Introduction
Lung cancer is a prevalent malignant tumor, with
annual prevalence and age-standardized mortality rate of 87.65 and
30.2 per 100,000 individuals (1,2).
Non-small cell lung cancer (NSCLC) is the most common form of lung
cancer, of which ~70% of cases are non-squamous (3,4).
Notably, non-squamous NSCLC in clinical tumor-node-metastasis
(cTNM) IIIA stage is a highly heterogeneous disease, and ~30–50% of
patients are inoperable (5,6). At present, neoadjuvant chemotherapy is
considered to increase the likelihood of surgery in inoperable
patients or reduce the risk of disease recurrence, which
contributes to certain survival benefits to patients with
non-squamous NSCLC in cTNM IIIA stage (4,6,7).
Unfortunately, current optional neoadjuvant chemotherapy regimens
are relatively limited with unsatisfactory efficacy for patients
with non-squamous NSCLC in cTNM IIIA stage, especially in those
with negative driver genes (8).
Therefore, searching for alternative neoadjuvant chemotherapy
regimens is crucial to manage patients with non-squamous NSCLC in
cTNM IIIA stage with negative driver genes.
Bevacizumab, as an inhibitor of vascular endothelial
growth factor (VEGF), restrains growth of tumors by inhibiting
angiogenesis, which is considered to contribute to the treatment of
NSCLC (9,10). Notably, adjuvant bevacizumab plus
platinum-based chemotherapy has provided certain clinical benefits
in patients with NSCLC (11,12).
For example, one study reported that adjuvant bevacizumab plus
platinum-based chemotherapy reduced the recurrences of NSCLC in the
brain (11). In addition, another
study reported that adjuvant bevacizumab plus platinum-based
chemotherapy improved overall survival (OS) to a certain extent in
patients with NSCLC (12). However,
the relevant research on the application of neoadjuvant bevacizumab
plus platinum-based chemotherapy in patients with NSCLC is
insufficient. Several studies preliminarily assessed the efficacy
of neoadjuvant bevacizumab in patients with NSCLC, which reported
that bevacizumab-based regimen as neoadjuvant is feasible and safe
in patients with stage III lung cancer (13,14).
Furthermore, another study performed with a Chinese cohort reported
that bevacizumab combined with platinum-containing neoadjuvant
therapy had acceptable efficacy and safety profiles in patients
with stage III lung cancer (15).
However, more studies are needed to form solid conclusions on
supporting the clinical usage of bevacizumab-based neoadjuvant
therapy in patients with stage III lung cancer.
Therefore, the present study aimed to compare the
efficacy and safety between neoadjuvant bevacizumab plus
platinum-based chemotherapy and platinum-based chemotherapy alone
in patients with non-squamous NSCLC in cTNM IIIA stage with
negative driver genes.
Materials and methods
Subjects
The present retrospective study included 110
patients with non-squamous NSCLC who underwent neoadjuvant therapy
(bevacizumab plus platinum-based chemotherapy or platinum-based
chemotherapy alone) and sequential surgical resection from January
2019 to October 2022 at Dazhou Central Hospital (Dazhou, China).
The inclusion criteria were as follows: i) Non-squamous NSCLC
diagnosis as per the guideline from National Comprehensive Cancer
Network (16); ii) presence of
negative driver genes involving epidermal growth factor receptor
(EGFR), anaplastic lymphoma kinase (ALK), c-ros oncogene 1 (ROS1)
fusion and v-raf murine sarcoma viral oncogene homolog B1 with
amino acid substitution for valine at position 600 (BRAF V600E)
mutation; iii) cTNM IIIA stage; iv) bevacizumab plus platinum-based
chemotherapy or platinum-based chemotherapy alone as neoadjuvant
therapy received; and v) surgical resection after neoadjuvant
therapy performed. The exclusion criteria were as follows: i) other
malignant diseases, such as other solid tumors or hematological
malignancies; ii) no available follow-up data; and iii) current
pregnancy or lactation. The Ethics Committee of Dazhou Central
Hospital (Dazhou, China) approved the present study (approval no.
2023100). Each subject or their guardian provided written informed
consent.
Study flow
Initially, 246 patients with stage IIIA NSCLC who
underwent surgical resection were screened. A total of 72 patients
who had positive driver genes, 34 patients who did not receive
neoadjuvant therapy, 14 patients with incomplete follow-up data, 7
patients (or their family) who could not be contacted, and 9
patients (or their family) who did not agree to participate in the
study or did not provide informed consent were excluded.
Subsequently, a total of 110 patients were considered eligible for
analysis.
Treatment
Patients received bevacizumab plus platinum-based
chemotherapy or platinum-based chemotherapy alone as neoadjuvant
therapy. In the present study, the administrated regimen of
platinum-based chemotherapy included: Paclitaxel-platinum (TP),
liposome-encapsulated paclitaxel-platinum (LP) and
pemetrexed-platinum (AP). The cycle of neoadjuvant therapy was 2–3
cycles (21-day cycle). The suggested doses were as follows: i) 7.5
mg/kg bevacizumab on the first day per cycle; ii) TP, 135–175
mg/m2 paclitaxel + 75 mg/m2 cisplatin or
carboplatin dosed to an area under the curve (AUC) of 5.0–6.0 on
the first day of each cycle; iii) LP, 135–175 mg/m2
liposome-encapsulated paclitaxel + 75 mg/m2 cisplatin or
carboplatin dosed to an AUC of 5.0–6.0 on the first day of each
cycle; iv) AP, 500 mg/m2 pemetrexed + 75
mg/m2 cisplatin or carboplatin dosed to an AUC of
5.0–6.0 on the first day of each cycle. Surgery was performed
within 3–4 weeks after neoadjuvant therapy.
