Introduction
Lung cancer is the most common malignant tumor type
in the world (1). Large cell
neuroendocrine carcinoma (LCNEC) of the lung is a rare and highly
aggressive malignancy, with an overall age-adjusted incidence rate
of 0.3/100,000 in the United States between 2000–2013 (2). Characterized by rapid progression and
poor prognosis, LCNEC presents limited therapeutic options
(3). Although classified as a
non-small cell lung cancer (NSCLC), LCNEC shares more similarities
in biological behavior with SCLC (4). The high aggressiveness of LCNEC, along
with the difficulties in diagnosis and nonspecific symptoms in the
early stage of LCNEC, often lead to a stage IV diagnosis, reducing
surgical opportunities (5,6). Furthermore, the rarity of LCNEC
contributes to the lack of randomized clinical trial data.
Consequently, the current first-line treatment for LCNEC typically
involves a combination of platinum-etoposide chemotherapy and
immunotherapy or palliative radiotherapy, resulting in unexpected
5-year OS rates ranging from 15 to 57% (7).
Historically, immunotherapy had been deemed
unsuitable for lung cancer treatment due to weak immune responses
(8). However, the discovery of
immune checkpoints (ICPs) has revolutionized lung cancer treatment,
enabling the integration of immunotherapy with various therapeutic
strategies (9). ICPs are proteins
produced by immune cells (such as T cells) and cancer cells, which
result in cancer cells escaping immune-mediated tumor cell death;
the application of checkpoint inhibitors (CKIs) targeting ICPs has
yielded beneficial outcomes (9).
Programmed cell death protein 1 (PD-1), one of the
most extensively studied ICPs, and its corresponding CKIs (PD-1
monoclonal antibodies) have been incorporated into lung cancer
immunotherapy (10). PD-1
monoclonal antibodies have shown promising prospects in lung cancer
