Introduction
Lung cancer is one of the most common malignancies
worldwide and the leading cause of cancer-associated death. As
shown in the cancer data of 2020 from the International Agency for
Research on Cancer, 19.3 million new cancer cases were estimated to
occur globally. Of these, 2,206,771 new cases (11.1%) in all ages
and sexes were attributed to lung cancer, which accounted for 18%
(n=1,796,144) of all cancer-related mortalities (1). Over the past few decades, lung cancer
treatment has undergone tremendous changes, with immunotherapy and
targeted therapies experiencing rapid advancement and becoming the
dominant treatments. Tyrosine kinase inhibitors (TKIs), checkpoint
inhibitors and chimeric antigen receptor T-cell therapy, among
others, have significantly improved the survival rate and quality
of life of patients with lung cancer beyond the abilities of
standard chemotherapeutic and radiotherapy regimens (2). However, limited by gene mutation,
individual differences, unclear mechanisms of tumor development and
other factors, the long-term efficacy of these therapies remains
unsatisfactory, as the survival time of patients with stage IV lung
cancer rarely exceeds 12 months (3). Therefore, the exploration of new
treatments for lung cancer remains necessary.
Since the global outbreak of the coronavirus disease
2019 (COVID-19) pandemic in 2020, the impact of severe acute
respiratory syndrome coronavirus 2 (SARS-CoV-2) on lung cancer has
been extensively studied. Similar to other respiratory viruses,
SARS-CoV-2 primarily spreads through droplets, thus infecting
respiratory mucosal epithelial cells, and causes a series of
inflammatory reactions, which include recruitment of macrophages,
and increased local secretion of inflammatory cytokines [e.g.,
interleukin (IL)-6 and IL-1β] and chemokines [e.g., interferon
(INF)-α, IFN-γ, monocyte chemoattractant protein-1 and IFNγ
inducible protein 10] into the peripheral blood. The cytokines and
chemokines induce more T helper 1 cells to enter into the lungs,
mediating immune responses that attack and kill virus-infected
cells (4). This reaction
undoubtedly aggravates the lung cancer burden, leading to more
complications. SARS-CoV-2 infection, based on the severity of
illness, is grouped into the following categories of asymptomatic
infection, viral pneumonia, acute respiratory distress syndrome and
death (5). However, a recent report
indicated that SARS-CoV-2 is not entirely harmful, and that it can
also bring some benefits with unknown mechanisms (6). A number of patients with malignancies
experienced tumor reduction or significant remission during
SARS-CoV-2 infection, even though their therapeutic regimens were
unchanged (7).
The present study reviewed the case of a patient
with advanced lung cancer who was initially prescribed TKI agents
but received poor efficacy. After the patient had tested negative
for SARS-CoV-2 for ~3 weeks, imaging showed significant tumor
reduction although the therapeutic schedule remained unchanged. To
the best of our knowledge, this is the first description of lung
cancer reduction after SARS-CoV-2 infection.
