Introduction
Glioblastoma (GBM) is the most common primary
malignant brain tumour in adults and among the most lethal of human
cancers (1). Treatment of GBM is
challenging due to its highly infiltrative nature, broad range of
genetic heterogeneity, and ability to establish an immunologically
‘cold’ microenvironment (2). The
standard of care consists of maximal surgical resection and 6 weeks
of concomitant radiation and temozolomide chemotherapy, followed by
six 5-day cycles of adjuvant temozolomide given every 28 days
(3). Outside of well-selected
patients in clinical trials, this protocol is associated with a
median survival of 12 to 15 months (4,5). Fewer
than 5% of GBM patients survive for longer than 3 years and are
known as ‘long-term survivors’ (6,7).
Cancer cells exhibit altered cell metabolism and
impaired mitochondrial biology (8,9). A
widespread feature of cancer cell metabolism is the Warburg effect,
which refers to a dramatic increase in the aerobic fermentation of
glucose that may act to compensate for damaged cell respiration
through the generation of fermentation energy (10). Another potential compensatory
mechanism is the Q-Effect, which involves a rewiring of glutamine
metabolism to generate fermentation energy (11). Cancer cells also rely on increased
growth signalling pathways involving insulin, insulin-like growth
factor-1 (IGF-1), and mammalian target of rapamycin (mTOR)
(12,13). Beyond these alterations, there is
evidence of impaired mitochondrial biology in human gliomas,
including GBM (14). Glioma cells
show a reduced mitochondria content (15), a low frequency of fusion and fission
(16), and a plethora of
mitochondrial DNA mutations (17,18).
Moreover, GBM mitochondria display a 56–92% reduction in the
activities of respiratory chain complexes I to IV (19), increased oxidative damage (20), and frequent swelling and cristolysis
(16,20).
Given the metabolic and mitochondrial profile of
GBM, the standard treatment protocol may benefit from ketogenic
metabolic therapy (KMT) (21).
Common strategies include fasting and ketogenic diet regimens,
which restrict glucose availability and generate fat-derived
ketones leading to a lowered blood glucose-to-ketone
(beta-hydroxybutyrate) ratio, or glucose ketone index (GKI)
(22). Lowering the GKI also evokes
differential stress resistance and sensitization, with normal cells
showing enhanced resistance to stressors (including radiation and
chemotherapy), whereas cancer cells are sensitized (23). Beyond lowering the GKI, KMT tempers
insulin, IGF-1, and mTOR availability (12,24).
Moreover, KMT increases the efficiency of ATP production, decreases
oxidative stress, and stimulates mitogenesis and mitophagy. Despite
these theoretical advantages, supportive interventional evidence in
patients with GBM remains limited (25). Only one study has incorporated a
combined fasting and ketogenic diet protocol in a rigorous manner
(26). This study showed that an
intensive, combined KMT program was feasible and safe in patients
with GBM. However, it did not specifically time KMT with the
standard treatments to take advantage of differential stress
resistance and sensitization.
Given the collective evidence, we hypothesized that
applying the standard treatment protocol in conjunction with an
intensive, long-term, multimodal KMT program, specifically timed to
maximize the tolerability and efficacy of the standard treatments,
would be feasible and potentially beneficial for survival in a
patient with GBM.
Case report
We report the case of a 64-year-old female teacher
of European background who attended Rotorua Hospital (Rotorua, New
Zealand) in December 2020 with 2 days of left upper and lower limb
numbness and weakness. Medical history included hypothyroidism and
gastro-oesophageal reflux, for which she was taking levothyroxine
and omeprazole. She was a non-smoker and lived with her husband.
