Introduction
Distant metastases commonly occur in the bone
(1). Bone metastases (BMs) occur in
approximately 30% of metastatic renal cell cancers (RCC), and most
patients with BMs do not expect a good prognosis (1-year OS,
approximately 30%) (2,3).
Hypofractionated RT (e.g., a single fraction of 8
Gy) is as effective as fractionated RT for pain relief and
metastatic spinal cord compression (MSCC) in the BMs from RCC
(4). However, the radiographic
local control of BMs receiving RT is insufficient with
hypofractionated RT, and the re-RT rate is higher for
hypofractionated RT than for fractionated RT (4–9).
Therefore, hypofractionated RT may be unsuitable for patients with
an expected long-term prognosis, and the prediction of life
expectancy is crucial at the time of RT for BMs from RCC.
Several scoring systems have been developed for
patients with bone metastasis (10–13).
In one of these scoring systems, Katagiri et al devised a
very precise prognostic scoring system for patients with BMs from
various primary cancers (10).
However, the recent remarkable progress in systemic therapy,
tyrosine kinase inhibitors (TKI), and immune checkpoint inhibitors
(ICI) has improved the prognosis of patients with advanced RCC
(14–18). Although several disease-specific
prognostic scoring systems for BMs have been devised. In addition,
the International Metastatic Renal Cell Carcinoma Database
Consortium (IMDC) scoring system (19,20)
and the Memorial Sloan-Kettering Cancer Center (MSKCC) prognostic
scoring systems (21) are
well-known prognostic scoring systems for metastatic RCC. However,
few prognostic scoring systems are useful for the radiation
oncologists when selecting the RT dose for BMs from RCC (22–25).
Therefore, to select the optimal RT dose for BMs from RCC, we
assessed the prognostic factors in RCC patients with BMs and
devised a prognostic scoring system.
Materials and methods
Study population
Between January 2010 and March 2023, 109 consecutive
RCC patients were treated with initial RT for BMs at our
institutions (Ehime University Hospital, Toon, Japan, n=50; Ehime
Prefectural Central Hospital, Matsuyama, Japan, n=38; National
Hospital Organization Shikoku Cancer Center, Matsuyama, Japan,
n=21). RCC patients with BMs were referred by an attending
physician to a radiation oncologist for palliative RT for the
following reasons: i) Pain relief with or without prevention of
pathological fractures, and ii) metastatic spinal cord compression
(MSCC) with or without pain and/or neurological symptoms. The
Ethics Committee of National Hospital Organization Shikoku Cancer
Center approved this retrospective study (registration no.
2023-525).
BMs was detected using computed tomography (CT,
n=109), bone scintigraphy (n=22), 18F fluorodeoxyglucose
positron-emission tomography and CT (n=31), or magnetic resonance
(MR, n=28) scans. Performance status (PS) was evaluated using the
Eastern Cooperative Oncology Group scale.
Radiotherapy
The patients received three-dimensional conformal RT
delivered using 4–10 MV photons with a linear accelerator (Clinac
21EX, Clinac iX, or TrueBeam, Varian Medical Systems).
The most common RT dose was 30 Gy in 10 fractions
(n=39, 34.9%). The other fraction schedules were as follows: 1×8 Gy
(n=8), 5×4 Gy (n=4), 4×5 Gy (n=1), 13–15×3 Gy (n=32), 15–20×2.5 Gy
(n=9), 20–25×2 Gy (n=5), and 5×4 Gy + 8×2 Gy (n=1).
Statistical analyses
The survival rate was calculated using the
Kaplan-Meier method with log-rank test. The Cox proportional hazard
model was used for univariate and multivariate analyses to
determine the hazard ratios (HRs), including 95% confidence
intervals (CIs) and P-values. Factors such as age, sex, PS,
histologic type, control of the primary tumor of the kidney
(primary site), brain metastasis, liver metastasis, lung
metastasis, disseminated metastasis, lymph node metastasis, number
of bone metastatic lesions, bone metastatic site, RT site,
pathological fracture, neurological symptoms, use of bone-modifying
agents (BMAs), pre-RT targeted therapies (TTs), and pre-RT
laboratory data were analyzed using univariate analysis. Because
the important factors were not clear in the previous studies,
factors with P<0.10 on univariate analysis were subjected to
multivariate analysis. In multivariate analysis and log-rank tests,
P<0.05 was considered to indicate a statistically significant
difference and a scoring system based on regression coefficients in
the multivariate analysis was devised. Statistical analyses were
performed using JMP software (JMP version 14.3.0; SAS Institute,
Cary, North Carolina, United States).
