Introduction
In the past, breast cancer (BC) was the most
prevalent illness affecting women globally (1–4). Since
the time of the ancient Egyptians, attempts have been made to
eradicate BC. More advanced surgical procedures are now being used
to decrease the psychological toll of treatment, while medicinal
therapies such as mastectomy and chemotherapy have significantly
enhanced patient survival (5–7).
However, preventative effectiveness and treatment options can never
be fully effective without a thorough grasp of the pathophysiology
and underlying mechanisms.
BC is a form of cancer that manifests itself
differently in various individuals (8). Women all across the world are affected
by BC, a prevalent type of cancer (9). BC can be classified into three groups
based on molecular and histological evidence: BC expressing human
epidermal growth factor receptor 2 (HER2+), triple-negative BC
[TNBC; estrogen receptor (ER)-, progesterone receptor (PR)- and
HER2-] and BC expressing hormone receptors, ER+ or PR+ (10,11).
The methods of treatment should be determined by the
molecular features of BC. TNBC has also been classified into six
groups: Luminal androgen receptor, immunomodulatory, mesenchymal,
mesenchymal stem cell-like, basal-like 1 (BL-1) and BL-2 (10). These categories are not always
useful because a single BC may have a variety of distinct cell
types (4,12–15).
The majority of BC-related fatalities result from
metastases. At 3 years after the main tumor was first discovered,
10–15% of patients with BC had distant metastases (16). At 10 years after the first
diagnosis, it is common to observe the appearance of
micrometastases in distant places. As a result, individuals with BC
have a lifetime risk of developing metastases (16–18).
Third-generation cyclin-dependent kinase (CDK)4/6
inhibitor ribociclib (LEE011) is highly selective and inhibits
CDK4/6 by competitively interacting with its ATP binding sites. By
inhibiting the CDK4/6-cyclin D-retinoblastoma (Rb)-E2F axis, this
strategy may stop unchecked cell division and tumor progression.
CDK4 and 6 are crucial for the development and division of cancer
cells, and ribociclib may limit the development of cancerous cells
and stop the spread of the disease by inhibiting these enzymes
(19).
Poly(ADP-ribose) polymerase (PARP) has crucial roles
in DNA repair, apoptosis, cell regulation, cell division,
differentiation, transcriptional regulation and chromosome
maintenance (20,21). PARP1 repairs single-strand breaks in
DNA through a base truncation repair mechanism. PARP-1 inhibition
is a potent cancer death pathway (21). DNA damage occurs with cancer
treatments such as temozolamide, platinum compounds, topoisomerase
inhibitors and radiation therapy. Treatment resistance develops due
to PARP, which is involved in DNA repair. Therefore, inhibiting the
PARP enzyme may increase the treatment efficacy. Tumor suppressor
genes, such as mutated BRCA1 and BRCA2, are highly sensitive to
PARP1 inhibition, leading to cell cycle arrest and apoptosis
(22). It has been proposed that
PARP limits the spread of improperly repaired DNA, serving as a
connection between severe DNA damage and cell death. Berger was the
first to postulate this idea, also referred to as the ‘PARP suicide
hypothesis’ (23).
In this study, the antiproliferative and anticancer
effects of Ribociclib and PARP1 inhibitor alone and together on the
Luminal A type breast cancer MCF-7 cell line and triple negative
cancer type MDA-MB-231 cell line were investigated.
Materials and methods
Cell culture
The TNBC cell line MDA-MB-231 and the luminal A BC
cell line MCF-7 (European Cell Culture Collection) were both
employed. The two cell lines were kept in DMEM (Gibco: Thermo
Fisher Scientific, Inc.) tissue culture medium containing 100 µg/ml
Streptomycin sulfate (I.E. Ulugay), 100 IU/ml penicillin (Pronapen;
Pfizer), Amphotericin B (Sigma-Aldrich; Merck KGaA), 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.) with the pH
adjusted to 7.2 with 4.4% NaHCO3.
Ribociclib and PARP1
concentration
In the experiments, for both cell lines, 40, 80 and
160 µg/ml concentrations of VLM (FARMANOVA), which contains the
active ingredient ribociclib, and 3, 6 and 9 µg/ml concentrations
of DPQ (Sigma-Aldrich; Merck KGaA), a PARP1 inhibitor, were used.
These concentrations are based on preliminary experiments performed
by our group.
Cell viability assay
The MTT assay was used to investigate the
cytotoxicity of VLM and DPQ on the cells as a consequence of the
application of the planned doses. For this application, MCF-7 cells
were seeded into 96-well plates at a density of 2×104
cells per well and incubated overnight. The cells were then treated
with VLM and DPQ concentrations for 24 h. At the end of the
experimental period, the medium in each well was removed and 40 µl
fresh MTT solution (Sigma-Aldrich; Merck KGaA) was added into each
well and cells were incubated at 37°C for 4 h. Subsequently, 160 µl
DMSO was added. By using the 690 nm wavelength as a reference, a
spectrophotometer was used to measure the absorbance values of the
experimental groups at 570 nm.
Cell index
Cell index values determine the cytotoxic effect of
agents applied to the cell by monitoring cell reproduction, cell
size or morphology in real time. The cytotoxic impact of VLM and
DPQ on the designated cell lines was assessed by xCELLigence DP
(Acea Biosciences, Inc.). Before taking any measurements, each well
of a 16-well E-plate was filled with 100 µl of the appropriate
medium. Subsequently, 100 µl cell suspension was added to each well
of the E-plate. In each well, 10.000 cells for the MCF-7 cell line
and 5.000 cells for the MDA-MB-231 cell line were seeded. The
E-plates were kept at room temperature in a sterile environment for
20 min and then placed in the stations in the device and the
experiment was continued at 37°C and 5% CO2 under
saturated humidity ambient conditions. The device was set to
measure every 15 min. After an overnight incubation, drugs were
added and measurements were continued (24).
Bromodeoxyuridine (BrdU) incorporation
assay
VLM and DPQ were used in the experiments in line
with the kit's protocol (BrdU Cell Proliferation Assay Kit; cat.
no. 2750; EMD Millipore) at the optimum combination
concentrations.
Mitotic index
Experimental results were obtained following the kit
protocol with VLM and DPQ at the specified doses (Mitotic assay
kit; cat. no. 18021; Active Motif).
Caspase activity
In line with the kit instructions (CaspaTag Caspase
3, 7 in situ assay kit; cat. no. APT403; EMD Millipore),
experimental findings were obtained.
Statistical analysis
All parameters of cell kinetics were evaluated
comparing multiple independent groups. Comparisons between groups
were performed using one-way ANOVA and Dunnett's tests. Statistical
evaluations of the cell index were made by the xCelligence device.
