Introduction
Renal cell carcinoma (RCC) is the most common type
of kidney cancer and its incidence has increased over the last
decade. At present, the incidence of RCC is estimated to be ~3%
among all types of cancer, accounting for >130,000 fatalities
each year worldwide (1,2). RCC is commonly accompanied by
localized tumors (65% of all cases), lymphatic node metastasis (16%
of all cases) and distant metastases (16% of all cases) (3,4).
Patients with RCC are at high risk of vascular invasion, as the
tumor is able to extend from the kidney and through the venous
drainage pathway. The malignancy can extend as a venous tumor
thrombus (VTT) from the renal vein to the inferior vena cava (IVC).
Furthermore, ~8.8% of patients with RCC have VTT at the time of
diagnosis.
The most common treatment approach for RCC is
complete surgical excision in the form of partial nephrectomy (PN)
or radical nephrectomy. For metastatic disease, surgical excision
of the original tumor (cytoreductive nephrectomy) can be performed
as palliative therapy prior to systemic treatment, which aims to
reduce tumor invasiveness and preserve renal function (5,6).
Furthermore, systemic treatment of RCC can also be applied during
the early stages of the disease. Tyrosine kinase inhibitors (TKIs),
such as sunitinib, sorafenib, pazopanib and axitinib, that target
vascular endothelial growth factor receptors (VEGFR) or monoclonal
antibodies against VEGF are considered as significant strategies
for the systemic therapy of RCC (7,8). The
effect of neoadjuvant TKI therapy on patients with RCC has been
investigated in numerous prospective and retrospective trials
(9–12), and it was shown to enhance
resectability of the tumor and reduce the need for renal
replacement therapy by enabling nephron-sparing surgery. However,
whether patients administered neoadjuvant TKI therapy encounter
more operative complications due to the effects of TKI on wound
healing remains controversial. Moreover, the utility of inducing
regression of neoadjuvant TKI therapy on IVC thrombus is unclear
(13). At present, there is a lack
of systematic meta-analyses regarding the effect of neoadjuvant TKI
therapy on the surgery of patients with RCC.
The present meta-analysis aimed to summarize the
surgical outcomes of patients with RCC treated with neoadjuvant TKI
therapy compared with patients treated with surgery alone, and to
evaluate the effect of neoadjuvant TKI therapy on surgery, thus
providing novel insights into the potential advances of neoadjuvant
TKI therapy in the treatment of RCC.
Materials and methods
Preferred Reporting Items for
Systematic Reviews and Meta-Analyses (PRISMA)
The present meta-analysis was conducted based on the
PRISMA criteria (14) and the
review protocol was registered on PROSPERO (registration no.
CRD42023387617; http://www.crd.york.ac.uk/PROSPERO/).
Search strategy
The Embase (https://www.embase.com/landing?status=grey), PubMed
(https://pubmed.ncbi.nlm.nih.gov/) and
Cochrane Library databases (https://www.cochranelibrary.com/) were screened for
eligible studies between the review inception and December, 2022.
Only articles in English were included in the present review
article. The detailed search strategies are shown in Data S1. The relevant cited references
from the selected studies were also retrieved to ascertain
additional potentially acceptable literature.
Study selection
Only observational or randomized controlled studies
published as conference abstracts or full papers were screened in
the present analysis. The inclusion criteria according to the
PRISMA guidelines were as follows: i) Studies comparing neoadjuvant
with non-neoadjuvant therapy prior to surgery in patients with
pathologically confirmed RCC; ii) randomized controlled trials
(RCTs) or retrospective comparative studies written in English;
iii) studies with at least one evaluable surgical outcome; and iv)
studies providing sufficient data to support comparisons.
Data extraction
In the present meta-analysis two reviewers
independently extracted the data using a standardized extraction
form. The differences were then compared by another independent
reviewer. The information extracted from the eligible studies,
included publication year, study design, first author, country,
clinical intervention, number of subjects, age (mean or median),
perioperative outcomes (blood loss, operative time and
complication), postoperative length of hospital stay or total
length of hospital stay and the proportion of patients who
underwent partial nephrectomy after neoadjuvant therapy. For
studies reporting median and range [or interquartile range (IQR)]
values, a validated mathematical model was used to convert the
median, range or IQR values to mean ± standard deviation (15,16).
For studies that provided statistical charts without values, these
values were estimated using a professional graphic processing
software (Adobe Photoshop 2021; Adobe Systems, Inc.).
Quality assessment
The quality of the RCTs was assessed using the
Cochrane risk of bias tool (17),
while the Newcastle-Ottawa Scale (NOS) (18) was adopted for evaluating the cohort
studies. The NOS categories included selection, comparability of
study groups and outcome (four, two and three stars maximally,
respectively). Studies that were rated more than six stars were
considered to be of high quality. In addition, two independent
reviewers evaluated the risk of bias in all included studies and
any inconsistency was discussed and resolved by another independent
reviewer to reach an agreement.
Statistical analysis
All statistical analyses were performed using
ReviewManager Software (version 5.4; http://training.cochrane.org/online-learning/core-software/revman).
The standardized mean difference (SMD) was used as a summary
measure for continuous outcomes, while the risk ratio (RR) with 95%
confidence interval (CI) was calculated for binary variables. The
random-effects model was used for all meta-analyses. Subgroup and
sensitivity analyses were conducted to assess the robustness of the
findings in studies with high heterogeneity. P<0.05 was
considered to indicate a statistically significant difference.
