Introduction
SWI/SNF-related matrix-associated actin-dependent
regulator of chromatin subfamily A member 4 (SMARCA4)-deficient
tumors are prone to misdiagnosis because of their lack of specific
differentiation, and are associated with a rapid progression and
poor prognosis (1). Previous
reports of SMARCA4-deficient tumors mainly refer to individual
cases in multiple organs and sites, such as the nasal cavity and
sinuses (2), chest and lung
(3), gastrointestinal tract
(4,5) and uterus (6). The main types of SMARCA4-deficient
tumors in the uterus are: Undifferentiated and dedifferentiated
endometrial cancer, and SMARCA4-deficient undifferentiated uterine
tumors. The former accounts for 1–2% of endometrial cancer cases,
mostly occurs during the perimenopause and the prognosis is poor;
notably, the prognosis of undifferentiated endometrial cancer is
worse in SMARCA4-deficient cases. SMARCA4-deficient
undifferentiated uterine tumors tends to occur in young people and
have a worse prognosis than undifferentiated and dedifferentiated
endometrial cancer. At present, the absence of SMARCA4 in poorly
differentiated uterine malignancies can be detected by
immunohistochemistry, which helps to identify SMARCA4-deficient
tumors and make a differential diagnosis with other malignancies.
SMARCA4-deficient tumors are highly aggressive tumors that do not
respond well to conventional treatment (3–5). The
present study describes the case of a SMARCA4-deficient tumor in
the adnexal region of the uterus with ascites. The present case
report may improve the understanding of this novel group of
diseases.
Case report
Ethic approval
The present study was approved by the Institutional
Review Board of Weifang People's Hospital (First Affiliated
Hospital of Shandong Second Medical University) (Weifang, China;
approval no. KYLL20240105-2). Written informed consent was obtained
from the patient for the publication of this case report and the
accompanying images. This study was conducted in accordance with
the principles of the Declaration of Helsinki.
Clinical history
A 64-year-old woman presented at Weifang People's
