Multiple myeloma (MM) is a neoplasm malignant
characterized by the clonal proliferation of abnormal plasma cells
(PCs) in the bone marrow and recurrent cytogenetic abnormalities
that can lead to bone marrow failure, bone destruction,
hypercalcemia, anaemia, infection, kidney dysfunction and
neurologic symptoms (1,2). The incidence of MM worldwide is on the
rise. In 2020, the age-standardized incidence rate of MM was 1.78
per 100,000 people globally, and the age-standardized mortality
rate was 1.14 per 100,000 people globally (3–5). The
occurrence, progression, treatment, resistance and prognosis of MM
are all associated with cytogenetic abnormalities (6–8).
Previous studies revealed that cytogenetic abnormalities, such as
t(4;14), t(14;16), t(14;20), 1q21+, del(1p), and
del(17p), can be used as predictors of poor prognosis (9–12).
Among them, 1q21+ is one of the most common chromosomal
abnormalities in MM, including 1q21 gain (three copies) and 1q21
amplification (≥4 copies) (13).
1q21+ has been found in ~30-40% of patients with newly
diagnosed MM (NDMM) (14,15).
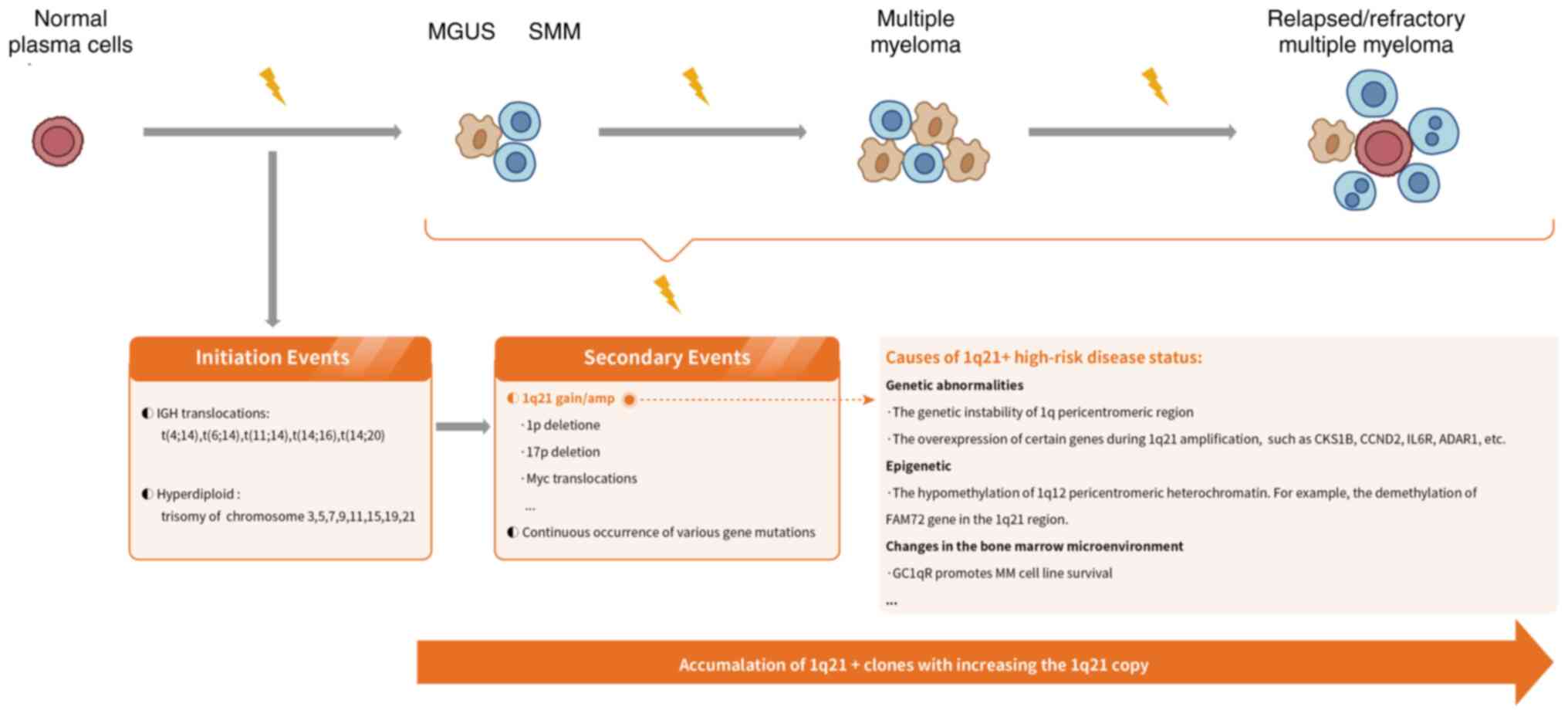
The evolution of MM is a complex and progressive
process in which initial carcinogenic events, namely primary
immunoglobulin heavy chain (IGH) translocations and hyper-diploid
karyotypes, have occurred in the early stages of monoclonal
gammopathy of uncertain significance and smouldering MM, followed
by disease progression to symptomatic MM (29,30)
(Fig. 1). Chromosome arm
1q21+ is a secondary genomic event occurring in patients
with MM (16). 1q21+
occurs in subclones of cells with primary translocations,
manifesting as chromosome 1q duplications, unbalanced whole-arm
translocations of chromosome 1q, or jumping translocations detected
by G-banding (30–33). Among them, jumping translocations
involve unbalanced translocations between non-homologous
chromosomes, caused by the exchange of the pericentromeric region
of 1q with other chromosomal sites, which involves the
de-condensation of pericentromeric chromatin of 1q and the
intranuclear replication of 1q, and the subsequent fusion of the
acquired region with the centromere or telomere end of another
chromosome (34,35). The jumping translocation can
increase the copy number of 1q, which may be the potential
mechanism of 1q21+ (30–36).
The genetic instability of 1q pericentromeric region
of 1q12 and its neighbor 1q21, as well as the overexpression of
certain genes that may be present during 1q21 amplification, are
closely associated with disease progression in MM (14,36).
Previous studies have found proliferation and dysregulation of G1/S
cell cycle checkpoints in most MM cells with 1q21+
(30). 1q21+ can
upregulate the activity of cyclin D by driving the oncogene CDC28
protein kinase regulatory subunit 1B (CKS1B), to further drive the
proliferation of downstream cells and promote the circulation of
PCs (37–39). 1q21+ is a copy number
alteration that is most relevant to transcriptome, and corresponds
to genome-wide transcriptional regulation, rather than being
restricted to this genomic region. It is associated with the
upregulation of cyclin D2 (CCND2) and other oncogene-driven
proliferation programs, such as PDL1 downregulation and Slam family
member 7 (SLAMF7), G protein-coupled receptor class c group
5-member D (GPRC5D), and apoptosis regulator BCL2 family member
(MCL1) upregulation predicted by 1q21 gain or amplification
(40). Interleukin-6 receptor
(IL6R) and ADAR1 (an RNA editing enzyme in 1q21 region near IL6R)
are critical genes located within the minimally amplified 1q21
region. The 1q21 state is closely related to the levels of IL6R and
ADAR1 (41,42). IL6R and ADAR1 collaborate to induce
a hyper-activation of the STAT3 pathway, and high levels of IL6 in
MM cells result in sustained activation of the STAT3 pathway
(43–45). The amplification of 1q21 leads to
elevated expression of IL6R and ADAR1. High IL6R confers
hypersensitivity to IL6 stimulation, leading to constitutive
activation of the STAT3 pathway. However, high expression of
ADAR1-P150 results in a hyper-activation of the STAT3 pathway, and
ultimately increases the survival rate of MM cells (42). In addition, the expression of some
genes (EANP32E, ARNT, BCL9, 2ILF2, MUC1, MCL1, NEK2, PSMD4, PDZK1
and SETDB1) is associated with 1q21+, promoting cell
proliferation (31,46–55).
In terms of epigenetic regulation, copy number gains
of 1q21 originate in part by the hypomethylation of 1q12
pericentromeric heterochromatin (56). A total of 25% of the genes
associated with MM-specific methylated regions lie within the
1q21.1 region. The demethylation of Family with sequence similarity
72 (FAM72) gene in the 1q21 region increases the expression of
FAM72, while FAM72 promotes MM cell proliferation through the
forkhead box M1 (FOXM1) transcription factor signaling pathway
(57). Moreover, the transcription
factor PBX homeobox (PBX)1 is ectopically expressed by genetic
amplification and epigenetic activation of its own preserved
regulatory domain. By binding to reprogrammed super-enhancers, PBX3
directly regulates critical oncogenic pathways and FOXM1-dependent
transcription programs to activate MM cell proliferation (58).
In addition to gene drive, changes in the bone
marrow microenvironment further contribute to the formation of a
high-risk disease state with 1q21+ (30). Complement factor C1q, as an
activator protein in the classical activation pathway, participates
in the recognition stage of classical activation pathway and has
the ability to regulate various immune cell responses (59). C1q has two classical cell surface
receptors (C1qRs): cC1qR binds to the collagen ‘stalks’ tail,
whereas gC1qR binds to the globular ‘heads’ (60). GC1qR promotes MM cell line survival
by suppressing the MM-inhibiting role of C1q and contributing to
the stabilization of the CKS1BmRNA through insulin-like growth
factor 2 mRNA binding protein 3 (IGF2BP3) (61).
With the deepening understanding of MM, the
influence or weight of various baseline risk factors in predicting
the outcomes of patients with MM may change. Recently, two major
prognostic scoring systems have been successively released; namely,
the second revision of the International Staging System (R2-ISS)
issued by the European Myeloma Network (EMN) and the Mayo Additive
Staging System (MASS) developed by the Mayo Clinic based on
single-center data (21,22). The R2-ISS stratifies patients with
NDMM into the following risk groups: i) low (0), ii)
low/intermediate (0.5–1), iii) intermediate/high (1.5–2.5) and iv)
high (3–5) by assigning different weight scores to
the six included variables: ISS II, ISS III, del(17p), high LDH,
t(4;14) and 1q+ (22).
