Introduction
Midkine (MK) is a pleiotropic growth binding protein
that is highly upregulated during embryogenesis, thereby playing a
key role in neuronal differentiation (1,2).
Furthermore, MK exhibits antiapoptotic and angiogenic activities
and can lead to enhanced cell proliferation in tumors. Since MK is
a soluble cytokine, its serum levels strongly correspond to protein
expression levels in tumors (3).
Serum MK (s-MK) has been proposed as a potential biomarker for
different tumors, including hepatocellular carcinoma (HCC).
Serum α-fetoprotein (AFP) is the only diagnostic
marker recommended in the HCC guidelines. However, its diagnostic
performance is unsatisfactory, with low sensitivity and
specificity. To improve the diagnosis of HCC, advances in biomarker
detection techniques have led to the identification of several new
biomarkers, such as autoantibodies and s-MK (4–6). s-MK,
an emerging serum biomarker, activates several cell surface
receptors to modulate various biological activities and is
significantly increased in HCC (7).
s-MK has been proposed as a promising serum biomarker for HCC
diagnosis. Although several studies have estimated the diagnostic
value of s-MK for HCC, the results are inconsistent (8–12).
Precise clinicopathological analyses including AFP and protein
induced by vitamin K absence-II (PIVKA-II) have not been
published.
An s-MK-positive status has been reported to be
associated with poor prognosis in some solid tumors, such as
colorectal cancer and non-small cell lung cancer, but not in
esophageal and gastric cancers (13–16).
The correlation between an s-MK-positive status and prognosis of
patients with HCC has not been published.
Therefore, this study aimed to clarify the
clinicopathological and prognostic significance of an s-MK-positive
status in patients with HCC.
Materials and methods
Patients
This study was registered as UMIN000014530. Serum
samples were obtained before surgery from 123 patients with HCC who
had undergone surgery at Omori Medical Center, Toho University
School of Medicine, between January 2012 and December 2020. In
total, 123 patients with histologically proven primary HCC were
enrolled. The patient cohort consisted of 87 male (70.7%) and 36
female (29.3%) patients, with a median age of 69 (range, 40–85)
years. To ensure complete absence of the influence of previous
cancer, those with active coexisting cancer, i.e., synchronous
coexisting cancer or metachronous cancer within 5 disease-free
years, were excluded. The final HCC stage was assessed
pathologically following the tumor-node-metastasis classification
criteria of the eighth edition of the International Union against
Cancer (17). Tumors associated
with distant metastasis, including peritoneal dissemination, were
considered unresectable. Hepatectomy was performed according to the
treatment algorithm described in Japanese guidelines (18,19).
The degree of liver damage is defined by the following factors:
Ascites, serum total bilirubin level, serum albumin level, ICG R15,
prothrombin activity value (20).
Data collection and serum biomarker
analyses
Serum samples were obtained before surgery and
stored at −80°C until analysis. Serum samples of healthy controls,
with no previous malignant disease and hepatitis B or C infection,
were obtained from Biobank Japan. The average age of the control
group (n=77) was 52 years, with a male-to-female ratio of
50:27.
Clinicopathological characteristics, AFP, and
PIVKA-II were analyzed. Preoperative variables, pathological
characteristics, postoperative status, and survival were entered
into a spreadsheet and imported to a dedicated database. The
prognostic value and clinical utility of s-MK for HCC diagnosis
were estimated. Overall survival was calculated from the time of
surgery until death or study conclusion.
Enzyme-linked immunosorbent assay kits for human MK
(CDYELISA, Immuno-probe Ltd., Saitama, Japan) were used for
detecting s-MK according to the manufacturer's protocol. The cutoff
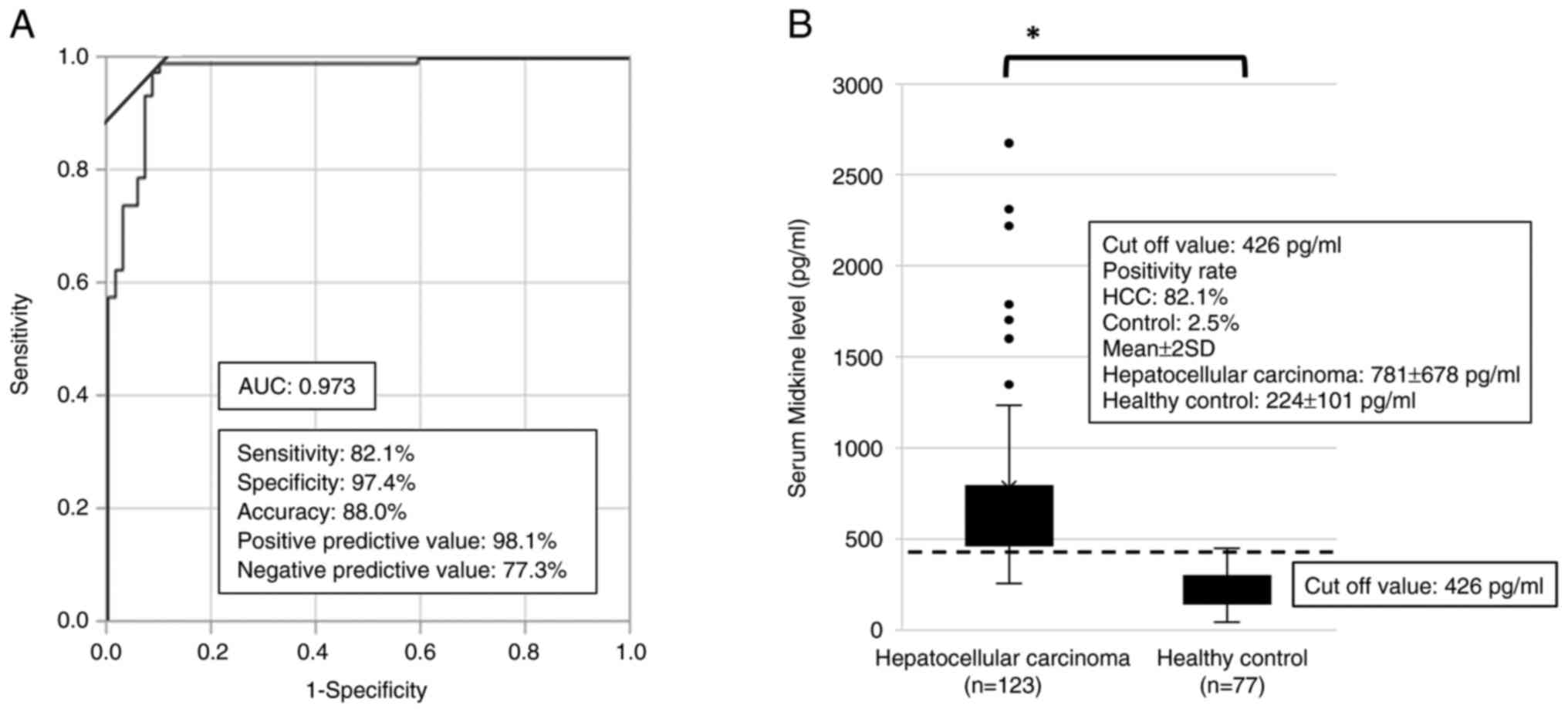
value for s-MK was fixed at 426 pg/ml based on the receiver
operating characteristic curve (Fig.
