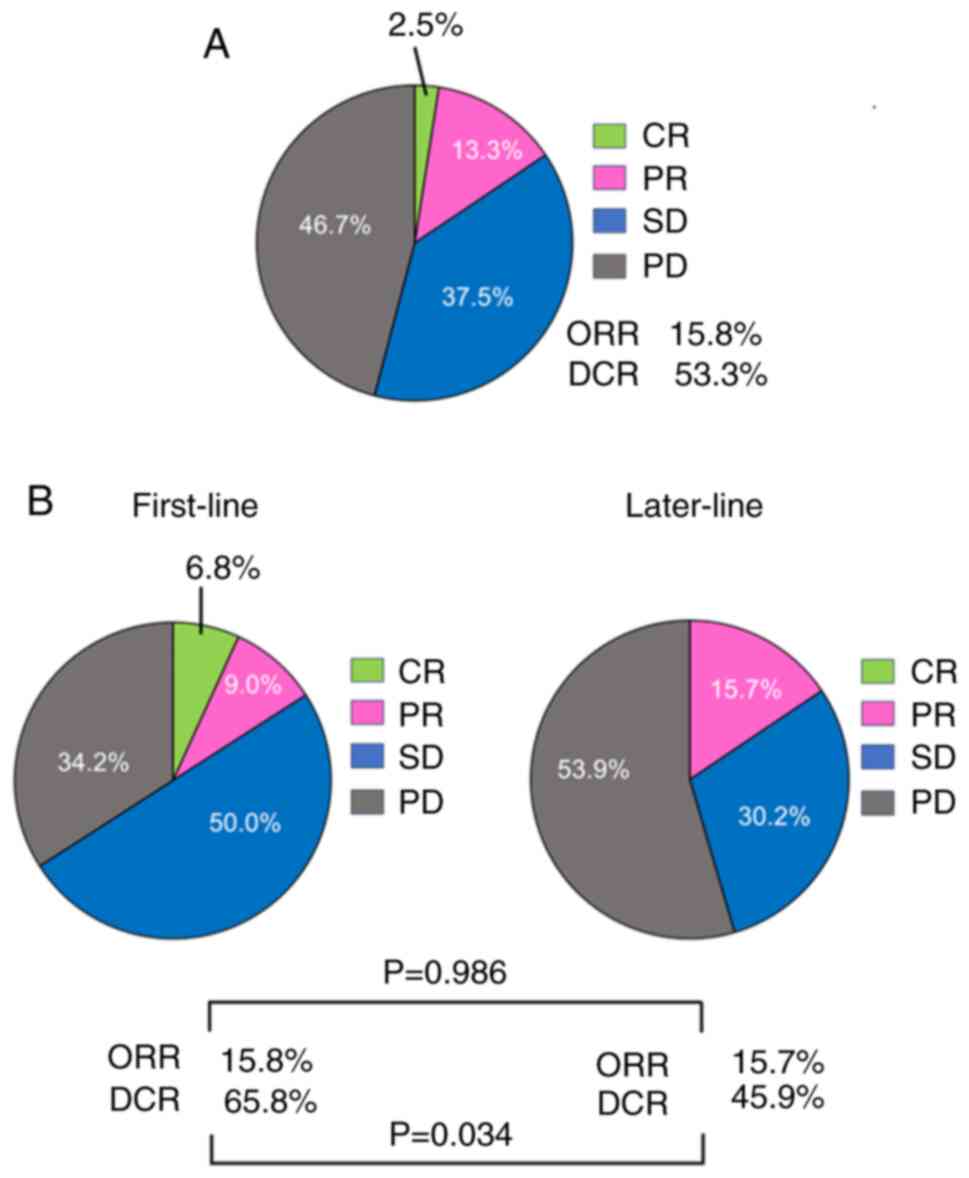
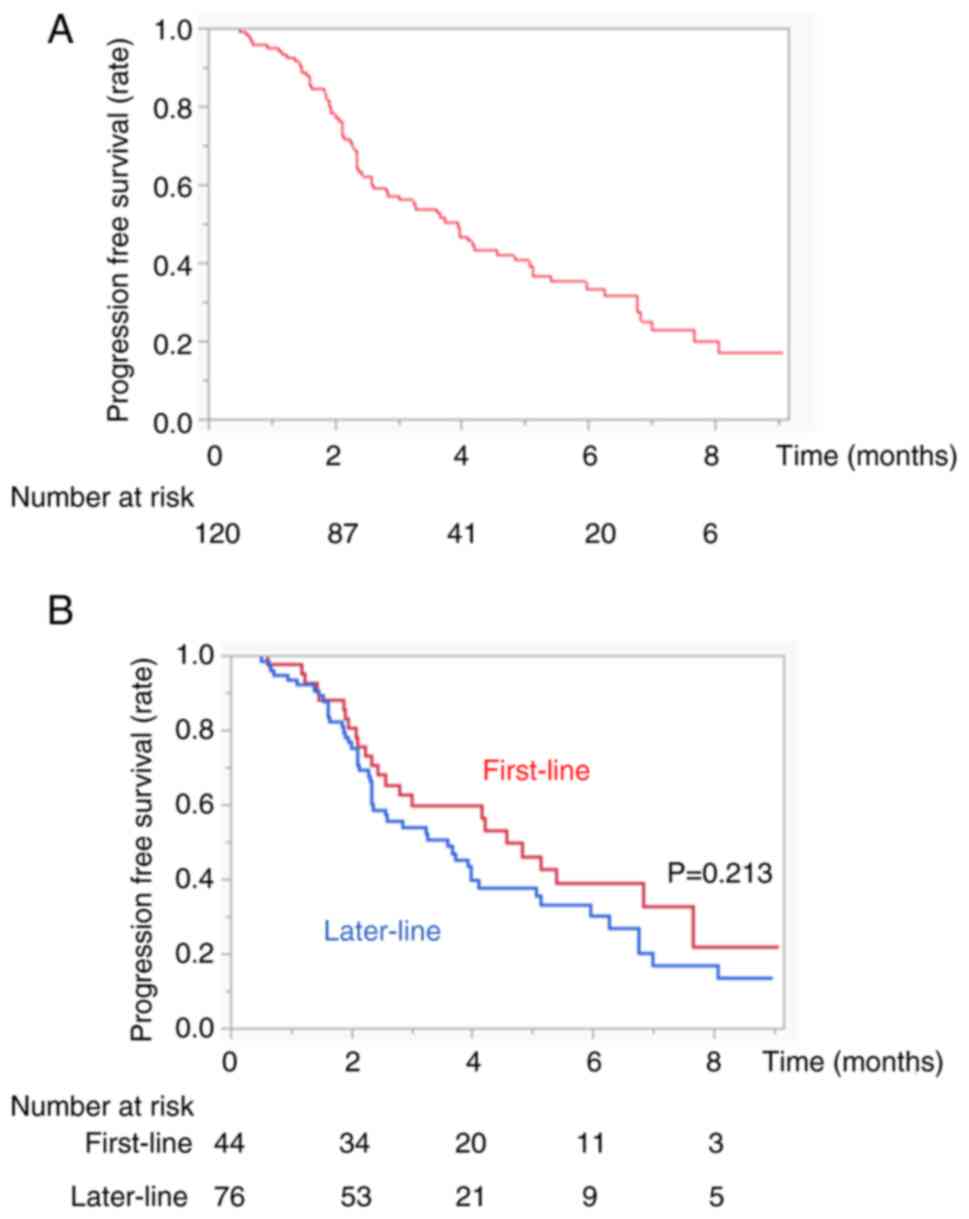
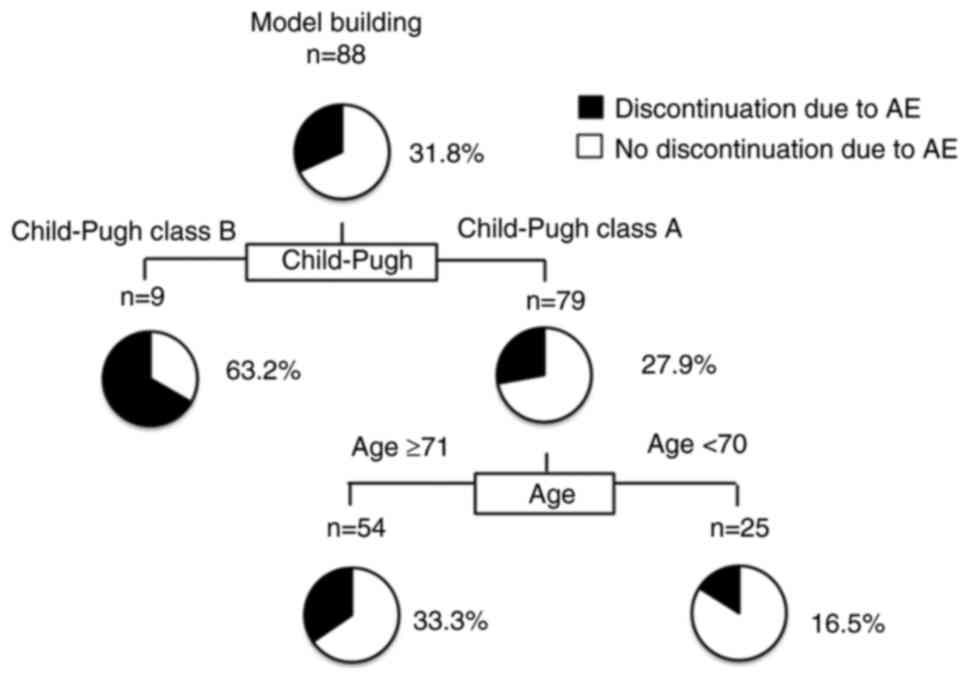
|
1
|
Rumgay H, Arnold M, Ferlay J, Lesi O,
Cabasag CJ, Vignat J, Laversanne M, McGlynn KA and Soerjomataram I:
Global burden of primary liver cancer in 2020 and predictions to
2040. J Hepatol. 77:1598–1606. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vogel A, Meyer T, Sapisochin G, Salem R
and Saborowski A: Hepatocellular carcinoma. Lancet. 400:1345–1362.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Villanueva A: Hepatocellular carcinoma. N
Engl J Med. 380:1450–1462. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kudo M: Recent advances in systemic
therapy for hepatocellular carcinoma in an aging society: 2020
update. Liver Cancer. 9:640–662. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rimassa L, Finn RS and Sangro B:
Combination immunotherapy for hepatocellular carcinoma. J Hepatol.
79:506–515. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singal AG, Llovet JM, Yarchoan M, Mehta N,
Heimbach JK, Dawson LA, Jou JH, Kulik LM, Agopian VG, Marrero JA,
et al: AASLD practice guidance on prevention, diagnosis, and
treatment of hepatocellular carcinoma. Hepatology. 78:1922–1965.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abou-Alfa GK, Lau G, Kudo M, Chan SL,
Kelley RK, Furuse J, Sukeepaisarnjaroen W, Kang YK, Van Dao T, De
Toni EN, et al: Tremelimumab plus durvalumab in unresectable
hepatocellular carcinoma. NEJM Evid. 1:EVIDoa21000702022.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reig M, Forner A, Rimola J, Ferrer-Fàbrega
