Introduction
Globally, pancreatic ductal adenocarcinoma (PDAC) is
the seventh leading cause of cancer-related deaths among men and
women (1). Unfortunately, 80–85% of
patients present with unresectable PDAC (UR-PDAC) [unresectable
locally advanced cancer (UR-LA) or unresectable cancer with distant
metastasis (UR-M)] and have a poor prognosis (2). However, the recent implementation of
intense regimens, such as FOLFIRINOX (3) and gemcitabine + nab-paclitaxel (GnP)
(4), has provided better clinical
response rates, and the number of patients who can expect long-term
survival is increasing. Moreover, the number of long-term survival
cases after such intense chemotherapy or chemoradiotherapy followed
by conversion surgery (CS) has increased in recent years (5). Currently, the National Comprehensive
Cancer Network Guidelines (6)
suggest that CS is an option for patients with UR-LA if resectable
after a successful multidisciplinary treatment. Conversely, some
reports (7,8) have shown that CS may prolong the
prognosis of UR-M patients, but the actual benefit of CS in
patients with UR-M remains controversial.
Previous studies (9–16) have
reported several prognostic factors associated with the survival
time of CS or the recurrence after CS in patients with UR-PDAC.
However, few pretherapeutic parameters have been reported to
predict the attainability of CS in patients with UR-PDAC.
Therefore, this study aimed to explore the pretherapeutic factors
predicting the attainability of CS in patients with UR-PDAC. In
this study, we focused on examining predictors for CS rather than
the overall survival (OS). The reason was that the association of
intermediate treatment, including CS, from the start of treatment
to death is too strong to ensure that baseline factors are accurate
in their prediction for OS. Previous reports have shown that CS is
beneficial for survival in patients with UR-PDAC. Therefore, by
examining factors that predict the attainability of CS, we
considered that these factors could influence the prognosis of
patients with UR-PDAC.
Materials and methods
Study design
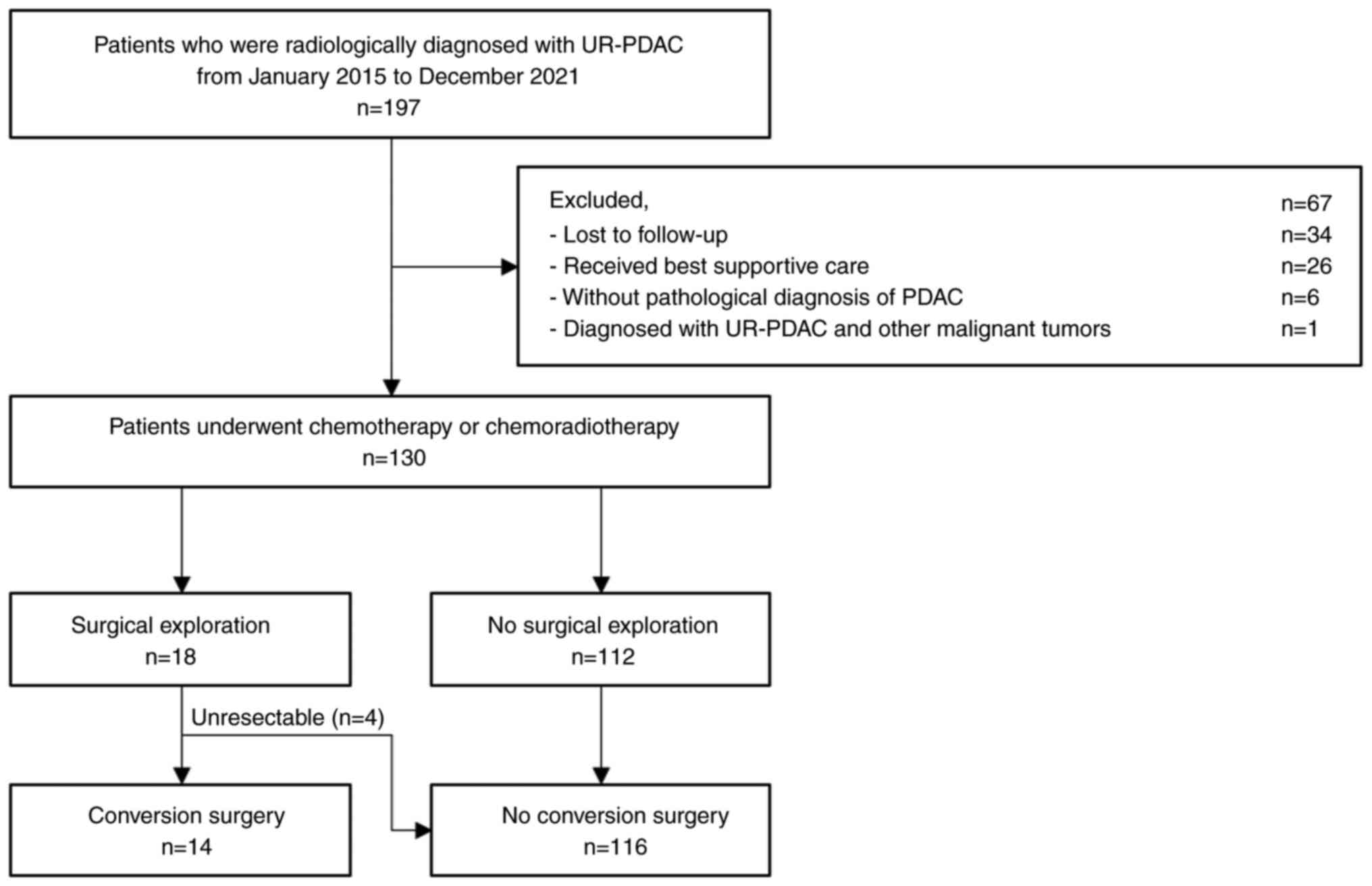
This retrospective study included patients treated
at a single tertiary care center (Gifu University Hospital, Gifu,
Japan) from January 2015 to December 2021, who were radiologically
diagnosed with UR-PDAC according to the Classification of
Pancreatic Cancer by the Japan Pancreas Society (4th English
Edition) (17). We excluded
patients who were lost to follow-up, underwent only the best
supportive care, were not diagnosed with PDAC, and patients with
UR-PDAC and other malignant tumors (Fig. 1). The participants provided informed
consent by an opt-out option before enrollment in the study. The
study was conducted following the human and ethical principles of
the Declaration of Helsinki, and the study protocol was approved by
the Institutional Review Board of the Gifu University Hospital
(approval number: 2022-285).