Results
Search results and description of
eligible studies
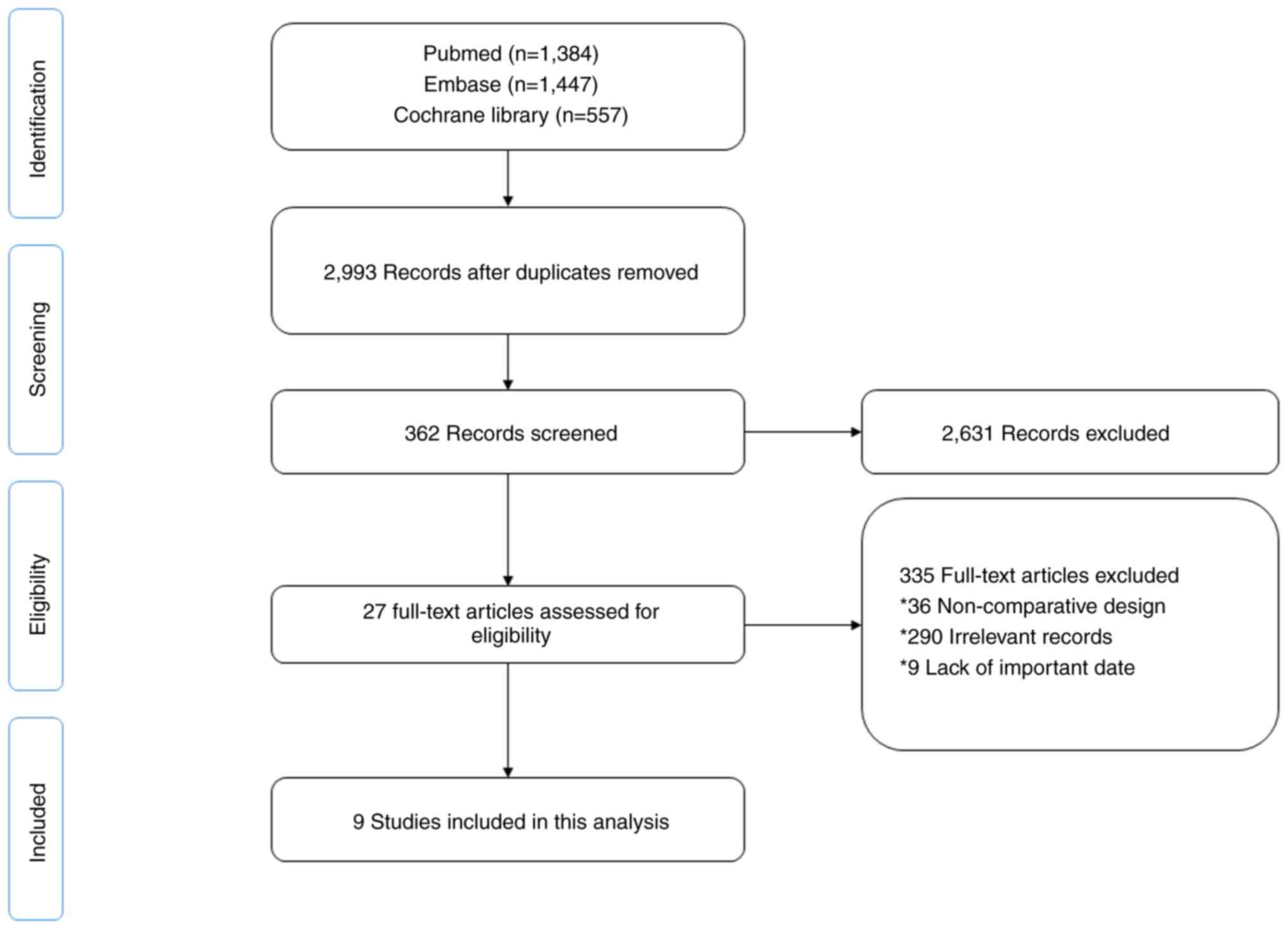
The detailed process of literature retrieval and
screening is shown in Fig. 1. In
total, 9 articles (19–27) were selected after the title,
abstract and full texts were screened according to the selection
criteria. The time span of publications covered 2011 to 2021,
involving 829 patients (336 in the neoadjuvant + surgery group; 493
in the surgery group).
The specific information retrieved from each study
is shown in Tables I and SI. Among the 9 included studies, 3 were
randomized controlled trials (19,20,22)
and the remaining studies were retrospective, non-randomized
studies (21,23–27).
In the included studies, the patients in the neoadjuvant group had
all received the targeted therapy before surgery, and the reported
median or average ages of patients ranged from 55.7–71.4 years old.
It should be noted that 2 articles (20,22)
reported overlapping series of patients, but the type of outcomes
were different, so both articles were included in the present
analysis without direct comparison.
 | Table I.Characteristics of the studies
included in the present meta-analysis. |
Table I.
Characteristics of the studies
included in the present meta-analysis.
