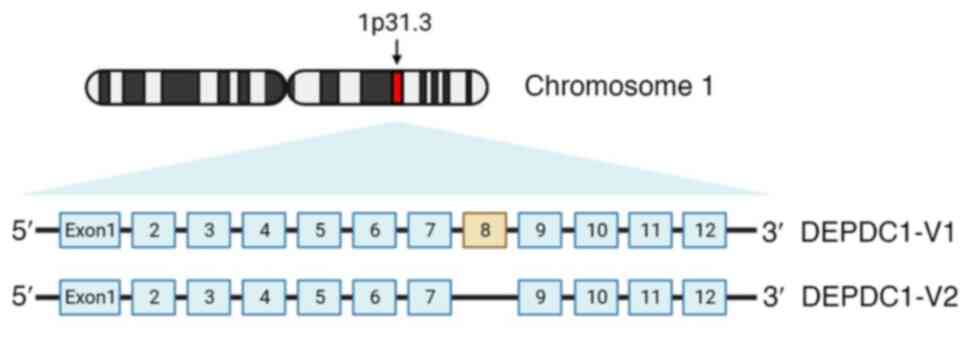
The disheveled, EGL-10 and pleckstrin (DEP) globular
protein domain interacts with phospholipids and membrane receptors
to recruit proteins to the plasma membrane for participation in
signal transduction (1). As first
described by Kanehira et al (2) in 2007, knockdown of upregulated DEP
domain-containing 1 (DEPDC1) was shown to significantly suppress
the proliferation of bladder cancer (BC) cells. Subsequent studies
(3–9) have implicated DEPDC1 in multiple
signaling pathways associated with tumorigenesis.
DEPDC1 serves critical roles in cell mitosis,
proliferation, migration and invasion, as well as angiogenesis,
autophagy and apoptosis (3,12–15).
Therefore, DEPDC1 may be considered a potential marker of prognosis
and response to therapy. In the present review, the roles of DEPDC1
in tumorigenesis and its related molecular mechanisms, as well as
its potential as a peptide vaccine are summarized to emphasize the
importance of DEPDC1 in future cancer research.
Upregulation of DEPDC1 has been associated with poor
prognosis and survival in hepatocellular carcinoma (HCC) (16–21),
lung adenocarcinoma (LUAD) (22,23),
colorectal cancer (CRC) (24–26),
breast cancer (BrC) (27–30), ovarian cancer (31), renal cancer (32), osteosarcoma (33,34),
anaplastic thyroid carcinoma (35),
gastric cancer (36),
cholangiocarcinoma (37), head and
neck squamous cell carcinoma (38),
esophageal squamous cell carcinoma (39), endometrial carcinoma (40), multiple myeloma (4,41),
glioma (42), diffuse large B-cell
lymphoma (43) and soft-tissue
sarcoma (44). Furthermore, DEPDC1
has been reported to be significantly upregulated in 29 out of 33
types of human cancer, and to be associated with overall survival,
disease-specific survival and progress-free interval prognosis in
numerous tumor types (45). The
upregulation of DEPDC1 may be related to apoptosis, autophagy,
proliferation, migration, invasion and angiogenesis in various
types of cancer (46–49). Hence, the present study reviewed the
molecular mechanisms underlying the multiple functions of DEPDC1 in
tumorigenesis.
BC is the 10th most common cancer and the second
most common cancer in men worldwide (50). The role of DEPDC1 in BC was first
described in 2007. Harada et al (3) reported that DEPDC1 forms a complex
with zinc finger protein 224 (ZNF224) that suppresses transcription
of A20, resulting in inhibition of apoptosis via activation of
NF-κB and the subsequent proliferation of BC cells, whereas a
peptide mimic formed by coupling 11 arginine residues at the amino
terminal of DEPDC1 (nucleotides 611–628) was able to inhibit
formation of the DEPDC1-ZNF224 complex and induce apoptosis of BC
cells both in vivo and in vitro. Wang et al
(51) revealed that overexpression
of DEPDC1 could alleviate or even reverse the inhibition of
proliferation and migration of BC cells induced by knockdown of
α-protein kinase 2.
Globally, LC remains a leading cause of
cancer-related death in both men and women (52). The two main types of LC are small
cell and non-small cell subtypes. Similar to BC, Wang et al
(9) reported activation of the
DEPDC1-ZNF224/A20/NF-κB axis in A549 LUAD cells. Maternal embryonic
leucine zipper kinase (MELK) is a is a highly conserved
serine/threonine kinase in various stages of the cell cycle
(53,54). Knockout of MELK in A549 cells has
been reported to significantly reduce DEPDC1 protein levels without
any change to transcription levels (55). In addition, MELK-induced
phosphorylation can regulate DEPDC1 protein stability (55), similar to the findings of a previous
report in myeloma cells (56). Wang
et al (15) reported that
DEPDC1 could upregulate the expression of RAS and suppress
autophagy via the RAS-ERK pathway in LUAD cells, thus revealing a
potential relationship between DEPDC1 and autophagy.
MicroRNAs (miRNAs/miRs) are short non-coding RNAs
that serve crucial roles in oncogenesis and have been investigated
as potential diagnostic and prognostic markers. A study by Liu
et al (57) found that
miR-23b could downregulate DEPDC1 in non-small cell LC. Multiple
miRNAs have been reported to inhibit the expression of DEPDC1 in
various types of cancer, as described below.
HCC is one of the most common causes of
cancer-related mortality worldwide, accounting for >800,000
deaths annually, with a 1-year survival rate of <20% (58). A study by Guo et al (14) revealed that upregulation of DEPDC1
induced the expression of phosphorylated (p)-Akt, c-myc and cyclin
E1 in HCC cells, whereas knockdown of chemokine ligand 20 (CCL20)
or chemokine receptor 6 (CCR6; receptor for CCL20) reversed these
effects. In addition, knockdown of CCL20 or CCR6 reversed
DEPDC1-related angiogenesis and the invasive capabilities of human
umbilical vein endothelial cells. These results indicated that
DEPDC1 may promote angiogenesis in HCC.
