Introduction
Nasopharyngeal carcinoma (NPC), which originates
from the nasopharyngeal mucosal lining, is endemic to Southeast
Asia, North Africa and South China, and more than 70% of new cases
are in east and southeast Asia (1,2). Due
to improvement of radiotherapy techniques, development of drugs and
the accuracy of cancer staging systems, the survival of patients
with NPC has notably improved, and the 5-year overall survival rate
of early nasopharyngeal carcinoma is 86.6 to 93.2% (3). However, certain patients still
experience treatment failure and 11–36% of patients with NPC
develop distant metastasis (2).
Therefore, it is key to elucidate novel biomarkers to better
stratify prognosis and predict treatment outcomes in NPC.
To date, most biomarkers studied have been
associated with tumors, whereas less attention has been paid to
host-associated factors, such as serum alpha-fetoprotein in
Hepatocellular carcinoma (4),
carbohydrate antigen 125 in ovarian cancer (5). However, certain blood-derived and
easily obtained immune-inflammatory biomarkers (IIBs) have been
studied in patients with malignancy, including NPC (6–14). The
neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio
(PLR), monocyte-to-lymphocyte ratio (MLR) and neutrophil platelet
and monocyte counts have prognostic relevance in NPC (6–14).
Furthermore, high NLR may be used to predict positive immune
response to radiotherapy in patients with NPC (15). However, the low discriminative
ability of these single biomarkers limits their clinical
application. Considering the interaction between immunity,
inflammation, and cancer, which depends on complicated networks,
more stable and robust prognostic power may be achieved using
composite biomarkers that encompass diverse immune-inflammatory
populations and reflect the overall inflammatory state. Notably,
systemic immune-inflammation index (SII), which includes
neutrophil, lymphocyte and platelet counts, but excludes monocytes,
has been applied to stratify the prognosis of patients with NPC
(8,10,12,16,17).
Furthermore, pan-immune-inflammation value (PIV), a novel biomarker
encompassing subsets of peripheral blood immune cells (neutrophils
platelets, monocytes and lymphocytes) has the potential to reflect
immunity and systemic inflammation in a patient (18–27).
Based on previous studies and meta-analyses, the PIV has been
identified as a strong prognostic indicator of outcomes in patients
with advanced cancer treated with surgery, cytotoxic chemotherapy,
immunotherapy and targeted therapy, such as in breast, esophageal
and colorectal cancer (CRC) and metastatic melanoma (18–27).
To the best of our knowledge, however, the role of the PIV in NPC
has not been studied.
The present study aimed to assess the prognostic
ability of the PIV as a novel biomarker, including all
immune-inflammatory populations from peripheral blood in
non-metastatic NPC.
Materials and methods
Study population
The electronic records of patients with
biopsy-proven, non-metastatic NPC admitted to Panyu Central
Hospital (Guangzhou, China) between January 2014 and December 2019
were reviewed. All patients detected Epstein-Barr virus
(EBV)-encoded RNA (EBER) in situ hybridization. A thorough
review of the medical records of the patients was performed. The
following data were extracted from the medical records of the
participants: Age, sex, histological type, smoking status, baseline
hematological profile and imaging data. Factors known to affect
routine blood tests were also reviewed, including evidence of
bacterial infection or abscess, acute or chronic inflammation,
current use of corticosteroids and coexisting hematological
malignancy (7). Furthermore, the
clinical features of the patients (for example, fever, rash and
arthritis), past medical history (for example, coexisting
hematological malignancy and current use of corticosteroids) and
the results of blood and stool tests, urinalysis, chest X-ray or
computed tomography, especially when leukocytes were above the
normal range requiring ruled out for infections, hormone use, were
reviewed (7). Patients who
underwent incomplete treatment, patients who were aged <18 year,
had a history of malignancies at other sites, hematological
disease, incomplete data were excluded. Finally, 319 patients with
pathologically confirmed nasopharyngeal carcinoma, no metastasis,
and completion of standard treatment were included in the present
study. The absolute counts of neutrophils, lymphocytes, monocytes
and platelets were used to estimate PIV and SII.
All patients were re-staged based on the eighth
edition of the American Joint Committee on Cancer (AJCC)/Union for
International Cancer Control Staging System for NPC (28). The therapeutic strategy for all
patients was determined according to guidelines of the National
Comprehensive Cancer Network (29)
and the Karnofsky performance status score (30). All patients were treated by
definitive intensity modulated radiation therapy, with or without
chemotherapy (2). The total
radiation doses were 66–70 Gy to the primary tumor, 60–66 Gy to the
involved cervical lymph nodes and ≥54 Gy to potential sites of
local infiltration and bilateral cervical lymphatics as 30–33
fractions (2). Induction
chemotherapy (IC) was used to treat 280 (87%) patients. IC regimens
included docetaxel + cisplatin (TP; 75 mg/m2 docetaxel
on days 1 and 75 mg/m2 cisplatin on day 1) or
gemcitabine + cisplatin (GP; 1,000 mg/m2 gemcitabine on
days 1 and 8, and 80 mg/m2 cisplatin on day 1) for 2–3
courses. Among patients who received IC, 80.4% (225/280) received
TP and 19.6% (55/280) received GP.
Statistical analysis
To represent the weight of the mutual effect between
inflammatory pro-tumor populations (neutrophils, monocytes and
platelets) and anticancer immune populations (lymphocytes), PIV was
measured using the following equation: (Neutrophil count × platelet
count × monocyte count)/lymphocyte count (18–27).
SII, which was regarded as an outperforming inflammatory biomarker,
was measured using the following equation: (Neutrophil count ×
platelet count)/lymphocyte count (8,10,12,16,17).