|
|
|
|
| Sample size, n | Age, years |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Author, year | Country | Study design | Interventions | Neo + Sur | Sur | Neo + Sur | Sur | (Refs.) |
|---|
| Hatiboglu et
al, 2017 | Germany | RCT | Sorafenib + surgery
vs. placebo + surgery | 9 | 3 | 55.7a | 62.1a | (19) |
| Voylenko et
al, 2020 | Ukraine | RCT | Pazopanib + surgery
vs. surgery alone | 75 | 77 | Na | Na | (20) |
| Semko et al,
2021 | Ukraine | RCT | Pazopanib + surgery
vs. surgery alone | 83 | 84 | Na | Na | (22) |
| McDonald et
al, 2018 | America | Retrospective | Sunitinib + surgery
vs. surgery alone | 47 | 78 | 59b | 61b | (25) |
| Harshman et
al, 2013 | America | Prospective | sunitinib or
sorafenib + surgery vs. surgery alone | 14 | 73 | 59a | 65a | (27) |
| Okamura et
al, 2019 | Japan | Retrospective | Pazopanib + surgery
vs. surgery alone | 9 | 10 | 71.4b | 69.8b | (24) |
| Chapin et
al, 2011 | America | Retrospective | Targeted therapy +
surgery vs. surgery alone | 70 | 103 | 61.4a | 59.7a | (26) |
| Tanaka et
al, 2018 | Japan | Retrospective | Axitinib + surgery
vs. surgery alone | 10 | 31 | 64a | 65a | (23) |
| Field et al,
2019 | America | Retrospective | Sunitinib + surgery
vs. surgery alone | 19 | 34 | 63b | 61b | (21) |
Quality evaluation
The quality assessment for the 3 RCTs included in
the present meta-analysis is shown in Fig. S1. According to the Cochrane
risk-of-bias tool, none of the trials were rated with a high risk
of bias. For the 6 retrospective observational studies, the quality
was assessed following the NOS guidelines. The quality of the
studies varied from a NOS score of 7 to 9 (Table SII). Therefore, all 9 studies were
included in the subsequent analysis.
Meta-analysis
The differences in operative time, blood loss,
complication, postoperative length of hospital stay or the total
length of stay in the hospital and the proportion of patients who
underwent partial nephrectomy were compared between the two groups.
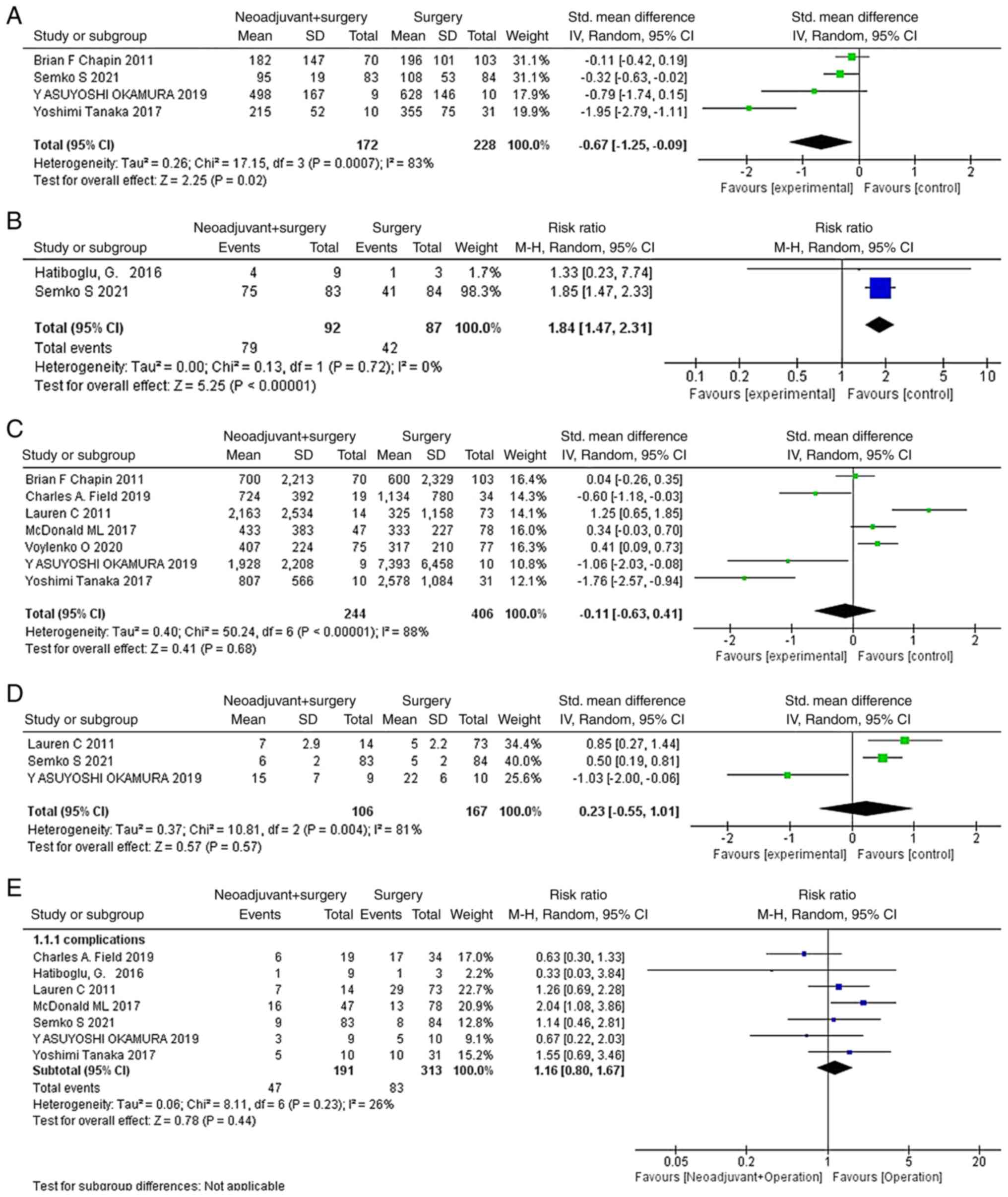
As illustrated in Fig. 2,
neoadjuvant treatment before surgery significantly reduced the
operation time (SMD=−0.67; 95% CI, −1.25- −0.09; P=0.02; Fig. 2A) and resulted in a greater
proportion of patients choosing partial nephrectomy (RR=1.84; 95%
CI, 1.47–2.31; P<0.00001; Fig.