Case report
A 60-year-old Chinese male, with no significant
previous history or family history of illness, who had smoked ~10
cigarettes per day for 25 years, presented to the Department of
Respiratory and Critical Care Medicine, Eighth Medical Center of
the Chinese People's Liberation Army General Hospital (Beijing,
China) complaining of right chest pain and discomfort, fatigue,
anorexia and weight loss for 3 weeks in March 2022. Although a
physical examination suggested no significant abnormalities, serum
tumor marker levels [carcinoembryonic antigen (CEA), 1,352 ng/ml;
carbohydrate antigen 125 (CA125), 83.45 U/l; CA15-3, 66.98 U/l;
CA724, 33.45 U/l; and serum keratin 19 fragment (CA21-1), 42.91
ng/l) were obviously beyond the normal ranges (CEA, 0–5 ng/ml;
CA125, 0–35 U/ml; CA15-3, 0–25 U/l; CA724, 0–6 U/l; and CA21-1, 0–3
ng/l). Moreover, a chest computed tomography (CT) scan showed a
17.0×14.5-mm space-occupying lesion in the center of the inferior
lobes of the right lung, with mixed ground-glass nodules and
irregular margins. A core needle biopsy was performed on the lung
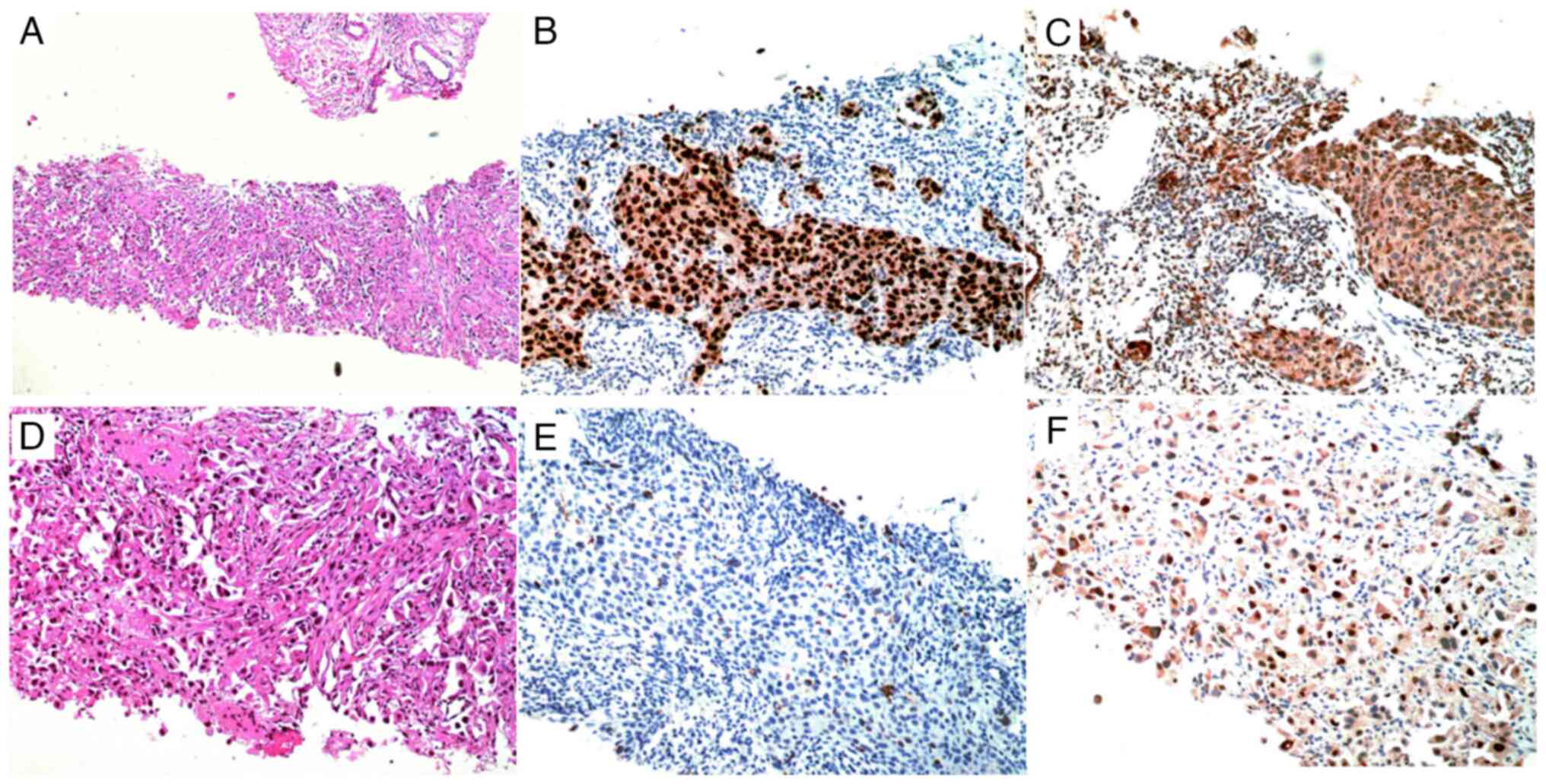
lesion using B-ultrasound guidance. The pathological diagnosis
based on hematoxylin-eosin staining (Data S1) and immunohistochemistry
(Data S1) demonstrated staining
characteristics of lung adenocarcinoma, with positivity for thyroid
transcription factor-1 and napsin A, and negativity for cytokeratin
5/6 (Fig. 1).
