As the main active ingredient of an ancient Chinese
herb that has been widely proven to be effective in cancer
treatment, some related reviews have summarized the pharmacological
effects of ginsenoside Rg3 (GS-Rg3) (1–4). With
more in-depth research in recent years, more anticancer mechanisms
of GS-Rg3 continue to be discovered. Therefore, the present article
reviews the newly discovered anticancer mechanisms of GS-Rg3,
aiming to highlight the current challenges and future prospects of
GS-Rg3 in the field of cancer therapy.
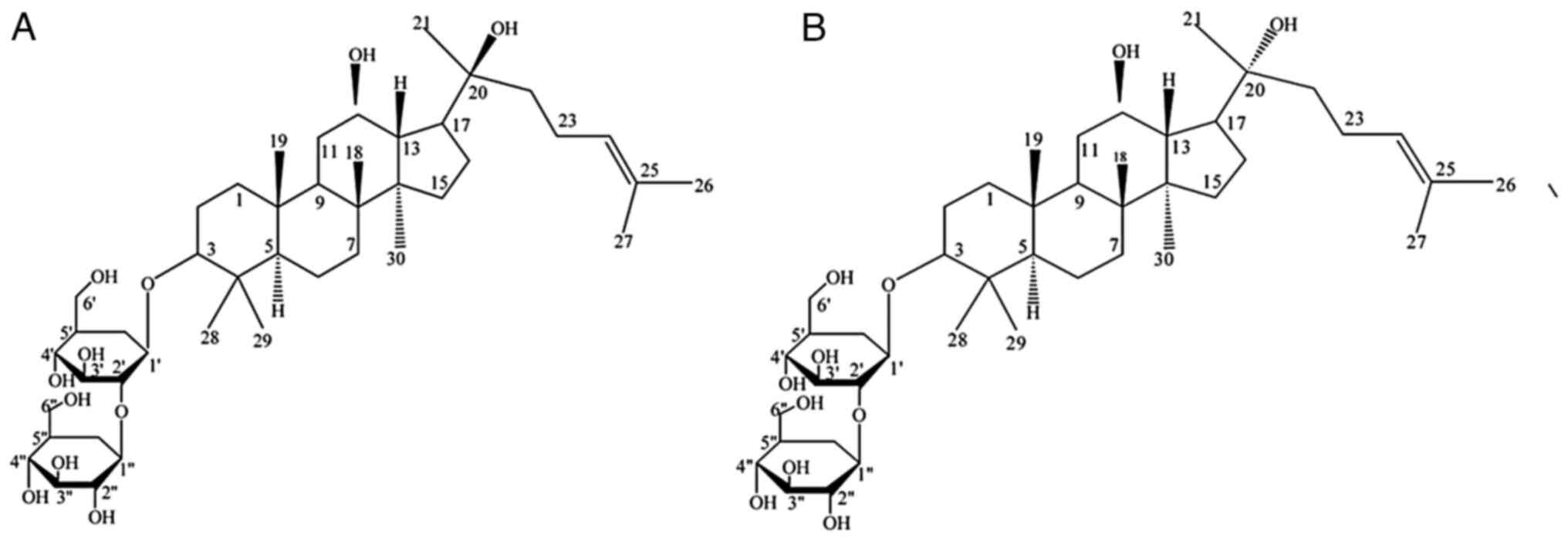
GS-Rg3 is a tetracyclic triterpene saponin derived
from red ginseng. Due to the chiral nature of C20, GS-Rg3 exists in
two isomers: 20 (S) and 20 (R), the chemical structures of which
are depicted in Fig. 1. Both
20(S)-GS-Rg3 and 20(R)-GS-Rg3 are white amorphous powders. The
former is soluble in cold H2O, ethanol, methanol and
acetonitrile, while the latter is only soluble in DMSO, with trace
amounts being soluble in H2O and acetonitrile (5).
Rapidly growing tumor cells experience a constant
deprivation of oxygen and nutrients, necessitating the development
of new vascular networks to sustain their growth. Angiogenesis, the
formation of new blood vessels, occurs when endothelial progenitor
cells (EPCs) or bone marrow-derived hematopoietic cells are
recruited to the tumor (8). EPCs
play a crucial role in early tumor growth by inhibiting angiogenic
switches while simultaneously releasing angiogenic molecules to
promote tumor neovascularization (9). Tumor cells secrete large amounts of
pro-angiogenic substances to promote the growth of new vascular
networks, which often results in the formation of immature,
disorganized and leaky blood vessels. These aberrant blood vessels
contribute to disease progression and increase resistance to
therapy (10). GS-Rg3 can inhibit
the proliferation of tumor vascular endothelial cells in two ways.
First, it attenuates the Akt/endothelial nitric oxide synthase
signaling pathway, which is dependent on vascular growth factors,
and inhibits vascular progenitor cell migration and angiogenesis,
thereby inhibiting the differentiation of EPCs and the formation of
tumor blood vessels (11). Second,
it blocks the PI3K/Akt and ERK1/2 pathways, which reduces the
expression of vascular endothelial growth factor (VEGF) and
hypoxia-inducible factor (HIF)-1α at the mRNA and protein levels of
bone marrow stromal cell (12).
Cells can undergo various types of death in response
to pathological or physiological stimuli, including apoptosis,
autophagy, cell necrosis, iron death and pyrolysis. While excessive
cell death can be detrimental in certain contexts, cancer cells
exhibit uncontrolled proliferation and growth rates that render the
promotion of cell death a therapeutic goal in the treatment of
neoplastic diseases.
Tumor metastasis is a leading cause of mortality in
individuals with tumors. Previous studies have linked tumor
metastasis to various factors, including E-cadherin, HIF,
tumor-associated macrophages, retinoid acid receptor responder 3,
and exosomes carrying miRNA (27,28).
Notably, research has shown that GS-Rg3 can impede tumor cell
metastasis by inhibiting the Wnt/β-catenin pathway (29). Additionally, GS-Rg3 inhibits the
expression of nuclear factor κB (NF-κB), c-Myc, cyclooxygenase-2
and MMP-9, all of which are regulated by NF-κB (30). These findings suggest that GS-Rg3
has the potential to act as a potent therapeutic agent for
preventing tumor metastasis.
GS-Rg3 exhibits a significant effect on cancer cell
proliferation. A previous study demonstrated that 20(R)-GS-Rg3
effectively inhibits tumor cell proliferation by increasing the
levels of IL-2 and IFN-γ (31).
20(R)-GS-Rg3 can also upregulate Rho GTPase activating protein 9,
which is implicated in cell proliferation and metastasis, to impede
tumor cell proliferation (32).
