Introduction
Immunotherapy has led to great progress in
anticancer therapy (1).
Immunotherapy activates the patient's own immune system to fight
cancer (1,2). Therefore, compared with conventional
chemotherapy or targeted therapies, reactivation of T cells by
immunotherapy can more efficiently kill tumor cells and prevent
cells from escaping the immune system, and is less prone to drug
resistance (2).
Immune checkpoint inhibitors (ICIs) are currently
used as an alternative treatment option for patients with advanced
lung adenocarcinoma (3).
Sintilimab, a recently developed human IgG4 monoclonal antibody,
can bind to programmed cell death receptor 1 (PD-1) and block
related pathways (4). It has been
approved in China for the treatment of advanced lung adenocarcinoma
(5). According to clinical trial
data, sintilimab also exhibits considerable antitumor effects in
non-small-cell lung cancer (NSCLC) (6). The expression of programmed
death-ligand 1 (PD-L1), a PD-1 receptor and immune checkpoint
mainly expressed on the surface of activated T cells, can be
evaluated by immunohistochemistry and is the only prognostic marker
approved for clinical use to evaluate the efficiency of anti-PD1
antibody-based lung cancer therapy (7). A low tumor proportion score (TPS) of
PD-L1 always indicates that the response to PD-1 inhibitors will be
deficient (8). Generally, a PD-L1
TPS >1% is regarded as the lower threshold for use of anti-PD1
antibody-based adjuvant therapy according to global guidelines such
as the National Comprehensive Cancer Network (NCCN) or the European
Society for Medical Oncology (ESMO) (9,10).
Malignant pleural effusion (MPE) is a common
complication of advanced lung cancers (11). It occurs in 30% of lung cancer cases
and indicates poor prognosis (12).
MPE also contributes to immunosuppression in advanced lung cancers,
although the detailed mechanism by which this occurs remains
obscure (13). Tumor-associated
macrophages (TAMs), which are normally detected in the MPE, can
impair the activation of T lymphocytes and natural killer cells and
exert an immunosuppressive function (14). In addition, a series of
immunosuppressive cytokines, such as IFN-γ, IL-4, IL-6, IL-1β and
CXCL1, secreted by cancer cells suppress the host immune response
and thereby promote tumor progression (15). CD8+ T cells from MPE
samples display insufficient differentiation and show a partial
response to anti-PD-L1 therapy (16). Intrapleural perfusion hyperthermia
(IPH) is a relatively new technique that involves intrapleural