1A).
Patients' clinicopathological variables,
demographics, tumor characteristics, and overall survival were
compared between the s-MK-positive group and s-MK-negative group.
The cutoff values were 10.0 ng/ml and 40.0 mAU/ml for AFP and
PIVKA-II, respectively, following the assay kit manufacturer's
instructions.
Statistical analysis
Statistical analyses were performed using JMP
version 12 (SAS Institute, Cary, NC, USA). The comparison of s-MK
levels in the HCC and healthy control groups was performed using
unpaired t-test. A multiple comparison test of ANOVA was performed
to compare the positivity rates of s-MK, AFP, and PIVKA-II
according to TNM stages. We selected the Bonferroni post hoc test
as multiple comparison test. Between-group comparisons of the
clinicopathological variables were performed using Fisher's exact
probability test. Overall survival was calculated using the
Kaplan-Meier product limit estimate. Between-group differences in
survival were compared using the log-rank test. Significant
predictors were identified via univariate and multivariate analyses
using Cox proportional hazard models, and hazard ratios with 95%
confidence intervals (CIs) were calculated. A P value of <0.05
was considered statistically significant.
Results
Sensitivity and specificity of serum
MK levels
Based on the ROC curve, the best cutoff point was
determined to distinguish the HCC group using s-MK. The area under
the curve for s-MK was 0.973 (95% CI 0.903–0.992) (Fig. 1A). According to the curve, the best
cutoff value for s-MK in differentiating HCC from healthy cases was
426 pg/ml. At this value, the sensitivity, specificity, and
accuracy were 82, 97, and 88%, respectively. The mean s-MK levels
in the HCC and healthy control groups were 781±678 and 224±101
pg/ml, respectively (Fig. 1B,
P<0.05).
Comparison of clinicopathological
characteristics between the s-MK-positive group and s-MK-negative
group
Of the 123 patients enrolled, 101 (82%) were
positive for s-MK (>426 pg/ml) (Table I). An s-MK-positive status was
significantly associated with hepatitis B virus negativity and
number of tumors (≥2) but not with the liver reserve or liver
background.
 | Table I.Comparisons between serum midkine
level according to clinicopathological factors and various
biomarkers. |
Table I.
Comparisons between serum midkine
level according to clinicopathological factors and various
biomarkers.
| Variables | Groups | Number of patients
(n=123) | Midkine level
(median) (pg/ml) | P valuea | No. of
Midkine-positive patients (%)b | P-valuec |
|---|
| Sex | Male | 87 | 605 (452–786) | 0.740 | 71 (81) | 0.819 |
|