J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V,
Salem R, et al: BCLC strategy for prognosis prediction and
treatment recommendation: The 2022 update. J Hepatol. 76:681–693.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abou-Alfa GK, Lau G, Kudo M, Chan SL,
Kelley RK, Furuse J, Sukeepaisarnjaroen W, Kang YK, Dao TV, De Toni
EN, et al: Plain language summary of the HIMALAYA study:
Tremelimumab and durvalumab for unresectable hepatocellular
carcinoma (liver cancer). Future Oncol. 19:2505–2516. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Johnson ML, Cho BC, Luft A,
Alatorre-Alexander J, Geater SL, Laktionov K, Kim SW, Ursol G,
Hussein M, Lim FL, et al: Durvalumab with or without tremelimumab
in combination with chemotherapy as first-line therapy for
metastatic non-small-cell lung cancer: The phase III POSEIDON
study. J Clin Oncol. 41:1213–1227. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Psyrri A, Fayette J, Harrington K,
Gillison M, Ahn MJ, Takahashi S, Weiss J, Machiels JP, Baxi S,
Vasilyev A, et al: Durvalumab with or without tremelimumab versus
the EXTREME regimen as first-line treatment for recurrent or
metastatic squamous cell carcinoma of the head and neck: KESTREL, a
randomized, open-label, phase III study. Ann Oncol. 34:262–274.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Horvat TZ, Adel NG, Dang TO, Momtaz P,
Postow MA, Callahan MK, Carvajal RD, Dickson MA, D'Angelo SP, Woo
KM, et al: Immune-related adverse events, need for systemic
immunosuppression, and effects on survival and time to treatment
failure in patients with melanoma treated with ipilimumab at