perfusion with a hyperthermic liquid combined with chemotherapeutic
agents (17). IPH can efficiently
exhaust the MPE and inhibit the malignant progression of cancers
(17,18).
In the present case report, a clinical case with
advanced lung adenocarcinoma who was refractory to sintilimab-based
chemotherapy but benefited from IPH therapy, is reported.
Subsequently, the patient continued to receive sintilimab and
pemetrexed combination treatment and maintained a stable disease
(SD) state for >36 months. This outcome highlights the potential
of IPH as an alternative method to overcome deficient response to
PD-I inhibitors and improve the overall survival of patients.
Case report
A 50-year-old woman was admitted to The Affiliated
Hospital of Hebei University, Baoding, China) in March 2020. The
clinical presentation of the patient was characterized by
hemoptysis and persistent fever, and the patient complained of pain
in the left scapular area. The patient did not have a history of
smoking or drinking. In April 2020, the patient was diagnosed with
lung adenocarcinoma with a small amount of MPE, following emission
computed tomography (CT) whole-body bone imaging; enhanced CT of
the chest, abdomen, and pelvic cavity and histopathological
examination (hematoxylin-eosin staining) of tissues from a thoracic
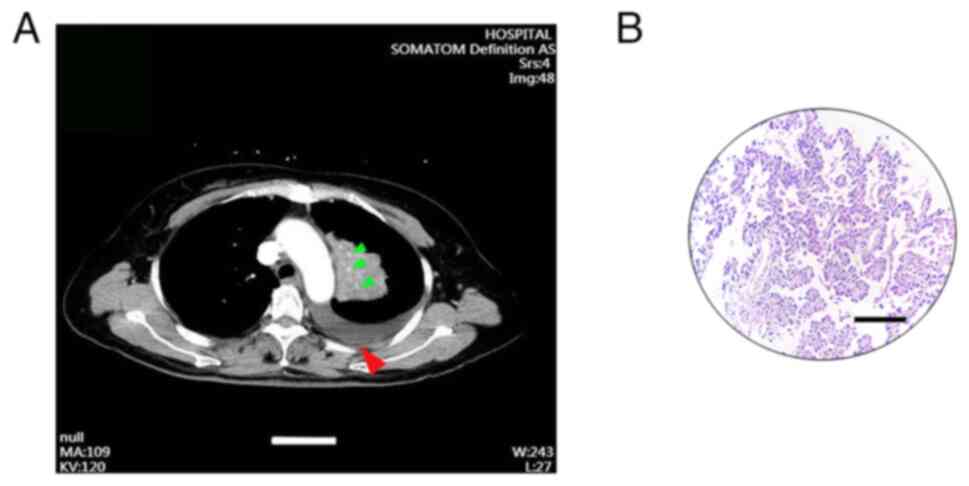
puncture tissue biopsy. Furthermore, the TNM and clinical stage
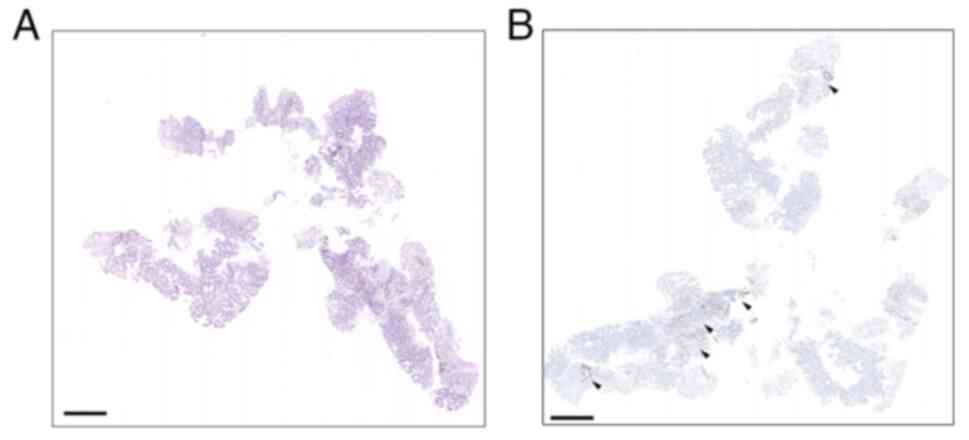
were identified as cT2N3M0 and stage IIIB, respectively (Fig. 1A and B). Immunohistochemical
assessment showed PD-L1 expression with 2% TPS (Fig. 2A and B). The biopsies were then
subjected to genetic testing via the target region sequence of 520
cancer-related genes which contained 520 cancer-driven and
sensitive genes (OncoScreen Plus panel; Burning Rock Medical
Laboratory Co., Ltd.). The panel was designed according to the
information of the OncoKB (Burning Rock Medical Laboratory Co.,
Ltd.) (19). The Genomic DNA was
extracted by the Genomic DNA Isolation Kit (cat. no. K281-50;
BioVision, Inc.). The DNA was then fragmented using ultrasound.
Subsequently, the DNA was separated using 2% agarose gel
electrophoresis. The fragment (range from 200 to 500 bp) was
harvested by QIAquick Gel Extraction Kit (cat. no. 28704; Qiagen,
Inc.). The DNA integrity and concentration number was determined
using the Lab-on-a-Chip-System Bio-analyzer 2100 (Agilent
Technologies, Inc.). The targeted DNA region was captured by the
