Introduction
Gastric cancer (GC) is one of the most prevalent
cancers, and it still accounts for over one million new cases
worldwide (1). Several new
treatments, including immune checkpoint inhibitors, have improved
the survival outcomes of GC (2–4).
However, the prognosis of patients with advanced GC, especially
stage IV, remains dismal, and the underlying molecular mechanism of
its progression should be uncovered for treating these
patients.
Deregulation of the Hippo pathway has been reported
in different cancer types, and the role of the Hippo signaling
pathway is attracting attention in terms of cancer progression
(5). The Hippo pathway is an
evolutionally conserved regulator of tissue growth and comprises a
kinase cassette (MST and LATS). LATS phosphorylates Yes-associated
protein (YAP), which is the main effector in this pathway.
Unphosphorylated YAP enters the nucleus and promotes tissue growth
and cell viability by regulating the activity of different
transcription factors. One of the targeted genes of this
transcription regulation is connective tissue growth factor (CTGF)
(6).
CTGF is a secretory protein that belongs to the CCN
family, consisting of six members: CTGF, nephroblastoma
overexpressed (NOV), cysteine-rich angiogenic protein 61 (CYR61),
WNT1-inducible signaling pathway protein 1 (WISP1), and WISP2. CCN
proteins are biologically active when binding and/or activating
cell surface integrins, and they are related to cell proliferation,
migration, adhesion, and extracellular matrix formation in tumor
tissues (7). CTGF in breast cancer
improves the motility of cancer cells via an
integrin-αvβ3-ERK1/2-dependent S100A4-upregulated pathway (8). Conversely, CTGF is a favorable
prognostic factor in GC because it inhibits peritoneal metastasis
by blocking integrinα3β1 dependent adhesion (9). These data indicate the variable
functions of CTGF in different cancer types.
This report focused on the prognostic significance
of CTGF in GC. GC has been histologically classified into
intestinal and diffuse types by Lauren (10), and diffuse-type GC usually has a lot
of stromal components in the tumor tissue (11–13).
Generally, the interaction between cancer and stromal cells is
crucial for the progression of diffuse-type GC (14,15).
However, Chen et al (9) did
not pay attention to the difference in histological type.
Furthermore, the differential role of CTGF expression in cancer and
stromal cells remains unknown. Thus, this study aimed to clarify
the clinical significance of CTGF expression in cancer and stromal
cells in patients with GC, depending on histological type, using
the Lauren classification.
Patients and methods
Patients
A total of 589 patients who underwent resection of
primary GC from January 2000 to December 2006 at Department of
Gastroenterological Surgery, Osaka City University (currently,
Osaka Metropolitan University; Osaka, Japan) were retrospectively
reviewed. The age range of the recruited patients was 21–88 years
old, and median age was 67. We generated tissue microarrays (TMA)
from these patients and used them for immunohistochemical (IHC)
staining. Each TMA core was selected at the invasion front of the
cancer. The pathologic diagnoses and classifications were made
according to the Japanese Classification of Gastric Carcinoma,
fifteenth edition. The study protocol conformed to the ethical
guidelines of the Declaration of Helsinki. The Osaka Metropolitan
University ethics committee approved this study in 2022 [approval
number (approval date): 2022-077 (2022/08/10)]. Informed consent
was obtained in the form of opt-out.
IHC determination of the CTGF
IHC staining was performed using 589 GC samples.
Slides were deparaffinized and then heated for 10 min at 105°C in
an autoclave in Target Retrieval Solution (Dako, Carpinteria, CA).
After blocking endogenous peroxidase activity using 3% hydrogen
peroxide, the specimens were incubated with CTGF antibody (1:200;
Life Technologies) for 1 h at room temperature and were incubated
with biotinylated goat antirabbit IgG for 10 min. The slides were
treated with streptavidin–peroxidase reagent, followed by
counterstaining with Mayer's hematoxylin. Slides were scanned using
a Leica Aperio CS2 scanner (Leica Biosystems), and subsequent
expression evaluations at each core were performed using the
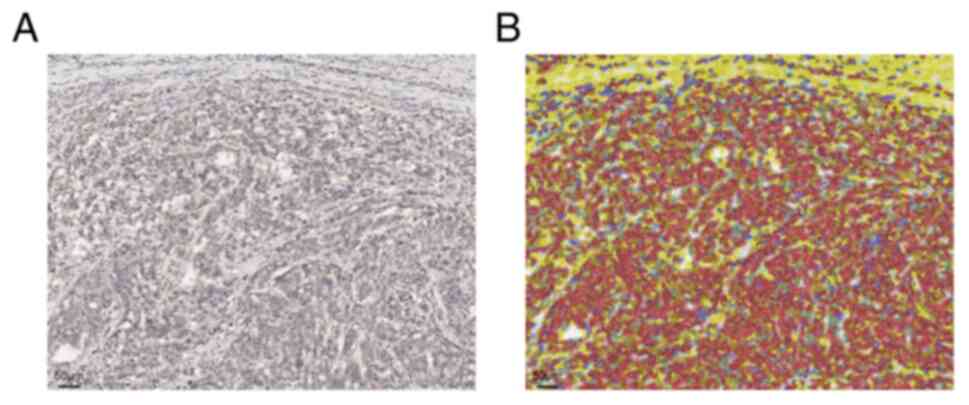
open-source software QuPath (http://qupathe.github.io). Cell detection and IHC
quantification were performed using 30 cores (Fig. 1). Furthermore, an objective
classifier was trained to classify individual cells as stromal or
epithelial cells. After these trainings, CTGF expression in all TMA
cores was assessed in every tumor and stromal cell. The percentage
of positive cells among total tumor and stromal cells was
automatically calculated using the threshold value of 0.15. We
diagnosed a case as CTGF positive when the positive percentage of
tumor and stromal cells was more than the first quartile of all
analyzed cases.