Results
Clinical characteristics
The patient characteristics are listed in Table I. A total of 41 (37.6%), 22 (20.2%),
and 46 (42.2%) patients had single, 2–3, and >3 BMs,
respectively. A total of 54 (50.5%) patients underwent pre-RT TTs
such as sorafenib, sunitinib, axitinib, pazopanib, everolimus,
temsirolimus, cabozantinib, nivolumab, and ipilimumab.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Value |
|---|
| Median age, years
(range) | 69 (42–89) |
| Age, n (%) |
|
| <70
years | 59 (54.1) |
| ≥70
years | 50 (45.9) |
| Sex, n (%) |
|
| Male | 84 (77.1) |
|
Female | 25 (22.9) |
| ECOG-PS, n (%) |
|
|
<2 | 54 (49.5) |
| 2 | 29 (26.6) |
|
>2 | 26 (23.9) |
| Histologic type, n
(%) |
|
| Clear
cell | 101 (92.7) |
| Clear
cell with spindle cell | 5 (4.6) |
|
Papillary | 2 (1.8) |
|
Collecting duct | 1 (1.0) |
| Primary site, n
(%) |
|
| Control
of primary site | 72 (66.0) |
|
Surgery | 65 (59.6) |
|
ATs | 7 (6.4) |
| No
control of primary site | 10 (9.2) |
|
Surgery | 4 (3.7) |
|
ATs | 6 (5.5) |
| De
novo | 27 (24.8) |
| Brain metastasis, n
(%) |
|
| Yes | 10 (9.2) |
| No | 99 (90.8) |
| Liver metastasis, n
(%) |
|
| Yes | 17 (15.6) |
| No | 92 (84.4) |
| Lung metastasis, n
(%) |
|
|
Yes | 66 (60.6) |
| No | 43 (39.4) |
| Lymph metastasis, n
(%) |
|
|
Yes | 35 (32.1) |
| No | 74 (67.9) |
| Disseminated
metastasis, n (%) |
|
|
Yes | 12 (11.0) |
| No | 97 (89.0) |
| Number of bone
metastatic lesions, n (%) |
|
| 1 | 41 (37.6) |
|
2-3 | 22 (20.2) |
|
>3 | 46 (42.2) |
| Bone metastatic
site, n (%) |
|
| Only
vertebral | 32 (29.4) |
| Only
non-vertebral | 33 (30.3) |
|
Others | 44 (40.4) |
| Pathological
fracture, n (%) |
|
|
Yes | 15 (13.8) |
| No | 94 (86.2) |
| RT dose (BED10), n
(%) |
|
|
<39.0 Gy | 12 (11.0) |
| 39.0 Gy
(=3 Gy × 10 fraction) | 36 (33.0) |
|
>39.0 Gy | 61 (56.0) |
| RT sites, n
(%) |
|
|
Vertebral | 65 (59.6) |
|
Others | 44 (40.4) |
| BMAs, n (%) |
|
|
Yes | 60 (55.0) |
| No | 49 (45.0) |
| Pre-RT TTs, n
(%) |
|
|
Yes | 54 (50.5) |
| No | 55 (49.5) |
| Pre-RT laboratory
data |
|
| Median
CRP, mg/dl (range) | 0.98
(0.02–23.33) |
| Median
LDH, U/l (range) | 197
(116–1,017) |
| Median
albumin, g/dl (range) | 3.6 (1.8–4.6) |
| Median
platelet, ×104/µl (range) | 25.2 (3.4–63) |
| Median
Ca, mg/dl (range) | 9.1 (7.6–11.3) |
| Median
T-Bil, mg/dl (range) | 0.5 (0.2–1.2) |
Pre-RT laboratory data were collected using the
Katagiri scoring system (10). Only
eight patients showed critically abnormal laboratory data
(platelets, 3; serum calcium, 5; total bilirubin, 0). Therefore,
abnormal [CRP ≥0.4 mg/dl, lactate dehydrogenase (LDH) ≥250 IU/l, or
serum albumin <3.7 g/dl] and critically abnormal (platelet
<100,000/l, serum calcium ≥10.3 mg/dl, or total bilirubin ≥1.4
mg/dl) laboratory data were included within the same group as
abnormal laboratory data.