specific probe sets (the probe sets were designed by Burning Rock
Medical Laboratory Co., Ltd. and synthetized by Agilent
Technologies, Inc.). PCR was used to amplify the captured DNA
region. The paired-end adaptors with nucleotide barcodes were
linked to the enriched DNA to prepare the sequencing library using
the NEB Next Ultra II DNA Library Prep Kit (cat. no. E7645L; New
England BioLabs, Inc.). The average insert fragment was 299 bp
which was measured using Bio-analyzer 2100. The final concentration
of the library was determined using Qubit 2.0 Fluorometer (Thermo
Fisher Scientific, Inc.). The loading concentration of the library
was then adjusted to 10 ng/µl. The library was then sequenced using
HiSeq X Ten (Illumina, Inc.). The paired-end 150-bp (PE150)
sequencing model was converted using bcl2fastq Conversion software
(v2.20.0.422), to convert raw sequencing data to fastq format
(Illumina, Inc.). The FastQC (v0.10.0, (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/)
software was used to control the data quality. The SNP calling was
performed using MuTect (v1.1.4;
broadinstitute.org/cancer/cga/mutect) software. The relationship
between the mutations and chemotherapy drug susceptibility referred
to the NCCN Guidelines (https://www.nccn.org/) and OncoKB. The results were
summarized as follows: the KRAS gene mutation abundance was
38.2%; the CDK2A gene was partially deleted; and the
MYC and RICTO genes were amplified. Immunotherapy
combined with chemotherapy was considered. Initially, the patient
received four courses of palliative chemotherapy from April to June
2020. The regimen for each course consisted of 200 mg sintilimab
every 3 weeks, with 800 mg/m2 pemetrexed on day 1 and 50
mg lobaplatin on day 2. Subsequently, enhanced CT examination of
the chest showed increased pleural effusion and left upper lobe
atelectasis in the left thoracic cavity. The main symptoms were a
choking sensation in the chest, expiratory dyspnea, and a
persistent fever. The best overall response (BOR) assessment of the
local lesion was SD. Pleural puncture for hydrops outflow was then
carried out. Hydrothorax cells were harvested by
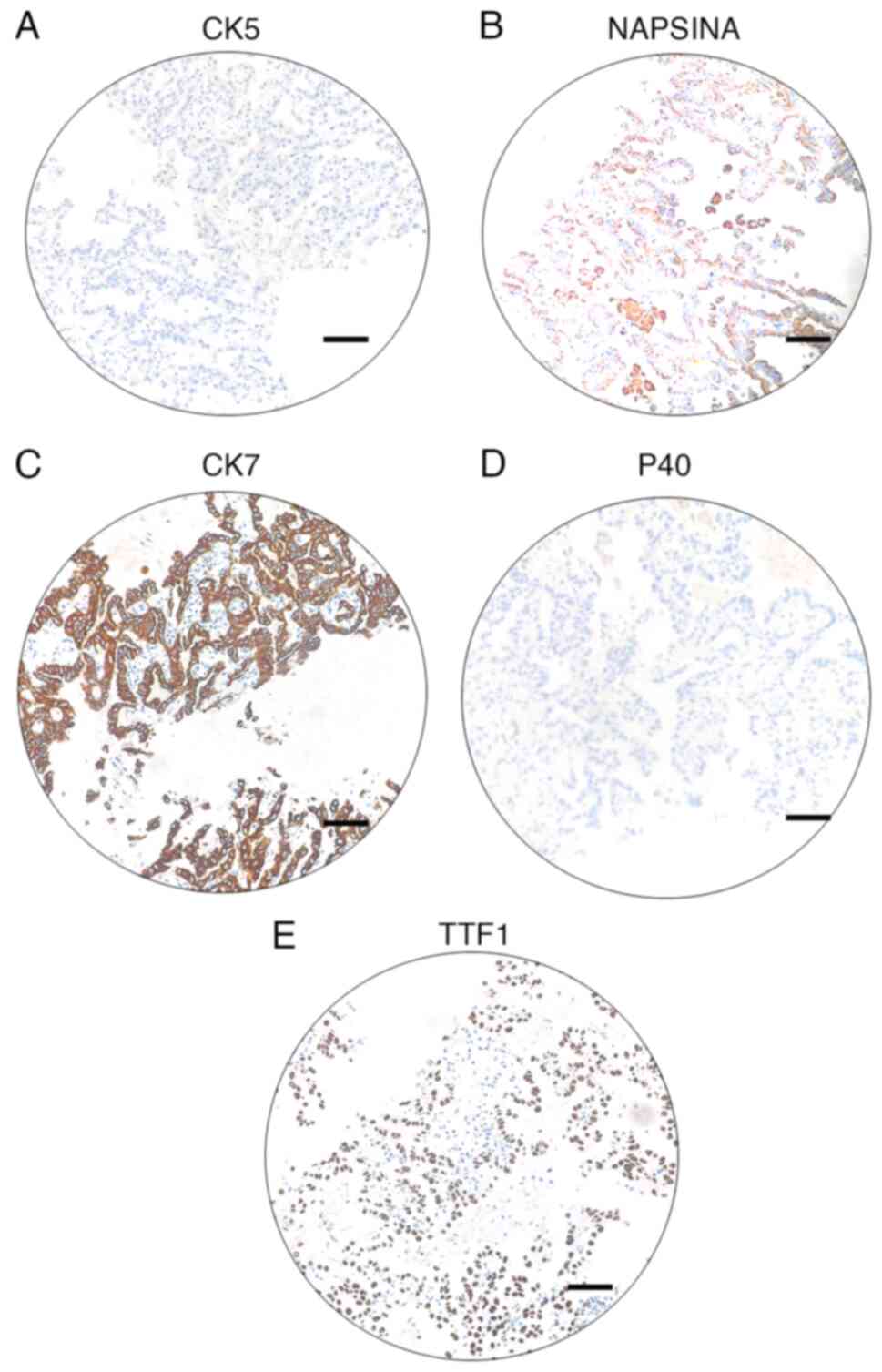
centrifugation(1200 g, 4°C, 5 min). Immunohistochemical staining of
biomarkers of the embedding cells indicated CK5−,
NapsinA+, P40−, CK7+ and
TTF-1+ (Fig. 3A-E).
Furthermore, pathological examination of cell proportions indicated
that large number of lymphocytes and severely dyskaryotic cells
were present in the hydrothorax.