In order to determine the mutation that could be
treated with targeted therapy, a next-generation sequencing (NGS)
assay (GeneseeqPrime™; Geneseeq Technology, Inc.; Data S1) was performed to detect 437
cancer-related genes, including a total of 1.53 MB bases involving
exons, fusion-related introns and microsatellite regions. The
result revealed that six genes (EGFR, TP53, WAS, DOT1L, EPHA2 and
SMAD4) had tumor-specific mutations. Immunotherapy-associated gene
mutations of lung cancer only occurred in EGFR, with the exception
of TP53, rather than other genes [such as BRAF V600, Kirsten rat
sarcoma viral oncogene homolog, mesenchymal epithelial transition
factor (MET), human epidermal growth factor receptor 2, anaplastic
lymphoma kinase, ret proto-oncogene and ROS proto-oncogene 1].
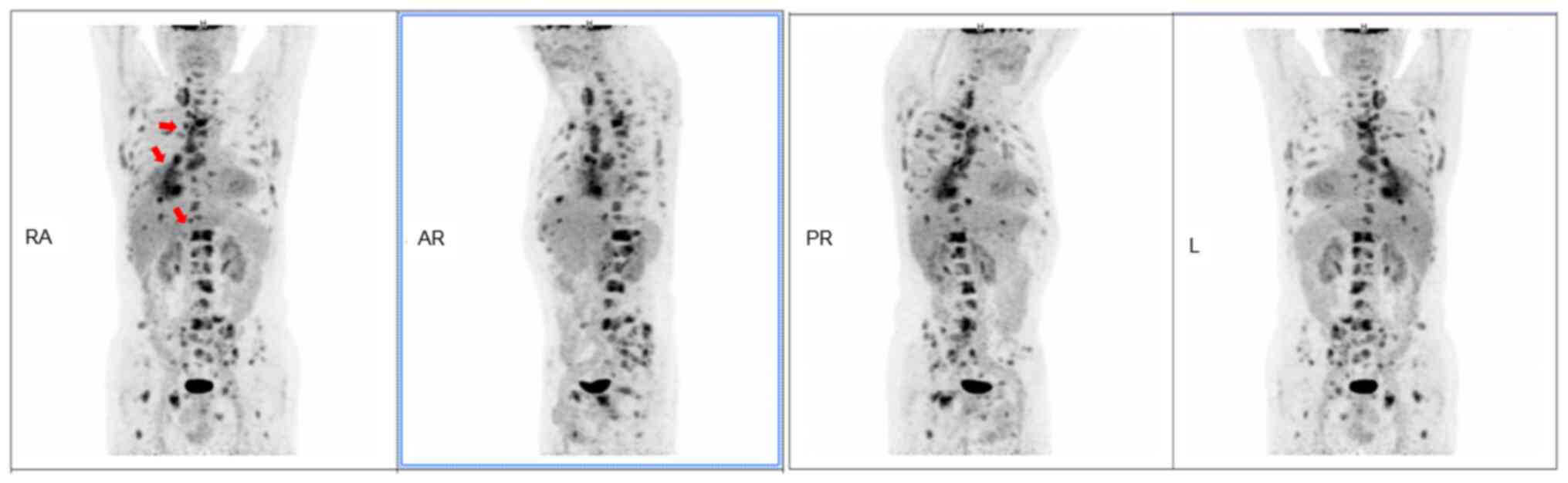
Subsequently, to further evaluate the general
condition of the patient, a fluorine-18-fluorodeoxyglucose positron
emission tomography-CT scan was performed, which revealed multiple
metastases, including those in the pleura, frontal lobe of the
brain and liver (maximum diameter, 1.1 cm) (Fig. 2). Given the overall condition of the
patient and the sequencing results, in order that there was the