Hospital (Weifang, China) in May 2023 with abdominal distension and
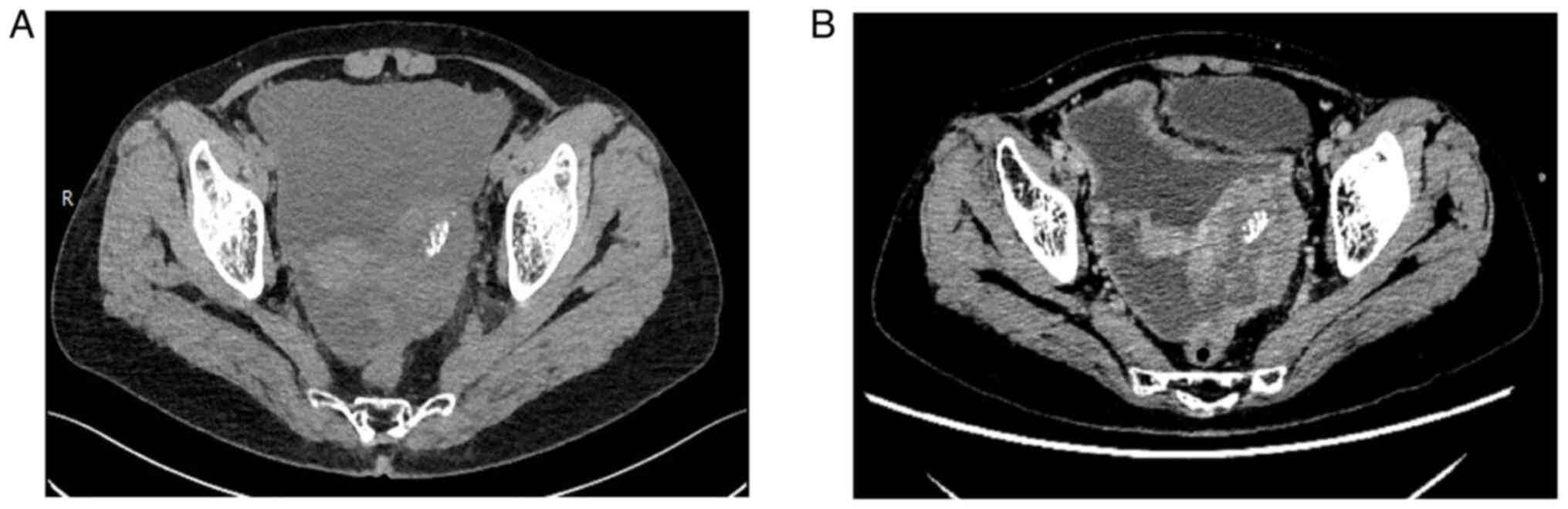
increased stool frequency for >10 days. Computed tomography
evaluation showed dense soft tissue in the left adnexal area, with
the interior showing a spot-like high density; the tumor was
~6.6×4.1 cm in size (Fig. 1). No
abnormalities were observed in the liver, gallbladder, pancreas,
bladder, bilateral kidneys or adrenal glands. The uterus was normal
in size and shape. The retroperitoneal and omental adipose spaces
were blurred; nodules were observed, and there were large amounts
of fluid in the abdominal and pelvic cavities. The blood calcium
concentration was slightly lower than normal at 1.95 mmol/l (normal
range, 2.11–2.58 mmol/l), tumor marker CA125 level (32.30 U/ml) was
slightly higher than normal (0–16 U/ml), and tumor marker CA199
14.47 U/ml (normal range, 0–34 U/ml) and carcinoembryonic antigen
(CEA) 1.04 ng/ml (0–5 ng/ml) levels were normal. After preoperative
examination, laparoscopic exploration was performed; during the
operation, a large number of bleeding ascites in the pelvic cavity
and abdominal cavity was observed, the surface of the uterus was
covered with crumbly-textured lesions, the left fallopian tube and
ovary were wrapped together with a diameter of ~10 cm, the right
ovary was atrophied with small lesions on the surface and the right
fallopian tube appeared normal. Because extensive lesions were
observed, the left and right adnexal lesions were biopsied
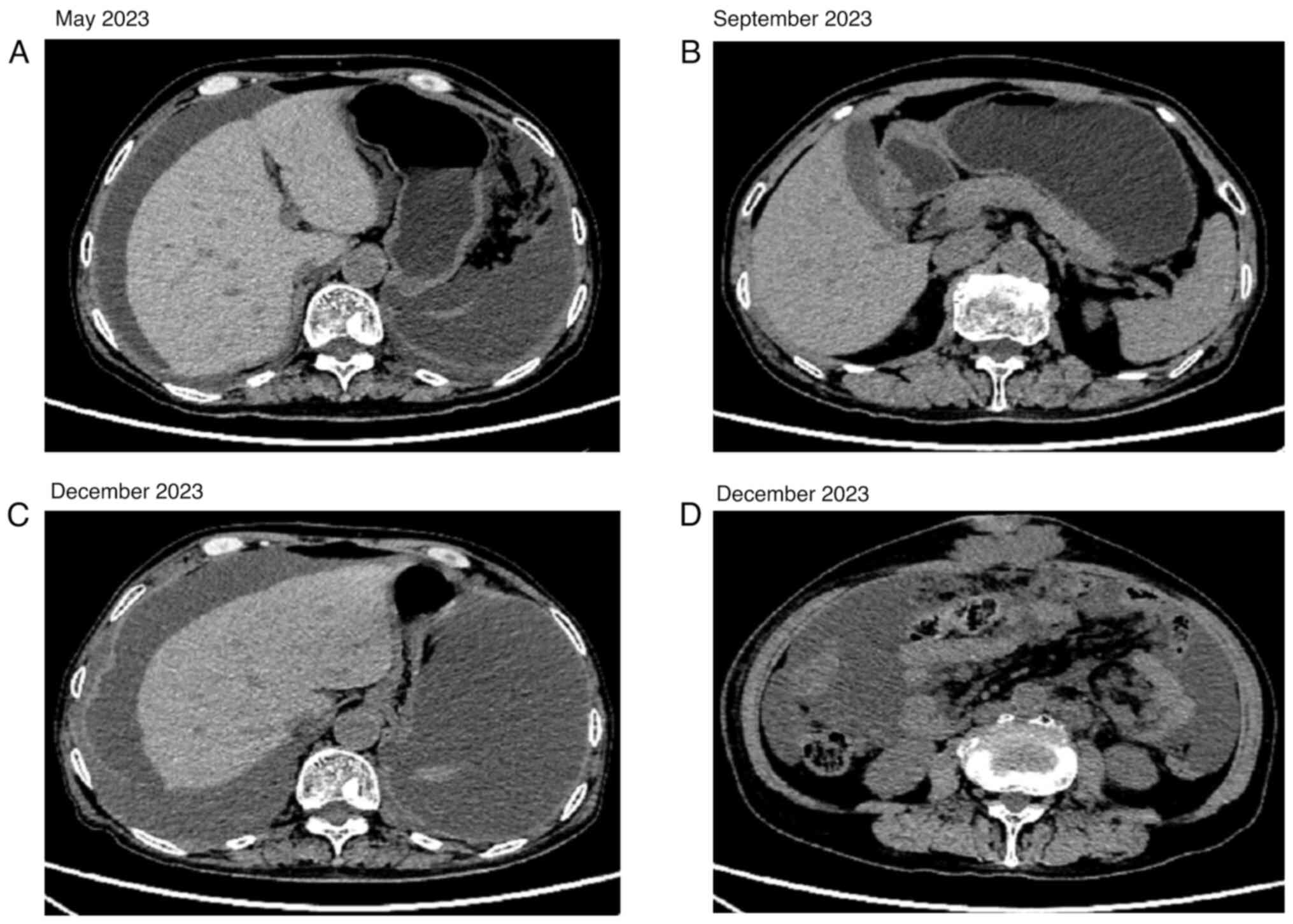
(Fig. 2A). The pelvic effusion
disappeared after four cycles of peritoneal thermoperfusion
chemotherapy with 30 mg cisplatin (Fig.
3A and B). Bevacizumab injection (500 mg on day 0), docetaxel
(90 mg on day 1) and carboplatin (400 mg on day 2) was administered
for three cycles, after which the disease was advanced (Fig. 3C and D). After one cycle of
bevacizumab (500 mg on day 0), sindilizumab (500 mg on day 0) and
carboplatin (500 mg on day 1), no further treatment was
administered. The patient died of multiple organ metastasis 9
months after the start of treatment.