| Female | 36 | 561 (486–937) |
| 30 (83) |
|
| Hepatitis B
virus | Positive | 24 | 561 (410–791) | 0.268 | 16 (67) | 0.038 |
|
| Negative | 99 | 616 (486–791) |
| 85 (85) |
|
| Hepatitis C
virus | Positive | 57 | 654 (469–791) | 0.504 | 49 (86) | 0.297 |
|
| Negative | 66 | 568 (461–792) |
| 52 (79) |
|
| Child-Pugh
classification | A | 118 | 588 (466–787) | 0.034 | 96 (81) | 0.155 |
|
| B | 5 | 812 (731–901) |
| 5 (100) |
|
| Liver damage | A | 99 | 616 (452–791) | 0.652 | 81 (81) | 0.861 |
|
| B | 24 | 555 (492–792) |
| 20 (83) |
|
| Liver
background | Normal | 29 | 730 (452–953) | 0.809 | 23 (79) | 0.153 |
|
| CH | 85 | 795 (481–787) |
| 69 (81) |
|
|
| LC | 9 | 721 (407–937) |
| 9 (100) |
|
| Tumor size, mm | <20 | 37 | 537 (486–687) | 0.138 | 31 (83) | 0.749 |
|
| ≥20 | 86 | 616 (452–855) |
| 70 (81) |
|
| Tumor number | 1 | 100 | 561 (446–730) | 0.004 | 78 (78) | 0.001 |
|
| 2- | 23 | 786 (574–1198) |
| 23 (100) |
|
|
Differentiation | Well | 18 | 795 (567–937) | 0.597 | 18 (100) | 0.054 |
|
| Moderate | 101 | 726 (609–844) |
| 79 (78) |
|
|
| Other | 4 | 763 (415–1111) |
| 4 (100) |
|
| Microvascular
invasion | Positive | 53 | 771 (603–939) | 0.763 | 41 (77) | 0.233 |
|
| Negative | 70 | 788 (614–962) |
| 60 (85) |
|
| Stage | I, II | 63 | 554 (486–687) | 0.056 | 53 (84) | 0.550 |
|
| III, IV | 60 | 696 (446–949) |
| 48 (80) |
|
| AFP, ng/ml | ≤10 | 71 | 554 (452–738) | 0.311 | 57 (80) | 0.533 |
|
| >10 | 52 | 629 (511–799) |
| 44 (84) |
|
| PIVKA-II,
mAU/ml | ≤40 | 63 | 580 (452–873) | 0.391 | 51 (81) | 0.730 |
|
| >40 | 60 | 597 (486–731) |
| 50 (83) |
|
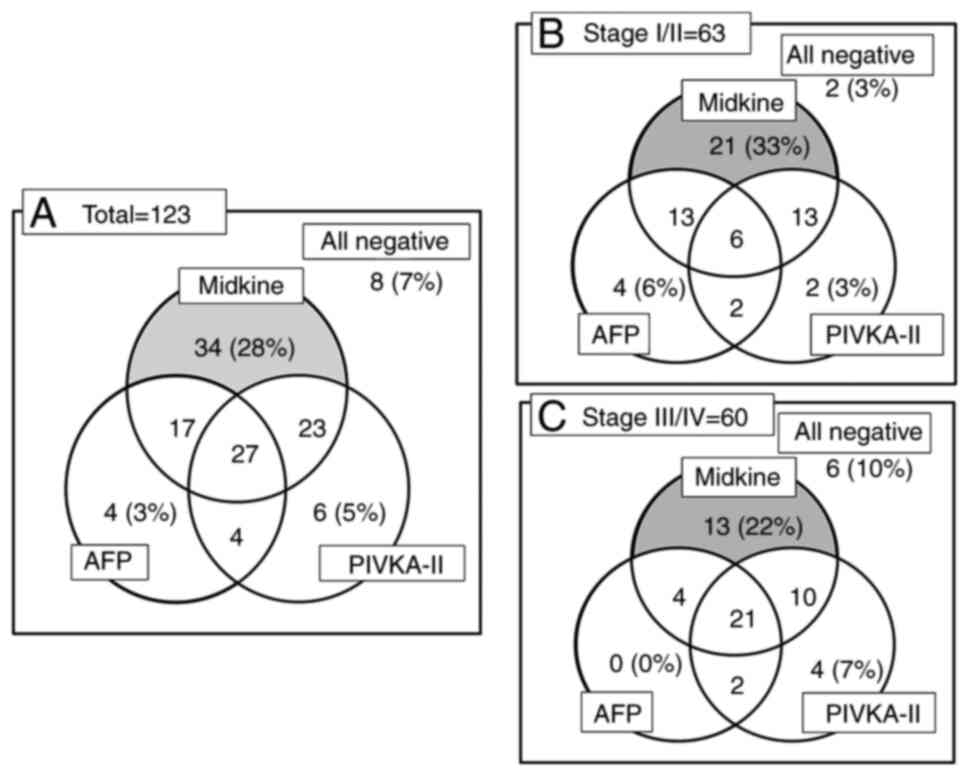
Positivity rates of s-MK, AFP, and
PIVKA-II according to TNM stages
The positivity rates of s-MK were significantly
higher than those of AFP and PIVKA-II (P<0.05, Fig. 2A). In total, only 28% (34 of 123) of
the patients were positive for s-MK. Among patients with stage
I/II, only 33% (21 of 63) were positive for s-MK (Fig. 2B). Even among patients with stage
III/IV, only 22% (13 of 60) were positive for s-MK (Fig. 2C).
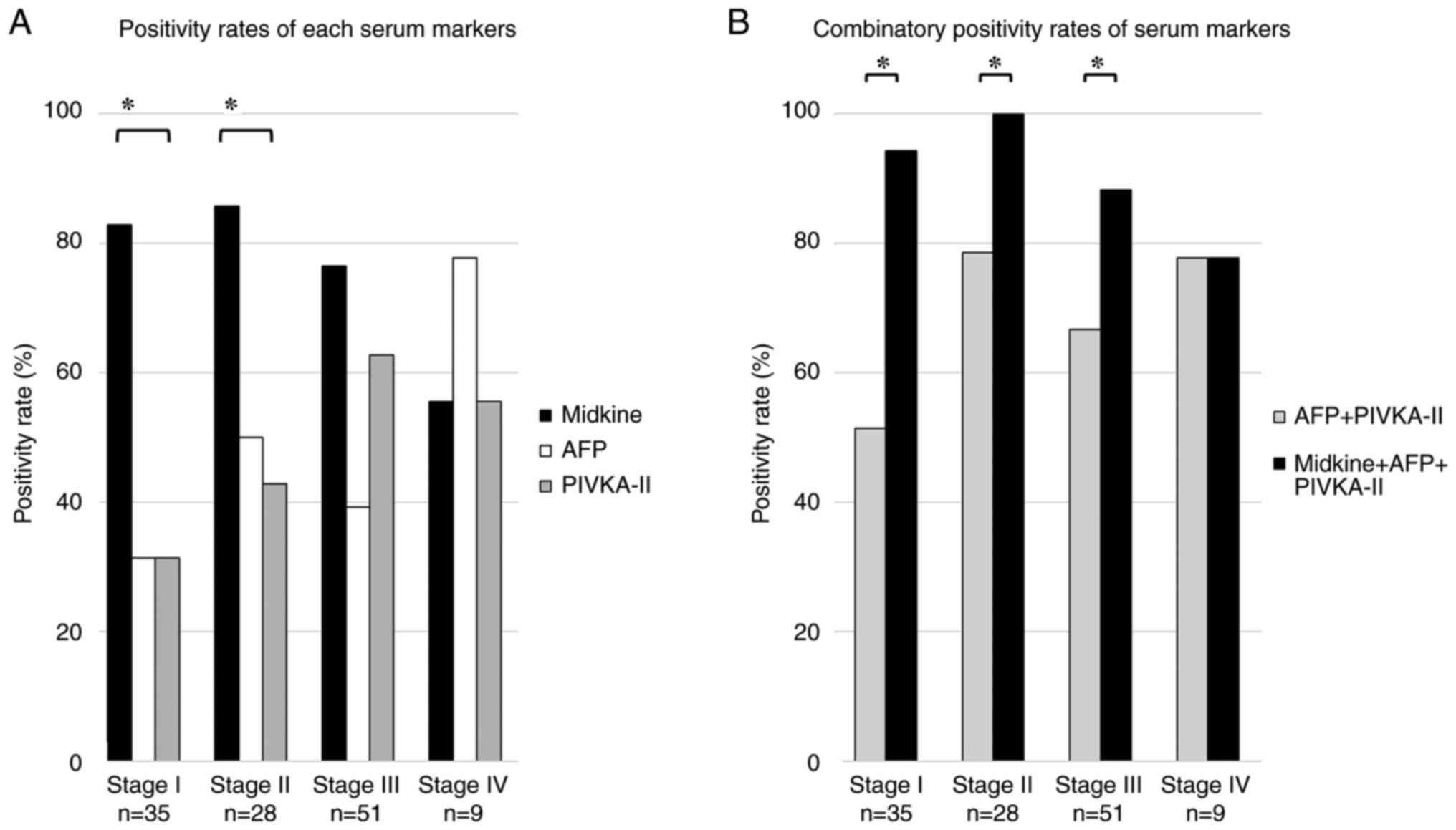
Fig. 3A shows the
positivity rates for s-MK, AFP, and PIVKA-II at each TNM stage. In
stage I, the positivity rate for s-MK was significantly higher than
that for AFP and PIVKA-II (83% vs. 31% vs. 31%, P<0.05). In
stage II, the positivity rates for s-MK, AFP, and PIVKA-II were 86,
50, and 43%, respectively (P<0.05). In stage III, the positivity
rates for s-MK, AFP, and PIVKA-II were 76, 39, and 63%,
respectively (not significant). In stage IV, the positivity rates
for s-MK, AFP, and PIVKA-II were 56, 78, and 56% (not significant),
respectively.