TCGA data
CTGF mRNA expression data and corresponding clinical
information of GC samples were collected from The Cancer Genome
Atlas (TCGA) database (http://tcga-data.nci.nih.gov/tcga/). We used data from
the ‘tcga_pan_cancer_atlas_2018’ study. The third quartile of the
mRNA expression level was defined as the cut-off, and we
categorized patients into high- and low-expression groups.
Statistical analysis
Associations between CTGF expression and
clinicopathological results were analyzed using the chi-square
test. Overall survival (OS) was the time from surgery to death from
any cause. The Kaplan-Meier method and the log-rank test were used
to estimate and compare the OS, respectively. Disease-free survival
was the time from surgery to recurrence or death from any cause.
Multivariate analysis was performed using the Cox proportional
hazards model. Statistical Package for the Social Sciences
statistical software (version 29.0; IBM) was used for all
statistical analyses. Two-sided P<0.05 was considered to
indicate a statistically significant difference.
Results
Association between CTGF expression
and clinicopathological factors in GC
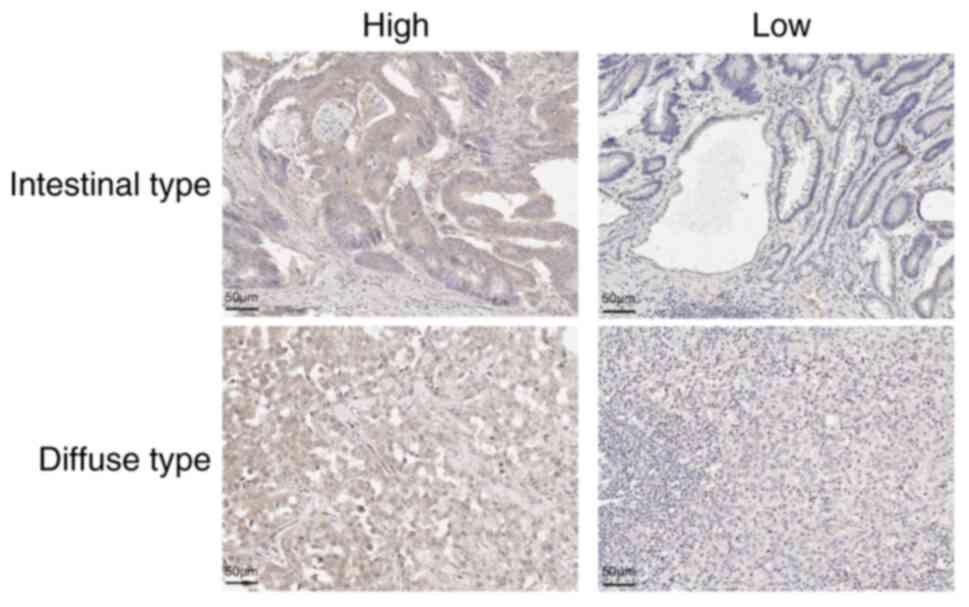
CTGF expression was mainly observed in the cytoplasm
of cancer and stromal cells (Fig.
1). We investigated the CTGF expressions of cancer and stromal
cells in 589 GC tissues using software (Fig. 2). Of 589 patients, 444 and 442 had
CTGF-positive cancer and stromal cells, respectively. Table I shows the association between CTGF
expression and clinicopathological factors. CTGF expression in
stromal cells was significantly associated with CTGF expression in
cancer cells (P<0.001). CTGF positivity in cancer cells was
significantly associated with sex (female), tumor depth (T1-2),
lymph node metastasis (N0-1), and tumor size (<3 cm). CTGF
positivity in stromal cells was significantly associated with
intestinal type, non-scirrhous type, tumor depth (T1-2), lymph node
metastasis (N0-1), lymphatic invasion (negative), and tumor size
(<3 cm).
 | Table I.Association between CTGF expression
and clinicopathologic factors in 589 patients with gastric
cancer. |
Table I.