memorial sloan kettering cancer center. J Clin Oncol. 33:3193–3198.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Postow MA, Sidlow R and Hellmann MD:
Immune-related adverse events associated with immune checkpoint
blockade. N Engl J Med. 378:158–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cholongitas E, Papatheodoridis GV, Vangeli
M, Terreni N, Patch D and Burroughs AK: Systematic review: The
model for end-stage liver disease-should it replace Child-Pugh's
classification for assessing prognosis in cirrhosis? Aliment
Pharmacol Ther. 22:1079–1089. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Johnson PJ, Berhane S, Kagebayashi C,
Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A,
Palmer D, et al: Assessment of liver function in patients with
hepatocellular carcinoma: A new evidence-based approach-the ALBI
grade. J Clin Oncol. 33:550–558. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hatanaka T, Kakizaki S, Hiraoka A, Tada T,
Hirooka M, Kariyama K, Tani J, Atsukawa M, Takaguchi K, Itobayashi
E, et al: Comparing the impact of atezolizumab plus bevacizumab and
lenvatinib on the liver function in hepatocellular carcinoma
patients: A mixed-effects regression model approach. Cancer Med.
12:21680–21693. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hayakawa Y, Tsuchiya K, Kurosaki M, Yasui
Y, Kaneko S, Tanaka Y, Ishido S, Inada K, Kirino S, Yamashita K, et
al: Early experience of atezolizumab plus bevacizumab therapy in
Japanese patients with unresectable hepatocellular carcinoma in