Dunnett's post-hoc test was used for all other parameters.
Statistical analyses were reviewed twice in line with referee
opinions. Experiments were performed in triplicate. The statistical
analyses were performed using SPSS statistics software (v22.0;
IBM). P<0.05 was considered to indicate a statistically
significant difference.
Results
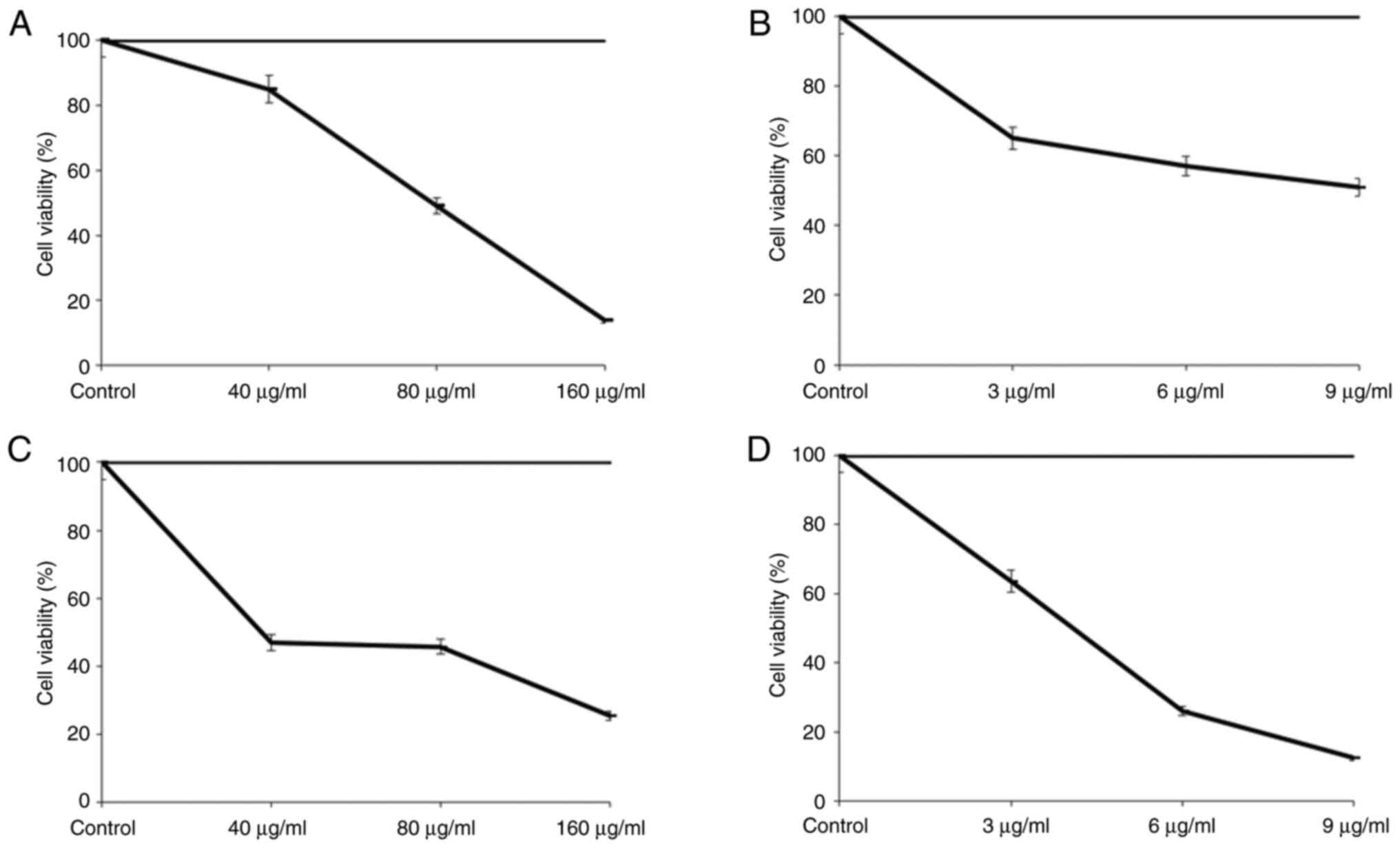
Cell viability
When the MCF-7 cell absorbance levels following
treatment for 40, 80 and 160 µg/ml VLM for 24 h were analyzed, it
was observed that the 40 µg/ml VLM decreased the viability of MCF-7
cells to 85%, the 80 µg/ml VLM concentration decreased cell
viability to 49.19% and the 160 µg/ml VLM concentration decreased
cell viability to 13.79% compared to the control group, which was
set as 100% (Fig. 1A).
Analysis of the absorbance values following DPQ
treatment of MCF-7 cells at concentrations of 3, 6 and 9 µg/ml for
24 h showed that the 3 µg/ml DPQ concentration decreased the
viability of MCF-7 cells to 65%, the 6 µg/ml DPQ concentration
decreased cell viability to 57% and the 9 µg/ml DPQ concentration
decreased cell viability to 51% compared to the control group,
which was set as 100% (Fig.
1B).
Analysis of the absorbance rates after 40, 80 and
160 µg/ml VLM treatment of MDA-MB-231 cells for 24 h indicated that
the 40 µg/ml VLM concentration decreased the viability of
MDA-MB-231 cells to 46.86% compared to the control group, which was
considered 100%. The 80 µg/ml VLM concentration reduced the cell
viability to 45.71%, while the 160 µg/ml VLM concentration reduced
the cell viability to 25.42% (Fig.
1C).
When the absorbance values were analyzed after DPQ
was applied to MDA-MB-231 cells at concentrations of 3, 6 and 9
µg/ml for 24 h, it was observed that 3 µg/ml MDA-MB-231 cells had a
63.52% vitality after exposure to DPQ, the 6 µg/ml DPQ
concentration decreased cell viability to 25.93% and the 9 µg/ml
DPQ concentration decreased cell viability to 12.48% compared to
the control group, which was set as 100% (Fig. 1D).