Magnetic resonance imaging (MRI) brain with contrast revealed a
large enhancing mass (33×30×39 mm) in the right parietal lobe with
local mass effect. She was transferred to neurosurgery at Waikato
Hospital (Hamilton, New Zealand) for surgical resection. Biopsy
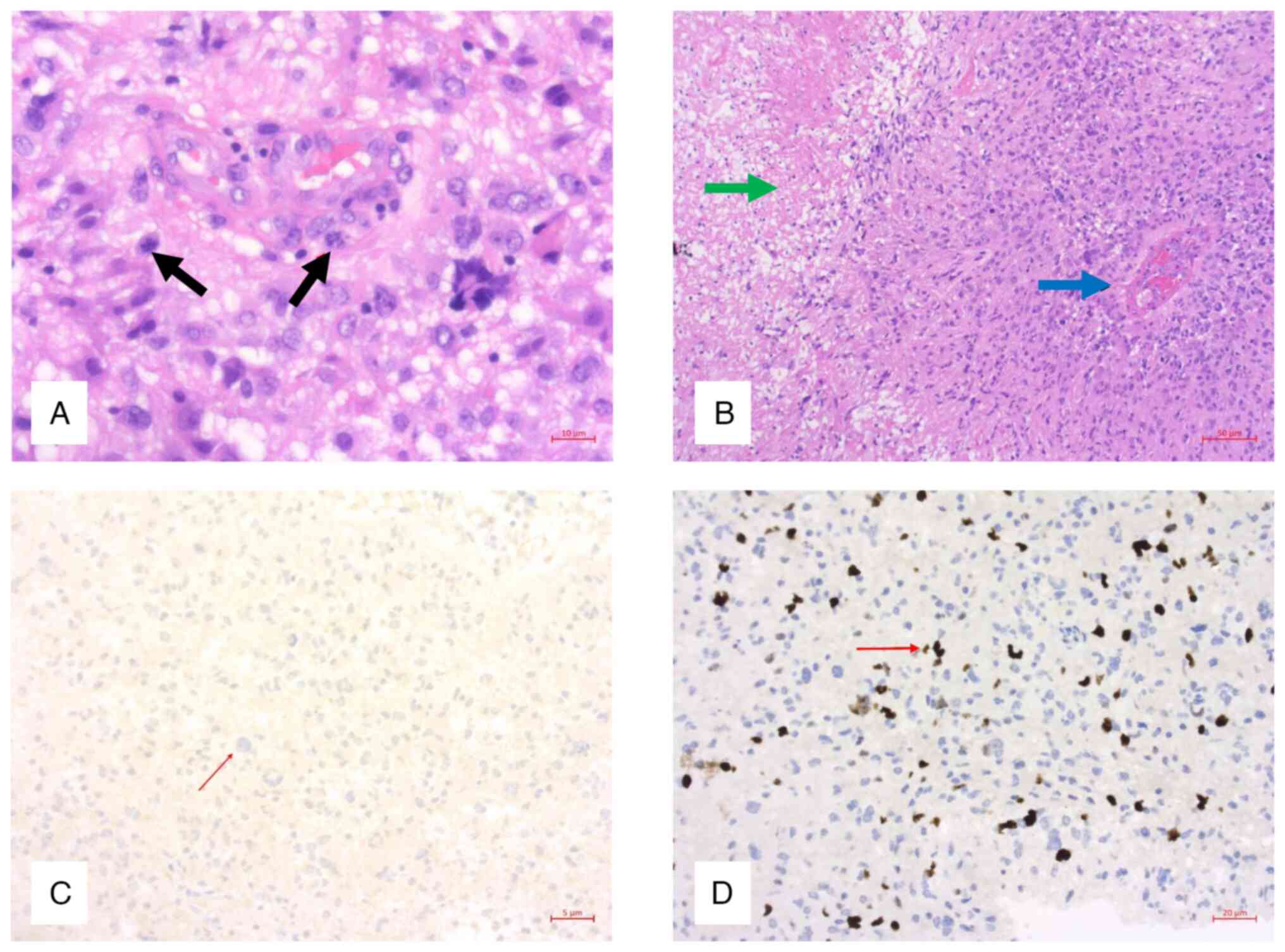
evaluation revealed areas of pleomorphic cells with brisk mitotic
activity, geographic and palisading necrosis, and endothelial
proliferation, with immunochemistry showing an isocitrate
dehydrogenase (IDH)-wildtype, borderline methylated GBM (average
CpG site methylation rate 8.3%) (Fig.
1). Post-operative MRI demonstrated no residual enhancing
tumour and she was discharged home on tapering dexamethasone. She
was reviewed by oncology as an outpatient and planned for 6 weeks
of concomitant radiation therapy (60 Gy in 30 fractions) and
temozolomide (75 mg/m2 daily), followed by six cycles of
adjuvant temozolomide (150 mg/m2 daily on cycle 1,
increasing to 200 mg/m2 daily for cycles 2–5). The
treating oncologist also referred her to a neurologist for
consideration of KMT.