2B). However, there were no significant differences in blood
loss (SMD=−0.11; 95% CI, −0.63–0.41; P=0.68; Fig. 2C), postoperative length of hospital
stay or the total length of stay in the hospital (SMD=0.23; 95% CI,
−0.55–1.01; P=0.57; Fig. 2D) or
complication (RR=1.16; 95% CI, 0.80–1.67; P=0.44; Fig. 2E) with or without neoadjuvant
therapy.
Subgroup analysis
Since RCC is a dynamic disease, patient data
originating from different phases of the disease may result from
different complexities of surgery, which may be a potential source
of heterogeneity. Therefore, a subgroup meta-analysis was conducted
where patients were divided into ‘patients with localized
RCC/underwent PN’, ‘patients with advanced RCC and/or metastatic
RCC’ and ‘patients with RCC and IVC tumor thrombus’. The results
demonstrated that neoadjuvant therapy significantly reduced the
blood loss during surgery for patients with RCC and tumor thrombus
in the IVC (SMD=−1.10; 95% CI, −1.82- −0.38; P=0.003; Fig. 3C). In addition, the proportion of
complications graded as ≥3 [according to the Clavien-Dindo
classification system (28)] for
all complications were compared between the two treatment groups.
As shown in Fig. 3D, neoadjuvant
therapy did not increase the proportion of complications with a
grade of ≥3 in all identified complications (RR=0.48; 95% CI,
0.20–1.15; P=0.10).
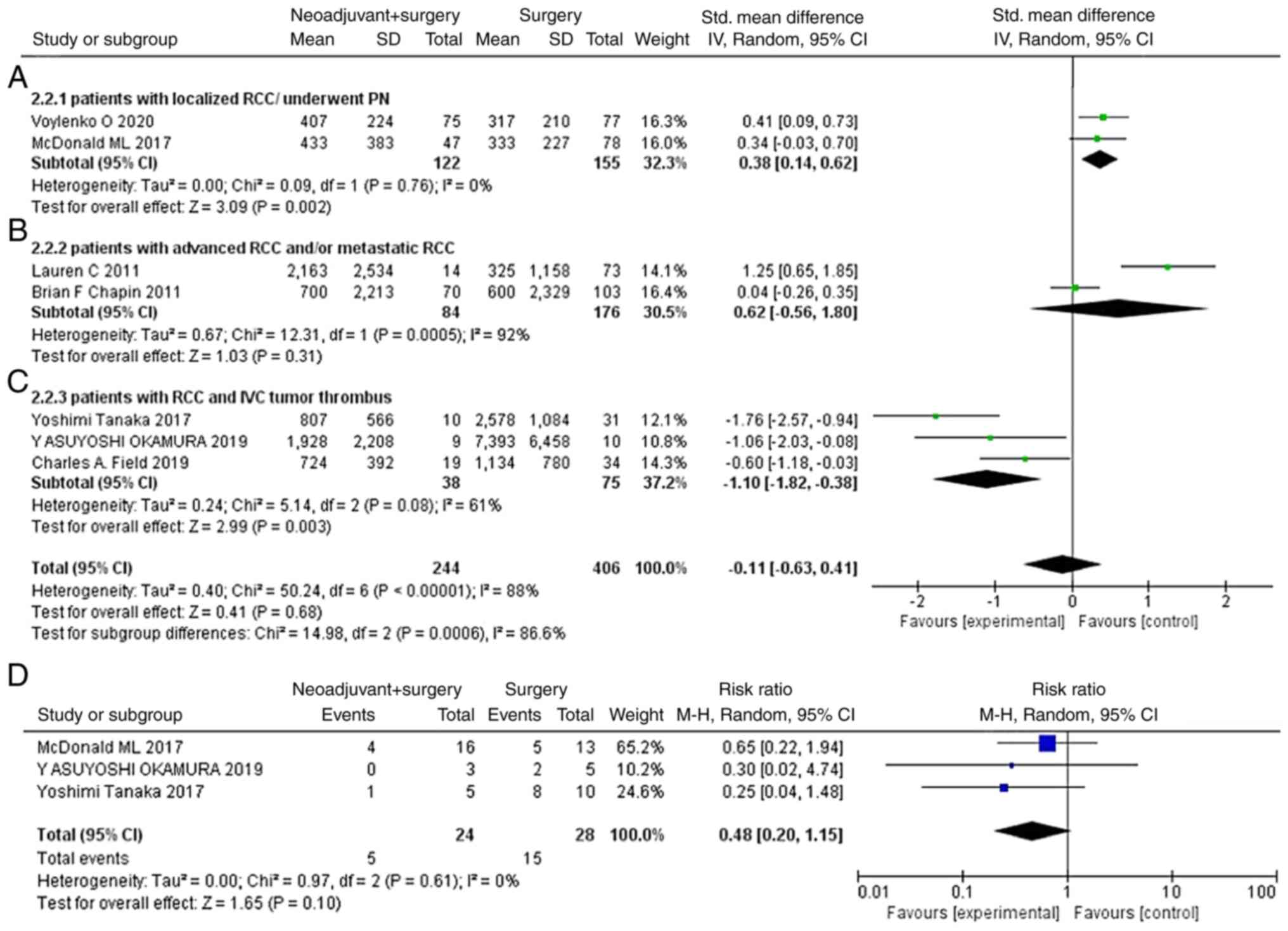 | Figure 3.Subgroup analysis on the estimated
blood loss. Analysis of the (A) patients with localized
RCC/underwent PN, (B) patients with advanced RCC and/or metastatic
RCC, (C) patients with RCC and IVC tumor thrombus and (D)
complications with a grade of ≥3 (Clavien-Dindo) in all
complications. CI, confidence interval; df, degree of freedom; IV,
inverse variance; M-H, Mantel-Haenszel; IVC, inferior vena cava;
RCC, renal cell carcinoma; SD, standard deviation; PN, partial
nephrectomy. |
Sensitivity analysis
Sensitivity analysis was performed in analyses with
high heterogeneity (postoperative length of hospital stay or the
total length of stay in the hospital). After excluding the study by
Okamura et al (24), the
heterogeneity was significantly reduced and the final result was
altered, with neoadjuvant therapy significantly increasing the
total length of stay in hospital (SMD=0.59; 95% CI, 0.28–0.89;
P=0.0001; Fig. S2).