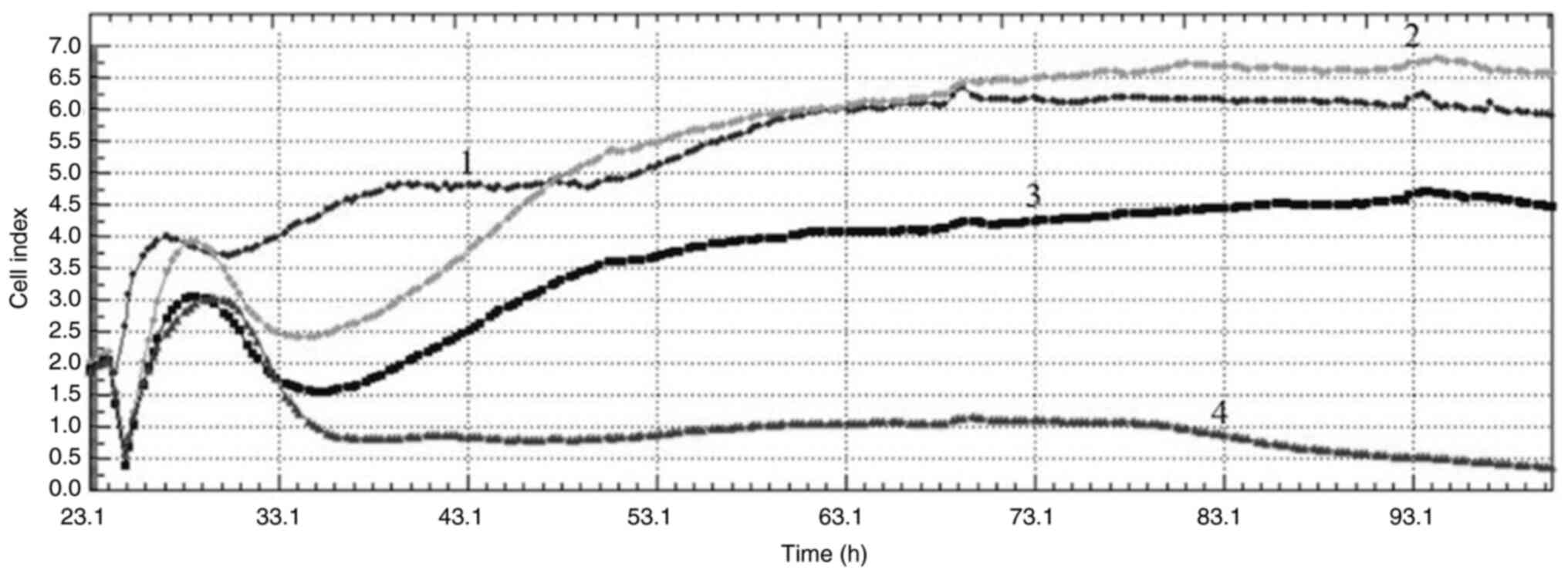
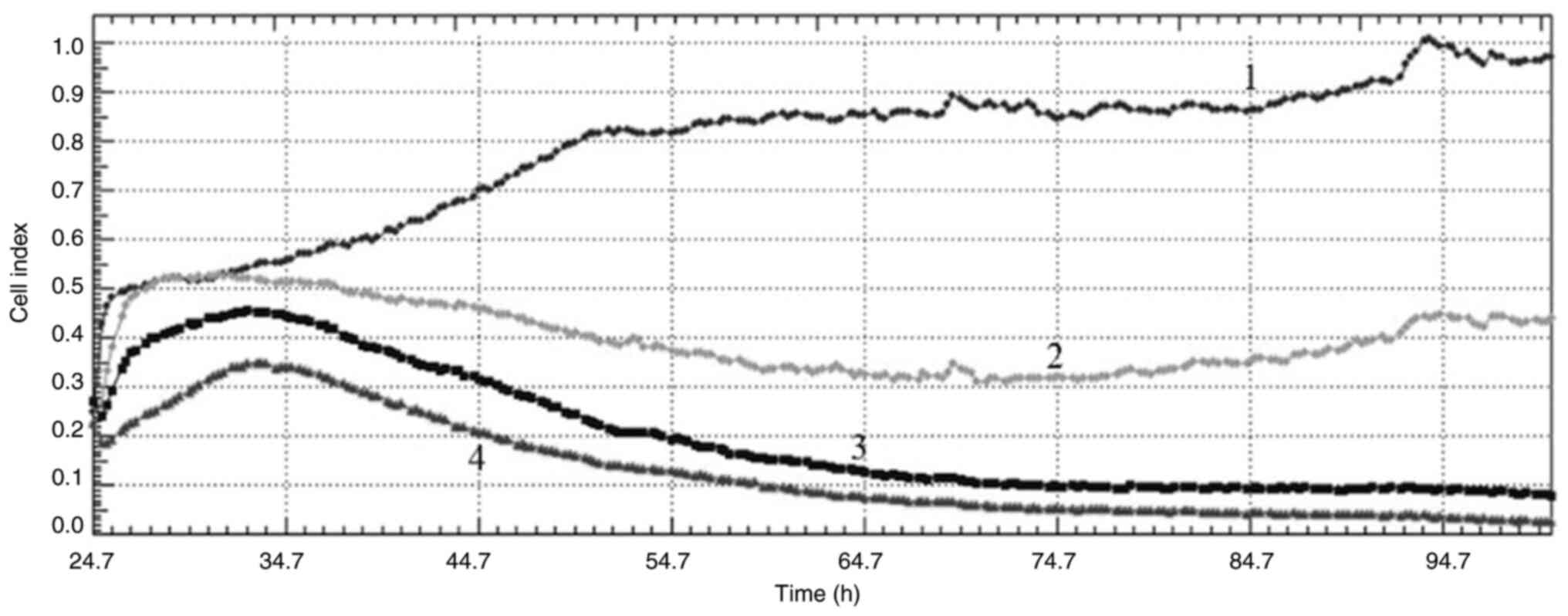
Cell index
Cell index values obtained from the xCelligence
real-time cell analysis system (Acea Biosciences Inc.) monitor cell
reproduction, cell size or morphology in real time. Acquired cell
index values from the real-time cell analysis (RTCA) after
treatment with VLM in MCF-7 and MDA-MB-231 cells at 40, 80 and 160
µg/ml showed that the drug had antiproliferative effects in both
cell lines. When the curves obtained from the cell index graphs of
MCF-7 cells are examined, different effects are observed at
different concentrations. While no effect was observed at a
concentration of 40 µg/ml, an antimitotic effect was observed at a
concentration of 80 µg/ml and DNA damage was observed at a
concentration of 160 µg/ml (Fig.