Upon review by neurology, the only residual
neurological symptom was mild left lower limb weakness. Our patient
measured 166 cm in height and 77.5 kg in weight, resulting in an
overweight body-mass index of 28.1 kg/m2. She had an
Eastern Cooperative Oncology Group (ECOG) score of 1, with 5-/5
power in all left lower limb movements. Following the assessment,
she was given the option of a KMT protocol that incorporated
prolonged fasting, time-restricted feeding, and a modified
ketogenic diet in a manner intended to maximize the tolerability
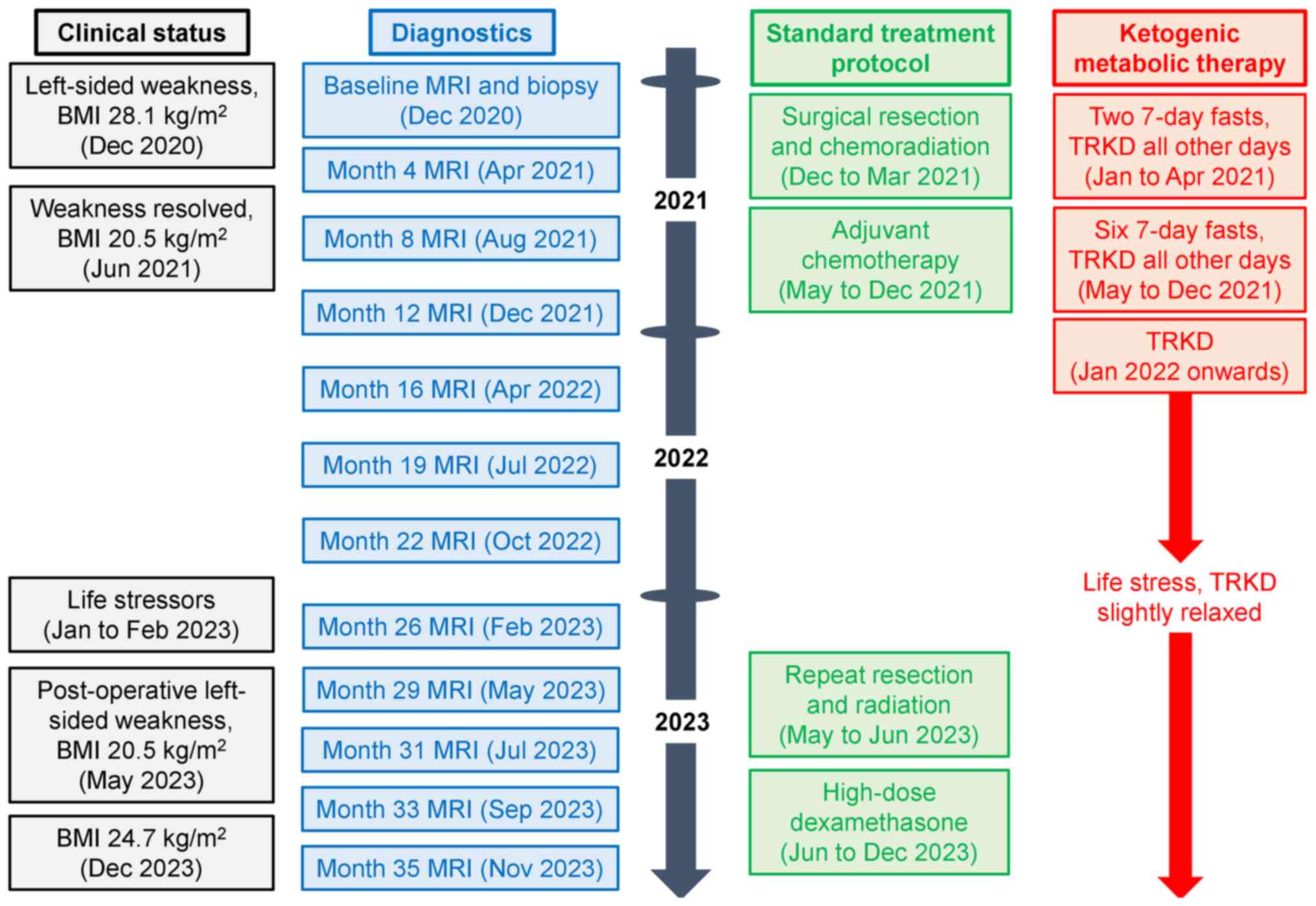
and efficacy of the standard treatments (Fig. 2). The prolonged fasts consisted of
eight 7-day, fluid-only (water, tea, or coffee) fasts, with the
first fast commencing 4 days prior to chemoradiation, the second
fast occurring in the third week of chemoradiation, and the latter
six fasts commencing 4 days prior to each cycle of temozolomide. On
all other days, she was instructed to undergo a time-restricted
ketogenic diet (TRKD), which involved reducing feeding times to two
meals per day, with no food intake in the intervening hours. She
could choose the timing of the two meals every day and was
permitted 1 h per meal to ensure that food intake was restricted to
2 h per day, with the remaining 22 h dedicated to fasting. All
meals consisted of a modified ketogenic diet, which was 60% fat,
30% protein, 5% fibre, and 5% net carbohydrate by weight and
comprised of whole foods (green vegetables, meats, eggs, nuts,
seeds, creams, and natural oils). She was instructed to eat until
satiated. Written informed consent was obtained and a booklet
provided with guidelines, recipes, and space to record daily
(bedtime) blood glucose and ketone levels, which she measured using
a blood glucose and ketone monitor (CareSens Dual, Pharmaco
Diabetes, Auckland, New Zealand). The lead investigator provided
regular email support.