Measures
The demographic and clinical variables included age,
sex, body mass index (BMI), tumor location, tumor size,
carbohydrate antigen 19-9 (CA19-9), total protein (TP), albumin,
C-reactive protein, hemoglobin, neutrophil, lymphocyte, monocyte,
prognostic nutritional index (18),
modified Glasgow prognostic score (19), neutrophil-to-lymphocyte ratio (NLR),
lymphocyte-to-monocyte ratio (LMR), and platelet-to-lymphocyte
ratio. These variables were measured before the initial treatment.
The tumor size was measured using pretherapeutic multidetector CT.
Response Evaluation Criteria in Solid Tumors version 1.1 (20) was used for radiologic tumor response
evaluation.
Outcome
In this study, the primary outcome was CS, defined
as surgical resection following chemotherapy or chemoradiotherapy
for patients initially diagnosed with UR-PDAC. At our institute,
tumor resectability is determined case-by-case through discussions
among surgeons, physicians, and radiologists. We considered
surgical exploration if the eligibility criteria met after
chemotherapy or chemoradiotherapy were as follows: i) Decrease or
normalization of tumor markers; ii) Clinical response (stable
disease/partial response/complete response) on multidetector CT;
iii) Technically resectable on imaging; iv) Decreased or absent
accumulation of the primary tumor on fluorine-18-fluorodeoxyglucose
positron emission tomography (FGD-PET); v) In cases of UR-M,
metastases shrank or disappeared on MDCT with no accumulation on
FGD-PET; vi) No appearance of new metastatic sites; and vii) Fine
performance status (0–1).
Statistical analysis
The patient characteristics are presented as
frequencies and percentages for categorical variables, and as
medians with interquartile ranges or means with standard deviations
for continuous variables. When comparing the OS between patients
with CS and non-CS, we treated CS as a time-varying covariate to
avoid immortal time bias. The survival rate was estimated using the
Simon and Makuch modified Kaplan-Meier method (21,22).
The hazard ratio (HR) was estimated using a time-varying Cox
regression model. The association between each pretherapeutic
predictor and CS was evaluated using the univariable and
age-adjusted Fine-Gray sub-distribution hazard model, considering
death and disease progression as competing risks. No correction for
the multiplicity of statistically significant tests was performed
as the analysis was exploratory. The bootstrap bias-corrected area
under the receiver operating characteristic curve (AUC-ROC) was
reported as a measure of the predictive performance of the
pretherapeutic predictors. In total, 10,000 bootstrap samples were
generated, and the AUCs obtained from each ROC were averaged to
calculate the bootstrap AUC-ROC. The cut-off values for predicting
the CS obtained from ROC were determined based on the Youden Index.
An appropriate cut-off value for predicting CS was calculated by
averaging the thresholds obtained from each ROC from the bootstrap
sample. The cumulative incidence rate was calculated with CS as the
outcome when divided into two groups based on the cut-off value of
each pretherapeutic predictor. Fisher's exact test was performed
for categorical variables. The Mann-Whitney U test was applied for
the comparison of continuous variables. All P-values are two-sided,
with the significance level set at P<0.05. All analyses,
including only data for patients with assessed pretherapeutic
predictor variables, were performed using R 4.2.2 software (The R
Project for Statistical Computing).
Results
Patient characteristics
This study retrospectively analyzed a total of 130
consecutive patients with UR-PDAC who were treated at our
institute. Table I summarizes the
patients' characteristics; 66 patients (51%) were men, with a
median age of 69 years. We used multidetector CT or laparotomy to
diagnose 36 patients (28%) with UR-PDAC and 94 patients (72%) with
UR-M. In patients with UR-LA, the median size of the main lesion
was 3.7 cm at the initial diagnosis, and the median CA19-9 level
before the initial treatment was 746 U/ml. The most frequently used
first-line treatment regimen was modified FOLFIRINOX (50%),
followed by GnP (31%). In patients with UR-M, the median size of
the main lesion was 3.4 cm at the initial diagnosis, and the median
CA19-9 level before the initial treatment was 888 U/ml. The most
frequently used first-line treatment regimen was modified
FOLFIRINOX (62%), followed by GnP (30%).
 | Table I.Patients' characteristics. |
Table I.
Patients' characteristics.