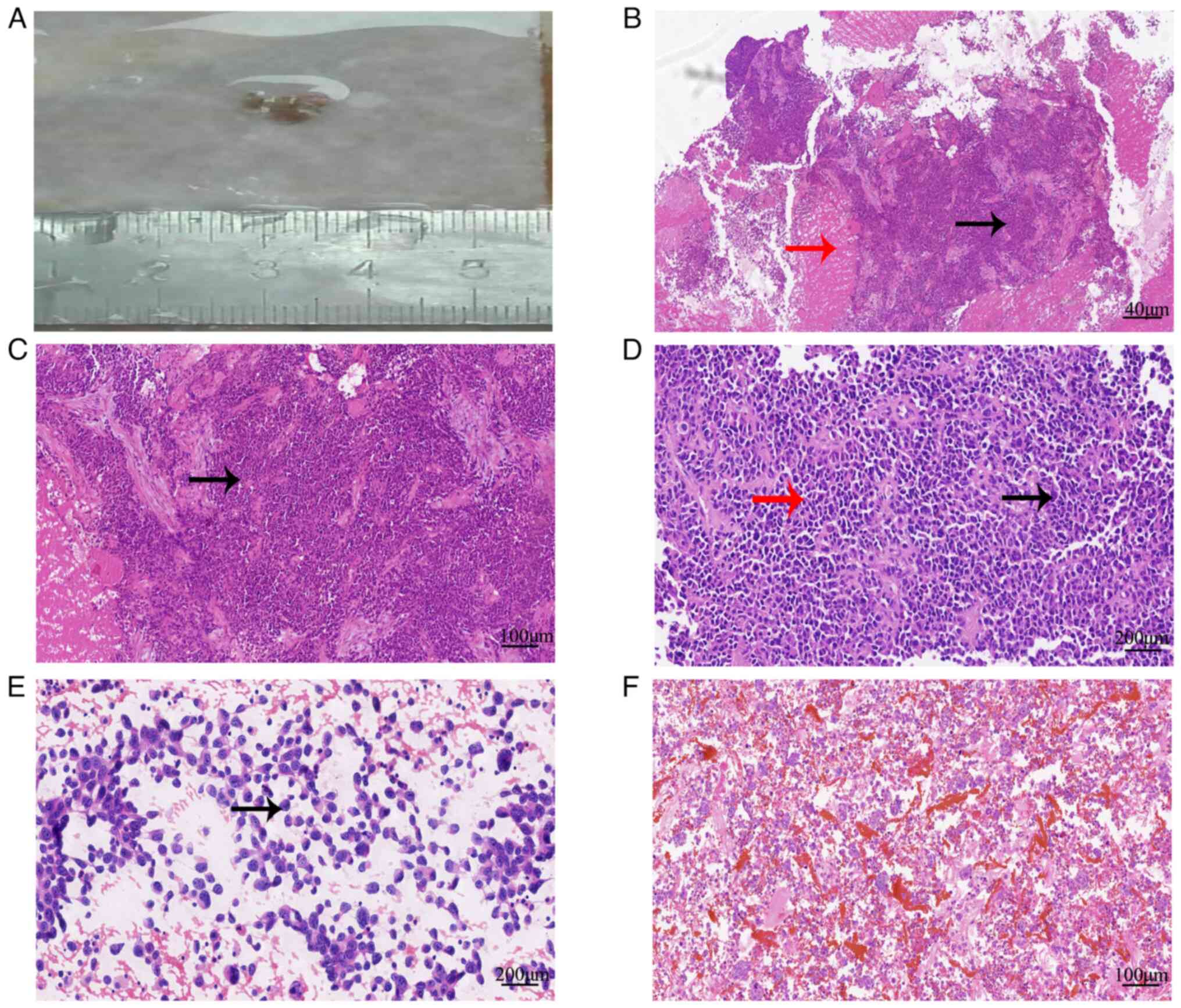 | Figure 2.Histological features associated with
the SWI/SNF-related matrix-associated actin-dependent regulator of
chromatin subfamily A member 4-deficient tumor in the adnexal area
of the uterus. (A) Biopsied tissues of the lesions. (B)
Histological morphology showed infiltrative growth of tumor cells,
accompanied by necrosis (red arrow), tumor arrangement disorder and
a local pseudochrysanthemum-like structure (black arrow)
(hematoxylin and eosin, ×40). (C) Tumor cells showed obvious
atypia, diverse shapes (round and oval, black arrow) (hematoxylin
and eosin, ×100). (D) Tumor cells had clear nucleolus, abundant
cytoplasm (black arrow), some cells had eosinophilic cytoplasm (red
arrow), and mitotic figures were detected (hematoxylin and eosin,
×200). (E) Smear of ascites tumor cells showed cytoplasmic
eosinophilic cells (black arrow) (hematoxylin and eosin, ×200).
Cancer cells were observed in both (E) ascites smears and (F) cell
blocks (hematoxylin and eosin, ×100). |
Immunohistochemical staining and gene
sequencing
The biopsied specimens were fixed in 10%
neutral-buffered formalin for 24 h at room temperature, embedded in
paraffin blocks and cut into 4-µm sections. The sections were
stained with hematoxylin and eosin (H&E; (cat. no. G1120;
Beijing Solarbio Science & Technology Co., Ltd.) at room
temperature for histological assessment. The slides were dewaxed
and stained with ready-to-use hematoxylin for 3 min, then rinsed
with distilled water for 5 min and differentiated with 1%
hydrochloric acid alcohol for 10 sec, followed by washing in
distilled water for 1 min. The slides were then stained with
ready-to-use eosin (water-soluble) for 1 min followed by washing in
distilled water for 20 sec. The H&E-stained sections were
observed under a light microscope (Nikon Corporation).
