reveal any metastasis.
Tumor marker assessment revealed mildly elevated
levels of intact human chorionic gonadotropin (hCG; 29.6 mIU/ml)
and normal levels of lactate dehydrogenase (137 IU/l),
α-fetoprotein (3.0 ng/ml) and hCG-β (0.1 ng/ml). Hormonal level
assessment demonstrated high testosterone (24.69 ng/ml) and
estradiol (115.5 pg/ml) levels and low luteinizing hormone (LH;
<0.1 mIU/ml) and follicle-stimulating hormone (FSH; <0.1
mIU/ml) levels. Semen analysis was performed according to the WHO
2010 manual (8). Semen analysis
revealed severe oligoasthenozoospermia (semen volume, 2.8 ml; sperm
density, 0.1×106 sperm/ml; 15 motile sperm were observed
in all fields; Table I).
 | Table I.Pre- and post-surgery blood and semen
parameters. |
Table I.
Pre- and post-surgery blood and semen
parameters.
| Value | Pre-surgery | Post-surgery | Reference value | (Refs.) |
|---|
| LDH, IU/l | 137 | 114 | 124-222 | a |
| AFP, ng/ml | 3.0 | 2.9 | <10.0 | a |
| hCG-β, ng/ml | 0.1 | <0.1 | <0.1 | a |
| Intact hCG,
mIU/ml | 29.6 | <0.5 | <5.0 | a |
| Testosterone,
ng/ml | 24.69 | 5.24 | 1.32–8.71 | a |
| Estradiol, pg/ml | 115.5 | 10.5 | 14.6–48.8 | a |
| LH, mIU/ml | <0.1 | 6.5 | 2.2–8.4 | a |
| FSH, mIU/ml | <0.1 | 5.6 | 1.8–12.0 | a |
| Semen volume, ml | 2.8 | 5.0 | ≥1.4 | 20 |
| Sperm density,
×106/ml | 0.1 | 29.0 | ≥16.0 | 20 |
| Motility, % | <0.1 | 44.8 | ≥42.0 | 20 |
The preoperative diagnosis was left testicular
cancer and severe oligoasthenozoospermia. The patient underwent
left high orchiectomy and oncological testicular sperm extraction
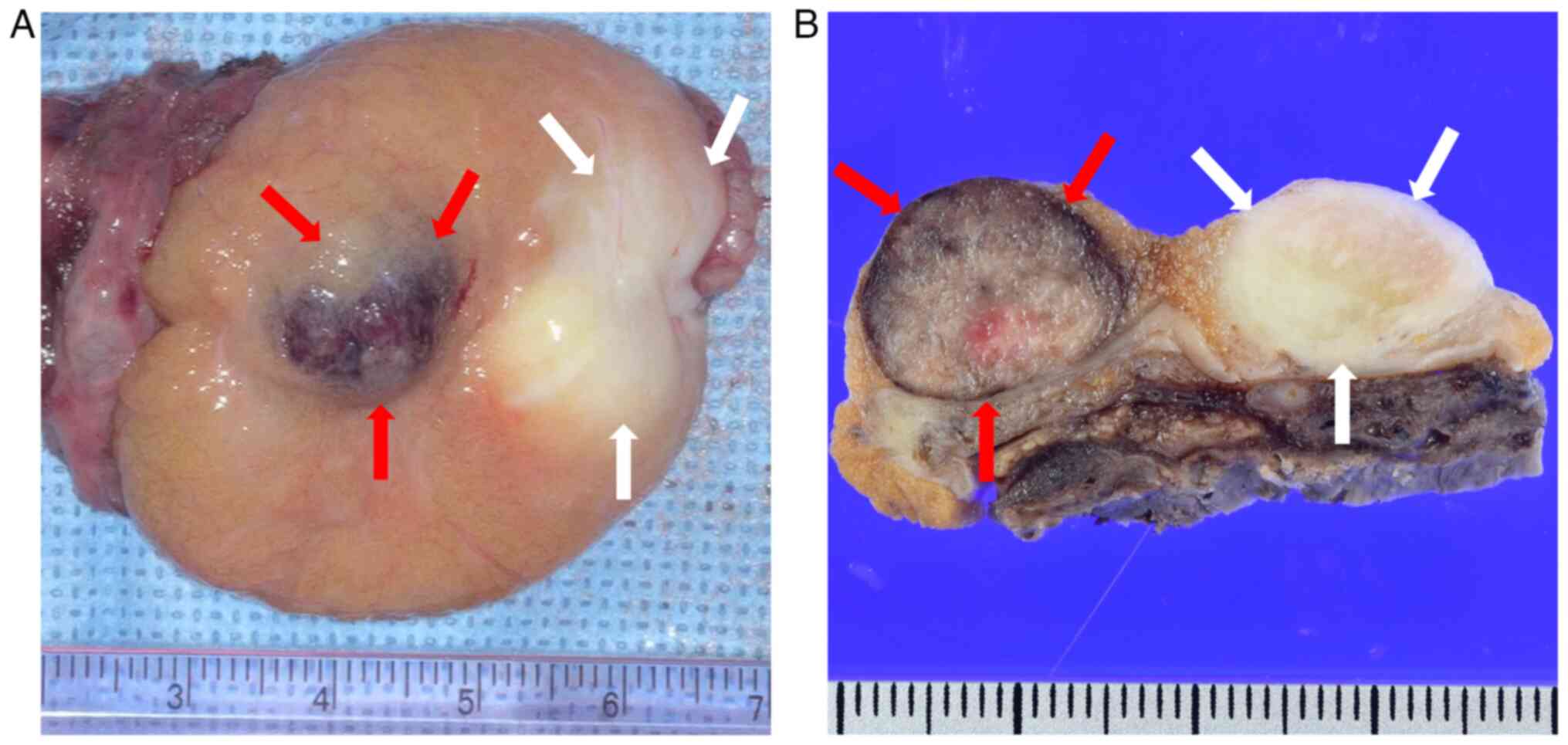
(onco-TESE). Gross examination of the extracted left testis
revealed a reddish-brown mass in the cranial and a grayish-white
mass in the caudal part (Fig. 3).
Pathological assessment was performed on the formalin-fixed,