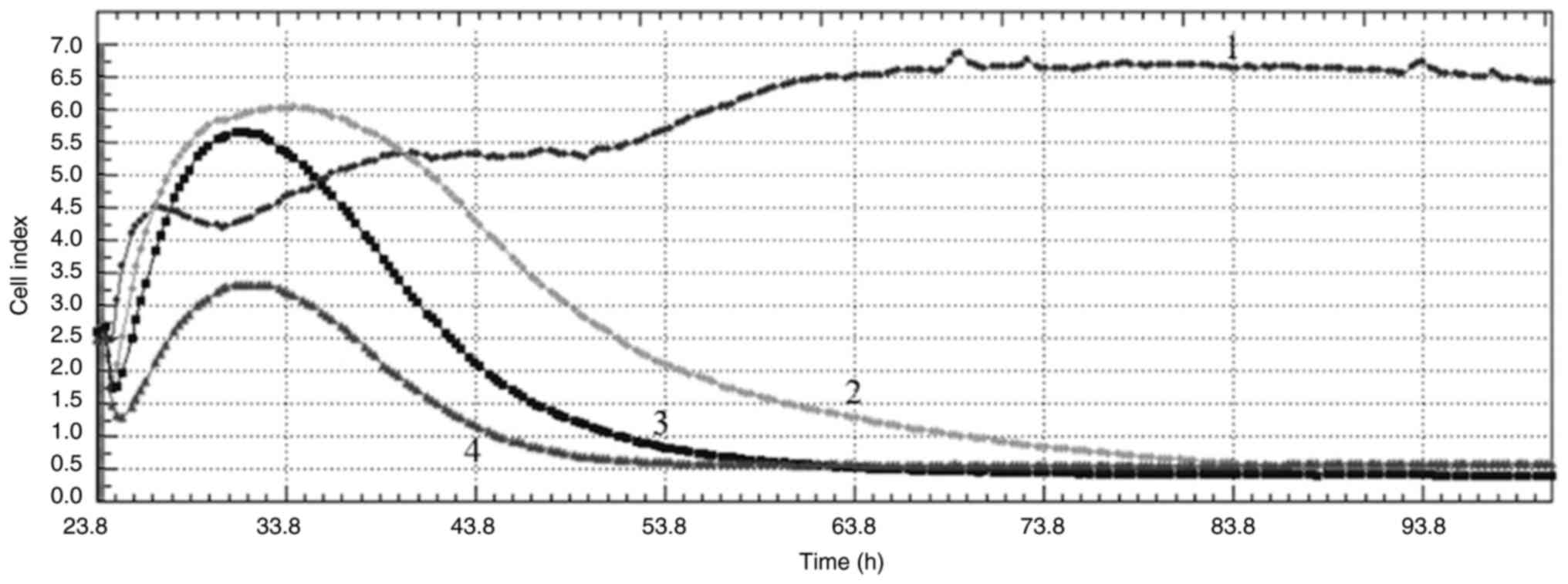
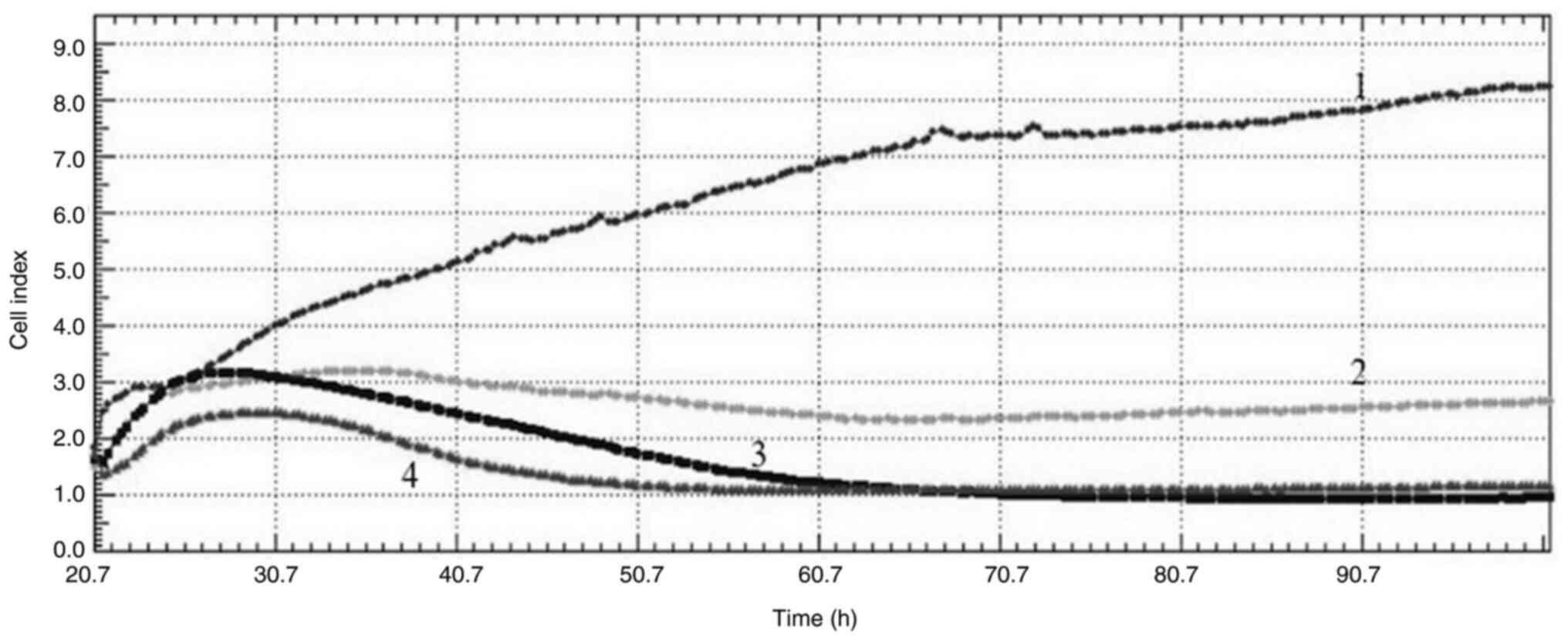
2). When the curves obtained from the cell index graphs of
MDA-MB-231 cells are examined, all concentrations applied appeared
to have an antiproliferative effect and cause DNA damage to the
cells (Fig. 3).