clinical trials and are recommended in oncological guidelines in
multiple countries (11). However,
due to the rarity of LCNEC, clinical trials evaluating the efficacy
of immunotherapy are currently focused on lung adenocarcinoma and
squamous lung cancer.
The present report presents a successful case of
LCNEC treatment, involving a perioperative treatment with
immunochemotherapy, which resulted in a pathological complete
response (pCR) post-surgery.
Case report
In March 2023, a 45-year-old man visited Fujian
Medical University Union Hospital (Fuzhou, China) to evaluate a
pulmonary mass incidentally identified during a routine medical
examination. The patient smoked an average of 30 cigarettes per day
for 30 years but denied any respiratory or systemic symptoms. The
patient experienced a significant weight loss of ~5 kg over the
previous 3 months, and clinical assessment revealed a body mass
index of 27.1 with an overweight status. The patient reported no
family history of cancer or chronic diseases and considered himself
generally healthy. Physical examination did not reveal any notable
abnormalities. Comprehensive laboratory tests indicated a markedly
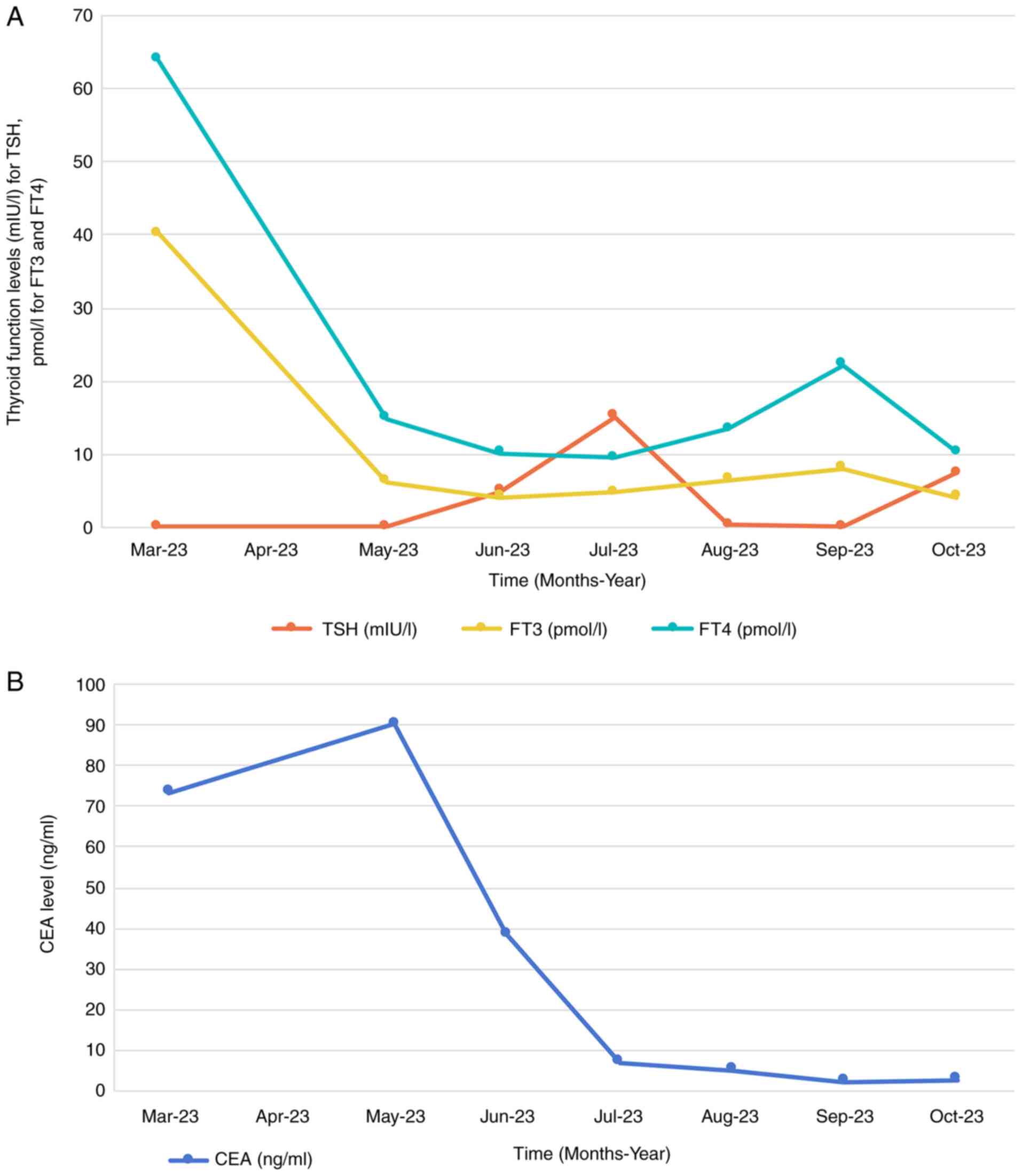
elevated carcinoembryonic antigen (CEA) level at 73.3 ng/ml
(Fig. 1B), 10-fold higher compared
with the upper normal limit (UNL) of the normal reference range of
<5 ng/ml. Thyroid function tests revealed suppressed thyroid
stimulating hormone (TSH) levels (<0.01 mIU/l) and elevated free
triiodothyronine (FT3) at 40.41 pmol/l (5-fold of UNL) and free
thyroxine (FT4) at 63.89 pmol/l (3-fold of UNL), suggestive of
hyperthyroidism (Fig. 1A). Chest
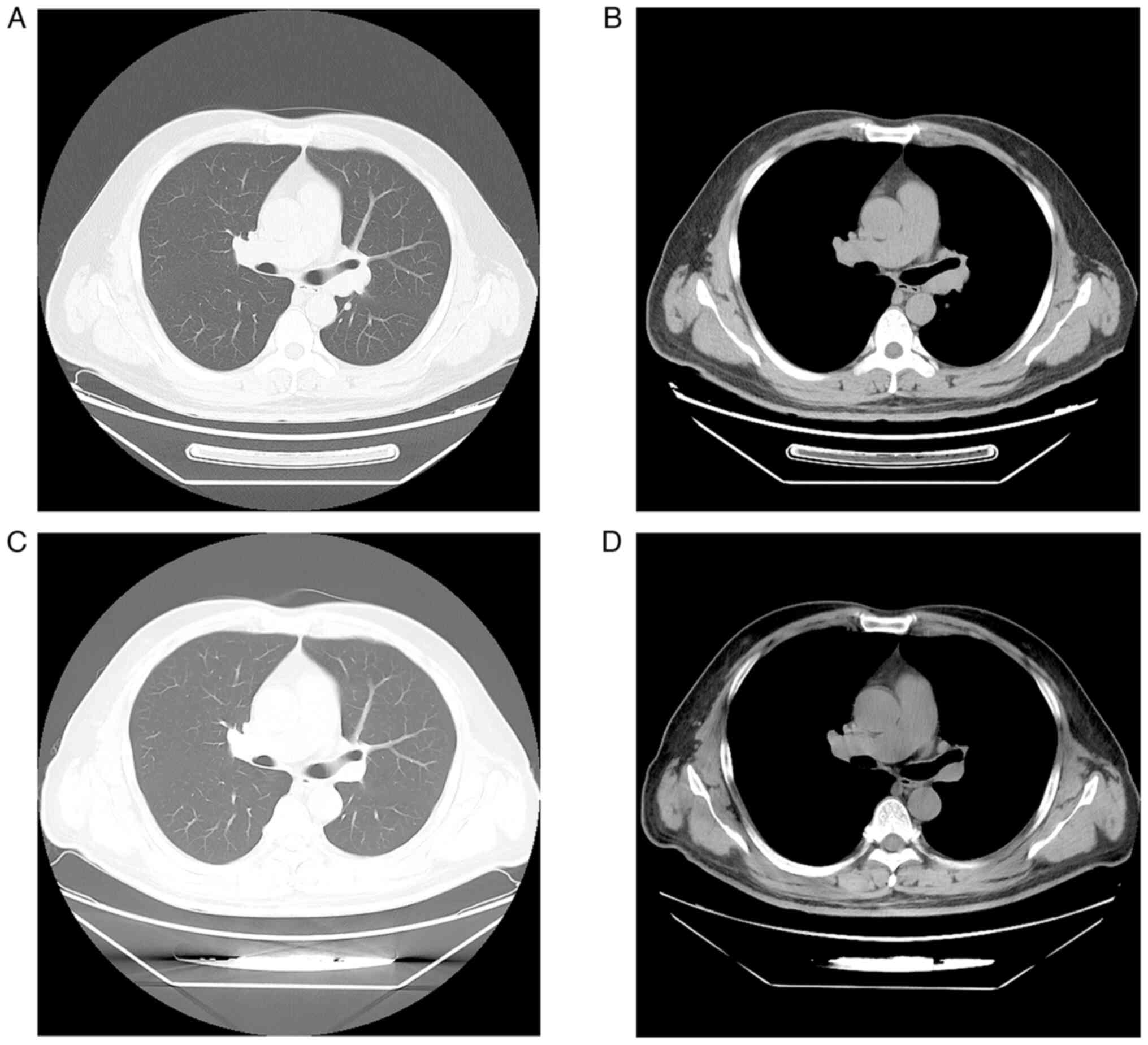
computed tomography (CT) scan revealed a mass in the dorsal segment
of the left lower lobe, measuring 0.8 cm, with enlarged lymph nodes
at the left hilum and mediastinum (Fig.