The optimal cut-offs for PIV and SII were determined using receiver
operating characteristic (ROC) analysis, as in previous studies
(8,10,12,16,19,20,25).
Overall survival (OS) was defined as time from
treatment initiation to last follow-up and/or death;
progression-free survival (PFS) was defined as time from treatment
initiation to disease progression and/or death.
Independent samples t, χ2 and Kruskal-Wallis H tests
were used to assess continuous and categorical variables.
Kaplan-Meier analysis was used for survival analyses and the
log-rank test was used for comparison of survival times between
prognostic subgroups. Multivariate analyses were performed using
Cox regression analyses to calculate hazard ratios (HRs) and 95%
confidence intervals (CIs). All statistical analyses were performed
using IBM SPSS3 version 22.0 (IBM Corp.) and SigmaPlot 14.0 (Systat
Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics according to
PIV
A total of 319 patients were included in the present
analysis. All patients were immunohistochemically confirmed as
Epstein-Barr virus (EBV)-encoded RNA+. Among them, 213 (66.7%)
patients were tested for EBV DNA and only 73 (34.6%) were positive.
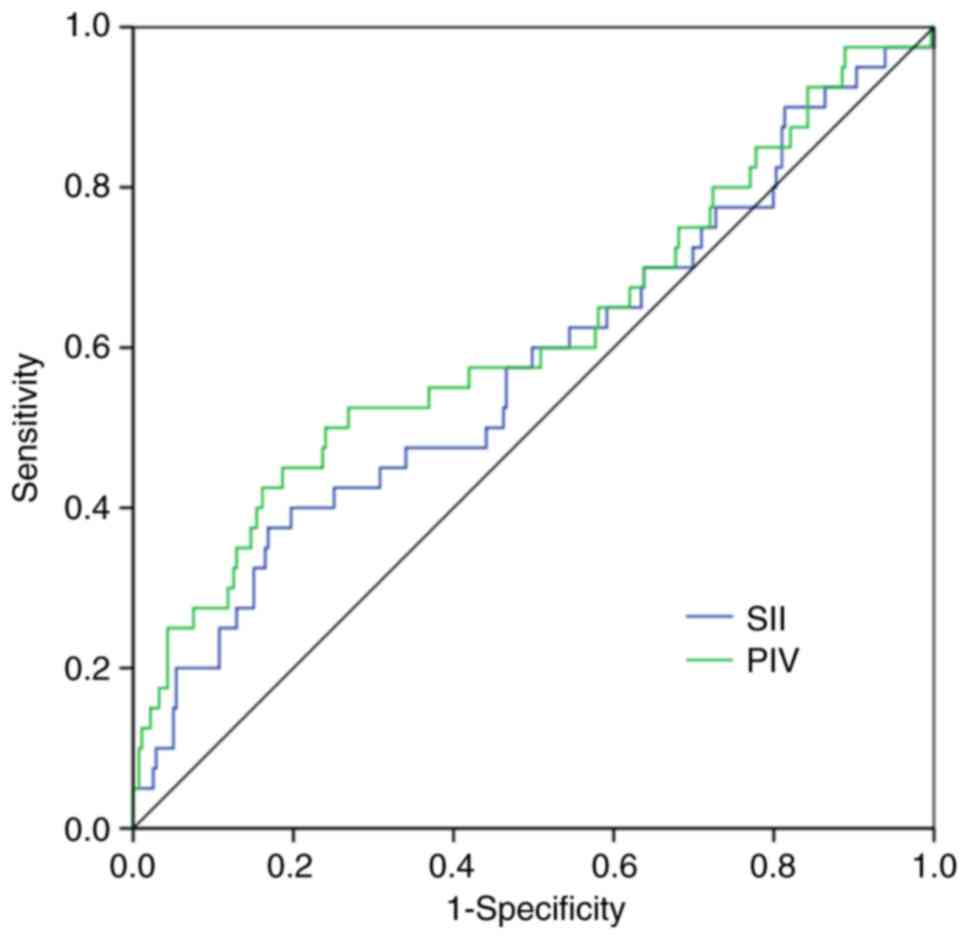
Table I and Fig. 1 present the values of the area under
the curve, sensitivity and specificity generated using ROC
analysis. The optimal cut-off values for the PIV and SII using ROC
analysis were 428.0 and 1032.7, respectively.
 | Table I.Receiver operating characteristic
curve analyses for overall survival. |
Table I.
Receiver operating characteristic
curve analyses for overall survival.
| Curve | Cut-off value | AUC | 95% CI | P-value | Sensitivity, % | Specificity, % |
|---|
| SII | 1,032.7 | 0.576 | 0.473–0.679 | 0.120 | 40.0 | 80.3 |
| PIV | 428.0 | 0.615 | 0.510–0.720 | 0.018 | 52.5 | 73.1 |
Overall, 96 patients (30.1%) had high PIV
(>428.0) and 223 patients (69.9%) had a low PIV (≤428.0)
(Table II). A high PIV was
significantly associated with more advanced T stage and a higher
SII and notably associated with NPC with a more advanced N stage
compared with low PIV. Moreover, patients with high PIV were
significantly more likely to receive IC compared with those with
low PIV.
 | Table II.Baseline characteristics in low and
high PIV groups. |
Table II.
