Introduction
Hepatocellular carcinoma (HCC) is one of the most
prevalent types of malignancy worldwide (1). To date, the primary therapeutic
approach for patients with HCC is surgical resection (2). The majority of patients with
early-stage HCC are eligible for a number of radical treatments,
such as surgical resection, local ablation and liver
transplantation, resulting in a median survival time of >5
years; however, a significant proportion of patients with HCC are
initially diagnosed at intermediate or advanced stages, due to the
subtle onset of symptoms (3,4). This
delayed diagnosis frequently results in these patients being
categorized as ‘unresectable’, precluding them from the benefits of
timely radical hepatectomy. Currently available non-surgical
treatment options for unresectable HCC (uHCC) include locoregional
therapy (LRT) and systemic antitumor therapy, which may improve
prognosis (5–8).
The primary aim of conversion therapy is to
transform uHCC into resectable HCC, so that patients can receive
radical treatment and achieve long-term survival (9). Supporting this, patients with uHCC
have previously been reported to experience tumor shrinkage and
downstaging following LRT and systemic therapies. Such changes
include reductions in primary tumor size, decreases in tumor count,
regression of portal vein tumor thrombus or even the complete
disappearance of metastases, ultimately meeting the ‘resectable’
criteria (10–13). However, guidelines from the National
Comprehensive Cancer Network and the European Association for the
Study of Liver suggest that surgical interventions are not
sufficient to fulfill a satisfactory role for patients with
advanced HCC (14,15). Therefore, it is recommended that
non-surgical conversion therapies, such as LRTs and systemic
therapy, are considered before surgical resection. LRT options for
conversion therapy include hepatic arterial infusion chemotherapy
(HAIC), transcatheter arterial radioembolization and transcatheter
arterial chemoembolization (TACE) (16–18).
Systemic options for conversion therapy typically consist of
targeted therapy, chemotherapy and immunotherapy.
Various types of therapeutic agents have been
proposed following the outcomes of various in-depth studies into
the mechanisms underlying tumor-related immune escape (19–21).
Lenvatinib (LEN) is one such agent, which has been recommended as a
first-line treatment strategy for the systemic treatment of HCC
(22). In a previous phase III,
multinational, randomized and non-inferiority trial (REFLECT), LEN
was comparable to sorafenib in terms of overall survival (OS),
whereas it achieved a higher objective response rate (ORR) and
superior progression-free survival (PFS) time (23). In addition, combining LEN with
immune checkpoint inhibitors (ICIs) has yielded promising results
in enhancing the conversion rate. As a result, explorations into
the efficacy of triple therapy involving LEN, ICIs and LRT has
intensified, with superior conversion rates being reported
(12,24–26).
Despite the accumulating evidence on conversion therapy, optimal
treatment approaches for uHCC remain elusive (27–30).
Therefore, a meta-analysis was performed to systematically assess
the safety and efficacy profile of LEN-based treatment regimens in
conversion therapy, by specifically measuring the conversion rate,
ORR, disease control rate (DCR) and adverse event (AE) incidence in
patients with uHCC. The aim of the present study was to provide a
basis for guiding clinical decision making for patients with
uHCC.
Materials and methods
Logistics
The present systematic review and meta-analysis was
performed according to the Preferred Reporting Items for Systematic
Reviews and Meta-Analyses guidelines (31), and have been registered with the
International Prospective Register of Systematic Reviews (PROSPERO)
database (https://www.crd.york.ac.uk/PROSPERO/) (registration
no. CRD42023411289). The present review was conducted by following
the methodological guidance outlined in the Cochrane Handbook for
Systematic Reviews of Interventions (32). Any amendments made to this protocol
during the study were documented and reported in PROSPERO.
Search strategy
Relevant studies were searched for in Medline (via
PubMed; http://pubmed.ncbi.nlm.nih.gov/), Web of Science
(https://clarivate.com.cn/solutions/web-of-science/),
EMBASE (https://www.embase.com/) and The
Cochrane Library (https://www.cochranelibrary.com/) databases. These
aforementioned databases were used to identify suitable studies
that were published up until September 1, 2023. In total, three
search terms were combined with the Boolean operator ‘and’ when
searching databases. The following search terms were used:
‘Lenvatinib’, ‘Conversion therapy’ and ‘Hepatocellular
Carcinoma’.
Inclusion and exclusion criteria
Studies were included if they fulfilled the
following criteria: i) Study participants included patients with
uHCC receiving a LEN-based treatment regimen; ii) outcomes assessed
included conversion rate or the number of individuals successfully
converted, ORR, DCR and grade ≥3 treatment-associated AE rate; and
iii) the studies were either a randomized controlled trial (RCT),
high-quality case-control study or cohort study.
The exclusion criteria were as follows: i)
Incomplete or unavailable outcome data; ii) duplicate reports, case
reports, comments and letters to the editors, systematic reviews or
meta-analyses; and iii) studies with the same population or
multiple publications from the same research series. For iii), if
multiple studies were found, then the study with the most direct
interventions or the largest sample size was adopted. A total of
two reviewers (ZZ and SL) independently assessed the articles
according to the inclusion criteria. Any disagreements were
resolved through discussions with a third reviewer (ZW).
Data extraction and quality
analyses
Data extraction from the included studies was
performed independently by two reviewers (ZZ and DL), with any
discrepancies resolved by a third reviewer (MS). In cases of
unclear or insufficient information, attempts were made to contact
the authors of the primary studies by email to obtain the missing
data. The extracted data included the first author, publication
year, country, study type, sample size, clinical outcomes,
conversion rate or the number of individuals successfully
converted, ORR, DCR and grade ≥3 AE rate. Tumor response was
assessed using the modified Response Evaluation Criteria in Solid
Tumors (mRECIST), which categorizes tumor responses into complete
remission (CR), partial remission (PR), stable disease (SD) or
progressive disease. ORR was defined as the percentage of patients
achieving CR or PR, whereas DCR was defined as the percentage of
patients achieving the best tumor response of CR, PR or SD
(33).
Literature quality evaluation
Eligible studies underwent assessment by two
independent reviewers using the Methodological Index for
Non-randomized Studies (MINORS) tool to evaluate the methodological
quality of both RCTs and non-RCTs treated as single-arm studies
(34). Additionally, the Institute
of Health Economics Quality Appraisal (IHEQA) checklist was used to
evaluate the methodological quality of cohort and case-control
studies, treating them as case series (35).
Statistical analysis
Data analysis was performed using Stata version 17.0
(StataCorp LP). The pooled event rates (conversion rate, ORR, DCR
and grade ≥3 AE rate) are expressed as risk ratio and corresponding
95% confidence interval (CI). P<0.05 was considered to indicate
statistical significance. Forest plots were used to visualize the
pooled estimates and the extent of heterogeneity among studies. The
I2 statistic was used to assess statistical
heterogeneity among the included studies, with >50% considered
to indicate significance, and a random-effects model (the
DerSimonian and Laird method) was used to analyze the outcomes
(36). Sensitivity analysis was
performed to assess the robustness and reliability of the pooled
results of the meta-analysis. Funnel plots and Egger's tests were
used to assess publication bias (37).