CRC is the second most common malignant tumor
worldwide, the incidence rate of which has increased annually
(63). Wang et al (48) reported that DEPDC1-induced enhanced
expression of suppressor of zest 12 could promote the
proliferation, invasion and epithelial-mesenchymal transition of
CRC cells. Sharen et al (64) revealed that overexpression of the
large ribosomal subunit protein eL31 promoted the proliferation and
migration of CRC cells by targeting DEPDC1, and upregulated the
expression of p-Akt, cyclin D1, cyclin-dependent kinase 6,
phosphatidylinositol-4,5-bisphosphate 3-kinase and catalytic
subunit α.
BrC has been reported to be the most commonly
diagnosed type of cancer, accounting for 2.26 million cases
worldwide in 2020, and is the leading cause of cancer-related death
of women (67). Zhao et al
(68) demonstrated that DEPDC1
promoted the proliferation, migration and invasion of BrC cells via
the PI3K/Akt/mammalian target of rapamycin (mTOR) signaling
pathway.
OSCC is a major public health concern, accounting
for >90% of all oral malignancies worldwide (72). Guo et al (46) indicated that the tobacco-specific
nitrosamine carcinogen
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone could lead to DNA
methyltransferase 1-regulated DNA methylation, which may promote
DEPDC1 expression; this upregulated DEPDC1 expression can stimulate
the proliferation of OSCC cells by inhibiting the expression of
cytochrome P450 family 27 subfamily B member 1. Qiu et al
(73) suggested that DEPDC1 may
positively regulate the expression of FOXM1, resulting in nuclear
localization of β-catenin, which is the most important indicator of
Wnt pathway activation. Huang et al (74) revealed that DEPDC1 promoted
activation of the Wnt/β-catenin signaling pathway in OSCC.
NB, or Wilms tumor, is the most common renal cell
malignancy and the second most common extracranial solid tumor in
children after neuroblastoma, (75). Geng et al (76) demonstrated that, in NB cells,
overexpression of FOXO3a inhibited the protein expression of
p-GSK-3β, Wnt3a and β-catenin, whereas overexpression of DEPDC1 had
the opposite effects. In addition, FOXO3a inhibited the
transcription of DEPDC1, which was associated with NB-like
proliferation, migration and invasion. This previous study also
revealed that knockout of S100 calcium-binding protein A16
(S100A16) inhibited the migration, invasiveness and angiogenesis of
NB cells, which was partially reversed by overexpression of DEPDC1.
Furthermore, a study by Geng et al (77) demonstrated that S100A16 and DEPDC1
promoted the progression and angiogenesis of NB through the
PI3K/Akt/mTOR pathway.
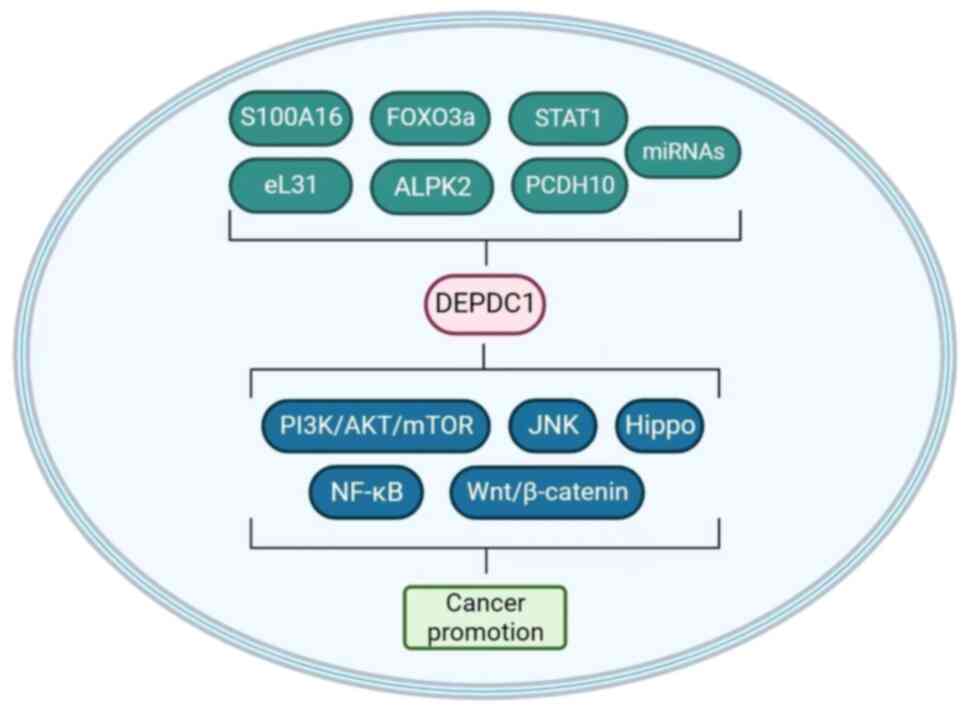
These studies have indicated that DEPDC1 serves a
complex and critical role in several important signaling pathways
in various types of cancer (Fig. 2;
Table I); however, the molecular
mechanisms require further clarification.
LncRNAs are involved in a variety of cell signaling
pathways. Some lncRNAs act as carcinogens in tumors, whereas others
act as tumor suppressors (81).
Antisense lncRNAs are transcribed from the DNA lagging strand as
complementary sequences to the leading strand (82).
As aforementioned, high DEPDC1 expression is
associated with the poor prognosis of various types of cancer.
Therefore, DEPDC1 may have potential as a diagnostic and prognostic
marker for clinical diagnosis and response to treatment. For
example, a significant proportion of LUAD cases are caused by
mutations to epidermal growth factor receptor, K-Ras or anaplastic
lymphoma kinase; however, these three genes are not suitable as
markers of triple-negative LUAD. Alternatively, high expression of
DEPDC1 has been associated with the poor prognosis of
triple-negative LUAD (5) and may
thus be a promising target for cancer prognosis.