After a multidisciplinary discussion, the patient
received four courses of IPH followed by chemotherapy with
cisplatin (a total of 60 mg, circulation 20 mg and retained 600 mg)
from August to September 2020. Following the treatment, the
hydrothorax was found to have been effectively controlled. Then,
six courses of immunotherapy and chemotherapy were performed from
September to October 2020. The regimen for each course was as
follows: 200 mg sintilimab was administered at day 0, 800
mg/m2 pemetrexed at day 1, and lobaplatin 50 mg at day
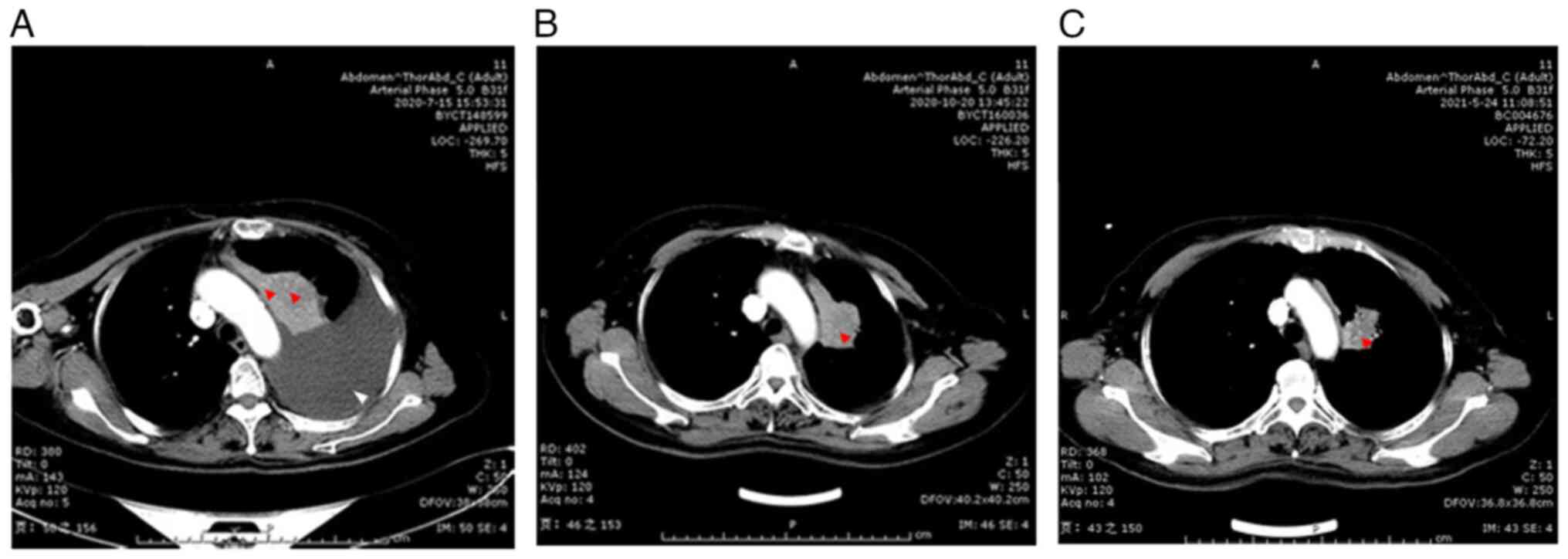
2. Lung CT was performed after the adjuvant chemotherapy and
indicated that the stigmatal tubercle had shrunk, the left upper
lobe atelectasis was released, and hydrothorax content was
significantly reduced (Fig. 4A-C).
No other symptoms were observed after this therapy.
Given that the IPH-adjuvant chemotherapy obviously
weakened the patient, 26 courses of immunotherapy-adjuvant
monotherapy were subsequently performed from October 2020 to
September 2023. The regimen for each course was as follows: 200 mg
sintilimab was administered at day 0, and 800 mg/m2
pemetrexed at day 1. Lobaplatin was excluded from the regimen.
Following courses 6, 8, 11, 14, 17, 20 and 26, a BOR assessment was
performed based on enhanced CT of the thoracic and abdominal
tumors. Data obtained from evaluation of the CT images at different
treatment stages are summarized in Table I. The BOR evaluation results were
all SD, indicating that the malignant progression of the tumor was
under control. To date, the patient remains alive, with a BOR stage
of SD.
 | Table I.Image evaluation of the CT
imaging. |
Table I.
Image evaluation of the CT
imaging.
| CT scan
findings | First visit | Following the first
phase of sintilimab treatment | Following IPH
treatment | Following the
fourth phase of sintilimab-based treatment after IPH treatment |
|---|
| Relative
representative area of MPE (%) | 13% | 45% | Not detected | Not detected |
| Location of the
MPE | Left | Left | N/A | N/A |
| Number of diffuse
pulmonary nodules | 4 | 13 | 8 | 8 |
| Estimated tumor
diameter (cm) | 1-2 | 2-3 | 2-3 | 2-3 |
| Mediastinal pleural
thickening | Observed | Observed | Observed | Observed |
| Circumferential
pleural thickening | Observed | Observed | Observed | Observed |
Discussion
ICI-based adjuvant chemotherapy has been previously
developed and adopted for treatment of advanced lung cancer. PD-1
inhibitor sintilimab, which has pharmacokinetic properties similar
to those of nivolumab, has been approved by the Chinese National
Medical Products Administration and the US Food and Drug
Administration to treat NSCLC (4).