Association between CTGF expression
and clinicopathologic factors in 589 patients with gastric
cancer.
|
| CTGF expression in
tumor cells |
| CTGF expression in
stromal cells |
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
factors | Low (n=145) | High (n=444) | P-value | Low (n=147) | High (n=442) | P-value |
|---|
| Age, years | | | 0.414 | | | 0.121 |
|
<70 | 94 (25.8%) | 271 (74.2%) |
| 99 (27.1%) | 266 (72.9%) |
|
| ≥70 | 51 (22.8%) | 173 (77.2%) |
| 48 (21.4%) | 176 (78.6%) |
|
| Sex | | | 0.034 | | | 0.058 |
|
Female | 53 (20.4%) | 207 (79.6%) |
| 55 (21.2%) | 205 (78.8%) |
|
| Male | 92 (28.0%) | 237 (72.0%) |
| 92 (28.0%) | 237 (72.0%) |
|
| Macroscopic type | | | 0.154 | | | 0.003 |
| 0-3 | 126 (23.8%) | 404 (76.2%) |
| 123 (23.2%) | 407 (76.8%) |
|
| 4
(scirrhous type) | 19 (32.2%) | 40 (67.8%) |
| 24 (40.7%) | 35 (59.3%) |
|
| Histologic type | | | 0.119 | | | 0.034 |
|
Intestinal | 63 (21.8%) | 226 (78.2%) |
| 61 (21.1%) | 228 (78.9%) |
|
|
Diffuse | 82 (27.3%) | 218 (72.7%) |
| 86 (28.7%) | 214 (71.3%) |
|
| Tumor depth | | | 0.013 | | | <0.001 |
| T1-2 | 71 (20.9%) | 269 (79.1%) |
| 65 (19.1%) | 275 (80.9%) |
|
| T3-4 | 74 (30.7%) | 175 (69.3%) |
| 82 (32.9%) | 167 (67.1%) |
|
| Lymph node
metastasis | | | 0.047 | | | 0.008 |
| N0-1 | 88 (22.2%) | 309 (77.8%) |
| 86 (21.7%) | 311 (78.3%) |
|
| N2-3 | 57 (29.7%) | 135 (70.3%) |
| 61 (31.8%) | 131 (68.2%) |
|
| Lymphatic
invasiona | | | 0.071 | | | 0.014 |
|
Negative | 55 (21.1%) | 206 (78.9%) |
| 52 (19.9%) | 209 (80.1%) |
|
|
Positive | 90 (27.5%) | 237 (72.5%) |
| 94 (28.7%) | 233 (71.3%) |
|
| Venous invasion | | | 0.054 | | | 0.282 |
|
Negative | 112 (23.0%) | 374 (77.0%) |
| 117 (24.1%) | 369 (75.9%) |
|
|
Positive | 33 (32.0%) | 70 (68.0%) |
| 30 (29.1%) | 73 (70.9%) |
|
| Tumor
sizea | | | 0.001 | | | 0.002 |
| <3
cm | 38 (17.4%) | 181 (82.6%) |
| 39 (17.8%) | 180 (82.2%) |
|
| ≥3
cm | 107 (29.2%) | 260 (70.8%) |
| 107 (29.2%) | 260 (70.8%) |
|
| CTGF expression in
stromal cells | | | <0.001 | | | |
|
Negative | 79 (53.7%) | 68 (46.6%) |
|
|
|
|
|
Positive | 66 (14.9%) | 376 (85.1%) |
|
|
|
|
Multivariate analysis
Table II shows the
univariate and multivariate analyses using the proportional hazards
model. Univariate analysis revealed that the OS of patients was
significantly associated with CTGF expression in stromal cells.
Multivariate logistic regression analysis revealed that CTGF
expression in stromal cells as well as age, tumor depth (T3-4),
lymph node metastasis (N2–3), and tumor size (≥3 cm) were
independent predictive parameters for OS.
 | Table II.Univariate and multivariate analyses
with respect to overall survival after surgery in 597 patients with
gastric cancer. |
Table II.