The MASS includes IgH translocation, 1q+, chromosome 17
abnormality, ISS stage III, and elevated LDH as high risk (HR)
factors. Patients with 0, 1, or ≥2 HR abnormalities are considered
as stage I, II, or III, respectively (21). Both staging systems take
1q+ as a HR factor and use it as a basis for
stratification, which has been supported by several recent studies
(68,74). A meta-analysis involving 2,596
patients with MM found that 1q gain was associated with inferior
survival in NDMM, irrespective of current standard therapies, and
should be considered as an independent risk factor (68). Similarly, in a post-hoc analysis of
another clinical study, 1q gain at relapse was associated with
shorter OS, independent of other risk markers or time of relapse
(74). This means that 1q gain can
be an independent poor prognostic factor for both NDMM and relapsed
MM. A retrospective study of 248 Chinese patients with NDMM
detected that 1q21+ was an independent poor prognostic
factor in patients with NDMM. According to the mSMART 3.0 stages,
double-hit and triple-hit groups had the worst prognosis, with a
median progression-free survival (PFS) of 22 months and a median OS
of 32 months, respectively (69).
Another retrospective study of 505 Chinese NDMM patients (47% with
1q21+) confirmed the prognostic stratification value of
R2-ISS staging system in Chinese patients with NDMM and evaluated
the impact of 1q21+ on survival. The median PFS and OS
of patients carrying the 1q21+ mutation were 33.3 and
62.6 months, respectively, significantly shorter than those without
1q21 abnormality [PFS, 50.8 months; OS, not reached (NR);
P<0.001] (75).
1q21 gain has long been considered as an early
driver event, and 1q21 amplification a progression-related late
event, both of which have negative prognostic implications,
although the prognostic implications of increased copy number of
1q21+ are still controversial (Table II) (76–85).
In a cohort study of 912 symptomatic patients with MM, patients
with 1q21+ showed inferior PFS (34 vs. 20 months,
P<0.001) and OS (75 vs. 44 months, P<0.001) compared with
those without 1q21+, but increased copies of
1q21+ had no effect on prognosis (18). Increasing evidence has identified
that patients with MM and three copies of 1q21 had comparable
survival with patients with more than three copies. A cohort study
demonstrated that in 290 cases of patients with NDMM treated with
bortezomib-based therapy and 1q21 copy number ≥3 significantly
worse outcomes were observed; and there was no statistically
significant difference between median PFS and OS in patients who
had three, four, or at least five copies of 1q21 (86). This conclusion was confirmed in
another study with an enlarged sample (667 patients) and extended
follow-up (87). A real-world study
which included 1,068 diagnosed MM Chinese patients reported that
the variation in copy number of 1q+ (copy number <3)
had no significant impact on the survival of MM patients with 1q
abnormalities (88). Similarly, in
a meta-analysis, there was no significant difference in the
prognosis between 1q21 gain and 1q21 amplification for patients
with thalidomide and those with bortezomib, both being associated
with adverse outcome (68). There
are also differences with the aforementioned views. Several studies
have revealed that the prognosis of patients with MM is worse when
1q21 copy number ≥4; for instance, You et al (28) revealed that 1q21 amplification had
worse PFS than 1q21 gain (24 months vs. not reached, P=0.0403).
Neben et al (77) discovered
that a 1q21 copy number of three has a marginal negative effect,
and having more than three copies significantly reduces PFS and OS.
Similarly, Schmidt et al (82) and Gao et al (89) both revealed that copy number of 1q21
≥4 led to a poor prognosis. In a recent retrospective study of 794
patients with MM, the median PFS times of normal copy number of 1q,
1q gain and 1q amplification were 49, 50 and 26 months,
respectively (P=0.268), and the median OS times were NR, NR and 41
months, respectively (P=0.001), suggesting that 1q21 amplification
had a greater negative impact on prognosis than 1q21 gain (90). This might be related to the dose
response of genes in the chromosome 1q region.
Currently, different graded treatment strategies are
adopted for MM in clinical practice. The treatment strategies for
NDMM are determined by ASCT and risk stratification (93). ASCT uses a very large dose of
chemoradiotherapy to clear MM cells, destroy the autoimmune system,
and transfuse autologous hematopoietic stem cells to rebuild
hematopoietic and immune function, so as to achieve the purpose of
further eliminating MM cells (94–96).
ASCT is the upfront choice for newly diagnosed patients with MM. If
the patient is younger than 70 years old with favourable
performance status, or older than 70 years old with favourable
general performance status, ASCT should be the upfront choice after
effective therapy (13,97). Previously, a real-world study of
1,068 Chinese patients with NDMM revealed that upfront ASCT could
eliminate the adverse prognostic effect of 1q21 gain but not 1q21
amplification (98). Therefore, in
clinical practice, ASCT is often combined with other medications as
part of a normative overall treatment for NDMM patients (88,98–100).
A recent retrospective study included patients with NDMM who
received ASCT combined with VRD (V, bortezomib; R, lenalidomide; D,
dexamethasone) or VRD only for 8 cycles (69). The aforementioned study identified
that the proportion of patients with 1q21+ was 54.4% and
they showed a poor prognosis with the VRD regimen. However, ASCT
could overcome the adverse effects significantly, and it played an
important role in the prognosis of patients with 1q21+
(P<0.05) (69). Similarly, in a
retrospective study of 1,491 patients with NDMM (100 patients in
the 1q+/1p group) who received induction with IMiD combined with PI
or bortezomib-cyclophosphamide-dexamethasone, with a pretreatment
regimen of Busulfan in combination with melphalan or melphalan
only, and who received ASCT, a median OS was not reached in the
1q+/1p-group compared with 81.1 months in the control group (HR,
1.25; CI, 0.3–4.6; P=0.73); the objective response rate (ORR) was
94% in both groups, and ASCT had a positive effect on ORR and OS
compared with the historical control (98).
NCCN guidelines state that VRD is recommended as a
standard first-line therapy for patients with NDMM, regardless of
suitability for transplantation (93,101,102). Thalidomide, lenalidomide and
pomalidomide are all IMiDs. IMiDs exert their anti-myeloma activity
mainly by binding cereblon (CRBN), the substrate receptor protein
of the CRL4 E3 ubiquitin ligase (CRL4CRBN) complex, leading to
rapid ubiquitination and degradation of two specific B-cell
transcription factors, the Ikaros family of zinc-finger proteins
Ikaros (IKZF1) and Aiolos (IKZF3) (103–105). Thus, the direct target of IMiDs is
CRBN (106,107). IMiDs can directly eliminate MM
cells, promote the apoptosis of neovascular cells and enhance the
immune reaction of natural killer cells (108–110). PIs can covalently bind to the
hydroxyl group of the N-terminal threonine of the proteasome and
inhibit the normal degradation of intracellular proteins by the
proteasome, resulting in myeloma cell cycle arrest and promoting MM
cell apoptosis (111,112). The target of PI was the proteasome
β5 subunit (113,114). Among them, bortezomib could
reversibly inhibit the chymoprotease-like activity of proteasome β5
subunit (115). Carfilzomib
inhibits chymotrypsin-like activity by irreversibly binding to the
proteasome β5 subunit (116).
Ixazomib preferentially binds to the 20S proteasome β5 subunit to
inhibit chymotrypsin-like activity (114). A summary of studies on IMiDs and
PIs in NDMM patients with 1q21+ over the past 3 years is
presented in Table III (81,117–121). In a previous study, patients with
1q21 gain receiving bortezomib-based treatment had significantly
improved the OS and PFS when compared with non-bortezomib treatment
(3-year PFS, 62.8 vs. 8.75%; P=0.0385; 3-year OS, 82.3 vs. 18.8%;
P=0.0154) (81). In a cohort study,
the combination of PIs and IMiDs produced improved outcomes in
patients with 1q21+, but only partially alleviated the
effects of 1q21+ (18).
However, An et al (86)
discovered that 1q21 gain had no significant impact on the
prognosis of thalidomide-treated patients and that 1q21 gain had a
median PFS (22.4 months vs. 20.0 months; P=0.625) and OS (30.0 vs.
22.0 months, P=0.355) compared with patients without
1q21+. The prognosis was significantly worse in patients
treated with bortezomib, compared with patients without 1q21,
patients with 1q21 had significant shorter PFS (13.5 months vs.
43.0 months; P<0.001) and OS (24.0 vs. 54.0 months, P<0.001)
(86). Similarly, in the ENDURANCE
ECOG-ACRIN E1A11 trial, 1q21+ was associated with poorer
outcome either with VRD or carfilzomib + lenalidomide +
dexamethasone (KRD) (118). This
may be due to the high expression of 26S proteasome non-ATPase
regulatory subunit 4 (PSMD4) in patients with 1q21+ as
PSMD4 may mediate resistance to bortezomib by enhancing proteasome
activity, resulting in enhanced protein degradation, decreased
protein load and reduced apoptosis (51,122).
Subclonal cereblon mutations in patients treated
with IMiDs are associated with MM recurrence and prognosis. The
NCRI Myeloma XI study enrolled 178 patients with NDMM (19% of
patients with 1q+) who received induction therapy with
thalidomide, lenalidomide, or carfilzomib and lenalidomide combined
with dexamethasone and cyclophosphamide. The aforementioned study
found that progressive clonal expansion was a feature of 17.5% 1q
gain cases, whereby 1q gain at diagnosis evolved into 1q
amplification at relapse. Compared with normal 1q+,
1q+ from presentation and evolution of new gain
(1q+) at relapse were both associated with significantly
shorter OS (HR, 2.11; P=0.0040; and HR, 2.00; P=0.021,
respectively) (71).