Our patient pursued the standard treatments
alongside KMT for 3 years, during which we documented her blood
glucose and ketone levels (± standard deviation) in a spreadsheet
(Table SI). We used this data to
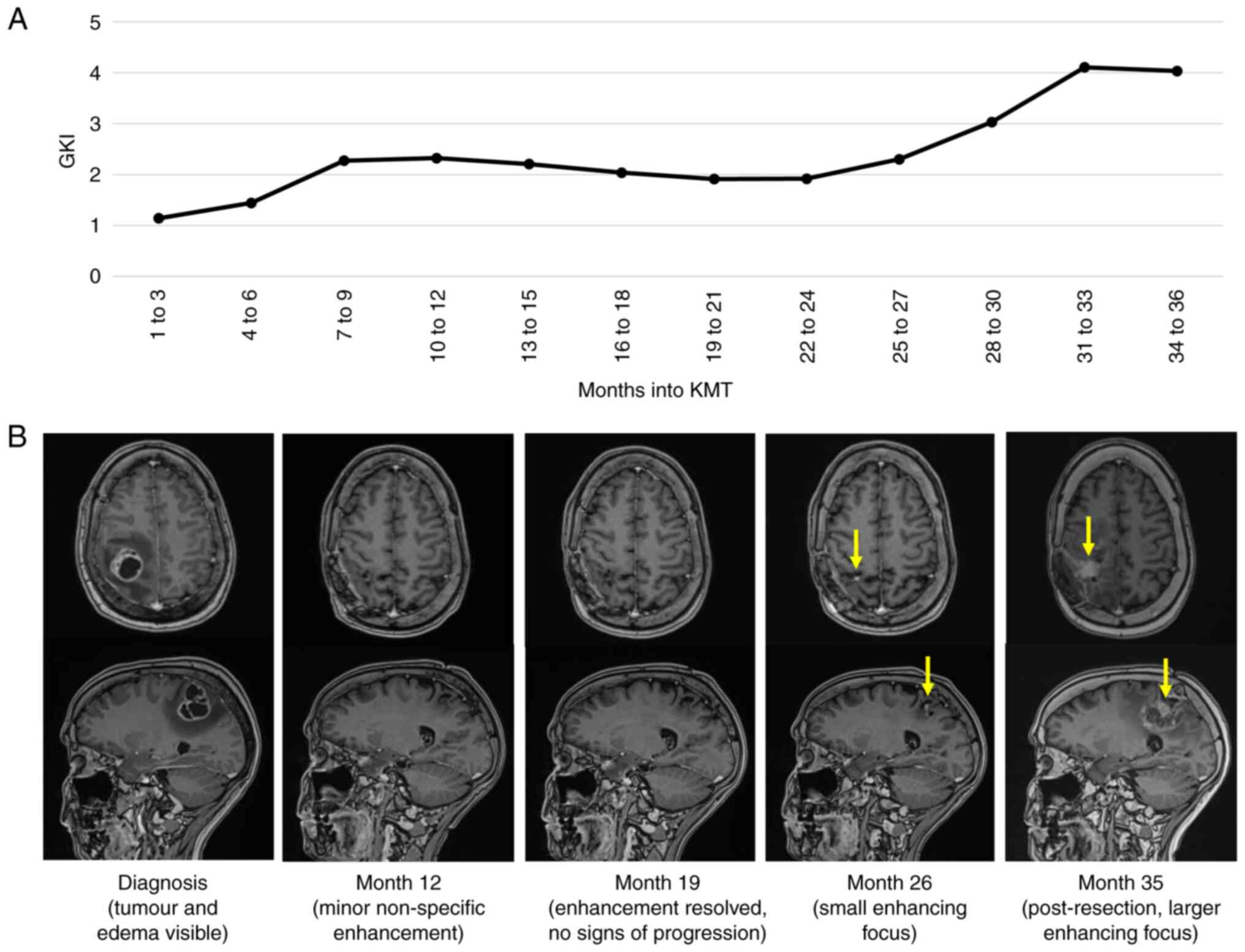
calculate her mean GKI, which we plotted alongside the tumour
features on imaging (Fig. 3). She
commenced her chemoradiation therapy approximately 6 weeks after
symptom onset (4 weeks after surgery).
During the first and second treatment years, our
patient maintained a low GKI, clinically improved, and there were
no signs of cancer progression on imaging. In the first year, her
average weekly blood glucose level was 4.65 ± 0.38 mmol/l and her
blood ketone level was 2.82 ± 1.43 mmol/l, resulting in a GKI of
1.65 (ranging from 0.52 to 5.97 during any week). In the second
year, her average blood glucose level was 4.68 ± 0.19 mmol/l and
her blood ketone level was 2.32 ± 0.67 mmol/l, resulting in a GKI
of 2.02 (range, 1.16 to 5.38). Clinically, her mild left lower limb
weakness resolved, leading to an ECOG score of 0 by month 6. Her
weight decreased to 56.4 kg (27% reduction from baseline),
resulting in a normal body-mass index of 20.5 kg/m2 by
month 6. Serial MRIs revealed no visible tumour from month 4
onwards. The ECOG, BMI, and MRI measurements remained stable until
the end of the second treatment year, without any clinical or
radiological evidence of cancer progression.