opportunity of surgery after neoadjuvant chemotherapy, the patient
decided to accept targeted therapy instead of a conventional
regimen. The patient initially underwent treatment with orally
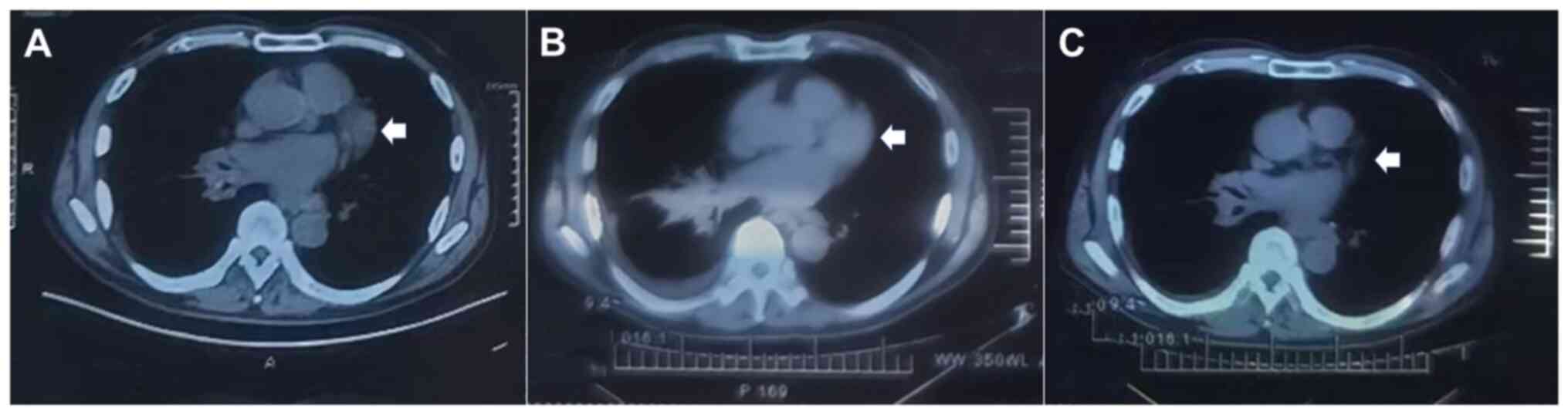
administered gefitinib (250 mg once daily, for 5 months). However,
follow-up CT showed a continually deteriorating condition, with an
enlarged primary lesion (18.6×17.2 mm) and multiple metastatic
lesions after 5 months. Given the possibility of gefitinib
resistance, a tumor individualized treatment molecular assay
(Anzekong®; Anhui Anlong Gene Technology, Inc.), which
covered 550 cancer-related gene sites, revealed EGFR T790M
resistance but EGFR L858R sensitivity, and the patient was
recommended to receive an orally administration of osimertinib (80
mg once daily) instead of gefitinib. A distinctive curative effect
was not present, nor was a partial response, after 1 month of
medication. A follow-up CT scan showed that the size of the primary
lesion had not significantly decreased (17.4×15 mm at the inferior
lobe of the right lung).
In October 2022 (~45 days from the start of
osmertinib administration), the patient was diagnosed with COVID-19
[by serum reverse transcription (RT)-PCR test; Data S1] and was isolated in a hotel.