Data collection
Demographics, disease-related data and neoadjuvant
therapy-linked data were collected. Simultaneously, imaging
examination results of patients were collected every 2 months for
the first 6 months and every 3 months thereafter. The best overall
response was taken to be the best radiological response recorded
during the duration of the whole treatment, which was appraised via
the Response Evaluation Criteria In Solid Tumors guidelines version
1.1 (17). Subsequently, the
overall response rate (ORR) was calculated. Additionally,
pathological response was assessed, including major pathologic
response (MPR) and pathological complete response (pCR) status. MPR
was defined as ≤10% of residual viable tumor present in the
resection specimen, and pCR was defined as the lack of any viable
tumor cells in the resected lung cancer specimen (including all
sampled regional lymph nodes) (18,19).
Moreover, follow-up data (including disease status and
corresponding time periods) was collected, then disease-free
survival (DFS) and OS were assessed in line with it. The definition
of DFS was the time from surgery to relapse or death, whilst OS was
defined as the period from neoadjuvant therapy initiation to death.
Furthermore, adverse events were counted for safety analysis, which
was graded by the Common Terminology Criteria for Adverse Events
version 5.0 (https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm).
Statistical analysis
Unpaired Student's t-test, χ2 test or
Mann-Whitney U test were used to perform comparison analysis
according to the appropriate conditions. Kaplan-Meier curves were
used to assess the DFS or OS, and the log-rank test was used to
compared DFS or OS between two groups. In addition, multivariate
logistic regression or Cox regression models were used to evaluate
independent factors related to pCR or DFS/OS, in which the enter
method (where all the factors were forced into the regression
model) was used. SPSS v.26.0 (IBM Corp.) was used for data
processing and GraphPad Prism v.7.0 (Dotmatics) was used for figure
construction. P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinical features
The bevacizumab plus chemotherapy group included 14
(28.0%) female and 36 (72.0%) male patients, with a mean age of
55.8±7.9 years. The chemotherapy alone group included 21 (35.0%)
female and 39 (65.0%) male patients, with a mean age of 58.9±11.0
years. Notably, there were no significant differences in baseline
features between groups, such as age, clinical tumor staging and
sex (all P>0.05). Characteristics of the two groups are
presented in Table I. The negative
driver genes defined in the present study include EGFR, ALK, ROS1
fusion and BRAF V600E mutation. All genetic mutations of the
patients are listed in Table
SI.
 | Table I.Characteristics of patients with
non-squamous non-small cell lung cancer. |