Entering the third treatment year, our patient
experienced the death of an immediate family member, which led to
several weeks of dramatically increased life stress and poor sleep
followed by a slight relaxation of KMT, clinical decline, and
radiological features concerning for cancer progression. Her
average weekly blood glucose level was 5.24 ± 0.62 mmol/l and her
blood ketone level was 1.64 ± 0.65 mmol/l throughout the year,
resulting in a GKI of 3.20 (range, 1.14 to 17.20). Although she
remained clinically stable, MRI at the end of month 26 showed a
small new enhancing focus in the resection bed considered to
represent possible cancer progression. Monitoring with serial MRIs
demonstrated a significant change of this enhancing focus, which
increased in size from 5 mm to 20 mm over 8 weeks. This prompted a
repeat resection in month 29. Biopsy evaluation revealed areas of
pleomorphic cells, necrosis, and hyalinized blood vessels
consistent with previous radiation therapy, with immunochemistry
showing an IDH-wildtype, unmethylated GBM (average CpG site
methylation rate of 2.5%). Following surgery, our patient retained
3/5 power in the left upper limb and 1/5 power in the left lower
limb, neither of which recovered in the following weeks, leading to
a persisting ECOG score of 2 and the gradual emergence of
depressive symptoms. Post-operative MRI demonstrated several small
enhancing foci bordering the surgical cavity, the largest of which
measured 10 mm, consistent with residual tumour. She was commenced
on dexamethasone 4 mg daily and underwent a further 2 weeks of
radiation therapy (35 Gy in 10 fractions). Despite this regimen,
MRI in month 33 showed an increase in the size of the enhancing
mass within the primary resection cavity with associated mass
effect. Due to the ongoing weakness and imaging findings,
dexamethasone was increased to 8 mg daily and remained at a
variable dose of 4 to 16 mg daily from months 30 to 36, during
which the GKI increased to 4.07. Weight increased during this time
to 68.0 kg, resulting in a body-mass index of 24.7 kg/m2
by month 36. MRI in month 35 showed an increase in size and avidity
of the enhancing mass, a new small enhancing nodule involving the
contralateral splenium, and progressive non-enhancing white matter
changes. These findings were considered to represent tumour
progression. Second-line systemic options such as bevacizumab
combined with irinotecan chemotherapy were discussed with our
patient, but she declined due to financial constraints and opted
for best supportive care.
There were several adverse effects during treatment.
Adverse effects attributed to chemoradiation by our patient and her
oncologists included mild fatigue, headache, and alopecia. Adverse
effects attributed to adjuvant temozolomide included nausea and a
low platelet count of 45,000 µl after cycle 3, which resulted in a
12-week break from chemotherapy and a dose reduction to 200 mg
temozolomide for the last three cycles, followed by resolution of
the nausea and low platelet count. Adverse effects attributed to
the prolonged fasts included mild fatigue, diarrhoea, and cold
intolerance. No significant adverse effects were attributed to the
TRKD, although our patient and her family found it to be a
significant commitment. Positive effects attributed to KMT included
reduced chronic neck, back, and bilateral shoulder pain. Our
patient also experienced a general feeling of well-being until she
lost her functional independence following the second surgical
resection. Moreover, she found KMT to be inexpensive compared with
further systemic options, which were cost-prohibitive.
Coming into the fourth year, in the setting of her
ongoing post-surgical weakness and subsequent depression, our
patient opted to significantly relax her KMT adherence, which
subsequently led to a rapid and progressive clinical decline. She
passed away 6 weeks later, during month 38.
Discussion
This case study is novel in that a patient with
IDH-wildtype GBM pursued the standard of care (surgery,
chemoradiation, adjuvant temozolomide) in conjunction with an
intensive, long-term, multimodal KMT program (prolonged fasting,
time-restricted feeding, ketogenic diet), which was specifically
timed to maximize the tolerability and efficacy of the standard
treatments. By the end of the second treatment year, she achieved a
complete clinical improvement, a stable body-mass index in the
healthy range, and a high quality of life, with no signs of cancer
progression on imaging. During the third year, following a period
of dramatically increased life stress and a slight relaxation of
KMT adherence, slow cancer progression occurred. Although the KMT
program required a significant commitment, adverse effects were
mild.
Given their differing mechanisms, integrating the
standard treatments with KMT can lead to synergistic therapeutic
effects in cancer (23). Broadly
speaking, surgery, radiation, and chemotherapy are designed to
target and eliminate cancer cells by directly removing tumour bulk
and damaging DNA to induce cytotoxicity (27). Rather than directly eliminating
cancer cells, KMT is designed to reinforce the resistance of normal
cells to radiation and chemotherapy by depriving them of nutrients,
which diverts energy and resources into maintenance and repair
processes (28). By contrast, since
cancer cells cannot slow their growth due to the uncontrolled
activation of growth pathways and mutations in tumour suppressor
genes (29), KMT may enhance the
sensitivity of these cells to the standard treatments by
restricting their access to glucose and growth factors (28). We attempted to maximize the
tolerability and efficacy of the standard GBM treatments by
administering KMT in a ‘press-pulse’ manner, which is designed to
induce a chronic, low-grade stress on cancer cell metabolism (the
‘press’) that is capitalized upon by a more acute, high-grade
stress (the ‘pulse’) (30). In our
patient, the press consisted of the TRKD, whereas the pulse was
comprised of the prolonged fasts, which were additionally timed to
maximize differential stress resistance and sensitization to the
standard treatments (23). The
administration of press-pulse KMT may have contributed to the
long-term survival experienced by our patient.