Furthermore, GS-Rg3 activates the VRK1/tumor protein p53 binding
protein 1 pathway, thereby preserving DNA integrity and inhibiting
non-small cell lung cancer cell proliferation (33). GS-Rg3 can also arrest A549 cell
proliferation by halting the cell cycle at the G0/G1 phase through
the EGFR/Ras/Raf/MEK/ERK pathway (34). Additionally, by inhibiting the
expression of miR-4425 via the tumor suppressor gene,
farnesyl-diphosphate farnesyltransferase 1 (35), GS-Rg3 reduces the methylation of
p53, p16 and hMLH1 promoter regions, promotes their mRNA and
protein levels and restricts ovarian cancer cell proliferation
(25). Moreover, GS-Rg3 can
suppress PC3 prostate cancer cell proliferation by arresting the
ROS-mediated cell cycle (36,37).
These findings suggest that GS-Rg3 has promise as a potent
therapeutic agent for inhibiting cancer cell proliferation. By
contrast, GS-Rg3 can stimulate cell proliferation even at low
concentrations through the mTORC1 pathway and mitochondrial
biogenesis (38).
Red ginseng-derived GS-Rg3 extract has been found to
induce apoptosis and mitochondrial autophagy in lung cancer cells
by producing ROS (39). In
colorectal cancer, GS-Rg3 treatment exerts antitumor effects by
activating the PTEN-induced kinase 1-Parkin signaling pathway,
increasing the ubiquitination of GAPDH and promoting mitochondrial
autophagy (40).
GS-Rg3 can be synergistically combined with other
treatments such as radiotherapy, chemotherapy and targeted therapy,
to enhance its antitumor effects. For instance, combining GS-Rg3
with gefitinib can increase gefitinib efficacy in treating
malignancy, as depicted in Table
II. This combination can elevate the levels of anti-apoptotic
protein, Bcl-2, pro-apoptotic protein, Bax, and caspase-3, while
reducing the levels of migration-promoting factors, SNAIL and SLUG,
and increasing the level of anti-migration protein, E-cadherin,
thereby enhancing the pro-apoptotic effect on lung cancer cells and
inhibiting metastasis (41). GS-Rg3
can also potentiate the efficacy of drugs in patients with advanced
non-small cell lung cancer and reduce chemotherapy-induced drug
toxicity (42). Furthermore, GS-Rg3
can reduce the cisplatin resistance of gastric cancer cells by
upregulating miR-2 and inhibiting SRY-box transcription factor 3
and the PI3K/Akt/mTOR signaling axis (43). GS-Rg3 can also increase the
sensitivity of pancreatic cancer to gemcitabine by decreasing
ZFP91-mediated TSPY like 2 instability (44). Moreover, GS-Rg3 can enhance the
radiosensitivity of cancer cells in different tumor types, such as
lung, breast and nasopharyngeal cancer (45–47).
Additionally, GS-Rg3 combined with artemisinin can inhibit STAT3
signal transduction in hepatocellular carcinoma cancer cells,
synergistically reduce the viability of cells, induce apoptosis and
inhibit the growth of mouse hepatocellular carcinoma (48). Similarly, the combined therapy of
GS-Rg3 and sorafenib can mitigate the progression of hepatocellular
carcinoma by inhibiting hexokinase 2-mediated glycolysis and the
PI3K/Akt signaling pathway (49).
Moreover, a meta-analysis has demonstrated that transarterial
chemoembolization combined with GS-Rg3 can effectively enhance the
objective response rate and disease control rate of hepatocellular
carcinoma while reducing adverse reactions to treatment (50).
GS-Rg3 can modulate the expression of non-coding
RNAs, such as miRNAs, circular (circ)RNAs and long non-coding RNAs,
in various types of tumor types, thereby inhibiting tumorigenesis
and progression by regulating the corresponding signaling pathways.
For example, in hepatocellular carcinoma, GS-Rg3 can inhibit the
PI3K/Akt signaling pathway by downregulating expression of the long
non-coding RNA, HOTAIR, which inhibits the proliferation and
metastasis of hepatocellular carcinoma (51). GS-Rg3 can impede osteosarcoma
progression by modulating the circ_0003074/miR-516b-5p/karyopherin
subunit α4 (KPNA4) axis. GS-Rg3 significantly reduces the
expression of circ_0003074, elevates the expression of miR-516b-5p
and downregulates the expression of KPNA4 (52). In breast cancer, GS-Rg3 can
counteract the inhibitory effect of the oncogenic long non-coding
RNA, ATXN8OS, on the tumor suppressive miR-424-5p, thereby
increasing apoptosis and inhibiting the proliferation of breast
cancer cells (26). Furthermore,
GS-Rg3 can exert antitumor effects on ovarian cancer by inhibiting
expression of the long non-coding RNA, H19, which impedes the
proliferation, migration and invasion of ovarian cancer cells
(53). These findings suggest that
GS-Rg3 is a promising therapeutic agent for modulating non-coding
RNAs and thus regulating the corresponding signaling pathways to
inhibit tumorigenesis and tumor progression.
The TME plays a crucial role in tumor proliferation,
metastasis and response to therapy. Typically, immune suppression
mediated by the TME leads to poor antitumor responses to therapy
(54). However, GS-Rg3 can exert
antitumor effects by modulating the TME. Current research in this
area is primarily focused on breast cancer. Innovative drug
delivery modalities such as GS-Rg3-based liposomes, can achieve
targeted localization to human breast cancer paclitaxel-resistant
cells and their TME, resulting in the repolarization of M2
macrophages from a tumor-promoting phenotype to an antitumor M1
phenotype. Through dual action of targeting tumor cells and
remodeling the TME, to 90.3% of paclitaxel-resistant breast cancer
cells are killed (55). In addition
to paclitaxel, GS-Rg3-based liposomes can significantly enhance the
antitumor effects of docetaxel in triple-negative breast cancer
(56). Moreover, GS-Rg3-modified
nanoparticles can enhance the immunogenic cell death (ICD) effect
induced by doxorubicin (57). When
combined with programmed death-ligand 1 (PD-L1) blockade,
significant antitumor effects can be achieved in breast cancer
through the recruitment of memory T cells and decreased adaptive
PD-L1 enrichment (57). For lung
metastases, the combination of GS-Rg3-based liposomes with
chemotherapy drugs allows for improved capture of circulating tumor
cells. Upon reaching the lungs, the immunosuppressive
microenvironment is reversed, leading to the inhibition of breast
cancer lung metastasis (58).
Paclitaxel-loaded GS-Rg3-based liposomes can activate the immune
microenvironment in glioblastoma, expanding the population of CD8 T
cells to promote T cell immune responses, increasing the M1/M2
ratio and reducing the number of regulatory T cells and
myeloid-derived suppressor cells, significantly prolonging the
survival time of mice with glioblastoma (7). Furthermore, GS-Rg3 and quercetin
nanoparticles can enhance tumor targeting in colorectal cancer
mice, with GS-Rg3 serving as an inducer of ICD. This allows for the
recruitment, activation, migration and cross-presentation of
antigen-presenting cells in lymph nodes and tertiary lymphoid
tissues, markedly modulating the immunosuppressive TME, and
remodeling ‘cold’ (non-T cell-inflamed) tumors into ‘hot’ (T
cell-inflamed) tumors (59). These
findings suggest that GS-Rg3-based drug delivery systems remodel
the TME and enhance antitumor effects by reversing immune
suppression, activating immune responses and promoting ICD.