Table I.
Characteristics of patients with
non-squamous non-small cell lung cancer.
| Characteristic | Chemotherapy alone
(n=60) | Bevacizumab plus
chemotherapy (n=50) | P-value |
|---|
| Age, years | 58.9±11.0 | 55.8±7.9 | 0.101 |
| Sex |
|
| 0.433 |
|
Female | 21 (35.0) | 14 (28.0) |
|
| Male | 39 (65.0) | 36 (72.0) |
|
| Smoking history |
|
| 0.230 |
| No | 38 (63.3) | 26 (52.0) |
|
| Yes | 22 (36.7) | 24 (48.0) |
|
| Histological
type |
|
| 0.372 |
|
Adenocarcinoma | 52 (86.7) | 46 (92.0) |
|
|
Others | 8 (13.3) | 4 (8.0) |
|
| cT stage |
|
| 0.278 |
| cT1 | 0 (0.0) | 3 (6.0) |
|
| cT2 | 26 (43.3) | 26 (52.0) |
|
| cT3 | 30 (50.0) | 13 (26.0) |
|
|
cT4 | 4 (6.7) | 8 (16.0) |
|
| cN stage |
|
| 0.142 |
|
cN0 | 1 (1.7) | 1 (2.0) |
|
|
cN1 | 33 (55.0) | 20 (40.0) |
|
|
cN2 | 26 (43.3) | 29 (58.0) |
|
| cTNM stage
IIIA |
|
| 0.284 |
|
cT1N2M0 | 0 (0.0) | 3 (6.0) |
|
|
cT2N2M0 | 26 (43.3) | 26 (52.0) |
|
|
cT3N1M0 | 30 (50.0) | 13 (26.0) |
|
|
cT4N0M0 | 1 (1.7) | 1 (2.0) |
|
|
cT4N1M0 | 3 (5.0) | 7 (14.0) |
|
| ECOG PS score |
|
| 0.253 |
| 0 | 37 (61.7) | 36 (72.0) |
|
| 1 | 23 (38.3) | 14 (28.0) |
|
| Chemotherapy
regimen |
|
| 0.174 |
| TP | 36 (60.0) | 22 (44.0) |
|
| LP | 18 (30.0) | 18 (36.0) |
|
| AP | 6 (10.0) | 10 (20.0) |
|
Radiological and pathological
responses between groups
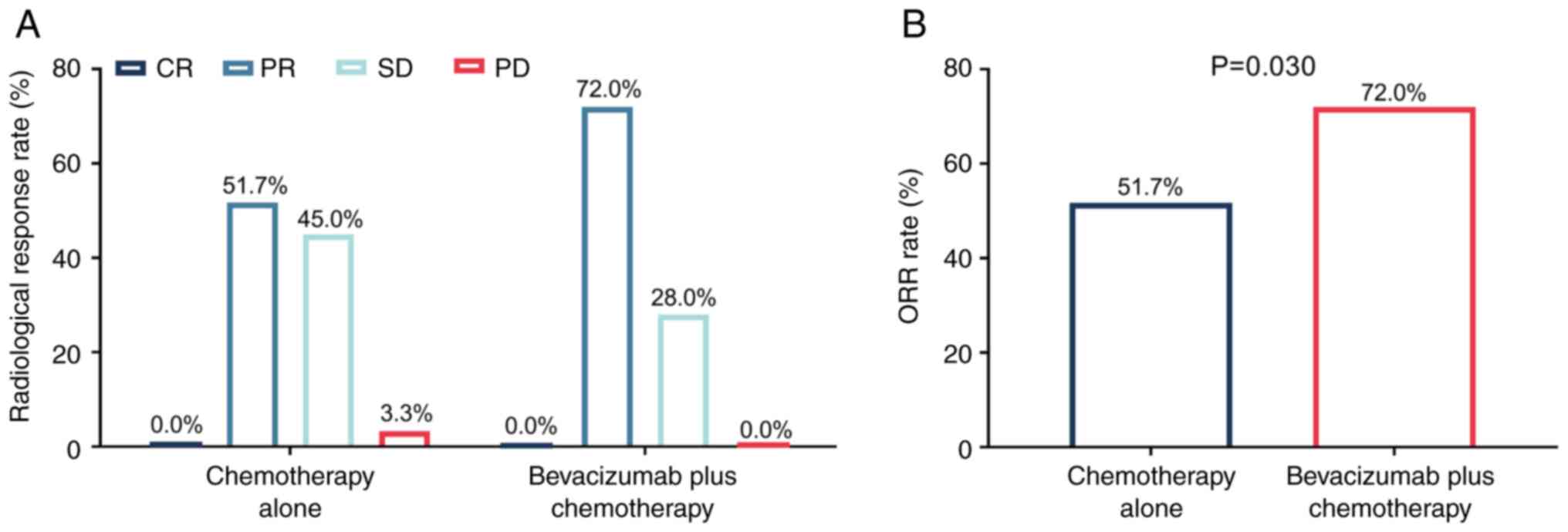
Complete response, partial response, stable disease
and progressive disease rates in the bevacizumab plus chemotherapy
group were demonstrated to be 0.0, 72.0, 28.0 and 0.0%,
respectively. Meanwhile, these rates in the chemotherapy alone
group were 0.0, 51.7, 45.0 and 3.3%, respectively (Fig. 1A). Notably, ORR significantly
increased in the bevacizumab plus chemotherapy group compared with
the chemotherapy alone group (72.0 vs. 51.7%; P=0.030; Fig. 1B).
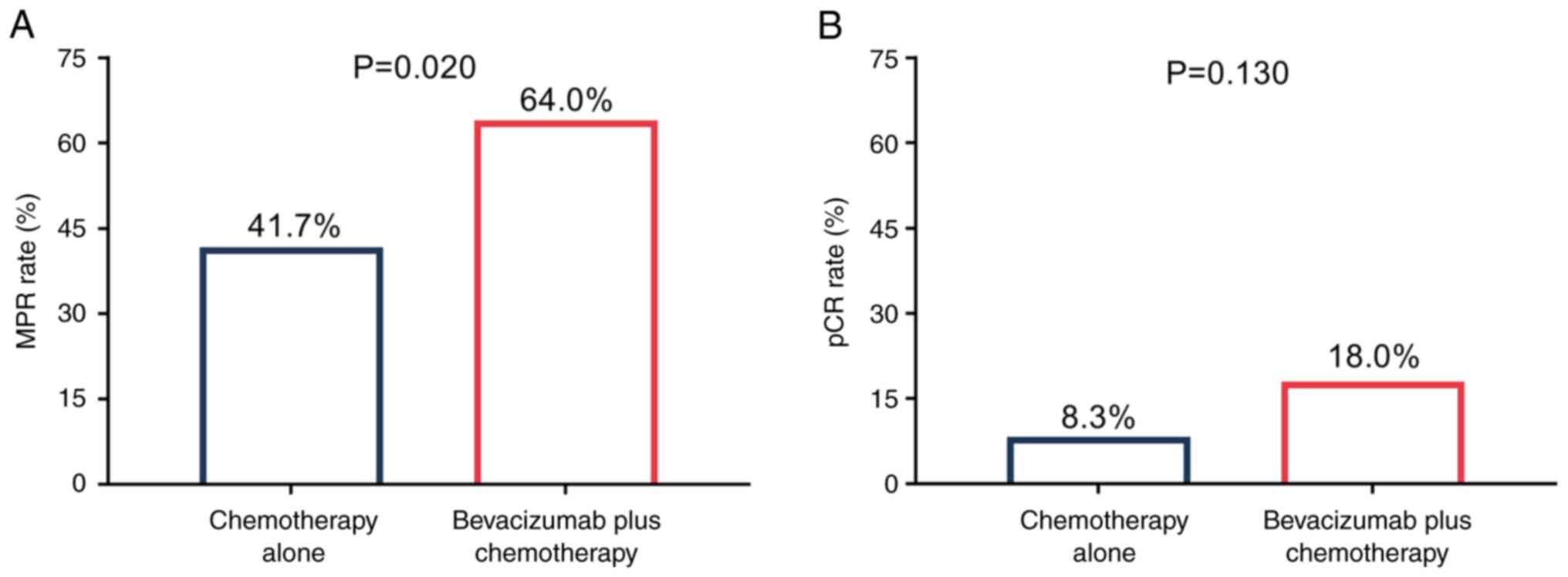
Notably, the MPR rate was significantly increased in
the bevacizumab plus chemotherapy group compared with the
chemotherapy alone group (64.0 vs. 41.7%; P=0.020; Fig. 2A). However, the pCR rate was not
significantly different between the bevacizumab plus chemotherapy
group and the chemotherapy alone group (18.0 vs. 8.3%; P=0.130;
Fig. 2B).
Factors associated with pCR
The multivariate logistic regression model revealed
that bevacizumab plus chemotherapy (vs. chemotherapy alone) was not
significantly independently associated with pCR in patients with
non-squamous NSCLC [odds ratio (OR)=2.897; P=0.117]. The Eastern
Cooperative Oncology Group Performance Status of 1 (vs. 0) was
notably independently associated with a lower pCR rate in patients
with non-squamous NSCLC, however this was not statistically
significant (OR=0.112; P=0.053; Table
II).
 | Table II.Multivariate logistic regression
model of pathological complete response in patients with
non-squamous non-small cell lung cancer. |
Table II.