Owing to its safety and pharmacoeconomic advantages, it has widely
been adopted for clinical practice in China. However, there are
numerous issues that can result in drug resistance and failure of
ICI treatment (20). The tumor
microenvironment (TME) that surrounds the tumor cells contains
various types of immune cells, including macrophages, natural
killer cells, myeloid-derived suppressor cells, and T lymphocytes
(21). The activation of T
lymphocytes in the TME affects the efficiency of ICI-based
chemotherapy; continuous antigenic stimulation of T cells has been
linked to increased expression of PD-1, which leads to T-cell
exhaustion (22). Increased
expression of the PD-1/PD-L1 axis in tumor cells is associated with
poorer prognosis (20). ICIs can
block the binding between PD-1 and PD-L1/L2 and restore the
endogenous antitumor T-cell response (20,22).
MPE occurs in 30% of lung cancers and is regarded as
signature for poor prognosis that promotes the malignant
progression of the cancer (20).
The immune impairment caused by immune suppressors in the MPE has
been reported previously. A number of cytokines and chemokines in
the MPE can serve as prognostic markers (23). For example, CD163+ TAMs
were identified as potential diagnostic and prognostic biomarkers
for MPE that could be used to evaluate the effects of therapy
(23,24). Furthermore, TAM-derived TGF-β can
lead to dysfunction of CD4+ and CD81+ cells
and impair T-cell cytotoxicity in the MPE. Generally,
PD-L1-expressing tumor cells in the MPE are considered to inhibit
the cytotoxic potential of CD8+ T cells (25). Therefore, ICIs can block PD-L1 and
reduce the volume of the MPE. However, the mechanism underlying ICI
resistance in the MPE remains unclear. In the present case report,
a patient who experienced ICI treatment failure and increased
volume of MPE, was reported. The findings indicate that the
clinical, immunological, and pathological indices in the MPE may
provide insight into the potential mechanism by which MPE
contributes to ICI resistance.
The treatment of advanced lung cancer with MPE is a
major challenge for thoracic surgeons (26), and no superior clinical strategy has
yet been identified. Pleurodesis and pleurectomy are commonly used
surgical methods (18,26); however, the condition of the patient
may limit the feasibility and efficiency of this operation. A
retrospective study explored the reasons for the poor prognosis of
patients with disseminated pleural cancer with MPE when treated by
lung resection. Kodama et al (27) first observed favorable clinical
outcomes of the patients with MPE who received IPH combined with
chemotherapy, and a case with a mean survival time of 20 months
after lung resection and IPH was reported in another study
(28). In the present study, IPH
combined with chemotherapy similarly extended the survival of the
patient to 30 months following lobaplatin and pemetrexed combined
therapy. In recent decades, IPH-based therapies have become a
standard strategy for treatment of MPE (29,30),
although in most reports, IPH therapy is combined with chemotherapy
or surgery (30–32). The efficiency and safety of these
methods depend strongly on the physical condition of the patient
and the resectability of tumors (29,31,33).
There have been few reports of the relationship between IPH and
immunotherapy. The patient in the present case report also had a
low PD-L1 positive ratio (accounting for ~2%), which is regarded as
a prognostic factor indicating poor response to ICI treatment.
However, the histopathology, genetic mutations, and clinical
features of this tumor did not suggest a poor prognosis. Increased
MPE was observed after phase 1 treatment and may be a possible
explanation for the poor efficacy of the sintilimab-based
chemotherapy. Following IPH treatment for nearly 1 month, MPE was
no longer generated during the subsequent sintilimab-based
chemotherapy. This clinical presentation indicates that IPH-based
chemotherapy may improve the sensitivity of cancer cells to the
ICIs. It also supports a previous finding that demonstrated that
IPH could convert the phase of T cells from Th1 to Th2 in patients
with lung cancer (34). Therefore,
effective and safe activation of the immune system in response to
ICIs may be the key to treatment.
Several local treatment methods are used as standard
treatments for unresectable tumors (35). There is a consensus among some
specialists that appropriate radiation therapy (RT) can enhance the
efficacy of ICIs, with manageable toxicity, in patients with lung
cancer (36). The efficiency of RT
largely depends on the clinical stage and on personalized tumor
histology and molecular status. In addition, different radiation
doses in a single-fraction or short-course fraction regimen, such
as hypofractionated radiation, particle implantation, and
radiofrequency ablation, may induce diverse immunogenic effects. In
a hypofractionated radiation model, researchers demonstrated that
3×8 Gy was the most effective scheme compared with other
fractionation protocols (18×2 or 1×16.4 Gy) when combined with
anti-PD-L1 therapy (37).