Ixazomib combined with lenalidomide-dexamethasone
therapy has been shown to be beneficial in patients with
relapsed/refractory MM. In a double-blind, randomized and
placebo-controlled phase III clinical (TOURMALINE-MM1) study, 722
patients with relapsed/refractory MM were randomized 1:1 to IRD
group (ixazomib + lenalidomide + dexamethasone) and RD group
(lenalidomide + dexamethasone). The results revealed a 40%
prolongation of PFS in the IRD group compared with the RD group
(HR, 0.74; 95% CI, 0.59–0.94; P=0.01) (123). Post hoc analysis revealed a
significant PFS benefit for the IRD group in the ‘expanded
high-risk’ cohort including partial 1q21 amplification patients
(HR, 0.66; 95% CI: 0.47–0.93). However, no significant difference
was observed between the two groups in patients with 1q21
amplification only (HR, 0.78; 95% CI, 0.49–1.24 for a detection
threshold of 3% for 1q21+ by fluorescence in situ
hybridization (FISH); HR 0.682; 95% CI, 0.413–1.123 for a detection
threshold of 20% for 1q21+ by FISH; HR, 0.683; 95% CI,
0.381–1.224 for a detection threshold of 60% for 1q21+
by FISH (124). This suggests that
ixazomib has limited efficacy in patients with 1q21
amplification.
CD38 antibodies have become a critical part of
relapsed MM and its first-line therapy. CD38 is highly expressed by
MM cells and is a cell surface receptor target for antibody therapy
in patients with MM (126). The
eliminating effect of CD38 antibody on tumor cells is mainly
achieved through the Fc-mediated immune-effector mechanisms,
including complement-dependent cytotoxicity, antibody-dependent
cellular cytotoxicity, antibody-dependent cellular phagocytosis and
secondary crosslinking-induced apoptosis (127). Daratumumab and Isatuximab, both
CD38-targeting antibodies, have been approved by FDA. Daratumumab,
an IgG1κ humanized monoclonal antibody that binds to CD38, reduces
levels of myeloid-derived suppressor cells (CD38+MDSCs),
regulatory T cells (CD38+Tregs), and B cells
(CD38+Bregs) (128,129). Isatuximab is a chimeric humanized
IgG1 monoclonal antibody that binds to a specific epitope on the
human cell surface antigen CD38 (130). Isatuximab can induce MM cell death
by caspase-dependent apoptosis and lysosome-mediated non-apoptotic
cell elimination (131). A summary
of studies on CD38 antibodies in patients with 1q21+
over the past 3 years is presented in Table IV.
Several studies have revealed that Daratumumab may
improve the rate of MRD negativity and PFS in patients with newly
diagnosed 1q21+, but has limited effect on patients with
relapsed 1q21+. The GRIFFIN study included 207
transplantation-eligible patients with NDMM who were randomized to
the D-RVD group (n=104) and the RVD group (n=103), and revealed
that for patients with 1q21+, the rates of MRD
negativity (10−5) in the D-RVD group and the RVD group
were 61.8 and 28.6% (OR, 4.04; 95% CI, 1.38–11.81), respectively,
and that the median PFS in the D-RVD group was higher (NR vs. 47.9
months) (OR, 0.42; 95% CI, 0.14–1.27) (132). This result suggests that the
combination of D-RVD induction/consolidation with ASCT and R + DARA
maintenance therapy increased the rate of MRD negativity and PFS in
the 1q21+ subgroup. However, the poor prognosis of
1q21+ may be difficult to be overcome with Daratumumab
in patients with MM and relapsed 1q21+. A study that
investigated the outcomes of all patients with refractory MM
receiving Daratumumab found that before initiating the treatment
with Daratumumab, patients who were 1q21+ positive and
classified as high-risk for GEP70 had the worst outcomes (0.3 and
0.8 years, respectively for PFS and OS), while patients without
1q21+ and low-risk for GEP70 did not reach the median
PFS and OS (133). This
observation indicates that a poor prognosis associated with
1q21+ may not be overcome by Daratumumab. Another
multicenter retrospective study enrolled 232 RRMM patients who
received a DARA-based regimen and underwent FISH following 1st-3rd
line therapies (134). At a median
follow-up of 35.7 months in the study, the median PFS for patients
with 1q21+ using the DARA-based regimen was 24.6 months,
and the ORR was 57.9%. There were no significant differences in PFS
and ORR between patients with 1q21+ using the DARA
regimen and patients without 1q21+. Similarly, there
were also no significant differences in PFS or ORR among patients
with 1q21+ using different treatment regimens. The
prognosis and response profiles in patients with 1q21+
using the DARA regimen were similar to those with standard-risk
cytogenetics (SRCyto) or other high-risk cytogenetics (HRCyto)
(134). Although the prognosis in
patients with 1q21+ using the DARA regimen in this study
was similar to that in patients without 1q21+, given
that median PFS and ORR were lower in patients with
1q21+ compared with historical controls (80,119,125) further studies are required to
validate the role of Daratumumab in patients with relapsed
1q21+.
Unlike Daratumumab, the efficacy of Isatuximab in
the treatment of relapsed/refractory MM with 1q21+ has
been demonstrated in several studies. In the phase III studies
ICARIA-MM and IKEMA, the addition of Isatuximab (Isa) to the
backbone of pomalidomide-dexamethasone (PD) or
carfilzomib-dexamethasone (KD), respectively, improved PFS among
patients with relapsed/refractory MM, and subgroup analyses
suggested benefit among patients with 1q21+ (136,137). Recently, four 1q21-related
subgroups from ICARIA-MM and IKEMA were analyzed and showed
improvement in median PFS and OS in patients with 1q21+
in the Isa-PD group when compared with the PD group. The median PFS
was 9.5 and 3.8 months (HR, 0.40; 95% CI, 0.25–0.63) in patients
with 1q21+, and the median OS was 21.3 and 13.9 months
(HR, 0.72; 95% CI, 0.48–1.07). The median PFS and OS of patients
with 1q21+ and patients without 1q21+ were
similar in the Isa-PD group, with a median PFS of 9.5 and 11.6
months, respectively in both groups (HR=1.19; 95% CI, 0.68–2.10)
and a median OS of 21.3 and 21.2 months, respectively (HR, 1.30;
95% CI, 0.80–2.14). In patients with 1q21+, the median
PFS was improved in the Isa + KD group compared with the KD group
(NR vs. 16.2 months, HR, 0.57; 95% CI, 0.33–0.98) (138). The aforementioned study has shown
that Isatuximab combined with PD or KD can all improve the poor
prognosis of 1q21+ in patients with relapsed/refractory
MM.
Elotuzumab is a monoclonal antibody targeting
signaling lymphocytic activation molecule family member 7 (SLAMF7),
which is highly expressed by MM cells (139). Elotuzumab can not only directly
act on SLAMF7 that is highly expressed on the surface of PCs to
inhibit intercellular adhesion, thereby reducing the growth
stimulation effect of stromal cells on myeloma cells, but also
target, bind to and label SLAMF7 on the surface of myeloma cells,
and enhance antibody-dependent cellular cytotoxicity in myeloma
cells mediated by NK cells (140,141).
The SWOG-1211 study is a randomized, multicenter
phase II clinical study that included 100 ASCT-ineligible patients
with high-risk NDMM, where high-risk MM is defined as: High risk
based on gene expression profiling analysis, t(14;16), t(14;20),
del(17p) or 1q21+, primary plasma cell leukaemia and
elevated serum LDH (≥2 times the upper limit of normal). The
proportion of patients with 1q21+ was 47%. In the
aforementioned study, there was no significant difference in
prognosis between the RVD + elotuzumab group and the RVd group,
with a PFS of 31 and 34 months, respectively (HR=0.968; 80% CI,
0.697–1.344; P=0.45). Similarly, in patients with 1q21+,
the addition of elotuzumab did not improve the prognosis, with a
median PFS of 41 months (95% CI, 22-NR) in the RVd group and 32
months (95% CI, 18-NR) in the RVD + elotuzumab group, with no
statistical difference between the two groups (142).
Recently, a multicenter, single-arm phase II
clinical study analyzed the efficacy and safety of elotuzumab
combined with KRD (Elo-KRD) regimen in NDMM patients without an
intent for ASCT. The study included 46 patients with NDMM,
including 35% of patients with 1q21+. After 8 courses of
Elo-KRD, a total of 26 (58%) patients achieved stringent complete
response (sCR) and/or MRD negativity. Finally, 17 (38%) patients
achieved sCR, 21 (47%) patients achieved CR or better, 38 (84%)
patients achieved VGPR or better, and 39 (87%) patients achieved PR
or better. In the evaluation of MRD, 26 (63%) patients of achieved
MRD negativity following 8 courses of Elo-KRD, among whom 19 (44%)
patients remained MRD negativity during the 8th to 12th courses and
15 (50%) patients sustained MRD negativity lasting for >1 year.
This study demonstrated a significant increase in sCR in NDMM
patients treated with elotuzumab + KRD but did not specifically
evaluate the actual efficacy of elotuzumab in patients with
1q21+ (143).
Selinexor is an oral selective inhibitor of nuclear
export that has been approved by FDA for the treatment of
relapsed/refractory MM. The target of Selinexor was exportin 1
(XPO1)/chromosome region maintenance 1, and selinexor reversibly
inhibits tumor suppressor proteins (TSPs), growth regulators and
oncogenic protein RNAs from the nucleus by blocking XPO1, which
leads to nuclear accumulation of TSPs, reduction of oncoproteins
such as c-myc and cyclinD1, cell cycle arrest and tumor cell
apoptosis (144–147).