Results
Search results and eligibility
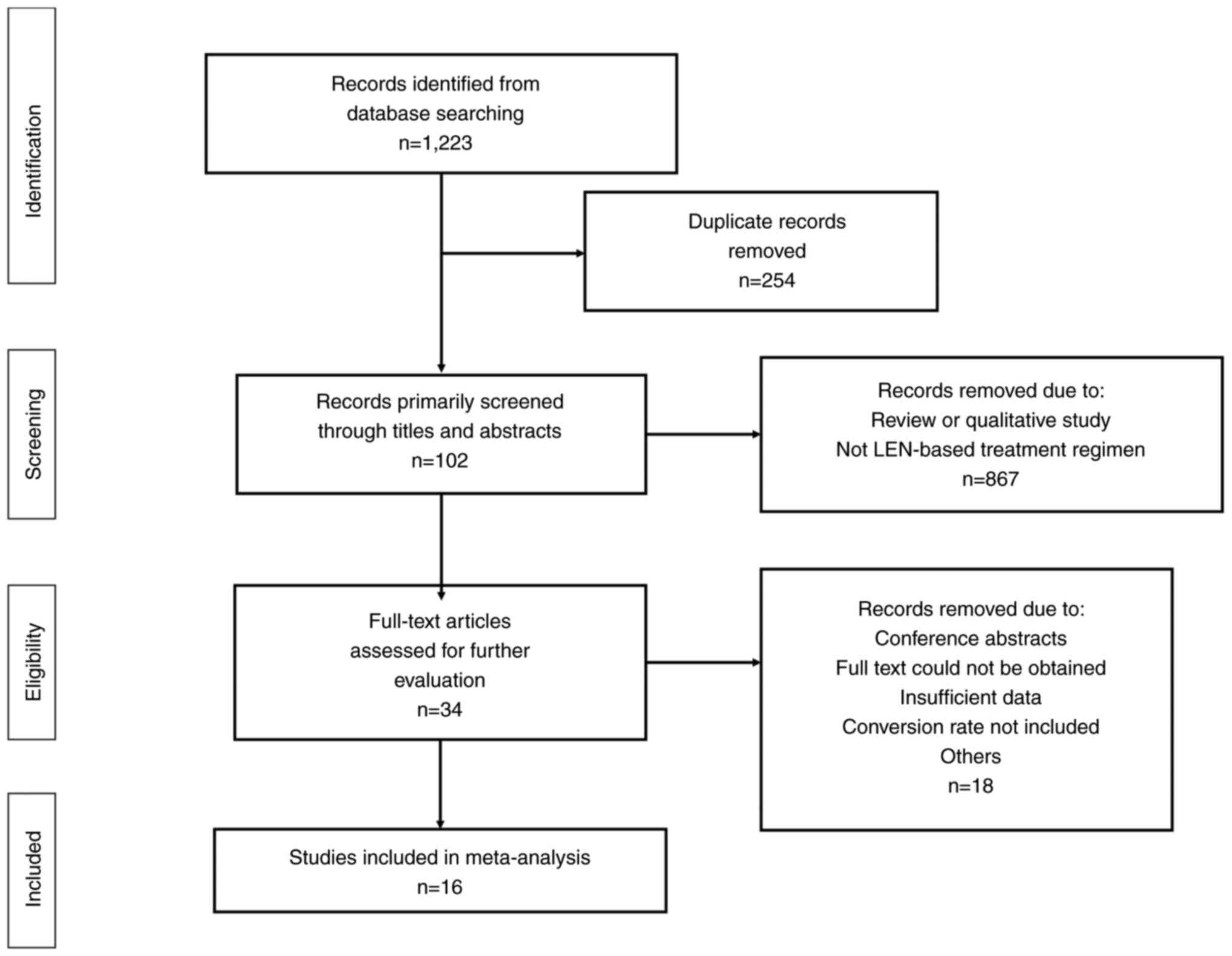
The database searches returned 1,223 results, 254 of
which were excluded due to duplication (Fig. 1). Furthermore, 867 studies were
excluded due to being reviews, qualitative studies or not being
relevant to the topic studied. The remaining articles were then
read in full. In total, 16 studies were included in the final
meta-analysis (12,24–26,38–49).
Characteristics of the included
studies
The characteristics of the included studies are
summarized in Table I, all of which
were published between 2021 and 2023. The sample size reported by
the included studies ranged from 16 to 187, encompassing a total of
1,650 cases with uHCC. According to the IHEQA checklist, 13 studies
were deemed to be of acceptable quality (Table SI) (12,24–26,40,42–49),
whereas the remaining three studies were deemed to be of high
quality by the MINORS tool (Table
SII) (38,39,41).
 | Table I.Characteristics of included
studies. |
Table I.
Characteristics of included
studies.
| First author,
year | Design | Group of
interventions | Subgroup of
interventions | Sample size | Reasons for
unresectability | Definition of
successful conversion | (Refs.) |
|---|
| Wang, 2023 | Prospective
single-arm | ST | LEN + ICI | 36 | Poor location;
inability to perform R0/R1 resection; insufficient FLR | R0/R1 resection;
adequate FLR; sufficient physical condition | (38) |
| Peng, 2023 | RCT | ST | LEN | 168 | Advanced HCC | Downstaging for
resection | (39) |
| Yi, 2022 | Retrospective
cohort | ST | LEN + ICI | 107 | Intermediate to
advanced HCC; insufficient FLR | R0 resection;
adequate FLR | (40) |
| Zhu, 2022 | Prospective
single-arm | ST | LEN + ICI | 101 | Intermediate to
advanced HCC; insufficient FLR | Downstaging to
resectable; R0 resection | (41) |
| Shindoh, 2021 | Retrospective
cohort | ST | LEN | 107 | Advanced HCC | R0 resection | (42) |
| Xu, 2022 | Retrospective
cohort | ST | LEN + ICI | 187 | Oncological
reasons; BCLC stage B/C | R0 resection;
adequate FLR | (43) |
| He, 2021 | Retrospective
cohort | ST | LEN | 86 | Advanced HCC; BCLC
stage C; extrahepatic spread | Tumor shrinkage to
resectable | (24) |
| Niizeki, 2022 | Retrospective
cohort | ST | LEN | 152 | Advanced HCC | Downstaging to
resectable | (44) |
| Peng, 2023 | RCT | LRT + ST | LEN + DEB-TACE | 170 | Advanced HCC | Downstaging for
resection | (39) |
| Qu, 2022 | Retrospective
cohort | LRT + ST | LEN + TACE | 21 | Inability to
perform R0 resection; insufficient FLR; insufficient resection
margin | R0 resection | (12) |
| Chen, 2022 | Retrospective
cohort | LRT + ST | LEN + TACE | 72 | Intermediate to
advanced HCC; insufficient FLR | Downstaging for
resection | (25) |
| Mu, 2023 | Retrospective
cohort | LRT + ST | LEN + ICI +
TACE | 16 | Insufficient FLR;
ECOG PS score of 0 or 1 | Downstaging for
resection | (26) |
| Wu, 2023 | Retrospective
cohort | LRT + ST | LEN + ICI +
TACE | 35 | Advanced HCC | Downstaging for
resection | (45) |
| Li, 2023 | Retrospective
cohort | LRT + ST | LEN + ICI +
TACE | 97 | Inability to
perform R0 resection; insufficient FLR; major vascular invasion;
intrahepatic metastases; extrahepatic metastases | R0 resection;
adequate FLR | (46) |
| Gan, 2023 | Retrospective
cohort | LRT + ST | LEN + ICI +
HAIC | 37 | Inability to
perform R0 resection; insufficient FLR | Downstaging for
resection | (47) |
| Qu, 2022 | Retrospective
cohort | LRT + ST | LEN + ICI +
TACE | 30 | Inability to
perform R0 resection; insufficient FLR; insufficient resection
margin | R0 resection | (12) |
| Zhang, 2021 | Retrospective
cohort | LRT + ST | LEN + ICI +
HAIC | 25 | Advanced HCC | R0 resection;
adequate FLR | (48) |
| He, 2021 | Retrospective
cohort | LRT + ST | LEN + ICI +
HAIC | 71 | Advanced HCC; BCLC
stage C; extrahepatic spread | Tumor shrinkage to
resectable | (24) |
| Wu, 2021 | Retrospective
cohort | LRT + ST | LEN + ICI +
DEB-TACE | 62 | Advanced HCC; major
vascular invasion | R0 resection;
adequate FLR | (49) |
| Chen, 2022 | Retrospective
cohort | LRT + ST | LEN + ICI +
TACE | 70 | Intermediate to
advanced HCC; insufficient FLR | Downstaging for
resection | (25) |
Systemic therapy
In total, seven studies adopted systemic therapy and
reported the conversion rate, the ORR and the DCR, and six studies
reported grade ≥3 AEs (38–44). The seven studies comprised 944
patients with uHCC (38–44). Among these studies, three studies
adopted LEN alone (39,42,44).
By contrast, the remaining four adopted LEN + ICI, including
various types of anti-programmed cell death protein 1 (PD-1)
antibodies (such as sintilimab, toripalimab, tislelizumab and
pembrolizumab) (38,40,41,43).