During this period, the patient took ibuprofen (0.4 g, twice per
day, for 2 weeks), dextromethorphan (20 mg, twice per day, for 3
weeks) and some Chinese patent medicines [lianhua qingwen granules
(1 bag, three times per day, for 2 weeks) and compound glycyrrhiza
oral solution (5 ml, as needed, for 3 weeks)] to treat a fever and
cough, but the administration of osimertinib was not changed. By
the middle of November 2022 (~65 days from the start of osmertinib
administration), blood samples tested negative for SARS-CoV-2
(RT-PCR). Unexpectedly, the patient experienced chest pain relief
and a continuous remission of the other symptoms, such as fatigue
and poor appetite, leading to an improved quality of life. The
values of several lung carcinoma-related tumor markers (CA125,
64.61 U/ml; CA15-3, 31.45 U/ml; CA724, 3.81 U/ml; and CA21-1, 9.281
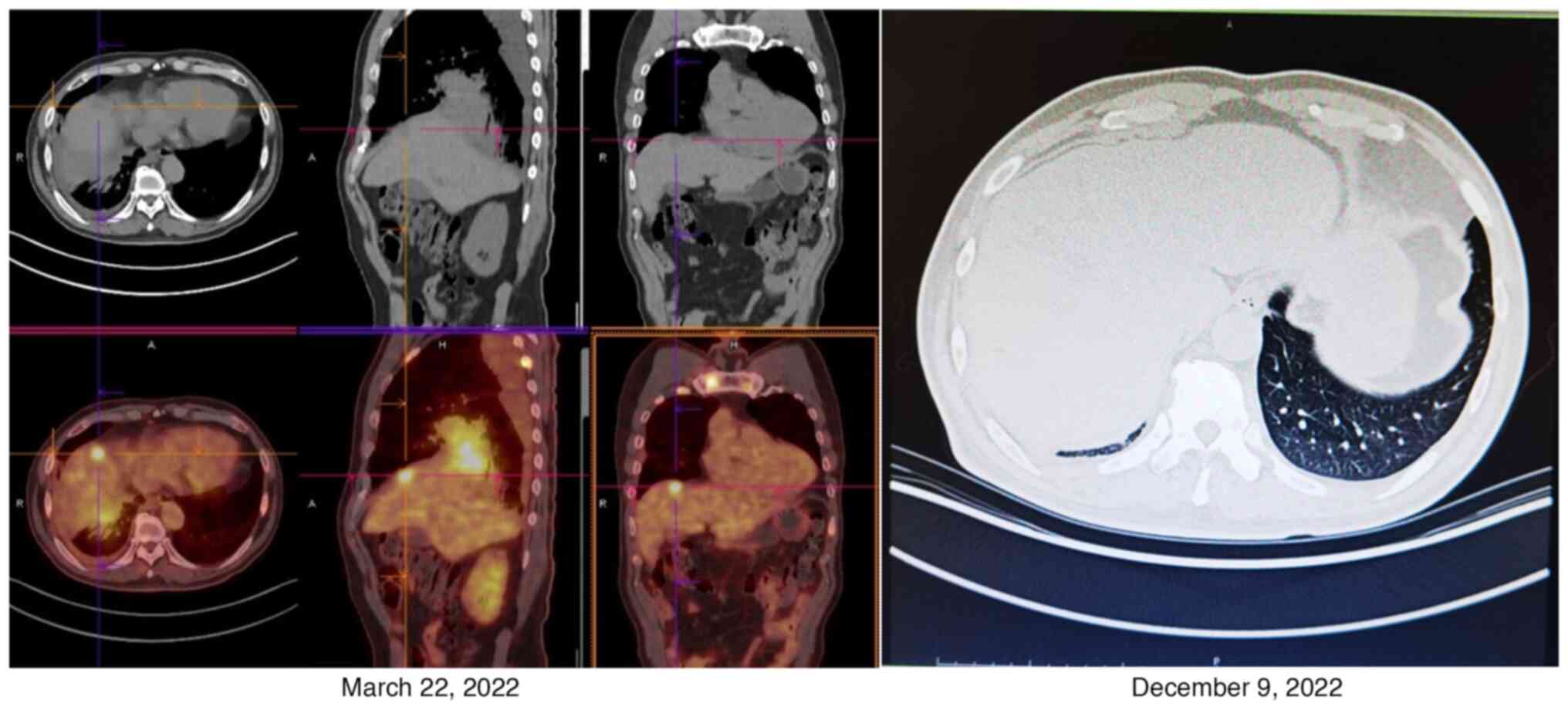
ng/ml) in December 2022, compared with those in March 2022, were
markedly decreased, and a CT scan revealed that the primary and
metastatic lesions were also significantly reduced in size,
especially the lung lesion (0.8×0.7 cm), and metastatic lesions
were significantly reduced in size or had even locally disappeared
(Figs. 3 and 4). The patient was still alive and being
followed up every 2 weeks at the time of publication of the present
study.