The MARCH study is a multicenter, single-arm phase
II clinical study that included 82 RRMM patients who had disease
refractory to PI and IMiD, including 64.6% of patients with
1q21+ (148). In the
aforementioned study, selinexor 80 mg combined with dexamethasone
20 mg was administered orally in patients on day 1 and day 3 of
each week in 4-week cycle for multiple cycles. The results
demonstrated an ORR of 29.3% (95% CI, 19.7–40.4), a median duration
of response of 4.7 months, and median PFS and OS of 3.7 and 13.2
months, respectively (148). The
aforementioned study did not analyze patients with 1q21+
separately but had a high proportion of patients with
1q21+. Similarly, the STOMP-XKd study, which involved 32
patients with RRMM, evaluated the safety and efficacy of selinexor
(80 or 100 mg) + carfilzomib (56 or 70 mg/m2) +
dexamethasone (40 mg) (XKD). The results showed that 53% of
patients had high-risk cytogenetics del(17p), t(4;14), t(14;16)
and/or gain 1q. The ORR was 78% after treatment with XKD, and
median PFS was 15 months. In addition, XKD was well tolerated
(149). Therefore, selinexor
combined with dexamethasone could be used as a clinical option for
the treatment of patients with RRMM, including
1q21+.
The interaction of MM cells with the bone marrow
microenvironment generates a high-risk ecosystem that facilitates
MM cell survival and immune response failure. 1q21+ is a
secondary genomic event that occurs following primary IGH
translocations. Therefore, the occurrence of 1q21+ is
crucial for the evolution of myeloma cells to a high-risk state. In
addition to the internal reasons such as genetic instability and
gene driving in the pericentromeric region of chromosome 1, the
genetic mechanism of 1q21+ also includes the protective
effect of bone marrow microenvironment on MM cells. Therefore, the
presence of 1q21+ predisposes to the risk of drug
resistance and disease progression. The precise molecular
mechanisms of acquiring 1q21+ and its effect on MM
pathophysiology are yet to be fully elucidated. Further studies are
required to assess the impact of the ecosystem formed by the bone
marrow microenvironment and MM cells on 1q21+-induced
resistance and aggressiveness.
The application of CD138 immunomagnetic bead
sorting combined with FISH technology is the most reliable method
for the detection of 1q21+, but the threshold for
1q21+ in FISH testing varies significantly from
<5-30% in various studies at present (89,154).
EMN recommended 20% as the threshold for FISH testing (15), while most clinical trials generally
do not report the threshold for 1q21+ by FISH testing or
use a laboratory-defined threshold for evaluation. Inconsistencies
in the thresholds for 1q21+ by FISH testing may result
in differences in prognostic indicators such as PFS. If using 20%
as the threshold for positive 1q21+, it might miss some
patients with minor clones of 1q21+. For example, the
retrospective study used 20 healthy donors' bone marrow specimens
to set the thresholds for 1q21+ by MACS-FISH and
direct-FISH and establish by a ‘mean + 3× standard deviation’
calculation, the cut-off values for each abnormality were 5% for
1q21+. The result indicated that 1q21+ clone
sizes of 5–20% had clear adverse prognostic significance, and 5%
was a reliable cut-off value for 1q21+ (89). If the EMN
threshold had been applied to that study, then 25% of patients who
tested positive for 1q21+ would have been considered
negative. Therefore, there is currently a lack of reasonable
threshold criteria for 1q21+ by FISH testing to improve
evaluation of the prognostic status of patients with
1q21+.
The therapeutic limitation of MM is determined by
clonal heterogeneity. 1q21+ is highly heterogeneous due
to its association with the expression of multiple driver genes,
posing numerous challenges to its treatment. IMiD/PI induction
regimens alleviated the PFS disadvantage of 1q gain in some studies
but did not improve the prognosis of 1q amplification. IMiD/PI
induction, upfront high-dose therapy, and ASCT had a positive, but
limited effect on the ORR and OS in patients with 1q21+.
Daratumumab may improve the rate of MRD negativity and PFS in
patients with newly diagnosed 1q21+, but there is a lack
of clinical evidence that would be beneficial for patients with
relapsed 1q21+. The actual efficacy in patients with
1q21+ was not specifically evaluated in studies of
elotuzumab and selinexor. Isatuximab may improve the rate of MRD
negativity in patients with newly diagnosed high-risk
1q21+, and in patients with RRMM, Isatuximab combined
with PD or KD can all enhance the poor prognosis of
1q21+. Based on the existing results in the field of
1q21+, Isatuximab combined with IMiD and PI may be the
therapy of the highest potential to improve the prognosis of
patients with 1q21+.
Not applicable.
The present study was supported by Sanofi.
Not applicable.
LW was responsible for designing the theme of the
review and ensuring that the descriptions are accurate and agreed
by all authors. NL and ZX were responsible for ideas, collecting
relevant studies from databases and writing of the manuscript. HL
made critical modifications to important knowledge content within
the manuscript. Data authentication is not applicable. All authors
read and approved the final version of the manuscript.
Not applicable.
Not applicable.
ZX is a Sanofi employee and may hold shares and/or
stock options in the company. The other authors declare that they
have no competing interests.
|
1
|
van de Donk NWCJ, Pawlyn C and Yong KL:
Multiple myeloma. Lancet. 397:410–427. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pop VS, Tomoaia G and Parvu A: Modern
imaging techniques for monitoring patients with multiple myeloma.
Med Pharm Rep. 95:377–384. 2022.PubMed/NCBI
|
|
3
|
Huang J, Chan SC, Lok V, Zhang L,
Lucero-Prisno DE III, Xu W, Zheng ZJ, Elcarte E, Withers M, Wong
MCS, et al: The epidemiological landscape of multiple myeloma: A
global cancer registry estimate of disease burden, risk factors,
and temporal trends. Lancet Haematol. 9:e670–e677. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rajkumar SV: Multiple myeloma: 2022 Update
on diagnosis, risk stratification, and management. Am J Hematol.
97:1086–1107. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hemminki K, Försti A, Houlston R and Sud
A: Epidemiology, genetics and treatment of multiple myeloma and
precursor diseases. Int J Cancer. 149:1980–1996. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dutta AK, Alberge JB,
Sklavenitis-Pistofidis R, Lightbody ED, Getz G and Ghobrial IM:
Single-cell profiling of tumour evolution in multiple
myeloma-opportunities for precision medicine. Nat Rev Clin Oncol.
19:223–236. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bolli N, Avet-Loiseau H, Wedge DC, Van Loo
P, Alexandrov LB, Martincorena I, Dawson KJ, Iorio F, Nik-Zainal S,
Bignell GR, et al: Heterogeneity of genomic evolution and
mutational profiles in multiple myeloma. Nat Commun. 5:29972014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rajkumar SV: Updated diagnostic criteria
and staging system for multiple myeloma. Am Soc Clin Oncol Educ
Book. 35:e418–e423. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Perrot A, Lauwers-Cances V, Tournay E,
Hulin C, Chretien ML, Royer B, Dib M, Decaux O, Jaccard A, Belhadj
K, et al: Development and validation of a cytogenetic prognostic
index predicting survival in multiple myeloma. J Clin Oncol.
37:1657–1665. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hanamura I: Multiple myeloma with
high-risk cytogenetics and its treatment approach. Int J Hematol.
115:762–777. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Caro J, Al Hadidi S, Usmani S, Yee AJ,
Raje N and Davies FE: How to treat high-risk myeloma at diagnosis
and relapse. Am Soc Clin Oncol Educ Book. 41:291–309. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Neuse CJ, Lomas OC, Schliemann C, Shen YJ,
Manier S, Bustoros M and Ghobrial IM: Genome instability in
multiple myeloma. Leukemia. 34:2887–2897. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schmidt TM, Fonseca R and Usmani SZ:
Chromosome 1q21 abnormalities in multiple myeloma. Blood Cancer J.
11:832021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hanamura I, Stewart JP, Huang Y, Zhan F,
Santra M, Sawyer JR, Hollmig K, Zangarri M, Pineda-Roman M, van
Rhee F, et al: Frequent gain of chromosome band 1q21 in plasma-cell
dyscrasias detected by fluorescence in situ hybridization:
Incidence increases from MGUS to relapsed myeloma and is related to
prognosis and disease progression following tandem stem-cell
transplantation. Blood. 108:1724–1732. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ross FM, Avet-Loiseau H, Ameye G,
Gutiérrez NC, Liebisch P, O'Connor S, Dalva K, Fabris S, Testi AM,
Jarosova M, et al: Report from the European myeloma network on
interphase FISH in multiple myeloma and related disorders.
Haematologica. 97:1272–1277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Burroughs Garcìa J, Eufemiese RA, Storti
P, Sammarelli G, Craviotto L, Todaro G, Toscani D, Marchica V and
Giuliani N: Role of 1q21 in multiple myeloma: From pathogenesis to
possible therapeutic targets. Cells. 10:13602021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bisht K, Walker B, Kumar SK, Spicka I,
Moreau P, Martin T, Costa LJ, Richter J, Fukao T, Macé S and van de
Velde H: Chromosomal 1q21 abnormalities in multiple myeloma: A
review of translational, clinical research, and therapeutic
strategies. Expert Rev Hematol. 14:1099–1114. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kastritis E, Migkou M, Dalampira D,
Gavriatopoulou M, Fotiou D, Roussou M, Kanellias N,
Ntanasis-Stathopoulos I, Malandrakis P, Theodorakakou F, et al:
Chromosome 1q21 aberrations identify ultra high-risk myeloma with
prognostic and clinical implications. Am J Hematol. 97:1142–1149.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li X, Chen W, Wu Y, Li J, Chen L, Fang B,
Feng Y, Liu J, Chen M, Gu J, et al: 1q21 gain combined with
high-risk factors is a heterogeneous prognostic factor in newly
diagnosed multiple myeloma: A multicenter study in China.