The positivity rate for the combined use of s-MK and
AFP + PIVKA-II was significantly higher than that for AFP +
PIVKA-II (93% vs. 65%, P<0.05, Fig.
3B). In stage I, the positivity rate for the combined use of
s-MK and AFP + PIVKA-II was significantly higher than that for AFP
+ PIVKA-II (94% vs. 51%, P<0.05). Moreover, in stage II, the
positivity rate for the combined use of s-MK and AFP + PIVKA-II was
significantly higher than that for AFP + PIVKA-II (100% vs. 79%,
P<0.05). In stage III, the positivity rate for the combined use
of s-MK and AFP + PIVKA-II was significantly higher than that for
AFP + PIVKA-II (88% vs. 67%, P<0.05).
Prognostic effect of s-MK, AFP, and
PIVKA-II status on overall survival
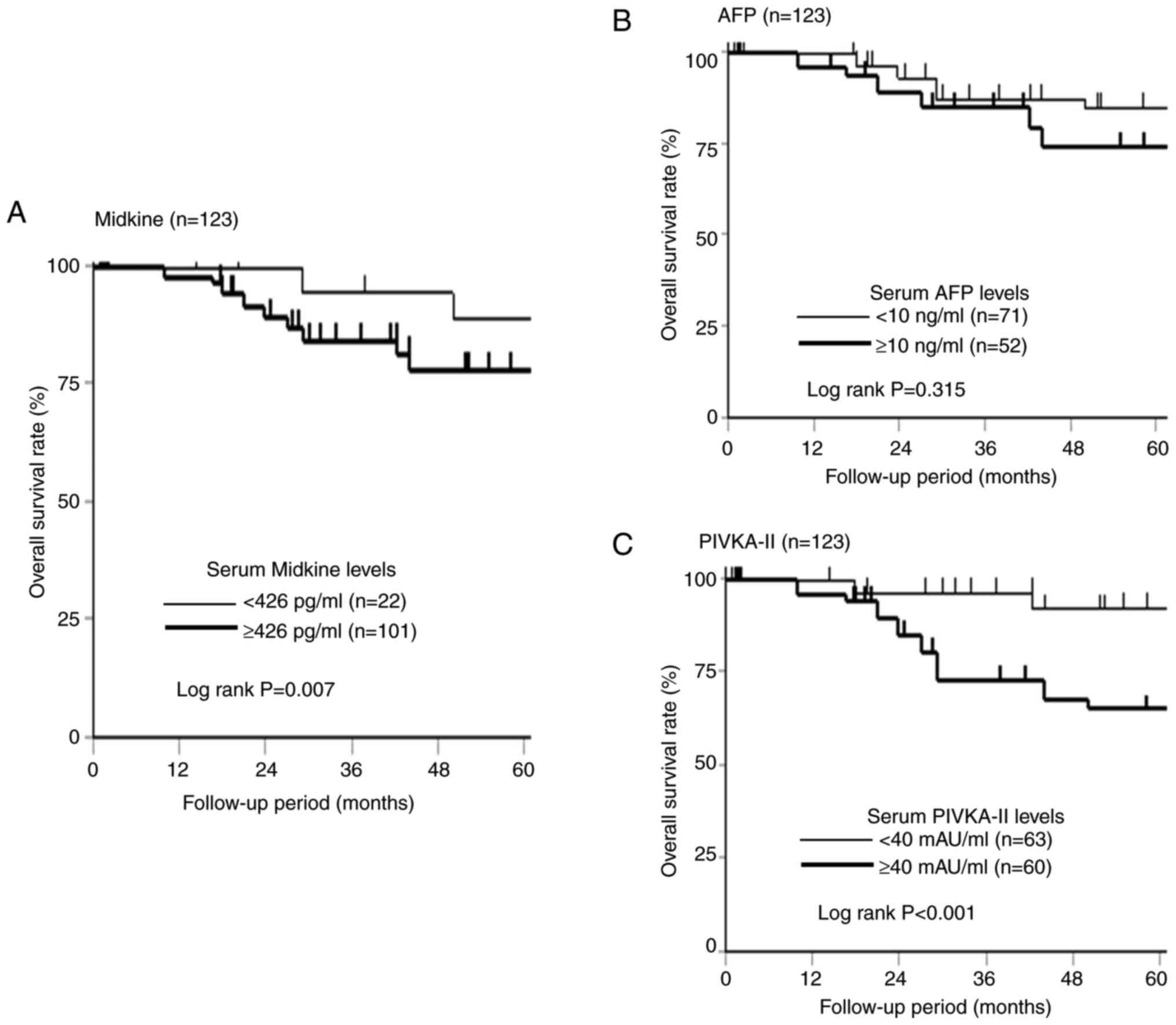
The 5-year overall survival according to the s-MK,
AFP, and PIVKA-II status is shown in Fig. 4. Although no significant difference
was observed in the overall survival according to the AFP status
(Fig. 4B, P=0.315), the
s-MK-positive group showed significantly worse overall survival
than the s-MK-negative group (Fig.