| Variable | Total (n=130) | UR-LA (n=36) | UR-M (n=94) |
|---|
| Male sex, n (%) | 66 (51) | 20 (56) | 46 (49) |
| Median age, years
(IQR) | 69 (62–74) | 71 (68–76) | 68 (62–73) |
| Tumor location Ph, n
(%) | 56 (43) | 23 (64) | 33 (35) |
| Median tumor size at
diagnosis, cm (IQR) | 3.5 (2.8–4.4) | 3.7 (2.9–4.1) | 3.4 (2.7–4.4) |
| Median CA19-9, U/ml
(IQR) | 877 (140–5,375) | 746 (169–2,426) | 888 (124–7,793) |
| Mean TP, g/dl
(SD) | 6.5 (0.6) | 6.6 (0.5) | 6.5 (0.6) |
| Median albumin,
g/dl (IQR) | 3.8 (3.5–4.1) | 4.0 (3.5–4.3) | 3.8 (3.5–4.0) |
| Median total
cholesterol, mg/dl (IQR) | 177 (154–208) | 172 (139–204) | 178 (158–211) |
| Median LDH, IU/l
(IQR) | 186 (159–230) | 179 (159–220) | 189 (160–240) |
| Median CRP, mg/dl
(IQR) | 0.45
(0.14–1.56) | 0.38
(0.10–1.12) | 0.47
(0.15–2.31) |
| Mean hemoglobin,
g/dl (SD) | 12.1 (1.3) | 12.0 (1.4) | 12.1 (1.3) |
| Median neutrophils,
cells/µl (IQR) | 4,200
(2,961–5,333) | 3,415
(2,773–4,975) | 4,280
(3,268–5,445) |
| Median lymphocytes,
cells/µl (IQR) | 1,266
(1,000–1,528) | 1,369
(1,151–1,570) | 1,210
(972–1,508) |
| Median monocytes,
cells/µl (IQR) | 401 (308–513) | 411 (318–532) | 399 (298–512) |
| Median platelets,
×104/µl (IQR) | 23.1
(18.0–29.2) | 20.0
(17.3–27.8) | 23.4
(18.5–29.2) |
| Median PNI IQR | 38.9
(35.7–41.8) | 40.2
(35.7–43.8) | 38.9
(35.7–41.3) |
| Modified GPS,
0/1/2 | 60/52/18 | 17/16/3 | 43/36/15 |
| Median NLR
(IQR) | 3.10
(2.25–4.38) | 2.65
(1.95–3.66) | 3.42
(2.55–4.70) |
| Median LMR
(IQR) | 3.16
(2.33–4.17) | 3.25
(2.37–4.08) | 3.15
(2.26–4.23) |
| Median PLR
(IQR) | 178 (133–263) | 156 (123–223) | 189 (144–274) |
| First-line
treatment |
|
|
|
|
mFFX/GnP/GEM/S-1/Other | 76/39/11/2/2 | 18/11/4/1/2 | 58/28/7/1/0 |
| Best response based
on imaging studies |
|
|
|
|
CR/PR/SD/PD/unknown | 1/45/53/29/2 | 0/14/14/7/1 | 1/31/39/22/1 |
Clinical characteristics and outcome
of conversion surgery
During this study, CS was performed for six patients
with UR-LA and eight patients with UR-M (Table II). The first-line treatment
regimens were modified FOLFIRINOX for 12 cases and GnP for two
cases. The median duration of first-line treatment was 7.4 months.
Treatment responses included stable disease in four cases, partial
response in nine, and complete response in one. In all cases, the
preoperative CA19-9 was lower than before first-line treatment,
with unresectable factors disappearing or shrinking afterwards. We
performed pancreaticoduodenectomy for six patients, distal
pancreatectomy (DP) for six, DP with en-bloc celiac axis resection
for one, and total pancreatectomy for one patient. One patient
initially presenting with liver metastases underwent partial
hepatectomy. There were no residual cancer cells in the resected
liver. R0 (no residual tumor) resection was achieved in 12 patients
(86%), and 11 received postoperative chemotherapy. Recurrence was
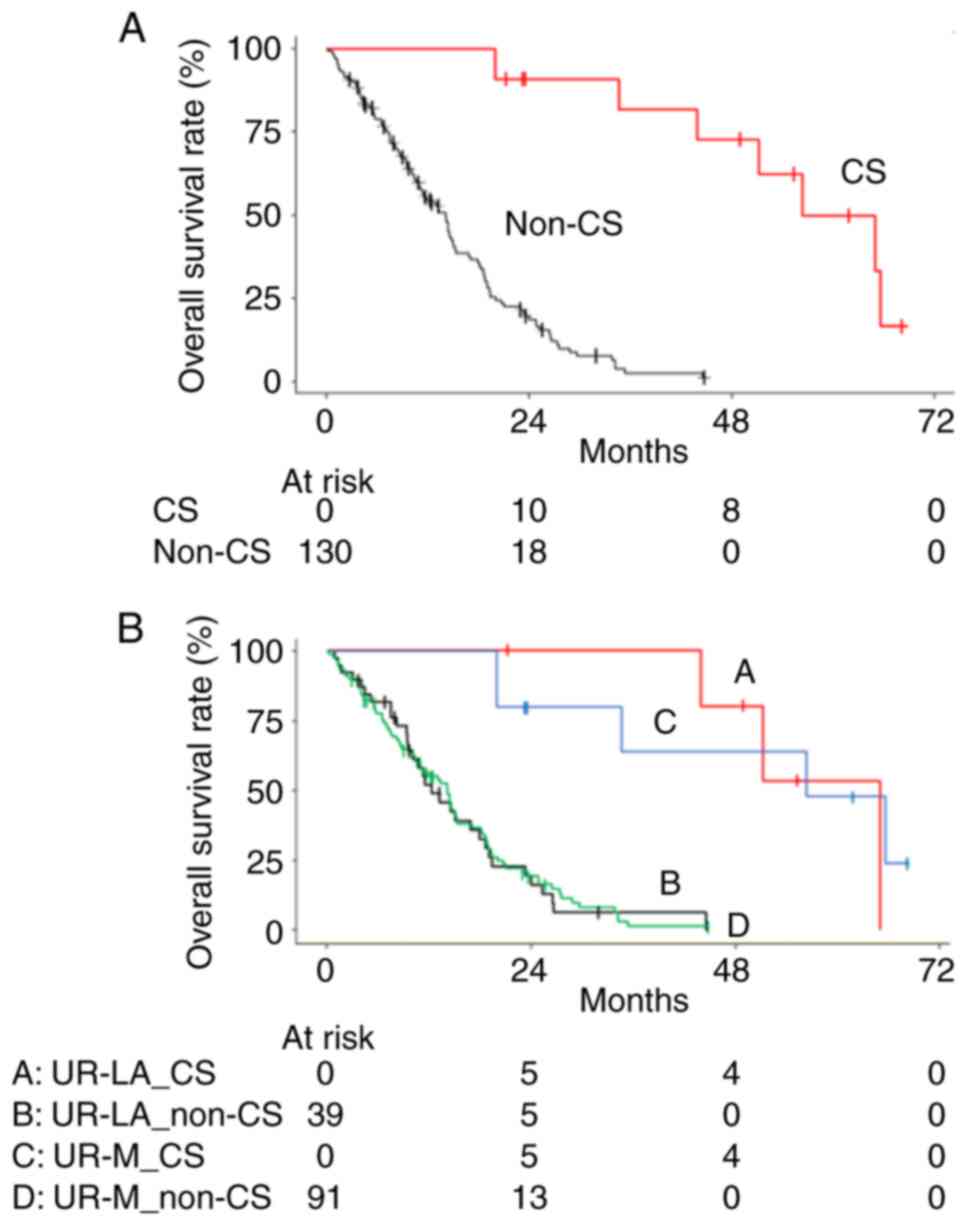
confirmed in eight patients (57%). The median OS in patients who
underwent CS was estimated as 56.3 months and was significantly
longer than that in all patients without CS (median OS of 14.1
months, HR 0.08, 95% CI 0.03–0.21; P<0.001) (Fig. 2A). In patients with UR-LA and UR-M,
the CS group had significantly longer OS than the non-CS group
(UR-LA: 64.9 vs. 12.3 months; HR 0.05, 95% CI 0.01–0.28; P=0.001
and UR-M: 56.3 vs. 14.2 months; HR 0.12, 95% CI 0.03–0.40; P=0.001,
respectively) (Fig. 2B). The median
OS from CS was estimated as 44.4 months in patients with UR-LA and
62.8 months in patients with UR-M.