The ascites (1,000 ml extracted during surgery) were
placed in a centrifuge tube and centrifuged at 6,000 × g for 10 min
at room temperature. After centrifugation, the supernatant was
poured away. The cell sediment deposited at the bottom was smeared
on a slide and fixed with 95% alcohol at room temperature for 30
min for H&E staining and microscopic observation, which was
performed as aforementioned. In addition, the cell sediment formed
by centrifugation was fixed with 95% alcohol for 1 h at room
temperature and centrifuged at 6,000 × g for 10 min, at room
temperature. The sediment was then poured out, wrapped in filter
paper, dehydrated, dipped in paraffin wax and embedded to prepare
cell wax blocks (4 µm). The wax blocks were cut into 4-µm sections
and prepared for immunohistochemical staining as follows. After
washing three times in 0.01 M PBS (pH 7.4) for 5 min each time at
room temperature, the sections were incubated with 3% hydrogen
peroxide at room temperature for 10 min. The sections were then
washed a further three times with 0.01 M PBS (pH 7.4) for 5 min
each time at room temperature. Antigen retrieval was performed with
EDTA at 100°C for 2.5 min followed by washing with PBS. The
sections were then incubated with undiluted primary antibody at
37°C for 60 min and ready-to-use secondary antibody at 37°C for 20
min. The following primary antibodies were used: Ready-to-use
primary antibodies against broad-spectrum cytokeratin (CK; cat. no.
MAB-0671); vimentin (cat. no. MAB-0735); SWI/SNF-related,
matrix-associated, actin-dependent regulator of chromatin,
subfamily B, member 1 (INI-1; cat. no. MAB-0696); synaptophysin
(Syn; cat. no. MAB-0742); calretinin (cat. no. MAB-0716); thyroid
transcription factor-1 (TTF-1; cat. no. MAB-0677); SOX10 (cat. no.
RMA-0726); CK7 (cat. no. MAB-0828); CK8/18 (cat. no. MAB-1002);
NapsinA (cat. no. MAB-0704); CK20 (cat. no. MAB-0834), CDX-2 (cat.
no. MAB-1056); special AT-rich sequence-binding protein 2 (SATB-2;
cat. no. RMA-0750); Villin (cat. no. MAB-0710); GATA3 (cat. no.
MAB-0695); P63 (cat. no. MAB-0694); CEA (cat. no. MAB-0852); Wilms
tumor gene (WT-1; cat. no. MAB-0678); paired box gene 8 (PAX-8;
cat. no. MAB-0837); CD34 (cat. no. Kit-0004); ERG (cat. no.
RMA-0748); CD56 (cat. no. MAB-0743); desmin (cat. no. MAB-0766);
myogenic differentiation 1 (MyoD1; cat. no. MAB-0822); epithelial
membrane antigen (EMA; cat. no. Kit-0011); CD30 (cat. no.
MAB-0868); S-100 (cat. no. Kit-0007); SMARCA4 (cat. no. RMA-1063);
mutL homolog 1 (MLH1; cat. no. MAB-0838); mutS homolog 2 (MSH2;
cat. no. MAB-0836); mutS homolog 6 (MSH6; cat. no. MAB-0831);
postmeiotic segregation increased 2 (PMS2; cat. no. MAB-0859); P53
(cat. no. MAB-0674) and Ki-67 (cat. no. MAB-0672) (Fuzhou Maixin
Biotech Co., Ltd.). Biotinylated goat anti-mouse and rabbit
secondary antibodies (cat. no. KIT-9710) were obtained from Fuzhou
Maixin Biotech Co., Ltd. Finally, tissue sections were stained with
3,3′-diaminobenzidine at room temperature for 5 min, counterstained
with hematoxylin at room temperature for 5 min and images were
captured using a light microscope (Nikon Corporation).
Next-generation sequencing (NGS) was performed to
detect mutations in SMARCA4 using a kit from Yuanma Gene Technology
(Beijing) Co., Ltd. DNA was extracted from the appropriate
paraffin-embedded tumor tissue for high-throughput gene sequencing
to evaluate gene mutations, base replacements, insertions,
deletions, copy number changes and fusion/rearrangement patterns. A
paraffin tissue DNA kit (cat. no. FFPE DNA; Amoy Diagnostics Co.,
Ltd.) was used to extract DNA from the tumor sections.
Subsequently, the extracted DNA concentration was measured with a
spectrophotometer. The DNA concentration used for sequencing was 20
pM. The tumor exome was sequenced by high-throughput sequencing
using the kit [cat. no. YMFW-102; Yuanma Gene Technology (Beijing)
Co., Ltd.]. Paired-end sequencing was performed and the nucleotide
length was 150–600 bp. Finally, the data were analyzed using
trimmomatic software (version: 0.38; http://www.usadellab.org/cms/index.php?page=trimmomatic).
Morphological and immunohistochemical
findings, and the results of NGS analysis
Microscopically, tumor cells showed infiltrative
growth with local pseudochrysanthemum-like structures accompanied
by necrosis. The tumor cells showed obvious atypia and diverse
shapes, such as round and oval nuclei, and abundant cytoplasm, and
some of the cells had eosinophilic cytoplasm and mitotic figures.