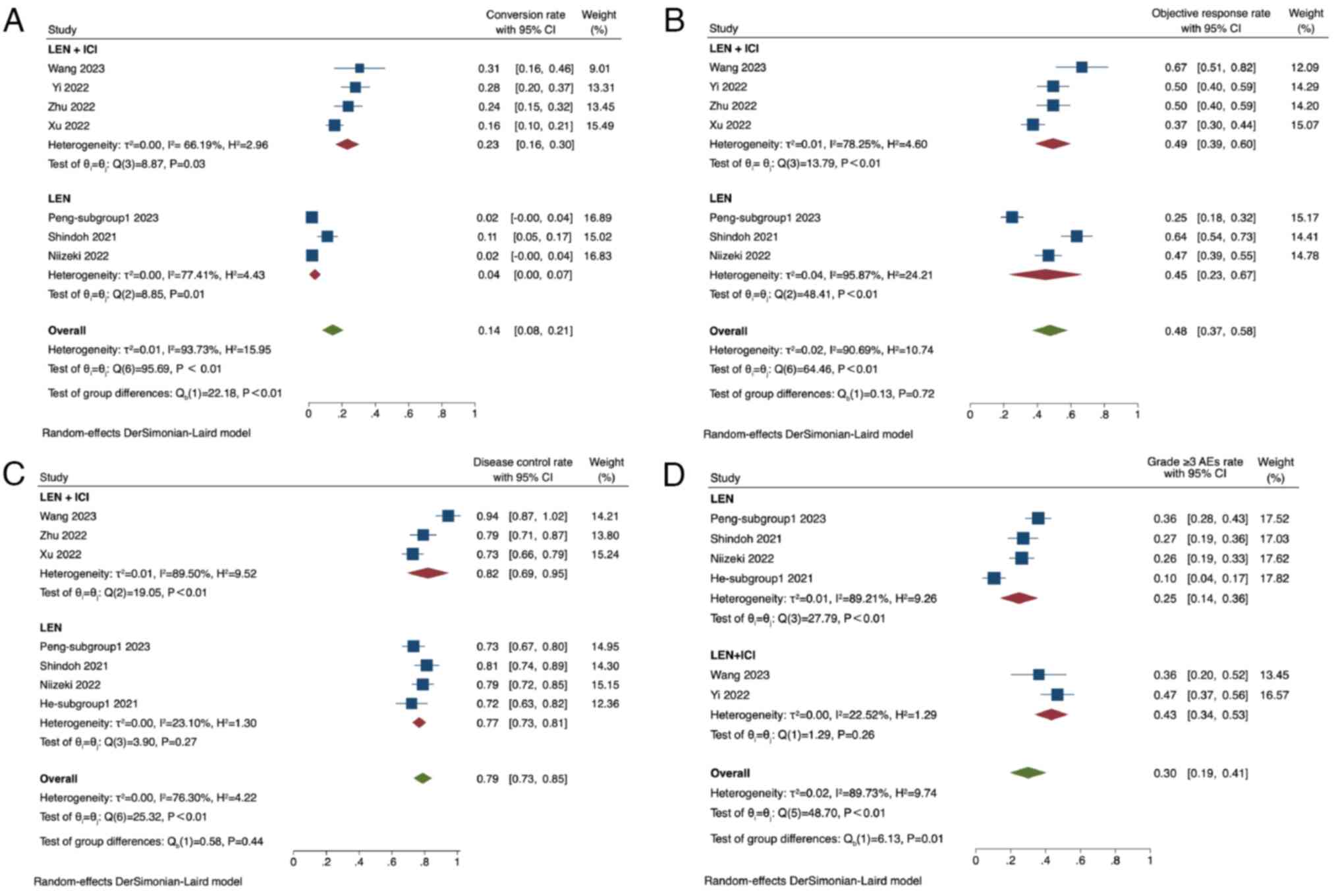
The conversion rate among the included studies
varied from 0.02 to 0.31, with a pooled conversion rate of 0.14
(95% CI, 0.08–0.21; I2=94%) (38–44).
Subgroup analysis comparing LEN + ICI treatment with LEN alone
revealed a conversion rate of 0.23 (95% CI, 0.16–0.30;
I2=66%) in the group receiving LEN + ICI and 0.04 (95%
CI, 0.00–0.07; I2=77%) in the LEN-alone group. The
conversion rate in the LEN + ICI group was found to be
significantly higher compared with that in the LEN-alone group
(P<0.01; Fig. 2A).
The ORR ranged from 0.25 to 0.67, with a pooled ORR
of 0.48 (95% CI, 0.37–0.58; I2=91%). The ORR was 0.49
(95% CI, 0.39–0.60; I2=78%) in the group receiving LEN +
ICI, whilst the LEN-alone group had an ORR of 0.45 (95% CI,
0.23–0.67; I2=96%). No significant difference could be
found in the ORR between the LEN + ICI group and the LEN-alone
group (P=0.72; Fig. 2B).
In terms of DCR, it ranged from 0.72 to 0.94, with a
pooled DCR of 0.79 (95% CI, 0.73–0.85; I2=76%). In the
subgroup analysis, the LEN + ICI group exhibited a DCR of 0.82 (95%
CI, 0.69–0.95; I2=90%), whilst the LEN-alone group had a
DCR of 0.77 (95% CI, 0.73–0.81; I2=23%). No significant
difference was found in the DCR between the two subgroups (P=0.44;
Fig. 2C).
For the grade ≥3 AEs, the rate ranged from 0.10 to
0.47, with a pooled rate of 0.30 (95% CI, 0.19–0.41;
I2=90%). Within the subgroups, the LEN + ICI group had a
grade ≥3 AE rate of 0.43 (95% CI, 0.34–0.53; I2=23%),
whereas the LEN-alone group had a rate of 0.25 (95% CI, 0.14–0.36;
I2=89%). The grade ≥3 AE incidence in the LEN-alone
group was significantly higher compared with that in the LEN + ICI
group (P<0.01; Fig. 2D).
Combined with LRT and systemic
therapy
In 10 studies, a total of 706 patients with uHCC
were included, 12 subgroups explored the efficacy of adding LRT
into the LEN therapy regimen for uHCC. All 12 subgroups of studies
reported the conversion rate and ORR, whereas 11 subgroups from
nine studies reported the DCR, and eight studies provided data on
the incidence of grade ≥3 AEs. Regarding the treatment strategies,
three subgroups adopted LEN + LRT (12,25,39)
and nine subgroups adopted LEN + ICI + LRT (12,24–26,45–49).
Among the nine studies that used TACE, two utilized drug-eluting
bead TACE (39,49). In addition, three studies
implementing HAIC used the FOLFOX regimen [oxaliplatin 85
mg/m2; leucovorin 400 mg/m2; 5-fluorouracil
bolus (400 mg/m2) on day 1 and 5-fluorouracil infusion
(2,400 mg/m2) for 46 h; every 3 weeks] (24,47,48).
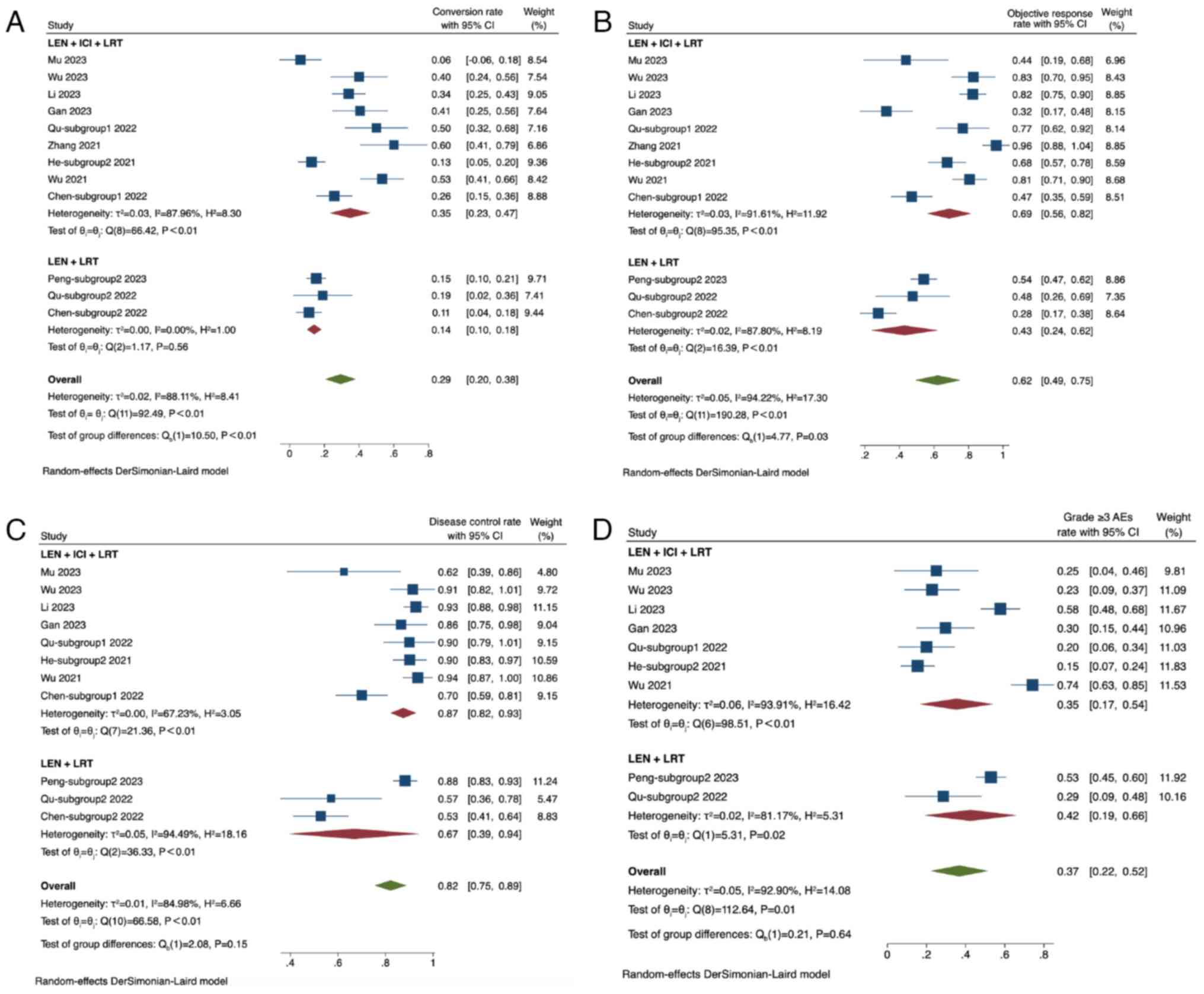
The conversion rate across the included studies
ranged from 0.06 to 0.60, resulting in a pooled conversion rate of
0.29 (95% CI, 0.20–0.38; I2=88%). Subgroup analysis
based on the combination of treatments revealed a conversion rate
of 0.35 (95% CI, 0.23–0.47; I2=88%) for the LEN + ICI +
LRT groups and 0.14 (95% CI, 0.10–0.18; I2=0%) for the
LEN + LRT group. The conversion rate in the LEN + ICI + LRT group
was significantly higher compared with that in the LEN + LRT group
(P<0.01; Fig. 3A).