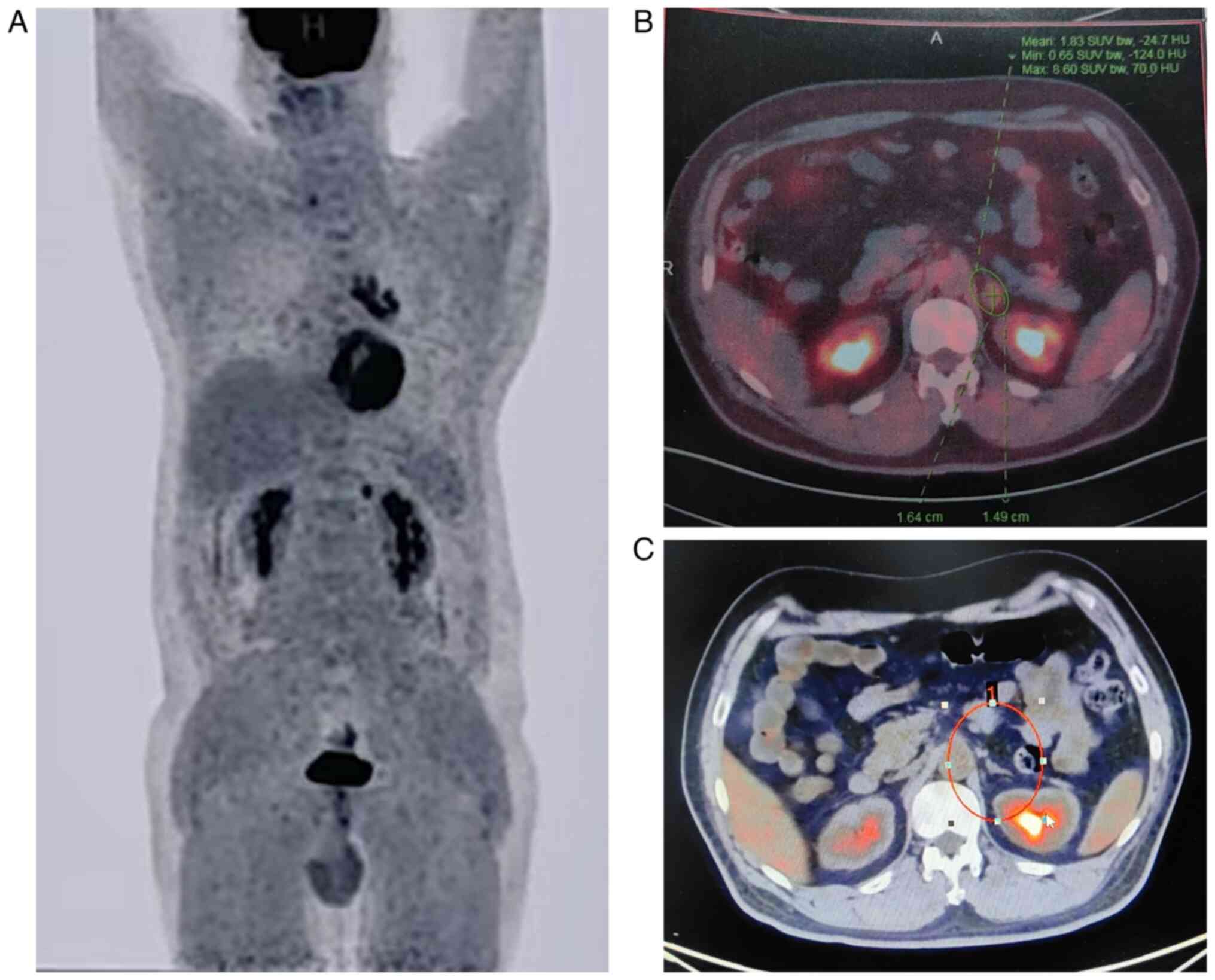
2A and B). Positron emission tomography-CT (PET-CT) scan showed
increased metabolic activity in the nodules in the left lower lobe,
mediastinal and left hilar lymph nodes, as well as a hypermetabolic
nodule in the left adrenal gland (Fig.
3A and B). Considering the small size of the pulmonary lesion
and the concurrent hyperthyroidism of the patient, which posed a
high anesthetic risk, an endobronchial ultrasound-guided
transbronchial needle aspiration was performed to obtain
mediastinal lymph node biopsy. However, the sample was insufficient
for a definitive diagnosis. After multidisciplinary consultation it
was decided to initially manage the thyroid hormone levels of the
patient with thiamazole tablets to minimize medical harm.
Subsequent thoracoscopic biopsy would be considered once the
thyroid condition was controlled.
In May 2023, thyroid function tests revealed
normalized levels of FT3 and FT4, with TSH remaining below
detectable limits, indicating effective management of the
hyperthyroidism (Fig. 1A). A slight
elevation in CEA levels was noted compared with 2 months prior
(Fig. 1B). Repeated chest and
abdominal CT scans showed no significant changes in the pulmonary
lesion, lymph nodes and adrenal nodule; brain MRI scans were
unremarkable (data not shown). Subsequently, the patient underwent
a thoracoscopic mediastinal lymph node biopsy. The tissue samples
were fixed in a 10% neutral formalin solution for 24 h, followed by
paraffin embedding and sectioning at a thickness of 4 µm. After
hematoxylin and eosin staining, the sections were examined using a
light microscope at a magnification of 200×. Histopathological
examination revealed heterogeneous tissue with poorly
differentiated, large cells and scattered necrotic areas (Fig. 4A). Tissue paraffin sections were
immersed in citrate buffer (pH 6.0) and heated in a microwave oven
for 15 min to induce epitope repair. The slides were then cooled
for 20 min, rinsed with distilled water and rehydrated in a
descending-gradient alcohol series. To block non-specific binding,
sections were incubated with 5% BSA (cat. no. A8850; Beijing
Solarbio Science & Technology Co., Ltd.) in PBS for 30 min at
room temperature. Antibodies for immunohistochemical analysis were
obtained from MXB Biotechnologies. thyroid transcription factor-1
(TTF-1) (1:200 dilution; cat. no. MAB-0677), cytokeratin (CK)
(1:200 dilution; cat. no. Kit-0009), chromogranin A (CgA) (1:100
dilution; cat. no. MAB-0707) and synaptophysin (Syn) (1:150
dilution; cat. no. MAB-0742) antibodies were diluted in PBS and
applied to the sections, which were then incubated at 4°C
overnight. After washing, sections were incubated with goat
anti-rabbit IgG H&L (HRP) (cat. no. ab6721; 1:200 dilution;
Abcam) for 1 h at room temperature. The sections were stained with
hematoxylin and observed under a light microscope at a
magnification of ×200. Immunohistochemical staining confirmed the
pulmonary origin and neuroendocrine characteristics of the tumor
cells: Positive for TTF-1, CK, CgA and Syn, but negative for
programmed death-ligand 1 (PD-L1) evaluated by 22C3 (cat. no.