Various types of ginsenoside nanomedicines have
shown potential in the treatment of different tumors, including
triple-negative breast cancer (60). The in vivo delivery results
of a multifunctional black phosphorus (BP) nanoreagent,
BPs/G-Rg3@PLGA, demonstrated its notable therapeutic effect on lung
metastases from breast cancer, along with significant
biocompatibility with various organs/tissues (61). GS-Rg3-loaded carbon nanotubes have
been shown to reduce IFN-γ-induced upregulation of PD-L1 in breast
cancer cells, thereby decreasing the programmed cell death
protein-1/PD-L1 axis in the T cell/triple-negative breast cancer
cell coculture system (62). GS-Rg3
loaded on a biomimetic nanosystem has been shown to enhance the
sensitivity of tumors to doxorubicin, thereby initiating antitumor
immune activation and effectively combating leukemia cells harbored
in the bone marrow (63).
Nanoparticles constructed using graphene oxide (GO) linked with the
photosensitizer, indocyanine green (ICG), folic acid and
polyethylene glycol (PEG), and loaded with GS-Rg3
(PEG-GO-FA/ICG-Rg3) can inhibit the proliferation, invasion and
migration of osteosarcoma cells, enhance the apoptosis and
autophagy of osteosarcoma cells and suppress the stemness of
osteosarcoma cell-derived cancer stem cells (64). Ursolic acid and GS-Rg3 co-loaded
liposomes can significantly reduce the proliferation of liver
cancer cells, while increasing the apoptotic rate and the
proportion of cells in the G0/G1 phase (65). A novel nanomedicine was recently
developed by combining metal-based nanoenzymes (Fe@Fe3O4) with
GS-Rg3. The glycosidic chains of GS-Rg3 formed a hydrophilic layer
on the outermost surface of the nanomedicine, improving
biocompatibility and pharmacokinetics, thus promoting the apoptosis
of cancer cells (66). These
studies suggest that ginsenoside nanomedicines have great potential
in the treatment of a variety of tumor types, with improved
biocompatibility, targeted delivery and enhanced therapeutic
efficacy.
A number of extracts from Chinese medicinal herbs
are used to treat immunological issues, and immune modulation is a
key mode of action of GS-Rg3. GS-Rg3 enhances cellular immunity by
upregulating CD4+, CD4+/CD8+, IgG,
IgM and IL-2, and downregulating CD8+ and IL-6 in a
dose-dependent manner (67). This
effect may be related to its stimulation of concanavalin A
(ConA)-induced lymphocyte proliferation and increased levels of the
Type 1 T helper (Th1)-type cytokines, IL-2 and interferon (IFN)-γ
(31). Furthermore, 20(R)-GS-Rg3
promotes natural killer (NK) cell activity via activation of the
MAPK/ERK pathway, suggesting that 20(R)-GS-Rg3 may be used as an
activator of NK cell cytotoxicity to treat various types of cancer
(68). Cyclophosphamide decreases
T-bet and IFN-γ expression in the thymus and spleen, while
increasing GATA-3 and IL-4 expression, thereby altering the Th1/Th2
balance and resulting in immunosuppression (69). GS-Rg3 can antagonize this effect by
regulating the ratio of T lymphocyte subsets (70). For autoimmune neuroinflammation,
GS-Rg3 has a minor effect on the dendritic cell production of
Th17-promoting cytokines such as IL-6, IL-12/23p40 and TNFα.
Specifically, it upregulates the expression of TNFα and IL-12 and
downregulates the expression of IL-6. Instead, it notably reduces
the induction of RAR-related orphan nuclear receptor γt expression
in the CD4+ T cells, and therefore inhibits the
differentiation of Th17 cells from their precursors (71). These finding suggest that GS-Rg3 may
be a promising therapeutic agent for the treatment of Th17-related
autoimmune disorders.
GS-Rg3 has two chiral isomers with notably distinct
therapeutic properties. An investigation was conducted to compare
the immunological response of 20(S)-GS-Rg3 and 20(R)-GS-Rg3 as
adjuvants to ovalbumin (OVA). The results revealed that both
20(R)-GS-Rg3 and 20(S)-GS-Rg3 can serve as adjuvants for the
immunological response induced by OVA. However, 20(R)-GS-Rg3
significantly increased OVA-specific IgG and IgG subtypes in the
blood, relative to 20(S)-GS-Rg3, and was accompanied by a marked
rise in serum IFN-γ and IL-5 levels (72). 20(R)-Rg3 significantly enhanced
splenocyte proliferative responses to Con A, LPS and OVA as well as
mRNA expression of IFN-γ, IL-12, IL-4 and IL-10 and transcription
factors T-bet and GATA-3 by splenocytes when compared with the
20(S)-Rg3 (67).
GS-Rg3 has anti-fatigue properties. GS-Rg3 can
increase the physical strength of older rats by increasing serum
total cholesterol, triglyceride and lactate dehydrogenase
concentrations, increasing superoxide dismutase concentrations,
decreasing malondialdehyde release in skeletal muscle and
increasing phosphoenolpyruvate carboxykinase mRNA expression
(73). GS-Rg3 can also relieve the
pain and discomfort associated with cancer and anti-cancer drugs,
improving patient comfort (74,75).
GS-Rg3 has also been found to stimulate sirtuin 1, which protects
skeletal muscles from damage caused by reactive oxygen species and
thus acts as an anti-fatigue agent (73). In rats, fatigue can decrease the
production and phosphorylation of tyrosine hydroxylase, which
results in a decrease in dopamine. GS-Rg3 has been shown to
effectively reverse this by increasing the phosphorylation of
synuclein, protein kinase A, ERK1/2 and Akt (76). These findings suggest that GS-Rg3
has potential as a natural anti-fatigue agent and may have
beneficial effects in improving physical strength and relieving the
pain and discomfort associated with cancer.