4A, P=0.007). Similarly, the PIVKA-II-positive group showed
significantly poorer overall survival than the PIVKA-II-negative
group (Fig. 4C, P<0.001).
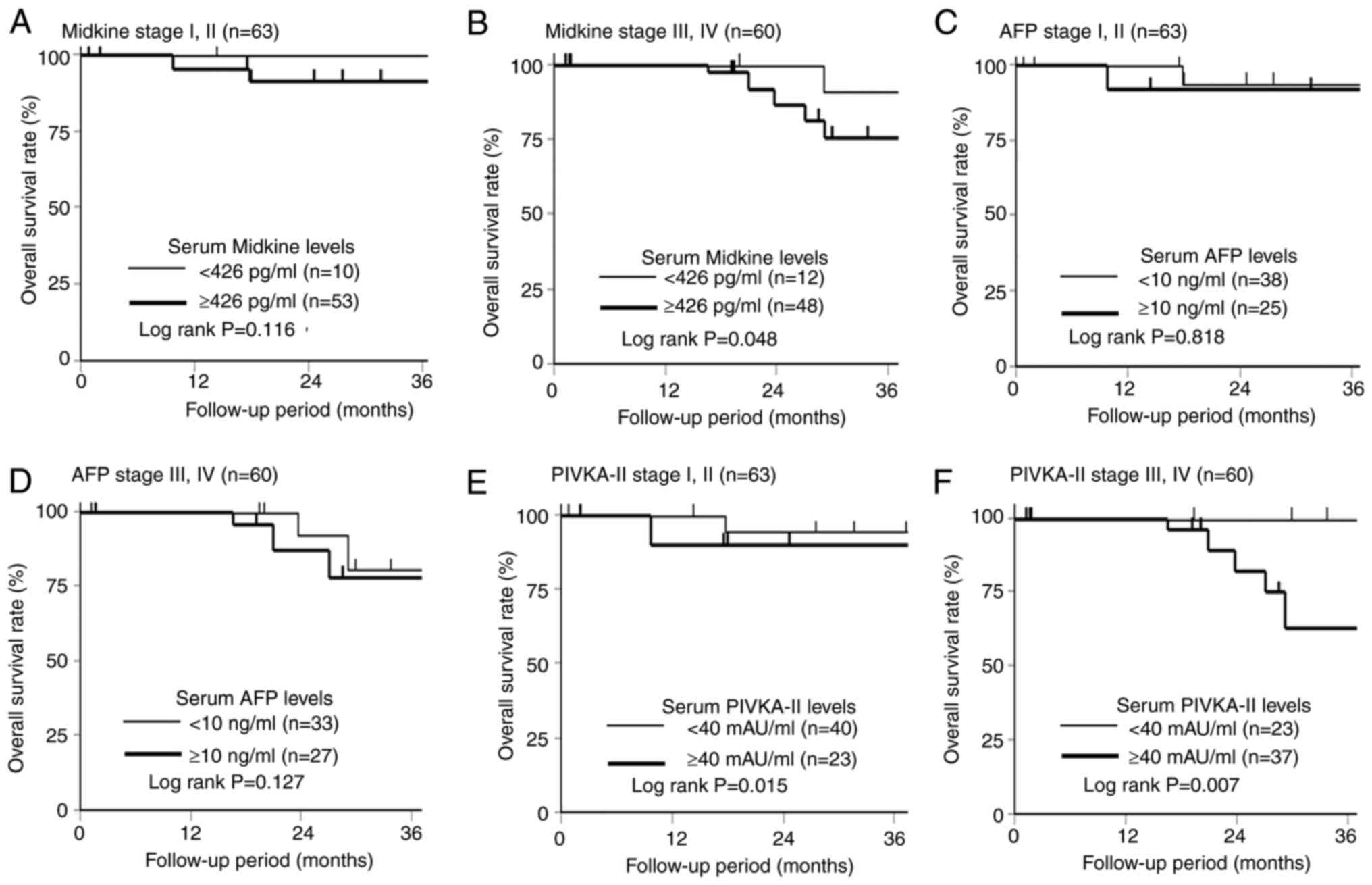
Fig. 5 shows the
comparison of overall survival at stages I/II and III/IV according
to the s-MK, AFP, and PIVKA-II status. Regarding the prognostic
effect of the s-MK status, the s-MK-positive group in stage I/II
showed slightly worse overall survival than the s-MK-negative group
(Fig. 5A, P=0.116). The
s-MK-positive group in stage III/IV showed significantly worse
overall survival than the s-MK-negative group (Fig. 5B, P=0.048). No significant
difference was observed in the overall survival according to the
AFP status (Fig. 5C and D, P=0.818,
P=0.127). In contrast, a significant difference was observed in
overall survival according to the PIVKA-II status (Fig. 5E and F, P=0.015, P=0.007).