Evidence from interventional studies in animals and
humans indicates that lower GKIs lead to more effective growth
suppression in brain tumours. In animal models of astrocytoma and
glioma, a GKI <6 in combination with radiation or chemotherapy
leads to lower brain tumour volumes and increased survival
(22). Although the human evidence
is not as robust, several case reports have indicated that
intensive KMT may suppress cancer growth in grade 4 astrocytoma and
GBM, whereas a loosening of KMT can facilitate progression
(31–33). Similarly, our patient's KMT
adherence and GKI status broadly correlated with the behaviour of
her tumour. During the first and second treatment years, she
received the standard treatment protocol while sustaining a GKI of
1.65 and 2.02, respectively, which coincided with clinical
improvement and no visible tumour on imaging. Entering the third
treatment year, she slightly relaxed her adherence and sustained a
GKI of 3.20, which coincided with slow radiological progression of
the tumour. As this is a case study, we cannot draw conclusions
regarding the initial focus of cancer progression. However, one
possibility is that tight adherence to the TRKD in the second year
helped suppress the growth of the tumour, whereas the relaxed
adherence in the third year contributed to (slow) progression. The
weekly GKI ranged as high as 17.20 during the third year, which
resulted from a combination of hyperglycaemia and hypoketonemia.
Importantly, this value was far higher than the maximal GKI
measured in either of the previous treatment years, which indicates
that sustaining a consistently low GKI, with minimal variability,
may be important. Alternatively, it is possible that the slight
relaxation in adherence was not clinically relevant and that the
TRKD ‘press’ on its own was unable to halt tumour growth. Given the
latter possibility, long-term GBM suppression in some patients
might require additional ‘pulses’ of prolonged fasting alongside
further radiation, chemotherapy, or metabolic drugs such as
glutamine blockers (11).
Regardless of mechanism, since it has been suggested that
dexamethasone compromises survival in GBM (34), which occurs in part through elevated
blood glucose levels (35), the
administration of high daily doses of this drug may have blunted
the potential therapeutic efficacy of the KMT program during the
latter half of the third year.
During the first and second treatment years, our
patient experienced substantial weight loss (27% of her initial
body weight). This technically represents a CTCAE grade 3 event.
However, it is crucial to recognize that she was initially
overweight, her weight loss was intentional, and her body-mass
index stabilized within a healthy range. While unintentional weight
loss can precipitate sarcopenia and cachexia in patients with
advanced cancer, intentional weight loss is associated with a lower
risk of many types of cancer (36,37).
Moreover, when correctly executed, fasting and ketogenic diet
protocols can comfortably meet a patient's nutritional needs while
exerting a sparing effect on lean mass (38), with some studies indicating that
ketogenic diets induce weight gain in cancer patients with cachexia
(39). Furthermore, several case
reports involving KMT have shown that significant intentional
weight loss (up to 28% of initial body weight) can be associated
with positive outcomes in patients with GBM and other advanced
cancers (31,32,40,41).
We cannot draw firm conclusions with respect to the
mechanism of KMT in our patient, or its impact on her long-term
survival. With respect to mechanism, a potential weakness of our
KMT program is that it did not specifically target glutamine, which
may also be utilized as a fermentable fuel in GBM (11). Since prolonged fasting reduces the
activity of glutaminase, which catalyses the conversion of
glutamine to glutamate as an energy source for the TCA cycle
(42), the prolonged fasts may have
non-specifically lowered glutamine availability; however, the
addition of a glutaminase inhibitor, such as
6-diazo-5-oxo-L-norleucine (DON), would probably be more effective
(43). Beyond DON, it is also
possible that our patient's KMT could have been augmented by
incorporating drugs that can alter metabolism and have been
associated with improved cancer outcomes, such as metformin, which
can suppress gluconeogenesis (44),
and atorvastatin, which may enhance ketogenesis (45,46).
With respect to the potential impact of KMT on survival, the
long-term survivor status experienced by our patient is in keeping
with preliminary findings from other studies that have investigated
KMT in glioma patients (47).
However, it is important to point out that our patient displayed
several positive prognostic features such as her gender, complete
resection, and borderline methylation status (48), all of which may have contributed to
her long-term survival.
In conclusion, this case study is novel in that a
patient with IDH-wildtype GBM pursued the standard of care in
conjunction with an intensive, long-term, multimodal KMT program,
which was specifically timed to maximize the tolerability and
efficacy of the standard treatments. By the end of the second
treatment year, she achieved complete clinical improvement, a
healthy body-mass index, and a high quality of life, with no
visible progressive tumour detected on imaging. In the setting of
dramatically increased life stress and slightly relaxed KMT
adherence, slow cancer progression occurred during the third year.
Adverse effects attributed to KMT were mild. Despite the
limitations of this case study, it highlights the feasibility of
implementing the standard treatment protocol in conjunction with an
intensive, long-term, multimodal, and specifically timed KMT
program, the potential therapeutic efficacy of which may depend
upon achieving as low a GKI as possible.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MCLP contributed to the conceptualization, design,
analysis, and writing of both the original and subsequent drafts.
PHD, ZT, FZ and BJM contributed to data collection, and reviewed
and edited the manuscript. MCLP and BJM confirm the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent for
participation
Ethics approval was not required for this study in
accordance with our local and institutional requirements. The study
was conducted in accordance with local legislation and
institutional requirements. The participant provided written
informed consent to participate in this study.