real-world practice. Invest New Drugs. 40:392–402. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Y, Zhang Y, Iwamoto H, Hosaka K, Seki
T, Andersson P, Lim S, Fischer C, Nakamura M, Abe M, et al:
Discontinuation of anti-VEGF cancer therapy promotes metastasis
through a liver revascularization mechanism. Nat Commun.
7:126802016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sangro B, Chan SL, Kelly RK, Lau G, Kudo
M, Sukeepaisarnjaroen W, Yarchoan M, De Toni EN, Furuse J, Kang YK,
et al: Four-year overall survival update from the phase 3 HIMALAYA
study of tremelimumab plus durvalumab in unresectable
hepatocellular carcinoma. Ann Oncol. 35:448–457. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tanabe N, Saeki I, Aibe Y, Matsuda T,
Hanazono T, Nishi M, Hidaka I, Kuwashiro S, Shiratsuki S, Matsuura
K, et al: Early prediction of response focused on tumor markers in
atezolizumab plus bevacizumab therapy for hepatocellular carcinoma.
Cancers (Basel). 15:29272023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Waterhouse D, Lam J, Betts KA, Yin L, Gao
S, Yuan Y, Hartman J, Rao S, Lubinga S and Stenehjem D: Real-world
outcomes of immunotherapy-based regimens in first-line advanced
non-small cell lung cancer. Lung Cancer. 156:41–49. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Buchbinder EI and Desai A: CTLA-4 and PD-1
pathways: Similarities, differences, and implications of their
inhibition. Am J Clin Oncol. 39:98–106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Robert L, Tsoi J, Wang X, Emerson R, Homet
B, Chodon T, Mok S, Huang RR, Cochran AJ, Comin-Anduix B, et al:
CTLA4 blockade broadens the peripheral T-cell receptor repertoire.