DEPDC1 is related to multiple signaling pathways and
may be a signaling hub for tumor development, and could thus be
considered a promising new therapeutic target. Arumugam et
al (88) suggested that DEPDC1,
as a consistently differentially expressed gene in TNBC, may
interact well with doxorubicin and anethole, which is a
phytocompound, suggesting that DEPDC1 could be a possible
therapeutic target of TNBC.
DEPDC1 is also a potential marker of the response to
medications. Microtubule-targeted chemotherapy induces apoptosis of
cancer cells by promoting phosphorylation and degradation of
induced myeloid leukemia cell differentiation protein Mcl (MCL1), a
member of the anti-apoptotic Bcl-2 family (89). Sendoel et al (6) concluded that DEPDC1 may regulate
vincristine (a tubulin-targeted chemotherapy drug)-induced cell
death by promoting JNK-dependent degradation of MCL1, whereas
knockdown of DEPDC1 mediated resistance to vincristine-induced cell
death, suggesting that vincristine may serve an antitumor role
through DEPDC1. Therefore, whether the expression levels of DEPDC1
can predict the efficacy of anti-tubulin drugs on tumors may be a
meaningful research direction.
As aforementioned, multiple miRNAs can inhibit the
expression of DEPDC1 and enhance the sensitivity of tumors to
chemotherapy drugs to address drug resistance. Therefore, the use
of miRNAs targeting DEPDC1 may be a promising research direction to
reduce chemotherapy resistance.
Peptide-based anticancer vaccines use
tumor-associated or tumor-specific peptides to induce and activate
T cells. These peptides are present on human leukocyte antigen
(HLA) molecules on the cell surface and are recognized by T-cell
receptors. Peptide vaccines have the advantages of low side
effects, but must be optimized prior to clinical application
(92).
Shortly after being described in BC, DEPDC1 was
investigated as a new target for peptide vaccines. Obara et
al (93) conducted a clinical
trial of possible peptide fragments that would bind to the
HLA-A*2402 molecule, and administered the M phase phosphoprotein 1
(MPHOSPH1)-278 and DEPDC1-294 peptides to six patients with BC.
Four of the six patients vaccinated with DEPDC1-294 achieved
cytotoxic T lymphocyte (CTL)-positive responses to DEPDC1-294, with
one achieving a partial response. This sample was expanded in a
further study to 32 patients who received this combined peptide
vaccine, and the study concluded that the 2-year survival rate was
slightly better than in response to treatment with vinflunine
(94). However, this previous study
did not include a control group, but rather compared the efficacy
of vinflunine with other studies, which may lead to errors due to
different backgrounds of the patients (94). In addition, the ability of the
peptide vaccine to prevent recurrence of non-muscular-invasive BC
was evaluated after transurethral resection of bladder tumors in
127 patients (95). Combined with
intravesical Bacillus Calmette-Guerin (BCG), the overall 2-year
recurrence-free survival rate was 74% for patients in the
HLA-A*2402-positive group, which was higher than the negative
control group (95). However, both
groups received the BCG vaccine, which might have masked the
therapeutic effect of the peptide vaccine. Therefore, an additional
study with three groups (peptide alone, BCG alone and peptide
combined with BCG vaccine) is required.
There have also been more successful treatments. For
example, a 2016 study conducted by Murahashi et al (99) enlisted 18 patients treated with five
HLA-A*2402 restricted tumor-associated antigen epitope peptides
from the novel chloroplast outer membrane kinase KOC1, threonine
tyrosine kinase, URLC10, DEPDC1 and MPHOSPH1 vaccines. A total of 4
days before vaccination, the patients received increasing dosages
of cyclophosphamide. In this study, the overall survival of nine
patients with CRC was 9.4 months, which was better than the effect
of cetuximab in another study (6.1 months) (100), indicating the advantage of peptide
vaccines with DEPDC1 as one of the epitope peptides. In addition, a
40-year-old male patient with esophageal cancer (celiac lymph node
metastasis) achieved a complete response with no disease recurrence
after 5 years.
DEPDC1 is a newly discovered tumor-related gene that
is upregulated in various malignant tumors and may be involved in
tumorigenesis as a marker of poor prognosis. Therefore, the
potential of DEPDC1 for detection and prognosis of cancer should be
considered.
DEPDC1 is involved in the regulation of tumor cell
proliferation, metastasis, autophagy, apoptosis and angiogenesis by
mediating different signaling pathways. Some of these signaling
pathways (NF-κB, PI3K/Akt, Wnt/β-catenin and Hippo) may promote
cell proliferation and survival. However, since intracellular
signal transduction is complex and there is cross-dialogue between
different pathways, further studies are needed to clarify the
signal transduction mechanism of DEPDC1.
Future studies may also focus on: i) Comparing the
sensitivity and specificity of DEPDC1 with other common diagnostic
and prognostic markers in different types of tumors; ii)
investigating the unique role of DEPDC1 in predicting therapeutic
response; and iii) evaluating the practical effectiveness of DEPDC1
as a marker in clinical applications.
Owing to the multifaceted characteristics and
effects of cancer-testis antigens (3,102),
DEPDC1 is a potential therapeutic target. Treatment targeting
DEPDC1 could inhibit the proliferation and metastasis of tumor
cells and improve patient outcomes. Furthermore, the efficacy of
DEPDC1-derived peptides in immunotherapy as cancer vaccines has
been confirmed in clinical trials, thus warranting further studies
to identify effective peptide segments, determine appropriate
dosages and assess efficacy in combination with other peptide
vaccines, as well as radiotherapy and chemotherapy. DEPDC1 also
provides a direction for the study of the molecular mechanisms of
antitumor drugs. Clarification of the mechanism of DEPDC1 could
provide a new therapeutic target for the prevention and treatment
of malignant tumors.