SK006; 1:40 dilution; Dako; Agilent Technologies, Inc.) assays/kit
(Fig. 4B-E). Due to economic
factors, the patient underwent a few genetic tests against targeted
therapeutic markers including ALK, ROS1, RET, EGFR, KRAS, BRAF,
PIK3CA, HER2 and MET mutations, all of which were
negative.
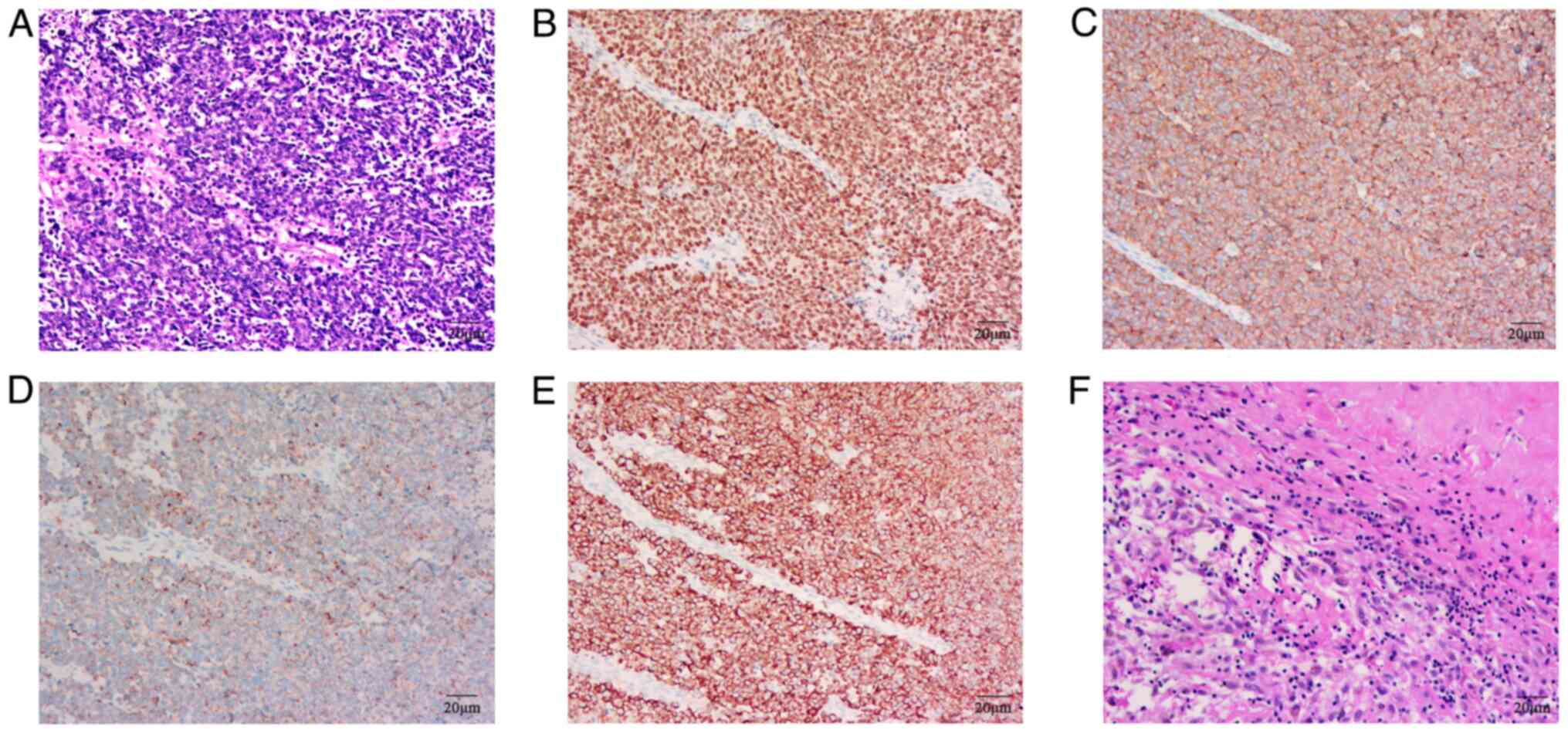 | Figure 4.HE and IHC staining of tumor. (A) A
HE-stained section of the lymph node biopsy at diagnosis. Positive
expression of (B) TTF-1, (C) Syn, (D) CgA and (E) CK through IHC,
respectively. (F) Surgically resected lymph node. Magnification,
200×. HE, hematoxylin-eosin; IHC, immunohistochemistry; TTF-1,
thyroid transcription factor-1; Syn, synaptophysin; CgA,
chromogranin A; CK, cytokeratin. |
Combining radiological and pathological findings,
the patient was diagnosed with stage IV LCNEC of the lung,
classified as cT1N2M1, with an Eastern Cooperative Oncology Group
performance status of 1.
Treatment and outcome
Typically, patients with stage IV cancer are not
considered surgical candidates. However, in the present case, the
patient expressed a strong desire for surgery. Given the slow
progression of the primary lesion and the general condition of the
patient, a multidisciplinary team decided to proceed with
neoadjuvant immunochemotherapy. The specific regimen included
nab-paclitaxel (400 mg intravenously) plus cisplatin (150 mg
intravenously) in combination with serplulimab (300 mg
intravenously) every 3 weeks for three cycles. Concurrently, the
patient continued thiamazole 20 mg per day to manage his thyroid
function. Post-neoadjuvant therapy, a repeat CT scan of the chest
and abdomen revealed no detectable nodule in the left lower lobe of
the lung where the primary lesion was located, a significant
reduction in mediastinal lymph nodes, and a shrunk of the adrenal
lesion (Fig. 2C and D). Another
multidisciplinary consultation considered that the patient might
benefit from surgical resection of the primary lung tumor. The
treatment strategy for the adrenal metastasis was left to be
determined based on the response of the tumor to the
immunochemotherapy. In August 2023, 31 days after completing three
cycles of the neoadjuvant immunochemotherapy, the patient underwent
left lower lobectomy and mediastinal lymphadenectomy. Postoperative
pathology revealed no residual cancer cells in the tumor bed or
lymph nodes (Fig. 4F).