Discussion
The current patient presented with a reduction in
tumor size on imaging, decreased serum tumor marker levels,
decreased pain and relief from fatigue after the SARS-CoV-2
infection, without a change to the therapeutic schedule. Combining
data on the clinical manifestations, serological test results and
the patient's symptoms, it was concluded that the tumor in this
patient with advanced lung cancer had spontaneously reduced, and
the patient's medical condition had improved.
Spontaneous tumor reduction after SARS-CoV-2
infection is rare. Generally, patients with cancer, as a
immunocompromised group, showed a higher mortality rate when
infected (7). However, recent
reports have demonstrated that the imaging and serological symptoms
of certain cancer patients were relieved after SARS-CoV-2 infection
(8). This effect of SARS-CoV-2
infection occurs not only in hematological malignancies, such as
Epstein-Barr virus-positive classical Hodgkin lymphoma (9), follicular lymphoma (10), acute myeloid leukemia (11) and chronic lymphocytic leukemia
(12), but also in solid tumors
such as colon cancer (13,14) and renal cell carcinoma (15). Although the underlying mechanism has
not been entirely understood, researchers have speculated that
SARS-CoV-2 can activate the body's innate immunity. By binding to
the angiotensin-converting enzyme 2/neuropilin-1 complex on the
surface of infected cell membranes through the surface spike
proteins, it changes the natural tolerance of innate immunity
through non-major histocompatibility complex-restricted pathways,
enhances the recognition of macrophages and dendritic cells, thus
inducing excessive inflammatory responses, and activates more
cytotoxic T cells to destroy infected cells (16). In other words, SARS-CoV-2 infection
makes tumor cells more susceptible to attack by immune cells and
destruction. Furthermore, some researchers believe that
cross-reactivity may occur between SARS-CoV-2 antigens and specific
tumor antigens, thus activating the tumor-specific anti-tumor
response of T cells to mediate the oncolytic effect (17). Additionally, activating natural
killer cells is also reported to be an essential factor involved in
oncolysis (18). We hypothesized
(despite it not being confirmed) that SARS-CoV-2 infection plays an
indispensable role in superior drug sensitivity and lower
resistance to antineoplastic agents. Therefore, SARS-CoV-2 maybe
exert the effect of an oncolytic virus (OV) on mediating the
enhancement of the sensitivity of targeted anticancer agents,
improving their efficacy, and promoting tumor dissolution and
regression.