Therefore, a series of hypofractionated radiation therapies such as
particle implantation and radiofrequency ablation have been
developed for ICI combination treatment. The implantation of
radioactive particles has been demonstrated to be highly efficient
in the treatment of middle- to late-stage lung cancer. For
instance, 125I combined with chemotherapy is widely used
for lung cancer therapy (38). To
date, most reports of radiofrequency ablation treatment for cancer
have involved colorectal cancer, and it has shown significant
clinical value in the treatment of colorectal cancer and liver
metastasis (39). Palussière et
al (40) demonstrated that the
combination of ICIs with percutaneous thermal ablation is an
important therapeutic option for NSCLC treatment. However, the
safety and efficiency of such methods still needs to be explored.
The development of efficient and low-toxicity delivery methods is
another aspect to be considered with respect to hypofractionated
radiation. In recent decades, the development of nanomedicine has
resulted in new methods and perspectives for local treatment.
Nanoparticles have been widely used in medical imaging,
photothermal therapy and photodynamical therapy (41). Owing to their excellent properties
(including good biocompatibility and biodegradation, low toxicity
and high specificity), bionanoparticles have emerged as a new type
of anticancer adjuvant in recent years (41). For instance, a dendrimer formed from
several nanoparticles was shown to activate specific immune cells
and facilitate cancer immunotherapy (42). A nanoparticle-based laser
desorption/ionization mass spectrometry platform also greatly
improved the specificity of metabolic fingerprinting in lung
adenocarcinoma; when integrated in a deep-learning model with other
classical cancer hallmarks, it could efficiently detect lung
adenocarcinoma at an early stage (43). These findings indicate that
combining the appropriate nanoparticles with a radioactive element
may greatly improve the specificity and sensitivity of RT and may
lead to the development of an optimal delivery carrier for RT
treatment in future.
In summary, combination of a dosage-controlled RT
agent with IPH may be a more efficient means of achieving a
favorable therapeutic outcome than IPH combined with chemotherapy.
This finding has special significance for patients for whom
chemotherapy or surgical treatment is not suitable and may indicate
an alternative direction for the development of IPH-based treatment
in future. A limitation of the present case report is that only a
single patient was reported. Therefore, studies with more subjects
or prospective/retrospective cohort studies are required to verify
the findings of the present study in the future. In addition,
mechanistic research, clinical trials and real-world studies are
also warranted to investigate the feasibility of combining various
ICIs, RT-based local treatment, nanomedicine and IPH for patients
with various pathologies, genetic mutations and clinical
features.
Acknowledgements
Not applicable.
Funding
The research was partially supported by the fund of Affiliated
hospital of Hebei University (no. 2022ZB04)
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the SRA repository, (https://www.ncbi.nlm.nih.gov/sra/PRJNA1063833).
Authors' contributions
XW, JS, LH, GR, NG and ZS made substantial
contributions to the conception and design, acquisition of data, as
well as analysis and interpretation of data. XW and JS made
substantial contributions to develop the treatment protocol,
acquisition of data and analysis, and wrote the manuscript. LH
carried out the collection of samples and managed the information
received from the patient and performed the follow-up. GR
participated in the data analysis. GR and NG contributed to the
experimental design and the literature review. ZS revised the
manuscript. XW and ZS confirm the authenticity of all the raw data.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study conformed to the ethical standards
for human subjects involved in medical research. The Ethics
Committee of the Affiliated Hospital of Hebei University (Baoding,
China) approved (approval no. 20220923) the present study. The
research was carried out in accordance with the World Medical
Association Declaration of Helsinki. Written informed consent was
obtained from the patient for participating in the present
study.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Abbott M and Ustoyev Y: Cancer and the
immune system: The history and background of immunotherapy. Semin
Oncol Nurs. 35:1509232019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Knaus HA, Kanakry CG, Luznik L and Gojo I:
Immunomodulatory drugs: Immune checkpoint agents in acute leukemia.
Curr Drug Targets. 18:315–331. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie W, Hu N and Cao L: Immune
thrombocytopenia induced by immune checkpoint inhibitrs in lung
cancer: Case report and literature review. Front Immunol.
12:7900512021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang L, Mai W, Jiang W and Geng Q:
Sintilimab: A promising anti-tumor PD-1 antibody. Front Oncol.
10:5945582020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang L, Qian Y, Li J, Cui C, Chen L, Qu S
and Lu S: Indirect comparison of sintilimab and other PD-L1
inhibitors for first-line treatment of non-squamous non-small-cell
lung cancer. Future Oncol. 18:1896–1905. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ru CH and Zhuang YB: Efficacy and safety
of addition of anti-PD1 to chemotherapy in treatment of non-small
cell lung cancer. Comb Chem High Throughput Screen. 21:711–717.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Patel SP and Kurzrock R: PD-L1 expression
as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther.