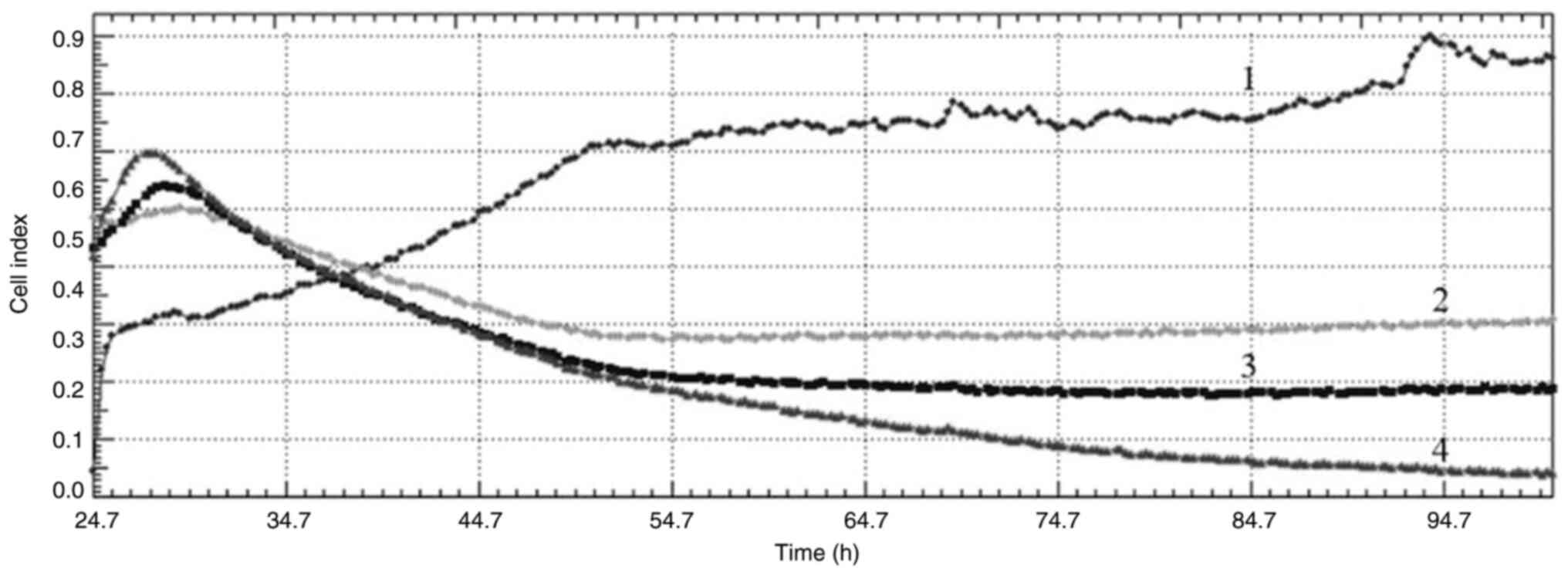
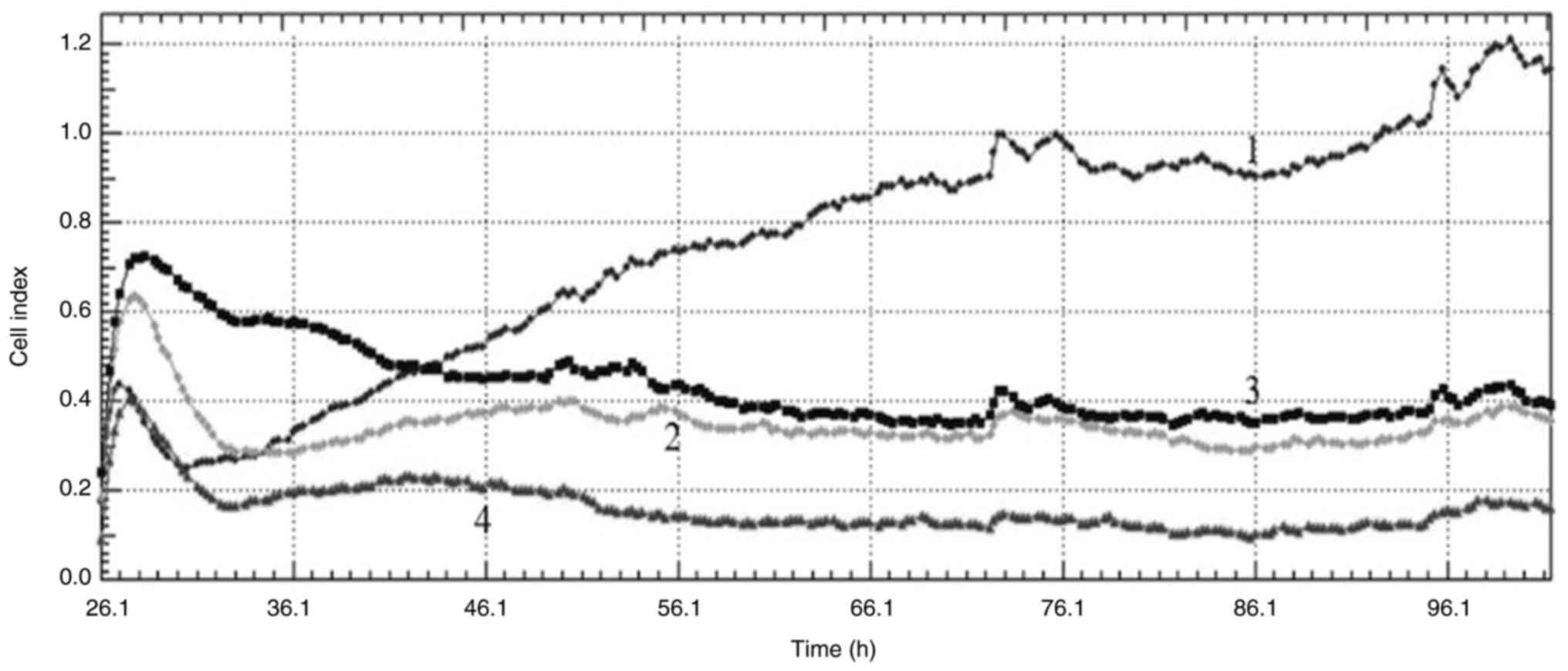
The cell index values obtained from the RTAC system
after DPQ treatment of cells at concentrations of 3, 6 and 9 µg/ml
showed that the PARP inhibitor had antiproliferative effects on
both cell lines. When the curves obtained from the cell index
graphs of MCF-7 cells were examined, DNA damage was observed at all
concentrations (Fig. 4). When the
curves obtained from the cell index graphs of MDA-MB-231 cells were
examined, it was observed that all concentrations applied had
antiproliferative effects and caused DNA damage to the cells
(Fig. 5).
Cell index values obtained from the RTAC system
showed antiproliferative effects on both MCF-7 and MDA-MB-231 cells
as a result of the combined application of VLM and DPQ at
concentrations of 2.5 µg/ml VLM + 1.5 µg/ml DPQ, 20 µg/ml VLM + 4.5
µg/ml DPQ and 10 µg/ml VLM + 4.5 µg/ml DPQ. The combined
concentrations showed DNA damage in MCF-7 cells (Fig. 6) and DNA damage in MDA-MB-231 cells
(Fig. 7).