 | Table II.Clinical characteristics and outcome
of 14 patients who underwent conversion surgery. |
Table II.
Clinical characteristics and outcome
of 14 patients who underwent conversion surgery.
| No. | Age, years | Sex | Tumor location | UR status (UR
factor) | First-line
treatment | Duration,
months | RECIST | CA19-9 before/after
treatment, U/ml | UR factor after
treatment | Surgery | R | Evans grade | Post-operative
therapy | Recurrence
site | Time to recurrence
from CS, months | Prognosis,
monthsa |
|---|
| 1 | 72 | M | Ph | LA (SMV) | mFFX | 8.0 | PR | 1,462/43 | Disappeared | PD | R0 | IIb | S-1 | Remnant
pancreas | 20 | Dead (66/58) |
| 2 | 73 | M | Ph | LA (CHA, PHA) | mFFX | 3.7 | SD | 354/89 | Disappeared | DP-CAR | R0 | IIa | None | None |
| Dead (45/41) |
| 3 | 58 | M | Ph | LA (Ao) | mFFX | 6.8 | PR | 171/12 | Shrank | PD | R0 | IIb | S-1 | Local | 29 | Alive (52/45) |
| 4 | 56 | F | Ph | LA (SMA, SMV) | mFFX | 13.4 | PR | 5,451/116 | Shrank | PD+PVR | R0 | IV | None | Lung | 14 | Alive (56/43) |
| 5 | 70 | F | Ph | LA (CHA, PHA) | GnP | 9.8 | PR | 228/20 | Shrank | PD+PVR+HAR | R0 | IIb | S-1 | Remnant
pancreas | 18 | Alive (50/40) |
| 6 | 57 | M | Ph | LA (Ao) | mFFX | 6.8 | PR | 10,890/26 | Disappeared | PD | R0 | IIb | S-1 | None |
| Alive (22/15) |
| 7 | 72 | M | Pt | M (eLN) | mFFX | 2.8 | SD | 24,469/3,333 | Shrank | DP | R1 | IIa | mFFX | LN | 50 | Dead (67/64) |
| 8 | 63 | M | Pb | M (Per) | mFFX | 4.5 | SD | 481/370 | Disappeared | DP | R0 | IIa | S-1 | Lung, LN | 24 | Dead (35/31) |
| 9 | 59 | M | Pb | M (Per) | mFFX | 24.0 | PR | 250/17 | Disappeared | DP | R0 | IIa | mFFX | PER | 19 | Dead (57/33) |
| 10 | 63 | F | Pb | M (Liver-Multi,
Bone-Multi) | mFFX | 23.3 | PR | 4,379/81 | Disappeared | DP | R0 | IIb | S-1 | None |
| Alive (69/46) |
| 11 | 79 | M | Pht | M (Per) | GnP | 4.6 | SD | 159/52 | Disappeared | TP | R1 | IIa | S-1 | Liver | 5 | Dead (20/16) |
| 12 | 61 | F | Ph | M
(Liver-Multi) | mFFX | 26.0 | CR | 10,424/36 | Disappeared | PD+Hep | R0 | IV | None | None |
| Alive (63/37) |
| 13 | 62 | M | Pb | M (eLN) | mFFX | 9.1 | PR | 96/48 | Shrank | DP | R0 | IIb | mFFX | None |
| Alive (24/15) |
| 14 | 59 | M | Pt | M (eLN) | mFFX | 5.5 | PR | 13/12 | Shrank | DP | R0 | IV | mFFX | None |
| Alive (24/18) |
Association between pretherapeutic
parameters and the attainability of CS
Age-adjusted Fine-Gray regression showed that TP (HR
2.81; P=0.018), NLR (HR 0.53; P=0.020), and LMR (HR 1.28; P=0.006)
were significant pretherapeutic markers predicting the
attainability of CS (Table III).