paraffin-embedded (FFPE) tissue block of surgical specimen stained
with hematoxylin and eosin. Immunohistochemical staining for
octamer binding transcription factor (OCT)-3/4, D2-40, hCG, SALL4
and testosterone was performed on the FFPE tissue block. Samples
were fixed in 10% neutral PBS at room temperature for 24 to 48 h;
thickness of section, 4 µm. Antigen retrieval was performed using
EnVision FLEX Target Retrieval Solution, High pH (Agilent
Technologies, 97°C, 20 min). Quenching step was performed using
EnVision FLEX peroxidase blocking reagent, Hydrogen peroxide
solution (ready to use, Agilent Technologies); v) the following
primary antibodies were used: OCT-3/4 (1:100, NCL-L-OCT3/4, Leica
Biosystems), D2-40 (ready to use, 713451, Nichirei Biosciences),
hCG (ready to use, IS508, Dako), SALL4 (1:1,000, H6271-6E3,
Sigma-Aldrich), and testosterone (1:400, cat. no. ab217912, Abcam)
incubated at room temperature for 30 min; vi) the following
secondary antibodies were used: EnVision FLEX/HRP (ready to use,
K8000, Agilent Technologies), incubated at room temperature for 20
min; vii) EnVision FLEX DAB+ Substrate Chromogen System (Agilent
Technologies) was used for chromogen detection, while Mayer's
Hematoxylin Solution (room temperature, 30 sec) was used for
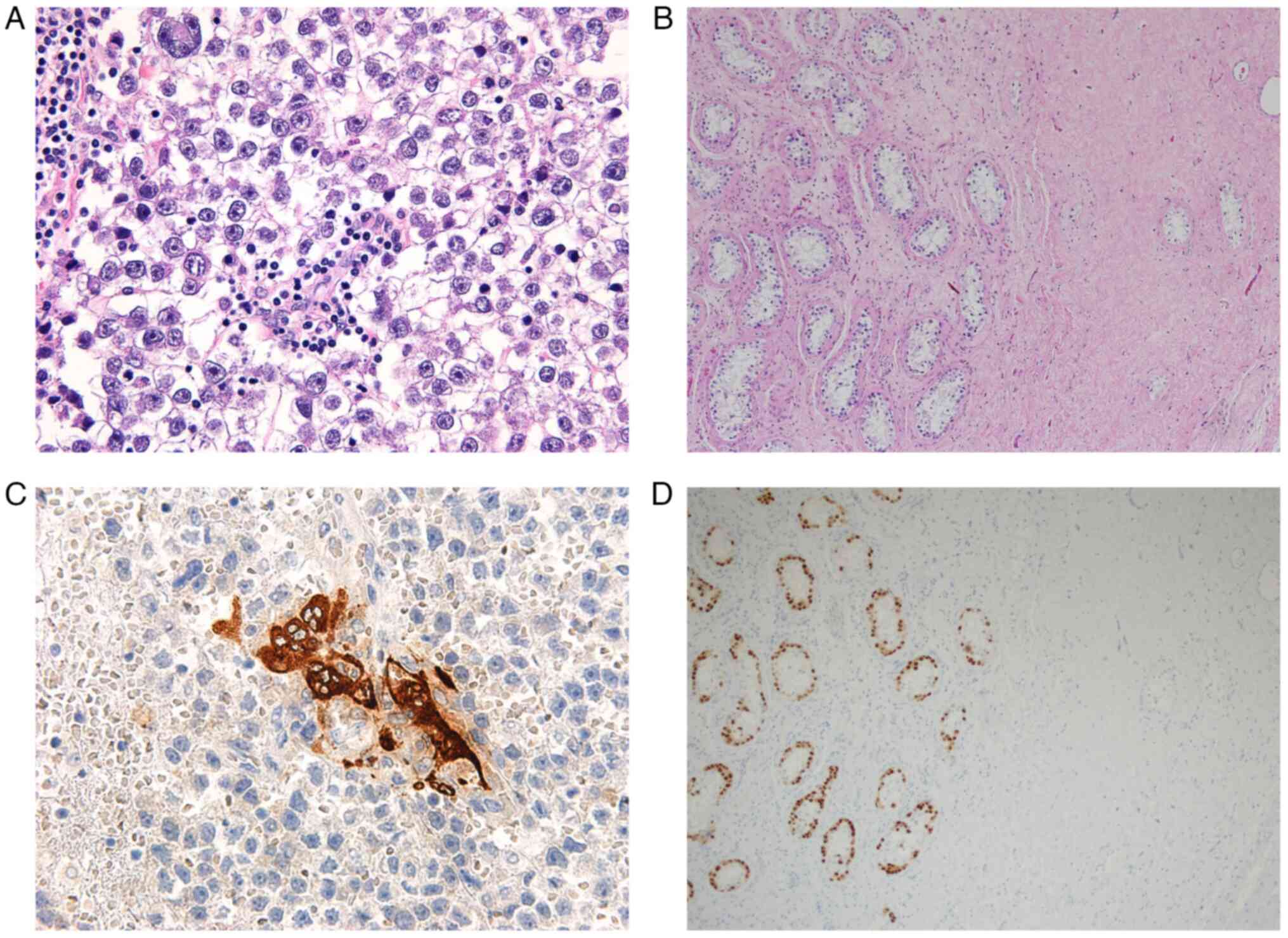
counterstain. Pathological assessment of the cranial tumor
demonstrated a proliferation of tumor cells with round nuclei and
well-defined nucleoli. The tumor cells were OCT-3/4+ and
D2-40+, and had characteristics of a seminoma with
numerous hCG+ trophoblastic cells. The caudal tumor was
composed of vitrified material with few cellular components and no
evidence of malignancy. Dysplastic cells with round nuclei and