Oncologist. 24:e1132–e1140. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Garifullin A, Voloshin S, Shuvaev V,
Martynkevich I, Kleina E, Chechetkin A, Bessmeltcev S, Kuzyaeva A,
Sсhmidt A, Kuvshinov A and Pavlova I: Significance of modified risk
stratification msmart 3.0 and autologous stem cell transplantation
for patients with newly diagnosed multiple myeloma. Blood. 134
(Suppl 1):S55932019. View Article : Google Scholar
|
|
21
|
Abdallah NH, Binder M, Rajkumar SV, Greipp
PT, Kapoor P, Dispenzieri A, Gertz MA, Baughn LB, Lacy MQ, Hayman
SR, et al: A simple additive staging system for newly diagnosed
multiple myeloma. Blood Cancer J. 12:212022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
D'Agostino M, Cairns DA, Lahuerta JJ,
Wester R, Bertsch U, Waage A, Zamagni E, Mateos MV, Dall'Olio D,
van de Donk NWCJ, et al: Second revision of the international
staging system (R2-ISS) for overall survival in multiple myeloma: A
European myeloma network (EMN) report within the HARMONY project. J
Clin Oncol. 40:3406–3418. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pasvolsky O, Milton DR, Rauf M, Tanner MR,
Bashir Q, Srour S, Tang G, Saini N, Ramdial J, Masood A, et al:
Lenalidomide-based maintenance after autologous hematopoietic stem
cell transplantation for patients with high-risk multiple myeloma.
Transplant Cell Ther. 28:752.e1–752.e6. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Villalba A, Gonzalez-Rodriguez AP,
Arzuaga-Mendez J, Puig N, Arnao M, Arguiñano JM, Jimenez M, Canet
M, Teruel AI, Sola M, et al: Single versus tandem autologous
stem-cell transplantation in patients with newly diagnosed multiple
myeloma and high-risk cytogenetics. A retrospective, open-label
study of the PETHEMA/Spanish myeloma group (GEM). Leuk Lymphoma.
63:3438–3447. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barbieri E, Maccaferri M, Leonardi G,
Giacobbi F, Corradini G, Lagreca I, Barozzi P, Potenza L, Marasca R
and Luppi M: Adverse outcome associated with daratumumab-based
treatments in relapsed/refractory multiple myeloma patients with
amplification of chromosome arm 1q21: A single-center retrospective
experience. Ann Hematol. 101:2777–2779. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen L, Li Z, Li S, Fu W and Li R:
Prognostic value and efficacy evaluation of novel drugs for
multiple myeloma patients with 1q21 amplification (Amp1q21) only: A
systematic review of randomized controlled trials. J Cancer.
11:2639–2644. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang YT, Bao L, Chu B, Chen XH, Lu MQ, Shi
L, Gao S, Fang LJ, Xiang QQ and Ding YH: Amp 1q21 is more
predictable with dismal survival than gain 1q21 of newly diagnosed
multiple myeloma in real-world analysis. J Clin Lab Anal.
36:e243752022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
You H, Jin S, Wu C, Wang Q, Yan S, Yao W,
Shi X, Shang J, Yan L, Yao Y, et al: The independent adverse
prognostic significance of 1q21 gain/amplification in newly
diagnosed multiple myeloma patients. Front Oncol. 12:9383922022.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Manier S, Salem KZ, Park J, Landau DA,
Getz G and Ghobrial IM: Genomic complexity of multiple myeloma and
its clinical implications. Nat Rev Clin Oncol. 14:100–113. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pawlyn C and Morgan GJ: Evolutionary
biology of high-risk multiple myeloma. Nat Rev Cancer. 17:543–556.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sawyer JR, Tricot G, Lukacs JL, Binz RL,
Tian E, Barlogie B and Shaughnessy J Jr: Genomic instability in
multiple myeloma: Evidence for jumping segmental duplications of
chromosome arm 1q. Genes Chromosomes Cancer. 42:95–106. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Walker BA, Wardell CP, Murison A, Boyle
EM, Begum DB, Dahir NM, Proszek PZ, Melchor L, Pawlyn C, Kaiser MF,
et al: APOBEC family mutational signatures are associated with poor
prognosis translocations in multiple myeloma. Nat Commun.
6:69972015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Saxe D, Seo EJ, Bergeron MB and Han JY:
Recent advances in cytogenetic characterization of multiple
myeloma. Int J Lab Hematol. 41:5–14. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sawyer JR, Tian E, Heuck CJ, Epstein J,
Johann DJ, Swanson CM, Lukacs JL, Johnson M, Binz R, Boast A, et
al: Jumping translocations of 1q12 in multiple myeloma: A novel
mechanism for deletion of 17p in cytogenetically defined high-risk
disease. Blood. 123:2504–2512. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sawyer JR, Tricot G, Mattox S, Jagannath S
and Barlogie B: Jumping translocations of chromosome 1q in multiple
myeloma: Evidence for a mechanism involving decondensation of
pericentromeric heterochromatin. Blood. 91:1732–1741. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sawyer JR, Tian E, Walker BA, Wardell C,
Lukacs JL, Sammartino G, Bailey C, Schinke CD, Thanendrarajan S,
Davies FE, et al: An acquired high-risk chromosome instability
phenotype in multiple myeloma: Jumping 1q syndrome. Blood Cancer J.
9:622019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bergsagel PL, Kuehl WM, Zhan F, Sawyer J,
Barlogie B and Shaughnessy J Jr: Cyclin D dysregulation: An early
and unifying pathogenic event in multiple myeloma. Blood.
106:296–303. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hose D, Rème T, Hielscher T, Moreaux J,
Messner T, Seckinger A, Benner A, Shaughnessy JD Jr, Barlogie B,
Zhou Y, et al: Proliferation is a central independent prognostic
factor and target for personalized and risk-adapted treatment in
multiple myeloma. Haematologica. 96:87–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shaughnessy J: Amplification and
overexpression of CKS1B at chromosome band 1q21 is associated with
reduced levels of p27Kip1 and an aggressive clinical course in
multiple myeloma. Hematology. 10 (Suppl 1):S117–S126. 2005.
View Article : Google Scholar
|
|
40
|
Ziccheddu B, Da Vià MC, Lionetti M, Maeda
A, Morlupi S, Dugo M, Todoerti K, Oliva S, D'Agostino M, Corradini
P, et al: Functional impact of genomic complexity on the
transcriptome of multiple myeloma. Clin Cancer Res. 27:6479–6490.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Barillé S, Bataille R and Amiot M: The
role of interleukin-6 and interleukin-6/interleukin-6
receptor-alpha complex in the pathogenesis of multiple myeloma. Eur
Cytokine Netw. 11:546–551. 2000.PubMed/NCBI
|
|
42
|
Teoh PJ, Chung TH, Chng PYZ, Toh SHM and
Chng WJ: IL6R-STAT3-ADAR1 (P150) interplay promotes oncogenicity in
multiple myeloma with 1q21 amplification. Haematologica.
105:1391–1404. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gadó K, Domján G, Hegyesi H and Falus A:
Role of interleukin-6 in the pathogenesis of multiple myeloma. Cell
Biol Int. 24:195–209. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Song Z, Ren D, Xu X and Wang Y: Molecular
cross-talk of IL-6 in tumors and new progress in combined therapy.
Thorac Cancer. 9:669–675. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Quintanilla-Martinez L, Kremer M, Specht
K, Calzada-Wack J, Nathrath M, Schaich R, Höfler H and Fend F:
Analysis of signal transducer and activator of transcription 3
(Stat 3) pathway in multiple myeloma: Stat 3 activation and cyclin
D1 dysregulation are mutually exclusive events. Am J Pathol.
162:1449–1461. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Treon SP, Maimonis P, Bua D, Young G, Raje
N, Mollick J, Chauhan D, Tai YT, Hideshima T, Shima Y, et al:
Elevated soluble MUC1 levels and decreased anti-MUC1 antibody
levels in patients with multiple myeloma. Blood. 96:3147–3153.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Inoue J, Otsuki T, Hirasawa A, Imoto I,
Matsuo Y, Shimizu S, Taniwaki M and Inazawa J: Overexpression of
PDZK1 within the 1q12-q22 amplicon is likely to be associated with
drug-resistance phenotype in multiple myeloma. Am J Pathol.
165:71–81. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Legartova S, Krejci J, Harnicarova A,
Hajek R, Kozubek S and Bartova E: Nuclear topography of the 1q21
genomic region and Mcl-1 protein levels associated with
pathophysiology of multiple myeloma. Neoplasma. 56:404–413. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Slomp A, Moesbergen LM, Gong JN, Cuenca M,
von dem Borne PA, Sonneveld P, Huang DCS, Minnema MC and Peperzak
V: Multiple myeloma with 1q21 amplification is highly sensitive to
MCL-1 targeting. Blood Adv. 3:4202–4214. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Walker BA, Leone PE, Chiecchio L, Dickens
NJ, Jenner MW, Boyd KD, Johnson DC, Gonzalez D, Dagrada GP,
Protheroe RK, et al: A compendium of myeloma-associated chromosomal
copy number abnormalities and their prognostic value. Blood.
116:e56–e65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shaughnessy JD Jr, Qu P, Usmani S, Heuck
CJ, Zhang Q, Zhou Y, Tian E, Hanamura I, van Rhee F, Anaissie E, et
al: Pharmacogenomics of bortezomib test-dosing identifies
hyperexpression of proteasome genes, especially PSMD4, as novel
high-risk feature in myeloma treated with total therapy 3. Blood.
118:3512–3524. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhou W, Yang Y, Xia J, Wang H, Salama ME,
Xiong W, Xu H, Shetty S, Chen T, Zeng Z, et al: NEK2 induces drug
resistance mainly through activation of efflux drug pumps and is
associated with poor prognosis in myeloma and other cancers. Cancer
Cell. 23:48–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Marchesini M, Ogoti Y, Fiorini E, Aktas
Samur A, Nezi L, D'Anca M, Storti P, Samur MK, Ganan-Gomez I,
Fulciniti MT, et al: ILF2 is a regulator of RNA splicing and DNA
damage response in 1q21-amplified multiple myeloma. Cancer Cell.