BrdU incorporation
Combining VLM with DPQ to treat MCF-7 and MDA-MB-231
cells (2.5 µg/ml VLM + 1.5 µg/ml DPQ) for 0–72 h resulted in
labeling of cells with BrdU at the synthesis stage and the
absorbance values obtained are presented in Tables I and II, respectively. There was a significant
difference between the proliferation of control and experimental
groups (P<0.05).
 | Table I.Absorbance values at 450–655 nm
(emission) of MCF-7 cells treated with 2.5 µg/ml VLM + 1.5 µg/ml
DPQ in the bromodeoxyuridine assay (×10−3). |
Table I.
Absorbance values at 450–655 nm
(emission) of MCF-7 cells treated with 2.5 µg/ml VLM + 1.5 µg/ml
DPQ in the bromodeoxyuridine assay (×10−3).
| Time, h | Control | Combination
treatment |
|---|
| 24 | 502±3 | 398±2a |
| 48 | 507±4 | 257±3a |
| 72 | 496±4 | 203±2a |
 | Table II.Absorbance values at 450–655 nm
(emission) of MDA-MB-231 cells treated with 2.5 µg/ml Valamor + 1.5
µg/ml DPQ in the bromodeoxyuridine assay (×10−3). |
Table II.
Absorbance values at 450–655 nm
(emission) of MDA-MB-231 cells treated with 2.5 µg/ml Valamor + 1.5
µg/ml DPQ in the bromodeoxyuridine assay (×10−3).
| Time, h | Control | Combination
treatment |
|---|
| 24 | 625±3 | 609±2a |
| 48 | 639±4 | 355±3a |
| 72 | 635±4 | 300±2a |
Mitotic activity
The absorbance values reflecting mitotic activity of
MCF-7 and MDA-MB-231 cells treated with a combination of VLM and
DPQ (2.5 µg/ml VLM + 1.5 µg/ml DPQ) for 0–72 h are presented in
Tables III and IV, respectively. There was a significant
decrease in the experimental group according to the control
(P<0.05).
 | Table III.Absorbance values at 450–655 nm
(emission) of MCF-7 cells treated with 2.5 µg/ml Valamor + 1.5
µg/ml DPQ reflecting mitotic activity (×10−3). |
Table III.
Absorbance values at 450–655 nm
(emission) of MCF-7 cells treated with 2.5 µg/ml Valamor + 1.5
µg/ml DPQ reflecting mitotic activity (×10−3).
| Time, h | Control | Combination
treatment |
|---|
| 24 | 169±3 | 97±2a |
| 48 | 165±04 | 83±3a |
| 72 | 163±04 | 32±2a |
 | Table IV.Absorbance values at 450–655 nm
(emission) of MDA-MB-231 cells treated with 2.5 µg/ml Valamor + 1.5
µg/ml DPQ reflecting mitotic activity (×10−3). |
Table IV.
Absorbance values at 450–655 nm
(emission) of MDA-MB-231 cells treated with 2.5 µg/ml Valamor + 1.5
µg/ml DPQ reflecting mitotic activity (×10−3).
| Time, h | Control | Combination
treatment |
|---|
| 24 | 84±3 | 52±2a |
| 48 | 85±4 | 28±3a |
| 72 | 82±4 | 16±2a |
Caspase activity
In Tables V and
VI, the respective fluorescence
amounts of caspase activity of MCF-7 and MDA-MB-231 cells after
treatment with a combination of VLM and DPQ (2.5 µg/ml VLM + 1.5
µg/ml DPQ) for 0–72 h are presented. There was a significant
increase in experimental group according to control
(P<0.05).
 | Table V.Fluorescence values at 450–655 nm
(emission) of MCF-7 cells treated with 2.5 µg/ml Valamor + 1.5
µg/ml DPQ reflecting caspase activity. |
Table V.
Fluorescence values at 450–655 nm
(emission) of MCF-7 cells treated with 2.5 µg/ml Valamor + 1.5
µg/ml DPQ reflecting caspase activity.
| Time, h | Control | Combination
treatment |
|---|
| 24 | 204±12 | 302±14a |
| 48 | 212±11 | 328±15a |
| 72 | 307±11 | 518±13a |
 | Table VI.Fluorescence values at 450–655 nm
(emission) of MDA-MB-231 cells treated with 2.5 µg/ml Valamor + 1.5
µg/ml DPQ reflecting caspase activity. |
Table VI.
Fluorescence values at 450–655 nm
(emission) of MDA-MB-231 cells treated with 2.5 µg/ml Valamor + 1.5
µg/ml DPQ reflecting caspase activity.
| Time, h | Control | Combination
treatment |
|---|
| 24 | 224±12 | 361±14a |
| 48 | 226±11 | 427±15a |
| 72 | 231±11 | 453±13a |
Discussion
The primary goal of treatment for advanced or
metastatic BC is to slow the disease's progression, ideally using
patient-friendly anticancer medications that do not cause any undue
damage (25). Cell cycle
progression is deregulated in cancer, which is characterized by
unchecked cell proliferation (26).
The mitotic cell division cycle consists of four phases: Mitosis
(M), the phase during which cellular DNA is synthesized (S), the
first gap phase (G1) between the M and S phases, and the G2 phase
between the S and M phases. To facilitate cell cycle progression,
which is essential for mammalian cell cycle regulation, cell
cycle-related proteins are phosphorylated by cyclins A, B, D and E,
and their associated CDK1, −2, −4 and −6 (27,28).
Although several distinct genes encode the 3 D-type cyclins, D1, D2
and D3 have a common set of amino acids (on average 57% across the
coding area) (29). CDK4/6
inhibitors are a novel family of pharmaceuticals that decrease cell
cycle progression. Tumor cell growth is halted in this manner.
Palbociclib, ribociclib and abemaciclib are three such inhibitors
that have been authorized lately for the treatment of BC in
different contexts and combination regimens (30).
After having demonstrated noticeably improved
progression-free survival outcomes in comparison to standard
therapy, ribociclib is one of three selective small-molecule
inhibitors of CDK4/6 that are currently approved for the treatment
of advanced hormone receptor-positive, HER2-negative BC (31–33).
Third-generation CDK4/6 inhibitor ribociclib (LEE011) is highly
selective and inhibits CDK4/6 by competitively interacting with its
ATP binding sites (19). Ribociclib
can halt the spread of cancer by inhibiting these enzymes, which
also reduce the proliferation of cancer cells (34).