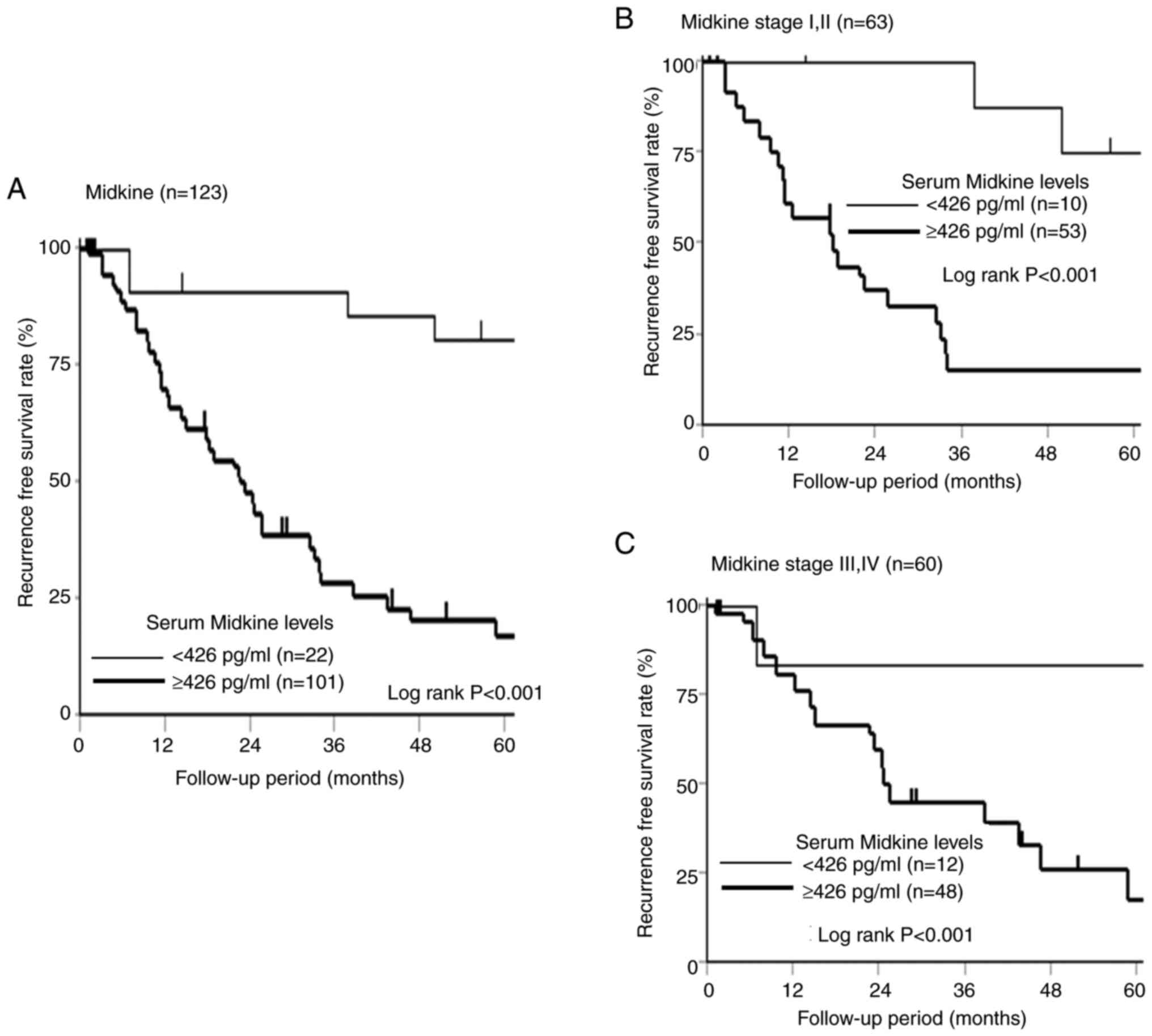
Recurrence effect of s-MK status on
recurrence-free survival
The 5-year recurrence-free survival according to the
s-MK status is shown in Fig. 6. The
s-MK-positive group showed significantly worse recurrence-free
survival than the s-MK-negative group (Fig. 6A, P<0.001). The s-MK-positive
group in stage I/II and III/IV showed significantly worse
recurrence-free survival than the s-MK-negative group (Fig. 6B and C, P<0.001).
Univariate and multivariate analyses
of overall survival
In the univariate analysis, the Child-Pugh
classification (B), liver damage (B), PIVKA-II-positive status, and
s-MK-positive status were significantly associated with poor
prognosis (Table II). In the
multivariate analysis, PIVKA-II-positive status (P=0.002; HR=3.759;
95% CI 1.600–9.603) and s-MK-positive status (P=0.006; HR=5.157;
95% CI 1.483–32.553) were independently associated with poor
prognosis.
 | Table II.Univariate and multivariate analysis
of risk factors for overall survival in 123 patients. |
Table II.
Univariate and multivariate analysis
of risk factors for overall survival in 123 patients.
|
|
|
| Multivariate |
|---|
|
|
| Univariate
P-valuea |
|
|---|
| Variables | Groups | HRb | 95% CIc |
P-valued |
|---|
| Hepatitis B
virus |
Positive/negative | 0.128 |
|
|
|
| Hepatitis C
virus |
Positive/negative | 0.339 |
|
|
|
| Child-Pugh
classification | B/A | <0.001 | 2.007 | 0.526–7.556 | 0.298 |
| Liver damage | B/A | 0.029 | 2.100 | 0.738–5.248 | 0.153 |
| Liver
background | LC/CH/normal | 0.599 |
|
|
|
| Tumor size, mm | ≥20/<20 | 0.667 |
|
|
|
| Tumor number | ≥2/1 | 0.072 |
|
|
|
|
Differentiation |
Well/moderate/other | 0.614 |
|
|
|
| Microvascular
invasion |
Positive/negative | 0.355 |
|
|
|
| AFP, ng/ml | >10/≤10 | 0.315 |
|
|
|
| PIVKA-II,
mAU/ml | >40/≤40 | <0.001 | 3.759 | 1.600–9.603 | 0.002 |
| Serum midkine,
pg/ml | >426/≤426 | 0.007 | 5.157 | 1.483–32.553 | 0.006 |
Discussion
The positivity rate for s-MK was 82% in patients
with HCC. The positivity rate for the combined use of s-MK and AFP
+ PIVKA-II was significantly higher than that for AFP + PIVKA-II.
An s-MK-positive status was associated with the number of tumors.
The s-MK-positive group showed poor overall survival.
An s-MK-positive rate was not associated with stage,
and this tendency was similar to the pattern of serum
autoantibodies, as previously reported (5,6). s-MK
is induced not only by cancer but also by various factors such as
inflammation and hemodynamics (21). At present, even in HCC, which has
multistage carcinogenesis, the stage at which s-MK is induced is
unclear. Shaheen et al reported that the s-MK level was
significantly elevated in the HCC group compared with the healthy
control group and liver cirrhosis group (22). These findings suggest that s-MK can
be used to detect early-stage cancer follow up patients with
cirrhosis.