32:88–100.e6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wu C, Yang T, Liu Y, Lu Y, Yang Y, Liu X,
Liu X, Ye L, Sun Y, Wang X, et al: ARNT/HIF-1β links high-risk 1q21
gain and microenvironmental hypoxia to drug resistance and poor
prognosis in multiple myeloma. Cancer Med. 7:3899–3911. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xiang J, Chen X, Chen M and Hou J:
Increased expression of SETDB1 predicts poor prognosis in multiple
myeloma. Biomed Res Int. 2022:33078732022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sawyer JR, Tian E, Heuck CJ, Johann DJ,
Epstein J, Swanson CM, Lukacs JL, Binz RL, Johnson M, Sammartino G,
et al: Evidence of an epigenetic origin for high-risk 1q21 copy
number aberrations in multiple myeloma. Blood. 125:3756–3759. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chatonnet F, Pignarre A, Sérandour AA,
Caron G, Avner S, Robert N, Kassambara A, Laurent A, Bizot M,
Agirre X, et al: The hydroxymethylome of multiple myeloma
identifies FAM72D as a 1q21 marker linked to proliferation.
Haematologica. 105:774–783. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Trasanidis N, Katsarou A, Ponnusamy K,
Shen YA, Kostopoulos IV, Bergonia B, Keren K, Reema P, Xiao X,
Szydlo RM, et al: Systems medicine dissection of chr1q-amp reveals
a novel PBX1-FOXM1 axis for targeted therapy in multiple myeloma.
Blood. 139:1939–1953. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Reid KBM: Complement component C1q:
Historical perspective of a functionally versatile, and
structurally unusual, serum protein. Front Immunol. 9:7642018.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Peerschke EI and Ghebrehiwet B: cC1qR/CR
and gC1qR/p33: Observations in cancer. Mol Immunol. 61:100–109.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Xu J, Sun Y, Jiang J, Xu Z, Li J, Xu T and
Liu P: Globular C1q receptor (gC1qR/p32/HABP1) suppresses the
tumor-inhibiting role of C1q and promotes tumor proliferation in
1q21-amplified multiple myeloma. Front Immunol. 11:12922020.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chen H, Zhou N, Shi H, Yu W, Wu L and Zhou
F: Presentation and outcomes of patients with multiple myeloma
harboring gain or amplification of 1q21 and receiving novel agent
therapies: Results from a single-center study. Hematology.
28:21779792023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yang P, Chen H, Liang X, Xu W, Yu S, Huang
W, Yi X, Guo Q, Tian M, Yue T, et al: Proposed risk-scoring model
for estimating the prognostic impact of 1q gain in patients with
newly diagnosed multiple myeloma. Am J Hematol. 98:251–263. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Avet-Loiseau H, Attal M, Moreau P,
Charbonnel C, Garban F, Hulin C, Leyvraz S, Michallet M,
Yakoub-Agha I, Garderet L, et al: Genetic abnormalities and
survival in multiple myeloma: The experience of the Intergroupe
Francophone du Myélome. Blood. 109:3489–3495. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wu KL, Beverloo B, Lokhorst HM, Segeren
CM, van der Holt B, Steijaert MM, Westveer PH, Poddighe PJ, Verhoef
GE, Sonneveld P, et al: Abnormalities of chromosome 1p/q are highly
associated with chromosome 13/13q deletions and are an adverse
prognostic factor for the outcome of high-dose chemotherapy in
patients with multiple myeloma. Br J Haematol. 136:615–623. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Bang SM, Kim YR, Cho HI, Chi HS, Seo EJ,
Park CJ, Yoo SJ, Kim HC, Chun HG, Min HC, et al: Identification of
13q deletion, trisomy 1q, and IgH rearrangement as the most
frequent chromosomal changes found in Korean patients with multiple
myeloma. Cancer Genet Cytogenet. 168:124–132. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Bock F, Lu G, Srour SA, Gaballa S, Lin HY,
Baladandayuthapani V, Honhar M, Stich M, Shah ND, Bashir Q, et al:
Outcome of patients with multiple myeloma and CKS1B gene
amplification after autologous hematopoietic stem cell
transplantation. Biol Blood Marrow Transplant. 22:2159–2164. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Weinhold N, Salwender HJ, Cairns DA, Raab
MS, Waldron G, Blau IW, Bertsch U, Hielscher T, Morgan GJ, Jauch A,
et al: Chromosome 1q21 abnormalities refine outcome prediction in
patients with multiple myeloma-a meta-analysis of 2,596 trial
patients. Haematologica. 106:2754–2758. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
You H, Jin S, Wu C, Wang Q, Yan S, Zhai Y,
Yao W, Shi X, Shang J, Yan L, et al: The independent adverse
prognostic significance of 1q21 gain/amplification in newly
diagnosed multiple myeloma patients. Blood. 140 (Suppl
1):S10056–S10057. 2022. View Article : Google Scholar
|
|
70
|
Kyle RA, Gertz MA, Witzig TE, Lust JA,
Lacy MQ, Dispenzieri A, Fonseca R, Rajkumar SV, Offord JR, Larson
DR, et al: Review of 1027 patients with newly diagnosed multiple
myeloma. Mayo Clin Proc. 78:21–33. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Krhovska P, Pika T, Proskova J, Balcarkova
J, Zapletalova J, Bacovsky J and Minarik J: Bone metabolism
parameters and their relation to cytogenetics in multiple myeloma.
Eur J Haematol. 109:75–82. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
D'Oronzo S, Brown J and Coleman R: The
role of biomarkers in the management of bone-homing malignancies. J
Bone Oncol. 9:1–9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kowalska M, Druzd-Sitek A, Fuksiewicz M,
Kotowicz B, Chechlinska M, Syczewska M, Walewski J and Kaminska J:
Procollagen I amino-terminal propeptide as a potential marker for
multiple myeloma. Clin Biochem. 43:604–608. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Croft J, Ellis S, Sherborne AL, Sharp K,
Price A, Jenner MW, Drayson MT, Owen RG, Chown S, Lindsay J, et al:
Copy number evolution and its relationship with patient outcome-an
analysis of 178 matched presentation-relapse tumor pairs from the
Myeloma XI trial. Leukemia. 35:2043–2053. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yan W, Fan H, Xu J, Liu J, Li L, Du C,
Deng S, Sui W, Xu Y, Zou D, et al: Prognostic value of the second
revision of the international staging system (R2-ISS) in a
real-world cohort of patients with newly-diagnosed multiple
myeloma. Chin Med J (Engl). 136:1744–1746. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Nemec P, Zemanova Z, Greslikova H,
Michalova K, Filkova H, Tajtlova J, Kralova D, Kupska R, Smetana J,
Krejci M, et al: Gain of 1q21 is an unfavorable genetic prognostic
factor for multiple myeloma patients treated with high-dose
chemotherapy. Biol Blood Marrow Transplant. 16:548–554. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Neben K, Lokhorst HM, Jauch A, Bertsch U,
Hielscher T, van der Holt B, Salwender H, Blau IW, Weisel K,
Pfreundschuh M, et al: Administration of bortezomib before and
after autologous stem cell transplantation improves outcome in
multiple myeloma patients with deletion 17p. Blood. 119:940–948.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Nahi H, Våtsveen TK, Lund J, Heeg BM,
Preiss B, Alici E, Møller MB, Wader KF, Møller HE, Grøseth LA, et
al: Proteasome inhibitors and IMiDs can overcome some high-risk
cytogenetics in multiple myeloma but not gain 1q21. Eur J Haematol.
96:46–54. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Shah GL, Landau H, Londono D, Devlin SM,
Kosuri S, Lesokhin AM, Lendvai N, Hassoun H, Chung DJ, Koehne G, et
al: Gain of chromosome 1q portends worse prognosis in multiple
myeloma despite novel agent-based induction regimens and autologous
transplantation. Leuk Lymphoma. 58:1823–1831. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Shah V, Sherborne AL, Walker BA, Johnson
DC, Boyle EM, Ellis S, Begum DB, Proszek PZ, Jones JR, Pawlyn C, et
al: Prediction of outcome in newly diagnosed myeloma: A
meta-analysis of the molecular profiles of 1905 trial patients.
Leukemia. 32:102–110. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Chen D, Zhou D, Xu J, Zhou R, Ouyang J and
Chen B: Prognostic value of 1q21 gain in multiple myeloma. Clin
Lymphoma Myeloma Leuk. 19:e159–e164. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Schmidt TM, Barwick BG, Joseph N, Heffner
LT, Hofmeister CC, Bernal L, Dhodapkar MV, Gupta VA, Jaye DL, Wu J,
et al: Gain of chromosome 1q is associated with early progression
in multiple myeloma patients treated with lenalidomide, bortezomib,
and dexamethasone. Blood Cancer J. 9:942019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Walker BA, Mavrommatis K, Wardell CP,
Ashby TC, Bauer M, Davies F, Rosenthal A, Wang H, Qu P, Hoering A,
et al: A high-risk, double-hit, group of newly diagnosed myeloma
identified by genomic analysis. Leukemia. 33:159–170. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Abdallah N, Greipp P, Kapoor P, Gertz MA,
Dispenzieri A, Baughn LB, Lacy MQ, Hayman SR, Buadi FK, Dingli D,
et al: Clinical characteristics and treatment outcomes of newly
diagnosed multiple myeloma with chromosome 1q abnormalities. Blood
Adv. 4:3509–3519. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Gao W, Jian Y, Du J, Li X, Zhou H, Zhang
Z, Yang G, Wang G, Tian Y, Li Y, et al: Gain of 1q21 is an adverse
prognostic factor for multiple myeloma patients treated by
autologous stem cell transplantation: A multicenter study in China.
Cancer Med. 9:7819–7829. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
An G, Xu Y, Shi L, Shizhen Z, Deng S, Xie
Z, Sui W, Zhan F and Qiu L: Chromosome 1q21 gains confer inferior
outcomes in multiple myeloma treated with bortezomib but copy
number variation and percentage of plasma cells involved have no
additional prognostic value. Haematologica. 99:353–359. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Du C, Mao X, Xu Y, Yan Y, Yuan C, Du X,
Liu J, Fan H, Wang Q, Sui W, et al: 1q21 gain but not t(4;14)
indicates inferior outcomes in multiple myeloma treated with
bortezomib. Leuk Lymphoma. 61:1201–1210. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wang Y, Xu J, Xu B, Li P, Yang Y, Wang W,
Xu T, Maihemaiti A, Lan T, Wang P, et al: The prognostic role of
1q21 gain/amplification in newly diagnosed multiple myeloma: The
faster, the worse. Cancer. 129:1005–1016. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Gao L, Liu Y, Li Y, Feng L, Wang Z, Wen L,
Wang F, Huang X, Lu J and Lai Y: The importance of FISH signal
cut-off value and copy number variation for 1q21 in newly diagnosed
multiple myeloma: Is it underestimated? Clin Lymphoma Myeloma Leuk.