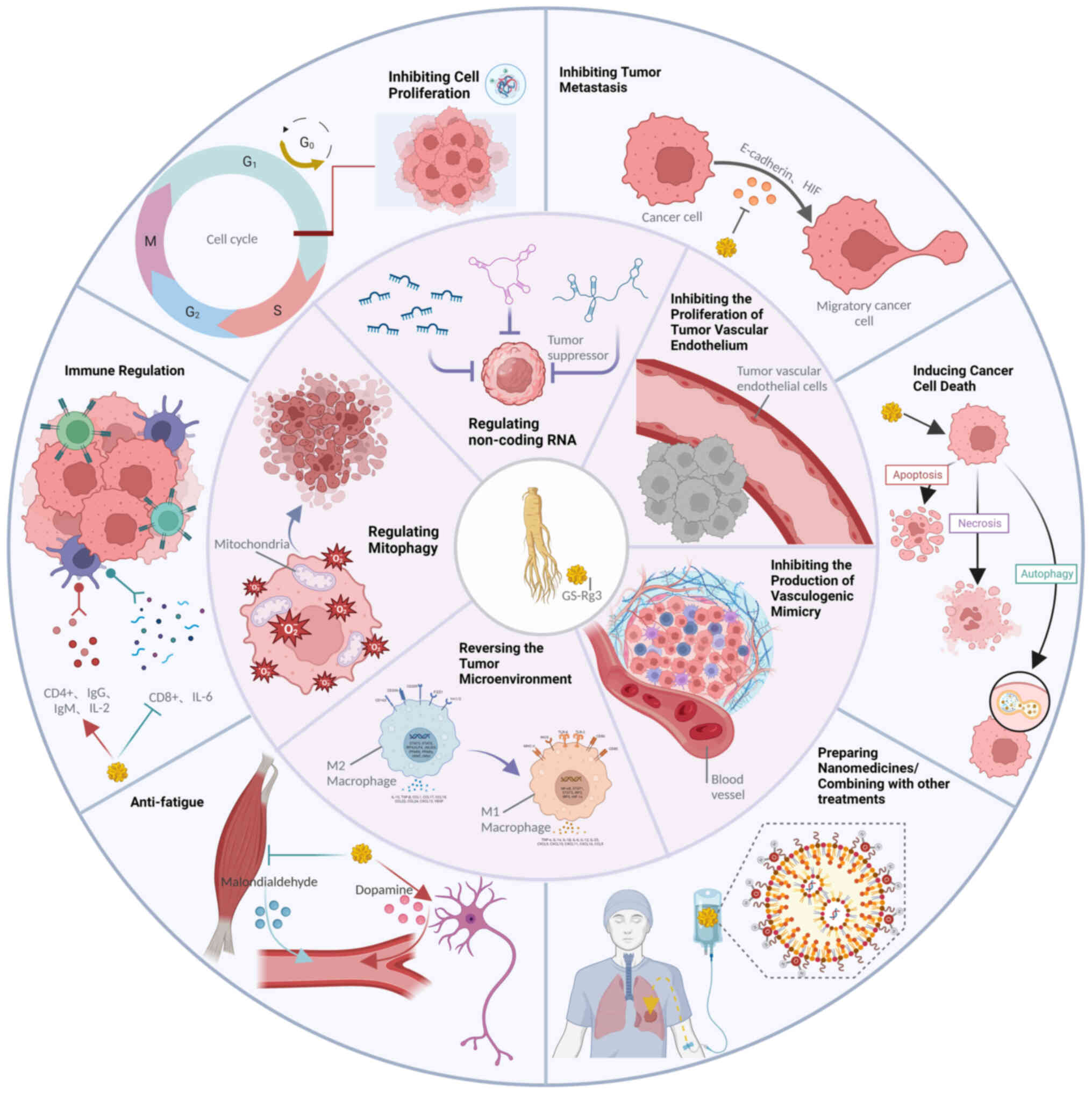
GS-Rg3 has emerged as a promising new anticancer
agent with multifaceted and heterogeneous anticancer properties
across various cancer types. Notably, GS-Rg3 has demonstrated
therapeutic benefits in the treatment of liver, lung, gastric,
breast, ovarian and prostate cancer. Recent literature reports have
shed light on the diverse cancer-fighting mechanisms of GS-Rg3,
including induction of tumor apoptosis, inhibition of tumor
metastasis, proliferation and angiogenesis, enhancement of immune
function, reversal of inhibitory TMEs and promotion of
mitochondrial autophagy (Fig. 2).
The use of immune checkpoint inhibitors has resulted in significant
improvements in the treatment paradigm for various solid tumors,
leading to notable enhancements in overall efficacy and patient
prognosis (77). Nevertheless, the
issue of resistance to immune therapy remains a significant
challenge. Tumor cells exploit their inhibitory immune
microenvironments to evade the effects of immune checkpoint
inhibitors, a phenomenon that has been demonstrated by numerous
studies to be reversible through the use of GS-Rg3. Ginseng, an
ancient and valuable Chinese herbal medicine, has a notable impact
on the immune system. The gut microbiota maintains a symbiotic
relationship with the intestinal mucosa, which is the largest
immune organ in the human body (78). Through the integration of
environmental factors such as diet, with genetic and immune
signals, the gut microbiota can influence the metabolism, immune
function, neurodevelopment and pathogen colonization of the host.
Similarly, alterations in the host immune environment can also
impact the gut microbiota through different immune cells such as
dendritic cells, regulatory T cells, monocytes, etc (79,80)
Dysbiosis of the gut microbiota can result in changes in intestinal
permeability, disrupting the existing equilibrium and affecting the
metabolism and immune function of the host, thereby contributing to
the development of various diseases, such as inflammatory bowel
disease, depression, and cancer (80,81).
In recent years, there has been increasing recognition of the
importance of the gut microbiota in the diagnosis and treatment of
diseases. As such, investigating whether the interaction between
orally administered GS-Rg3 and the gut microbiota affects the
anticancer effects of GS-Rg3 is a worthwhile endeavor.
Mitochondria, which serve as cellular energy
factories, play a crucial role in cells, particularly cancer cells.
Mitochondrial autophagy may exert a bidirectional regulatory effect
on the occurrence and development of cancer; it can promote cancer
progression by enabling cancer cells to survive under stress or it
can induce carcinogenesis by affecting cell signaling transduction
or promoting intracellular toxicity when mutations or abnormalities
occur (82). Recent studies have
also demonstrated that abnormal mitochondrial function is linked to
the cellular immune response (83,84).
At present, there are limited investigations regarding whether
GS-Rg3 can influence cancer cell proliferation by modulating
mitochondrial autophagy or other mitochondrial functions and
activities. This area may be a future research focus to identify
new properties that GS-Rg3 may offer in the field of antitumor
therapy.
However, the low oral bioavailability of GS-Rg3,
given its extensive pro-systemic metabolism and poor membrane
permeability, prevents the attainment of high working
concentrations in vivo (38). Given that reaching the concentration
of GS-Rg3 required to inhibit cancer cell growth in vivo is
challenging, efforts should also be focused on investigating new
methods to improve the solubility of GS-Rg3 without decreasing the
efficacy of the drug, such as in the development of cofactors to
aid in solubilization. The combination of GS-Rg3 with cholesterol
transport liposomes as an alternative approach has shown greater
therapeutic efficacy, reduced toxicity and the potential for
overcoming drug resistance, exhibiting certain synergistic effects
with anticancer drugs (60).