Clin Cancer Res. 20:2424–2432. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shimose S, Hiraoka A, Tanaka M, Iwamoto H,
Tanaka T, Noguchi K, Aino H, Yamaguchi T, Itano S, Suga H, et al:
Deterioration of liver function and aging disturb sequential
systemic therapy for unresectable hepatocellular carcinoma. Sci
Rep. 12:170182022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Delire B, De Martin E, Meunier L, Larrey D
and Horsmans Y: Immunotherapy and gene therapy: New challenges in
the diagnosis and management of drug-induced liver injury. Front
Pharmacol. 12:7861742021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ito T, Ishigami M, Yamamoto T, Mizuno K,
Yamamoto K, Imai N, Ishizu Y, Honda T, Kawashima H, Yasuda S, et
al: Clinical course of liver injury induced by immune checkpoint
inhibitors in patients with advanced malignancies. Hepatol Int.
15:1278–1287. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dudek M, Pfister D, Donakonda S, Filpe P,
Schneider A, Laschinger M, Hartmann D, Hüser N, Meiser P, Bayerl F,
et al: Auto-aggressive CXCR6+ CD8 T cells cause liver
immune pathology in NASH. Nature. 592:444–449. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pfister D, Núñez NG, Pinyol R, Govaere O,
Pinter M, Szydlowska M, Gupta R, Qiu M, Deczkowska A, Weiner A, et
al: NASH limits anti-tumour surveillance in immunotherapy-treated
HCC. Nature. 592:450–456. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tani J, Senoh T, Moriya A, Ogawa C,
Deguchi A, Sakamoto T, Takuma K, Nakahara M, Oura K, Tadokoro T, et
al: Long-term outcomes and evaluation of hepatocellular carcinoma
recurrence after hepatitis C virus eradication by direct-acting
antiviral treatment: All kagawa liver disease group (AKLDG) study.
Cancers (Basel). 13:22572021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Persano M, Rimini M, Tada T, Suda G,
Shimose S, Kudo M, Cheon J, Finkelmeier F, Lim HY, Presa J, et al:
Sequential therapies after atezolizumab plus bevacizumab or
lenvatinib first-line treatments in hepatocellular carcinoma
patients. Eur J Cancer. 189:1129332023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takeuchi Y, Nouso K, Fujioka SI, Kariyama
K, Kobashi H, Uematsu S, Moriya A, Hagihara H, Takabatake H,
Nakamura S, et al: The prediction of early progressive disease in
patients with hepatocellular carcinoma receiving atezolizumab plus
bevacizumab. Cancer Med. 12:17559–17568. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tomonari T, Tani J, Sato Y, Tanaka H,
Tanaka T, Taniguchi T, Asahiro M, Okamoto K, Sogabe M, Miyamoto H,
et al: Initial therapeutic results of atezolizumab plus bevacizumab
for unresectable advanced hepatocellular carcinoma and the
importance of hepatic functional reserve. Cancer Med. 12:2646–2657.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hatanaka T, Kakizaki S, Hiraoka A, Tada T,
Hirooka M, Kariyama K, Tani J, Atsukawa M, Takaguchi K, Itobayashi
E, et al: Comparative analysis of the therapeutic outcomes of
atezolizumab plus bevacizumab and lenvatinib for hepatocellular
carcinoma patients aged 80 years and older: Multicenter study.
Hepatol Res. 54:382–391. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Iavarone M, Cabibbo G, Biolato M, Corte
CD, Maida M, Barbara M, Basso M, Vavassori S, Craxì A, Grieco A, et
al: Predictors of survival in patients with advanced hepatocellular
carcinoma who permanently discontinued sorafenib. Hepatology.
62:784–791. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shimose S, Iwamoto H, Niizeki T, Shirono
T, Noda Y, Kamachi N, Okamura S, Nakano M, Suga H, Kuromatsu R, et
al: Clinical significance of adverse events for patients with
unresectable hepatocellular carcinoma treated with lenvatinib: A
multicenter retrospective study. Cancers (Basel). 12:18672020.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dale W, Mohile SG, Eldadah BA, Trimble EL,
Schilsky RL, Cohen HJ, Muss HB, Schmader KE, Ferrell B, Extermann
M, et al: Biological, clinical, and psychosocial correlates at the
interface of cancer and aging research. J Natl Cancer Inst.
104:581–589. 2012. View Article : Google Scholar : PubMed/NCBI
|