Cancer cells were observed in both ascites smears and cell blocks
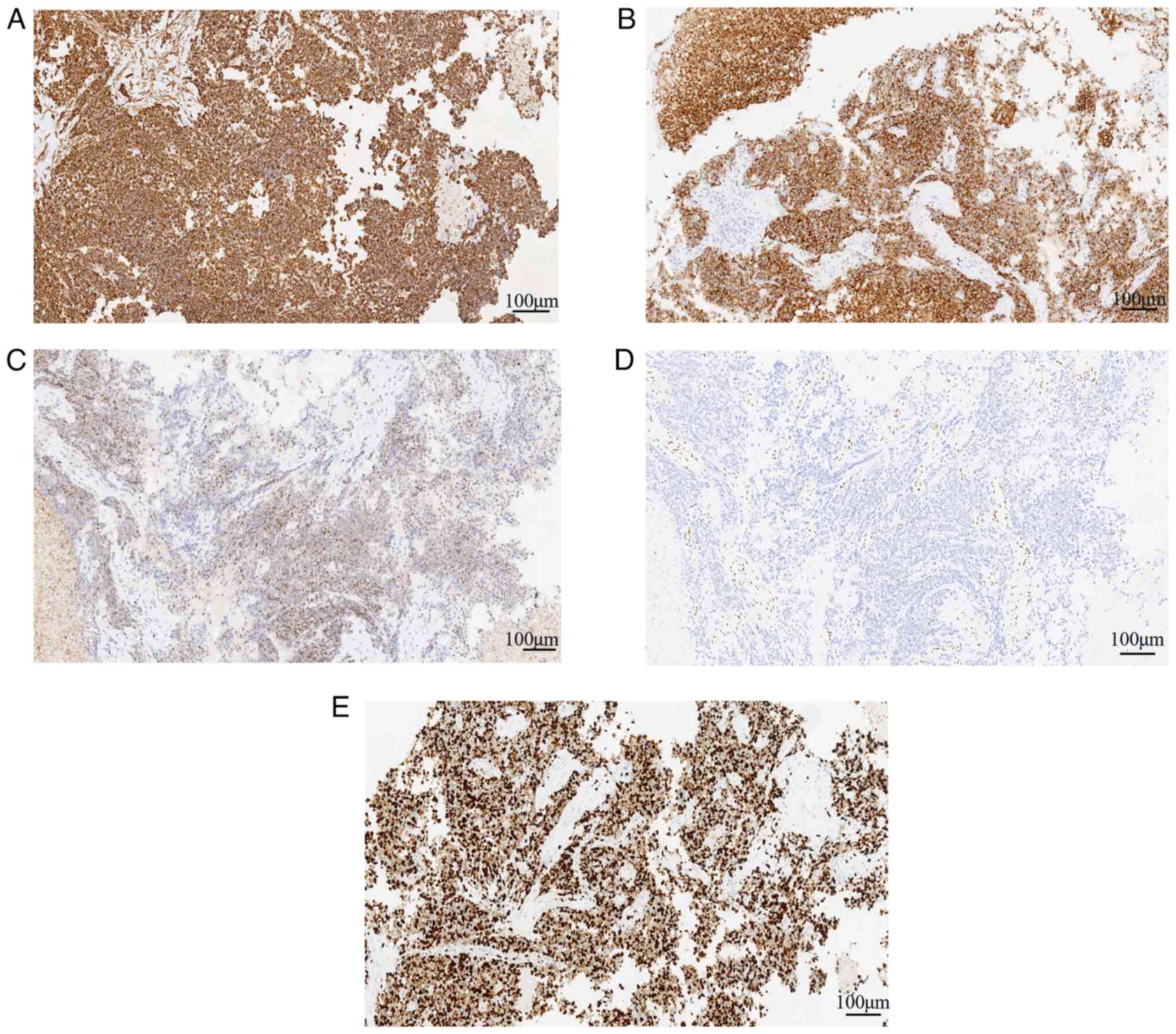
(Fig. 2B-F). Immunohistochemically,
the tumor cells showed positive expression of vimentin (Fig. 4A), INI-1 (Fig. 4C) and Syn (Fig. 4B), and scattered expression of CK,
TTF-1, SOX10 and CK7; the tumor was negative for CK8/18, NapsinA,
CK20, CDX-2, SATB-2, Villin, GATA3, P63, CEA, WT-1, PAX-8, CD34,
ERG, CD56, desmin, MyoD1, calretinin, EMA, CD30, S-100 and SMARCA4
(Fig. 4D). Mismatch repair proteins
(MLH1, MSH2, MSH6 and PMS2) were positive. P53 was negative. The
Ki-67 index of the tumor cells was ~85% (Fig. 4E). Notably, with the exception of
vimentin, INI-1, Syn, SMARCA4 and Ki-67, the images of
immunohistochemical staining are not shown.
The NGS results showed no SMARCA4 gene mutation, a
TP53 gene frameshift deletion and a Fanconi anemia protein A
(FANCA) gene missense mutation (exon 33 p.S1088F) (Table SI). Other gene mutations were
negative.
Based on the aforementioned clinical information,
morphological features and immunohistochemical results, the
pathological diagnosis was primary SMARCA4-deficient tumor in the
adnexal region.
Discussion
SMARCA4 is on chromosome 19q13.2 and is one of two
catalytic subunits of the SWI/SNF complex. Deletion or mutation of
the catalytic and core subunits of the SWI/SNF complex in somatic
cells or germline backgrounds can result in inactivation of coding
proteins and an abnormal overall function of the complex. The
SWI/SNF complex is a key component of chromatin remodeling, which
can bind to nucleosomes and use the energy of ATP to disrupt DNA
and histone interactions, move or eject histones, alter nucleosome
structure, and alter transcription and regulatory mechanisms,
leading to tumorigenesis (7,8). Peng
et al (1) reported that
SMARCA4 expression was significantly higher in various malignant
solid tumors than in normal tissues, whereas its expression level
in renal clear cell carcinoma and eosinophilic renal tumors was
revealed to be significantly lower than that in normal tissues.