The cut-off value of each marker for CS based on the ROC curve is
presented in Table IV. The optimal
cut-off values for useful parameters using the bootstrap ROC curve
were 6.8 (AUC=0.70), 2.84 (AUC=0.74), and 3.87 (AUC=0.72) for TP,
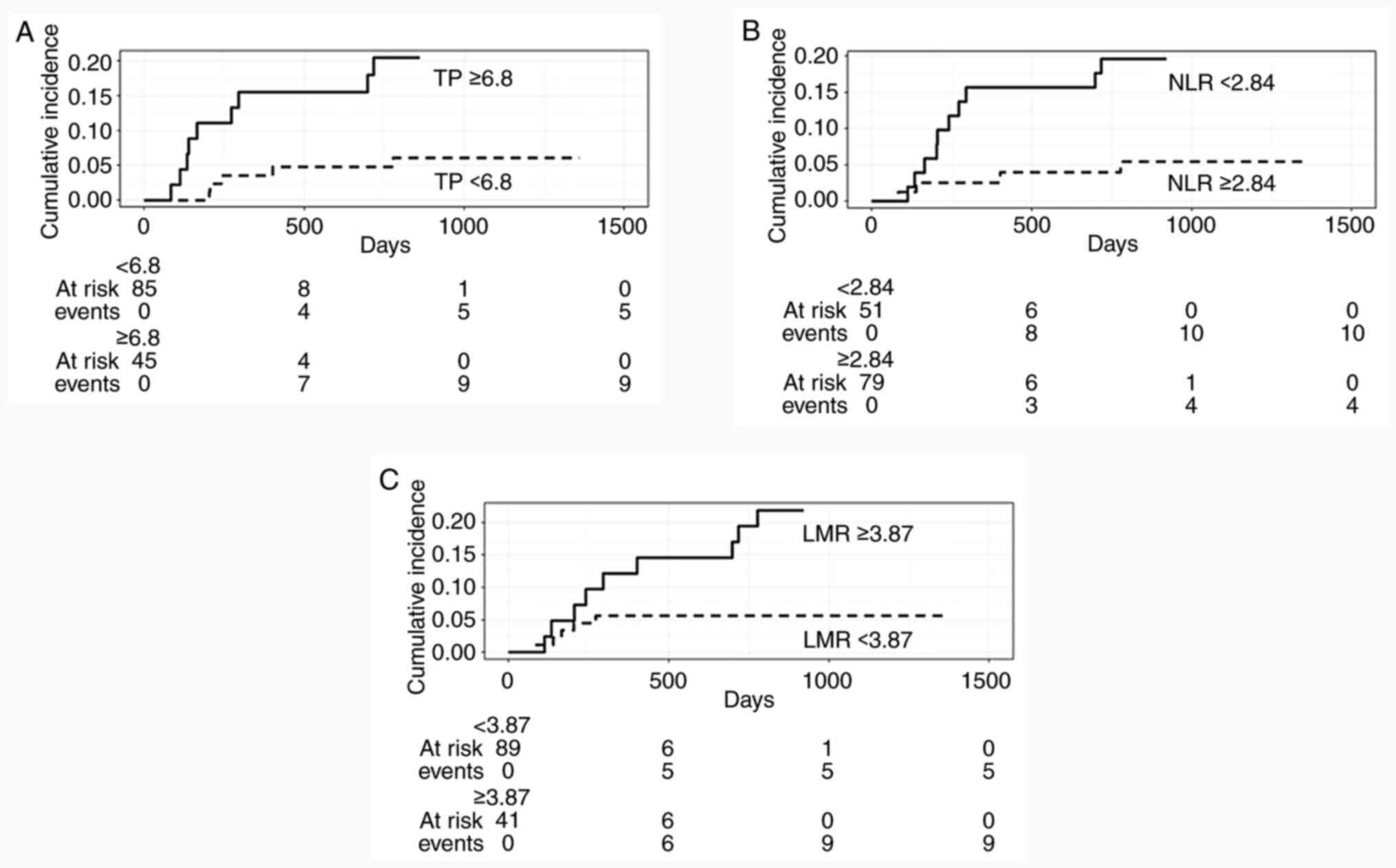
NLR, and LMR, respectively. Fig. 3
shows the cumulative incidence of these parameters with each
cut-off value using the bootstrap ROC curve. The percentage of
patients who underwent CS was higher in the TP ≥6.8 (20%, 9/45),
NLR <2.84 (20%, 10/51), and LMR ≥3.87 (22%, 9/41) groups
compared with the TP <6.8 (6%, 5/85), NLR ≥2.84 (5%, 4/79), and
LMR <3.87 (6%, 5/89) groups, respectively. In patients who
underwent CS, the changes in TP, NLR, and LMR before and after
first-line treatment were 0.6 (95% CI 0.28–0.86), 0.27 (95%
CI-0.55–1.08), and 1.27 (95% CI 0.28–2.25), respectively. In
patients who did not undergo CS, the changes in TP, NLR, and LMR
between and after first-line treatment were 0.3 (95% CI 0.20–0.40),
0.31 (95% CI-0.54–1.16), and 0.25 (95% CI-0.34–0.83),
respectively.
 | Table III.Pretherapeutic predictors for the
attainability of conversion surgery (univariate analysis and
age-adjusted Fine-Gray sub-distribution hazard regression). |
Table III.
Pretherapeutic predictors for the
attainability of conversion surgery (univariate analysis and
age-adjusted Fine-Gray sub-distribution hazard regression).