Multivariate logistic regression
model of pathological complete response in patients with
non-squamous non-small cell lung cancer.
| Factor | OR | 95% CI | P-value |
|---|
| Bevacizumab plus
chemotherapy vs. chemotherapy alone | 2.897 | (0.765–10.966) | 0.117 |
| Age, ≥60 vs. <60
years | 0.608 | (0.138–2.672) | 0.510 |
| Sex, male vs.
female | 1.751 | (0.377–8.133) | 0.475 |
| Smoking history,
yes vs. no | 0.694 | (0.181–2.661) | 0.594 |
| Histological type,
adenocarcinoma vs. others | 0.443 | (0.061–3.195) | 0.419 |
| Higher cT
stage | 0.373 | (0.055–2.547) | 0.314 |
| Higher cN
stage | 0.188 | (0.014–2.591) | 0.212 |
| ECOG PS, 1 vs.
0 | 0.112 | (0.012–1.027) | 0.053 |
| Chemotherapy
regimen |
|
|
|
| TP
(reference) | 1.000 |
|
|
| LP vs.
TP | 1.445 | (0.400–5.222) | 0.575 |
| AP vs.
TP | <0.001 | (0.000-NR) | 0.998 |
Accumulating DFS and OS rates between
groups
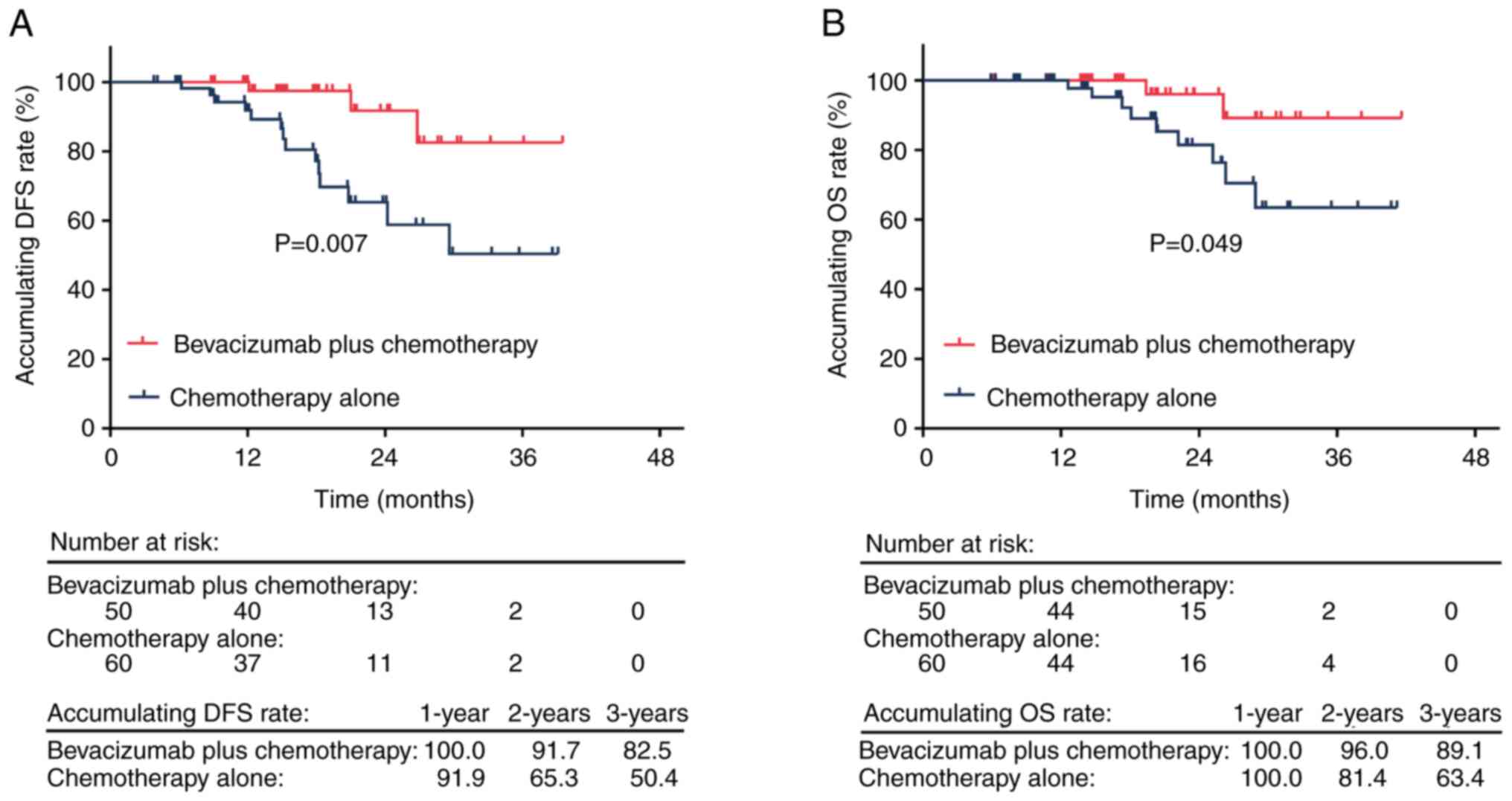
The accumulating DFS rate was significantly higher
in the bevacizumab plus chemotherapy group in comparison with the
chemotherapy alone group (P=0.007). The 1-, 2- and 3-year
accumulating DFS rates were 100.0, 91.7 and 82.5% in the
bevacizumab plus chemotherapy group, respectively, and 91.9, 65.3
and 50.4% in the chemotherapy alone group, respectively (Fig. 3A). Furthermore, the accumulating OS
rate was significantly higher in the bevacizumab plus chemotherapy
group in comparison with the chemotherapy alone group (P=0.049).