Baseline characteristics in low and
high PIV groups.
| Characteristic | Low PIV
(n=223) | High PIV
(n=96) | P-value |
|---|
| Median age (range),
years | 52.0 (23–80) | 53.5 (19–78) | 0.730 |
| Sex |
|
| 0.136 |
|
Male | 151 | 73 |
|
|
Female | 72 | 23 |
|
| Smoking status |
|
| 0.032 |
|
Yes | 43 | 29 |
|
| No | 180 | 67 |
|
| T stage |
|
| 0.001 |
| T1 | 39 | 8 |
|
| T2 | 57 | 15 |
|
| T3 | 86 | 36 |
|
| T4 | 41 | 37 |
|
| N stage |
|
| 0.059 |
| N0 | 14 | 8 |
|
| N1 | 102 | 38 |
|
| N2 | 77 | 26 |
|
| N3 | 30 | 24 |
|
| Induction
chemotherapy |
|
| 0.004 |
|
Yes | 188 | 92 |
|
| No | 35 | 4 |
|
| Chemotherapy
regimen |
|
| 0.208 |
| GP | 33 | 22 |
|
| TP | 155 | 70 |
|
| SII |
|
| <0.001 |
|
Low | 209 | 39 |
|
|
High | 14 | 57 |
|
Outcomes
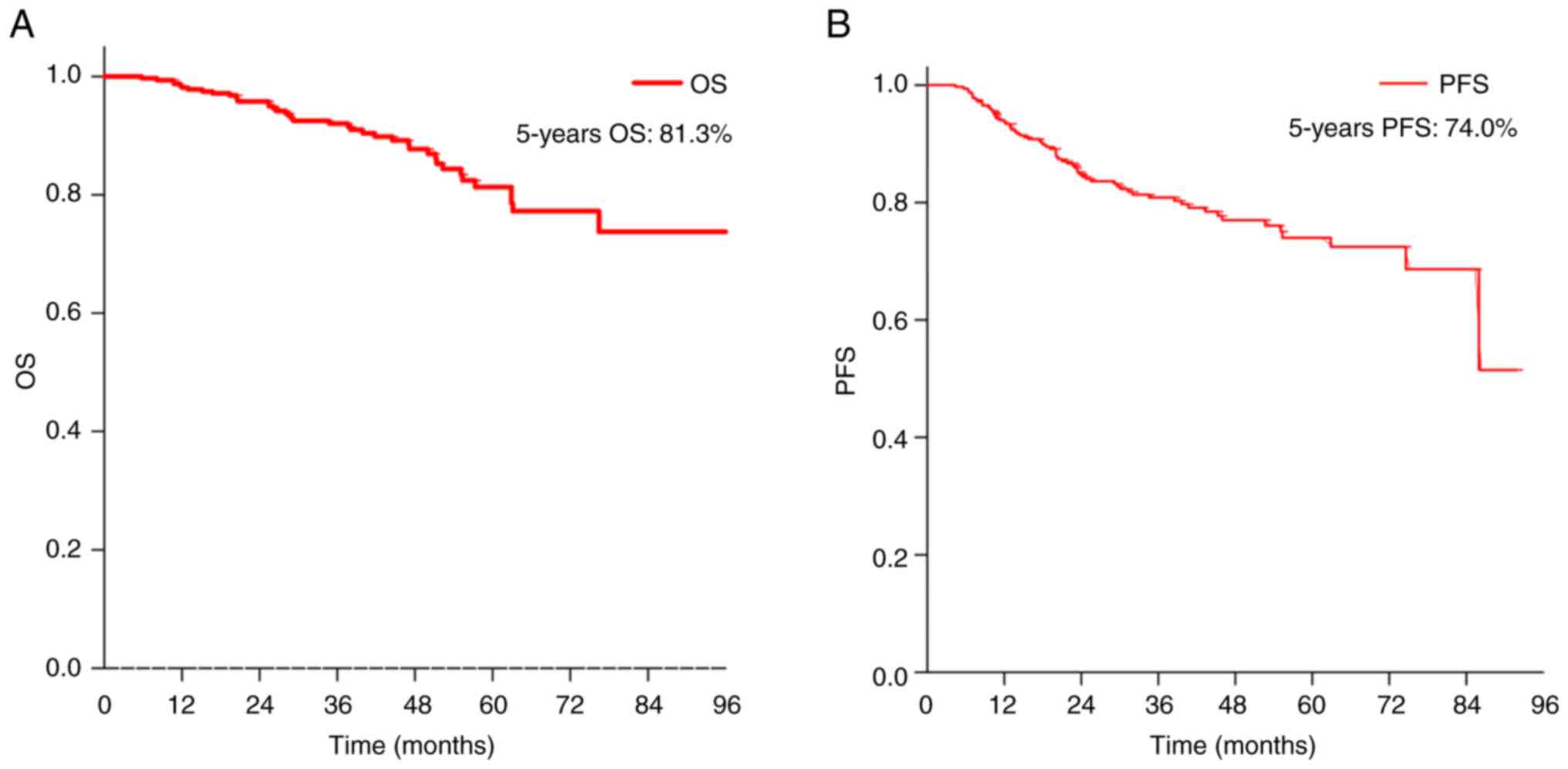
The median follow-up time was 40.4 months (range,
5.75–98.79 months). Median OS and PFS were not reached. The 5-year
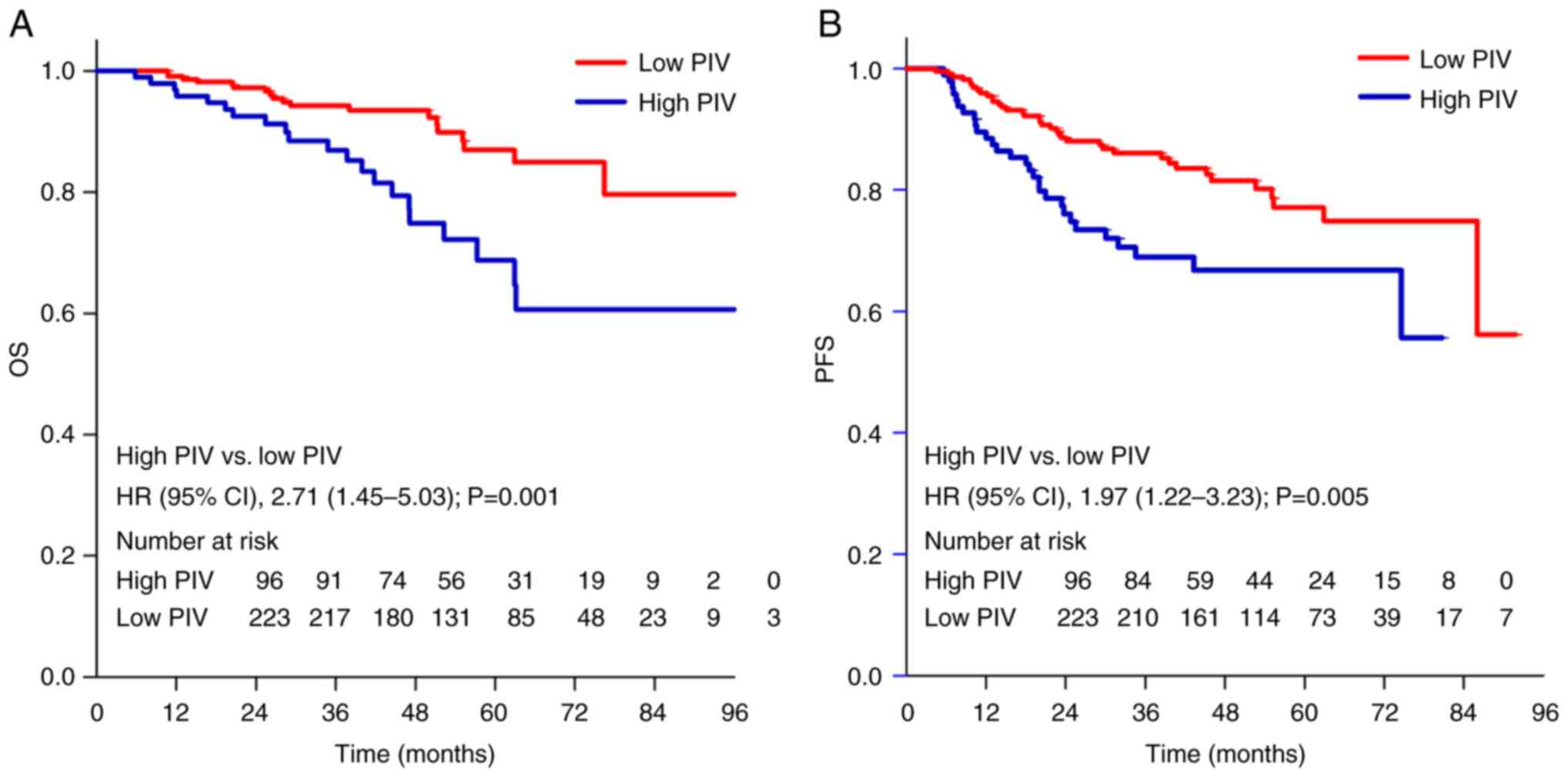
OS and PFS values were 81.3 and 74.0% (Fig. 2), respectively. Patients with high
PIV had a worse PFS (5-year PFS, 66.8 vs. 77.1%; HR, 1.97; 95% CI,
1.22–3.23) and a worse OS (5-year OS, 68.7 vs. 86.9%; HR, 2.71; 95%
CI, 1.45–5.03) in comparison with patients with low PIV (Fig. 3).