Discussion
Surgery remains the cornerstone for treatment of
localized RCC at present. Surgery-related outcomes such as blood
loss, operative time and surgical complications, are crucial
factors affecting decisions regarding surgery, as well as the
operative risk and postoperative management of patients (29,30).
Neoadjuvant TKI therapy preliminarily demonstrated promising
results with downsizing and/or downstaging of the primary tumor in
patients with unresectable tumors or poor surgical candidates
(31). Although the study by Bex
et al (32) demonstrated
that for some patients who underwent cytoreductive nephrectomy,
preoperative neoadjuvant TKI therapy extended the median overall
survival time (32.4 vs. 15.0 months), large-scale clinical studies
investigating the effect of neoadjuvant TKI therapy on the
prognosis of patients with RCC are still lacking. At present,
studies (25,26,31,33–37)
have largely focused on the feasibility of neoadjuvant TKI therapy
to facilitate surgery and as well as the adverse events associated
with this treatment modality. The study by Assi et al
(38) suggested that the incidence
of adverse events following preoperative therapy for RCC might be
acceptable. To the best of our knowledge, no study has yet
summarized and evaluated the surgery-related outcomes, such as the
blood loss, procedure-related complications and operation time, in
patients with RCC following neoadjuvant therapy. The present study
therefore aimed to explore the effect of neoadjuvant TKI therapy on
surgery in patients with RCC compared with patients who underwent
surgery alone. The results demonstrated that neoadjuvant therapy
could shorten the operation time, reduce blood loss in patients
with IVC tumor thrombus and enable more patients to choose partial
nephrectomy for treating RCC. Additionally, although neoadjuvant
therapy did not reduce the overall incidence of complications, it
did decrease the proportion of complications with a grade of
≥3.
Targeted therapy for RCC has been greatly explored
due to an improved understanding of cancer pathophysiology. A
number of studies have investigated the efficacy and safety of
neoadjuvant TKI therapy in patients with locally advanced disease
(19,31,39–41).
The results revealed that neoadjuvant therapy may reduce tumor
volume to promote the surgical treatment of advanced RCC.
Another motive for investigating the utility of
neoadjuvant therapy is in the facilitation of nephron-sparing
surgery (25,31,33,34,36,37).
It has been reported that nephrectomy can result in dialysis, more
particularly in patients with bilateral kidney lesions, single
kidney tumors or RCC with simultaneous opposite kidney pathology.
Neoadjuvant therapy seeks to increase the possibility of partial
nephrectomy, which can prevent the oversubscription of dialysis
beds and high cost (42). In the
present study, more patients underwent partial nephrectomy after
neoadjuvant therapy, which was particularly appealing from the
perspective of renal function preservation (43).
The effectiveness of neoadjuvant therapy prior to
radical nephrectomy and IVC tumor thrombectomy is controversial. In
a retrospective study including 25 patients with IVC thrombi, 12
were treated with sunitinib as a neoadjuvant therapy and the
remaining 13 were treated with alternative targeted therapies
(44). The results demonstrated
that thrombus height was reduced in 44% of patients, while 7
patients (28%) exhibited a measurable increase in thrombus height.
Furthermore, treatment with sunitinib induced tumor thrombus
regression from level IV to level III in only 1 patient (4%).
Similarly, in the study by Bigot et al (9), only 1 patient (7%) had thrombus level
downstaging and 1 patient (7%) had thrombus level upstaging.
Furthermore, stable thrombus level was observed in 12 patients
(85%). The aforementioned findings suggested that neoadjuvant
therapy may exert a limited effect on the feasibility of surgical
extirpation. Since a considerable proportion of patients
experienced tumor thrombi progression during treatment, this
strategy could potentially expose patients to an increased risk of
tumor thrombi progression and render the tumor inextirpable.
However, other studies came to a different conclusion. In a
multicenter retrospective study, including patients with RCC, after
neoadjuvant therapy with sunitinib, tumor thrombi decreased by 1.3
cm (IQR, 0.7–1.5) and 8/19 (42.1%) patients had a lower thrombus
stage, while partial response, according to the RECIST criteria,
was reported in 5 patients (26.3%) (21). Furthermore, the NAXIVA trial
evaluated the response of VTT to axitinib prior to surgery and
reported that 15/20 (75%) patients had a reduction in VTT length,
while 7/17 (41.2%) patients who underwent surgery experienced a
less invasive surgery compared with that originally planned
(45). This aforementioned study
provided the first level II evidence that axitinib could downstage
VTT in a large proportion of patients and reduce the extent of
surgery. Another two studies indicated that preoperative
neoadjuvant therapy was beneficial in reducing surgical risk and
improving surgical outcomes (23,24).