Patient consent for publication
Written informed consent for publication of this
article was obtained from the participant.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ostrom QT, Price M, Neff C, Cioffi G,
Waite KA, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical
report: Primary brain and other central nervous system tumors
diagnosed in the United States in 2015–2019. Neuro Oncol. 24 (Suppl
5):v1–v95. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wen PY, Weller M, Lee EQ, Alexander BM,
Barnholtz-Sloan JS, Barthel FP, Batchelor TT, Bindra RS, Chang SM,
Chiocca EA, et al: Glioblastoma in adults: A society for
neuro-oncology (SNO) and european society of neuro-oncology (EANO)
consensus review on current management and future directions. Neuro
Oncol. 22:1073–1113. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Delgado-López PD and Corrales-García EM:
Survival in glioblastoma: A review on the impact of treatment
modalities. Clin Transl Oncol. 18:1062–1071. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krex D, Klink B, Hartmann C, von Deimling
A, Pietsch T, Simon M, Sabel M, Steinbach JP, Heese O, Reifenberger
G, et al: Long-term survival with glioblastoma multiforme. Brain.
130:2596–2606. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scott JN, Rewcastle NB, Brasher PM, Fulton
D, MacKinnon JA, Hamilton M, Cairncross JG and Forsyth P: Which
glioblastoma multiforme patient will become a long-term survivor? A
population-based study. Ann Neurol. 46:183–188. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carew JS and Huang P: Mitochondrial
defects in cancer. Mol Cancer. 1:92002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seyfried TN and Mukherjee P: Targeting
energy metabolism in brain cancer: Review and hypothesis. Nutr
Metab. 2:302005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chinopoulos C and Seyfried TN:
Mitochondrial substrate-level phosphorylation as energy source for
glioblastoma: Review and hypothesis. ASN Neuro.
10:17590914188182612018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Cabo R and Mattson MP: Effects of
intermittent fasting on health, aging, and disease. N Engl J Med.
381:2541–2551. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nencioni A, Caffa I, Cortellino S and
Longo VD: Fasting and cancer: Molecular mechanisms and clinical
application. Nat Rev Cancer. 18:707–719. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seyfried TN, Arismendi-Morillo G,
Mukherjee P and Chinopoulos C: On the origin of ATP synthesis in
cancer. iScience. 23:1017612020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oudard S, Boitier E, Miccoli L, Rousset S,
Dutrillaux B and Poupon MF: Gliomas are driven by glycolysis:
Putative roles of hexokinase, oxidative phosphorylation and
mitochondrial ultrastructure. Anticancer Res. 17:1903–1911.
1997.PubMed/NCBI
|
|
16
|
Arismendi-Morillo GJ and
Castellano-Ramirez AV: Ultrastructural mitochondrial pathology in
human astrocytic tumors: Potentials implications pro-therapeutics
strategies. J Electron Microsc. 57:33–39. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liang BC: Evidence for association of
mitochondrial DNA sequence amplification and nuclear localization
in human low-grade gliomas. Mutat Res. 354:27–33. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liang BC and Hays L: Mitochondrial DNA
copy number changes in human gliomas. Cancer Lett. 105:167–173.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feichtinger RG, Weis S, Mayr JA,
Zimmermann F, Geilberger R, Sperl W and Kofler B: Alterations of
oxidative phosphorylation complexes in astrocytomas. Glia.
62:514–525. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deighton RF, Le Bihan T, Martin SF, Gerth
AMJ, McCulloch M, Edgar JM, Kerr LE, Whittle IR and McCulloch J:
Interactions among mitochondrial proteins altered in glioblastoma.
J Neurooncol. 118:247–256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Winter SF, Loebel F and Dietrich J: Role
of ketogenic metabolic therapy in malignant glioma: A systematic
review. Crit Rev Oncol Hematol. 112:41–58. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meidenbauer JJ, Mukherjee P and Seyfried
TN: The glucose ketone index calculator: A simple tool to monitor
therapeutic efficacy for metabolic management of brain cancer. Nutr
Metab. 12:122015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
de Groot S, Pijl H, van der Hoeven JJM and
Kroep JR: Effects of short-term fasting on cancer treatment. J Exp
Clin Cancer Res. 38:2092019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miller VJ, Villamena FA and Volek JS:
Nutritional ketosis and mitohormesis: Potential implications for
mitochondrial function and human health. J Nutr Metab.
2018:51576452018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dal Bello S, Valdemarin F, Martinuzzi D,
Filippi F, Gigli GL and Valente M: Ketogenic diet in the treatment
of gliomas and glioblastomas. Nutrients. 14:38512022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Phillips MCL, Leyden J, McManus EJ, Lowyim
DG, Ziad F, Moon BG, Haji Mohd Yasin NAB, Tan A, Thotathil Z and
Jameson MB: Feasibility and safety of a combined metabolic strategy
in glioblastoma multiforme: A prospective case series. J Oncol.