The ORR ranged from 0.28 to 0.96, with a pooled ORR
of 0.62 (95% CI, 0.49–0.75; I2=94%). In the subgroup
analysis, the group receiving LEN + ICI + LRT achieved an ORR of
0.69 (95% CI, 0.56–0.82; I2=92%), whereas the LEN + LRT
group had an ORR of 0.43 (95% CI, 0.24–0.62; I2=88%).
The ORR in the LEN + ICI + LRT group was significantly higher
compared with that in the LEN + LRT group (P=0.03; Fig. 3B).
For the DCR, the range spanned from 0.53 to 0.94,
with a pooled DCR of 0.82 (95% CI, 0.75–0.89; I2=85%).
Subgroup analysis indicated a DCR of 0.87 (95% CI, 0.82–0.93;
I2=67%) for the LEN + ICI + LRT group and 0.67 (95% CI,
0.39–0.94; I2=94%) for the LEN + LRT group. No
significant difference in DCR was observed between the two groups
(P=0.15; Fig. 3C).
In terms of grade ≥3 AEs, the rate ranged from 0.15
to 0.74, with a pooled rate of 0.37 (95% CI, 0.22–0.52;
I2=93%). The LEN + ICI + LRT group had a grade ≥3 AEs
rate of 0.35 (95% CI, 0.17–0.54; I2=94%), whereas the
LEN + LRT group had a rate of 0.42 (95% CI, 0.19–0.66;
I2=81%). No significant difference in the grade ≥3 AEs
rate could be found between the two groups (P=0.64; Fig. 3D).
Publication bias and sensitivity
analysis
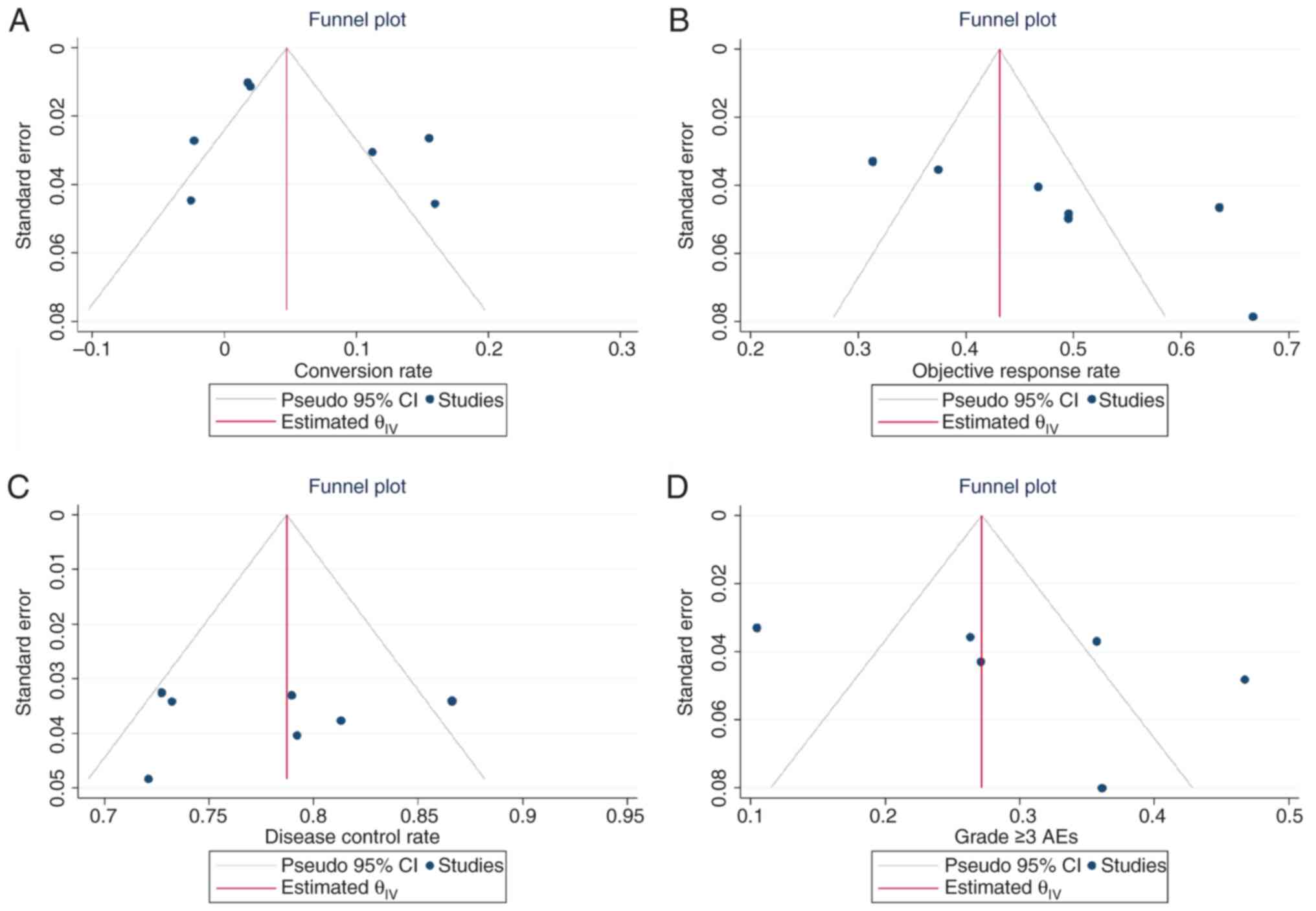
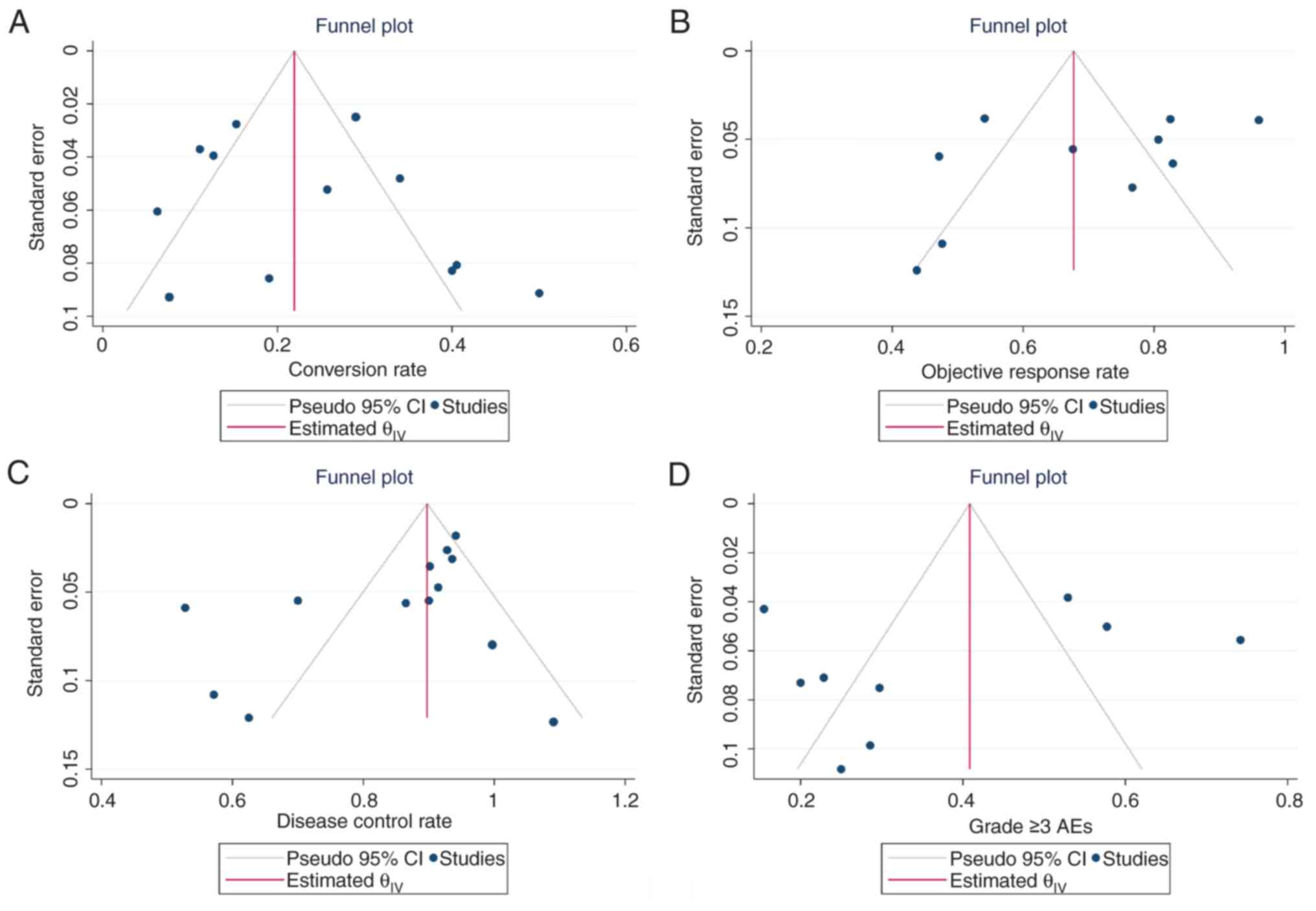
Egger's test and funnel plots were used to evaluate
the publication bias. No indication of publication bias was
observed for the conversion rate (Egger's test, P=0.05), ORR
(Egger's test, P=0.17), DCR (Egger's test, P=0.38) and grade ≥3 AE
rate (Egger's test, P=0.34) of systemic therapy. In addition, no
indication of publication bias was observed for the conversion rate
(Egger's test, P=0.08), ORR (Egger's test, P=0.19), DCR (Egger's
test, P=0.31) and grade ≥3 AE rate (Egger's test, P=0.27) of LEN
combined with LRT and/or systemic therapy. Funnel plots were
visually examined to assess the symmetry of all outcomes reported,
and no publication bias was found (Figs. 4 and 5). Furthermore, sensitivity analysis was
performed for conversion rate. The pooled rates did not markedly
fluctuate after the removal of any single study that used systemic
therapy and LEN combined with LRT and/or systemic therapy (Fig. S1).
Discussion
Conversion therapy holds promise for enhancing the
OS and tumor-free survival of patients with uHCC, with advancements
in tyrosine kinase inhibitor (TKI) use and immunotherapy (17). LEN has emerged as a cornerstone of
HCC treatment since its introduction in 2018. ICIs, such as PD-1
and cytotoxic T-lymphocyte-associated protein 4 antibodies, have
also been incorporated into the HCC treatment schedule (29). Combining LEN, ICIs and LRT may
confer potential synergistic effects, given the distinct reported
anti-malignancy mechanisms exhibited by each modality (50). This combination therefore holds
promise for achieving efficacy in patients with advanced HCC
(51). However, while multiple
conversion therapy options do exist, the optimal option remains
elusive (52). To the best of our
knowledge, the present meta-analysis is the first to assess the
safety and efficacy of LEN-based treatment regimens in conversion
therapy for uHCC, thus bridging the knowledge gap in the field.
In the present meta-analysis, 16 studies focusing on
the safety and efficacy of LEN-based treatment regimens in
conversion therapy for uHCC were systematically reviewed. In terms
of efficacy, the conversion rate, ORR and DCR between systemic
therapy and LEN combined with LRT were compared. In systemic
therapy, LEN + ICI yielded a markedly higher conversion rate
compared with that in the LEN-alone group, whereas the most
favorable outcomes were achieved when LRT was added alongside LEN,
surpassing LEN alone or LEN + ICIs. In addition, the conversion
rate of LEN + LRT + ICIs was found to reach 35%. Similarly, ORR and
DCR could be improved by combining LEN with LRT and ICIs, offering
potential surgical opportunities for patients with uHCC. However,
LEN + LRT (43%) resulted in comparable ORR compared with LEN alone