Not applicable.
The present study was supported by the National Natural Science
Foundation of China (grant no. 82104603) and the Peking University
People's Hospital Research and Development Fund (grant nos.
RS2021-12 and RDX2021-08).
Not applicable.
DL performed the literature review and wrote the
draft. HL was responsible for reviewing the literature, and
reviewed and revised the draft. JO conceived and designed the
study, acquired funding, and reviewed and revised the draft. Data
authentication is not applicable. All authors read and approved the
final version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Consonni SV, Maurice MM and Bos JL: DEP
domains: Structurally similar but functionally different. Nat Rev
Mol Cell Biol. 15:357–362. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kanehira M, Harada Y, Takata R, Shuin T,
Miki T, Fujioka T, Nakamura Y and Katagiri T: Involvement of
upregulation of DEPDC1 (DEP domain containing 1) in bladder
carcinogenesis. Oncogene. 26:6448–6455. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harada Y, Kanehira M, Fujisawa Y, Takata
R, Shuin T, Miki T, Fujioka T, Nakamura Y and Katagiri T:
Cell-permeable peptide DEPDC1-ZNF224 interferes with
transcriptional repression and oncogenicity in bladder cancer
cells. Cancer Res. 70:5829–5839. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kassambara A, Schoenhals M, Moreaux J,
Veyrune JL, Rème T, Goldschmidt H, Hose D and Klein B: Inhibition
of DEPDC1A, a bad prognostic marker in multiple myeloma, delays
growth and induces mature plasma cell markers in malignant plasma
cells. PLoS One. 8:e627522013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okayama H, Kohno T, Ishii Y, Shimada Y,
Shiraishi K, Iwakawa R, Furuta K, Tsuta K, Shibata T, Yamamoto S,
et al: Identification of genes upregulated in ALK-positive and
EGFR/KRAS/ALK-negative lung adenocarcinomas. Cancer Res.
72:100–111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sendoel A, Maida S, Zheng X, Teo Y,
Stergiou L, Rossi CA, Subasic D, Pinto SM, Kinchen JM, Shi M, et
al: DEPDC1/LET-99 participates in an evolutionarily conserved
pathway for anti-tubulin drug-induced apoptosis. Nat Cell Biol.
16:812–820. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang Y, Jiang Y, Jiang M, Zhang J, Yang B,
She Y, Wang W, Deng Y and Ye Y: Protocadherin 10 inhibits cell
proliferation and induces apoptosis via regulation of DEP domain
containing 1 in endometrial endometrioid carcinoma. Exp Mol Pathol.
100:344–352. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang L, Chen K, Cai ZP, Chen FC, Shen HY,
Zhao WH, Yang SJ, Chen XB, Tang GX and Lin X: DEPDC1 promotes cell
proliferation and tumor growth via activation of E2F signaling in
prostate cancer. Biochem Biophys Res Commun. 490:707–712. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Q, Li A, Jin J and Huang G: Targeted
interfering DEP domain containing 1 protein induces apoptosis in
A549 lung adenocarcinoma cells through the NF-κB signaling pathway.
Onco Targets Ther. 10:4443–4454. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Johannsdottir HK, Jonsson G,
Johannesdottir G, Agnarsson BA, Eerola H, Arason A, Heikkila P,
Egilsson V, Olsson H, Johannsson OT, et al: Chromosome 5 imbalance
mapping in breast tumors from BRCA1 and BRCA2 mutation carriers and
sporadic breast tumors. Int J Cancer. 119:1052–1060. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zuo X, Wang D, Tao C, Dou X, Zhao Z, Zhang
J, Huang S, Li Y, Zhang X, Bu Y and Wang Y: DEPDC1B is a novel
direct target of B-Myb and contributes to malignant progression and
immune infiltration in lung adenocarcinoma. Front Biosci (Landmark
Ed). 29:2042024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen D, Ito S, Hyodo T, Asano-Inami E,
Yuan H and Senga T: Phosphorylation of DEPDC1 at Ser110 is required
to maintain centrosome organization during mitosis. Exp Cell Res.
358:101–110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mi Y, Zhang C, Bu Y, Zhang Y, He L, Li H,
Zhu H, Li Y, Lei Y and Zhu J: DEPDC1 is a novel cell cycle related
gene that regulates mitotic progression. BMB Rep. 48:413–418. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo W, Li H, Liu H, Ma X, Yang S and Wang
Z: DEPDC1 drives hepatocellular carcinoma cell proliferation,
invasion and angiogenesis by regulating the CCL20/CCR6 signaling
pathway. Oncol Rep. 42:1075–1089. 2019.PubMed/NCBI
|
|
15
|
Wang W, Li A, Han X, Wang Q, Guo J, Wu Y,
Wang C and Huang G: DEPDC1 up-regulates RAS expression to inhibit
autophagy in lung adenocarcinoma cells. J Cell Mol Med.
24:13303–13313. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Amisaki M, Yagyu T, Uchinaka EI, Morimoto
M, Hanaki T, Watanabe J, Tokuyasu N, Sakamoto T, Honjo S and
Fujiwara Y: Prognostic value of DEPDC1 expression in tumor and
non-tumor tissue of patients with hepatocellular carcinoma.
Anticancer Res. 39:4423–4430. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuan SG, Liao WJ, Yang JJ, Huang GJ and
Huang ZQ: DEP domain containing 1 is a novel diagnostic marker and
prognostic predictor for hepatocellular carcinoma. Asian Pac J
Cancer Prev. 15:10917–10922. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bin X, Luo Z, Wang J and Zhou S:
Identification of a five immune term signature for prognosis and
therapy options (immunotherapy versus targeted therapy) for
patients with hepatocellular carcinoma. Comput Math Methods Med.