22:535–544. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Liu X, Jia S, Chu Y, Tian B, Gao Y, Zhang
C, Zheng Y, Jia W, Liu X, Yuan R, et al: Chromosome 1q21 gain is an
adverse prognostic factor for newly diagnosed multiple myeloma
patients treated with bortezomib-based regimens. Front Oncol.
12:9385502022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Schavgoulidze A, Perrot A, Cazaubiel T,
Leleu X, Montes L, Jacquet C, Belhadj K, Brechignac S, Frenzel L,
Chalopin T, et al: Prognostic impact of translocation t(14;16) in
multiple myeloma according to the presence of additional genetic
lesions. Blood Cancer J. 13:1602023. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Pasvolsky O, Ghanem S, Milton DR, Rauf M,
Tanner MR, Bashir Q, Srour S, Saini N, Lin P, Ramdial J, et al:
Outcomes of patients with multiple myeloma and 1q
gain/amplification receiving autologous hematopoietic stem cell
transplant: The MD Anderson cancer center experience. Blood Cancer
J. 14:42024. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Cowan AJ, Green DJ, Kwok M, Lee S, Coffey
DG, Holmberg LA, Tuazon S, Gopal AK and Libby EN: Diagnosis and
management of multiple myeloma: A review. JAMA. 327:464–477. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Rajkumar SV, Dimopoulos MA, Palumbo A,
Blade J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E,
Richardson P, et al: International myeloma working group updated
criteria for the diagnosis of multiple myeloma. Lancet Oncol.
15:e538–e548. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Kumar SK and Rajkumar SV: The multiple
myelomas-current concepts in cytogenetic classification and
therapy. Nat Rev Clin Oncol. 15:409–421. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Bazarbachi AH, Al Hamed R, Malard F,
Bazarbachi A, Harousseau JL and Mohty M: Induction therapy prior to
autologous stem cell transplantation (ASCT) in newly diagnosed
multiple myeloma: An update. Blood Cancer J. 12:472022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Al Hamed R, Bazarbachi AH, Malard F,
Harousseau JL and Mohty M: Current status of autologous stem cell
transplantation for multiple myeloma. Blood Cancer J. 9:442019.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Varma A, Sui D, Milton DR, Tang G, Saini
N, Hasan O, Mukherjee A, Joseph JJ, Bashir Q, Rondon G, et al:
Outcome of multiple myeloma with chromosome 1q gain and 1p deletion
after autologous hematopoietic stem cell transplantation:
Propensity score matched analysis. Biol Blood Marrow Transplant.
26:665–671. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Kumar SK, Buadi FK and Rajkumar SV: Pros
and cons of frontline autologous transplant in multiple myeloma:
The debate over timing. Blood. 133:652–659. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ntanasis-Stathopoulos I, Gavriatopoulou M,
Kastritis E, Terpos E and Dimopoulos MA: Multiple myeloma: Role of
autologous transplantation. Cancer Treat Rev. 82:1019292020.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Dimopoulos MA, Moreau P, Terpos E, Mateos
MV, Zweegman S, Cook G, Delforge M, Hájek R, Schjesvold F, Cavo M,
et al: Multiple myeloma: EHA-ESMO clinical practice guidelines for
diagnosis, treatment and follow-up†. Ann Oncol.
32:309–322. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Brown PA, Shah B, Advani A, Aoun P, Boyer
MW, Burke PW, DeAngelo DJ, Dinner S, Fathi AT, Gauthier J, et al:
Acute lymphoblastic leukemia, version 2.2021, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
19:1079–1109. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Ito T, Ando H, Suzuki T, Ogura T, Hotta K,
Imamura Y, Yamaguchi Y and Handa H: Identification of a primary
target of thalidomide teratogenicity. Science. 327:1345–1350. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Lu G, Middleton RE, Sun H, Naniong M, Ott
CJ, Mitsiades CS, Wong KK, Bradner JE and Kaelin WG Jr: The myeloma
drug lenalidomide promotes the cereblon-dependent destruction of
Ikaros proteins. Science. 343:305–309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Krönke J, Udeshi ND, Narla A, Grauman P,
Hurst SN, McConkey M, Svinkina T, Heckl D, Comer E, Li X, et al:
Lenalidomide causes selective degradation of IKZF1 and IKZF3 in
multiple myeloma cells. Science. 343:301–305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Wang S and Jin FY: Advances on
immunomodulatory drugs against multiple myeloma. Zhonghua Xue Ye
Xue Za Zhi. 37:262–264. 2016.(In Chinese). PubMed/NCBI
|
|
107
|
Abe Y and Ishida T: Immunomodulatory drugs
in the treatment of multiple myeloma. Jpn J Clin Oncol. 49:695–702.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Parman T, Wiley MJ and Wells PG: Free
radical-mediated oxidative DNA damage in the mechanism of
thalidomide teratogenicity. Nat Med. 5:582–585. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Moreau P: How I treat myeloma with new
agents. Blood. 130:1507–1513. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Quach H, Ritchie D, Stewart AK, Neeson P,
Harrison S, Smyth MJ and Prince HM: Mechanism of action of
immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia.
24:22–32. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Cao Y, Zhu H, He R, Kong L, Shao J, Zhuang
R, Xi J and Zhang J: Proteasome, a promising therapeutic target for
multiple diseases beyond cancer. Drug Des Devel Ther. 14:4327–4342.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Thibaudeau TA and Smith DM: A practical
review of proteasome pharmacology. Pharmacol Rev. 71:170–197. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Nunes AT and Annunziata CM: Proteasome
inhibitors: Structure and function. Semin Oncol. 44:377–380. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Ito S: Proteasome inhibitors for the
treatment of multiple myeloma. Cancers (Basel). 12:2652020.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Moreau P, Richardson PG, Cavo M, Orlowski
RZ, San Miguel JF, Palumbo A and Harousseau JL: Proteasome
inhibitors in multiple myeloma: 10 Years later. Blood. 120:947–959.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Herndon TM, Deisseroth A, Kaminskas E,
Kane RC, Koti KM, Rothmann MD, Habtemariam B, Bullock J, Bray JD,
Hawes J, et al: U.S. food and drug administration approval:
Carfilzomib for the treatment of multiple myeloma. Clin Cancer Res.
19:4559–4563. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Chen Q, Han X, Zheng G, Yang Y, Li Y,
Zhang E, Yang L, Dong M, He D, He J and Cai Z: The adverse impact
of a gain in chromosome 1q on the prognosis of multiple myeloma
treated with bortezomib-based regimens: A retrospective
single-center study in China. Front Oncol. 12:10846832022.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Kapoor P, Schmidt TM, Jacobus S, Wei Z,
Fonseca R, Callander NS, Lonial S, Rajkumar SV and Kumar S:
OAB-052: Impact of chromosome 1 abnormalities on newly diagnosed
multiple myeloma treated with proteasome inhibitor,
immunomodulatory drug, and dexamethasone: Analysis from the
ENDURANCE ECOG-ACRIN E1A11 trial. Clin Lymphoma Myeloma Leuk. 21
(Suppl 2):S33–S34. 2021. View Article : Google Scholar
|
|
119
|
Tang HKK, Fung CY, Morgan GJ, Kumar S, Siu
L, Ip HWA, Yip SF, Lau KNH, Lau CK, Lee H, et al: The impact of
bortezomib-based induction in newly diagnosed multiple myeloma with
chromosome 1q21 gain. Ther Adv Hematol. 13:204062072210820432022.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Panopoulou A, Cairns DA, Holroyd A,
Nichols I, Cray N, Pawlyn C, Cook G, Drayson M, Boyd K, Davies FE,
et al: Optimizing the value of lenalidomide maintenance by extended
genetic profiling: An analysis of 556 patients in the Myeloma XI
trial. Blood. 141:1666–1674. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
D'Agostino M, Ruggeri M, Aquino S,
Giuliani N, Arigoni M, Gentile M, Olivero M, Vincelli ID, Capra A,
Mussatto C, et al: Impact of gain and amplification of 1q in newly
diagnosed multiple myeloma patients receiving carfilzomib-based
treatment in the forte trial. Blood. 136 (Suppl 1):S38–S40. 2020.
View Article : Google Scholar
|
|
122
|
Misiewicz-Krzeminska I, de Ramón C,
Corchete LA, Krzeminski P, Rojas EA, Isidro I, García-Sanz R,
Martínez-López J, Oriol A, Bladé J, et al: Quantitative expression
of Ikaros, IRF4, and PSMD10 proteins predicts survival in
VRD-treated patients with multiple myeloma. Blood Adv. 4:6023–6033.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Moreau P, Masszi T, Grzasko N, Bahlis NJ,
Hansson M, Pour L, Sandhu I, Ganly P, Baker BW, Jackson SR, et al:
Oral ixazomib, lenalidomide, and dexamethasone for multiple
myeloma. N Engl J Med. 374:1621–1634. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Avet-Loiseau H, Bahlis NJ, Chng WJ, Masszi
T, Viterbo L, Pour L, Ganly P, Palumbo A, Cavo M, Langer C, et al:
Ixazomib significantly prolongs progression-free survival in
high-risk relapsed/refractory myeloma patients. Blood.