SMARCA4-deficient tumors are common in non-small cell lung cancer,
colorectal adenocarcinoma, bladder urothelial carcinoma and
invasive breast ductal carcinoma, and are associated with a poor
prognosis (9).
The most commonly observed tumors with SMARCA4
mutations in the female reproductive system are small cell
carcinoma of the ovary hypercalcemic type (SCCOHT) (10), undifferentiated and dedifferentiated
endometrial carcinoma, and SMARCA4-deficient undifferentiated
tumors of the uterus (11).
SMARCA4-mutated tumors in the female reproductive system are often
clinically asymptomatic, present with abdominal pain and
distension, and are confirmed using imaging data and pathological
examination (12,13). Because of the aggressive nature of
SMARCA4-deficient tumors, they are often treated in the middle and
late stages, and their progression is rapid. Microscopically, these
tumors are distributed in sheets or nests, with epithelioid and
rhabdomyoid morphology. Mitotic figures are easily observed, with
large necrosis and loss of SMARCA4 expression. Due to differences
in tumor treatment and prognosis, adnexal SMARCA4-deficient tumors
must be differentiated from ovarian SCCOHT and high-grade serous
carcinoma. SCCOHT is most common in adolescents and young women,
occurring at a mean age of 24 years, and is associated with
elevated blood calcium levels. The tumor cells are generally small,
round and uniform in size, and are arranged in sheets, nests,
islands, beams or ropes, similar to neuroendocrine cells. Cases
showing large cells in the tumor are referred to as macrocellular
SCCOHT (14). The SCCOHT tumor
cells are often positive for expression of EMA, CK and
neuroendocrine markers (CD56, CgA and Syn). However, unilateral or
bilateral SCCOHT is more common in middle-aged and older women. The
high-grade serous carcinoma tumor is solid, papillary, glandular or
cribriform, and the tumor cells are pleomorphic with obvious atypia
and mitotic figures. Immunohistochemistry has shown that WT-1 is
positive and P53 is mutated in high-grade serous carcinoma.
Table I shows the differential
diagnosis of adnexal SMARCA4-deficient tumors from SCCOHT of the
ovary and high-grade serous carcinoma (15).
 | Table I.Differential diagnosis of
SMARCA4-deficient adnexal tumors from SCCOHT and high-grade serous
carcinoma. |
Table I.
Differential diagnosis of
SMARCA4-deficient adnexal tumors from SCCOHT and high-grade serous
carcinoma.
| Tumor Type | Common age | Blood calcium
level | Microscopic
appearance | Tumor expression |
|---|
|
|
|---|
| CK | Vimentin | CD56 | CgA | Syn | P53 | WT-1 | SMARCA4 | (Refs.) |
|---|
|
SMARCA4-deficient | No consensus | Normal | Lamellar and/or
nest-like tumors distribution, with epithelioid and rhabdomyoid
morphology, mitotic figures are visible, and necrosis is often
detected. | +/- | + | - | - | +/- | No consensus | - | - | (11,13) |
| SCCOHT | Under 40 years
old | High | Similar
neuroendocrine cells of uniform size arranged in sheets, nests,
islands, beams or cords. | + | - | + | + | + | No mutation | - | +/- | (12) |
| High-grade serous
carcinoma | Middle-aged and
elderly | Normal | Solid, papillary,
glandular or cribriform structure; cells are polymorphic and
heteromorphic, mitotic figures are obvious. | + | - | - | - | - | Mutated | + | + | (14,15) |
SMARCA4 mutations are mainly stop/nonsense,
frameshift, splicing and missense mutations, and in-frame
deletions, and no specific mutation site has been defined (16–19).
Uterine SMARCA4-deficient tumors show the highest frequency of
SMARCA4 mutations, whereas the SMARCA4-amplified type is the main
form of ovarian cancer, with a frequency of 9% (1). In the present case, the NGS results
showed that no SMARCA4 mutation was detected, whereas the FANCA
gene contained a missense mutation. SMARCA4 mutations lead to a
loss of SMARCA4, which can be detected using immunohistochemical
methods (1). Mutations in SMARCA4
are often accompanied by TP53 mutations. In the present case,
immunohistochemistry showed that SMARCA4 protein expression was
absent, and the NGS sequencing results indicated that SMARCA4 was
not mutated, possibly because of structural variations in the
intron region of the gene or mutations in other proteins of the
SWI/SNF family. Additionally, a frameshift deletion of TP53 was
detected. FANCA, a homologous recombination repair pathway gene,
encodes Fanconi anemia protein A and belongs to the Fanconi anemia
family. The most common changes in FANCA in cancer have been
reported to be FANCA mutations (2.31%), FANCA loss (0.24%), FANCA
nonsense mutations (0.14%), FANCA frameshift mutations (0.10%) and
FANCA amplifications (0.07%) (20).
FANCA changes occur in 2.82% of malignant solid tumors and in 2.22%
of patients with ovarian cancer. Notably, loss of FANCA function is
associated with hereditary breast and ovarian cancer (21,22).
Currently, there is no consensus on the best
treatment plan for SMARCA4-mutated tumors, and radical surgery,
chemotherapy and radiotherapy can be used as treatment methods.
Anti-programmed cell death-1/programmed cell death-ligand 1
immunotherapy has a curative effect on SMARCA4-deficient thoracic
tumors and SMARCA4-deficient undifferentiated tumors of the
gastrointestinal tract (23,24).