Among the 109 patients, 62 (56.9%) died and 47
(43.1%) survived at the latest follow-up. The median follow-up time
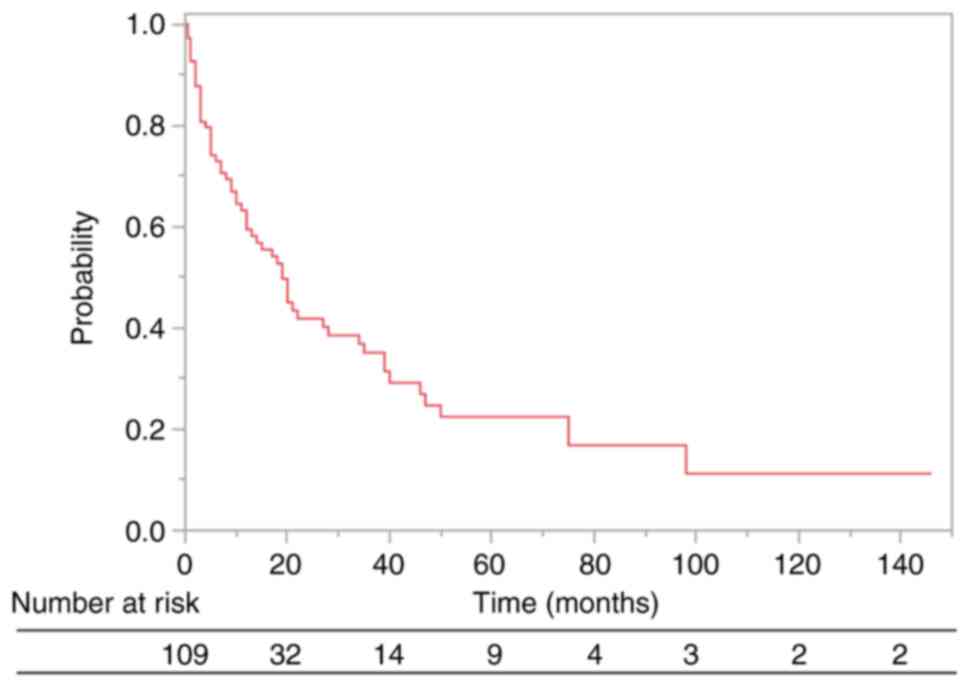
of OS was 9.0 months (range, 0.5–146.0 months), and the 0.5- and
1-year OS rates were 73.0 and 59.4%, respectively (Fig. 1). In addition, the median follow-up
time for the survival of living and dead patients at the final
follow-up was 10.0 months (range, 1.0–146.0 months) and 9.0 months
(range, 0.5–98.0 months), respectively.
Prognostic factors for patients with
BMs from RCC
Because de novo, which means ‘RCC patients with BMs
at the time of initial diagnosis, and the primary site control
cases did not differ significantly in OS (HR, 1.40; 95% CI,
0.70–2.80; P=0.33), the control evaluation of the primary site was
classified into two groups (control or de novo vs. no control).
In the univariate analysis, PS (<2 vs. ≥2; HR,
2.16; 95% CI, 1.30–3.61; P<0.01), primary site (control or de
novo vs. no control; HR, 2.67; 95% CI, 1.30–5.47; P=0.01), lung
metastasis (no vs. yes; HR, 1.68; 95% CI, 0.99–2.86; P=0.05),
disseminated metastasis (no vs. yes; HR, 3.00; 95% CI, 1.54–5.88;
P<0.01), lymph node metastasis (no vs. yes; HR, 2.23; 95% CI,
1.31–3.80; P<0.01), number of bone metastasis (single vs.
multiple; HR, 2.31; 95% CI, 1.29–4.14; P<0.01), and pre-RT
laboratory data (normal vs. abnormal; HR, 2.40; 95% CI, 1.13–5.12;
P=0.02) were significantly associated with OS (Table II).
 | Table II.Survival rates after RT and results
of univariate and multivariate analyses. |
Table II.
Survival rates after RT and results
of univariate and multivariate analyses.
|
|
| Univariate
analysis | Multivariate
analysis |
|
|---|
|
|
|
|
|
|
|---|
| Characteristic | 1-year survival,
% | HR (95% CI) | P-value | HR (95% CI) | P-value | Regression
coefficient |
|---|
| Age, <70 years
vs. ≥70 years | 61.0 vs. 57.2 | 1.39
(0.83–2.33) | 0.21 | - | - | - |
| Sex, male vs.
female | 64.2 vs. 47.3 | 1.53
(0.87–2.69) | 0.14 | - | - | - |
| ECOG-PS, 0–1 vs.
2–4 | 78.0 vs. 41.1 | 2.16
(1.30–3.61) | <0.01 | 1.84
(1.06–3.18) | 0.03 | 0.31 |
| Histologic type,
clear cell vs. others | 61.8 vs. 37.5 | 1.51
(0.65–3.53) | 0.34 | - | - | - |
| Primary site,
control or de novo vs. no control | 64.1 vs. 20.0 | 2.67
(1.30–5.47) | 0.01 | 3.24
(1.51–6.95) | <0.01 | 0.59 |
| Brain metastasis,
no vs. yes | 59.5 vs. 58.3 | 1.17
(0.53–2.59) | 0.70 | - | - | - |
| Liver metastasis,
no vs. yes | 62.5 vs. 44.8 | 1.43
(0.76–2.70) | 0.27 | - | - | - |
| Lung metastasis, no
vs. yes | 62.9 vs. 57.1 | 1.68
(0.99–2.86) | 0.05 | 1.31
(0.74–2.30) | 0.36 | 0.13 |
| Disseminated
metastasis, no vs. yes | 63.4 vs. 30.0 | 3.00
(1.54–5.88) | <0.01 | 2.36
(1.12–4.97) | 0.02 | 0.43 |
| Lymph node
metastasis, no vs. yes | 64.7 vs. 48.7 | 2.23
(1.31–3.80) | <0.01 | 1.91
(1.04–3.51) | 0.04 | 0.32 |
| Number of bone
metastatic lesions, single vs. multiple | 73.4 vs. 51.8 | 2.31
(1.29–4.14) | <0.01 | 2.56
(1.38–4.75) | <0.01 | 0.47 |
| Bone metastatic
site, only spine vs. others | 57.2 vs. 60.3 | 1.33
(0.73–2.42) | 0.35 | - | - | - |
| RT sites, only
spine vs. others | 57.4 vs. 62.8 | 1.19
(0.71–2.01) | 0.50 | - | - | - |
| Pathological
fracture, no vs. yes | 58.7 vs. 64.3 | 1.03
(0.53–1.99) | 0.93 | - | - | - |
| Neurological
symptom, no vs. yes | 61.4 vs. 58.3 | 1.22
(0.71–2.08) | 0.47 | - | - | - |
| Use of BMAs, no vs.