130:2610–2618. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Gay F, Musto P, Rota-Scalabrini D,
Bertamini L, Belotti A, Galli M, Offidani M, Zamagni E, Ledda A,
Grasso M, et al: Carfilzomib with cyclophosphamide and
dexamethasone or lenalidomide and dexamethasone plus autologous
transplantation or carfilzomib plus lenalidomide and dexamethasone,
followed by maintenance with carfilzomib plus lenalidomide or
lenalidomide alone for patients with newly diagnosed multiple
myeloma (FORTE): A randomised, open-label, phase 2 trial. Lancet
Oncol. 22:1705–1720. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Wu HT and Zhao XY: Regulation of CD38 on
multiple myeloma and NK cells by monoclonal antibodies. Int J Biol
Sci. 18:1974–1988. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
van de Donk NWCJ, Richardson PG and
Malavasi F: CD38 antibodies in multiple myeloma: Back to the
future. Blood. 131:13–29. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Krejcik J, Casneuf T, Nijhof IS, Verbist
B, Bald J, Plesner T, Syed K, Liu K, van de Donk NW, Weiss BM, et
al: Daratumumab depletes CD38+ immune regulatory cells, promotes
T-cell expansion, and skews T-cell repertoire in multiple myeloma.
Blood. 128:384–394. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
van de Donk NWCJ and Usmani SZ: CD38
antibodies in multiple myeloma: Mechanisms of action and modes of
resistance. Front Immunol. 9:21342018. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Gozzetti A, Ciofini S, Simoncelli M,
Santoni A, Pacelli P, Raspadori D and Bocchia M: Anti CD38
monoclonal antibodies for multiple myeloma treatment. Hum Vaccin
Immunother. 18:20526582022. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Jiang H, Acharya C, An G, Zhong M, Feng X,
Wang L, Dasilva N, Song Z, Yang G, Adrian F, et al: SAR650984
directly induces multiple myeloma cell death via
lysosomal-associated and apoptotic pathways, which is further
enhanced by pomalidomide. Leukemia. 30:399–408. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Chari A, Kaufman JL, Laubach JP, Sborov
DW, Reeves B, Rodriguez C, Silbermann R, Costa LJ, Anderson LD Jr,
Nathwani N, et al: Daratumumab plus lenalidomide, bortezomib, and
dexamethasone (D-RVd) in transplant-eligible newly diagnosed
multiple myeloma (NDMM) patients (Pts): Final analysis of griffin
among clinically relevant subgroups. Blood. 140 (Suppl
1):S7278–S7281. 2022. View Article : Google Scholar
|
|
133
|
Mohan M, Weinhold N, Schinke C,
Thanedrarajan S, Rasche L, Sawyer JR, Tian E, van Rhee F and
Zangari M: Daratumumab in high-risk relapsed/refractory multiple
myeloma patients: Adverse effect of chromosome 1q21
gain/amplification and GEP70 status on outcome. Br J Haematol.
189:67–71. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Parrondo RD, Gardner LB, Alhaj Moustafa M,
Roy V, Sher T, Rasheed A, Warsame RM, Larsen JT, Gonsalves EI,
Kourelis T, et al: Therapeutic outcomes of relapsed-refractory
multiple myeloma patients with 1q21+treated with daratumumab-based
regimens: A retrospective analysis. Blood. 140 (Suppl
1):S7237–S7238. 2022. View Article : Google Scholar
|
|
135
|
Weisel K, Besemer B, Haenel M, Lutz R,
Mann C, Munder M, Goerner M, Reinhardt HC, Nogai A, Ko YD, et al:
Isatuximab, carfilzomib, lenalidomide, and dexamethasone (Isa-KRd)
in patients with high-risk newly diagnosed multiple myeloma:
Planned interim analysis of the GMMG-concept trial. Blood. 140
(Suppl 1):S1836–S1838. 2022. View Article : Google Scholar
|
|
136
|
Harrison SJ, Perrot A, Alegre A, Simpson
D, Wang MC, Spencer A, Delimpasi S, Hulin C, Sunami K, Facon T, et
al: Subgroup analysis of ICARIA-MM study in relapsed/refractory
multiple myeloma patients with high-risk cytogenetics. Br J
Haematol. 194:120–131. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Spicka I, Moreau P, Martin TG, Facon T,
Martinez G, Oriol A, Koh Y, Lim A, Mikala G, Rosiñol L, et al:
Isatuximab plus carfilzomib and dexamethasone in relapsed multiple
myeloma patients with high-risk cytogenetics: IKEMA subgroup
analysis. Eur J Haematol. 109:504–512. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Martin T, Richardson PG, Facon T, Moreau
P, Perrot A, Spicka I, Bisht K, Inchauspé M, Casca F, Macé S, et
al: Primary outcomes by 1q21+ status for isatuximab-treated
patients with relapsed/refractory multiple myeloma: Subgroup
analyses from ICARIA-MM and IKEMA. Haematologica. 107:2485–2491.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Hsi ED, Steinle R, Balasa B, Szmania S,
Draksharapu A, Shum BP, Huseni M, Powers D, Nanisetti A, Zhang Y,
et al: CS1, a potential new therapeutic antibody target for the
treatment of multiple myeloma. Clin Cancer Res. 14:2775–2784. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Ishibashi M, Morita R and Tamura H: Immune
functions of signaling lymphocytic activation molecule family
molecules in multiple myeloma. Cancers (Basel). 13:2792021.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Pazina T, James AM, MacFarlane AW IV,
Bezman NA, Henning KA, Bee C, Graziano RF, Robbins MD, Cohen AD and
Campbell KS: The anti-SLAMF7 antibody elotuzumab mediates NK cell
activation through both CD16-dependent and -independent mechanisms.
Oncoimmunology. 6:e13398532017. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Usmani SZ, Hoering A, Ailawadhi S, Sexton
R, Lipe B, Hita SF, Valent J, Rosenzweig M, Zonder JA, Dhodapkar M,
et al: Bortezomib, lenalidomide, and dexamethasone with or without
elotuzumab in patients with untreated, high-risk multiple myeloma
(SWOG-1211): Primary analysis of a randomised, phase 2 trial.
Lancet Haematol. 8:e45–e54. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Derman BA, Kansagra A, Zonder J, Stefka
AT, Grinblatt DL, Anderson LD Jr, Gurbuxani S, Narula S, Rayani S,
Major A, et al: Elotuzumab and weekly carfilzomib, lenalidomide,
and dexamethasone in patients with newly diagnosed multiple myeloma
without transplant intent: A phase 2 measurable residual
disease-adapted study. JAMA Oncol. 8:1278–1286. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Turner JG, Kashyap T, Dawson JL, Gomez J,
Bauer AA, Grant S, Dai Y, Shain KH, Meads M, Landesman Y and
Sullivan DM: XPO1 inhibitor combination therapy with bortezomib or
carfilzomib induces nuclear localization of IkappaBalpha and
overcomes acquired proteasome inhibitor resistance in human
multiple myeloma. Oncotarget. 7:78896–78909. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Gandhi UH, Cornell RF, Lakshman A, Gahvari
ZJ, McGehee E, Jagosky MH, Gupta R, Varnado W, Fiala MA, Chhabra S,
et al: Outcomes of patients with multiple myeloma refractory to
CD38-targeted monoclonal antibody therapy. Leukemia. 33:2266–2275.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Tai YT, Landesman Y, Acharya C, Calle Y,
Zhong MY, Cea M, Tannenbaum D, Cagnetta A, Reagan M, Munshi AA, et
al: CRM1 inhibition induces tumor cell cytotoxicity and impairs
osteoclastogenesis in multiple myeloma: Molecular mechanisms and
therapeutic implications. Leukemia. 28:155–165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Argueta C, Kashyap T, Klebanov B, Unger
TJ, Guo C, Harrington S, Baloglu E, Lee M, Senapedis W, Shacham S
and Landesman Y: Selinexor synergizes with dexamethasone to repress
mTORC1 signaling and induce multiple myeloma cell death.
Oncotarget. 9:25529–25544. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Qiu L, Xia Z, Fu C, Chen W, Chang C, Fang
B, An G, Wei Y, Cai Z, Gao S, et al: Selinexor plus low-dose
dexamethasone in Chinese patients with relapsed/refractory multiple
myeloma previously treated with an immunomodulatory agent and a
proteasome inhibitor (MARCH): a phase II, single-arm study. BMC
Med. 20:1082022. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Gasparetto C, Schiller GJ, Tuchman SA,
Callander NS, Baljevic M, Lentzsch S, Rossi AC, Kotb R, White D,
Bahlis NJ, et al: Once weekly selinexor, carfilzomib and
dexamethasone in carfilzomib non-refractory multiple myeloma
patients. Br J Cancer. 126:718–725. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Chng WJ, Dispenzieri A, Chim CS, Fonseca
R, Goldschmidt H, Lentzsch S, Munshi N, Palumbo A, Miguel JS,
Sonneveld P, et al: IMWG consensus on risk stratification in
multiple myeloma. Leukemia. 28:269–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Palumbo A, Avet-Loiseau H, Oliva S,
Lokhorst HM, Goldschmidt H, Rosinol L, Richardson P, Caltagirone S,
Lahuerta JJ, Facon T, et al: Revised international staging system
for multiple myeloma: A report from international myeloma working
group. J Clin Oncol. 33:2863–2869. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Sonneveld P, Avet-Loiseau H, Lonial S,
Usmani S, Siegel D, Anderson KC, Chng WJ, Moreau P, Attal M, Kyle
RA, et al: Treatment of multiple myeloma with high-risk
cytogenetics: A consensus of the International myeloma working
group. Blood. 127:2955–2962. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Rajkumar SV: Multiple myeloma: 2020 Update
on diagnosis, risk-stratification and management. Am J Hematol.
95:548–567. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Hanamura I: Gain/amplification of
chromosome arm 1q21 in multiple myeloma. Cancers (Basel).
13:2562021. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Goldman-Mazur S, Vesole DH and Jurczyszyn
A: Clinical implications of cytogenetic and molecular aberrations
in multiple myeloma. Acta Haematol Pol. 52:18–28. 2021. View Article : Google Scholar
|