In the univariate analysis (Table III), advanced T and N stage and
high SII and PIV were significantly associated with a poor OS.
Advanced N stage and high SII and PIV were significantly associated
with a poor PFS.
 | Table III.Univariate cox regression analyses
for OS and PFS in patients with nasopharyngeal carcinoma. |
Table III.
Univariate cox regression analyses
for OS and PFS in patients with nasopharyngeal carcinoma.
| A, OS |
|---|
|
|---|
| Factor | HR (95% CI) | P-value |
|---|
| Age | 1.02
(0.99–1.05) | 0.209 |
| Sex (Female vs.
male) | 0.67
(0.32–1.40) | 0.284 |
| Smoking (Yes vs.
no) | 1.80
(0.93–3.50) | 0.082 |
| T stage (T3-4 vs.
T1-2) | 1.42
(1.01–2.01) | 0.044 |
| N stage (N2-3 vs.
N0-1) | 2.17
(1.46–3.23) | <0.001 |
| Induction
chemotherapy | 7.10
(0.97–51.7) | 0.053 |
| (Yes vs. no) |
|
|
| SII (high vs.
low) | 2.23
(1.19–4.21) | 0.013 |
| PIV (high vs.
low) | 2.71
(1.45–5.03) | 0.002 |
|
| B, PFS |
|
| Factor | HR (95%
CI) | P-value |
|
| Age | 1.01
(0.98–1.03) | 0.535 |
| Sex (Male vs.
female) | 1.08
(0.64–1.08) | 0.775 |
| Smoking (Yes vs.
no) | 1.37
(0.79–2.35) | 0.259 |
| T stage (T3-4 vs.
T1-2) | 1.18
(0.92–1.52) | 0.198 |
| N stage (N2-3 vs.