Univariate and multivariate analyses
with respect to overall survival after surgery in 597 patients with
gastric cancer.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | Odds ratio (95%
CI) | P-value | Odds ratio (95%
CI) | P-value |
|---|
| CTGF in tumor cells
(High) | 0.93
(0.65–1.32) | 0.68 | 1.36
(0.91–2.02) | 0.125 |
| CTGF in stromal
cells (High) | 0.63
(0.45–0.87) | 0.006 | 0.68
(0.47–0.99) | 0.048 |
| Age (≥70
years) | 1.71
(1.25–2.34) | <0.001 | 1.76
(1.27–2.44) | <0.001 |
| Sex (Male) | 1.26
(0.91–1.75) | 0.15 | 0.98
(0.70–1.36) | 0.75 |
| Lymph node
metastasis (≥N2) | 5.72
(4.11–7.97) | <0.001 | 3.36
(1.87–6.02) | <0.001 |
| Distant metastasis
(Positive) | 6.06
(3.49–10.5) | <0.001 | 3.59
(2.49–5.17) | <0.001 |
| Tumor size (≥3
cm) | 5.68
(3.47–9.29) | <0.001 | 2.79
(1.63–4.78) | <0.001 |
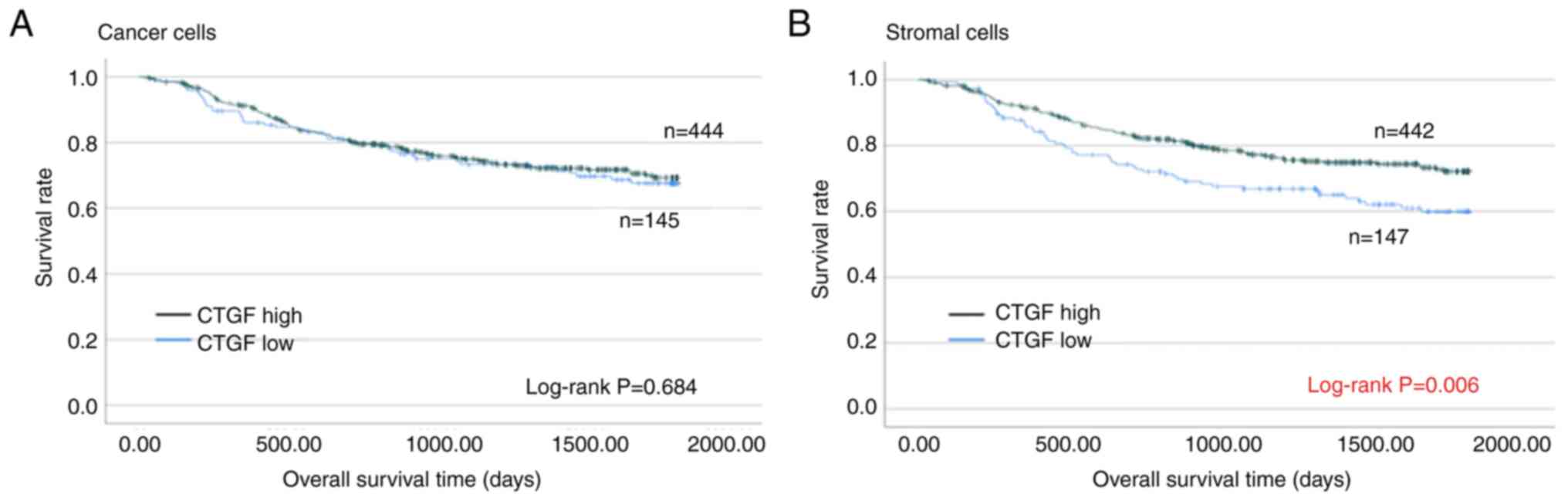
Survival analysis of the
subgroups
Fig. 3 shows the OS
curves of patients by CTGF expression using the Kaplan-Meier
method. The 5-year OS rates of patients with high and low CTGF
expression in cancer cells were 69.3 and 67.6%, respectively.
Regarding the analyses of cancer cells, OS outcomes between
patients with high and low CTGF expression were not statistically
significant (log-rank; P=0.684). Conversely, the analyses of
stromal cells revealed that patients with low CTGF expression
demonstrated significantly worse OS than those with high CTGF
expression (log-rank; P=0.006). The 5-year OS rates of patients
with high and low CTGF expression in stromal cells were 72.1 and
59.8%, respectively.