Immunohistochemical staining results were negative for CK, Syn,
CgA, CD56, TTF-1 and P40, indicating a pCR.
Despite the clear benefits of neoadjuvant and
surgical treatments, the adrenal metastasis of the patient remained
a concern. Therefore, the treatment plan was extended to include
three additional cycles of adjuvant therapy using nad-paclitaxel
(400 mg intravenously, D1, q3w), cisplatin (150 mg intravenously,
D1, q3w) and serplulimab (300 mg intravenously, D1, q3w). This was
followed by maintenance therapy with serplulimab alone. Throughout
the treatment period, the patient experienced hypothyroidism
(decreased FT3 and FT4) in October 2023 and April 2024, and
hyperthyroidism (increased FT3 and FT4) in January 2024, both of
which were corrected with dose adjustments of the hyperthyroid
medication. Otherwise, the patient had no other adverse
effects.
As of July 2024, the patient has completed 17 cycles
of the serplulimab maintenance therapy, and the PET-CT scan
revealed a complete metabolic response of the adrenal metastasis,
without any specialized intervention (Fig. 3C). Event-free survival has exceeded
11 months, and overall survival has exceeded 14 months. The
radiological scans showed no visible adrenal metastasis, no
recurrence of the pulmonary lesion, and no evidence of distant
metastasis, indicating a clinical cure. The treatment journey of
this patient underscores the potential of tailored, multimodal
oncological approaches in managing advanced lung cancer cases, even
in cases that are traditionally deemed non-operable.
Discussion
LCNEC of the lung, originating from pulmonary
argentaffin cells, is a rare and aggressive malignant tumor
characterized by neuroendocrine morphology and differentiation
(12,13). In 2021, the World Health
Organization (WHO) classified LCNEC as a neuroendocrine carcinoma,
highlighting its molecular subtyping, potentially contributing to
the diagnosis and treatment (14).
Although LCNEC is categorized as a type of NSCLC, it exhibits
increased invasiveness and malignancy compared with other NSCLCs,
with biological behavior similar to SCLC (15). Additionally, LCNEC is prone to
chemoresistance, metastasis and recurrence, leading to poor
prognosis (16). Due to its rarity,
research on LCNEC is limited, resulting in a lack of
epidemiological data and standardized treatment. Therefore, further
research and clinical trials are necessary to explore effective
treatment strategies and improve prognosis for patients with
LCNEC.
Biomarkers play a pivotal role in the diagnosis of
malignancies. In LCNEC, serum biomarkers such as CEA and
pro-gastrin-releasing peptide are elevated (17). Additionally, neuron-specific enolase
(NSE), a specific neuroendocrine marker for lung neuroendocrine
tumors, is also increased in LCNEC cases (17). A study by Iyoda et al
(18) analyzed serum biomarkers in
LCNEC, identifying significant elevations in lactate dehydrogenase
(LDH), tissue polypeptide antigen, CEA and NSE, with a minority of
patients showing increased levels of α-fetoprotein (AFP), CA199 and
CA125. These changes in biomarkers may contribute to diagnosing
LCNEC, although further epidemiological validation is required. The
histology of LCNEC typically reveals distinct necrotic areas, large
cells with abundant cytoplasm and high proliferative nuclei
features (19). IHC analysis
frequently demonstrates diffuse positivity for specific
neuroendocrine markers such as CgA, Syn and CD56 in LCNEC (17,19,20).
Approximately 84.4% of LCNEC cases express CK, and 54.8% express
TTF-1 (21). Sturm et al
(22) have suggested that TTF-1
expression may differentiate LCNEC from basaloid carcinoma. In the
present case, the tumor histopathology revealed prominent large
cells with scattered necrotic areas, and positive staining for
TTF-1, CK, CgA and Syn. Considering the presence of hypermetabolic
nodules in the mediastinum and adrenal gland, the patient was
diagnosed with stage IV LCNEC based on the 2021 WHO criteria for
LCNEC (14). Additionally, the
patient did not exhibit any definitive association of genotypic
profiles with targeted therapies.
Currently, research on genetic mutation in LCNEC
remains limited and lacks representativeness. Given the reliance of
tumor cells on aberrant signaling pathways and survival mechanisms
conferred by mutated genes, the disruption of these pathways
typically results in cell cycle arrest or apoptosis (23). Statistics indicate the potential
presence of mutations in genes such as TP53, RB1, STK11, KEAP1,
KRAS, MEN1 and EGFR in LCNEC, and the mutation frequencies are
markedly different in a previous study (21), with TP53 (82.3%) being the most
common. In 2021, the WHO underscored the importance of genotypic
profiling in LCNEC, suggesting that more studies on LCNEC
genotyping are crucial as they could provide valuable insights and
direction for subsequent treatment approaches (14). Additionally, the association between
high tumor mutational burden (TMB) value and survival benefit of
patients with lung cancer treated with ICIs has been shown in
CheckMate 026 (24) and CheckMate
227 (25). Smoking is a significant
risk factor for lung cancer, and previous study has shown that the
TMB value in smoking patients was significantly higher (26). Garassino et al (27) have indicated that patients who smoke
may benefit more from second-line immunotherapy than non-smoking
patients. However, the relationships between tobacco exposure, TMB
and survival benefits have not been yet fully elucidated.
Currently, there is no standardized treatment for
LCNEC. Treatment for LCNEC is based on multidisciplinary strategies
(including surgery, chemotherapy, radiotherapy, immunotherapy and
targeted therapy), and platinum-based chemotherapy plays a pivotal
role (16). Surgical intervention
is generally suitable for stage I–III patients (28); however, the high rate of local and
distant metastases (29), coupled
with the insidious onset of the disease, leads to most patients
being diagnosed at stage IV, resulting in adjuvant or multimodal
treatments (16,30). Studies have shown that
platinum-based regimens achieve an objective response rate of 59.1
to 73% in patients with LCNEC, significantly higher compared with
other chemotherapy and offering improved OS benefits (30–32).