In the present study, s-MK was associated with the
number of tumors but not with liver background or tumor size. Among
the 23 patients with multiple tumors, the positivity rates for
s-MK, AFP, and PIVKA-II were 100, 69, and 43%, respectively. This
may be because MK plays an important role in cell proliferation,
survival, migration, angiogenesis, and carcinogenesis (23,24).
Whether s-MK is a cause or a consequence of multiple tumors is
unclear. However, given that an s-MK-positive status is a poor
prognostic factor, an s-MK-positive status may reflect the
biological grade of the tumor.
The prognostic effect of s-MK on various cancers was
not consistent. In this study, we first evaluated the prognostic
effect of s-MK on HCC. An s-MK-positive status was an independent
risk factor for poor overall survival. The poor prognostic effect
of an s-MK-positive status in HCC suggests the high biological
malignancy of s-MK-positive HCC cells, given the lack of
correlation between an s-MK-positive status and cirrhosis.
MK-positive cancer cells have been reported to be associated with
antiapoptotic function, and resistance to chemotherapy after HCC
recurrence may contribute to poor prognosis (25). Considering that miRNA519d, an
exosome derived from HCC, can inhibit apoptosis and distinguish
between cirrhotic patients without HCC and cirrhotic patients with
early-stage HCC, miRNA519d and s-MK may have a common mechanism
(26). Considering the results of
the IMbrave050 trial, patients with an s-MK positive status who are
at a high risk of recurrence may be able to effectively prolong
their recurrence-free survival by receiving adjuvant atezolizumab
plus bevacizumab (27).
This study had some limitations. First, the sample
size was not large enough. Assuming a 95% confidence level and a 5%
confidence interval, we were unable to collect a sample size large
enough for this study. Second, no data were available for
evaluating the association between s-MK positivity and the
immunoreactivity of cancer cells. Since several previous studies
have reported that s-MK concentrations are significantly associated
with immunoreactivity, MK expression in cancer cells may similarly
be associated with s-MK (28,29).
Third, we did not analyze the other cytokines, such as serum
vascular endothelial growth factor (VEGF), in this study. Alzamzamy
et al reported that in patients with HCV, serum VEGF and
VEGF/PLT separately or in combination with AFP are reliable
biomarkers for early and accurate HCC diagnosis (30). Furthermore, Mamdouh et al
reported that the serum VEGF levels in patients with HCC and
cirrhosis were significant compared with the control group
(31). It is possible that s-MK,
together with cytokines such as VEGF, will play a major role in the
diagnosis of hepatocellular carcinoma in the future. Fourth, this
study only focused on preoperative s-MK and had no data of
postoperative monitoring. Therefore, we could not capture changes
in s-MK levels before and after surgery. The s-MK level was
reported to decrease significantly after surgery in esophageal
cancer (28).
In conclusion, s-MK was a convenient and useful
serum biomarker to detect HCC even in patients with stage I/II
regardless of LC. An s-MK-positive status was associated with the
number of tumors and was an independent prognostic risk factor.
Considering the malignant potential of s-MK-positive HCC, more
intensive follow-up is necessary after surgery.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
RO and HS confirmed the authenticity of all the raw
data. RO conceptualized and designed the study, performed the
statistical analysis and prepared the manuscript. YO, YK, TM, JI,
KK, YM, YI and KF acquired the data. RO and YO performed the
quality control of data and algorithms. RO, YO and HS analyzed and
interpreted the data. RO and HS edited the manuscript. All authors
reviewed the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
All study participants provided consent for future
analyses of their blood samples for research. The protocol for this
study was approved by the Ethics Committee of Toho University
(approval nos. M22211, M21038_20197_19213 and
A18103_A17052_A16035_A16001_26095_25024_24038_22047_22112).
Patients provided written informed consent before enrolment. The
study was registered in the UMIN Clinical Trials Registry (clinical
trial no. UMIN000014530) and was conducted following the guidelines
of the Declaration of Helsinki and the Japanese Ethical Guidelines
for Clinical Research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AFP
|
α-fetoprotein
|
|
HCC
|
hepatocellular carcinoma
|
|
MK
|
midkine
|
|
PIVKA-II
|
protein-induced by vitamin K
absence-II
|
References
|
1
|
Kadomatsu K: Midkine, a heparin-binding
growth factor: Its discovery and functions. Seikagaku.
70:1315–1325. 1998.(In Japanese). PubMed/NCBI
|
|
2
|
Muramatsu T: Midkine, a heparin-binding
cytokine with multiple roles in development, repair and diseases.
Proc Jpn Acad Ser B Phys Biol Sci. 86:410–425. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jones DR: Measuring midkine: The utility
of midkine as a biomarker in cancer and other diseases. Br J
Pharmacol. 171:2925–2939. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsuchiya N, Sawada Y, Endo I, Saito K,
Uemura Y and Nakatsura T: Biomarkers for the early diagnosis of
hepatocellular carcinoma. World J Gastroenterol. 21:10573–10583.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okada R, Otsuka Y, Wakabayashi T, Shinoda
M, Aoki T, Murakami M, Arizumi S, Yamamoto M, Aramaki O, Takayama
T, et al: Six autoantibodies as potential serum biomarkers of
hepatocellular carcinoma: A prospective multicenter study. Int J
Cancer. 147:2578–2586. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Okada R, Otsuka Y, Yokosuka O, Kato N,
Imazaki F, Hoshino I, Sugiura N, Mizumoto H, Azemoto R, Kato K and
Shimada H: Six autoantibodies as potential differential biomarkers
of hepatocellular carcinoma vs. liver cirrhosis and chronic
hepatitis: A prospective multi-institutional study. Oncol Lett.
24:3672022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kadomatsu K and Muramatsu T: Midkine and
pleiotrophin in neural development and cancer. Cancer Lett.
204:127–143. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mashaly AH, Anwar R, Ebrahim MA, Eissa LA
and El Shishtawy MM: Diagnostic and prognostic value of talin-1 and
midkine as tumor markers in hepatocellular carcinoma in Egyptian
patients. Asian Pac J Cancer Prev. 19:1503–1508. 2018.PubMed/NCBI
|
|
9
|
Hodeib H, ELshora O, Selim A, Sabry NM and
El-Ashry HM: Serum midkine and osteopontin levels as diagnostic
biomarkers of hepatocellular carcinoma. Electron Physician.