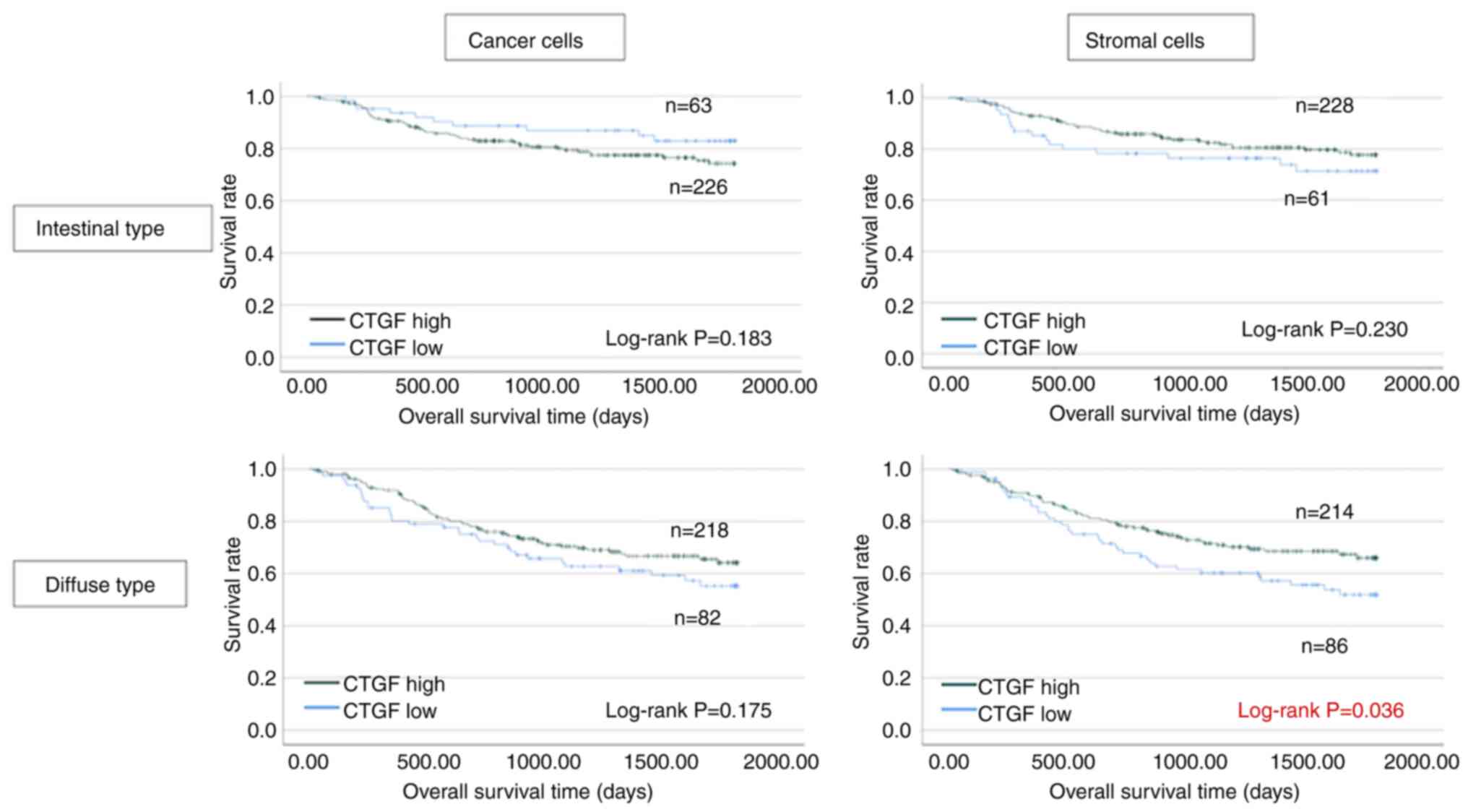
Fig. 4 shows the
analyses for each histological type. The OS rates in terms of the
intestinal case were not significantly different depending on CTGF
expression in cancer and stromal cells (P=0.183 and P=0.230,
respectively). In contrast, among the cases with diffuse type, the
OS of patients with low CTGF expression in stromal cells was
significantly worse than that of patients with high CTGF expression
(P=0.036). The cancer cell analysis in the diffuse type revealed no
statistically significant difference, but CTGF-negative cases
demonstrated a worse prognosis (P=0.175).
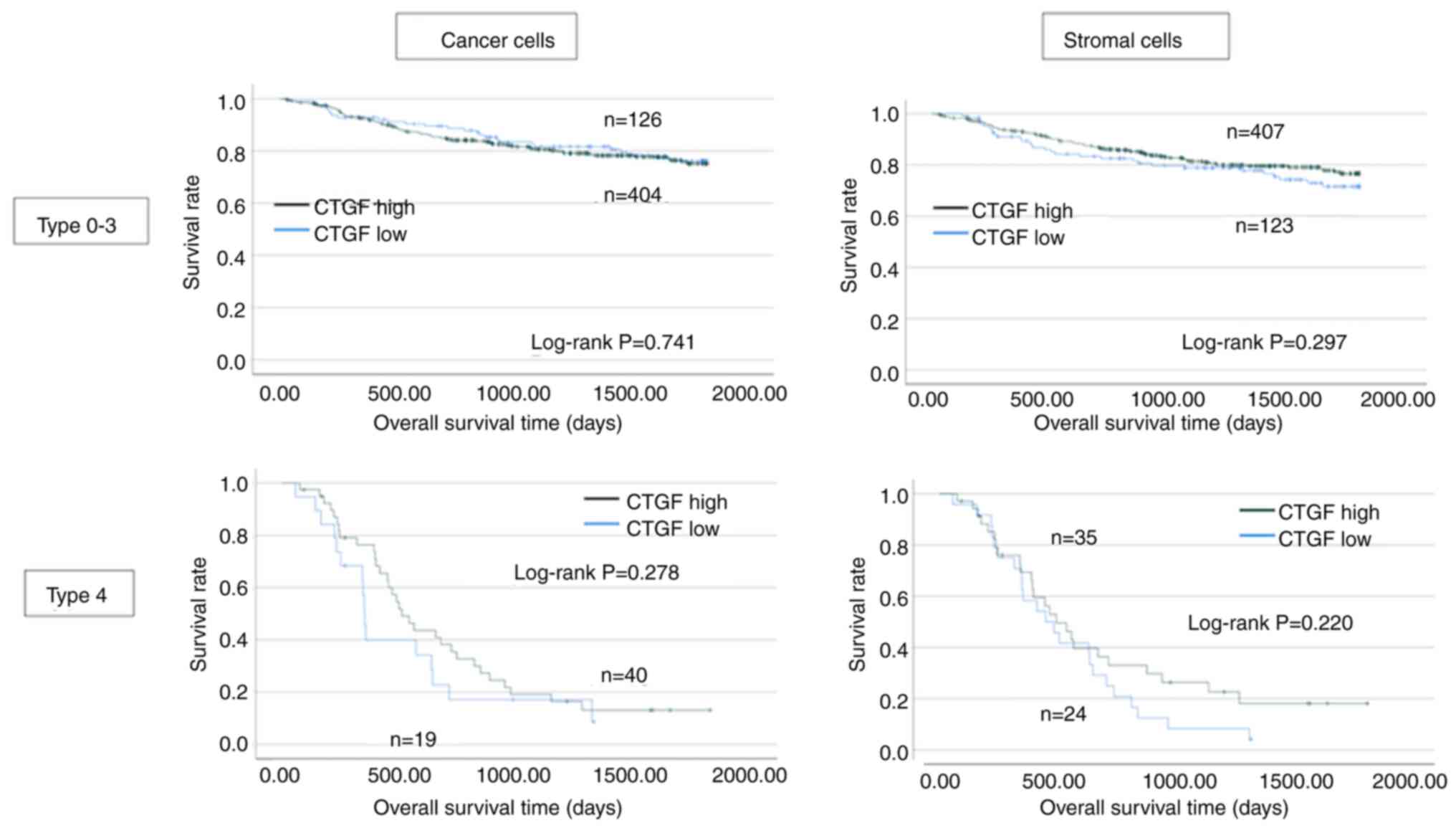
Fig. 5 shows the
analyses for each macroscopic type. Among the scirrhous type GC,
patients with CTGF-negative cases demonstrated worse survival in
both cancer and stromal cell analyses although the difference was
not significant.
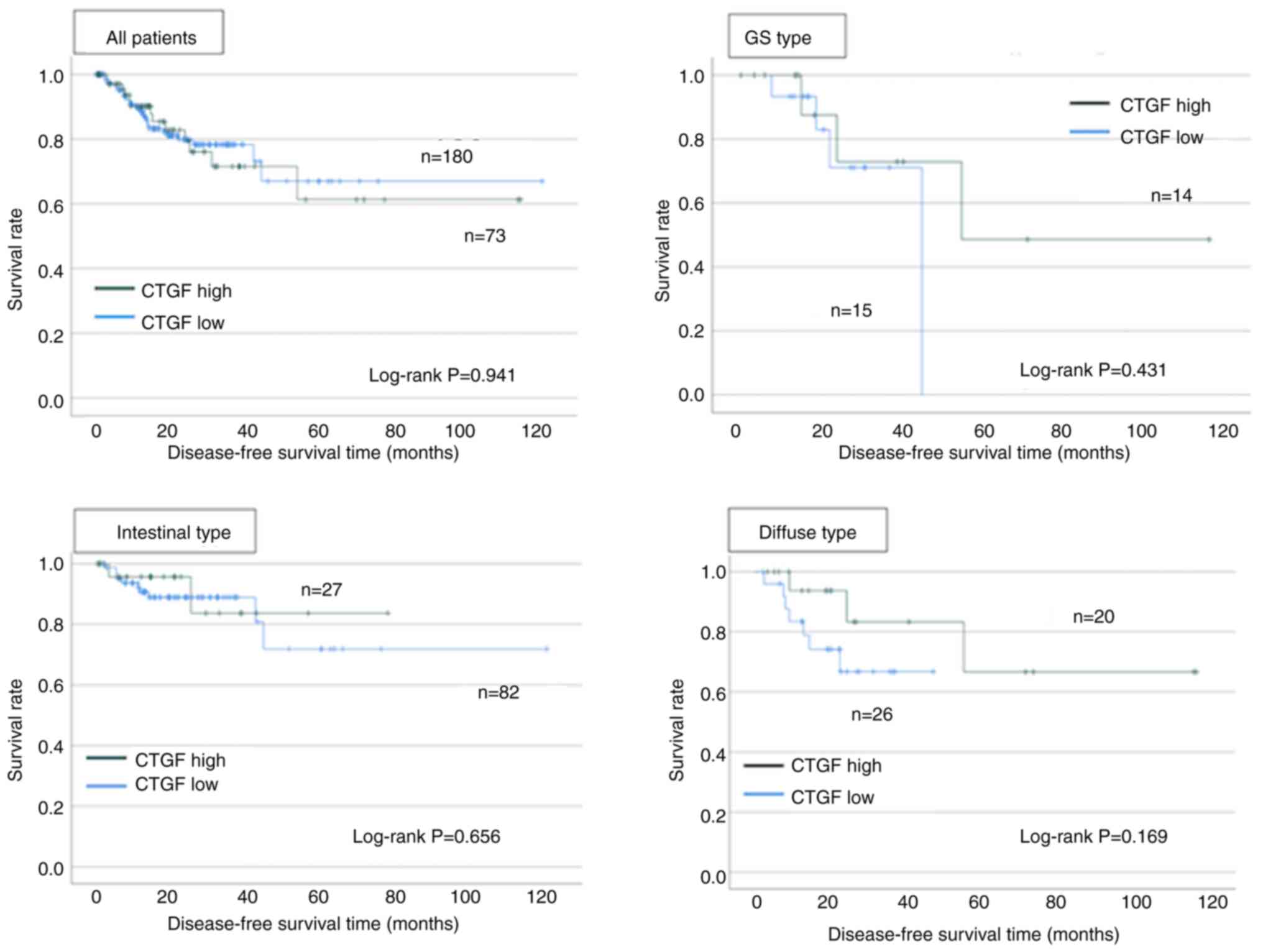
TCGA data
Fig. 6 shows the
survival analyses using TCGA. Among all cases, CTGF positivity did
not affect disease-free survival (log-rank; P=0.941). Low CTGF
expression cases demonstrated worse prognosis than high CTGF
expression cases in genomically stable cases and diffuse type,
although the differences were not significant (log-rank; P=0.431
and P=0.169).
Discussion
This study revealed that low CTGF expression of
stromal cells in diffuse GC was significantly associated with a
worse prognosis. This trend is similar in the scirrhous type, which
is a special subgroup in the diffuse type.