The 2021 National Comprehensive Cancer Network guidelines recommend
chemotherapy combinations for locally unresectable or metastatic
lung neuroendocrine carcinomas (including LCNEC), and noted the
limited efficacy of second-line and subsequent therapies (33). Targeted treatments for LCNEC [such
as EGFR-TKIs (34,35), ALK inhibitors (36,37),
anti-VEGF drugs and anti-c-KIT drugs (38)] are still under investigation, with
their effectiveness in patients with LCNEC remaining contentious.
Moreover, due to the genetic heterogeneity among LCNEC patients,
targeted therapies are limited to personalized treatments (21).
PD-1 and its ligand PD-L1 combined with chemotherapy
are standard treatment strategies for most advanced NSCLC and SCLC
(39,40). However, the immunotherapy for LCNEC
remains uncertain, and the benefits of CKIs still require
exploration. Due to the rarity of LCNEC, there is little
information on PD-1/PD-L1 expression and the efficacy of CKIs in
LCNEC, and no clinical trials of immunotherapy have been conducted
in patients with LCNEC, so reports and studies on the use of PD-1
monoclonal antibodies in LCNEC are limited (41). Prospective data on ICP in LCNEC are
still lacking, and only some small-sample studies have evaluated
PD-1 and PD-L1 expression in patients with LCNEC. Some studies have
shown that the majority of patients included in LCNEC exhibit
PD-1/PD-L1 positivity (42,43), but another portion of the studies
have shown PD-L1 positivity in only a minority of samples (44,45). A
retrospective study found only 10.4% of patients with LCNEC express
PD-L1 (44). Such a discrepancy is
most likely due to the small number of LCNEC samples included and
warrants further study to determine the potential value of PD-1
applied to immunotherapy.
In the absence of specific clinical trials for
patients with LCNEC, current data on the efficacy of immunotherapy
for LCNEC come primarily from a small number of studies in a small
sample of patients treated with CKIs. The University of Kentucky
treated three patients with LCNEC with nivolumab after
platinum-based chemotherapy progression disease, and all of whom
achieved either a complete radiological response or stable disease
(46). Levra et al (47) reviewed prognostic data on 10
patients with LCNEC treated with CKIs, of whom 6/10 had partial
responses and 1/10 had stable disease with a median PFS of 57
weeks. A retrospective study reported the efficacy of nivolumab in
17 pretreated patients with stage III–IV LCNEC, showing a prolonged
OS as second-line treatment or beyond (48). In addition, Zhang et al
(49) described a case of a
PD-L1-negative patient whose disease progressed rapidly after
surgery and adjuvant chemotherapy, but the patient achieved a
complete response during nivolumab treatment. Sato et al
(50) reported another patient with
stage IV LCNEC negative for PD-L1 expression who responded to
nivolumab. A retrospective study involving 37 patients with LCNEC,
11 were PD-L1 positive and received nivolumab or pembrolizumab
treatment, with 60% achieving partial response (51). Another retrospective study that
included 661 stage IV patients with LCNEC, 37 of whom underwent
immunotherapy, evaluated the efficacy of immunotherapy and showed
that immunotherapy is associated with improved overall survival,
but propensity scores on the overall survival analysis show a
non-significant trend in favor of immunotherapy (52). Although all of these studies have
limitations and the relevance of LCNEC to immunotherapy is still
under investigation, the results of all of the aforementioned
studies suggest that PD-1/PD-L1 expression and the use of CKIs have
potential in the treatment of LCNEC.
Additionally, Wang et al (39) reported a case of a PD-1-positive but
PD-L1-negtive patient with LCNEC with disease progression after
postoperative adjuvant chemotherapy, who was subsequently treated
with pembrolizumab, with all visible lesions shrinking after one
cycle and no new lesions; at 6 months later, the patient was still
on pembrolizumab and continued to improve. This parallels the
present case, where the PD-L1 negative patient showed sensitivity
to perioperative immunochemotherapy with serplulimab, achieving
complete response after three cycles of neoadjuvant therapy.
Notably, the present patient with stage IV LCNEC was a
controversial candidate for surgical intervention. However, with
the favorable response of neoadjuvant treatment, as well as the
patient's strong desire for surgery, the present study conducted
the surgical resection of the primary lung tumor. Postoperative
pathology confirmed a pCR, demonstrating the therapeutic potential
of immunochemotherapy in the neoadjuvant setting. Following
surgery, combined with adjuvant immunochemotherapy and radiotherapy
targeting the adrenal metastasis, the patient experienced the
disappearance of primary and metastatic lesions without recurrence,
achieving a clinical cure.
Abnormal thyroid function is one of the common
immune-related adverse reactions with an incidence of ~25%
(53). Its main manifestations are
hypothyroidism, hyperthyroidism or transient thyroiditis, but the
mechanism of occurrence remains unclear (54). It has been shown that the
interaction of PD-1-expressing lymphocytes and PD-L1-expressing
thyroid cells can inhibit T-cell-mediated autoimmune responses,
thereby protecting the thyroid gland (55). However, PD-1 monoclonal antibodies
may block this interaction, thereby inducing T-lymphocyte
infiltration within the thyroid gland, leading to abnormal thyroid
function (56). In the present
case, given that: i) The patient had a clear history of
hyperthyroidism; ii) the thyroid function abnormalities of the
patient during the treatment period showed alternating
hyperthyroidism and hypothyroidism; and iii) that the thyroid
function of the patient returned to normal after adjusting the
dosage of the hyperthyroid medication, the present study
hypothesized that the thyroid function abnormalities of the patient
were mainly related to the dosage adjustment of the hyperthyroid
medication, and that there was no clear and direct relationship
with the immunotherapy.