The 1-, 2- and 3-year accumulating OS rates were 100.0, 96.0 and
89.1% in the bevacizumab plus chemotherapy group, respectively, and
100.0, 81.4 and 63.4% in the chemotherapy alone group, respectively
(Fig. 3B).
Factors linked with DFS and OS
Bevacizumab plus chemotherapy (vs. chemotherapy
alone) was significantly independently associated with a longer DFS
in patients with non-squamous NSCLC [hazard ratio (HR)=0.251;
P=0.042; Table III]. However,
bevacizumab plus chemotherapy (vs. chemotherapy alone) was not
significantly independently associated with OS in patients with
non-squamous NSCLC (HR=0.297; P=0.158; Table IV). Furthermore, all other factors
were not demonstrated to be significantly independently associated
with DFS or OS in patients with non-squamous NSCLC (all P>0.05;
Tables III and IV).
 | Table III.Multivariate Cox regression model of
disease-free survival in patients with non-squamous non-small cell
lung cancer. |
Table III.
Multivariate Cox regression model of
disease-free survival in patients with non-squamous non-small cell
lung cancer.
| Factor | HR | 95% CI | P-value |
|---|
| Bevacizumab plus
chemotherapy vs. chemotherapy alone | 0.251 | (0.066–0.952) | 0.042a |
| Age, ≥60 vs. <60
years | 1.834 | (0.558–6.029) | 0.318 |
| Sex, male vs.
female | 0.528 | (0.139–2.006) | 0.348 |
| Smoking history,
yes vs. no | 1.149 | (0.284–4.640) | 0.846 |
| Histological type,
adenocarcinoma vs. others | 0.948 | (0.230–3.898) | 0.941 |
| Higher cT
stage | 1.858 | (0.080–42.880) | 0.699 |
| Higher cN
stage | 5.378 |
(0.170–170.378) | 0.340 |
| ECOG PS, 1 vs.
0 | 1.454 | (0.425–4.966) | 0.551 |
| Chemotherapy
regimen |
|
|
|
| TP
(reference) | 1.000 |
|
|
| LP vs.
TP | 0.406 | (0.091–1.822) | 0.239 |
| AP vs.
TP | 1.975 | (0.464–8.411) | 0.357 |
 | Table IV.Multivariate Cox regression model of
overall survival in patients with non-squamous non-small cell lung
cancer. |
Table IV.
Multivariate Cox regression model of
overall survival in patients with non-squamous non-small cell lung
cancer.
| Factor | HR | 95% CI | P-value |
|---|
| Bevacizumab plus
chemotherapy vs. chemotherapy alone | 0.297 | (0.055–1.603) | 0.158 |
| Age, ≥60 vs. <60
years | 1.263 | (0.290–5.505) | 0.756 |
| Sex, male vs.
female | 0.363 | (0.060–2.209) | 0.271 |
| Smoking history,
yes vs. no | 1.961 | (0.309–12.453) | 0.475 |
| Histological type,
adenocarcinoma vs. others | 1.335 | (0.232–7.668) | 0.746 |
| Higher cT
stage | 2.393 |
(0.037–155.710) | 0.682 |
| Higher cN
stage | 3.352 |
(0.035–323.152) | 0.604 |
| ECOG PS, 1 vs.
0 | 0.997 | (0.227–4.388) | 0.997 |
| Chemotherapy
regimen |
|
|
|
| TP (reference) | 1.000 | - | - |
| LP vs. TP | 0.159 | (0.016–1.564) | 0.115 |
| AP vs. TP | 2.008 | (0.322–12.506) | 0.455 |
Adverse events between groups
No significant differences were demonstrated for the
number of adverse events between both groups, such as for fatigue,
anemia and hand-foot syndrome (all P>0.05). In the bevacizumab
plus chemotherapy group, fatigue (46.0%), alopecia (40.0%), anemia
(36.0%), hand-foot syndrome (36.0%), neutropenia (36.0%), nausea
and vomiting (36.0%) and hypertension (36.0%) were the most
commonly reported adverse events. Moreover, in the chemotherapy
alone group, alopecia (33.3%), anemia (33.3%), fatigue (30.0%),
hand-foot syndrome (26.7%), neutropenia (25.0%), thrombopenia
(25.0%) and nausea and vomiting (23.3%) were the most common. The
incidence of hypertension was notably increased in the bevacizumab
plus chemotherapy group compared with the chemotherapy alone group
(36.0 vs. 20.0%), however the difference was not statistically
significant (P=0.061). Furthermore, the adverse events with grade
1–2 were the most commonly reported, compared with those that were
grade 3–4. Moreover, the incidence of delayed incision healing was
markedly increased in the bevacizumab plus chemotherapy group
compared with the chemotherapy alone group, however there was no
statistically significant difference (20.0 vs. 8.3%; P=0.076;
Table V).
 | Table V.Adverse events. |
Table V.
Adverse events.