well-defined nucleoli were observed in the adjacent intratubular
parenchyma. The dysplastic cells were OCT-3/4+ and
SALL4+ and had the characteristics of germ cell
neoplasia in situ (GCNIS; Fig.
4). Both tumors were negative for testosterone. The
pathological findings of onco-TESE were a small number of
spermatocytes and spermatozoa in a few seminiferous tubules
(Johnsen score, 5.4) (9). Based on
the pathology, cranial tumor was diagnosed as a seminoma with
syncytiotrophoblast cells, and the caudal tumor was diagnosed as
regressed GC tumor.
Following surgery, the patient was followed up
without medication. At 1 month post-surgery, hormone level
assessment demonstrated improvements in several hormone levels
(testosterone, 5.24 ng/ml; estradiol, 10.5 pg/ml; LH, 6.5 mIU/ml
and FSH, 5.6 mIU/ml). Furthermore, the intact hCG at 10 months
after surgery was almost undetectable (<0.5 mIU/ml). Semen
analysis 2 months after surgery demonstrated an improvement in
semen parameters (semen volume, 5.0 ml; sperm density,
29×106 sperm/ml and motility, 44.8%). The patient and
his partner achieved spontaneous conception 12 months after surgery
and a healthy baby was born 22 months post-surgery. As of November
2023, the patient had no recurrence at CT follow-up checks and no
elevation of serum tumor marker levels 30 months after surgery.
Discussion
The risk of testicular tumor is 4.8-fold higher in
patients with a history of cryptorchidism, which is an established
risk factor for testicular tumors (3); however, the mechanism underlying the
association between cryptorchidism and testicular tumors remains
unclear (10). In the present case,
two distinct tumors were noted in the left testis of a patient with
a history of cryptorchidism, suggesting that cryptorchidism may be
associated with tumor development.