Neoadjuvant therapy may improve venous flow in the IVC via
promoting tumor shrinking or thrombus reduction, which could
eliminate the fragile collateral venous flow and decrease
intraoperative blood loss. Furthermore, neoadjuvant therapy may
induce a sclerosing change in the thrombus and decrease potential
risks during surgery. However, the results of the subgroup analysis
of the present study demonstrated that neoadjuvant therapy could
lead to enhanced blood loss in patients with localized RCC
(20) and patients who underwent
partial nephrectomy (25), which
seemed to contradict previous conclusions. The anti-angiogenic
effects of VEGF-TKI may affect wound revascularization, strength
and epithelialization, as well as induce wound complications and
potentially impair renorrhaphy integrity (46). In general, surgery in patients with
RCC with tumor thrombus in the IVC is more complicated and bleeding
during the operation can be profuse. Therefore, the benefit of
neoadjuvant therapy using TKIs could overcome the shortcomings of
their anti-angiogenic effects and the defects in patients with
localized RCC who undergo partial nephrectomy.
In terms of perioperative complication, Margulis
et al (35) compared the
surgical outcomes of 48 patients who received preoperative therapy
with a cohort of 58 patients who underwent immediate surgery. The
results demonstrated that there were no statistically significant
differences in the incidence of perioperative (30 day) morbidity
and mortality, suggesting that a longer time may be required when
reporting complications. Another study showed that preoperative
therapy was not an independent predictor for overall postoperative
complication risk (P=0.064) (26).
These findings therefore supported the safety of preoperative
systemic therapy. The results of the present study showed that
neoadjuvant therapy could reduce severe complications
(Clavien-Dindo grade, ≥3). However, statistical significance was
not reached (P=0.06). Nevertheless, due to the limitation of the
small number of patients and the nature of the included studies,
the results should be interpreted with caution.
Although a considerable number of the aforementioned
studies explored the effects of neoadjuvant TKI therapy on RCC,
they did not meet the inclusion criteria of the present study due
to a lack of comparisons with patients who had surgery alone.
Clinical trials evaluating the benefits of neoadjuvant VEGF/TKI
therapy on the long-term survival of patients with RCC are unlikely
to be conducted in the future since VEGFR-TKI as a monotherapy
treatment is gradually being surpassed by or used in combination
with immunotherapy-based regimens (47). In the present study, the
meta-analysis evaluated the safety and effectiveness of neoadjuvant
VEGFR-TKI therapy alone. As such, it is considered that the
evidence provided by the present meta-analysis could provide some
benefits to clinical practice. Although combination therapies could
enhance the objective response of the tumor compared with the use
of single agents, the high toxic effects of the combined therapies
could compromise the fitness of the patient to undergo surgery
(3). Notably, radiographic
overestimation of tumor size and the fibrotic changes induced by
immunotherapy have an effect on the surgical decision-making
process and intimate some difficulties with the operation (48,49).
In addition, the combination therapies may become a challenge for
the operating surgeon (50).
Overall, further exploration of the effect of different neoadjuvant
therapies on the surgery of patients with RCC is still
required.
In conclusion, the results of the present
meta-analysis suggested that neoadjuvant VEGF-TKI treatment may
shorten the operation time and reduce blood loss, while it also did
not cause an increase in the incidence of severe complications. In
addition, neoadjuvant therapy-induced primary tumor shrinkage could
reduce the complexity of the surgery and therefore some patients
may get the chance to choose partial nephrectomy to preserve renal
function. However, more well-designed and high-quality prospective
randomized controlled trials with larger sample sizes are required
to provide additional evidence to validate these results.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MKZ performed the statistical analysis and wrote the
manuscript. MKZ, ZFL, YHZ, ZWJ, SZC and WFW were in charge of
acquisition of data. BKS and YFZ participated in the study design
and in revising the manuscript. All authors contributed to the
article and read and approved the final version of the manuscript.
MKZ, ZFL, YHZ, ZWJ, SZC and WFW confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hsieh JJ, Purdue MP, Signoretti S, Swanton
C, Albiges L, Schmidinger M, Heng DY, Larkin J and Ficarra V: Renal
cell carcinoma. Nat Rev Dis Primers. 3:170092017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Capitanio U, Bensalah K, Bex A, Boorjian
SA, Bray F, Coleman J, Gore JL, Sun M, Wood C and Russo P:
Epidemiology of renal cell carcinoma. Eur Urol. 75:74–84. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ingels A, Campi R, Capitanio U, Amparore
D, Bertolo R, Carbonara U, Erdem S, Kara Ö, Klatte T, Kriegmair MC,
et al: Complementary roles of surgery and systemic treatment in
clear cell renal cell carcinoma. Nat Rev Urol. 19:391–418. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prunty M, Bell S, Kutikov A and Bukavina
L: Review of robotic-assisted radical nephrectomy with inferior
vena cava thrombectomy in renal cell carcinoma. Curr Urol Rep Dec.
23:363–370. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rini BI, Campbell SC and Escudier B: Renal
cell carcinoma. Lancet. 373:1119–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ho TH, Serie DJ, Parasramka M, Cheville
JC, Bot BM, Tan W, Wang L, Joseph RW, Hilton T, Leibovich BC, et
al: Differential gene expression profiling of matched primary renal
cell carcinoma and metastases reveals upregulation of extracellular
matrix genes. Ann Oncol. 28:604–610. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pontes O, Oliveira-Pinto S, Baltazar F and
Costa M: Renal cell carcinoma therapy: Current and new drug
candidates. Drug Discov Today. 27:304–314. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gray RE and Harris GT: Renal cell
carcinoma: Diagnosis and management. Am Fam Physician. 99:179–184.
2019.PubMed/NCBI
|
|
9
|
Bigot P, Fardoun T, Bernhard JC, Xylinas
E, Berger J, Rouprêt M, Beauval JB, Lagabrielle S, Lebdai S, Ammi
M, et al: Neoadjuvant targeted molecular therapies in patients
undergoing nephrectomy and inferior vena cava thrombectomy: Is it
useful? World J Urol. 32:109–1014. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martini A, Fallara G, Pellegrino F,
Cirulli GO, Larcher A, Necchi A, Montorsi F and Capitanio U:
Neoadjuvant and adjuvant immunotherapy in renal cell carcinoma.