Additionally, immunosuppressive targets, such as monoclonal
antibodies against programmed death receptor 1 and programmed death
ligand 1, suitable for immunotherapy combined with chemotherapy
(platinum and/or paclitaxel) were revealed to be effective for
treating SMARCA4-deficient undifferentiated thoracic tumors
(21,25). Perioperative and palliative
radiotherapy may improve the prognosis of patients with
SMARCA4-deficient undifferentiated uterine sarcomas (24). In cases where standard therapy does
not work, novel therapies, such as enhancer of zeste homolog
inhibitors (21) and etoposide, as
well as targeted therapy with histone deacetylase inhibitors and
DNA methyltransferase inhibitors, may be considered (25). Another case report showed that
application of a poly ADP-ribose polymerase inhibitor was
beneficial for treating recurrent epithelial ovarian cancer with a
FANCA mutation (26). Most patients
with SMARCA4-deficient undifferentiated tumors are diagnosed in the
advanced stages, with rapid progression and poor prognosis, whereas
patients with lymph node metastasis of SMARCA4-deficient
undifferentiated tumors have a worse prognosis with a median
overall survival of 4–6 months (27).
Since the discovery of SMARCA4-deficient tumors in
recent years, no consensus has been reached on the diagnostic
criteria or treatment plan for this type of cancer. The present
case report provides relevant evidence for such tumors occurring in
the adnexal region of the uterus. Data on a larger number of cases
should be collected to improve the understanding of this disease
and improve prognosis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Natural Science Foundation of
Shandong Province (grant no. ZR2021MH261 to XFL).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article. The NGS data
generated in the present study may be found in the BioProject
database under accession number PRJNA1102919 or at the following
URL: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1102919.
Authors' contributions
XFL and MQY confirm the authenticity of all the raw
data. XFL was responsible for funding acquisition. XFL, YPZ, LLW,
ZJW and MQY designed the study. XFL, MQY and ZJW were responsible
for writing the original draft. XFL, MQY and ZJW were responsible
for editing the original draft. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The research protocol was reviewed and conducted
with the approval of the local institutional review board at the
Weifang People's Hospital (First Affiliated Hospital of Shandong
Second Medical University; Weifang, China; approval no.
KYLL20240105-2). The patient provided written consent to
participate in the study.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report and the
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SMARCA4
|
SWI/SNF-related matrix-associated
actin-dependent regulator of chromatin subfamily A member 4
|
|
NGS
|
next-generation sequencing
|
|
PAX-8
|
paired box gene 8
|
|
CK
|
cytokeratin
|
|
Syn
|
synaptophysin
|
|
TTF-1
|
thyroid transcription factor-1
|
|
EMA
|
epithelial membrane antigen
|
|
CEA
|
carcinoembryonic antigen
|
|
MyoD1
|
myogenic differentiation 1
|
|
WT-1
|
Wilms tumor gene
|
|
MLH1
|
mutL homolog 1
|
|
MSH2
|
mutS homolog 2
|
|
MSH6
|
mutS homolog 6
|
|
PMS2
|
postmeiotic segregation increased
2
|
|
SCCOHT
|
small cell carcinoma of the ovary
hypercalcemic type
|
References
|
1
|
Peng L, Li J, Wu J, Xu B, Wang Z, Giamas
G, Stebbing J and Yu Z: A Pan-Cancer Analysis of SMARCA4
alterations in human cancers. Front Immunol. 12:7625982021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kakkar A, Ashraf SF, Rathor A, Adhya AK,
Mani S, Sikka K and Jain D: SMARCA4/BRG1-Deficient Sinonasal
Carcinoma. Arch Pathol Lab Med. 146:1122–1130. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nambirajan A, Singh V, Bhardwaj N, Mittal
S, Kumar S and Jain D: SMARCA4/BRG1-Deficient non-small cell lung
carcinomas: A case series and review of the literature. Arch Pathol
Lab Med. 145:90–98. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ota T, Ishikawa T, Yasuda R, Yasuda T,
Okayama T, Inoue K, Dohi O, Yoshida N, Kamada K, Uchiyama K, et al:
The first case of SMARCA4-deficient sarcoma of stomach. Clin J
Gastroenterol. 15:531–536. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Duan H, Gao W, Wang L, Cao F and Teng L:
Undifferentiated colonic neoplasm with SMARCA4 germline gene
mutation and loss of SMARCA4 protein expression: A case report and
literature review. Diagn Pathol. 16:302021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang R, Chen L, Pan C and Fang X:
SMARCA4-deficient dedifferentiated endometrioid carcinoma: A case
report. Asian J Surg. 46:5484–5485. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Michel BC, D'Avino AR, Cassel SH,
Mashtalir N, McKenzie ZM, McBride MJ, Valencia AM, Zhou Q, Bocker
M, Soares LMM, et al: A non-canonical SWI/SNF complex is a
synthetic lethal target in cancers driven by BAF complex
perturbation. Nat Cell Biol. 20:1410–1420. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim SY, Shen Q, Son K, Kim HS, Yang HD, Na
MJ, Shin E, Yu S, Kang K, You JS, et al: SMARCA4 oncogenic
potential via IRAK1 enhancer to activate Gankyrin and AKR1B10 in
liver cancer. Oncogene. 40:4652–4662. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guerrero-Martínez JA and Reyes JC: High
expression of SMARCA4 or SMARCA2 is frequently associated with an
opposite prognosis in cancer. Sci Rep. 8:20432018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Witkowski L, Carrot-Zhang J, Albrecht S,
Fahiminiya S, Hamel N, Tomiak E, Grynspan D, Saloustros E, Nadaf J,
Rivera B, et al: Germline and somatic SMARCA4 mutations
characterize small cell carcinoma of the ovary, hypercalcemic type.