|
| Chemotherapy alone
(n=60) | Bevacizumab plus
chemotherapy (n=50) |
|
|---|
|
|
|
|
|
|---|
| Event | Total | Grade 1–2 | Grade 3–4 | Total | Grade 1–2 | Grade 3–4 | P-value |
|---|
| Fatigue | 18 (30.0) | 18 (30.0) | 0 (0.0) | 23 (46.0) | 21 (42.0) | 2 (4.0) | 0.084 |
| Alopecia | 20 (33.3) | 20 (33.3) | 0 (0.0) | 20 (40.0) | 20 (40.0) | 0 (0.0) | 0.469 |
| Anemia | 20 (33.3) | 18 (30.0) | 2 (3.3) | 18 (36.0) | 17 (34.0) | 1 (2.0) | 0.770 |
| Hand-foot
syndrome | 16 (26.7) | 16 (26.7) | 0 (0.0) | 18 (36.0) | 18 (36.0) | 0 (0.0) | 0.292 |
| Neutropenia | 15 (25.0) | 13 (21.7) | 2 (3.3) | 18 (36.0) | 13 (26.0) | 5 (10.0) | 0.210 |
| Nausea and
vomiting | 14 (23.3) | 13 (21.7) | 1 (1.7) | 18 (36.0) | 16 (32.0) | 2 (4.0) | 0.145 |
| Hypertension | 12 (20.0) | 12 (20.0) | 0 (0.0) | 18 (36.0) | 16 (32.0) | 2 (4.0) | 0.061 |
| Leukopenia | 13 (21.7) | 12 (20.0) | 1 (1.7) | 17 (34.0) | 16 (32.0) | 1 (2.0) | 0.148 |
| Rash | 13 (21.7) | 13 (21.7) | 0 (0.0) | 15 (30.0) | 15 (30.0) | 0 (0.0) | 0.318 |
| Constipation | 11 (18.3) | 11 (18.3) | 0 (0.0) | 13 (26.0) | 13 (26.0) | 0 (0.0) | 0.332 |
| Elevated
transaminase | 11 (18.3) | 10 (16.7) | 1 (1.7) | 13 (26.0) | 12 (24.0) | 1 (2.0) | 0.332 |
| Anorexia | 8 (13.3) | 8 (13.3) | 0 (0.0) | 12 (24.0) | 12 (24.0) | 0 (0.0) | 0.149 |
| Thrombopenia | 15 (25.0) | 15 (25.0) | 0 (0.0) | 10 (20.0) | 10 (20.0) | 0 (0.0) | 0.533 |
| Diarrhea | 8 (13.3) | 8 (13.3) | 0 (0.0) | 8 (16.0) | 8 (16.0) | 0 (0.0) | 0.693 |
| Elevated
bilirubin | 5 (8.3) | 5 (8.3) | 0 (0.0) | 6 (12.0) | 6 (12.0) | 0 (0.0) | 0.523 |
| Delayed incision
healing | 5 (8.3) | NA | NA | 10 (20.0) | NA | NA | 0.076 |
Discussion
VEGF is an important factor for promoting
angiogenesis, which participates in the progress of several
cancers, such as renal carcinomas, ovarian cancer, breast cancer
and NSCLC (20). Notably,
bevacizumab blocks the VEGF signaling pathway, which is considered
a favorable drug to suppress the growth and metastasis of NSCLC
(21). At present, adjuvant
bevacizumab plus platinum-based chemotherapy brings certain
clinical benefits to patients with non-squamous NSCLC (11,12).
For example, one study found that adjuvant bevacizumab plus
platinum-based chemotherapy reduced the risk of brain metastases in
patients with non-squamous NSCLC (11). Another study showed that adjuvant
bevacizumab plus platinum-based chemotherapy increased OS to some
extent in patients with non-squamous NSCLC (12). However, the efficacy of neoadjuvant
bevacizumab plus platinum-based chemotherapy in patients with
non-squamous NSCLC in cTNM IIIA stage is unclear. The present study
demonstrated that neoadjuvant bevacizumab plus platinum-based
chemotherapy compared with chemotherapy alone did not significantly
improve the pCR in these patients. This finding agrees with that of
a previous study, which reported that although neoadjuvant
bevacizumab plus chemotherapy was feasible, it did not improve the
pCR rate in patients with stage III NSCLC (13).
The survival rate of current neoadjuvant
chemotherapy is not ideal in patients with NSCLC (22,23).
For example, previous research revealed rates of 21.2 and 50.0% for
3-year DFS and OS, respectively, for patients with NSCLC in cTNM
IIIA stage who were treated with neoadjuvant chemotherapy (23). Another study reported that the
3-year OS rate ranged from 58–64% in patients with NSCLC who were
treated with neoadjuvant chemotherapy (22). Notably, the present study
demonstrated that the 3-year DFS was 82.5%, which was higher in
patients with non-squamous NSCLC in cTNM IIIA stage who received
neoadjuvant bevacizumab plus platinum-based chemotherapy in
comparison with those who received chemotherapy alone. The possible
explanations are as follows: Bevacizumab suppressed angiogenesis by
targeting the VEGF signaling pathway, thus suppressing the
progression of non-squamous NSCLC (24). However, it was demonstrated that
bevacizumab plus chemotherapy did not prolong OS in patients with
non-squamous NSCLC in cTNM IIIA stage after adjustment by the
multivariate Cox's regression analysis. This finding indicates that
the efficacy of bevacizumab plus chemotherapy in patients with
non-squamous NSCLC in cTNM IIIA stage is limited, and further
validation is needed.
Notably, the application of bevacizumab in the
treatment of patients with NSCLC may cause certain adverse events
(25,26). A previous study reported that
bevacizumab increased the incidences of hypertension, hemorrhagic
events, leukopenia, proteinuria, febrile neutropenia and
neutropenia in patients with advanced NSCLC (26). In the present study, the incidence
of delayed incision healing was markedly increased in patients with
non-squamous NSCLC in cTNM IIIA stage who received neoadjuvant
bevacizumab plus platinum-based chemotherapy compared with those
who received chemotherapy alone. This may be because bevacizumab
inhibited VEGF, which prevented wound healing. Nevertheless, there
was no statistical difference demonstrated. Moreover, there was no
significant difference in the number of adverse events between the
two groups. This may be due to the fact that the sample size was
inadequate and the dose of bevacizumab was relatively low in the
present study (27). Furthermore,
in the neoadjuvant bevacizumab plus platinum-based chemotherapy
group, grade 1–2 adverse events were the most commonly reported.
These findings indicate that the safety of neoadjuvant bevacizumab
plus platinum-based chemotherapy in patients with non-squamous
NSCLC in cTNM IIIA stage is reliable.