14:847–856. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jöhrens K and Rüschoff J: The challenge to
the pathologist of PD-L1 expression in tumor cells of
non-small-cell lung cancer-an overview. Curr Oncol. 28:5227–5239.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman J, Chirieac LR, D'Amico TA, DeCamp MM, Dilling TJ,
Dobelbower M, et al: Non-small cell lung cancer, version 5.2017,
NCCN clinical practice guidelines in oncology. J Natl Compr Canc
Netw. 15:504–535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hendriks LE, Kerr KM, Menis J, Mok TS,
Nestle U, Passaro A, Peters S, Planchard D, Smit EF, Solomon BJ, et
al: Electronic address: simpleclinicalguidelines@esmo.org.
Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO
clinical practice guideline for diagnosis, treatment and follow-up.
Ann Oncol. 34:358–376. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Simpson G and Judge DJ: Management of
malignant pleural effusion. Respirology. 20:1692015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ferreiro L, Suárez-Antelo J,
Álvarez-Dobaño JM, Toubes ME, Riveiro V and Valdés L: Malignant
pleural effusion: Diagnosis and management. Can Respir J.
2020:29507512020.PubMed/NCBI
|
|
13
|
Sun Y, Hu Y, Wan C, Lovell JF, Jin H and
Yang K: Local biomaterial-assisted antitumour immunotherapy for
effusions in the pleural and peritoneal cavities caused by
malignancies. Biomater Sci. 9:6381–6390. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ruffell B, Affara NI and Coussens LM:
Differential macrophage programming in the tumor micro environment.
Trends Immunol. 33:119–126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De Cicco P, Ercolano G and Ianaro A: The
new era of cancer immunotherapy: targeting myeloid-derived
suppressor cells to overcome immune evasion. Front Immunol.
11:16802020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li L, Yang L, Wang L, Wang F, Zhang Z, Li
J, Yue D, Chen X, Ping Y, Huang L, et al: Impaired T cell function
in malignant pleural effusion is caused by TGF-β derived
predominantly from macrophages. Int J Cancer. 139:2261–2269. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ba M, Long H, Wang Y, Tang Y, Wu Y, Zhang
X and Cui S: Intrapleural hyperthermic perfusion using distilled
water at 48°C for malignant pleural effusion. J Cancer Res Clin
Oncol. 139:2005–2012. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shigemura N, Akashi A, Ohta M and Matsuda
H: Combined surgery of intrapleural perfusion hyperthermic
chemotherapy and panpleuropneumonectomy for lung cancer with
advanced pleural spread: a pilot study. Interact Cardiovasc Thorac
Surg. 2:671–675. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chakravarty D, Gao J, Phillips SM, Kundra
R, Zhang H, Wang J, Rudolph JE, Yaeger R, Soumerai T, Nissan MH, et
al: OncoKB: A precision oncology knowledge base. JCO Precis Oncol.
2017.PO.17.00011. 2017. View Article : Google Scholar
|
|
20
|
Błach J, Wojas-Krawczyk K, Nicoś M and
Krawczyk P: Failure of immunotherapy-the molecular and
immunological origin of immunotherapy resistance in lung cancer.
Int J Mol Sci. 22:90302021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu W, Zheng YL, Tian LP, Lai JB, Hu CC,
Zhang P, Chen JK, Hu JB, Huang ML, Wei N, et al: Circulating T
lymphocyte subsets, cytokines, and immune checkpoint inhibitors in
patients with bipolar II or major depression: A preliminary study.
Sci Rep. 7:405302017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dolina JS, Van Braeckel-Budimir N, Thomas
GD and Salek-Ardakani S: CD8+ T cell exhaustion in cancer. Front
Immunol. 12:7152342021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang F, Yang L, Gao Q, Huang L, Wang L,
Wang J, Wang S, Zhang B and Zhang Y: CD163+CD14+ macrophages, a
potential immune biomarker for malignant pleural effusion. Cancer
Immunol Immunother. 64:965–976. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang L, Wang F, Wang L, Huang L, Wang J,
Zhang B and Zhang Y: CD163+ tumor-associated macrophage
is a prognostic biomarker and is associated with therapeutic effect
on malignant pleural effusion of lung cancer patients. Oncotarget.
6:10592–10603. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wahl SM, Swisher J, McCartney-Francis N
and Chen W: TGF-beta: The perpetrator of immune suppression by
regulatory T cells and suicidal T cells. J Leukoc Biol. 76:15–24.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Suzuki K, Funai K, Shundo Y, Asano K,
Takamochi K, Asai K, Kazui T and Miura K: Extrapleural
pneumonectomy after hyperthermo-chemotherapy for the lung cancer
patients with malignant pleural effusion. Kyobu Geka. 57:1023–1027.