9:3492–3498. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vongsuvanh R, van der Poorten D, Iseli T,
Strasser SI, McCaughan GW and George J: Midkine increases
diagnostic yield in AFP negative and NASH-related hepatocellular
carcinoma. PLoS One. 11:e01558002016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang BH, Li B, Kong LX, Yan LN and Yang
JY: Diagnostic accuracy of midkine on hepatocellular carcinoma: A
meta-analysis. PLoS One. 14:e02235142019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu Q, Li J, Cao H, Lv C, Wang X and Cao S:
Comparison of diagnostic accuracy of midkine and AFP for detecting
hepatocellular carcinoma: A systematic review and meta-analysis.
Biosci Rep. 40:BSR201924242020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kemper M, Hentschel W, Graß JK, Stüben BO,
Konczalla L, Rawnaq T, Ghadban T, Izbicki JR and Reeh M: Serum
midkine is a clinical significant biomarker for colorectal cancer
and associated with poor survival. Cancer Med. 9:2010–2018. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stern L, Mueller E, Bellon E, Reeh M,
Grotelueschen R, Guengoer C, Melling N, Goetz M, Perez DR, Izbicki
JR, et al: Serum midkine as non-invasive biomarker for detection
and prognosis of non-small cell lung cancer. Sci Rep. 11:146162021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shiratori F, Ito M, Yajima S, Suzuki T,
Oshima Y, Nanami T, Funahashi K and Shimada H: The effectiveness of
serum midkine in detecting esophageal squamous cell carcinoma.
Esophagus. 16:246–251. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ito M, Oshima Y, Yajima S, Suzuki T,
Nanami T, Shiratori F, Funahashi K and Shimada H: Diagnostic impact
of high serum midkine level in patients with gastric cancer. Ann
Gastroenterol Surg. 3:195–201. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Union for International Cancer Control, .
TNM Classification of Malignant Tumors. Brierley JD, Gospodarowicz
MK and Wittekind CH: 8th edition. UICC; Wiley, New York, NY:
2001
|
|
18
|
Makuuchi M and Kokudo N: Clinical practice
guidelines for hepatocellular carcinoma: The first evidence based
guidelines from Japan. World J Gastroenterol. 12:828–829. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kubota K, Makuuchi M, Kusaka K, Kobayashi
T, Miki K, Hasegawa K, Harihara Y and Takayama T: Measurement of
liver volume and hepatic functional reserve as a guide to
decision-making in resectional surgery for hepatic tumors.
Hepatology. 26:1176–1181. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kudo M, Izumi N, Kokudo N, Matsui O,
Sakamoto M, Nakashima O, Kojiro M and Makuuchi M; HCC Expert Panel
of Japan Society of Hepatology, : Management of hepatocellular
carcinoma in Japan: Consensus-Based Clinical Practice Guidelines
proposed by the Japan Society of Hepatology (JSH) 2010 updated
version. Dig Dis. 29:339–364. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ketenci S, Uygar Kalaycı M, Dündar B,
Duranay R and Şükrü Aynacıoğlu A: Elevated serum midkine levels in
severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
infected patients. Int Immunopharmacol. 110:1089392022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shaheen KY, Abdel-Mageed AI, Safwat E and
AlBreedy AM: The value of serum midkine level in diagnosis of
hepatocellular carcinoma. Int J Hepatol. 2015:1463892015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shin DH, Jo JY, Kim SH, Choi M, Han C,
Choi BK and Kim SS: Midkine is a potential therapeutic target of
tumorigenesis, angiogenesis, and metastasis in non-small cell lung
cancer. Cancers (Basel). 12:24022020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Karadeniz Z, Aynacıoğlu AŞ, Bilir A and
Tuna MY: Inhibition of midkine by metformin can contribute to its
anticancer effects in malignancies: A proposal mechanism of action
of metformin in context of endometrial cancer prevention and
therapy. Med Hypotheses. 134:1094202020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qi M, Ikematsu S, Ichihara-Tanaka K,
Sakuma S, Muramatsu T and Kadomatsu K: Midkine rescues Wilms' tumor
cells from cisplatin-induced apoptosis: Regulation of Bcl-2
expression by midkine. J Biochem. 127:269–277. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sasaki R, Kanda T, Yokosuka O, Kato N,
Matsuoka S and Moriyama M: Exosomes and hepatocellular carcinoma:
From bench to bedside. Int J Mol Sci. 20:14062019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qin S, Chen M, Cheng AL, Kaseb AO, Kudo M,
Lee HC, Yopp AC, Zhou J, Wang L, Wen X, et al: Atezolizumab plus
bevacizumab versus active surveillance in patients with resected or
ablated high-risk hepatocellular carcinoma (IMbrave050): A
randomised, open-label, multicentre, phase 3 trial. Lancet.
402:1835–1847. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamashita T, Shimada H, Tanaka S, Araki K,
Tomifuji M, Mizokami D, Tanaka N, Kamide D, Miyagawa Y, Suzuki H,
et al: Serum midkine as a biomarker for malignancy, prognosis, and
chemosensitivity in head and neck squamous cell carcinoma. Cancer
Med. 5:415–425. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shimada H, Nabeya Y, Tagawa M, Okazumi S,
Matsubara H, Kadomatsu K, Muramatsu T, Ikematsu S, Sakuma S and
Ochiai T: Preoperative serum midkine concentration is a prognostic
marker for esophageal squamous cell carcinoma. Cancer Sci.
94:628–632. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alzamzamy A, Elsayed H, Abd Elraouf M,
Eltoukhy H, Megahed T and Aboubakr A: Serum vascular endothelial
growth factor as a tumor marker for hepatocellular carcinoma in
hepatitis C virus-related cirrhotic patients. World J Gastrointest
Oncol. 13:600–611. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mamdouh S, Soliman A, Khorshed F and Saber
M: Glypican-3, vascular endothelial growth factor and golgi
protein-73 for differentiation between liver cirrhosis and
hepatocellular carcinoma. Asian Pac J Cancer Prev. 24:497–507.
2023. View Article : Google Scholar : PubMed/NCBI
|