Oncolytic virotherapy is a promising avenue of
immunotherapy that exerts its oncolytic effect by actively
identifying and infecting tumor cells, replicating and eventually
killing tumor cells with different regulatory mechanisms, such as
inducing an innate immune response, but with immune exemption in
normal cells (19). Although few
viruses have currently been successfully developed into Food and
Drug Administration-approved treatments for use in clinical
regimens, the discovery of OVs can be traced back as far as the mid
to late 19th century, when Dock (20) described a patient with myeloid
leukemia who has suffered a spontaneous reduction of abnormal
leukocyte counts after an influenza epidemic. Following this,
similar reports became more common, mainly finding patients with
hematological tumors who experienced transient remission after
coincidental viral infection. With more and more researchers
working to modify the natural wild-type lytic virus, further
modifications have been able to provide a more powerful anticancer
effect (21). Currently, various
viruses, including RNA viruses such as rhinovirus, poliovirus,
measles virus and vesicular stomatitis virus, and DNA viruses, such
as adenovirus and herpes simplex virus, have been used in cancer
treatment (22). Due to their
single-stranded structure, RNA viruses have some natural
disadvantages in use as preclinical virus models of anticancer
therapy compared with DNA viruses. The mutation rate of RNA viruses
is 1×10−6−10−4 per base per cell, while that
of DNA viruses is 1×10−8−10−6. Therefore, if
RNA viruses are used as vectors and injected into patients, the
patients may face more unpredictable risks (23). Compared with DNA viruses, RNA
viruses are more easily integrated into the host genome, which also
brings unpredictable risks to patients. Thus, if SARS-CoV-2 is to
be used as an oncolytic therapy vector, a series of genetically
engineered modifications need to be conducted. Similar to the case
of combining poliovirus and rhinovirus to form a recombinant
poliovirus chimera (24), it is
necessary to further attenuate pathogenicity and cytotoxicity, and
enhance specificity and security if SARS-CoV-2 is to play a role in
antitumor immunomodulation.
In conclusion, tumor reduction after SARS-CoV-2
infection is a rare condition. Although the exact cause and
mechanism are unknown, the clinical symptoms and diagnostic tests
in the present report suggest that SARS-CoV-2 infection may alter
the immunomodulatory response or drug resistance, leading to
improved antitumor effects. This case also provides a rationale and
inspiration for exploring SARS-CoV-2 as a viral vector in oncolytic
virotherapy, although further evidential studies are required. Due
to the present study reporting only a single case, there was no way
for a controlled trial to be conducted. Therefore, this case is
shared with other researchers and physicians to aid in further
elucidation of the mechanism involved.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Key Research and Development
Project of Hainan (grant no. ZDYF2023SHFZ117) and the Military
Medical Science and Technology Youth Cultivation Program (grant no.
2019NPY11)
Availability of data and materials
The NGS data generated in the present study are not
publicly available to preserve patient anonymity. All other
datasets used and/or analyzed during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
XMZ, JYC and JS drafted the manuscript and conceived
the study. SYG and FYZ participated in the analysis, collection and
interpretation of data, and NSQ obtained PET/CT images and analyzed
patient data from the NGS assay. NSQ and XMZ confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the case study to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Petty WJ and Paz-Ares L: Emerging
strategies for the treatment of small cell lung cancer: A review.
JAMA Oncol. 9:419–429. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vokes NI, Pan K and Le X: Efficacy of
immunotherapy in oncogene-driven non-small-cell lung cancer. Ther
Adv Med Oncol. 15:175883592311614092023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zong Z, Wei Y, Ren J, Zhang L and Zhou F:
The intersection of COVID-19 and cancer: Signaling pathways and
treatment implications. Mol Cancer. 20:76–82. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsamakis K, Gavriatopoulou M, Schizas D,
Stravodimou A, Mougkou A, Tsiptsios D, Sioulas V, Spartalis E,
Sioulas AD, Tsamakis C, et al: Oncology during the COVID-19
pandemic: Challenges, dilemmas and the psychosocial impact on
cancer patients. Oncol Lett. 20:441–447. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bounassar-Filho JP, Boeckler-Troncoso L,
Cajigas-Gonzalez J and Zavala-Cerna MG: SARS-CoV-2 as an oncolytic
virus following reactivation of the immune system: A review. Int J
Mol Sci. 24:243–249. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meo C, Palma G, Bruzzese F, Budillon A,
Napoli C and de Nigris F: Spontaneous cancer remission after
COVID-19: Insights from the pandemic and their relevance for cancer
treatment. J Transl Med. 21:273–278. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rahimmanesh I, Shariati L, Dana N,
Esmaeili Y, Vaseghi G and Haghjooy Javanmard S: Cancer occurrence
as the upcoming complications of COVID-19. Front Mol Biosci.