2023:89589622023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang L, Li Y, Dai Y, Wang D, Wang X, Cao
Y, Liu W and Tao Z: Glycolysis-related gene expression profiling
serves as a novel prognosis risk predictor for human hepatocellular
carcinoma. Sci Rep. 11:188752021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pu Z, Zhu Y, Wang X, Zhong Y, Peng F and
Zhang Y: Identification of prognostic biomarkers and correlation
with immune infiltrates in hepatocellular carcinoma based on a
competing endogenous RNA network. Front Genet. 12:5916232021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang J, Liu X, Zhou W, Lu S, Wu C, Wu Z,
Liu R, Li X, Wu J, Liu Y, et al: Identification of key genes
associated with the process of hepatitis B inflammation and cancer
transformation by integrated bioinformatics analysis. Front Genet.
12:6545172021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shao F, Ling L, Li C, Huang X, Ye Y, Zhang
M, Huang K, Pan J, Chen J and Wang Y: Establishing a
metastasis-related diagnosis and prognosis model for lung
adenocarcinoma through CRISPR library and TCGA database. J Cancer
Res Clin Oncol. 149:885–899. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen J and Xi M: DEPDC1 is highly
expressed in lung adenocarcinoma and promotes tumor cell
proliferation. Zhongguo Fei Ai Za Zhi. 24:453–460. 2021.(In
Chinese). PubMed/NCBI
|
|
24
|
Zhu Y, Sun L, Yu J, Xiang Y, Shen M, Wasan
HS, Ruan S and Qiu S: Identification of biomarkers in colon cancer
based on bioinformatic analysis. Transl Cancer Res. 9:4879–4895.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shen X and Han J: Overexpression of gene
DEP domain containing 1 and its clinical prognostic significance in
colorectal cancer. J Clin Lab Anal. 34:e236342020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miyata Y, Kumagai K, Nagaoka T, Kitaura K,
Kaneda G, Kanazawa H, Suzuki S, Hamada Y and Suzuki R:
Clinicopathological significance and prognostic value of Wilms'
tumor gene expression in colorectal cancer. Cancer Biomark.
15:789–797. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Colak D, Nofal A, Albakheet A, Nirmal M,
Jeprel H, Eldali A, Al-Tweigeri T, Tulbah A, Ajarim D, Malik OA, et
al: Age-specific gene expression signatures for breast tumors and
cross-species conserved potential cancer progression markers in
young women. PLoS One. 8:e632042013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kretschmer C, Sterner-Kock A, Siedentopf
F, Schoenegg W, Schlag PM and Kemmner W: Identification of early
molecular markers for breast cancer. Mol Cancer. 10:152011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sang M, Wu M, Meng L, Zheng Y, Gu L, Liu F
and Sang M: Identification of epithelial-mesenchymal
transition-related circRNA-miRNA-mRNA ceRNA regulatory network in
breast cancer. Pathol Res Pract. 216:1530882020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim J: In silico analysis of
differentially expressed genesets in metastatic breast cancer
identifies potential prognostic biomarkers. World J Surg Oncol.
19:1882021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ke Y, Zhuang X and You L: Identification
of core genes shared by endometrial cancer and ovarian cancer using
an integrated approach. Cell Mol Biol (Noisy-le-grand). 68:140–145.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu F, Guan Y, Xue L, Huang S, Gao K, Yang
Z and Chong T: The effect of a novel glycolysis-related gene
signature on progression, prognosis and immune microenvironment of
renal cell carcinoma. BMC Cancer. 20:12072020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma Y, Guo J, Li D and Cai X:
Identification of potential key genes and functional role of CENPF
in osteosarcoma using bioinformatics and experimental analysis. Exp
Ther Med. 23:802022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shen L, Li H, Liu R, Zhou C, Bretches M,
Gong X, Lu L, Zhang Y, Zhao K, Ning B, et al: DEPDC1 as a crucial
factor in the progression of human osteosarcoma. Cancer Med.
12:5798–5808. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang Z, Zou Z, Dai H, Ye R, Di X, Li R,
Ha Y, Sun Y and Gan S: Key genes involved in cell cycle arrest and
DNA damage repair identified in anaplastic thyroid carcinoma using
integrated bioinformatics analysis. Transl Cancer Res. 9:4188–4203.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gong Z, Chu H, Chen J, Jiang L, Gong B,
Zhu P, Zhang C, Wang Z, Zhang W, Wang J, et al: DEPDC1 upregulation
promotes cell proliferation and predicts poor prognosis in patients
with gastric cancer. Cancer Biomark. 30:299–307. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mishra NK, Niu M, Southekal S, Bajpai P,
Elkholy A, Manne U and Guda C: Identification of prognostic markers
in cholangiocarcinoma using altered DNA methylation and gene
expression profiles. Front Genet. 11:5221252020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang J, Lin H, Jiang H, Jiang H, Xie T,
Wang B, Huang X, Lin J, Xu A, Li R, et al: A key genomic signature
associated with lymphovascular invasion in head and neck squamous
cell carcinoma. BMC Cancer. 20:2662020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zheng L, Li L, Xie J, Jin H and Zhu N: Six
novel biomarkers for diagnosis and prognosis of esophageal squamous
cell carcinoma: Validated by scRNA-seq and qPCR. J Cancer.
12:899–911. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu J, Wu Z, Sun R, Nie S, Meng H, Zhong
Y, Nie X and Cheng W: Using mRNAsi to identify prognostic-related
genes in endometrial carcinoma based on WGCNA. Life Sci.
258:1182312020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Noll JE, Vandyke K, Hewett DR, Mrozik KM,
Bala RJ, Williams SA, Kok CH and Zannettino AC: PTTG1 expression is
associated with hyperproliferative disease and poor prognosis in
multiple myeloma. J Hematol Oncol. 8:1062015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stangeland B, Mughal AA, Grieg Z, Sandberg
CJ, Joel M, Nygård S, Meling T, Murrell W, Vik Mo EO and Langmoen
IA: Combined expressional analysis, bioinformatics and targeted
proteomics identify new potential therapeutic targets in
glioblastoma stem cells. Oncotarget. 6:26192–26215. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhu QY: Bioinformatics analysis of the
pathogenic link between Epstein-Barr virus infection, systemic
lupus erythematosus and diffuse large B cell lymphoma. Sci Rep.