yes | 51.4 vs. 63.3 | 0.71
(0.43–1.17) | 0.17 | - | - | - |
| Pre-RT TTs, no vs.
yes | 63.8 vs. 56.3 | 1.30
(0.78–2.16) | 0.31 | - | - | - |
| Pre-RT laboratory
data, normal vs. abnormal | 79.4 vs. 55.3 | 2.40
(1.13–5.12) | 0.02 | 1.83
(0.82–4.10) | 0.14 | 0.30 |
In the multivariate analysis, ECOG-PS (<2 vs. ≥2;
HR, 1.84; 95% CI, 1.06–3.18; P=0.03), primary site (control or de
novo vs. no control; HR, 3.24; 95% CI, 1.51–6.95; P<0.01),
disseminated metastasis (no vs. yes; HR, 2.36; 95% CI, 1.12–4.97;
P=0.02), lymph node metastasis (no vs. yes; HR, 1.91; 95% CI,
1.04–3.51; P=0.04), and the number of bone metastasis (single vs.
multiple; HR, 2.56; 95% CI, 1.38–4.75; P<0.01) were
significantly associated with reduced OS (Table II).
RT course length [long (>10 fractions) vs. short
(≤10 fractions); HR, 1.78; 95% CI, 1.08–2.94; P=0.02] was
significantly associated with OS in the univariate analysis, but
this factor was not included in the multivariate analysis because
of selection bias.
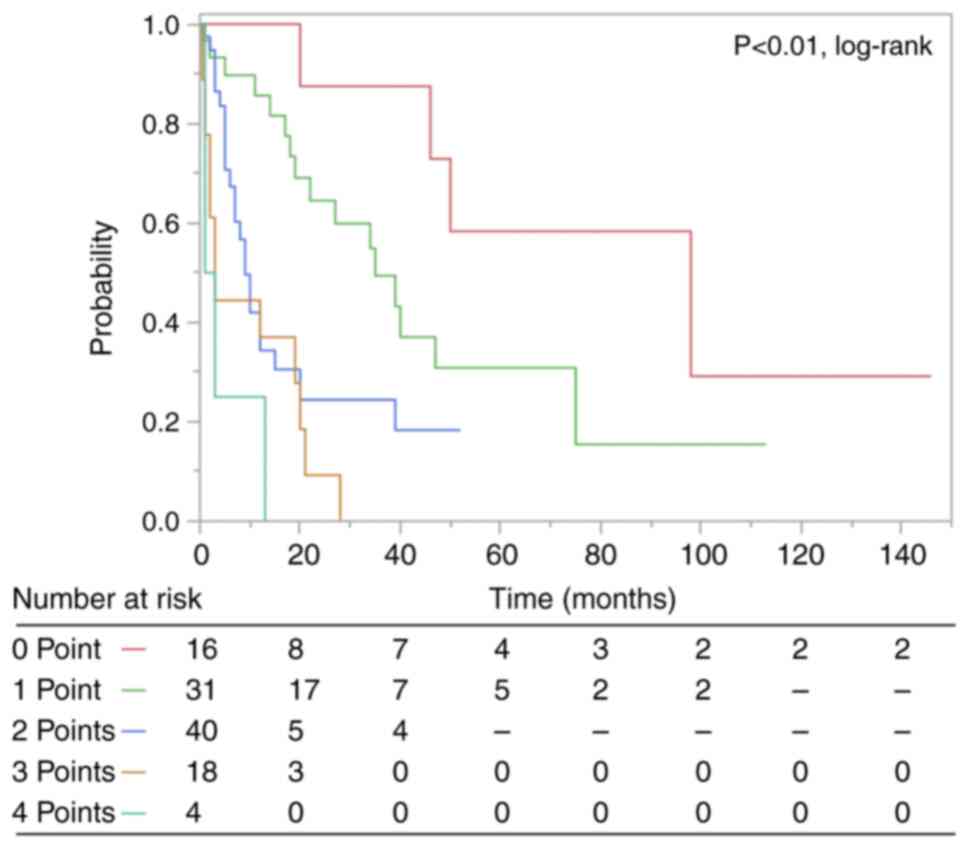
Prognosis according to the devised
prognostic scoring system
A prognostic scoring system using the regression
coefficients of significant prognostic factors in multivariate
analysis was developed (Tables II
and III). ECOG-PS, primary sites,
disseminated metastasis, lymph node metastasis, and number of bone
metastatic lesions were used to create a scoring system for the
estimation of survival. Because all regression coefficients were
between 0.3 and 0.6, one point was assigned to each factor. The
associations between the total points and the 0.5- and 1-year OS
rates are listed in Table IV, and
the corresponding Kaplan-Meier curves are shown in Fig. 2.
 | Table III.Points of significant prognostic
factors. |
Table III.