(45%) and LEN + ICI (49%). Regarding safety, the analysis focused
on the incidence of grade ≥3 AEs. It was observed that LEN + ICI
had the highest AE rate (43%). However, it is worth noting that
this combination also achieved a significantly higher conversion
rate (23%) compared with that in the LEN-alone group (4%).
Therefore, the decision to opt for combination therapy when
systemic therapy alone is also available should be carefully
weighed.
The development of conversion therapy for patients
with uHCC spans >50 years. In the 1970s, Hermann and Lonsdale
(53) reported the use of
chemotherapy and radiotherapy for shrinking giant hepatoblastomas,
followed by surgical resection. In the subsequent 50 years, various
approaches, including TACE, internal radionuclide radiotherapy,
external radiotherapy and chemotherapy, have been employed to
induce tumor shrinkage and downstaging, ultimately facilitating
surgical resection (54–56). The outcomes of conversion therapy
have been promising, with reported 5-year survival rates ranging
from 50 to 60%, comparable to those achieved through resection in
early-stage HCC (57,58). However, the success rate of
conversion therapy remains limited, ranging from 1.8 to 34.6%
(59,60). Consequently, conversion therapy can
benefit only a relatively small subset of patients with uHCC due to
the restricted range of available options.
Sorafenib previously held the position as the
primary first-line treatment option for uHCC until the approval of
LEN in 2018 (19). The pivotal
REFLECT study previously demonstrated the similar efficacy
conferred by LEN to that by sorafenib in terms of OS [13.6 vs. 12.3
months; hazards ratio (HR), 0.92; 95% CI, 0.79–1.06] when used as a
first-line treatment for uHCC. LEN also significantly improved
various secondary endpoints, including PFS (7.4 vs. 3.7 months; HR,
0.66; 95% CI, 0.57–0.77), time to progression (8.9 vs. 3.7 months;
HR, 0.63; 95% CI 0.53–0.73) and ORR (24.1 vs. 9.2%; OR, 3.1; 95%
CI, 2.2–4.6), according to the mRECIST. While LEN appeared to
confer notable advantages in terms of PFS and ORR compared with
sorafenib, it remains unsatisfactory that 75% patients with uHCC do
not respond to this treatment. Accumulating evidence supports the
combination of ICIs and TKIs for treating this malignancy (30,61,62).
In addition, the pooled data from the present meta-analysis
provided promising results, suggesting that LEN combined with ICIs
can achieve favorable efficacy and conversion rates whilst
maintaining acceptable toxicity. However, the selection of which
specific ICI remains an issue that requires further study. In
real-world clinical practice, LEN combined with various ICIs has
demonstrated superior outcomes in terms of OS, PFS and ORR
according to the RECIST version 1.1 compared with LEN alone. In
particular, subgroup analysis indicated that the type of ICI did
not have a notable impact on OS or PFS (24,38–43).
Further research into tumor-related mechanisms has
gradually validated the potential for enhanced conversion when
drugs with different reported antitumor mechanisms are used in
combination (63,64). This rationale has led to the
proposal of combining LRT and systemic therapy, which has resulted
in higher conversion rates in uHCC (65). In the present study, the assessment
of LEN + ICI + LRT demonstrated additional promising outcomes
compared with LEN + LRT. Notably, 35% patients receiving
triple-therapy compared with 14% of patients receiving
double-therapy achieved conversion. These findings suggested that
the inclusion of ICIs alongside LEN and LRT may further elevate the
rate of successful conversion therapy.
LEN can not only exert a direct antitumor effect,
but can also promote vessel normalization and prevent hypoxia in
the tumor tissue (66). In
addition, accumulating evidence suggested that LEN possesses
immunomodulatory effects, impacting the activity and number of
infiltrating immune cells, thereby indicating potential synergistic
effects with immunotherapy (50,67,68).