The present case report is limited to a single case,
and the detection of targeted therapeutic markers and ICPs in
patients is not perfect. From our results, genetic testing did not
provide a strong contribution to the subsequent treatment plan of
the patient. Also, the present study was unable to indicate whether
PD-1/PD-L1 could be used as a support for determining the treatment
plan of the patient in this case. In addition, the follow-up of
this patient was slightly shorter. Due to the rarity of LCNEC,
there is an urgent need to investigate clinical trials of
serplulimab in combination with chemotherapy in larger cohorts of
patients with LCNEC to clarify the relationship between ICPs, CKIs
and patient management and prognosis.
In conclusion, the present study provided novel
evidence for the efficacy of immunochemotherapy in the
perioperative treatment of patients with stage IV LCNEC, offering a
novel approach to LCNEC management.
Acknowledgements
Not applicable.
Funding
The study was supported by the Key Laboratory of Cardio-Thoracic
Surgery (Fujian Medical University) of Fujian Province University
(grant no. 2019-67) and Wu Jieping Medical Foundation (grant no.
320.6750.2022-17-21).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SZ, CL and JD conceived and designed the study,
analyzed and interpreted the data and approved the final version of
the manuscript. MC performed follow-up of the case and drafted and
critically revised the manuscript. YH and MC extracted the images
and acquired the laboratory and clinical data. CC wrote the
manuscript and acquired the laboratory and clinical data. BZ
critically revised the manuscript for intellectual content and
conceived and designed the study. All authors confirmed the
authenticity of all the raw data, and have read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient to publish this case report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
LCNEC
|
large cell neuroendocrine
carcinoma
|
|
pCR
|
pathological complete response
|
|
ICPs
|
immune checkpoints
|
|
CKIs
|
checkpoint inhibitors
|
|
PD-l
|
programmed cell death protein 1
|
|
CEA
|
carcinoembryonic antigen
|
|
UNL
|
upper normal limit
|
|
TSH
|
thyroid stimulating hormone
|
|
FT3
|
triiodothyronine
|
|
FT4
|
thyroxine
|
|
CT
|
computed tomography
|
|
PET-CT
|
positron emission tomography-computed
tomography
|
|
TTF-1
|
thyroid transcription factor-1
|
|
CK
|
cytokeratin
|
|
CgA
|
chromogranin A
|
|
Syn
|
synaptophysin
|
|
PD-L1
|
programmed death-ligand 1
|
|
NSCLC
|
non-small cell lung cancer
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Deng C, Wu SG and Tian Y: Lung large cell
neuroendocrine carcinoma: An analysis of patients from the
surveillance, epidemiology, and End-Results (SEER) database. Med
Sci Monit. 25:3636–3646. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fasano M, Della Corte CM, Papaccio F,
Ciardiello F and Morgillo F: Pulmonary large-cell neuroendocrine
carcinoma: From epidemiology to therapy. J Thorac Oncol.
10:1133–1141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kinslow CJ, May MS, Saqi A, Shu CA,
Chaudhary KR, Wang TJC and Cheng SK: Large-Cell neuroendocrine
carcinoma of the lung: A population-based study. Clin Lung Cancer.
21:e99–e113. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Derks JL, Hendriks LE, Buikhuisen WA,
Groen HJ, Thunnissen E, van Suylen RJ, Houben R, Damhuis RA, Speel
EJ and Dingemans AM: Clinical features of large cell neuroendocrine
carcinoma: A population-based overview. Eur Respir J. 47:615–624.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fisseler-Eckhoff A and Demes M:
Neuroendocrine tumors of the lung. Cancers (Basel). 4:777–798.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iyoda A, Hiroshima K, Moriya Y, Iwadate Y,
Takiguchi Y, Uno T, Nakatani Y and Yoshino I: Postoperative
recurrence and the role of adjuvant chemotherapy in patients with
pulmonary large-cell neuroendocrine carcinoma. J Thorac Cardiovasc
Surg. 138:446–453. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Raez LE, Cassileth PA, Schlesselman JJ,
Sridhar K, Padmanabhan S, Fisher EZ, Baldie PA and Podack ER:
Allogeneic vaccination with a B7.1 HLA-A gene-modified
adenocarcinoma cell line in patients with advanced non-small-cell
lung cancer. J Clin Oncol. 22:2800–2807. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lahiri A, Maji A, Potdar PD, Singh N,
Parikh P, Bisht B, Mukherjee A and Paul MK: Lung cancer
immunotherapy: Progress, pitfalls, and promises. Mol Cancer.
22:402023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Steven A, Fisher SA and Robinson BW:
Immunotherapy for lung cancer. Respirology. 21:821–833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, DeCamp M,
et al: NCCN Guidelines® Insights: Non-Small cell lung
cancer, version 2.2023. J Natl Compr Canc Netw. 21:340–350. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Travis WD: Advances in neuroendocrine lung
tumors. Ann Oncol. 21 (Suppl 7):vii65–vii71. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Asamura H, Kameya T, Matsuno Y, Noguchi M,
Tada H, Ishikawa Y, Yokose T, Jiang SX, Inoue T, Nakagawa K, et al:
Neuroendocrine neoplasms of the lung: A prognostic spectrum. J Clin
Oncol. 24:70–76. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nicholson AG, Tsao MS, Beasley MB, Borczuk
AC, Brambilla E, Cooper WA, Dacic S, Jain D, Kerr KM, Lantuejoul S,
et al: The 2021 WHO classification of lung tumors: Impact of
advances since 2015. J Thorac Oncol. 17:362–387. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kupeli M and Koseoglu RD: Large cell
carcinoma with adenocarcinoma in lung. J Coll Physicians Surg Pak.
28:240–242. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Welter S, Aigner C and Roesel C: The role
of surgery in high grade neuroendocrine tumours of the lung. J
Thorac Dis. 9 (Suppl 15):S1474–S1483. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eichhorn F, Dienemann H, Muley T, Warth A
and Hoffmann H: Predictors of survival after operation among
patients with large cell neuroendocrine carcinoma of the lung. Ann
Thorac Surg. 99:983–989. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iyoda A, Hiroshima K, Toyozaki T, Haga Y,
Fujisawa T and Ohwada H: Clinical characterization of pulmonary
large cell neuroendocrine carcinoma and large cell carcinoma with
neuroendocrine morphology. Cancer. 91:1992–2000. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Travis WD: Pathology and diagnosis of
neuroendocrine tumors: Lung neuroendocrine. Thorac Surg Clin.