Points of significant prognostic
factors.
| Characteristic | Point |
|---|
| ECOG-PS |
|
| ≥2 | 1 |
|
<2 | 0 |
| Primary site |
|
| No
control | 1 |
| Control
or de novo | 0 |
| Disseminated
metastasis |
|
|
Yes | 1 |
| No | 0 |
| Lymph node
metastasis |
|
|
Yes | 1 |
| No | 0 |
| Number of bone
metastatic lesions |
|
|
Multiple | 1 |
|
Single | 0 |
 | Table IV.Associations between the total
points, and 0.5- and 1-year OS rate. |
Table IV.
Associations between the total
points, and 0.5- and 1-year OS rate.
| Total points | n | 0.5-year OS rate,
% | 1-year OS rate,
% |
|---|
| 0 | 16 | 100 | 100 |
| 1 | 31 | 89.7 | 85.7 |
| 2 | 40 | 67.3 | 34.3 |
| 3 | 18 | 44.4 | 37.0 |
| 4 | 4 | 25.0 | 0 |
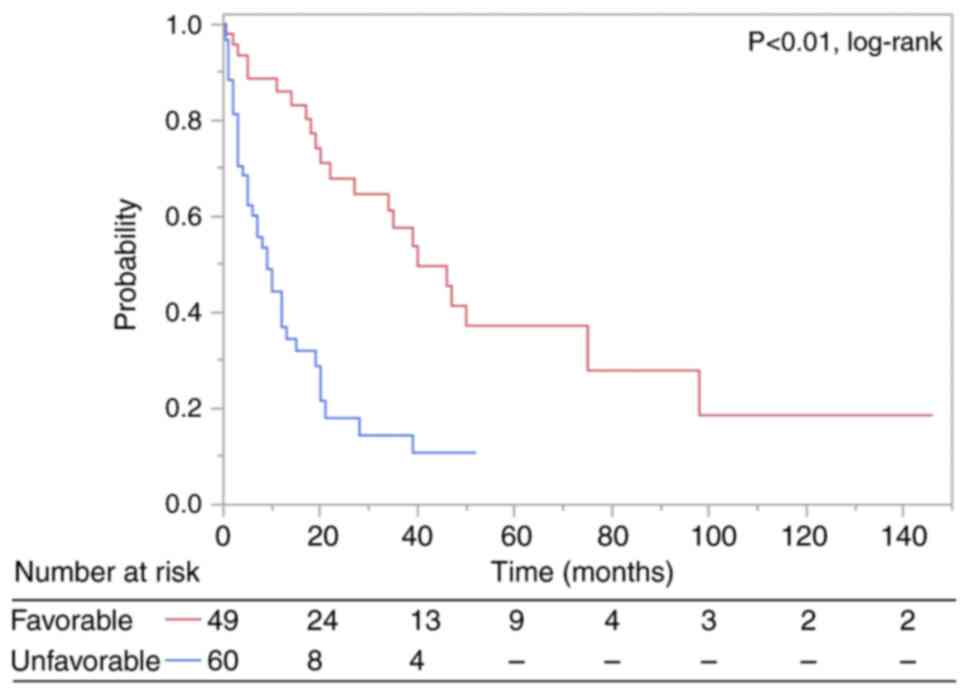
We classified patients with BMs from LC into two
groups and stratified them according to our scoring system. The
median OS was 19.0 months for the favorable group (total point
score 0–1) (n=49) and 5.0 months for the unfavorable group (total
point score 2–4) (n=60) (p<0.01, log-rank test). The OS curves
are shown in Fig. 3.
Discussion
This is the first study to identify the factors that
help radiation oncologists' selection the optimal RT dose for BMs
from RCC. Based on our multivariate analysis, a higher ECOG-PS
score, no control of the primary sites, disseminated metastasis,
lymph node metastasis, and multiple bone metastases were important
unfavorable prognostic factors for survival. Based on the number of
risk factors, patients with BMs from RCC were classified into two
groups [median OS: favorable (0–1 point), 19.0 months; unfavorable
(2–4 points), 5.0 months].
Katagiri et al proposed a scoring system for
predicting patients with BMs (10).
Although this scoring system is one of the most precise scoring
systems, previous study suggested that its prognostic factors may
be influenced by the high frequency of bone metastases from breast
and prostate cancer (23).