When combined with an anti-PD-1 antibody, LEN has been reported to
enhance antitumor activity in murine HCC models by increasing the
levels and cytotoxic activity of CD8+ T cells,
activating immune pathways, preventing regulatory T-cell
infiltration and downregulating programmed death-ligand 1 and PD-1
expression (69). In addition, such
combination therapy may elicit long-term immune memory. LRT for HCC
can shape tumor immunity by modifying the composition of the tumor
microenvironment (70). Following
thermal treatment using percutaneous techniques or other LRTs,
tumor cell necrosis typically results in the release of tumoral
neoantigens, facilitating the recruitment and activation of
dendritic cells within the microenvironment. This effect can be
utilized to shift an immunosuppressive microenvironment, which may
not favor checkpoint inhibitor therapy, into an immune-supportive
setting where systemic therapies may yield greater efficacy
(5,71). However, larger scale RCTs remain of
importance to definitively validate and elucidate these
findings.
The safety profiles of the combined treatment were
also investigated in the present study. In the case of LEN + ICI +
LRT, the grade ≥3 AE rate was 35%, which appears acceptable given
its substantial 35% conversion rate. AEs of grade ≥3 warrant
treatment interruption or discontinuation followed by prompt
corticosteroid administration, with escalation to
immunosuppressants in cases of the lack of response (72). AEs serve an intriguing role in the
assessment of survival benefits. A post hoc analysis performed by
the REFLECT study revealed that the presence of diarrhea,
hypertension, hypothyroidism and proteinuria was associated with
improved OS (73). Furthermore,
immune-related AEs induced by ICIs have been reported to be
associated with enhanced clinical benefits (74).
There were a number of limitations in the present
study. The present meta-analysis focused on conversion rate,
leading to the exclusion of studies that solely examined the
efficacy and safety of conversion therapy during the screening
process. The majority of the included studies did not utilize
conversion rate as the primary endpoint since conversion therapy
for HCC has only recently garnered attention. It is anticipated
that higher quality RCTs will emerge in the near future exploring
this aspect more comprehensively. Determining the optimal ICIs and
LRT in combination with LEN remains challenging, although several
phase II or III studies are currently evaluating the efficacy and
safety of LEN in combination with ICIs and LRT (https://clinicaltrials.gov/study/NCT04523493,
https://clinicaltrials.gov/study/NCT04194775 and
http://clinicaltrials.gov/study/NCT05312216). Whilst
these trials may assist clinicians in selecting among various
second-line therapeutic options, a comprehensive understanding of
this field necessitates additional studies. In addition, only two
articles included in the present study were RCTs, and the rest were
retrospective studies. This may impact the overall quality of
results. A high degree of heterogeneity remains among the included
studies in the present meta-analysis. Possible sources of this may
have been that the characteristics of patients varied among
included studies or that the determination of successful conversion
is relatively subjective, even if the criteria for successful
conversion are clearly defined.
LEN has transformed the treatment landscape of uHCC.
Since preclinical studies have progressively elucidated the
antitumor and resistance mechanisms of this drug, patients with
uHCC may benefit from LEN-based treatment regimens. To clarify the
significance of conversion therapy, it is necessary to compare the
long-term results of patients who underwent conversion surgery
because of a favorable tumor response to systemic therapy and
patients who did not despite a favorable tumor response. The search
for reliable prognostic biomarkers to guide the development of
targeted treatments or immunotherapy has been another focus of
research. However, consistent conclusions have remained elusive,
indicating the need for further progress in the screening and
validation of biomarkers. Ongoing clinical trials are actively
investigating additional combination strategies as options and
insights for the treatment of patients with uHCC (https://clinicaltrials.gov/study/NCT05312216 and
http://clinicaltrials.gov/study/NCT04740307).
In conclusion, the present meta-analysis provides
valuable insight and suggests that LEN-based combination strategies
may confer efficacy and acceptable tolerability for patients with
uHCC. In particular, LEN + ICI with or without LRT appears to
represent a highly effective conversion regimen with an acceptable
conversion rate and a well-characterized safety profile.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This research received grants from the Beijing Natural Science
Foundation (grant no. 7182063) and the Beijing Health System High
Level Health Technical Personnel (grant no. 2014-3-058).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ZW and KL developed the initial idea for the study.
SL and ZW designed the study. SL and ZZ analyzed some of the data
and wrote the manuscript. KW, DL and MS contributed to the
acquisition, analysis and interpretation of data for the work. ZZ,
ZW and KL revised the manuscript. SL, ZZ, ZW, KW, DL, MS and KL
confirm the authenticity of all the raw data. SL and ZZ contributed
equally to this paper and are co-first authors. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forner A, Reig M and Bruix J:
Hepatocellular carcinoma. Lancet. 391:1301–1314. 2018. View Article : Google Scholar
|
|
3
|
Vogel A, Cervantes A, Chau I, Daniele B,
Llovet JM, Meyer T, Nault JC, Neumann U, Ricke J, Sangro B, et al:
Hepatocellular carcinoma: ESMO clinical practice guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 29 (Suppl
4):iv238–iv255. 2018. View Article : Google Scholar
|
|
4
|
Dimitroulis D, Damaskos C, Valsami S,
Davakis S, Garmpis N, Spartalis E, Athanasiou A, Moris D,
Sakellariou S, Kykalos S, et al: From diagnosis to treatment of
hepatocellular carcinoma: An epidemic problem for both developed
and developing world. World J Gastroenterol. 23:5282–5294. 2017.
View Article : Google Scholar
|
|
5
|
Llovet JM, De Baere T, Kulik L, Haber PK,
Greten TF, Meyer T and Lencioni R: Locoregional therapies in the
era of molecular and immune treatments for hepatocellular
carcinoma. Nat Rev Gastroenterol Hepatol. 18:293–313. 2021.
View Article : Google Scholar
|
|
6
|
Huang A, Yang XR, Chung WY, Dennison AR
and Zhou J: Targeted therapy for hepatocellular carcinoma. Signal
Transduct Target Ther. 5:1462020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brown ZJ, Tsilimigras DI, Ruff SM, Mohseni
A, Kamel IR, Cloyd JM and Pawlik TM: Management of hepatocellular
carcinoma: A review. JAMA Surg. 158:410–420. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rizzo A, Ricci AD and Brandi G: Systemic
adjuvant treatment in hepatocellular carcinoma: Tempted to do
something rather than nothing. Future Oncol. 16:2587–2589. 2020.
View Article : Google Scholar
|
|
9
|
Matsuki R, Kogure M, Hasui N, Momose H,
Suzuki Y and Sakamoto Y: Development of conversion therapy for
advanced hepatocellular carcinoma. Hepatobiliary Surg Nutr.
12:453–456. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kudo M: Atezolizumab plus bevacizumab
followed by curative conversion (ABC conversion) in patients with
unresectable, TACE-unsuitable intermediate-stage hepatocellular
carcinoma. Liver Cancer. 11:399–406. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chiang CL, Chiu KWH, Chan KSK, Lee FAS, Li
JCB, Wan CWS, Dai WC, Lam TC, Chen W, Wong NSM, et al: Sequential
transarterial chemoembolisation and stereotactic body radiotherapy
followed by immunotherapy as conversion therapy for patients with
locally advanced, unresectable hepatocellular carcinoma
(START-FIT): A single-arm, phase 2 trial. Lancet Gastroenterol
Hepatol. 8:169–178. 2023. View Article : Google Scholar
|
|
12
|
Qu WF, Ding ZB, Qu XD, Tang Z, Zhu GQ, Fu
XT, Zhang ZH, Zhang X, Huang A, Tang M, et al: Conversion therapy
for initially unresectable hepatocellular carcinoma using a
combination of toripalimab, lenvatinib plus TACE: Real-world study.