CTGF expression was mainly observed in the cytoplasm
of both cancer and stromal cells. Notably, CTGF expression in
stromal cells significantly affected survival outcomes. The main
component of stromal cells is generally cancer-associated
fibroblasts (CAFs), and our data reveal that CTGF expression of
CAFs and its secretion to the stroma is strongly related to cancer
progression. To the best of our knowledge, this is the first study
to investigate the significance of CTGF expression in stromal
cells.
CTGF expression in stromal cells was significantly
associated with that in cancer cells. CTGF is known to be
controlled by the Hippo pathway (6). Surrounding mechanotransduction
controlled the pathway (16). As
described, diffuse-type GC consists of many stromal components,
which are composed of stromal cells and fibrosis by excessive
collagen deposition. This feature is less distinct in the
intestinal type. Thus, the different extracellular matrix stiffness
may be associated with the different roles of CTGF expression in
diffuse and intestinal types. The detailed mechanism remains
unknown, and factors that affect the Hippo pathway in CAFs and
cancer cells should be investigated in the future.
Previously, Chen et al (9) revealed that CTGF expression was
significantly associated with early TNM staging and better
survival. This is consistent with our current data. However, we
reveal the more prominent effect of CTGF on survival in the diffuse
type. Furthermore, we revealed that CTGF expression on stromal
cells was independently associated with a worse prognosis.