Therefore, predicting the prognosis of patients with BMs from RCC
alone is important for selecting the optimal palliative RT dose. In
this study, internal metastases (brain, liver, and lung metastases)
were not a prognostic factor in patients with BM from RCC, in
contrast to the Katagiri scoring system. In addition, lack of
control of primary sites and lymph node metastasis were new
prognostic factors for patients with BM from RCC, which were not
included in the Katagiri scoring system. Fan et al also
suggested that these are important prognostic factors for patients
with BMs from RCC (24). These
factors should be included as prognostic and predictive factors
when selecting the optimal RT dose for BMs from RCC. In addition,
similar to the study by Fan et al (24), pre-RT TTs did not appear to
influence the prognosis of RCC patients with BM. Because the pre-RT
chemotherapy in the Katagiri scoring system seemed to be influenced
by the aggressiveness of hormone-resistant prostate and breast
cancers, this factor did not seem to be important for predicting
the prognosis of RCC patients with BM. In contrast, abnormal
laboratory data and pre-RT TTs had a small impact on the prognosis
of RCC patients with BMs as included in the Katagiri scoring
system. In this study, only 16.5% (18/109) of the patients had
normal laboratory data, and only 7.3% (8/109) had critically
abnormal laboratory data. Most patients had abnormal laboratory
data, which may have influenced the small impact of abnormal
laboratory data on the prognosis of RCC patients with BM in this
study.
Specific scoring systems for single cancers are
important when considering the individual characteristics of a
primary cancer type. In this study, a scoring system was devised
for RCC patients with BM. Five factors [ECOG-PS (≥2:1 point),
primary site (no control: 1 point), disseminated metastasis (yes: 1
point), lymph node metastasis (yes: 1 point), and number of bone
metastatic lesions (multiple: 1 point)] were important in
predicting the survival time of patients with BMs from RCC. In
addition, two prognostic groups (favorable: 0–1 points and
unfavorable: 2–4 points) that were significantly correlated with
survival time were devised according to the regression coefficients
of these factors. The significance of the RT dose escalation for
BMs from RCC is controversial, but the re-irradiation rate is
higher in the lower RT dose group (4,9,26,27).
Therefore, although a higher RT dose may be preferable in the
favorable group (median OS, 19 months), a lower RT dose should be
administered aggressively in the unfavorable group (median OS, 5
months). Moreover, some studies have reported that stereotactic
body radiation therapy (SBRT), a precise irradiation technique with
an extremely high dose per fraction, has remarkably improved local
control of primary and metastatic RCC (28–30).
In Japan, SBRT for spinal metastases or oligometastases has been
available in routine clinical practice under the Japanese National
Health Insurance System since April 2020. However, currently, the
number of patients treated with SBRT for spinal metastases or
oligometastases at our institutions is very small, and no patients
has been performed for BMs from RCC. In the future, SBRT may become
an option over conventional higher RT doses for the patients in the
favorable group.
There are some limitations to our study owing to its
retrospective nature. First, the number of patients included in
this study was relatively small. Therefore, it is possible that
visceral metastasis (brain, liver, or lung metastases) may not be
an unfavorable prognostic factor. Large-scale prospective studies
are needed to validate the findings. Second, our study analyzed
almost all important factors with reference to the Katagiri scoring
system. However, the pathological grade, identified as important
for predicting the prognosis of RCC patients with BM (24), could not be examined because of
insufficient data. In this study, only 20 cases (grade 1, n=7;
grade 2, n=6; grade 3, n=7) could be evaluated for pathological
grade. However, in some cases, these data may not be described in
routine clinical practice. In addition, although the histological
type could not be evaluated in this study because most patients
[92.7% (101/109)] had clear cell cancer, important results are
useful in daily clinical practice because the majority of RCC were
clear cell cancer. Third, our scoring system cannot be accurately
compared with the IMDC and MSKCC scoring systems, which are
well-known prognostic scoring systems for metastatic RCC. This was
not only owing to the lack of detailed laboratory data (neutrophil
count) used in the IMDC scoring system but also because of the lack
of time from diagnosis of RCC used in the IMDC and MSKCC scoring
systems in some patients who were referred from other hospitals
only for palliative RT. With regard to the lack of detailed
laboratory data, detailed information is needed because the
laboratory data used in the IMDC or MSKCC scoring systems are used
to determine optimal treatment strategies. In contrast, in patients
referred to radiation oncologists for palliative RT, minimal
laboratory data are often obtained. This difference in the purpose
of examinations may have influenced the collected laboratory data.
Furthermore, the laboratory data for the Katagiri scoring system,
which was used as a reference in devising our scoring system,
showed abnormal C-reactive protein levels in most patients (67.9%,
n=74). In addition, the laboratory data for the MSKCC scoring
system showed abnormal hemoglobin values in most patients (77.1%,
n=84). This indicates that abnormal laboratory data were observed
in many patients who received palliative RT, regardless of the
scoring system used. Therefore, abnormal laboratory data may be
important for predicting the prognosis of bone metastatic RCC;
however, it was unlikely to emerge as an important factor in our
study. With regard to the time from RCC diagnosis, the date of RCC
diagnosis was unknown in some patients who were referred to
radiation oncologists for palliative RT from other hospitals, but
the MSKCC scoring system could be used if these patients were
assumed to be in the group of time from diagnosis to therapy
initiation ≥1 year. However, the prognostic classification using
the MSKCC scoring system based on this hypothesis was less optimal
than our prognostic scoring system (Figs. S1 and S2). Therefore, we believe that our
prognostic scoring system may be more useful for radiation
oncologists compared to the MSKCC scoring system for selecting
optimal RT doses. Finally, because this was a long-term,
multicenter, retrospective study, detailed information on the
reasons for selecting the RT schedule, palliative effects of
treatment, and adverse events was difficult to obtain. A higher RT
dose may be prescribed for some degree of local control even if
pain control is the main purpose of palliative RT. Owing to these
limitations, further large-scale studies based on more detailed
information are required.