N0-1) | 1.59
(1.19–2.13) | 0.002 |
| Induction
chemotherapy | 1.70
(0.73–3.93) | 0.217 |
| (Yes vs. no) |
|
|
| SII (high vs.
low) | 1.82
(1.09–3.03) | 0.022 |
| PIV (high vs.
low) | 1.97
(1.22–3.23) | 0.006 |
In the multivariate Cox regression analyses
(Table IV), advanced N stage (HR,
2.05; 95% CI, 1.39–3.02) and high PIV (HR, 2.19; 95% CI, 1.16–4.12)
were significant independent predictors for OS. Similar results
were observed for PFS, with advanced N stage (HR, 1.54; 95% CI,
1.15–2.05) and high PIV (HR, 1.86; 95% CI, 1.14–3.04) significant
independent predictors for PFS. The blood-based inflammation marker
SII was not significant in the multivariate analysis of OS and
PFS.
 | Table IV.Multivariate cox regression analyses
for OS and PFS in patients with nasopharyngeal carcinoma. |
Table IV.
Multivariate cox regression analyses
for OS and PFS in patients with nasopharyngeal carcinoma.
| A, OS |
|---|
|
|---|
| Factor | HR (95% CI) | P-value |
|---|
| N stage (N2-3 vs.
N0-1) | 2.05
(1.39–3.02) | <0.001 |
| PIV (high vs.
low) | 2.19
(1.16–4.12) | 0.016 |
|
| B, PFS |
|
| Factor | HR (95%
CI) | P-value |
|
| N stage (N2-3 vs.
N0-1) | 1.54
(1.15–2.05) | 0.003 |
| PIV (high vs.
low) | 1.86
(1.14–3.04) | 0.013 |
Discussion
The present study demonstrated that PIV, as a novel
biomarker, was an independent predictor for poor OS and PFS in
patients with non-metastatic NPC and outperformed the SII, another
blood-derived inflammation marker.
Inflammation is relevant in cancer: An inflammatory
microenvironment is a key constituent of the tumor
microenvironment. Chronic inflammation caused by sustained
infection or ongoing exposure to non-infectious factors, such as
smoke, asbestos or silica, may eventually lead to carcinogenesis
(31–33).
White blood cell counts reflect the overall and/or
local inflammatory status (34) and
each type of white blood cells serves a unique role. Firstly,
neutrophils serve a primary role in regulating inflammation and
cancer and they actively promote progression and metastasis
(35). Secondly, peripheral
monocyte count is associated with the density of the M2 phenotype
of tumor-associated macrophages (36,37),
which derive from circulating monocytes within the tumor
microenvironment and promote metastasis and immunosuppression
(38,39). A high absolute monocyte count
predicts low survival rate for patients with cancer (37). Thirdly, platelets promote tumor cell
proliferation and survival through via mechanisms, such as
aggregation with tumor cells, thereby protecting them from host
immune surveillance through physical shielding and induction of
‘platelet mimicry’. This provides an immunosuppressive tumor
microenvironment, which is a key source of TGF-β, a key cytokine
for immunosuppression in the tumor microenvironment, and
facilitates vascular evasion (40,41).
High platelet count is associated with unfavorable outcomes in
several kinds of cancer such as lung cancer, colon cancer, breast
and prostate cancer (42). However,
lymphocytes have notable positive effects in tumor-associated
immunology. High lymphocyte levels are associated with an improved
prognosis in numerous types of tumor, exerting a strong antitumor
immune function to suppress tumor development (43). Leukocytes serve a role in the
development and outcomes of cancer.
Previous studies have analyzed the association
between each type of leukocyte (lymphocytes, neutrophils, platelets
and monocytes) and clinical results and have constructed
computational models (nomograms or scores) driven by statistical
methods (6–14). To avoid fragmenting systemic
inflammation information, SII and systemic inflammation response
index (SIRI) have been evaluated (8,10–12,16,17,44,45).
SII and SIRI have been assessed as prognostic markers for NPC
(8,12,16,17,44,45);
prognostic value of SII is superior to that of PLR, NLR and MLR
(8). However, two studies reported
that SIRI was not an independent risk factor for OS in patients
with NPC (10,11). Thus, the role of SIRI requires more
investigation. To the best of our knowledge, no studies have
comprehensively evaluated the prognosis of lymphocytes,
neutrophils, platelets, and monocytes in nasopharyngeal
carcinoma.
PIV is proposed as a biomarker based on peripheral
blood count, which integrates different peripheral blood immune
cell subpopulations (neutrophils, platelets, monocytes and
lymphocytes). Considering its potential to mirror comprehensive
manifestations of systemic inflammation and immunity, PIV is
considered a powerful and robust prognostic indicator of survival
in patients with cancer undergoing surgery, cytotoxic chemotherapy,
immunotherapy and targeted therapy (18–27). A
study reported that baseline PIV is a predictor for a pathological
complete response and survival and has greater predictive abilities
than NLR, MLR and PLR in patients with breast cancer receiving
neoadjuvant chemotherapy (20).