2004.(In Japanese). PubMed/NCBI
|
|
27
|
Kodama K, Doi O, Higashiyama M, Yokouchi H
and Tatsuta M: Long-term results of postoperative intrathoracic
chemo-thermotherapy for lung cancer with pleural dissemination.
Cancer. 72:426–431. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matsuzaki Y, Shibata K, Yoshioka M, Inoue
M, Sekiya R, Onitsuka T, Iwamoto I and Koga Y: Intrapleural
perfusion hyperthermo-chemotherapy for malignant pleural
dissemination and effusion. Ann Thorac Surg. 59:127–131. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cao Y, Zhang Q, Huang Z, Chai Z, Liu J,
Wang J, Sun Z, Zhao T, Wang G, Chen G, et al: Better effect of
intrapleural perfusion with hyperthermic chemotherapy by
video-assisted thoracoscopic surgery for malignant pleural effusion
treatment compared to normothermic chemoperfusion of the pleural
cavity. Cancer Med. 11:348–357. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Işık AF, Sanlı M, Yılmaz M, Meteroğlu F,
Dikensoy O, Sevinç A, Camcı C, Tunçözgür B and Elbeyli L:
Intrapleural hyperthermic perfusion chemotherapy in subjects with
metastatic pleural malignancies. Respir Med. 107:762–767. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu L, Jing Y, Ma S, Li F and Zhang YF:
Cytoreductive surgery combined with hyperthermic intrapleural
chemotherapy to treat thymoma or thymic carcinoma with pleural
dissemination. Onco Targets Ther. 6:517–521. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang X, Kong M, Jin J, Lin Y, Jia L and Ye
M: The efficacy and safety of intrapleural hyperthermic perfusion
in patients with malignant pleural effusion undergoing
video-assisted thoracic surgery: A single-arm clinical trial. J
Thorac Dis. 14:1497–1503. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li J, Yao H, Lei Y and Ye Y: Establishment
of a human intrapleural hyperthermic perfusion model and analysis
of pleural malignancy treatment depth. Respir Med. 138:144–149.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kang MQ, Cao YP and Deng F: Impact of
intrapleural hyperthermic perfusion on immunologic reaction state
of cytokines TH1/TH2 of lung carcinoma patients with malignant
pleural effusion. Ai Zheng. 27:210–213. 2008.(In Chinese).
PubMed/NCBI
|
|
35
|
Lemjabbar-Alaoui H, Hassan OU, Yang YW and
Buchanan P: Lung cancer: Biology and treatment options. Biochim
Biophys Acta. 1856:189–210. 2015.PubMed/NCBI
|
|
36
|
Zhu Z, Ni J, Cai X, Su S, Zhuang H, Yang
Z, Chen M, Ma S, Xie C, Xu Y, et al: International consensus on
radiotherapy in metastatic non-small cell lung cancer. Transl Lung
Cancer Res. 11:1763–1795. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Grapin M, Richard C, Limagne E, Boidot R,
Morgand V, Bertaut A, Derangere V, Laurent PA, Thibaudin M, Fumet
JD, et al: Optimized fractionated radiotherapy with anti-PD-L1 and
anti-TIGIT: A promising new combination. J Immunother Cancer.
7:1602019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang S, Zheng Y, Yu P, Yu F, Zhang Q, Lv
Y, Xie X and Gao Y: The combined treatment of CT-guided
percutaneous 125I seed implantation and chemotherapy for
non-small-cell lung cancer. J Cancer Res Clin Oncol. 137:1813–1822.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Carditello A, Scisca C, David A, Littori
F, Stilo F and Allegra G: New perspectives in the treatment of
liver metastasis from colorectal cancer: Radiofrequency thermal
ablation. G Chir. 22:407–409. 2001.PubMed/NCBI
|
|
40
|
Palussière J, Catena V, Lagarde P, Cousin
S, Cabart M, Buy X and Chomy F: Primary tumors of the lung: Should
we consider thermal ablation as a valid therapeutic option? Int J
Hyperthermia. 36:46–52. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yan L, Qing B, Shuxu Y, Mingying Y and
Chuanbin M: Bionanoparticles in cancer imaging, diagnosis, and
treatment. View. 3:1–45. 2022.PubMed/NCBI
|
|
42
|
Gao Y, Shen M and Shi X: Interaction of
dendrimers with the immune system: An insight into cancer
nanotheranostics. View 2. 1–9. 2021.
|
|
43
|
Wang L, Zhang M, Pan X, Zhao M, Huang L,
Hu X, Wang X, Qiao L, Guo Q, Xu W, et al: Integrative serum
metabolic fingerprints based multi-modal platforms for lung
adenocarcinoma early detection and pulmonary nodule classification.
Adv Sci (Weinh). 9:e22037862022. View Article : Google Scholar : PubMed/NCBI
|