We have devised a new scoring system for patients
with bone metastases from RCC. Our prognostic model for RCC
patients with BMs may be useful for selecting an appropriate RT
dose.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KM designed the study concepts. KM, YH, HK, KN, YK
and TK collected patient data. KM and YH analyzed data. KM, YH, HK,
KN, YK and TK drafted the article. KM, YH, HK, KN, YK and TK
collaborated in the discussion. KM prepared the manuscript and YH
edited the manuscript. KM and YH confirm the authenticity of all
the raw data. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
All procedures in studies involving human
participants were performed in accordance with the ethical
standards of the institutional research committee, and The 1964
Declaration of Helsinki and its later amendments or comparable
ethical standards. The need for patient consent was waived by the
institutional ethics committee due to the retrospective nature of
the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Coleman RE: Metastatic bone disease:
Clinical features, pathophysiology and treatment strategies. Cancer
Treat Rev. 27:165–176. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsuya A, Kurata T, Tamura K and Fukuoka M:
Skeletal metastases in non-small cell lung cancer: A retrospective
study. Lung Cancer. 57:229–232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Svensson E, Christiansen CF, Ulrichsen SP,
Rørth MR and Sørensen HT: Survival after bone metastasis by primary
cancer type: A Danish population-based cohort study. BMJ Open.
7:e0160222017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rades D, Walz J, Stalpers LJA, Veninga T,
Schulte R, Obralic N, Wildfang I, Engenhart-Cabilic R, Hoskin PJ
and Schild SE: Short-course radiotherapy (RT) for metastatic spinal
cord compression (MSCC) due to renal cell carcinoma: Results of a
retrospective multi-center study. Eur Urol. 49:846–852. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chow R, Hoskin P, Schild SE, Raman S, Im
J, Zhang D, Chan S, Chiu N, Chiu L, Lam H, et al: Single vs
multiple fraction palliative radiation therapy for bone metastases:
Cumulative meta-analysis. Radiother Oncol. 141:56–61. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rades D, Lange M, Veninga T, Stalpers LJ,
Bajrovic A, Adamietz IA, Rudat V and Schild SE: Final results of a
prospective study comparing the local control of short-course and
long-course radiotherapy for metastatic spinal cord compression.
Int J Radiat Oncol Biol Phys. 79:524–530. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rades D, Segedin B, Conde-Moreno AJ,
Garcia R, Perpar A, Metz M, Badakhshi H, Schreiber A, Nitsche M,
Hipp P, et al: Radiotherapy with 4 Gy × 5 versus 3 Gy × 10 for
metastatic epidural spinal cord compression: Final results of the
SCORE-2 trial (ARO 2009/01). J Clin Oncol. 34:597–602. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Makita K, Hamamoto Y, Kanzaki H, Kataoka
M, Yamamoto S, Nagasaki K, Ishikawa H, Takata N, Tsuruoka S, Uwatsu
K and Kido T: Local control of bone metastases treated with
external beam radiotherapy in recent years: A multicenter
retrospective study. Radiat Oncol. 16:2252021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Makita K, Hamamoto Y, Kanzaki H, Nagasaki
K, Takata N, Tsuruoka S, Uwatsu K and Kido T: Factors affecting
local control of bone metastases from radioresistant tumors treated
with palliative external beam radiotherapy. Discov Oncol.