Patients with testicular tumors in one testis are
more likely to develop contralateral testicular tumors than
patients without testicular tumors (3). There are numerous reports of bilateral
testicular tumor development (3,11), but
no reports of two types of testicular tumors in one testis, to the
best of our knowledge. Therefore, the present case is rare.
In the present case, the tumor on the caudal side
had regressed, resulting in a lack of symptoms. Hence, it is
possible that the tumor on the cranial side (the seminoma) would
not have been detected until it increased in size. Early detection
was achieved via scrotal ultrasonography during an infertility
examination. As certain patients may have no symptoms, scrotal
ultrasonography should be performed in those with abnormal semen
results to rule out testicular tumors. The cranial seminoma may be
considered a metastatic lesion of the caudal tumor and the caudal
tumor, which had only scar tissue, may be considered a regressed GC
tumor. Pathological findings of regressed GC tumors typically
include scarring, decreased spermatogenesis and microlithiasis
(12). Notably, the findings of
GCNIS in the adjacent parenchyma, and coarse and large intratubular
calcifications have been suggested to be specific for GC tumor
regression rather than non-neoplastic scarring (12). Non-neoplastic scarring secondary to
ischemia, trauma or infection is typically seen in testes lacking
diffuse atrophy and is often multifocal. Additionally,
non-neoplastic scarring may be associated with vascular lesions
such as thrombi and vasculitis and is not associated with more
specific features of regression such as GCNIS, and coarse and large
intratubular calcifications. Nodular and stellate atrophy with
interstitial fibrosis in testicular regressed GC tumors are
distinguished from pure atrophy (13). The patient in the present case had a
distinct nodular scar with GCNIS in the adjacent parenchyma, which
may indicate a regressed GC tumor.
The association between male infertility and
testicular tumors is well-established, and ≤50% of patients with
testicular tumors prior to high orchiectomy have abnormal semen
parameters (4,14). In the present case, hormonal status
(high testosterone and low LH and FSH levels) and semen parameters
improved notably following resection of the testicular tumors. This
indicated that the testicular tumors caused hormonal abnormalities
and infertility. Pathological findings demonstrated no testosterone
production in either tumor; however, the seminoma contained
syncytiotrophoblast cells that were positive for hCG.
Previous studies have reported that in patients with
testicular tumors and elevated blood β-hCG levels, hCG has an
LH-like effect, gonadotropin production is suppressed and blood
testosterone and estradiol levels are increased (15,16).
In the present case, the blood hCG-β levels were within the normal
range; however, the intact hCG levels in the blood were mildly
elevated, which may have contributed to the increase in
testosterone levels. It is likely that hCG concentrations were
higher in the left testis than in the blood as hCG is produced by
the cranial tumor, a seminoma with syncytiotrophoblast cells
(17). The high hCG environment in
the left testis may have stimulated the production of testosterone
by Leydig cells, which in turn suppressed LH and FSH secretion by
the pituitary gland via negative feedback. As a result,
spermatogenesis may have been notably inhibited, causing severe
oligoasthenozoospermia. In addition, an increase in blood estradiol
levels has a negative feedback effect on activity of the
hypothalamic-pituitary-gonadal axis (18). In the present case, the estradiol
levels were elevated, and suppression of gonadotropin production
may have led to progressive dysfunction of spermatogenesis.
Testicular tumors promote production of several hormones (e.g.,
hCG-β, estradiol, and prolactin) and cytokines (e.g.,
interleukin-1, interleukin-6, and tumor necrosis factor-α) that
notably change the intratesticular environment (19). These changes cause spermatogenic
dysfunction. The present case is a good clinical example of changes
in multiple hormone levels due to testicular tumor treatment
improving semen parameters.
In conclusion, the present report describes the
first case, to the best of our knowledge, in which two types of
testicular tumors were found in a unilateral testis in a patient
with a history of cryptorchidism surgery. The present report
demonstrated that scrotal ultrasonography should be performed in
patients with abnormal semen results to rule out testicular
tumors.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
HT, KU, AO and TI participated in the conception,
design and data acquisition of the study. HT wrote the manuscript.
AF and SB performed the histological assessment of the testis and
wrote the manuscript. KU, HO and KS interpreted data and reviewed
and edited the manuscript. HT and KU confirm the authenticity of
all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study protocol was reviewed in accordance with
the Dokkyo Medical University Saitama Medical Center Ethics
Committee's regulations and approved by the Committee (Koshigaya,
Japan; approval no. 22096).