Similar results have been observed in patients with advanced human
epidermal growth factor receptor 2+ breast cancer (23). A proposed nomogram incorporating PIV
could be a feasible tool for individualized prognostic assessment
in patients with breast cancer receiving surgery (18). Furthermore, PIV is a strong
prognostic indicator of survival outcomes, outperforming other
IIBs, such as NLR and SII, not only in patients with metastatic CRC
receiving first-line therapy (24),
but also in patients with stage I–III CRC (19). PIV serves a role in patients with
advanced cancer receiving immune checkpoint inhibitors (21). Moreover, in patients with metastatic
melanoma, high PIV is significantly associated with primary
resistance to both targeted therapy [odds ratio (OR), 8.42; 95% CI,
2.50–34.5; P<0.001] and immunotherapy (OR, 3.98; 95% CI,
1.45–12.32; P=0.005). Therefore, PIV may be used to guide the
treatment decision process and the development of novel first-line
treatment strategies (22). In
esophageal cancer, high PIV is significantly associated with low
tumor-infiltrating lymphocyte status (P<0.001) and low CD8+ cell
count (P=0.011), which may allow response to treatment (25). In addition, high PIV is associated
with more advanced AJCC stage and younger age (46). Consistent with the aforementioned
studies, the present study demonstrated that patients with high PIV
had more advanced T stage and worse survival outcome than those
with a low PIV. Moreover, to the best of our knowledge, the present
study is the first to report that PIV may be a more reliable
predictor of OS and PFS than SII in patients with NPC.
The present study had limitations, including the
retrospective nature of the study and the fact that the population
was only recruited from a single center in an endemic area.
Furthermore, the EBV DNA data were missing in the present study.
Although EBV DNA has been established as a robust prognostic marker
in NPC for clinical outcomes, there is no internationally
recognized EBV DNA standardized testing process and comparatively
large interlaboratory variability has been observed, even for the
same assay using identical procedures without harmonization
(1,2,47,48).
In the present study, only 66.7% patients were tested for EBV DNA
because some patients refused to have their blood drawn again.
Among the tested patients, only 34.6% patients were positive. EBV
DNA testing is key but the accuracy of EBV DNA testing needs
improvement. NPC and laboratory medicine experts focused on
harmonization and validation of the assay, as well as adaptation of
new technologies to improve assay quantification, such as
next-generation sequencing or digital polymerase chain reaction
(1). However, tests for peripheral
blood immune cell are mature and robust. Moreover, they are
routine, cheap, convenient and stable and thus have good prospects
in clinical application (8,10–12,16,17,44,45).
To improve detection of EBV DNA, use of regular test markers to
predict the survival of patients with NPC should be performed, such
as using the PIV, in which a high PIV can predict worse survival
and identify patients that might need more intensive treatment. The
selection of appropriate patients requires exclusion of other
causes of abnormal blood results, such as fever. Although the
technology to detect peripheral blood immune cells is mature, the
cut-off value for the PIV (high vs. low) was different in the
present study and previous studies that the cut-off values raged
285.0 to 513.4 (18–27). In future studies, optimal PIV
cut-off for clinical application should be assessed. PIV is not
directly measured but calculated by a formula. In the future, PIV
results may be obtained directly through blood test instrument,
which may require a program to calculate PIV.
In conclusion, the present study demonstrated that
baseline PIV had a significant predictive value and outperformed
SII in patients with NPC. Therefore, the PIV may be a helpful tool
to tailor management of patients with NPC; however, further
research is needed to confirm the findings.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Panyu Science and
Technology Medical Project (Guangzhou, China; grant no.
2019-Z04-29) and Guangzhou Municipal Health Commission (grant no.
20211A011115).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ZS, JT, GRZ and XLC contributed to study conception
and design. Material preparation, data collection and analysis were
performed by ZS, JT, YH, WHZ and QY. The collection of pathological
data was performed by QY. ZS wrote the manuscript. ZS and JT
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the Declaration of Helsinki and was approved by the Ethics
Committee of Panyu Central Hospital (Guangzhou, China; approval no.
PYRC-2023-395). The data were anonymous and the requirement for
informed consent was waived.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NPC
|
nasopharyngeal carcinoma
|
|
IIB
|
immune-inflammatory biomarker
|
|
NLR
|
neutrophil-to-lymphocyte ratio
|
|
PLR
|
platelet-to-lymphocyte ratio
|
|
MLR
|
monocyte-to-lymphocyte ratio
|
|
SII
|
systemic immune-inflammation index
|
|
PIV
|
pan-immune-inflammation value
|
|
CRC
|
colorectal cancer
|
|
IC
|
induction chemotherapy
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
|
EBV
|
Epstein-Barr virus
|
|
ROC
|
receiver operating characteristic
|
|
SIRI
|
systemic inflammation response
index
|
References
|
1
|
Chen YP, Chan ATC, Le QT, Blanchard P, Sun
Y and Ma J: Nasopharyngeal carcinoma. Lancet. 394:64–80. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang LL, Chen YP, Chen CB, Chen MY, Chen
NY, Chen XZ, Du XJ, Fang WF, Feng M, Gao J, et al: The Chinese
Society of Clinical Oncology (CSCO) clinical guidelines for the
diagnosis and treatment of nasopharyngeal carcinoma. Cancer Commun
(Lond). 41:1195–1227. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Özdemir F and Baskiran A: The importance
of AFP in liver transplantation for HCC. J Gastrointest Cancer.
51:1127–1132. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang M, Cheng S, Jin Y, Zhao Y and Wang
Y: Roles of CA125 in diagnosis, prediction, and oncogenesis of
ovarian cancer. Biochim Biophys Acta Rev Cancer. 1875:1885032021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Au KH, Ngan RKC, Ng AWY, Poon DMC, Ng WT,
Yuen KT, Lee VHF, Tung SY, Chan ATC, Sze HCK, et al: Treatment
outcomes of nasopharyngeal carcinoma in modern era after intensity
modulated radiotherapy (IMRT) in Hong Kong: A report of 3328
patients (HKNPCSG 1301 study). Oral Oncol. 77:16–21. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Su L, Zhang M, Zhang W, Cai C and Hong J:
Pretreatment hematologic markers as prognostic factors in patients
with nasopharyngeal carcinoma: A systematic review and
meta-analysis. Medicine (Baltimore). 96:e63642017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Su Z, Mao YP, OuYang PY, Tang J and Xie
FY: Initial hyperleukocytosis and neutrophilia in nasopharyngeal
carcinoma: Incidence and prognostic impact. PLoS One.