BJS Open. 6:zrac1142022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Z and Zhang E: Conversion therapy
for advanced hepatocellular carcinoma with vascular invasion: A
comprehensive review. Front Immunol. 14:10735312023. View Article : Google Scholar
|
|
14
|
Benson AB, D'Angelica MI, Abbott DE, Anaya
DA, Anders R, Are C, Bachini M, Borad M, Brown D, Burgoyne A, et
al: Hepatobiliary cancers, version 2.2021, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 19:541–565. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
European Association for the Study of the
Liver. lectronic address, . simpleeasloffice@easloffice.eu;
European Association for the Study of the Liverr: EASL clinical
practice guidelines: Management of hepatocellular carcinoma. J
Hepatol. 69:182–236. 2018. View Article : Google Scholar
|
|
16
|
Lee JJX, Tai DWM and Choo SP: Locoregional
therapy in hepatocellular carcinoma: When to start and when to stop
and when to revisit. ESMO Open. 6:1001292021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Llovet JM, Pinyol R, Kelley RK,
El-Khoueiry A, Reeves HL, Wang XW, Gores GJ and Villanueva A:
Molecular pathogenesis and systemic therapies for hepatocellular
carcinoma. Nat Cancer. 3:386–401. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rizzo A, Ricci AD and Brandi G:
Trans-arterial chemoembolization plus systemic treatments for
hepatocellular carcinoma: An update. J Pers Med. 12:17882022.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018. View Article : Google Scholar
|
|
20
|
Abou-Alfa GK, Meyer T, Cheng AL,
El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park
JW, et al: Cabozantinib in patients with advanced and progressing
hepatocellular carcinoma. N Engl J Med. 379:54–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu AX, Kang YK, Yen CJ, Finn RS, Galle
PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, et al:
Ramucirumab after sorafenib in patients with advanced
hepatocellular carcinoma and increased α-fetoprotein concentrations
(REACH-2): A randomised, double-blind, placebo-controlled, phase 3
trial. Lancet Oncol. 20:282–296. 2019. View Article : Google Scholar
|
|
22
|
Al-Salama ZT, Syed YY and Scott LJ:
Lenvatinib: A review in hepatocellular carcinoma. Drugs.
79:665–674. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamashita T, Kudo M, Ikeda K, Izumi N,
Tateishi R, Ikeda M, Aikata H, Kawaguchi Y, Wada Y, Numata K, et
al: REFLECT-a phase 3 trial comparing efficacy and safety of
lenvatinib to sorafenib for the treatment of unresectable
hepatocellular carcinoma: An analysis of Japanese subset. J
Gastroenterol. 55:113–122. 2020. View Article : Google Scholar
|
|
24
|
He MK, Liang RB, Zhao Y, Xu YJ, Chen HW,
Zhou YM, Lai ZC, Xu L, Wei W, Zhang YJ, et al: Lenvatinib,
toripalimab, plus hepatic arterial infusion chemotherapy versus
lenvatinib alone for advanced hepatocellular carcinoma. Ther Adv
Med Oncol. 13:175883592110027202021. View Article : Google Scholar
|
|
25
|
Chen S, Wu Z, Shi F, Mai Q, Wang L, Wang
F, Zhuang W, Chen X, Chen H, Xu B, et al: Lenvatinib plus TACE with
or without pembrolizumab for the treatment of initially
unresectable hepatocellular carcinoma harbouring PD-L1 expression:
A retrospective study. J Cancer Res Clin Oncol. 148:2115–2125.
2022. View Article : Google Scholar
|
|
26
|
Mu C, Shen J, Zhu X, Peng W, Zhang X and
Wen T: The efficacy and safety of lenvatinib plus transarterial
chemoembolization in combination with PD-1 antibody in treatment of
unresectable recurrent hepatocellular carcinoma: A case series
report. Front Oncol. 13:10969552023. View Article : Google Scholar
|
|
27
|
Mollica V, Rizzo A, Marchetti A, Tateo V,
Tassinari E, Rosellini M, Massafra R, Santoni M and Massari F: The
impact of ECOG performance status on efficacy of immunotherapy and
immune-based combinations in cancer patients: The MOUSEION-06
study. Clin Exp Med. 23:5039–5049. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rizzo A, Mollica V, Tateo V, Tassinari E,
Marchetti A, Rosellini M, De Luca R, Santoni M and Massari F:
Hypertransaminasemia in cancer patients receiving immunotherapy and
immune-based combinations: The MOUSEION-05 study. Cancer Immunol
Immunother. 72:1381–1394. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sangro B, Sarobe P, Hervás-Stubbs S and
Melero I: Advances in immunotherapy for hepatocellular carcinoma.
Nat Rev Gastroenterol Hepatol. 18:525–543. 2021. View Article : Google Scholar
|
|
30
|
Pei Y, Li W, Wang Z and Liu J: Successful
conversion therapy for unresectable hepatocellular carcinoma is
getting closer: A systematic review and meta-analysis. Front Oncol.
12:9788232022. View Article : Google Scholar
|
|
31
|
Liberati A, Altman DG, Tetzlaff J, Mulrow
C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J
and Moher D: The PRISMA statement for reporting systematic reviews
and meta-analyses of studies that evaluate healthcare
interventions: Explanation and elaboration. BMJ. 339:b27002009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cumpston M, Li T, Page MJ, Chandler J,
Welch VA, Higgins JP and Thomas J: Updated guidance for trusted
systematic reviews: A new edition of the cochrane handbook for
systematic reviews of interventions. Cochrane Database Syst Rev.
10:ED0001422019.PubMed/NCBI
|
|
33
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Slim K, Nini E, Forestier D, Kwiatkowski
F, Panis Y and Chipponi J: Methodological index for non-randomized
studies (minors): Development and validation of a new instrument.
ANZ J Surg. 73:712–716. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guo B, Moga C, Harstall C and Schopflocher
D: A principal component analysis is conducted for a case series
quality appraisal checklist. J Clin Epidemiol. 69:199–207.e2. 2016.
View Article : Google Scholar
|
|
36
|
Higgins JPT and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang L, Wang H, Cui Y, Liu M, Jin K, Xu D,
Wang K and Xing B: Sintilimab plus Lenvatinib conversion therapy
for intermediate/locally advanced hepatocellular carcinoma: A phase
2 study. Front Oncol. 13:11151092023. View Article : Google Scholar
|
|
39
|
Peng Z, Fan W, Zhu B, Wang G, Sun J, Xiao
C, Huang F, Tang R, Cheng Y, Huang Z, et al: Lenvatinib combined
with transarterial chemoembolization as first-line treatment for
advanced hepatocellular carcinoma: A phase III, randomized clinical
trial (LAUNCH). J Clin Oncol. 41:117–127. 2023. View Article : Google Scholar
|
|
40
|
Yi Y, Sun BY, Weng JL, Zhou C, Zhou CH,
Cai MH, Zhang JY, Gao H, Sun J, Zhou J, et al: Lenvatinib plus
anti-PD-1 therapy represents a feasible conversion resection
strategy for patients with initially unresectable hepatocellular
carcinoma: A retrospective study. Front Oncol. 12:10465842022.
View Article : Google Scholar
|
|
41
|
Zhu XD, Huang C, Shen YH, Xu B, Ge NL, Ji
Y, Qu XD, Chen L, Chen Y, Li ML, et al: Hepatectomy after
conversion therapy using tyrosine kinase inhibitors plus anti-PD-1
antibody therapy for patients with unresectable hepatocellular
carcinoma. Ann Surg Oncol. 30:2782–2790. 2023. View Article : Google Scholar
|
|
42
|
Shindoh J, Kawamura Y, Kobayashi Y,
Kobayashi M, Akuta N, Okubo S, Suzuki Y and Hashimoto M: Prognostic
impact of surgical intervention after lenvatinib treatment for
advanced hepatocellular carcinoma. Ann Surg Oncol. 28:7663–7672.
2021. View Article : Google Scholar
|
|
43
|
Xu B, Zhu XD, Shen YH, Zhu JJ, Liu J, Li
ML, Tang PW, Zhou J, Fan J, Sun HC and Huang C: Criteria for
identifying potentially resectable patients with initially
oncologically unresectable hepatocellular carcinoma before
treatment with envatinib plus an anti-PD-1 antibody. Front Immunol.
13:10167362022. View Article : Google Scholar
|
|
44
|
Niizeki T, Tokunaga T, Takami Y, Wada Y,
Harada M, Shibata M, Nakao K, Sasaki R, Hirai F, Shakado S, et al:
Comparison of efficacy and safety of atezolizumab plus bevacizumab
and lenvatinib as first-line therapy for unresectable
hepatocellular carcinoma: A propensity score matching analysis.
Target Oncol. 17:643–653. 2022. View Article : Google Scholar
|
|
45
|
Wu SJ, Ruan DD, Wu QY, Tang Y, Zhang JH,
Cai SL, Zhou YF, Luo JW and Fang ZT: Safety and efficacy of
drug-eluting bead transarterial chemoembolization combined with
lenvatinib and anti-PD-1 antibodies for unresectable hepatocellular
carcinoma: A retrospective analysis. J Hepatocell Carcinoma.