13:63102023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pollino S, Benassi MS, Pazzaglia L, Conti
A, Bertani N, Righi A, Piccinni-Leopardi M, Picci P and Perris R:
Prognostic role of XTP1/DEPDC1B and SDP35/DEPDC1A in high grade
soft-tissue sarcomas. Histol Histopathol. 33:597–608.
2018.PubMed/NCBI
|
|
45
|
Jia B, Liu J, Hu X, Xia L and Han Y:
Pan-cancer analysis of DEPDC1 as a candidate prognostic biomarker
and associated with immune infiltration. Ann Transl Med.
10:13552022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Guo J, Zhou S, Huang P, Xu S, Zhang G, He
H, Zeng Y, Xu CX, Kim H and Tan Y: NNK-mediated upregulation of
DEPDC1 stimulates the progression of oral squamous cell carcinoma
by inhibiting CYP27B1 expression. Am J Cancer Res. 10:1745–1760.
2020.PubMed/NCBI
|
|
47
|
Hao S, Tian W, Chen Y, Wang L, Jiang Y,
Gao B and Luo D: MicroRNA-374c-5p inhibits the development of
breast cancer through TATA-box binding protein associated factor
7-mediated transcriptional regulation of DEP domain containing 1. J
Cell Biochem. 120:15360–15368. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang Q, Jiang S, Liu J, Ma G, Zheng J and
Zhang Y: DEP domain containing 1 promotes proliferation, invasion,
and epithelial-mesenchymal transition in colorectal cancer by
enhancing expression of suppressor of zest 12. Cancer Biother
Radiopharm. 36:36–44. 2021.PubMed/NCBI
|
|
49
|
Tian C, Abudoureyimu M, Lin X, Chu X and
Wang R: Linc-ROR facilitates progression and angiogenesis of
hepatocellular carcinoma by modulating DEPDC1 expression. Cell
Death Dis. 12:10472021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang L, Wang Y, Wang J, Li L and Bi J:
Identification of a prognosis-related risk signature for bladder
cancer to predict survival and immune landscapes. J Immunol Res.
2021:32363842021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang Y, Wu J, Luo W, Zhang H, Shi G, Shen
Y and Zhu Y, Ma C, Dai B, Ye D and Zhu Y: ALPK2 acts as tumor
promotor in development of bladder cancer through targeting
DEPDC1A. Cell Death Dis. 12:6612021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Barta JA, Powell CA and Wisnivesky JP:
Global epidemiology of lung cancer. Ann Glob Health. 85:82019.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nakano I, Paucar AA, Bajpai R, Dougherty
JD, Zewail A, Kelly TK, Kim KJ, Ou J, Groszer M, Imura T, et al:
Maternal embryonic leucine zipper kinase (MELK) regulates
multipotent neural progenitor proliferation. J Cell Biol.
170:413–427. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ganguly R, Hong CS, Smith LGF, Kornblum HI
and Nakano I: Maternal embryonic leucine zipper kinase: Key kinase
for stem cell phenotype in glioma and other cancers. Mol Cancer
Ther. 13:1393–1398. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chung S, Kijima K, Kudo A, Fujisawa Y,
Harada Y, Taira A, Takamatsu N, Miyamoto T, Matsuo Y and Nakamura
Y: Preclinical evaluation of biomarkers associated with antitumor
activity of MELK inhibitor. Oncotarget. 7:18171–18182. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bolomsky A, Heusschen R, Schlangen K,
Stangelberger K, Muller J, Schreiner W, Zojer N, Caers J and Ludwig
H: Maternal embryonic leucine zipper kinase is a novel target for
proliferation-associated high-risk myeloma. Haematologica.
103:325–335. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu C, Li X, Hao Y, Wang F, Cheng Z, Geng
H and Geng D: STAT1-induced upregulation of lncRNA KTN1-AS1
predicts poor prognosis and facilitates non-small cell lung cancer
progression via miR-23b/DEPDC1 axis. Aging (Albany NY).
12:8680–8701. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Johnson P, Zhou Q, Dao DY and Lo YMD:
Circulating biomarkers in the diagnosis and management of
hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol.
19:670–681. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li Y, Tian Y, Zhong W, Wang N, Wang Y,
Zhang Y, Zhang Z, Li J, Ma F, Zhao Z and Peng Y: Artemisia argyi
essential oil inhibits hepatocellular carcinoma metastasis via
suppression of DEPDC1 dependent Wnt/β-catenin signaling pathway.
Front Cell Dev Biol. 9:6647912021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Qu D, Cui F, Lu D, Yang Y and Xu Y: DEP
domain containing 1 predicts prognosis of hepatocellular carcinoma
patients and regulates tumor proliferation and metastasis. Cancer
Sci. 110:157–165. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhou C, Wang P, Tu M, Huang Y, Xiong F and
Wu Y: DEPDC1 promotes cell proliferation and suppresses sensitivity
to chemotherapy in human hepatocellular carcinoma. Biosci Rep.
39:BSR201909462019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Xu C, Luo L, Yu Y, Zhang Z, Zhang Y, Li H,
Cheng Y, Qin H, Zhang X, Ma H and Li Y: Screening therapeutic
targets of ribavirin in hepatocellular carcinoma. Oncol Lett.
15:9625–9632. 2018.PubMed/NCBI
|
|
63
|
Lewandowski M, Lipiński P, Bednarski I,
Mik M and Dziki A: Knowledge and awareness of colorectal cancer.