|
| Univariate
analysis | Age-adjusted |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| CA19-9 | 1.00
(1.00–1.00) | 0.260 | 1.00
(1.00–1.00) | 0.270 |
| TP | 2.59
(1.09–6.15) | 0.031 | 2.81
(1.19–6.65) | 0.018 |
| Albumin | 5.22
(0.90–30.3) | 0.066 | 4.65
(0.75–28.8) | 0.099 |
| Total
cholesterol | 1.00
(0.99–1.01) | 0.910 | 1.00
(0.99–1.01) | 0.820 |
| LDH | 0.99
(0.99–1.00) | 0.130 | 0.99
(0.99–1.00) | 0.079 |
| CRP | 0.72
(0.49–1.06) | 0.097 | 0.75
(0.54–1.04) | 0.085 |
| Hemoglobin | 1.09
(0.81–1.46) | 0.580 | 1.09
(0.80–1.47) | 0.600 |
| Neutrophil | 1.00
(1.00–1.00) | 0.027 | 1.00
(1.00–1.00) | 0.029 |
| Lymphocyte | 1.00
(1.00–1.00) | 0.030 | 1.00
(1.00–1.00) | 0.017 |
| Monocyte | 1.00
(0.99–1.00) | 0.310 | 1.00
(1.00–1.00) | 0.320 |
| Platelet | 1.01
(1.00–1.01) | <0.001 | 1.01
(1.00–1.01) | <0.001 |
| PNI | 1.18
(1.00–1.40) | 0.055 | 1.17
(0.98–1.39) | 0.081 |
| Modified GPS | 0.68
(0.31–1.49) | 0.340 | 0.75
(0.35–1.59) | 0.450 |
| NLR | 0.53
(0.31–0.91) | 0.020 | 0.53
(0.31–0.90) | 0.020 |
| LMR | 1.27
(1.07–1.51) | 0.007 | 1.28
(1.07–1.53) | 0.006 |
| PLR | 1.00
(1.00–1.00) | 0.024 | 1.00
(1.00–1.00) | 0.022 |
 | Table IV.Cutoff value and predictive
performance for each marker based on ROC curves. |
Table IV.
Cutoff value and predictive
performance for each marker based on ROC curves.
|
| Usual method | Bootstrap
method |
|---|
|
|
|
|
|---|
| Variable | AUC | 95% CI | Sensitivity | Specificity | Cutoff value | AUC | Sensitivity | Specificity | Cutoff value |
|---|
| TP | 0.70 | 0.54–0.86 | 0.86 | 0.50 | 6.6 | 0.70 | 0.76 | 0.66 | 6.8 |
| NLR | 0.74 | 0.61–0.87 | 0.86 | 0.55 | 3.08 | 0.74 | 0.83 | 0.65 | 2.84 |
| LMR | 0.72 | 0.59–0.86 | 0.64 | 0.77 | 4.12 | 0.72 | 0.77 | 0.70 | 3.87 |
Differences in the course of treatment
for each parameter
There was no difference in the first-line treatment
regimens or treatment-related adverse events for each parameter
(Table V). However, patients with
NLR <2.84 and LMR ≥3.87 had significantly longer overall
treatment time than those with NLR ≥2.84 (P=0.006) and LMR <3.87
(P<0.001), respectively.
 | Table V.Summary of the course of treatment
for each parameter. |
Table V.
Summary of the course of treatment
for each parameter.
| Variable | TP <6.8
(n=85) | TP ≥6.8 (n=45) | P-value | NLR <2.84
(n=51) | NLR ≥2.84
(n=79) | P-value | LMR <3.87
(n=89) | LMR ≥3.87
(n=41) | P-value |
|---|
| First-line
treatment |
|
| 0.335 |
|
| 0.359 |
|
| 0.286 |
|
mFFX/GnP/ | 51/24/0/8/2 | 25/15/2/3/0 |
| 30/16/2/3/0 | 46/23/0/8/2 |
| 53/24/1/10/1 | 23/15/1/1/1 |
|
|
S-1/GEM/Other |
|
|
|
|
|
|
|
|
|
| ≥Grade 3
toxicity | 75 (88.2) | 37 (82.2) | 0.498 | 44 (86.3) | 68 (86.1) | 0.999 | 77 (86.5) | 35 (85.4) | 0.999 |
| adverse event, |
|
|
|
|
|
|
|
|
|
| n (%) |
|
|
|
|
|
|
|
|
|
| Median overall | 11.2 | 10.8 | 0.854 | 13.3 | 8.5 | 0.006 | 8.5 | 15.8 | <0.001 |
| treatment
time, | (4.8–18.3) | (3.7–20.0) |
| (7.8–23.0) | (2.8–17.5) |
| (3.2–16.8) | (8.7–28.5) |
|
| months (IQR) |
|
|
|
|
|
|
|
|
|
Discussion
In this study, the median OS was significantly
longer in patients who underwent CS than in all patients without
CS. The study also indicated that patients who underwent CS with
UR-LA and UR-M had a significant difference in the median OS
compared to patients who did not undergo CS. Therefore, this study
demonstrated that CS significantly impacted the prognosis of
patients with UR-PDAC. We examined the factors that predict the
attainability of CS based on the pretherapeutic parameters in
patients with UR-PDAC. As a result, TP ≥6.8, NLR <2.84, and LMR
≥3.87 were associated with a higher cumulative incidence of CS.
The three parameters evaluated in this study were
associated with nutritional status and cancer-related inflammation.
TP is a predictor of postoperative prognosis in lung cancer
(23) and retroperitoneal sarcoma
(24). TP decreases with the
progression of the disease because of malnutritional and
inflammatory status, cachexia, and increased intracellular
catabolism by cancer cells. Furthermore, hypoproteinemia is
associated with decreased tolerance to chemotherapy (25).
NLR is a leading inflammation-related marker in
various cancers and is valuable for predicting the prognosis of
UR-PDAC (26–28). LMR also predicts poor outcomes in
patients with UR-PDAC undergoing chemotherapy (29). Moreover, both NLR and LMR are
associated with nutritional status (30,31).
The features of cancer-related inflammation include the following:
malignant cell proliferation and survival, inflammatory cell
infiltration and production of inflammatory mediators in tumor
tissues, tissue remodeling, promotion of tissue repair and
angiogenesis, disruption of adaptive immune responses, and altered
responses to chemotherapeutic agents (32). Therefore, TP, NLR, and LMR are
important indicators of nutritional status, cancer progression, and
tolerability of chemotherapy.
This study demonstrated that three pretherapeutic
markers reflecting nutritional and inflammatory status were
associated with the attainability of CS in patients with UR-PDAC
during treatment. Moreover, patients with low NLR and high LMR had
significantly longer overall treatment time than those with high
NLR and low LMR, respectively. This may indicate that patients with
better nutritional status and lower systemic inflammatory response
were more likely to receive relatively long-term treatment because
they tolerate chemotherapy better and their cancer progresses is
slower. Consequently, these patients are often able to receive
adequate doses of chemotherapy, potentially leading to CS.