Additionally, GS-Rg3 has displayed functions that are independent
of its anticancer effects, such as enhancing biocompatibility and
pharmacokinetics, which offer new directions for its optimal
utilization.
Overall, research in basic medicine has highlighted
the marked anticancer properties of GS-Rg3 across various tumor
types. However, its clinical use is predominantly restricted to
primary lung cancer and liver cancer chemotherapy. The ongoing
research efforts aim to fully harness the anticancer capabilities
of GS-Rg3 within biological systems, potentially broadening its
clinical applications to a wider range of cancer types. Meanwhile,
more clinical trials should be conducted to comprehensively
evaluate its safety and feasibility to realize its clinical
application and benefits to patients. In summary, the evidence
presented thus far suggests that GS-Rg3 is a promising anticancer
agent that warrants further investigation. Further clinical trials
are required to assess the effectiveness and safety of GS-Rg3 and
optimize its potential for clinical implementation in cancer
treatment.
Not applicable.
Funding: Not applicable.
Not applicable.
YZ edited the review. JN and GL wrote the review. YL
was a major contributor in revision of the manuscript. All authors
have read and approved the final manuscript. Data authentication is
not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Gao S, Fang C, Wang T, Lu W, Wang N, Sun
L, Fang W, Chen Y and Hu R: The effect of ginsenoside Rg3 combined
with chemotherapy on immune function in non-small cell lung cancer:
A systematic review and meta-analysis of randomized controlled
trials. Medicine (Baltimore). 102:e334632023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakhjavani M, Smith E, Townsend AR, Price
TJ and Hardingham JE: Anti-Angiogenic properties of ginsenoside
Rg3. Molecules. 25:49052020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang J, Qi F, Wang Z, Zhang Z, Pan N, Huai
L, Qu S and Zhao L: A review of traditional Chinese medicine for
treatment of glioblastoma. Biosci Trends. 13:476–487. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun M, Ye Y, Xiao L, Duan X, Zhang Y and
Zhang H: Anticancer effects of ginsenoside Rg3 (Review). Int J Mol
Med. 39:507–518. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim IW, Sun WS, Yun BS, Kim NR, Min D and
Kim SK: Characterizing a full spectrum of physico-chemical
properties of (20S)-and (20R)-ginsenoside Rg3 to be proposed as
standard reference materials. J Ginseng Res. 37:124–134. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ren Z, Chen X, Hong L, Zhao X, Cui G, Li
A, Liu Y, Zhou L, Sun R, Shen S, et al: Nanoparticle conjugation of
ginsenoside Rg3 inhibits hepatocellular carcinoma development and
metastasis. Small. 16:e19052332020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu Y, Liang J, Gao C, Wang A, Xia J, Hong
C, Zhong Z, Zuo Z, Kim J, Ren H, et al: Multifunctional ginsenoside
Rg3-based liposomes for glioma targeting therapy. J Control
Release. 330:641–657. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu ZL, Chen HH, Zheng LL, Sun LP and Shi
L: Angiogenic signaling pathways and anti-angiogenic therapy for
cancer. Sig Transduct Target Ther. 8:1982023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lugano R, Ramachandran M and Dimberg A:
Tumor angiogenesis: Causes, consequences, challenges and
opportunities. Cell Mol Life Sci. 77:1745–1770. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carmeliet P and Jain RK: Principles and
mechanisms of vessel normalization for cancer and other angiogenic
diseases. Nat Rev Drug Discov. 10:417–427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim JW, Jung SY, Kwon YH, Lee SH, Lee JH,
Lee BY and Kwon SM: Ginsenoside Rg3 inhibits endothelial progenitor
cell differentiation through attenuation of VEGF-Dependent Akt/eNOS
signaling. Phytother Res. 26:1286–1293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zeng D, Wang J, Kong P, Chang C and Li J
and Li J: Ginsenoside Rg3 inhibits HIF-1α and VEGF expression in
patient with acute leukemia via inhibiting the activation of
PI3K/Akt and ERK1/2 pathways. Int J Clin Exp Pathol. 7:2172–2178.
2014.PubMed/NCBI
|
|
13
|
Maniotis AJ, Folberg R, Hess A, Seftor EA,
Gardner LM, Pe'er J, Trent JM, Meltzer PS and Hendrix MJ: Vascular
channel formation by human melanoma cells in vivo and in vitro:
Vasculogenic Mimicry. Am J Pathol. 155:739–752. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Delgado-Bellido D, Zamudio-Martínez E,
Fernández-Cortés M, Herrera-Campos AB, Olmedo-Pelayo J, Perez CJ,
Expósito J, de Álava E, Amaral AT, Valle FO, et al: VE-Cadherin
modulates β-catenin/TCF-4 to enhance vasculogenic mimicry. Cell
Death Dis. 14:1352023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang F, Lin H, Cao K, Wang H, Pan J,
Zhuang J, Chen X, Huang B, Wang D and Qiu S: Vasculogenic mimicry
plays an important role in adrenocortical carcinoma. Int J Urol.
23:371–377. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Williamson SC, Metcalf RL, Trapani F,
Mohan S, Antonello J, Abbott B, Leong HS, Chester CP, Simms N,
Polanski R, et al: Vasculogenic mimicry in small cell lung cancer.
Nat Commun. 7:133222016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X, Xue Y, Liu X, Zheng J, Shen S, Yang
C, Chen J, Li Z, Liu L, Ma J, et al: ZRANB2/SNHG20/FOXK1 Axis
regulates Vasculogenic mimicry formation in glioma. J Exp Clin
Cancer Res. 38:682019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang S, Zhang Z, Qian W, Ji D, Wang Q, Ji
B, Zhang Y, Zhang C and Sun Y, Zhu C and Sun Y: Angiogenesis and
vasculogenic mimicry are inhibited by 8-Br-cAMP through activation
of the cAMP/PKA pathway in colorectal cancer. Onco Targets Ther.
11:3765–3774. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu MR, Wei PF, Suo MZ, Hu Y, Ding W, Su L,
Zhu YD, Song WJ, Tang GH, Zhang M and Li P: Brucine suppresses
vasculogenic mimicry in human triple-negative breast cancer cell
line MDA-MB-231. Biomed Res Int. 2019:65432302019.PubMed/NCBI
|
|
20
|
Treps L, Faure S and Clere N: Vasculogenic
mimicry, a complex and devious process favoring
tumorigenesis-Interest in making it a therapeutic target. Pharmacol
Ther. 223:1078052021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo JQ, Zheng QH, Chen H, Chen L, Xu JB,
Chen MY, Lu D, Wang ZH, Tong HF and Lin S: Ginsenoside Rg3
inhibition of vasculogenic mimicry in pancreatic cancer through
downregulation of VE-cadherin/EphA2/MMP9/MMP2 expression. Int J
Oncol. 45:1065–1072. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qi L, Song W, Liu Z, Zhao X, Cao W and Sun
B: Wnt3a promotes the vasculogenic mimicry formation of colon
cancer via Wnt/β-Catenin signaling. Int J Mol Sci. 16:18564–18579.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Junmin S, Hongxiang L, Zhen L, Chao Y and
Chaojie W: Ginsenoside Rg3 inhibits colon cancer cell migration by
suppressing nuclear factor kappa B activity. J Tradit Chin Med.