Patient consent for publication
Written informed consent was obtained from the
patient for publication of data and images in the present
report.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
FSH
|
follicle-stimulating hormone
|
|
hCG
|
human chorionic gonadotropin
|
|
LH
|
luteinizing hormone
|
|
onco-TESE
|
oncologic testicular sperm
extraction
|
|
GCNIS
|
germ cell neoplasia in situ
|
References
|
1
|
Cheng L, Albers P, Berney DM, Feldman DR,
Daugaard G, Gilligan T and Looijenga LHJ: Testicular cancer. Nat
Rev Dis Primers. 4:292018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chovanec M and Cheng L: Advances in
diagnosis and treatment of testicular cancer. BMJ. 379:e0704992022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dieckmann KP and Pichlmeier U: Clinical
epidemiology of testicular germ cell tumors. World J Urol. 22:2–14.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Raman JD, Nobert CF and Goldstein M:
Increased incidence of testicular cancer in men presenting with
infertility and abnormal semen analysis. J Urol. 174:1819–1822.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parekh NV, Lundy SD and Vij SC: Fertility
considerations in men with testicular cancer. Transl Androl Urol. 9
(Suppl 1):S14–S23. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Williams DH, Karpman E, Sander JC, Spiess
PE, Pisters LL and Lipshultz LI: Pretreatment semen parameters in
men with cancer. J Urol. 181:736–740. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tal R, Holland R, Belenky A, Konichezky M
and Baniel J: Incidental testicular tumors in infertile men. Fertil
Steril. 82:469–471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
WHO, . WHO laboratory manual for the
examination and processing of human semen. (5th edition). 2010.
|
|
9
|
Johnsen SG: Testicular biopsy score
count-a method for registration of spermatogenesis in human testes:
Normal values and results in 335 Hypogonadal males. Hormones.
1:2–25. 1970.PubMed/NCBI
|
|
10
|
Gurney JK, McGlynn KA, Stanley J, Merriman
T, Signal V, Shaw C, Edwards R, Richiardi L, Hutson J and Sarfati
D: Risk factors for cryptorchidism. Nat Rev Urol. 14:534–548. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Salazar-Mejía CE, Zayas-Villanueva O,
Gutiérrez AG, Martínez RJ, Cepeda AG, Wimer-Castillo BO,
Rodríguez-Calvillo HA, Chapa-Montalvo LP, Samaniego-Sáenz BA,
Hernández-Barajas D and Vidal-Gutiérrez O: Clinical characteristics
and treatment adherence among men with testicular germ cell tumors:
Real-world data from a referral center in Mexico. J Clin Oncol. 38
(Suppl 6):abstract 393. 2020. View Article : Google Scholar
|
|
12
|
Williamson SR, Delahunt B, Magi-Galluzzi
C, Algaba F, Egevad L, Ulbright TM, Tickoo SK, Srigley JR, Epstein
JI and Berney DM; the Member of the ISUP Testicular Tumour Panel, :
The World Health Organization 2016 classification of testicular
germ cell tumours: A review and update from the International
Society of Urological Pathology Testis Consultation Panel.
Histopathology. 70:335–346. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Balzer BL and Ulbright TM: Spontaneous
regression of testicular germ cell tumors: An analysis of 42 cases.
Am J Surg Pathol. 30:858–865. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Djaladat H, Burner E, Parikh PM, Beroukhim
Kay D and Hays K: The association between testis cancer and semen
abnormalities before orchiectomy: A systematic review. J Adolesc
Young Adult Oncol. 3:153–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bandak M, Jørgensen N, Juul A, Lauritsen
J, Gundgaard Kier MG, Mortensen MS and Daugaard G: Preorchiectomy
eydig cell dysfunction in patients with testicular cancer. Clin
Genitourin Cancer. 15:e37–e43. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Petersen PM, Skakkebaek NE, Rorth M and
Giwercman A: Semen quality and reproductive hormones before and
after orchiectomy in men with testicular cancer. J Urol.
161:822–826. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lempiainen A, Sankila A, Hotakainen K,
Haglund C, Blomqvist C and Stenman UH: Expression of human
chorionic gonadotropin in testicular germ cell tumors. Urol Oncol.
32:727–734. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Raven G, de Jong FH, Kaufman JM and de
Ronde W: In men, peripheral estradiol levels directly reflect the
action of estrogens at the Hypothalamo-Pituitary level to inhibit
gonadotropin secretion. J Clin Endocrinol Metab. 91:3324–3328.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ostrowski KA and Walsh TJ: Infertility
with testicular cancer. Urol Clin North Am. 42:409–420. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
WHO, . WHO laboratory manual for the
examination and processing of human semen. (6th edition). 2021.
|