Beyond the locally advanced/metastatic scenario, the
introduction of PARP inhibitors may offer advantages for the
treatment of BC (35). The oral
version of PARP inhibitors has the potential to enhance patient
experience and adherence (36).
The activity of CDKs is required for DNA end
resection. Numerous investigations revealed that CDKs were crucial
to PARP inhibitor resistance (37–42).
TNBC cells that had been resistant to niraparib were
made sensitive again by the CDK inhibitor dinaciclib. The
experiment, which included dinaciclib and niraparib, was effective
not just in TNBC cells but also in cells from the pancreas, ovary,
prostate, colon and lung cancers (43).
Furthermore, out of all the functioning cell-cycle
complexes, the CDK4/6 complex had the strongest negative connection
with mutations, indicating that combination suppression of CDK4/6
and PARP may work in concert. In addition, combined therapy
demonstrated a reactive oxygen species-dependent synergy in both
Rb-proficient and Rb-deficient BC cells. These results point to a
possible therapeutic approach to increase the effectiveness of PARP
and CDK4/6 inhibitors in the treatment of cancer (44).
In a study conducted with the HCC1937 cell line, a
CDK4/6 inhibitor was used together with a PARP inhibitor. The
results of the study showed that the combination of CDK4/6
inhibitor and PARP inhibitor may expand the use of these inhibitors
in patients with TNBC and potentially overcome PARP inhibitor
resistance (45). Several recent
studies have shown how CDK4/6 and PARP inhibitors work
synergistically in various cancer cells (46–48).
In the current study, consistent with the studies
mentioned above, VLM and DPQ showed antiproliferative effects on
both MCF-7 and MDA-MB-231 cells at the lowest concentration
combinations used. The combined concentrations were shown to induce
DNA damage in both target cell lines. In conclusion, the findings
of this research investigating the inhibitory effects of VLM and
demonstrated significant efficacy to reduce the viability of MCF-7
and MDA-MB231 cell lines, the cell index, mitotic signs and BrdU
labeling, as well as a significant increase in caspase activity in
these cell lines. The lack of cell proliferation after 72 h of
treatment may be due to the cells becoming stable, or it may be due
to the cells entering the process of apoptosis when caspase
activity is taken into account.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Scientific Research
Projects Coordination Unit of Istanbul University (project no.
FYL-2022-39390).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
EP and MT performed the experiments. EP and MT wrote
and edited the manuscript. EP and MT confirm the authenticity of
all the raw data. Both authors read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Veronesi U, Boyle P, Goldhirsch A,
Orecchia R and Viale G: Breast cancer. Lancet. 365:1727–1741. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cliffton EE and Young LE: Carcinoma of the
breast; five to twenty-year follow-up following radical mastectomy.
Am J Surg. 82:185–190. 1951. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Figueiredo MI, Cullen J, Hwang YT, Rowland
JH and Mandelblatt JS: Breast cancer treatment in older women: Does
getting what you want improve your long-term body image and mental
health? J Clin Oncol. 22:4002–4009. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mathews FS: The ten-year survivors of
radical mastectomy. Ann Surg. 98:635–643. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nagarajan D and McArdle SEB: Immune
landscape of breast cancers. Biomedicines. 6:202018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Makhoul I, Atiq M, Alwbari A and
Kieber-Emmons T: Breast cancer immunotherapy: An update. Breast
Cancer (Auckl). 12:11782234187748022018.PubMed/NCBI
|
|
10
|
Lehmann BD, Bauer JA, Chen X, Sanders ME,
Chakravarthy AB, Shyr Y and Pietenpol JA: Identification of human
triple-negative breast cancer subtypes and preclinical models for
selection of targeted therapies. J Clin Investig. 121:2750–2767.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liedtke C, Mazouni C, Hess KR, André F,
Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B,
Green M, et al: Response to neoadjuvant therapy and long-term
survival in patients with triple-negative breast cancer. J Clin
Oncol. 26:1275–1281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen X, Xu D, Li X, Zhang J, Xu W, Hou J,
Zhang W and Tang J: Latest overview of the cyclindependent kinases
4/6 inhibitors in breast cancer: The past, the present, and the
future. J Cancer. 10:6608–6617. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hulka BS: Epidemiology of susceptibility
to breast cancer. Prog Clin Biol Res. 395:159–174. 1996.PubMed/NCBI
|
|
14
|
Reinert T and Barrios CH: Optimal
management of hormone receptor positive metastatic breast cancer in
2016. Ther Adv Med Oncol. 7:304–320. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wuerstlein R and Harbeck N: Neoadjuvant
therapy for HER2-positive breast cancer. Rev Recent Clin Trials.
12:81–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Riggi N, Aguet M and Stamenkovic I: Cancer
metastasis: A reappraisal of its underlying mechanisms and their
relevance to treatment. Annu Rev Pathol. 13:117–140. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weigelt B, Peterse JL and van't Veer LJ:
Breast cancer metastasis: Markers and models. Nat Rev Cancer.
5:591–602. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Scully OJ, Bay BH, Yip G and Yu Y: Breast
cancer metastasis. Cancer Genomics Proteomics. 9:311–320.
2012.PubMed/NCBI
|
|
19
|
Poratti M and Marzaro G: Third-generation
CDK inhibitors: A review on the synthesis and binding modes of
palbociclib, ribociclib, and abemaciclib. Eur J Med Chem.
172:143–153. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luo X and Kraus WL: A one and a two …
expanding roles for poly(ADP-ribose) polymerases in metabolism.
Cell Metab. 13:353–355. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou D, Chu W, Xu J, Jones LA, Peng X, Li
S, Chen DL and Mach RH: Synthesis, [18F] radiolabeling,
and evaluation of poly (ADP-ribose) polymerase-1 (PARP-1)
inhibitors for in vivo imaging of PARP-1 using positron emission
tomography. Bioorg Med Chem Lett. 22:1700–1707. 2014. View Article : Google Scholar
|
|
22
|
Giannini G, Battistuzzi G, Vesci L,
Milazzo FM, Paolis FD, Barbarino M, Guglielmi MB, Carollo V, Gallo
G, Artali R and Dallavalle S: Novel PARP-1 inhibitors based on a
2-propanoyl-3H-quinazolin-4-one scaffold. Bioorg Med Chem Lett.
24:462–466. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Berger NA: Poly(ADP-ribose) in the
cellular response to DNA damage. Radiat Res. 101:4–15. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Topçul M, Çeti N İL, Özbaş Turan S and
Kolusayin Ozar MÖ: In vitro cytotoxic effect of PARP
inhibitor alone and in combination with nab-paclitaxel on
triple-negative and luminal A breast cancer cells. Oncol Rep.