35:440–444. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie Q, Wen H, Zhang Q, Zhou W, Lin X, Xie
D and Liu Y: Inhibiting PI3K-AKt signaling pathway is involved in
antitumor effects of ginsenoside Rg3 in lung cancer cell. Biomed
Pharmacother. 85:16–21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao L, Shou H, Chen L, Gao W, Fang C and
Zhang P: Effects of ginsenoside Rg3 on epigenetic modification in
ovarian cancer cells. Oncol Rep. 41:3209–3218. 2019.PubMed/NCBI
|
|
26
|
Kim H, Ji HW, Kim HW, Yun SH, Park JE and
Kim SJ: Ginsenoside Rg3 prevents oncogenic long noncoding RNA
ATXN8OS from inhibiting tumor-suppressive microRNA-424-5p in breast
cancer cells. Biomolecules. 11:1182021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao JY, Yuan XK, Luo RZ, Wang LX, Gu W,
Yamane D and Feng H: Phospholipase A and acyltransferase 4/retinoic
acid receptor responder 3 at the intersection of tumor suppression
and pathogen restriction. Front Immunol. 14:11072392023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu W, Pan HF, Yang LJ, Zhao ZM, Yuan DS,
Liu YL and Lin LZ: Panax ginseng C.A. Meyer (Rg3) ameliorates
gastric precancerous lesions in Atp4a-/- Mice via inhibition of
glycolysis through PI3K/AKT/miRNA-21 Pathway. Evid Based Complement
Alternat Med. 2020.2672648. 2020.
|
|
29
|
Mao X, Jin Y, Feng T, Wang H, Liu D, Zhou
Z, Yan Q, Yang H, Yang J, Yang J, et al: Ginsenoside Rg3 inhibits
the growth of osteosarcoma and attenuates metastasis through the
Wnt/β-Catenin and EMT signaling pathway. Evid Based Complement
Alternat Med. 2020:60651242020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bian S, Zhao Y, Li F, Lu S, Wang S, Bai X,
Liu M, Zhao D, Wang J and Guo D: 20(S)-Ginsenoside Rg3 Promotes
HeLa Cell Apoptosis by Regulating Autophagy. Molecules.
24:36552019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu R, Ru Q, Chen L, Ma B and Li C:
Stereospecificity of Ginsenoside Rg3 in the promotion of cellular
immunity in hepatoma H22-Bearing mice. J Food Sci. 79:H1430–H1435.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun MY, Song YN, Zhang M, Zhang CY, Zhang
LJ and Zhang H: Ginsenoside Rg3 inhibits the migration and invasion
of liver cancer cells by increasing the protein expression of
ARHGAP9. Oncol Lett. 17:965–973. 2019.PubMed/NCBI
|
|
33
|
Liu T, Zuo L, Guo D, Chai X, Xu J, Cui Z,
Wang Z and Hou C: Ginsenoside Rg3 regulates DNA damage in non-small
cell lung cancer cells by activating VRK1/P53BP1 pathway. Biomed
Pharmacother. 120:1094832019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liang Y, Zhang T, Jing S, Zuo P, Li T,
Wang Y, Xing S, Zhang J and Wei Z: 20(S)-Ginsenoside Rg3 inhibits
lung cancer cell proliferation by targeting EGFR-Mediated
Ras/Raf/MEK/ERK pathway. Am J Chin Med. 49:753–765. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lu J, Zhou Y, Zheng X, Chen L, Tuo X, Chen
H, Xue M, Chen Q, Chen W, Li X and Zhao L: 20(S)-Rg3 upregulates
FDFT1 via reducing miR-4425 to inhibit ovarian cancer progression.
Arch Biochem Biophys. 693:1085692020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Peng Y, Zhang R, Yang X, Zhang Z, Kang N,
Bao L, Shen Y, Yan H and Zheng F: Ginsenoside Rg3 suppresses the
proliferation of prostate cancer cell line PC3 through ROS-induced
cell cycle arrest. Oncol Lett. 17:1139–1145. 2019.PubMed/NCBI
|
|
37
|
Liu Z, Liu T, Li W, Li J, Wang C and Zhang
K: Insights into the antitumor mechanism of ginsenosides Rg3. Mol
Biol Rep. 48:2639–2652. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu W, Zhang SX, Ai B, Pan HF, Zhang D,
Jiang Y, Hu LH, Sun LL, Chen ZS and Lin LZ: Ginsenoside Rg3
promotes cell growth through activation of mTORC1. Front Cell Dev
Biol. 9:7303092021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hwang SK, Jeong YJ, Cho HJ, Park YY, Song
KH and Chang YC: Rg3-enriched red ginseng extract promotes lung
cancer cell apoptosis and mitophagy by ROS production. J Ginseng
Res. 46:138–146. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun X, Hong Y, Shu Y, Wu C, Ye G, Chen H,
Zhou H, Gao R and Zhang J: The involvement of Parkin-dependent
mitophagy in the anti-cancer activity of Ginsenoside. J Ginseng
Res. 46:266–274. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dai Y, Wang W, Sun Q and Tuohayi J:
Ginsenoside Rg3 promotes the antitumor activity of gefitinib in
lung cancer cell lines. Exp Ther Med. 17:953–959. 2019.PubMed/NCBI
|
|
42
|
Peng Z, Wu WW and Yi P: The efficacy of
ginsenoside Rg3 combined with first-line chemotherapy in the
treatment of advanced non-small cell lung cancer in China: A
systematic review and meta-analysis of randomized clinical trials.
Front Pharmacol. 11:6308252020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang X, He R, Geng L, Yuan J and Fan H:
Ginsenoside Rg3 alleviates cisplatin resistance of gastric cancer
cells through inhibiting SOX2 and the PI3K/Akt/mTOR signaling axis
by Up-Regulating miR-429. Front Genet. 13:8231822022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pan H, Yang L, Bai H, Luo J and Deng Y:
Ginsenoside Rg3 increases gemcitabine sensitivity of pancreatic
adenocarcinoma via reducing ZFP91 mediated TSPYL2 destabilization.
J Ginseng Res. 46:636–645. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li J and Yang B: Ginsenoside Rg3 enhances
the radiosensitivity of lung cancer A549 and H1299 cells via the
PI3K/AKT signaling pathway. In Vitro Cell Dev Biol Anim. 59:19–30.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Changizi V, Gharekhani V and Motavaseli E:
Co-treatment with Ginsenoside 20(S)-Rg3 and curcumin increases
radiosensitivity of MDA-MB-231 cancer cell line. Iran J Med Sci.