Nutrition and inflammation levels are known to affect the risk of
severe toxicity during cancer chemotherapy and the overall
progression of the disease. Malnutrition can impact the absorption,
protein binding, hepatic metabolism, and renal elimination of drugs
and their metabolites (33). In
malnourished patients, a reduced concentration of plasma protein
levels can heighten the toxicity risk from drugs that bind strongly
to proteins, such as prednisolone, etoposide, cisplatinum,
paclitaxel, and metabolites of irinotecan (34). Additionally, several studies have
linked inflammation-based scores with the prognosis of several
types of malignances (35).
Inflammation is causally related to cancer development, through
processes that involve genotoxicity, aberrant tissue repair,
proliferative responses, invasion, and metastasis (36). Elevated systemic inflammation can
also directly enhance cancer cell metastases in other parts of the
body and accelerate the overall progression of the disease
(15). While we found no
significant link between these pre-treatment markers and adverse
events during chemotherapy or chemoradiotherapy, assessing
patients' nutritional and inflammatory status before surgery for
UR-PDAC might be vital. This assessment aids in selecting
appropriate treatments and identifying patients with advanced
disease or those unsuitable for surgery, potentially improving
patient outcomes.
Our study had some limitations. First, this was a
retrospective study conducted at a single institution. Second, the
number of enrolled patients, particularly those who underwent CS,
was relatively small. As a result, the outcomes could not be
separately evaluated between UR-LA and UR-M, although these two
conditions might be different. Therefore, sufficiently large
cohorts should be analyzed in future multicenter collaborative
studies. Third, in this study, the criteria for surgical
indications of CS were defined as described above. However, the
surgical indications for CS have not been clearly defined
currently. Furthermore, the efficacy of CS in patients with UR-M
remains controversial. Further studies are needed to determine the
efficacy and surgical indications for CS.
In conclusion, our findings suggest that
pretherapeutic TP, NLR, and LMR in patients with UR-PDAC are
predictive factors for the attainability of CS. These parameters
may be useful to predict which patients with UR-PDAC will likely
undergo CS during multidisciplinary therapy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TH, KM, DW, TIs, RY, MK, MF, TIw, JYT, SK, YT, NO,
MS and NM conceived the study concept and study design. TH made the
initial proposal for this study, collected and analyzed the data,
and wrote and edited the manuscript. TH, KM, RY, MK, MF, TIw, JYT,
SK, YT and NO treated and monitored the patients. TH, DW and TIs
conducted the statistical analyses. KM, MS, and NM revised and
supervised the study. TH, KM and NM confirm the authenticity of all
the raw data. All authors read and approved the final manuscript,
and agreed to be accountable for the content of this work.
Ethics approval and consent to
participate
The study protocol was approved by the Institutional
Review Board of the Gifu University Hospital (approval number:
2022-285; Gifu, Japan). Participants provided informed consent by
an opt-out option before study enrollment.
Patient consent for publication
Not applicable.
Competing interests
NM received grants to his institute from Taiho
Pharma and Yakult Honsha. All the other authors have no conflicts
of interest.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Conroy T, Desseigne F, Ychou M, Bouche O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al: FOLFIRINOX versus gemcitabine for
metastatic pancreatic cancer. N Engl J Med. 364:1817–1825. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Satoi S, Yamaue H, Kato K, Takahashi S,
Hirono S, Takeda S, Eguchi H, Sho M, Wada K, Shinchi H, et al: Role
of adjuvant surgery for patients with initially unresectable
pancreatic cancer with a long-term favorable response to
non-surgical anti-cancer treatments: Results of a project study for
pancreatic surgery by the Japanese society of
hepato-biliary-pancreatic surgery. J Hepatobiliary Pancreat Sci.
20:590–600. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
National Comprehensive Cancer Network, .
NCCN Clinical practice guidelines in oncology: pancreatic
adenocarcinoma. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdfAugust
29–2023
|
|
7
|
Zhou Y, Liao S, You J and Wu H: Conversion
surgery for initially unresectable pancreatic ductal adenocarcinoma
following induction therapy: A systematic review of the published
literature. Updates Surg. 74:43–53. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanaka M, Heckler M, Mihaljevic AL, Sun H,
Klaiber U, Heger U, Buchler MW and Hackert T: CT response of
primary tumor and CA19-9 predict resectability of metastasized
pancreatic cancer after FOLFIRINOX. Eur J Surg Oncol. 45:1453–1459.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang M, Zhu P, Chen Z and Yang L:
Conversion therapy, palliative chemotherapy and surgery, which of
these is the best treatment for locally advanced and advanced
pancreatic cancer? Anticancer Drugs. 33:e686–e691. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yanagimoto H, Satoi S, Yamamoto T, Yamaki
S, Hirooka S, Kotsuka M, Ryota H, Ishida M, Matsui Y and Sekimoto
M: Benefits of conversion surgery after multimodal treatment for
unresectable pancreatic ductal adenocarcinoma. Cancers (Basel).
12:14282020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ushida Y, Inoue Y, Oba A, Mie T, Ito H,
Ono Y, Sato T, Ozak a, Sasaki T, Saiura A, et al: Optimizing
indications for conversion surgery based on analysis of 454
consecutive Japanese cases with unresectable pancreatic cancer who
received modified FOLFIRINOX or gemcitabine plus nab-paclitaxel: A
single-center retrospective study. Ann Surg Oncol. 29:5038–5050.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takano N, Yamada S, Sonohara F, Inokawa Y,
Takami H, Hayashi M, Koike M, Fujii T and Kodera Y: The impact of
early tumor shrinkage on conversion surgery and the survival in
patients with unresectable locally advanced pancreatic cancer. Surg
Today. 51:1099–1107. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee M, Kang JS, Kim H, Kwon W, Lee SH, Ryu
JK, Kim YT, Oh DY, Chie EK and Jang JY: Impact of conversion
surgery on survival in locally advanced pancreatic cancer patients
treated with FOLFIRINOX chemotherapy. J Hepatobiliary Pancreat Sci.