Pol Przegl Chir. 92:34–41. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sharen G, Li X, Sun J, Zhang L, Xi W, Zhao
X, Han F, Jia L, A R, Cheng H and Hou M: Silencing eL31 suppresses
the progression of colorectal cancer via targeting DEPDC1. J Transl
Med. 20:4932022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhao B, Wang Y, Zhao X, Ni J, Zhu X, Fu Y
and Yang F: SIRT1 enhances oxaliplatin resistance in colorectal
cancer through microRNA-20b-3p/DEPDC1 axis. Cell Biol Int.
46:2107–2117. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lou T, Zhang L, Jin Z, Miao C, Wang J and
Ke K: miR-455-5p enhances 5-fluorouracil sensitivity in colorectal
cancer cells by targeting PIK3R1 and DEPDC1. Open Med (Wars).
17:847–856. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wilkinson L and Gathani T: Understanding
breast cancer as a global health concern. Br J Radiol.
95:202110332022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhao H, Yu M, Sui L, Gong B, Zhou B, Chen
J, Gong Z and Hao C: High expression of DEPDC1 promotes malignant
phenotypes of breast cancer cells and predicts poor prognosis in
patients with breast cancer. Front Oncol. 9:2622019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhang L, Du Y, Xu S, Jiang Y, Yuan C, Zhou
L, Ma X, Bai Y, Lu J and Ma J: DEPDC1, negatively regulated by
miR-26b, facilitates cell proliferation via the up-regulation of
FOXM1 expression in TNBC. Cancer Lett. 442:242–251. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Sekhoacha M, Riet K, Motloung P, Gumenku
L, Adegoke A and Mashele S: Prostate cancer review: Genetics,
diagnosis, treatment options, and alternative approaches.
Molecules. 27:57302022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ramalho-Carvalho J, Martins JB, Cekaite L,
Sveen A, Torres-Ferreira J, Graça I, Costa-Pinheiro P, Eilertsen
IA, Antunes L, Oliveira J, et al: Epigenetic disruption of miR-130a
promotes prostate cancer by targeting SEC23B and DEPDC1. Cancer
Lett. 385:150–159. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Badwelan M, Muaddi H, Ahmed A, Lee KT and
Tran SD: Oral squamous cell carcinoma and concomitant primary
tumors, what do we know? A review of the literature. Curr Oncol.
30:3721–3734. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Qiu J, Tang Y, Liu L, Yu J, Chen Z, Chen H
and Yuan R: FOXM1 is regulated by DEPDC1 to facilitate development
and metastasis of oral squamous cell carcinoma. Front Oncol.
12:8159982022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Huang G, Chen S, Washio J, Paka Lubamba G,
Takahashi N and Li C: Glycolysis-related gene analyses indicate
that DEPDC1 promotes the malignant progression of oral squamous
cell carcinoma via the WNT/β-catenin signaling pathway. Int J Mol
Sci. 24:19922023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Pietras W: Advances and changes in the
treatment of children with nephroblastoma. Adv Clin Exp Med.
21:809–820. 2012.PubMed/NCBI
|
|
76
|
Geng G, Li Q, Guo X, Ni Q, Xu Y, Ma Z,
Wang Y and Ming M: FOXO3a-modulated DEPDC1 promotes malignant
progression of nephroblastoma via the Wnt/β-catenin signaling
pathway. Mol Med Rep. 26:7272022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Geng G, Xu Y, Li Q, Li Q, Yuan L, Dong M
and Ming M: S100A16 cooperates with DEPDC1 to promote the
progression and angiogenesis of nephroblastoma through
PI3K/Akt/mTOR pathway. Pol J Pathol. 74:182–193. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Feng X, Zhang C, Zhu L, Zhang L, Li H, He
L, Mi Y, Wang Y, Zhu J and Bu Y: DEPDC1 is required for cell cycle
progression and motility in nasopharyngeal carcinoma. Oncotarget.
8:63605–63619. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kikuchi R, Sampetrean O, Saya H, Yoshida K
and Toda M: Functional analysis of the DEPDC1 oncoantigen in
malignant glioma and brain tumor initiating cells. J Neurooncol.
133:297–307. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Yang M, Zhang H, Gao S and Huang W: DEPDC1
and KIF4A synergistically inhibit the malignant biological behavior
of osteosarcoma cells through Hippo signaling pathway. J Orthop
Surg Res. 18:1452023. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Peng WX, Koirala P and Mo YY:
LncRNA-mediated regulation of cell signaling in cancer. Oncogene.
36:5661–5667. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Cui XY, Zhan JK and Liu YS: Roles and
functions of antisense lncRNA in vascular aging. Ageing Res Rev.
72:1014802021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yang L, Wu Y, Xu H, Zhang J, Zheng X,
Zhang L, Wang Y, Chen W and Wang K: Identification and validation
of a novel six-lncRNA-based prognostic model for lung
adenocarcinoma. Front Oncol. 11:7755832022. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Li N, Yu K, Huang D, Li S, Zeng D, Li J
and Fan L: Molecular characterization of cuproptosis-related
lncRNAs: Defining molecular subtypes and a prognostic signature of
ovarian cancer. Biol Trace Elem Res. 202:1428–1445. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Lu L, Liu LP, Zhao QQ, Gui R and Zhao QY:
Identification of a ferroptosis-related LncRNA signature as a novel
prognosis model for lung adenocarcinoma. Front Oncol.
11:6755452021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Xu W, Wang J, Xu J, Li S, Zhang R, Shen C,
Xie M, Zheng B and Gu M: Long non-coding RNA DEPDC1-AS1 promotes
proliferation and migration of human gastric cancer cells HGC-27
via the human antigen R-F11R pathway. J Int Med Res.