46:291–297. 2021.PubMed/NCBI
|
|
47
|
Hu G, Luo N, Guo Q, Wang D, Peng P, Liu D,
Liu S, Zhang L, Long G and Sun W: Ginsenoside Rg3 sensitizes
nasopharyngeal carcinoma cells to radiation by suppressing
epithelial mesenchymal transition. Radiat Res. 199:460–467. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen YJ, Wu JY, Deng YY, Wu Y, Wang XQ, Li
AS, Wong LY, Fu XQ, Yu ZL and Liang C: Ginsenoside Rg3 in
combination with artesunate overcomes sorafenib resistance in
hepatoma cell and mouse models. J Ginseng Res. 46:418–425. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wei Q, Ren Y, Zheng X, Yang S, Lu T, Ji H,
Hua H and Shan K: Ginsenoside Rg3 and sorafenib combination therapy
relieves the hepatocellular carcinomaprogression through regulating
the HK2-mediated glycolysis and PI3K/Akt signaling pathway.
Bioengineered. 13:13919–13928. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhu H, Wang SY, Zhu JH, Liu H, Kong M, Mao
Q, Zhang W and Li SL: Efficacy and safety of transcatheter arterial
chemoembolization combined with ginsenosides in hepatocellular
carcinoma treatment. Phytomedicine. 91:1537002021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pu Z, Ge F, Wang Y, Jiang Z, Zhu S, Qin S,
Dai Q, Liu H and Hua H: Ginsenoside-Rg3 inhibits the proliferation
and invasion of hepatoma carcinoma cells via regulating long
non-coding RNA HOX antisense intergenic. Bioengineered.
12:2398–2409. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang T, Zhang C and Wang S: Ginsenoside
Rg3 inhibits osteosarcoma progression by reducing circ_0003074
expression in a miR-516b-5p/KPNA4-dependent manner. J Orthop Surg
Res. 16:7242021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhao L, Sun W, Zheng A, Zhang Y, Fang C
and Zhang P: Ginsenoside Rg3 suppresses ovarian cancer cell
proliferation and invasion by inhibiting the expression of lncRNA
H19. Acta Biochim Pol. 68:575–582. 2021.PubMed/NCBI
|
|
54
|
Bilotta MT, Antignani A and Fitzgerald DJ:
Managing the TME to improve the efficacy of cancer therapy. Front
Immunol. 13:9549922022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhu Y, Wang A, Zhang S, Kim J, Xia J,
Zhang F, Wang D, Wang Q and Wang J: Paclitaxel-loaded ginsenoside
Rg3 liposomes for drug-resistant cancer therapy by dual targeting
of the tumor microenvironment and cancer cells. J Adv Res.
49:159–173. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xia J, Zhang S, Zhang R, Wang A, Zhu Y,
Dong M, Ma S, Hong C, Liu S, Wang D and Wang J: Targeting therapy
and tumor microenvironment remodeling of triple-negative breast
cancer by ginsenoside Rg3 based liposomes. J Nanobiotechnol.
20:4142022. View Article : Google Scholar
|
|
57
|
Wu H, Wei G, Luo L, Li L, Gao Y, Tan X,
Wang S, Chang H, Liu Y, Wei Y, et al: Ginsenoside Rg3 nanoparticles
with permeation enhancing based chitosan derivatives were
encapsulated with doxorubicin by thermosensitive hydrogel and
anti-cancer evaluation of peritumoral hydrogel injection combined
with PD-L1 antibody. Biomater Res. 26:772022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Xia J, Ma S, Zhu X, Chen C, Zhang R, Cao
Z, Chen X, Zhang L, Zhu Y, Zhang S, et al: Versatile ginsenoside
Rg3 liposomes inhibit tumor metastasis by capturing circulating
tumor cells and destroying metastatic niches. Sci Adv.
8:eabj12622022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sun D, Zou Y, Song L, Han S, Yang H, Chu
D, Dai Y, Ma J, O'Driscoll CM, Yu Z and Guo J: A cyclodextrin-based
nanoformulation achieves co-delivery of ginsenoside Rg3 and
quercetin for chemo-immunotherapy in colorectal cancer. Acta Pharm
Sin B. 12:378–393. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zuo S, Wang J, An X, Wang Z, Zheng X and
Zhang Y: Fabrication of ginsenoside-based nanodrugs for enhanced
antitumor efficacy on triple-negative breast cancer. Front Bioeng
Biotechnol. 10:9454722022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Xiong J, Yuan H, Wu H, Cheng J, Yang S and
Hu T: Black phosphorus conjugation of chemotherapeutic ginsenoside
Rg3: enhancing targeted multimodal nanotheranostics against lung
cancer metastasis. Drug Deliv. 28:1748–1758. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Luo X, Wang H and Ji D: Carbon nanotubes
(CNT)-loaded ginsenosides Rb3 suppresses the PD-1/PD-L1 pathway in
triple-negative breast cancer. Aging (Albany NY). 13:17177–17189.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chen M, Qiao Y, Cao J, Ta L, Ci T and Ke
X: Biomimetic doxorubicin/ginsenoside co-loading nanosystem for
chemoimmunotherapy of acute myeloid leukemia. J Nanobiotechnol.
20:2732022. View Article : Google Scholar
|
|
64
|
Lu SL, Wang YH, Liu GF, Wang L, Li Y, Guo
ZY and Cheng C: Graphene oxide nanoparticle-loaded ginsenoside rg3
improves photodynamic therapy in inhibiting malignant progression
and stemness of osteosarcoma. Front Mol Biosci. 8:6630892021.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wang B, Xu Q, Zhou C and Lin Y: Liposomes
co-loaded with ursolic acid and ginsenoside Rg3 in the treatment of
hepatocellular carcinoma. Acta Biochim Pol. 68:711–715.
2021.PubMed/NCBI
|
|
66
|
Zhao X, Wu J, Zhang K, Guo D, Hong L, Chen
X, Wang B and Song Y: The synthesis of a nanodrug using metal-based
nanozymes conjugated with ginsenoside Rg3 for pancreatic cancer
therapy. Nanoscale Adv. 4:190–199. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wei X, Chen J, Su F, Su X, Hu T and Hu S:
Stereospecificity of ginsenoside Rg3 in promotion of the immune
response to ovalbumin in mice. Int Immunol. 24:465–471. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lee Y, Park A, Park YJ, Jung H, Kim TD,
Noh JY, Choi I, Lee S and Ran Yoon S: Ginsenoside 20(R)-Rg3
enhances natural killer cell activity by increasing activating
receptor expression through the MAPK/ERK signaling pathway. Int
Immunopharmacol. 107:1086182022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Cho M, Choi G, Shim I and Chung Y:
Enhanced Rg3 negatively regulates Th1 cell responses. J Ginseng
Res. 43:49–57. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liu X, Zhang Z, Liu J, Wang Y, Zhou Q,
Wang S and Wang X: Ginsenoside Rg3 improves
cyclophosphamide-induced immunocompetence in Balb/c mice. Int
Immunopharmacol. 72:98–111. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Park YJ, Cho M, Choi G, Na H and Chung Y:
A Critical Regulation of Th17 Cell Responses and Autoimmune
Neuro-Inflammation by Ginsenoside Rg3. Biomolecules. 10:1222020.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Sun J, Hu S and Song X: Adjuvant effects
of protopanaxadiol and protopanaxatriol saponins from ginseng roots
on the immune responses to ovalbumin in mice. Vaccine.