40:527–535. 2018.PubMed/NCBI
|
|
25
|
Silberholz J, Bertsimas D and Vahdat L:
Clinical benefit, toxicity and cost of metastatic breast cancer
therapies: Systematic review and meta-analysis. Breast Cancer Res
Treat. 176:535–543. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Malumbres M and Barbacid M: Cell cycle,
CDKs, and cancer: A changing paradigm. Nat Rev Cancer. 9:153–166.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sherr CJ, Beach D and Shapiro GI:
Targeting CDK4 and CDK6: From discovery to therapy. Cancer Discov.
6:353–367. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xiong Y, Menninger J, Beach D and Ward DC:
Molecular cloning and chromosomal mapping of CCND genes encoding
human D-type cyclins. Genomics. 13:575–584. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Braal CL, Jongbloed EM, Wilting SM,
Mathijssen RHJ, Koolen SLW and Jager A: Inhibiting CDK4/6 in breast
cancer with palbociclib, ribociclib, and abemaciclib: Similarities
and differences. Drugs. 81:317–331. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hortobagyi GN, Stemmer SM, Burris HA, Yap
YS, Sonke GS, Paluch-Shimon S, Campone M, Petrakova K, Blackwell
KL, Winer EP, et al: Updated results from MONALEESA-2, a phase III
trial of first-line ribociclib plus letrozole versus placebo plus
letrozole in hormone receptor-positive, HER2-negative advanced
breast cancer. Ann Oncol. 29:1541–1547. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Slamon DJ, Neven P, Chia S, Fasching PA,
De Laurentiis M, Im SA, Petrakova K, Bianchi GV, Esteva FJ, Martín
M, et al: Phase III randomized study of ribociclib and fulvestrant
in hormone receptor-positive, human epidermal growth factor
receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin
Oncol. 36:2465–2472. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tripathy D, Im SA, Colleoni M, Franke F,
Bardia A, Harbeck N, Hurvitz SA, Chow L, Sohn J, Lee KS, et al:
Ribociclib plus endocrine therapy for premenopausal women with
hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A
randomised phase 3 trial. Lancet Oncol. 19:904–915. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fang H, Huang D, Yang F and Guan X:
Potential biomarkers of CDK4/6 inhibitors in hormone
receptor-positive advanced breast cancer. Breast Cancer Res Treat.
168:287–297. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cortesi L, Rugo HS and Jackisch C: An
overview of PARP ınhibitors for the treatment of breast cancer.
Target Oncol. 16:255–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Eek D, Krohe M, Mazar I, Horsfeld A,
Pompilus F, Friebe R and Shields AL: Patient-reported preferences
for oral versus intravenous administration for the treatment of
cancer: A review of the literature. Patient Prefer Adherence.
10:1609–1621. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tomimatsu N, Mukherjee B, Catherine
Hardebeck M, Ilcheva M, Vanessa Camacho C, Louise Harris J, Porteus
M, Llorente B, Khanna KK and Burma S: Phosphorylation of EXO1 by
CDKs 1 and 2 regulates DNA end resection and repair pathway choice.
Nat Commun. 5:35612014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bajrami I, Frankum JR, Konde A, Miller RE,
Rehman FL, Brough R, Campbell J, Sims D, Rafiq R, Hooper S, et al:
Genome-wide profiling of genetic synthetic lethality identifies
CDK12 as a novel determinant of PARP1/2 inhibitor sensitivity.
Cancer Res. 74:287–297. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Joshi PM, Sutor SL, Huntoon CJ and Karnitz
LM: Ovarian cancer-associated mutations disable catalytic activity
of CDK12, a kinase that promotes homologous recombination repair
and resistance to cisplatin and poly(ADP-ribose) polymerase
inhibitors. J Biol Chem. 289:9247–9253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Johnson SF, Cruz C, Greifenberg AK, Dust
S, Stover DG, Chi D, Primack B, Cao S, Bernhardy AJ, Coulson R, et
al: CDK12 ınhibition reverses de novo and acquired PARP inhibitor
resistance in BRCA wild-type and mutated models of triple-negative
breast cancer. Cell Rep. 17:2367–2381. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ning JF, Stanciu M, Humphrey MR, Gorham J,
Wakimoto H, Nishihara R, Lees J, Zou L, Martuza RL, Wakimoto H and
Rabkin SD: Myc targeted CDK18 promotes ATR and homologous
recombination to mediate PARP inhibitor resistance in glioblastoma.
Nat Commun. 10:29102019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Militello AM, Zielli T, Boggiani D,
Michiara M, Naldi N, Bortesi B, Zanelli P, Uliana V, Giuliotti S
and Musolino A: Mechanism of action and clinical efficacy of CDK4/6
inhibitors in BRCA-mutated, estrogen receptor-positive breast
cancers: Case report and literature review. Front Oncol. 9:7592019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Carey JPW, Karakas C, Bui T, Chen X,
Vijayaraghavan S, Zhao Y, Wang J, Mikule K, Litton JK, Hunt KK and
Keyomarsi K: Synthetic lethality of PARP inhibitors in combination
with MYC blockade is independent of BRCA status in triple-negative
breast Cancer. Cancer Res. 78:742–757. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li S, Zhang Y, Wang N, Guo R, Liu Q, Lv C,
Wang J, Wang L and Yang Q: Pan-cancer analysis reveals synergistic
effects of CDK4/6i and PARPi combination treatment in RB-proficient
and RB-deficient breast cancer cells. Cell Death Dis. 11:2192020.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Eskiler GG, Ozman Z, Haciefendi A and
Cansaran-Duman D: Novel combination treatment of CDK 4/6 inhibitors
with PARP inhibitors in triple negative breast cancer cells. Naunyn
Schmiedebergs Arch Pharmacol. 396:1031–1041. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li H, Liu ZY, Wu N, Chen YC, Cheng Q and
Wang J: PARP inhibitor resistance: The underlying mechanisms and
clinical implications. Mol Cancer. 19:1072020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Klein FG, Granier C, Zhao Y, Pan Q, Tong
Z, Gschwend JE, Holm PS and Nawroth R: Combination of talazoparib
and palbociclib as a potent treatment strategy in bladder cancer. J
Pers Med. 11:3402021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhu X, Chen L, Huang B, Li X, Yang L, Hu
X, Jiang Y, Shao Z and Wang Z: Efficacy and mechanism of the
combination of PARP and CDK4/6 inhibitors in the treatment of
triple-negative breast cancer. J Exp Clin Cancer Res. 40:1222021.
View Article : Google Scholar : PubMed/NCBI
|