World J Urol. 39:1369–1376. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Posadas EM and Figlin RA: Kidney cancer:
Progress and controversies in neoadjuvant therapy. Nat Rev Urol.
11:254–255. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Westerman ME, Shapiro DD, Wood CG and
Karam JA: Neoadjuvant Therapy for locally advanced renal cell
carcinoma. Urol Clin North Am Aug. 47:329–343. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bindayi A, Hamilton ZA, McDonald ML, Yim
K, Millard F, McKay RR, Campbell SC, Rini BI and Derweesh IH:
Neoadjuvant therapy for localized and locally advanced renal cell
carcinoma. Urol Oncol. 36:31–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liberati A, Altman DG, Tetzlaff J, Mulrow
C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J
and Moher D: The PRISMA statement for reporting systematic reviews
and meta-analyses of studies that evaluate healthcare
interventions: Explanation and elaboration. BMJ. 339:b27002009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hozo SP, Djulbegovic B and Hozo I:
Estimating the mean and variance from the median, range, and the
size of a sample. BMC Med Res Methodol. 5:132005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wan X, Wang W, Liu J and Tong T:
Estimating the sample mean and standard deviation from the sample
size, median, range and/or interquartile range. BMC Med Res
Methodol. 14:1352014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Higgins JP, Altman DG, Gotzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The Cochrane Collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343:d59282011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hatiboglu G, Hohenfellner M, Arslan A,
Hadaschik B, Teber D, Radtke JP, Hallscheidt P, Tolstov Y, Roth W,
Grüllich C, et al: Effective downsizing but enhanced intratumoral
heterogeneity following neoadjuvant sorafenib in patients with
non-metastatic renal cell carcinoma. Langenbecks Arch Surg.
402:637–644. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Voylenko O, Pikul M, Stakhovsky E,
Stakhovskyi O, Semko S, Kononenko OA and Vitruk I: Influence of
neoadjuvant targeted therapy on perioperative complication rate.
Eur Urol Open Sci. 19:e2382020. View Article : Google Scholar
|
|
21
|
Field CA, Cotta BH, Jimenez J, Lane BR,
Yim K, Lee HJ, Ryan ST, Hamilton ZA, Patel S, Wang S, et al:
Neoadjuvant sunitinib decreases inferior vena caval thrombus size
and is associated with improved oncologic outcomes: A multicenter
comparative analysis. Clin Genitourin Cancer. 17:e505–e512. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Semko S, Pikul M, Stakhovsky E, Voylenko
O, Stakhovskyi O, Vitruk I, Hrechko B and Kononenko O: Oncological
outcome of neoadjuvant target therapy in patients with localized
RCC. Eur Urol. 79:S7702021. View Article : Google Scholar
|
|
23
|
Tanaka Y, Hatakeyama S, Hosogoe S, Tanaka
T, Hamano I, Kusaka A, Iwamura H, Fujita N, Yamamoto H, Tobisawa Y,
et al: Presurgical axitinib therapy increases fibrotic reactions
within tumor thrombus in renal cell carcinoma with thrombus
extending to the inferior vena cava. Int J Clin Oncol. 23:134–141.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Okamura Y, Terakawa T, Sakamoto M, Bando
Y, Suzuki K, Hara T, Furukawa J, Harada K, Hinata N, Nakano Y and
Fujisawa M: Presurgical pazopanib improves surgical outcomes for
renal cell carcinoma with High-level IVC tumor thrombosis. In Vivo.
33:2013–2019. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McDonald ML, Lane BR, Jimenez J, Lee HJ,
Yim K, Bindayi A, Hamilton ZA, Field CA, Bloch AS, Dey S, et al:
Renal functional outcome of partial nephrectomy for complex
R.E.N.A.L. score tumors with or without neoadjuvant sunitinib: A
multicenter analysis. Clin Genitourin Cancer. 16:e289–e295. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chapin BF, Delacroix SE Jr, Culp SH,
Nogueras Gonzalez GM, Tannir NM, Jonasch E, Tamboli P and Wood CG:
Safety of presurgical targeted therapy in the setting of metastatic
renal cell carcinoma. Eur Urol. 60:964–971. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Harshman LC, Yu RJ, Allen GI, Srinivas S,
Gill HS and Chung BI: Surgical outcomes and complications
associated with presurgical tyrosine kinase inhibition for advanced
renal cell carcinoma (RCC). Urol Oncol. 31:379–385. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Clavien PA, Barkun J, de Oliveira ML,
Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J,
Slankamenac K, Bassi C, et al: The Clavien-Dindo classification of
surgical complications: Five-year experience. Ann Surg.
250:187–196. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen K, Liu Z, Li Y, Zhao X, Wang G, Tian
X, Zhang H, Ma L and Zhang S: Prevention, incidence, and risk
factors of chyle leak after radical nephrectomy and thrombectomy.
Cancer Med. 13:e68582023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Colomer R, Saura C, Sanchez-Rovira P,
Pascual T, Rubio IT, Burgués O, Marcos L, Rodríguez CA, Martín M
and Lluch A: Neoadjuvant management of early breast cancer: A
clinical and investigational position statement. Oncologist.
24:603–611. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Karam JA, Devine CE, Urbauer DL, Lozano M,
Maity T, Ahrar K, Tamboli P, Tannir NM and Wood CG: Phase 2 trial
of neoadjuvant axitinib in patients with locally advanced
nonmetastatic clear cell renal cell carcinoma. Eur Urol.