8:8131752021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kurlapski M, Romanowicz G, Taszner M and
Zaucha JM: SARS-CoV-2-induced remission of advanced classical
Hodgkin lymphoma. Pol Arch Intern Med. 13:132–139. 2022.
|
|
10
|
Sollini M, Gelardi F, Carlo-Stella C and
Chiti A: Complete remission of follicular lymphoma after SARS-CoV-2
infection: from the ‘flare phenomenon’ to the ‘abscopal effect’.
Eur J Nucl Med Mol Imaging. 48:2652–2654. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barkhordar M, Rostami FT, Yaghmaie M,
Abbaszadeh M, Chahardouli B and Mousavi SA: Spontaneous complete
remission of acute myeloid leukemia in the absence of
disease-modifying therapy following severe pulmonary involvement by
coronavirus infectious disease-19. Case Rep Hematol.
2022:26036072022.PubMed/NCBI
|
|
12
|
Bulbul H, Nazli HE, Olgun A, Togay A and
Kahraman DS: Spontaneous remission of chronic lymphocytic leukemia
in a patient with SARS-CoV2. Leuk Res Rep. 18:103–106.
2022.PubMed/NCBI
|
|
13
|
Ottaiano A, Scala S, D'Alterio C, Trotta
A, Bello A, Rea G, Picone C, Santorsola M, Petrillo A and Nasti G:
Unexpected tumor reduction in metastatic colorectal cancer patients
during SARS-Cov-2 infection. Ther Adv Med Oncol.
13:175883592110114552021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ottaiano A, Santorsola M, Circelli L,
Cascella M, Petrillo N, Perri F, Casillo M, Granata V, Ianniello M,
Izzo F, et al: Genetic landscape of colorectal cancer patients
manifesting tumor shrinkage during SARS-Cov-2 infection. Ther Adv
Med Oncol. 14:175883592211383882022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Buchler T, Fiser L, Benesova J, Jirickova
H and Votrubova J: Spontaneous regression of metastatic renal cell
carcinoma after SARS-CoV-2 infection: A report of two cases. Curr
Oncol. 28:3403–3407. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Daly JL, Simonetti B, Klein K, Chen KE,
Williamson MK, Anton-Plagaro C, Shoemark DK, Simon-Gracia L, Bauer
M, Hollandi R, et al: Neuropilin-1 is a host factor for SARS-CoV-2
infection. Science. 370:861–865. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Challenor S and Tucker D:
SARS-CoV-2-induced remission of Hodgkin lymphoma. Br J Haematol.
192:415–421. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pasin F, Mascalchi Calveri M, Calabrese A,
Pizzarelli G, Bongiovanni I, Andreoli M, Cattaneo C and Rignanese
G: Oncolytic effect of SARS-CoV2 in a patient with NK lymphoma.
Acta Biomed. 91:Aug 10–2020.(Epub ahead of print).
|
|
19
|
Lin D, Shen Y and Liang T: Oncolytic
virotherapy: Basic principles, recent advances and future
directions. Signal Transduct Target Ther. 8:156–173. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dock G: The influence of complicating
diseases upon leukemia. Am J Med Sci. 127:563–592. 1904. View Article : Google Scholar
|
|
21
|
Kristin DP and Greg MD: Integrating innate
and adaptive immunity in oncolytic virus therapy. Trends Cancer.
23:190–196. 2023.
|
|
22
|
Yun CO, Hong J and Yoon AR: Current
clinical landscape of oncolytic viruses as novel cancer
immunotherapeutic and recent preclinical advancements. Front
Immunol. 13:95–104. 2022. View Article : Google Scholar
|
|
23
|
Muscolini M, Tassone E and Hiscott J:
Oncolytic immunotherapy: Can't start a fire without a spark.
Cytokine Growth Factor Rev. 56:94–101. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hamad A, Yusubalieva GM, Baklaushev VP,
Chumakov PM and Lipatova AV: Recent developments in glioblastoma
therapy: Oncolytic viruses and emerging future strategies. Viruses.
15:106–119. 2023. View Article : Google Scholar
|