30:111–121. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mataki Y, Kurahara H, Idichi T, Tanoue K,
Hozaka Y, Kawasaki Y, Iino S, Maemura K, Shinchi H and Ohtsuka T:
Clinical benefits of conversion surgery for unresectable pancreatic
ductal adenocarcinoma: A single-institution, retrospective
analysis. Cancers (Basel). 13:10572021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kokumai T, Aoki S, Mizuma M, Maeda S,
Ohtsuka H, Nakagawa K, Morikawa T, Motoi F, Kamei T and Unno M:
Prognostic value of an inflammation-based nutritional score for
patients with initially unresectable pancreatic adenocarcinoma
undergoing conversion surgery following chemo-/radiotherapy. Surg
Today. 51:1682–1693. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsuchiya N, Matsuyama R, Murakami T,
Yabushita Y, Sawada Y, Kumamoto T and Endo I: Role of conversion
surgery for unresectable pancreatic cancer after long-term
chemotherapy. World J Surg. 44:2752–2760. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Japan Pancreas Society, . Classification
of Pancreatic Carcinoma (4th English Edition). Kanehara & Co.;
Tokyo, Japan: 2017, http://www.suizou.org/pdf/Classification_of_Pancreatic_Carcinoma_4th_Engl_ed.pdfAugust
30–2023
|
|
18
|
Kanda M, Fujii T, Kodera Y, Nagai S,
Takeda S and Nakao A: Nutritional predictors of postoperative
outcome in pancreatic cancer. Br J Surg. 98:268–274. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Toiyama Y, Miki C, Inoue Y, Tanaka K,
Mohri Y and Kusunoki M: Evaluation of an inflammation-based
prognostic score for the identification of patients requiring
postoperative adjuvant chemotherapy for stage II colorectal cancer.
Exp Ther Med. 2:95–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Simon R and Makuch RW: A non-parametric
graphical representation of the relationship between survival and
the occurrence of an event: Application to responder versus
non-responder bias. Stat Med. 3:35–44. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schultz LR, Peterson EL and Breslau N:
Graphing survival curve estimates for time-dependent covariates.
Int J Methods Psychiatr Res. 11:68–74. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao J, Luo F, Zeng K, Ma W, Lu F, Huang Y,
Zhang L and Zhao H: Predictive value of high preoperative serum
total protein and elevated hematocrit in patients with
non-small-cell lung cancer after radical resection. Nutr Cancer.
74:3533–3545. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yao Y, Wang Z, Yong L, Yao Q, Tian X, Wang
T, Yang Q, Hao C and Zhou T: Longitudinal and time-to-event
modeling for prognostic implications of radical surgery in
retroperitoneal sarcoma. CPT Pharmacometrics Syst Pharmacol.
11:1170–1182. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abbasi B, Hayat A, Lyons M, Gupta A and
Gupta S: Serum protein and electrolyte imbalances are associated
with chemotherapy induced neutropenia. Heliyon. 8:e099492022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iwai N, Okuda T, Sakagami J, Harada T,
Ohara T, Taniguchi M, Sakai H, Oka K, Hara T, Tsuji T, et al:
Neutrophil to lymphocyte ratio predicts prognosis in unresectable
pancreatic cancer. Sci Rep. 10:187582020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Allen J, Cernik C, Bajwa S, Al-Rajabi R,
Saeed A, Baranda J, Williamson S, Sun W and Kasi A: Association of
neutrophil, platelet, and lymphocyte ratios with the prognosis in
unresectable and metastatic pancreatic cancer. J Clin Med.
9:32832020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Terao T, Kumagi T, Hyodo I, Yokota T,
Azemoto N, Miyata H, Kuroda T, Ohno Y, Tanaka Y, Shibata N, et al:
Simple prognostic markers for optimal treatment of patients with
unresectable pancreatic cancer. Medicine (Baltimore).
100:e275912021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shimizu T, Taniguchi K, Asakuma M, Tomioka
A, Inoue Y, Komeda K, Hirokawa F and Uchiyama K:
Lymphocyte-to-monocyte ratio and prognostic nutritional index
predict poor prognosis in patients on chemotherapy for unresectable
pancreatic cancer. Anticancer Res. 39:2169–2176. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kaya T, Acikgoz SB, Yildirim M, Nalbant A,
Altas AE and Cinemre H: Association between
neutrophil-to-lymphocyte ratio and nutritional status in geriatric
patients. J Clin Lab Anal. 33:e226362019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakamura Y, Imada A, Fukugaki A, Kanto S,
Yamaura T, Kinjo Y and Kuroda N: Association of nutritional risk
and systemic inflammation with survival in patients with colorectal
cancer who underwent curative surgery. Clin Nutr ESPEN. 49:417–424.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Santarpia L, Contaldo F and Pasanisi F:
Nutritional screening and early treatment of malnutrition in cancer
patients. J Cachexia Sarcopenia Muscle. 2:27–35. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Murry DJ, Riva L and Poplack DG: Impact of
nutrition on pharmacokinetics of anti-neoplastic agents. Int J
Cancer. (Suppl 11):48–51. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ikuta S, Sonoda T, Aihara T, Nakajima T
and Yamanaka N: The preoperative modified Glasgow prognostic score
for the prediction of survival after pancreatic cancer resection
following non-surgical treatment of an initially unresectable
disease. Contemp Oncol (Pozn). 22:229–235. 2018.PubMed/NCBI
|
|
36
|
Elinav E, Nowarski R, Thaiss CA, Hu B, Jin
C and Flavell RA: Inflammation-induced cancer: Crosstalk between
tumours, immune cells and microorganisms. Nat Rev Cancer.
13:759–771. 2013. View
Article : Google Scholar : PubMed/NCBI
|