66:874–880. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bex A, Mulders P, Jewett M, Wagstaff J,
van Thienen JV, Blank CU, van Velthoven R, Del Pilar Laguna M, Wood
L, van Melick HHE, et al: Comparison of immediate vs deferred
cytoreductive nephrectomy in patients with synchronous metastatic
renal cell carcinoma receiving sunitinib: The SURTIMe randomized
clinical trial. JAMA Oncol. 5:164–170. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lane BR, Derweesh IH, Kim HL, O'Malley R,
Klink J, Ercole CE, Palazzi KL, Thomas AA, Rini BI and Campbell SC:
Presurgical sunitinib reduces tumor size and may facilitate partial
nephrectomy in patients with renal cell carcinoma. Urol Oncol.
33:112.e15–e21. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lebacle C, Bensalah K, Bernhard JC,
Albiges L, Laguerre B, Gross-Goupil M, Baumert H, Lang H, Tricard
T, Duclos B, et al: Evaluation of axitinib to downstage cT2a renal
tumours and allow partial nephrectomy: A phase II study. BJU.
123:804–810. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Margulis V, Matin SF, Tannir N, Tamboli P,
Swanson DA, Jonasch E and Wood CG: Surgical morbidity associated
with administration of targeted molecular therapies before
cytoreductive nephrectomy or resection of locally recurrent renal
cell carcinoma. J Urol. 180:94–98. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rini BI, Plimack ER, Takagi T, Elson P,
Wood LS, Dreicer R, Gilligan T, Garcia J, Zhang Z, Kaouk J, et al:
A Phase II study of pazopanib in patients with localized renal cell
carcinoma to optimize preservation of renal parenchyma. J Urol.
194:297–303. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Silberstein JL, Millard F, Mehrazin R,
Kopp R, Bazzi W, DiBlasio CJ, Patterson AL, Downs TM, Yunus F, Kane
CJ and Derweesh IH: Feasibility and efficacy of neoadjuvant
sunitinib before nephron-sparing surgery. BJU Int. 106:1270–1276.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Assi T, El Rassy E, Farhat F and Kattan J:
Overview on the role of preoperative therapy in the management of
kidney cancer. Clin Transl Oncol. 22:11–20. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cowey CL, Amin C, Pruthi RS, Wallen EM,
Nielsen ME, Grigson G, Watkins C, Nance KV, Crane J, Jalkut M, et
al: Neoadjuvant clinical trial with sorafenib for patients with
stage II or higher renal cell carcinoma. J Clin Oncol.
28:1502–1507. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hellenthal NJ, Underwood W, Penetrante R,
Litwin A, Zhang S, Wilding GE, Teh BT and Kim HL: Prospective
clinical trial of preoperative sunitinib in patients with renal
cell carcinoma. J Urol. 184:859–864. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rini BI, Garcia J, Elson P, Wood L, Shah
S, Stephenson A, Salem M, Gong M, Fergany A, Rabets J, et al: The
effect of sunitinib on primary renal cell carcinoma and
facilitation of subsequent surgery. J Urol. 187:1548–1554. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shuch B, Linehan WM and Bratslavsky G:
Repeat partial nephrectomy: Surgical, functional and oncological
outcomes. Curr Opin Urol. 21:368–375. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Voylenko OA, Stakhovsky OE, Vitruk IV,
Kononenko OA, Pikul MV, Semko SL and Stakhovsky EO: Efficacy of
neoadjuvant targeted therapy in treatment of patients with
localised clear-cell renal cell carcinoma. Adv Urol.
2021:66746372021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cost NG, Delacroix SE Jr, Sleeper JP,
Smith PJ, Youssef RF, Chapin BF, Karam JA, Culp S, Abel EJ,
Brugarolas J, et al: The impact of targeted molecular therapies on
the level of renal cell carcinoma vena caval tumor thrombus. Eur
Urol. 59:912–918. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Stewart GD, Welsh SJ, Ursprung S,
Gallagher FA, Jones JO, Shields J, Smith CG, Mitchell TJ, Warren
AY, Bex A, et al: A Phase II study of neoadjuvant axitinib for
reducing the extent of venous tumour thrombus in clear cell renal
cell cancer with venous invasion (NAXIVA). Br J Cancer.
127:1051–1060. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Schmidinger M, Arnold D, Szczylik C,
Wagstaff J and Ravaud A: Optimizing the use of sunitinib in
metastatic renal cell carcinoma: An update from clinical practice.
Cancer Invest. 28:856–864. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ljungberg B, Albiges L, Abu-Ghanem Y,
Bensalah K, Dabestani S, Fernández-Pello S, Giles RH, Hofmann F,
Hora M, Kuczyk MA, et al: European association of urology
guidelines on renal cell carcinoma: The 2019 update. Eur Urol.
75:799–810. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dibajnia P, Cardenas LM and Lalani AA: The
emerging landscape of neo/adjuvant immunotherapy in renal cell
carcinoma. Hum Vaccin Immunother. 19:21782172023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Labbate C, Hatogai K, Werntz R, Stadler
WM, Steinberg GD, Eggener S and Sweis RF: Complete response of
renal cell carcinoma vena cava tumor thrombus to neoadjuvant
immunotherapy. J Immunother Cancer. 7:662019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zemankova A, Studentova H, Kopova A, Tichy
T, Student V and Melichar B: Neoadjuvant nivolumab and cabozantinib
in advanced renal cell carcinoma in a horseshoe kidney-how to
achieve a safe and radical resection? a case report and review of
the literature. Front Oncol. 13:11159012023. View Article : Google Scholar : PubMed/NCBI
|