10:807–820. 2023. View Article : Google Scholar
|
|
46
|
Li X, Chen J, Wang X, Bai T, Lu S, Wei T,
Tang Z, Huang C, Zhang B, Liu B, et al: Outcomes and prognostic
factors in initially unresectable hepatocellular carcinoma treated
using conversion therapy with lenvatinib and TACE plus PD-1
inhibitors. Front Oncol. 13:11106892023. View Article : Google Scholar
|
|
47
|
Gan L, Lang M, Tian X, Ren S, Li G, Liu Y,
Han R, Zhu K, Li H, Wu Q, et al: A retrospective analysis of
conversion therapy with lenvatinib, sintilimab, and
arterially-directed therapy in patients with initially unresectable
hepatocellular carcinoma. J Hepatocell Carcinoma. 10:673–686. 2023.
View Article : Google Scholar
|
|
48
|
Zhang J, Zhang X, Mu H, Yu G, Xing W, Wang
L and Zhang T: Surgical conversion for initially unresectable
locally advanced hepatocellular carcinoma using a triple
combination of angiogenesis inhibitors, anti-PD-1 antibodies, and
hepatic arterial infusion chemotherapy: A retrospective study.
Front Oncol. 11:7297642021. View Article : Google Scholar
|
|
49
|
Wu JY, Yin ZY, Bai YN, Chen YF, Zhou SQ,
Wang SJ, Zhou JY, Li YN, Qiu FN, Li B and Yan ML: Lenvatinib
combined with anti-PD-1 antibodies plus transcatheter arterial
chemoembolization for unresectable hepatocellular carcinoma: A
multicenter retrospective study. J Hepatocell Carcinoma.
8:1233–1240. 2021. View Article : Google Scholar
|
|
50
|
Zhao Y, Zhang YN, Wang KT and Chen L:
Lenvatinib for hepatocellular carcinoma: From preclinical
mechanisms to anti-cancer therapy. Biochim Biophys Acta Rev Cancer.
1874:1883912020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cai M, Huang W, Huang J, Shi W, Guo Y,
Liang L, Zhou J, Lin L, Cao B, Chen Y, et al: Transarterial
chemoembolization combined with lenvatinib plus PD-1 inhibitor for
advanced hepatocellular carcinoma: A retrospective cohort study.
Front Immunol. 13:8483872022. View Article : Google Scholar
|
|
52
|
Hatanaka T, Yata Y, Naganuma A and
Kakizaki S: Treatment strategy for intermediate-stage
hepatocellular carcinoma: Transarterial chemoembolization, systemic
therapy, and conversion therapy. Cancers (Basel). 15:17982023.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hermann RE and Lonsdale D: Chemotherapy,
radiotherapy, and hepatic lobectomy for hepatoblastoma in an
infant: Report of a survival. Surgery. 68:383–388. 1970.PubMed/NCBI
|
|
54
|
Tsurusaki M and Murakami T: Surgical and
locoregional therapy of HCC: TACE. Liver Cancer. 4:165–175. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sergio A, Cristofori C, Cardin R, Pivetta
G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A and
Farinati F: Transcatheter arterial chemoembolization (TACE) in
hepatocellular carcinoma (HCC): The role of angiogenesis and
invasiveness. Am J Gastroenterol. 103:914–921. 2008. View Article : Google Scholar
|
|
56
|
Forner A, Llovet JM and Bruix J:
Chemoembolization for intermediate HCC: Is there proof of survival
benefit? J Hepatol. 56:984–986. 2012. View Article : Google Scholar
|
|
57
|
Fan J, Tang ZY, Yu YQ, Wu ZQ, Ma ZC, Zhou
XD, Zhou J, Qiu SJ and Lu JZ: Improved survival with resection
after transcatheter arterial chemoembolization (TACE) for
unresectable hepatocellular carcinoma. Dig Surg. 15:674–678. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lau WY, Ho SKW, Yu SCH, Lai ECH, Liew C
and Leung TWT: Salvage surgery following downstaging of
unresectable hepatocellular carcinoma. Ann Surg. 240:299–305. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yamamura K and Beppu T: Conversion surgery
for hepatocellular carcinoma after multidisciplinary treatment
including lenvatinib. Hepatol Res. 51:1029–1030. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sun HC, Zhou J, Wang Z, Liu X, Xie Q, Jia
W, Zhao M, Bi X, Li G, Bai X, et al: Chinese expert consensus on
conversion therapy for hepatocellular carcinoma (2021 edition).
Hepatobiliary Surg Nutr. 11:227–252. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lei Q, Yan X, Zou H, Jiang Y, Lai Y, Ung
COL and Hu H: Efficacy and safety of monotherapy and combination
therapy of immune checkpoint inhibitors as first-line treatment for
unresectable hepatocellular carcinoma: A systematic review,
meta-analysis and network meta-analysis. Discov Oncol. 13:952022.
View Article : Google Scholar
|
|
62
|
Arita J, Ichida A, Nagata R, Mihara Y,
Kawaguchi Y, Ishizawa T, Akamatsu N, Kaneko J and Hasegawa K:
Conversion surgery after preoperative therapy for advanced
hepatocellular carcinoma in the era of molecular targeted therapy
and immune checkpoint inhibitors. J Hepatobiliary Pancreat Sci.
29:732–740. 2022. View Article : Google Scholar
|
|
63
|
Chen J, Zhang D and Yuan Y:
Anti-PD-1/PD-L1 immunotherapy in conversion treatment of locally
advanced hepatocellular carcinoma. Clin Exp Med. 23:579–590. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Killock D: Novel ICI-TKI combination
improves HCC outcomes. Nat Rev Clin Oncol. 20:7332023. View Article : Google Scholar
|
|
65
|
Kudo M: A novel treatment strategy for
patients with intermediate-stage HCC who are not suitable for TACE:
Upfront systemic therapy followed by curative conversion. Liver
Cancer. 10:539–544. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yamamoto Y, Matsui J, Matsushima T,
Obaishi H, Miyazaki K, Nakamura K, Tohyama O, Semba T, Yamaguchi A,
Hoshi SS, et al: Lenvatinib, an angiogenesis inhibitor targeting
VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft
models associated with microvessel density and pericyte coverage.
Vasc Cell. 6:182014. View Article : Google Scholar
|
|
67
|
Kato Y, Tabata K, Kimura T,
Yachie-Kinoshita A, Ozawa Y, Yamada K, Ito J, Tachino S, Hori Y,
Matsuki M, et al: Lenvatinib plus anti-PD-1 antibody combination
treatment activates CD8+ T cells through reduction of
tumor-associated macrophage and activation of the interferon
pathway. PLoS One. 14:e02125132019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kimura T, Kato Y, Ozawa Y, Kodama K, Ito
J, Ichikawa K, Yamada K, Hori Y, Tabata K, Takase K, et al:
Immunomodulatory activity of lenvatinib contributes to antitumor
activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci.
109:3993–4002. 2018. View Article : Google Scholar
|
|
69
|
Deng H, Kan A, Lyu N, Mu L, Han Y, Liu L,
Zhang Y, Duan Y, Liao S, Li S, et al: Dual vascular endothelial
growth factor receptor and fibroblast growth factor receptor
inhibition elicits antitumor immunity and enhances programmed cell
death-1 checkpoint blockade in hepatocellular carcinoma. Liver
Cancer. 9:338–357. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Jiang H, Meng Q, Tan H, Pan S, Sun B, Xu R
and Sun X: Antiangiogenic therapy enhances the efficacy of
transcatheter arterial embolization for hepatocellular carcinomas.
Int J Cancer. 121:416–424. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Pinato DJ, Murray SM, Forner A, Kaneko T,
Fessas P, Toniutto P, Mínguez B, Cacciato V, Avellini C, Diaz A, et
al: Trans-arterial chemoembolization as a loco-regional inducer of
immunogenic cell death in hepatocellular carcinoma: implications
for immunotherapy. J Immunother Cancer. 9:e0033112021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Brahmer JR, Lacchetti C, Schneider BJ,
Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner
JM, Ginex P, et al: Management of immune-related adverse events in
patients treated with immune checkpoint inhibitor therapy: American
society of clinical oncology clinical practice guideline. J Clin
Oncol. 36:1714–1768. 2018. View Article : Google Scholar
|
|
73
|
Llovet JM, Castet F, Heikenwalder M, Maini
MK, Mazzaferro V, Pinato DJ, Pikarsky E, Zhu AX and Finn RS:
Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol.
19:151–172. 2022. View Article : Google Scholar
|
|
74
|
Sung MW, Finn RS, Qin S, Han KH, Ikeda K,
Cheng AL, Kudo M, Tateishi R, Ikeda M, Breder V, et al: Association
between overall survival and adverse events with lenvatinib
treatment in patients with hepatocellular carcinoma (REFLECT). J
Clin Oncol. 37 (Suppl):S3172019. View Article : Google Scholar
|