Nat Genet. 46:438–443. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kolin DL, Quick CM, Dong F, Fletcher CDM,
Stewart CJR, Soma A, Hornick JL, Nucci MR and Howitt BE:
SMARCA4-deficient uterine sarcoma and undifferentiated endometrial
carcinoma are distinct clinicopathologic entities. Am J Surg
Pathol. 44:263–270. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Karanian-Philippe M, Velasco V, Longy M,
Floquet A, Arnould L, Coindre JM, Le Naoures-Méar C, Averous G,
Guyon F, MacGrogan G and Croce S: SMARCA4 (BRG1) loss of expression
is a useful marker for the diagnosis of ovarian small cell
carcinoma of the hypercalcemic type (ovarian rhabdoid tumor): A
comprehensive analysis of 116 rare gynecologic tumors, 9 soft
tissue tumors, and 9 melanomas. Am J Surg Pathol. 39:1197–1205.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin DI, Allen JM, Hecht JL, Killian JK,
Ngo NT, Edgerly C, Severson EA, Ali SM, Erlich RL, Ramkissoon SH,
et al: SMARCA4 inactivation defines a subset of undifferentiated
uterine sarcomas with rhabdoid and small cell features and germline
mutation association. Mod Pathol. 32:1675–1687. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Azzalini E, Stanta G, Canzonieri V and
Bonin S: Overview of tumor heterogeneity in high-grade serous
ovarian cancers. Int J Mol Sci. 24:150772023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brambs CE, Höhn AK, Klagges S, Gläser A,
Taubenheim S, Dornhöfer N, Einenkel J, Hiller GGR and Horn LC:
Clinico-pathologic characteristics and prognostic factors of
ovarian carcinoma with different histologic subtypes-A benchmark
analysis of 482 cases. Pathol Res Pract. 233:1538592022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Connor YD, Miao D, Lin DI, Hayne C, Howitt
BE, Dalrymple JL, DeLeonardis KR, Hacker MR, Esselen KM and Shea M:
Germline mutations of SMARCA4 in small cell carcinoma of the ovary,
hypercalcemic type and in SMARCA4-deficient undifferentiated
uterine sarcoma: Clinical features of a single family and
comparison of large cohorts. Gynecol Oncol. 157:106–114. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu B and Shi H: An in-depth look at small
cell carcinoma of the ovary, hypercalcemic type (SCCOHT): Clinical
implications from recent molecular findings. J Cancer. 10:223–237.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao J, Fan R, Chen D, Hou J, Chen H and Lu
M: Pathological characteristics and immune microenvironment of
SMARCA4-deficient undifferentiated uterine sarcoma. Diagn Pathol.
18:622023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Witkowski L, Goudie C, Ramos P, Boshari T,
Brunet JS, Karnezis AN, Longy M, Knost JA, Saloustros E, McCluggage
WG, et al: The influence of clinical and genetic factors on patient
outcome in small cell carcinoma of the ovary, hypercalcemic type.
Gynecol Oncol. 141:454–460. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shinno Y, Yoshida A, Masuda K, Matsumoto
Y, Okuma Y, Yoshida T, Goto Y, Horinouchi H, Yamamoto N, Yatabe Y
and Ohe Y: Efficacy of immune checkpoint inhibitors in
SMARCA4-Deficient thoracic tumor. Clin Lung Cancer. 23:386–392.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bhat V, Koneru M, Knapp K, Joneja U,
Morrison J and Hong YK: Identification and treatment of SMARCA4
deficient poorly differentiated gastric carcinoma. Am Surg.
89:4987–4989. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang P, Xiong F, Lin Y, Liang P and Tang
C: Effectiveness of tislelizumab when combined with etoposide and
carboplatin in patients with SMARCA4-deficient undifferentiated
thoracic tumor: A case report. Transl Cancer Res. 12:1041–1048.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kunimasa K, Okami J, Takenaka S, Honma K,
Kukita Y, Nagata S, Kawamura T, Inoue T, Tamiya M, Kuhara H, et al:
Conversion surgery for advanced thoracic SMARCA4-Deficient
undifferentiated tumor with atezolizumab in combination with
bevacizumab, paclitaxel, and carboplatin treatment: A case report.
JTO Clin Res Rep. 2:1002352021.PubMed/NCBI
|
|
24
|
Kurokawa M, Shimizuguchi T, Ito K, Takao
M, Motoi T, Taguchi A, Yasugi T and Karasawa K: Notable Response of
SMARCA4-Deficient undifferentiated uterine sarcoma to palliative
radiation therapy. Adv Radiat Oncol. 6:1007282021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Romero OA, Vilarrubi A, Alburquerque-Bejar
JJ, Gomez A, Andrades A, Trastulli D, Pros E, Setien F, Verdura S,
Farré L, et al: SMARCA4 deficient tumours are vulnerable to
KDM6A/UTX and KDM6B/JMJD3 blockade. Nat Commun. 12:43192021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qian B, Leng W, Yan Z, Lu J, Chen S, Yi H
and Jiang Z: Clinical Benefit With PARP inhibitor for pathogenic
germline FANCA-Mutated relapsed epithelial ovarian cancer: A case
report. Front Oncol. 12:7785452022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liang X, Gao X, Wang F, Li S, Zhou Y, Guo
P, Meng Y and Lu T: Clinical characteristics and prognostic
analysis of SMARCA4-deficient non-small cell lung cancer. Cancer
Med. 12:14171–14182. 2023. View Article : Google Scholar : PubMed/NCBI
|