Reportedly, CTGF inhibits cell adhesion through integrin α3β1 and
decreases the incidence of peritoneal metastasis. Diffuse-type GC
and its special subtype scirrhous type often metastasize to the
peritoneum. Therefore, CTGF function regarding cell adhesion during
peritoneal metastasis formation explained the poor survival of low
stromal CTGF expression in the diffuse type.
We used the downloaded data from the TCGA database
to validate our IHC data. The survival difference between the CTGF
high and low groups was not statistically significant although the
trend is similar to our IHC data. The TCGA data does not completely
include the histological type, so the number of cases we could
include was relatively small. Additionally, TCGA data are mRNA
level, which indicates that CTGF protein expression may be modified
post-transcriptionally.
As a limitation, this analysis was performed using
TMA, and a broad tissue area was not analyzed. However, we
attempted to generate the TMA using a representative core, thus the
result should be justified. Furthermore, we used automatic software
in the analyses, and the data were quite objective and less
biased.
In conclusion, CTGF expression in stromal cells
affects prognosis, especially in diffuse-type GC. The development
of a treatment to overcome peritoneal metastasis is strongly
awaited. Our data indicates CTGF and its control by the Hippo
pathway might be potential treatment targets in diffuse-type GC,
and future studies will be necessary to elucidate this issue.