2022:44967342022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu W, Klockow JL, Zhang M, Lafortune F,
Chang E, Jin L, Wu Y and Daldrup-Link HE: Glioblastoma multiforme
(GBM): An overview of current t9erapies and mechanisms of
resistance. Pharmacol Res. 171:1057802021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Raffaghello L, Lee C, Safdie FM, Wei M,
Madia F, Bianchi G and Longo VD: Starvation-dependent differential
stress resistance protects normal but not cancer cells against
high-dose chemotherapy. Proc Natl Acad Sci USA. 105:8215–8220.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Seyfried TN, Yu G, Maroon JC and
D'Agostino DP: Press-pulse: A novel therapeutic strategy for the
metabolic management of cancer. Nutr Metab. 14:192017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zuccoli G, Marcello N, Pisanello A,
Servadei F, Vaccaro S, Mukherjee P and Seyfried TN: Metabolic
management of glioblastoma multiforme using standard therapy
together with a restricted ketogenic diet: Case report. Nutr Metab.
7:332010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Elsakka AMA, Bary MA, Abdelzaher E,
Elnaggar M, Kalamian M, Mukherjee P and Seyfried TN: Management of
glioblastoma multiforme in a patient treated with ketogenic
metabolic therapy and modified standard of care: A 24-month
follow-up. Front Nutr. 5:202018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Seyfried TN, Shivane AG, Kalamian M,
Maroon JC, Mukherjee P and Zuccoli G: Ketogenic metabolic therapy,
without chemo or radiation, for the long-term management of
IDH1-mutant glioblastoma: An 80-month follow-up case report. Front
Nutr. 8:6822432021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pitter KL, Tamagno I, Alikhanyan K,
Hosni-Ahmed A, Pattwell SS, Donnola S, Dai C, Ozawa T, Chang M,
Chan TA, et al: Corticosteroids compromise survival in
glioblastoma. Brain. 139:1458–1471. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Klement RJ and Champ CE: Corticosteroids
compromise survival in glioblastoma in part through their elevation
of blood glucose levels. Brain. 140:e162017.PubMed/NCBI
|
|
36
|
Luo J, Hendryx M, Manson JE, Figueiredo
JC, LeBlanc ES, Barrington W, Rohan TE, Howard BV, Reding K, Ho GY,
et al: Intentional weight loss and obesity-related cancer risk.
JNCI Cancer Spectr. 3:kz0542019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
MacKintosh ML, Derbyshire AE, McVey RJ,
Bolton J, Nickkho-Amiry M, Higgins CL, Kamieniorz M, Pemberton PW,
Kirmani BH, Ahmed B, et al: The impact of obesity and bariatric
surgery on circulating and tissue biomarkers of endometrial cancer
risk. Int J Cancer. 144:641–650. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Phillips MCL: Fasting as a therapy in
neurological disease. Nutrients. 11:25012019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Weber DD, Aminzadeh-Gohari S, Tulipan J,
Catalano L, Feichtinger RG and Kofler B: Ketogenic diet in the
treatment of cancer-where do we stand? Mol Metab. 33:102–121. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Goldhamer AC, Klaper M, Foorohar A and
Myers TR: Water-only fasting and an exclusively plant foods diet in
the management of stage IIIa, low-grade follicular lymphoma. BMJ
Case Rep. 2015:bcr20152115822015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Phillips MCL, Murtagh DKJ, Sinha SK and
Moon BG: Managing metastatic thymoma with metabolic and medical
therapy: A case report. Front Oncol. 10:5782020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cruzat V, Macedo Rogero M, Noel Keane K,
Curi R and Newsholme P: Glutamine: Metabolism and immune function,
supplementation and clinical translation. Nutrients. 10:15642018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mukherjee P, Augur ZM, Li M, Hill C,
Greenwood B, Domin MA, Kondakci G, Narain NR, Kiebish MA, Bronson
RT, et al: Therapeutic benefit of combining calorie-restricted
ketogenic diet and glutamine targeting in late-stage experimental
glioblastoma. Commun Biol. 2:2002019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Takhwifa F, Aninditha T, Setiawan H and
Sauriasari R: The potential of metformin as an antineoplastic in
brain tumors: A systematic review. Heliyon. 7:e065582021.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jiang W, Hu J and He X, Jin W and He X:
Statins: A repurposed drug to fight cancer. J Exp Clin Cancer Res.
40:2412021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Baul PB, Deepak AD, Kakkar M and Modi S:
Effect of atorvastatin on blood ketone levels and glycemic control
in patients with type 2 diabetes mellitus: A single arm pilot
study. Diabetes Metab Syndr. 14:1333–1337. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Smith KA, Hendricks BK, DiDomenico JD,
Conway BN, Smith TL, Azadi A and Fonkem E: Ketogenic metabolic
therapy for glioma. Cureus. 14:e264572022.PubMed/NCBI
|
|
48
|
Brown NF, Ottaviani D, Tazare J, Gregson
J, Kitchen N, Brandner S, Fersht N and Mulholland P: Survival
outcomes and prognostic factors in glioblastoma. Cancers.
14:31612022. View Article : Google Scholar : PubMed/NCBI
|