14:742023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Katagiri H, Okada R, Takagi T, Takahashi
M, Murata H, Harada H, Nishimura T, Asakura H and Ogawa H: New
prognostic factors and scoring system for patients with skeletal
metastasis. Cancer Med. 3:1359–1367. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bollen L, van der Linden YM, Pondaag W,
Fiocco M, Pattynama BP, Marijnen CA, Nelissen RG, Peul WC and
Dijkstra PD: Prognostic factors associated with survival in
patients with symptomatic spinal bone metastases: A retrospective
cohort study of 1,043 patients. Neuro Oncol. 16:991–998. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Westhoff PG, de Graeff A, Monninkhof EM,
Bollen L, Dijkstra SP, van der Steen-Banasik EM, van Vulpen M, Leer
JW, Marijnen CA and van der Linden YM; Dutch Bone Metastasis Study
Group, : An easy tool to predict survival in patients receiving
radiation therapy for painful bone metastases. Int J Radiat Oncol
Biol Phys. 90:739–747. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Willeumier JJ, van der Linden YM, van der
Wal CWPG, Jutte PC, van der Velden JM, Smolle MA, van der Zwaal P,
Koper P, Bakri L, de Pree I, et al: An easy-to-use prognostic model
for survival estimation for patients with symptomatic long bone
metastases. J Bone Joint Surg Am. 100:196–204. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Escudier B, Eisen T, Stadler WM, Szczylik
C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA,
et al: Sorafenib in advanced clear-cell renal-cell carcinoma. N
Engl J Med. 356:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Motzer RJ, Hutson TE, Tomczak P,
Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik
C, Kim ST, et al: Sunitinib versus interferon alfa in metastatic
renal-cell carcinoma. N Engl J Med. 356:115–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Motzer RJ, Hutson TE, Tomczal P,
Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili
R, Bjarnason GA, et al: Overall survival and updated results for
sunitinib compared with interferon alfa in patients with metastatic
renal cell carcinoma. J Clin Oncol. 27:3584–3590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sternberg CN, Davis ID, Mardiak J,
Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA,
Kavina A, et al: Pazopanib in locally advanced or metastatic renal
cell carcinoma: Results of a randomized phase III trial. J Clin
Oncol. 28:1061–1068. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: Nivolumab versus everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1803–1813. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Heng DY, Xie W, Regan MM, Warren MA,
Golshayan AR, Sahi C, Eigl BJ, Ruether JD, Cheng T, North S, et al:
Prognostic factors for overall survival in patients with metastatic
renal cell carcinoma treated with vascular endothelial growth
factor-targeted agents: Results from a large, multicenter study. J
Clin Oncol. 27:5794–5799. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Heng DY, Xie W, Regan MM, Harshman LC,
Bjarnason GA, Vaishampayan UN, Mackenzie M, Wood L, Donskov F, Tan
MH, et al: External validation and comparison with other models of
the international metastatic renal-cell carcinoma database
consortium prognostic model: A population-based study. Lancet
Oncol. 14:141–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Motzer RJ, Bacik J, Murphy BA, Russo P and
Mazumdar M: Interferon-alfa as a comparative treatment for clinical
trials of new therapies against advanced renal cell carcinoma. J
Clin Oncol. 20:289–296. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Makita K, Kanzaki H, Hamamoto Y, Nagasaki
K, Kataoka M, Kido T and Ohsumi S: Prognostic assessment of
patients who receive radiotherapy for bone metastases from breast
cancer. Oncol Lett. 25:1882023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Makita K, Hamamoto Y, Kanzaki H, Nagasaki
K and Kozuki T: An easy tool to predict survival in patients with
bone metastatic lung cancer treated with palliative radiotherapy.
Thorac Cancer. 14:1795–1801. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fan Z, Huang Z and Huang X: Bone
metastasis in renal cell carcinoma patients: Risk and prognostic
factors and nomograms. J Oncol. 2021:55752952021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rades D, Haus R, Janssen S and Schild SE:
An easy-to-use scoring system to estimate the survival of patients
irradiated for bone metastases from lung cancer. Transl Lung Cancer
Res. 9:1067–1073. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rades D, Freundt K, Meyners T, Bajrovic A,
Basic H, Karstens JH, Adamietz IA, Wildfang I, Rudat V, Schild SE
and Dunst J: Dose escalation for metastatic spinal cord compression
in patients with relatively radioresistant tumors. Int J Radiat
Oncol Biol Phys. 80:1492–1497. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Freundt K, Meyners T, Bajrovic A, Basic H,
Karstens JH, Adamietz IA, Rudat V, Schild SE, Dunst J and Rades D:
Radiotherapy for oligometastatic disease in patients with spinal
cord compression (MSCC) from relatively radioresistant tumors.
Strahlenther Onkol. 186:218–223. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Siva S, Louie AV, Kotecha R, Barber MN,
Ali M, Zhang Z, Guckenberger M, Kim MS, Scorsetti M, Tree AC, et
al: Stereotactic body radiotherapy for primary renal cell
carcinoma: A systematic review and practice guideline from the
international society of stereotactic radiosurgery (ISRS). Lancet
Oncol. 25:e18–e28. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Amini A, Altoos B, Bourlon MT, Bedrick E,
Bhatia S, Kessler ER, Flaig TW, Fisher CM, Kavanagh BD, Lam ET and
Karam SD: Local control rates of metastatic renal cell carcinoma
(RCC) to the bone using stereotactic body radiation therapy: Is RCC
truly radioresistant? Pract Radiat Oncol. 5:e589–e596. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Altoos B, Amini A, Yacoub M, Bourlon MT,
Kessler EE, Flaig TW, Fisher CM, Kavanagh BD, Lam ET and Karam SD:
Local control rates of metastatic renal cell carcinoma (RCC) to
thoracic, abdominal, and soft tissue lesions using stereotactic
body radiotherapy (SBRT). Radiat Oncol. 10:2182015. View Article : Google Scholar : PubMed/NCBI
|