In recent years, the treatment strategies of NSCLC
have been continuously explored. Although neoadjuvant immunotherapy
has been successful in treating patients with NSCLC to a certain
extent, more neoadjuvant treatment options are required for
patients with non-squamous NSCLC in cTNM IIIA stage (28). Neoadjuvant chemotherapy is still one
of the predominant neoadjuvant options for the treatment of
patients with NSCLC (4). Current
optional neoadjuvant chemotherapy regimens report unsatisfactory
efficacy in patients with non-squamous NSCLC in cTNM IIIA stage
with negative driver genes (8).
Notably, previous studies have reported that bevacizumab (an
inhibitor of VEGF that inhibits the growth of tumors by inhibiting
angiogenesis) plus platinum-based chemotherapy as a neoadjuvant
chemotherapy regimen exhibits survival benefits to a certain extent
in patients with non-squamous NSCLC (13–15).
However, more studies are required to make a solid conclusion on
supporting the clinical usage of bevacizumab-based neoadjuvant
therapy in patients with stage III lung cancer.
The present study used a dose of 7.5 mg bevacizumab
plus platinum-based chemotherapy as a neoadjuvant treatment
regimen, and revealed that 7.5 mg bevacizumab was effective for the
treatment of patients with non-squamous NSCLC in cTNM IIIA stage.
This provides a potential optional treatment strategy with a
low-dose of bevacizumab. Moreover, the tumor driver gene detection
in the present study was based on a panel of the following genes:
EGFR, Kirsten rat sarcoma viral oncogene homolog, Harvey rat
sarcoma virus oncogene, neuroblastoma RAS viral oncogene homolog,
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit
alpha, ALK, ROS1, BRAF, human epidermal growth factor receptor 2,
rearranged during transfection, tumor protein p53, mesenchymal to
epithelial transition factor, mitogen-activated protein kinase
kinase 1, fibroblast growth factor receptor 1 (FGFR1), FGFR2, AKT
serine/threonine kinase 1, phosphatase and tensin homolog,
smoothened, frizzled class receptor, KIT proto-oncogene, receptor
tyrosine kinase, platelet derived growth factor receptor alpha,
discoidin domain receptor 2, Retinoblastoma transcriptional
corepressor 1, tuberous sclerosis complex 1, mitogen-activated
extracellular signal-regulated kinase 1, breast cancer
susceptibility gene, Tet methylcytosine dioxygenase 2, DNA
methyltransferase 3 alpha and G protein subunit alpha 11. In the
screening process of the present study, EGFR, ALK, ROS1 fusion and
BRAF V600E mutation in patients with non-squamous NSCLC in cTNM
IIIA stage were regarded as positive driver genes, and no mutation
in any of the above four genes was demonstrated to be negative. The
present study did not define the mutations of other driver genes as
positive as patients with lung cancer with mutations of other
driver genes lacked specific treatment drugs in Dazhou Central
Hospital.
Certain limitations exist for the present study: i)
Although the sample size in the present study was larger than in
previous studies (13,14), future studies with an even larger
sample size are required to verify the efficacy and safety of
neoadjuvant bevacizumab plus platinum-based chemotherapy in
patients with non-squamous NSCLC in cTNM IIIA stage; and ii) the
present study is retrospective, and therefore it may have
confounding factors (such as body mass index and diseases history)
causing a certain degree bias.
In conclusion, neoadjuvant bevacizumab plus
platinum-based chemotherapy is associated with improved DFS but has
limited efficacy in improving pCR and OS rates in comparison with
neoadjuvant chemotherapy alone in patients with stage-IIIA
non-squamous NSCLC. Therefore, a larger sample size and randomized
controlled studies are is required for further validation of the
findings of the present study.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Project of Dazhou Science
and Technology Bureau (grant no. 22ZDYF0020).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HW conceived and designed the study. DJ, YR, CZ, DW
and XJ performed data acquisition and data analysis. DJ and HW
confirm the authenticity of all the raw data. All authors wrote and
revised the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Dazhou Central Hospital
approved the present study [approval no. 2023100]. Each subject or
their guardian provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhu D, Shi X, Nicholas S, Ma Y and He P:
Estimated annual prevalence, medical service utilization and direct
costs of lung cancer in urban China. Cancer Med. 10:2914–2923.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li C, Lei S, Ding L, Xu Y, Wu X, Wang H,
Zhang Z, Gao T, Zhang Y and Li L: Global burden and trends of lung
cancer incidence and mortality. Chin Med J (Engl). 136:1583–1590.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, D'Amico
TA, et al: Non-small cell lung cancer, version 3.2022, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
20:497–530. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen P, Liu Y, Wen Y and Zhou C: Non-small
cell lung cancer in China. Cancer Commun (Lond). 42:937–970. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Daly ME, Singh N, Ismaila N, Antonoff MB,
Arenberg DA, Bradley J, David E, Detterbeck F, Fruh M, Gubens MA,
et al: Management of stage III non-small-cell lung cancer: ASCO
guideline. J Clin Oncol. 40:1356–1384. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoon SM, Shaikh T and Hallman M:
Therapeutic management options for stage III non-small cell lung
cancer. World J Clin Oncol. 8:1–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nagasaka M and Gadgeel SM: Role of
chemotherapy and targeted therapy in early-stage non-small cell
lung cancer. Expert Rev Anticancer Ther. 18:63–70. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bai R, Li L, Chen X, Chen N, Song W and
Cui J: Neoadjuvant and adjuvant immunotherapy: Opening new horizons
for patients with early-stage non-small cell lung cancer. Front
Oncol. 10:5754722020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhai J, Lu J, Zhang Z, Wang Y, Li X, Zhang
S, Mu S, Zhi X, Ge X, Lu D, et al: First-line PD-1/PD-L1 inhibitors
plus chemotherapy versus bevacizumab plus chemotherapy for advanced
non-squamous non-small cell lung cancer: A Bayesian network
meta-analysis of randomized controlled trials. Cancer Med.