10:e01367522015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang W, Chen Y, Huang J, Xi D, Chen J,
Shao Y, Xu G, Ying W, Wei J, Chen J, et al: Systemic
immune-inflammation index predicts the clinical outcome in patients
with nasopharyngeal carcinoma: A propensity score-matched analysis.
Oncotarget. 8:66075–66086. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takenaka Y, Kitamura T, Oya R, Ashida N,
Shimizu K, Takemura K, Yamamoto Y and Uno A: Prognostic role of
neutrophil-lymphocyte ratio in nasopharyngeal carcinoma: A
meta-analysis. PLoS One. 12:e01814782017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Q, Yu L, Yang P and Hu Q: Prognostic
value of inflammatory markers in nasopharyngeal carcinoma patients
in the intensity-modulated radiotherapy Era. Cancer Manag Res.
13:6799–6810. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen Y, Sun J, Hu D, Zhang J, Xu Y, Feng
H, Chen Z, Luo Y, Lou Y and Wu H: Predictive value of pretreatment
lymphocyte-to-monocyte ratio and platelet-to-lymphocyte ratio in
the survival of nasopharyngeal carcinoma patients. Cancer Manag
Res. 13:8767–8779. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiong Y, Shi LL, Zhu LS, Ding Q, Ba L and
Peng G: Prognostic efficacy of the combination of the pretreatment
systemic immune-inflammation index and epstein-barr virus DNA
status in locally advanced nasopharyngeal carcinoma patients. J
Cancer. 12:2275–2284. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zeng X, Liu G, Pan Y and Li Y: Development
and validation of immune inflammation-based index for predicting
the clinical outcome in patients with nasopharyngeal carcinoma. J
Cell Mol Med. 24:8326–8349. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang S, Zhao K, Ding X, Jiang H and Lu H:
Prognostic significance of hematological markers for patients with
nasopharyngeal carcinoma: A meta-analysis. J Cancer. 10:2568–2577.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang P, Zhao Y, Liang H, Zhou G, Youssef
B, Elhalawani H, Li M, Tan F, Jin Y, Jin H, et al:
Neutrophil-to-lymphocyte ratio trend: A novel prognostic predictor
in patients with nasopharyngeal carcinoma receiving radiotherapy.
Int J Biol Markers. 37:270–279. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oei RW, Ye L, Kong F, Du C, Zhai R, Xu T,
Shen C, Wang X, He X, Kong L, et al: Prognostic value of
inflammation-based prognostic index in patients with nasopharyngeal
carcinoma: A propensity score matching study. Cancer Manag Res.
10:2785–2797. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin C, Lin S, Guo QJ, Zong JF, Lu TZ, Lin
N, Lin SJ and Pan JJ: Systemic immune-inflammation index as a
prognostic marker in patients with newly diagnosed metastatic
nasopharyngeal carcinoma: A propensity score-matched study. Transl
Cancer Res. 8:2089–2098. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin F, Zhang LP, Xie SY, Huang HY, Chen
XY, Jiang TC, Guo L and Lin HX: Pan-Immune-Inflammation Value: A
new prognostic index in operative breast cancer. Front Oncol.
12:8301382022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sato S, Shimizu T, Ishizuka M, Suda K,
Shibuya N, Hachiya H, Iso Y, Takagi K, Aoki T and Kubota K: The
preoperative pan-immune-inflammation value is a novel prognostic
predictor for with stage I–III colorectal cancer patients
undergoing surgery. Surg Today. 52:1160–1169. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sahin AB, Cubukcu E, Ocak B, Deligonul A,
Oyucu Orhan S, Tolunay S, Gokgoz MS, Cetintas S, Yarbas G, Senol K,
et al: Low pan-immune-inflammation-value predicts better
chemotherapy response and survival in breast cancer patients
treated with neoadjuvant chemotherapy. Sci Rep. 11:146622021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guven DC, Yildirim HC, Bilgin E, Aktepe
OH, Taban H, Sahin TK, Cakir IY, Akin S, Dizdar O, Aksoy S, et al:
PILE: A candidate prognostic score in cancer patients treated with
immunotherapy. Clin Transl Oncol. 23:1630–1636. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fuca G, Beninato T, Bini M, Mazzeo L, Di
Guardo L, Cimminiello C, Randon G, Apollonio G, Bisogno I, Del
Vecchio M, et al: The pan-immune-inflammation value in patients
with metastatic melanoma receiving first-line therapy. Target
Oncol. 16:529–536. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ligorio F, Fuca G, Zattarin E, Lobefaro R,
Zambelli L, Leporati R, Rea C, Mariani G, Bianchi GV, Capri G, et
al: The pan-immune-inflammation-value predicts the survival of
patients with human epidermal growth factor receptor 2
(HER2)-Positive advanced breast cancer treated with first-line
taxane-trastuzumab-pertuzumab. Cancers (Basel). 13:19642021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fuca G, Guarini V, Antoniotti C, Morano F,
Moretto R, Corallo S, Marmorino F, Lonardi S, Rimassa L,
Sartore-Bianchi A, et al: The Pan-Immune-Inflammation Value is a
new prognostic biomarker in metastatic colorectal cancer: Results
from a pooled-analysis of the Valentino and TRIBE first-line
trials. Br J Cancer. 123:403–409. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baba Y, Nakagawa S, Toihata T, Harada K,
Iwatsuki M, Hayashi H, Miyamoto Y, Yoshida N and Baba H:
Pan-immune-inflammation value and prognosis in patients with
esophageal cancer. Ann Surg Open. 3:e1132021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guven DC, Sahin TK, Erul E, Kilickap S,
Gambichler T and Aksoy S: The association between the
pan-immune-inflammation value and cancer prognosis: A systematic
review and meta-analysis. Cancers (Basel). 14:26752022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang XC, Liu H, Liu DC, Tong C, Liang XW
and Chen RH: Prognostic value of pan-immune-inflammation value in
colorectal cancer patients: A systematic review and meta-analysis.