25:1114–1120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Yang QY, Lai XD, Ouyang J and Yang JD:
Effects of Ginsenoside Rg3 on fatigue resistance and SIRT1 in aged
rats. Toxicology. 409:144–151. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Park KT, Jo H, Kim B and Kim W: Red Ginger
Extract Prevents the Development of Oxaliplatin-Induced Neuropathic
Pain by Inhibiting the Spinal Noradrenergic System in Mice.
Biomedicines. 11:4322023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Suzuki T, Yamamoto A, Ohsawa M, Motoo Y,
Mizukami H and Makino T: Effect of ninjin'yoeito and ginseng
extracts on oxaliplatin-induced neuropathies in mice. J Nat Med.
71:757–764. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Xu Y, Zhang P, Wang C, Shan Y, Wang D,
Qian F, Sun M and Zhu C: Effect of ginsenoside Rg3 on tyrosine
hydroxylase and related mechanisms in the forced swimming-induced
fatigue rats. J Ethnopharmacol. 150:138–147. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang Y and Zhang Z: The history and
advances in cancer immunotherapy: Understanding the characteristics
of tumor-infiltrating immune cells and their therapeutic
implications. Cell Mol Immunol. 17:807–821. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wastyk HC, Fragiadakis GK, Perelman D,
Dahan D, Merrill BD, Yu FB, Topf M, Gonzalez CG, Van Treuren W, Han
S, et al: Gut-microbiota-targeted diets modulate human immune
status. Cell. 184:4137–4153.e14. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhou CB, Zhou YL and Fang JY: Gut
Microbiota in cancer immune response and immunotherapy. Trends
Cancer. 7:647–660. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Pickard JM, Zeng MY, Caruso R and Núñez G:
Gut microbiota: Role in pathogen colonization, immune responses,
and inflammatory disease. Immunol Rev. 279:70–89. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Adak A and Khan MR: An insight into gut
microbiota and its functionalities. Cell Mol Life Sci. 76:473–493.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Panigrahi DP, Praharaj PP, Bhol CS,
Mahapatra KK, Patra S, Behera BP, Mishra SR and Bhutia SK: The
emerging, multifaceted role of mitophagy in cancer and cancer
therapeutics. Semin Cancer Biol. 66:45–58. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zecchini V, Paupe V, Herranz-Montoya I,
Janssen J, Wortel IMN, Morris JL, Ferguson A, Chowdury SR,
Segarra-Mondejar M, Costa ASH, et al: Fumarate induces vesicular
release of mtDNA to drive innate immunity. Nature. 615:499–506.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Qiu S, Zhong X, Meng X, Li S, Qian X, Lu
H, Cai J, Zhang Y, Wang M, Ye Z, et al: Mitochondria-localized cGAS
suppresses ferroptosis to promote cancer progression. Cell Res.
33:299–311. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Tang YC, Zhang Y, Zhou J, Zhi Q, Wu MY,
Gong FR, Shen M, Liu L, Tao M, Shen B, et al: Ginsenoside Rg3
targets cancer stem cells and tumor angiogenesis to inhibit
colorectal cancer progression in vivo. Int J Oncol. 52:127–138.
2018.PubMed/NCBI
|
|
86
|
Song JH, Eum DY, Park SY, Jin YH, Shim JW,
Park SJ, Kim MY, Park SJ, Heo K and Choi YJ: Inhibitory effect of
ginsenoside Rg3 on cancer stemness and mesenchymal transition in
breast cancer via regulation of myeloid-derived suppressor cells.
PLoS One. 15:e02405332020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ge X, Zhen F, Yang B, Yang X, Cai J, Zhang
C, Zhang S, Cao Y, Ma J, Cheng H and Sun X: Ginsenoside Rg3
enhances radiosensitization of hypoxic oesophageal cancer cell
lines through vascular endothelial growth factor and hypoxia
inducible factor 1α. J Int Med Res. 42:628–640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Qu G and Li B: Inhibition of the
hypoxia-induced factor-1α and vascular endothelial growth factor
expression through ginsenoside Rg3 in human gastric cancer cells. J
Can Res Ther. 15:1642–1646. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Chang L, Huo B, Lv Y, Wang Y and Liu W:
Ginsenoside Rg3 enhances the inhibitory effects of chemotherapy on
esophageal squamous cell carcinoma in mice. Mol Clin Oncol.
2:1043–1046. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lee YJ, Lee S, Ho JN, Byun SS, Hong SK,
Lee SE and Lee E: Synergistic antitumor effect of ginsenoside Rg3
and cisplatin in cisplatin-resistant bladder tumor cell line. Oncol
Rep. 32:1803–1808. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zou J, Su H, Zou C, Liang X and Fei Z:
Ginsenoside Rg3 suppresses the growth of gemcitabine-resistant
pancreatic cancer cells by upregulating lncRNA-CASC2 and activating
PTEN signaling. J Biochem Mol Toxicol. 34:e224802020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Ahmmed B, Kampo S, Khan M, Faqeer A, Kumar
SP, Yulin L, Liu JW and Yan Q: Rg3 inhibits gemcitabine-induced
lung cancer cell invasiveness through ROS-dependent, NF-κB- and
HIF-1α-mediated downregulation of PTX3. J Cell Physiol.
234:10680–10697. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Yuan Z, Jiang H, Zhu X, Liu X and Li J:
Ginsenoside Rg3 promotes cytotoxicity of Paclitaxel through
inhibiting NF-κB signaling and regulating Bax/Bcl-2 expression on
triple-negative breast cancer. Biomed Pharmacother. 89:227–232.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Li L, Ni J, Li M, Chen J, Han L, Zhu Y,
Kong D, Mao J, Wang Y, Zhang B, et al: Ginsenoside Rg3 micelles
mitigate doxorubicin-induced cardiotoxicity and enhance its
anticancer efficacy. Drug Deliv. 24:1617–1630. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Shan K, Wang Y, Hua H, Qin S, Yang A and
Shao J: Ginsenoside Rg3 combined with oxaliplatin inhibits the
proliferation and promotes apoptosis of hepatocellular carcinoma
cells via downregulating PCNA and cyclin D1. Biol Pharm Bull.
42:900–905. 2019. View Article : Google Scholar : PubMed/NCBI
|