11:2043–2055. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Garcia J, Hurwitz HI, Sandler AB, Miles D,
Coleman RL, Deurloo R and Chinot OL: Bevacizumab
(Avastin®) in cancer treatment: A review of 15 years of
clinical experience and future outlook. Cancer Treat Rev.
86:1020172020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Varlotto JM, Wang Y, Sun Z, Wakelee HA,
Ramalingam S and Schiller J: Bevacizumab's association with a
decreased risk of brain metastases in ECOG-ACRIN E1505, a phase 3
randomized trial of adjuvant chemotherapy with or without
bevacizumab in surgically resected NSCLC. JTO Clin Res Rep.
3:1002742022.PubMed/NCBI
|
|
12
|
Wakelee HA, Dahlberg SE, Keller SM, Tester
WJ, Gandara DR, Graziano SL, Adjei AA, Leighl NB, Aisner SC,
Rothman JM, et al: Adjuvant chemotherapy with or without
bevacizumab in patients with resected non-small-cell lung cancer
(E1505): An open-label, multicentre, randomised, phase 3 trial.
Lancet Oncol. 18:1610–1623. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chaft JE, Rusch V, Ginsberg MS, Paik PK,
Finley DJ, Kris MG, Price KAR, Azzoli CG, Fury MG, Riely GJ, et al:
Phase II trial of neoadjuvant bevacizumab plus chemotherapy and
adjuvant bevacizumab in patients with resectable nonsquamous
non-small-cell lung cancers. J Thorac Oncol. 8:1084–1090. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ou W, Li N, Wang SY, Li J, Liu QW, Huang
QA and Wang BX: Phase 2 trial of neoadjuvant bevacizumab plus
pemetrexed and carboplatin in patients with unresectable stage III
lung adenocarcinoma (GASTO 1001). Cancer. 122:740–747. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie N, Qu YZ, Jiang HP and Yang XJ: Effect
of preoperative of bevacizumab combined with platinum-containing
neoadjuvant chemotherapy on the prognosis of patients with stage
IIIA-N2 stage lung cancer surgery. Chin J Surg Oncol. 14:371–375.
2022.
|
|
16
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman J, Chirieac LR, D'Amico TA, DeCamp MM, Dilling TJ,
Dobelbower M, et al: Non-small cell lung cancer, version 5.2017,
NCCN clinical practice guidelines in oncology. J Natl Compr Canc
Netw. 15:504–535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Travis WD, Dacic S, Wistuba I, Sholl L,
Adusumilli P, Bubendorf L, Bunn P, Cascone T, Chaft J, Chen G, et
al: IASLC multidisciplinary recommendations for pathologic
assessment of lung cancer resection specimens after neoadjuvant
therapy. J Thorac Oncol. 15:709–740. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pataer A, Weissferdt A, Correa AM,
Vaporciyan AA, Sepesi B, Heymach JV, Berezowska S, Cascone T and
Swisher SG: Major pathologic response and prognostic score predict
survival in patients with lung cancer receiving neoadjuvant
chemotherapy. JTO Clin Res Rep. 3:1004202022.PubMed/NCBI
|
|
20
|
Mabeta P and Steenkamp V: The VEGF/VEGFR
axis revisited: Implications for cancer therapy. Int J Mol Sci.
23:155852022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Le X, Nilsson M, Goldman J, Reck M,
Nakagawa K, Kato T, Ares LP, Frimodt-Moller B, Wolff K,
Visseren-Grul C, et al: Dual EGFR-VEGF pathway inhibition: A
promising strategy for patients with EGFR-mutant NSCLC. J Thorac
Oncol. 16:205–215. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dell'Amore A, Lomangino I, Tamburini N,
Bongiolatti S, Parri NSF, Grossi W, Catelli C, Lorenzoni G, Gregori
D, Nicotra S, et al: Video-assisted thoracoscopic lobectomy after
neoadjuvant chemotherapy for non-small cell lung cancer: A
multicenter propensity-matched study. Surg Endosc. 36:1466–1475.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao L, Zhang L, Gong F and Xu J:
Thoracoscopic radical resection in the treatment of NSCLC patients
(stage IIIA) after neoadjuvant chemotherapy. J BUON. 26:313–319.
2021.PubMed/NCBI
|
|
24
|
Trukhin D, Poddubskaya E, Andric Z,
Makharadze T, Bellala RS, Charoentum C, Yanez Ruiz EP, Fulop A,
Hyder Ali IA, Syrigos K, et al: Efficacy, safety and immunogenicity
of MB02 (bevacizumab biosimilar) versus reference bevacizumab in
advanced non-small cell lung cancer: A randomized, double-blind,
phase III study (STELLA). BioDrugs. 35:429–444. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Quintanilha JCF, Wang J, Sibley AB, Jiang
C, Etheridge AS, Shen F, Jiang G, Mulkey F, Patel JN, Hertz DL, et
al: Bevacizumab-induced hypertension and proteinuria: A genome-wide
study of more than 1000 patients. Br J Cancer. 126:265–274. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu Y, Li HM and Wang R: Effectiveness and
safety of adding bevacizumab to platinum-based chemotherapy as
first-line treatment for advanced non-small-cell lung cancer: A
meta-analysis. Front Med (Lausanne). 8:6163802021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou CH, Yang F, Jiang WJ, Zhang YC, Yang
HY, Zeng L, Liu L, Xiong Y, Zeng FX, Wang Z and Yang N: Efficacy
and safety of different doses of bevacizumab combined with
pemetrexed and platinum in first-line treatment of advanced NSCLC:
A retrospective-real world study. Front Pharmacol. 12:7271022021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, DeCamp M,
et al: NCCN guidelines® insights: Non-small cell lung
cancer, version 2.2023. J Natl Compr Canc Netw. 21:340–350. 2023.
View Article : Google Scholar : PubMed/NCBI
|