50:30006052210931352022. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Mattick JS, Amaral PP, Carninci P,
Carpenter S, Chang HY, Chen LL, Chen R, Dean C, Dinger ME,
Fitzgerald KA, et al: Long non-coding RNAs: Definitions, functions,
challenges and recommendations. Nat Rev Mol Cell Biol. 24:430–447.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Arumugam P, Ramesh V, Sampathkumar B,
Perumalsamy H, Balusamy SR, Suganya K, Balraj S, Nachimuthu SK and
Sundaravadivelu S: Integrative transcriptome analysis of triple
negative breast cancer profiles for identification of druggable
targets. J Biomol Struct Dyn. 41:12106–12119. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wertz IE, Kusam S, Lam C, Okamoto T,
Sandoval W, Anderson DJ, Helgason E, Ernst JA, Eby M, Liu J, et al:
Sensitivity to antitubulin chemotherapeutics is regulated by MCL1
and FBW7. Nature. 471:110–114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Xiang Y, Zhang Q, Wei S, Huang C, Li Z and
Gao Y: Paeoniflorin: A monoterpene glycoside from plants of
Paeoniaceae family with diverse anticancer activities. J Pharm
Pharmacol. 72:483–495. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Xie L, Zhao YX, Zheng Y and Li XF: The
pharmacology and mechanisms of platycodin D, an active triterpenoid
saponin from Platycodon grandiflorus. Front Pharmacol.
14:11488532023. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Nelde A, Rammensee HG and Walz JS: The
peptide vaccine of the future. Mol Cell Proteomics. 20:1000222021.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Obara W, Ohsawa R, Kanehira M, Takata R,
Tsunoda T, Yoshida K, Takeda K, Katagiri T, Nakamura Y and Fujioka
T: Cancer peptide vaccine therapy developed from oncoantigens
identified through genome-wide expression profile analysis for
bladder cancer. Jpn J Clin Oncol. 42:591–600. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Obara W, Eto M, Mimata H, Kohri K,
Mitsuhata N, Miura I, Shuin T, Miki T, Koie T, Fujimoto H, et al: A
phase I/II study of cancer peptide vaccine S-288310 in patients
with advanced urothelial carcinoma of the bladder. Ann Oncol.
28:798–803. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Obara W, Hara I, Kato Y, Kato R, Inoue K,
Sato F, Mimata H, Nakamura Y and Fujioka T: Immunotherapy with
cancer peptides in combination with intravesical bacillus
Calmette-Guerin for patients with non-muscle invasive bladder
cancer. Cancer Immunol Immunother. 67:1371–1380. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Fujiwara Y, Okada K, Omori T, Sugimura K,
Miyata H, Ohue M, Kobayashi S, Takahashi H, Nakano H, Mochizuki C,
et al: Multiple therapeutic peptide vaccines for patients with
advanced gastric cancer. Int J Oncol. 50:1655–1662. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Fujiwara Y, Sugimura K, Miyata H, Omori T,
Nakano H, Mochizuki C, Shimizu K, Saito H, Ashida K, Honjyo S, et
al: A pilot study of post-operative adjuvant vaccine for advanced
gastric cancer. Yonago Acta Med. 60:101–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Daiko H, Marafioti T, Fujiwara T,
Shirakawa Y, Nakatsura T, Kato K, Puccio I, Hikichi T, Yoshimura S,
Nakagawa T, et al: Exploratory open-label clinical study to
determine the S-588410 cancer peptide vaccine-induced
tumor-infiltrating lymphocytes and changes in the tumor
microenvironment in esophageal cancer patients. Cancer Immunol
Immunother. 69:2247–2257. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Murahashi M, Hijikata Y, Yamada K, Tanaka
Y, Kishimoto J, Inoue H, Marumoto T, Takahashi A, Okazaki T, Takeda
K, et al: Phase I clinical trial of a five-peptide cancer vaccine
combined with cyclophosphamide in advanced solid tumors. Clin
Immunol. 166–167. 48–58. 2016.
|
|
100
|
Jonker DJ, O'Callaghan CJ, Karapetis CS,
Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ, et
al: Cetuximab for the treatment of colorectal cancer. N Engl J Med.
357:2040–2048. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Kikuchi R, Ueda R, Saito K, Shibao S,
Nagashima H, Tamura R, Morimoto Y, Sasaki H, Noji S, Kawakami Y, et
al: A pilot study of vaccine therapy with multiple glioma
oncoantigen/glioma angiogenesis-associated antigen peptides for
patients with recurrent/progressive high-grade glioma. J Clin Med.
8:2632019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Tsuruta M, Ueda S, Yew PY, Fukuda I,
Yoshimura S, Kishi H, Hamana H, Hirayama M, Yatsuda J, Irie A, et
al: Bladder cancer-associated cancer-testis antigen-derived long
peptides encompassing both CTL and promiscuous HLA class
II-restricted Th cell epitopes induced CD4+ T cells
expressing converged T-cell receptor genes in vitro.
Oncoimmunology. 7:e14156872018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Tosi A, Dalla Santa S, Cappuzzello E,
Marotta C, Walerych D, Del Sal G, Zanovello P, Sommaggio R and
Rosato A: Identification of a HLA-A*0201-restricted immunogenic
epitope from the universal tumor antigen DEPDC1. Oncoimmunology.
6:e13133712017. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Yatsuda J, Irie A, Harada K, Michibata Y,
Tsukamoto H, Senju S, Tomita Y, Yuno A, Hirayama M, Abu Sayem M, et
al: Establishment of HLA-DR4 transgenic mice for the identification
of CD4+ T cell epitopes of tumor-associated antigens. PLoS One.
8:e849082013. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Slingluff CL Jr, Lee S, Zhao F,
Chianese-Bullock KA, Olson WC, Butterfield LH, Whiteside TL, Leming
PD and Kirkwood JM: A randomized phase II trial of multiepitope
vaccination with melanoma peptides for cytotoxic T cells and helper
T cells for patients with metastatic melanoma (E1602). Clin Cancer
Res. 19:4228–4238. 2013. View Article : Google Scholar : PubMed/NCBI
|