Acknowledgements
The authors would like to thank Mrs. Akiko Tsuda
(Osaka Metropolitan University Graduate School of Medicine) for
technical assistance.
Funding
This study was partially funded by KAKENHI Grant-in-Aid for
Scientific Research (grant no. 50866697) to YM.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YM was responsible for conceptualization, data
analysis and writing the manuscript. MYo, RM, HK, TF, TTa, MS, TTo,
SL, MYa and KM were involved in data collection. MYa and KM
supervised the study. YM and RM confirm the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by Osaka Metropolitan
University ethics committee on 2022/08/10 (approval no. 2022-077).
Informed consent was obtained in the form of opt-out.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Song Y, Liu X, Cheng W, Li H and Zhang D:
The global, regional and national burden of stomach cancer and its
attributable risk factors from 1990 to 2019. Sci Rep. 12:115422022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Janjigian YY, Shitara K, Moehler M,
Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T,
Campos Bragagnoli A, et al: First-line nivolumab plus chemotherapy
versus chemotherapy alone for advanced gastric, gastro-oesophageal
junction, and oesophageal adenocarcinoma (CheckMate 649): A
randomised, open-label, phase 3 trial. Lancet. 398:27–40. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ono H, Yao K, Fujishiro M, Oda I, Nimura
S, Yahagi N, Iishi H, Oka M, Ajioka Y, Ichinose M and Matsui T:
Guidelines for endoscopic submucosal dissection and endoscopic
mucosal resection for early gastric cancer. Dig Endosc. 28:3–15.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Etoh T, Ohyama T, Sakuramoto S, Tsuji T,
Lee SW, Yoshida K, Koeda K, Hiki N, Kunisaki C, Tokunaga M, et al:
Five-Year survival outcomes of laparoscopy-assisted vs open distal
gastrectomy for advanced gastric cancer: The JLSSG0901 Randomized
clinical trial. JAMA Surg. 158:445–454. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harvey KF, Zhang X and Thomas DM: The
Hippo pathway and human cancer. Nat Rev Cancer. 13:246–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li N, Xie C and Lu N: Crosstalk between
Hippo signalling and miRNAs in tumour progression. FEBS J.
284:1045–1055. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ramazani Y, Knops N, Elmonem MA, Nguyen
TQ, Arcolino FO, van den Heuvel L, Levtchenko E, Kuypers D and
Goldschmeding R: Connective tissue growth factor (CTGF) from basics
to clinics. Matrix Biol. 68–69. 44–66. 2018.
|
|
8
|
Chen PS, Wang MY, Wu SN, Su JL, Hong CC,
Chuang SE, Chen MW, Hua KT, Wu YL, Cha ST, et al: CTGF enhances the
motility of breast cancer cells via an
integrin-alphavbeta3-ERK1/2-dependent S100A4-upregulated pathway. J
Cell Sci. 120:2053–2065. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen CN, Chang CC, Lai HS, Jeng YM, Chen
CI, Chang KJ, Lee PH and Lee H: Connective tissue growth factor
inhibits gastric cancer peritoneal metastasis by blocking integrin
α3β1-dependent adhesion. Gastric Cancer. 18:504–515. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jarvi O and Lauren P: On the pathogenesis
of gastric cancer. Acta Unio Int Contra Cancrum. 8:393–394.
1952.PubMed/NCBI
|
|
11
|
Yashiro M, Chung YS, Nishimura S, Inoue T
and Sowa M: Fibrosis in the peritoneum induced by scirrhous gastric
cancer cells may act as ‘soil’ for peritoneal dissemination.
Cancer. 77 (8 Suppl):S1668–S1675. 1996. View Article : Google Scholar
|
|
12
|
Yashiro M and Hirakawa K: Cancer-stromal
interactions in scirrhous gastric carcinoma. Cancer Microenviron.
3:127–135. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miki Y, Yashiro M, Moyano-Galceran L,
Sugimoto A, Ohira M and Lehti K: Crosstalk between cancer
associated fibroblasts and cancer cells in scirrhous type gastric
cancer. Front Oncol. 10:5685572020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yashiro M, Chung YS, Kubo T, Hato F and
Sowa M: Differential responses of scirrhous and well-differentiated
gastric cancer cells to orthotopic fibroblasts. Br J Cancer.
74:1096–1103. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miki Y, Yashiro M, Okuno T, Kuroda K,
Togano S, Hirakawa K and Ohira M: Clinico-pathological significance
of exosome marker CD63 expression on cancer cells and stromal cells
in gastric cancer. PLoS One. 13:e02029562018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dupont S, Morsut L, Aragona M, Enzo E,
Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M,
Bicciato S, et al: Role of YAP/TAZ in mechanotransduction. Nature.
474:179–183. 2011. View Article : Google Scholar : PubMed/NCBI
|