Front Oncol. 12:10368902022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Amin MB, Greene FL, Byrd DR, Brookland RK,
Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC,
Jessup JM, et al: AJCC Cancer Staging Manual. 8th edition.
Springer; New York, NY: 2017
|
|
29
|
Pfister DC, Spencer S, Adelstein D, Adkins
D, Anzai Y, Brizel DM, Bruce JY, Busse PM, Caudell JJ, Cmelak AJ,
et al: Head and neck cancers, version 2.2020, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
18:873–898. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yates JW, Chalmer B and McKegney FP:
Evaluation of patients with advanced cancer using the Karnofsky
performance status. Cancer. 45:2220–2224. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Khandia R and Munjal A: Interplay between
inflammation and cancer. Adv Protein Chem Struct Biol. 119:199–245.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Singh N, Baby D, Rajguru JP, Patil PB,
Thakkannavar SS and Pujari VB: Inflammation and cancer. Ann Afr
Med. 18:121–126. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Murata M: Inflammation and cancer. Environ
Health Prev Med. 23:502018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dupre A and Malik HZ: Inflammation and
cancer: What a surgical oncologist should know. Eur J Surg Oncol.
44:566–570. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu L, Saxena S, Awaji M and Singh RK:
Tumor-Associated neutrophils in cancer: Going Pro. Cancers (Basel).
11:5642019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shibutani M, Maeda K, Nagahara H, Fukuoka
T, Nakao S, Matsutani S, Hirakawa K and Ohira M: The peripheral
monocyte count is associated with the density of tumor-associated
macrophages in the tumor microenvironment of colorectal cancer: A
retrospective study. BMC Cancer. 17:4042017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shigeta K, Kosaka T, Kitano S, Yasumizu Y,
Miyazaki Y, Mizuno R, Shinojima T, Kikuchi E, Miyajima A, Tanoguchi
H, et al: High absolute monocyte count predicts poor clinical
outcome in patients with castration-resistant prostate cancer
treated with docetaxel chemotherapy. Ann Surg Oncol. 23:4115–4122.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou J, Tang Z, Gao S, Li C, Feng Y and
Zhou X: Tumor-Associated macrophages: Recent insights and
therapies. Front Oncol. 10:1882020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen Y, Song Y, Du W, Gong L, Chang H and
Zou Z: Tumor-associated macrophages: An accomplice in solid tumor
progression. J Biomed Sci. 26:782019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sharma D, Brummel-Ziedins KE, Bouchard BA
and Holmes CE: Platelets in tumor progression: A host factor that
offers multiple potential targets in the treatment of cancer. J
Cell Physiol. 229:1005–1015. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schmied L, Hoglund P and Meinke S:
Platelet-Mediated protection of cancer cells from immune
surveillance-possible implications for cancer immunotherapy. Front
Immunol. 12:6405782021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Giannakeas V, Kotsopoulos J, Brooks JD,
Cheung MC, Rosella L, Lipscombe L, Akbari MR, Austin PC and Narod
SA: Platelet count and survival after cancer. Cancers (Basel).
14:5492022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Quigley DA and Kristensen V: Predicting
prognosis and therapeutic response from interactions between
lymphocytes and tumor cells. Mol Oncol. 9:2054–2062. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Feng Y, Zhang N, Wang S, Zou W, He Y, Ma
JA, Liu P, Liu X, Hu C and Hou T: Systemic inflammation response
index is a predictor of poor survival in locally advanced
nasopharyngeal carcinoma: A propensity score matching study. Front
Oncol. 10:5754172020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen Y, Jiang W, Xi D, Chen J, Xu G, Yin
W, Chen J and Gu W: Development and validation of nomogram based on
SIRI for predicting the clinical outcome in patients with
nasopharyngeal carcinomas. J Investig Med. 67:691–698. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Demir H, Demirci A, Eren SK, Beypinar I,
Davarcı SE and Baykara M: A new prognostic index in young breast
cancer patients. J Coll Physicians Surg Pak. 32:86–91. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kim KY, Le QT, Yom SS, Pinsky BA, Bratman
SV, Ng RH, El Mubarak HS, Chan KC, Sander M and Conley BA: Current
state of PCR-Based epstein-barr virus DNA testing for
nasopharyngeal cancer. J Natl Cancer Inst. 109:djx0072017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Le QT, Zhang Q, Cao H, Cheng AJ, Pinsky
BA, Hong RL, Chang JT, Wang CW, Tsao KC, Lo YD, et al: An
international collaboration to harmonize the quantitative plasma
Epstein-Barr virus DNA assay for future biomarker-guided trials in
nasopharyngeal carcinoma. Clin Cancer Res. 19:2208–2215. 2013.
View Article : Google Scholar : PubMed/NCBI
|