24:257–266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peifer M, Fernandez-Cuesta L, Sos ML,
George J, Seidel D, Kasper LH, Plenker D, Leenders F, Sun R, Zander
T, et al: Integrative genome analyses identify key somatic driver
mutations of small-cell lung cancer. Nat Genet. 44:1104–1110. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang L, Fan Y and Lu H: Pulmonary large
cell neuroendocrine carcinoma. Pathol Oncol Res. 28:16107302022.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sturm N, Lantuejoul S, Laverriere MH,
Papotti M, Brichon PY, Brambilla C and Brambilla E: Thyroid
transcription factor 1 and cytokeratins 1, 5, 10, 14 (34betaE12)
expression in basaloid and large-cell neuroendocrine carcinomas of
the lung. Hum Pathol. 32:918–925. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu C, Qin Q and Cong H: Research progress
on the relationship between mitochondrial deoxyguanosine kinase and
apoptosis and autophagy in lung adenocarcinoma cells. Cancer
Insight. 1:53–62. 2022. View Article : Google Scholar
|
|
24
|
Carbone DP, Reck M, Paz-Ares L, Creelan B,
Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F,
et al: First-Line nivolumab in Stage IV or recurrent Non-Small-Cell
lung cancer. N Engl J Med. 376:2415–2426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hellmann MD, Ciuleanu TE, Pluzanski A, Lee
JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers
S, Salman P, et al: Nivolumab plus ipilimumab in lung cancer with a
high tumor mutational burden. N Engl J Med. 378:2093–2104. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun LY, Cen WJ, Tang WT, Long YK, Yang XH,
Ji XM, Yang JJ, Zhang RJ, Wang F, Shao JY, et al: Smoking status
combined with tumor mutational burden as a prognosis predictor for
combination immune checkpoint inhibitor therapy in non-small cell
lung cancer. Cancer Med. 10:6610–6617. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Garassino MC, Gelibter AJ, Grossi F,
Chiari R, Soto Parra H, Cascinu S, Cognetti F, Turci D, Blasi L,
Bengala C, et al: Italian nivolumab expanded access program in
nonsquamous non-Small cell lung cancer patients: Results in
Never-Smokers and EGFR-mutant patients. J Thorac Oncol.
13:1146–1155. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Iyoda A, Makino T, Koezuka S, Otsuka H and
Hata Y: Treatment options for patients with large cell
neuroendocrine carcinoma of the lung. Gen Thorac Cardiovasc Surg.
62:351–356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoshida A, Kobayashi E, Kubo T, Kodaira M,
Motoi T, Motoi N, Yonemori K, Ohe Y, Watanabe SI, Kawai A, et al:
Clinicopathological and molecular characterization of
SMARCA4-deficient thoracic sarcomas with comparison to potentially
related entities. Mod Pathol. 30:797–809. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sarkaria IS, Iyoda A, Roh MS, Sica G, Kuk
D, Sima CS, Pietanza MC, Park BJ, Travis WD and Rusch VW:
Neoadjuvant and adjuvant chemotherapy in resected pulmonary large
cell neuroendocrine carcinomas: A single institution experience.
Ann Thorac Surg. 92:1180–1187. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tokito T, Kenmotsu H, Watanabe R, Ito I,
Shukuya T, Ono A, Nakamura Y, Tsuya A, Naito T, Murakami H, et al:
Comparison of chemotherapeutic efficacy between LCNEC diagnosed
using large specimens and possible LCNEC diagnosed using small
biopsy specimens. Int J Clin Oncol. 19:63–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun JM, Ahn MJ, Ahn JS, Um SW, Kim H, Kim
HK, Choi YS, Han J, Kim J, Kwon OJ, et al: Chemotherapy for
pulmonary large cell neuroendocrine carcinoma: Similar to that for
small cell lung cancer or non-small cell lung cancer? Lung Cancer.
77:365–370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shah MH, Goldner WS, Benson AB, Bergsland
E, Blaszkowsky LS, Brock P, Chan J, Das S, Dickson PV, Fanta P, et
al: Neuroendocrine and adrenal tumors, version 2.2021, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
19:839–868. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
De Pas TM, Giovannini M, Manzotti M,
Trifiro G, Toffalorio F, Catania C, Spaggiari L, Labianca R and
Barberis M: Large-cell neuroendocrine carcinoma of the lung
harboring EGFR mutation and responding to gefitinib. J Clin Oncol.
29:e819–e822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
George J, Walter V, Peifer M, Alexandrov
LB, Seidel D, Leenders F, Maas L, Muller C, Dahmen I, Delhomme TM,
et al: Integrative genomic profiling of large-cell neuroendocrine
carcinomas reveals distinct subtypes of high-grade neuroendocrine
lung tumors. Nat Commun. 9:10482018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Peters S, Camidge DR, Shaw AT, Gadgeel S,
Ahn JS, Kim DW, Ou SI, Perol M, Dziadziuszko R, Rosell R, et al:
Alectinib versus Crizotinib in untreated ALK-positive
non-small-cell lung cancer. N Engl J Med. 377:829–838. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hayashi N, Fujita A, Saikai T, Takabatake
H, Sotoshiro M, Sekine K and Kawana A: Large cell neuroendocrine
carcinoma harboring an anaplastic lymphoma kinase (ALK)
rearrangement with response to alectinib. Intern Med. 57:713–716.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Iyoda A, Travis WD, Sarkaria IS, Jiang SX,
Amano H, Sato Y, Saegusa M, Rusch VW and Satoh Y: Expression
profiling and identification of potential molecular targets for
therapy in pulmonary large-cell neuroendocrine carcinoma. Exp Ther
Med. 2:1041–1045. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang VE, Urisman A, Albacker L, Ali S,
Miller V, Aggarwal R and Jablons D: Checkpoint inhibitor is active
against large cell neuroendocrine carcinoma with high tumor
mutation burden. J Immunother Cancer. 5:752017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Weber MM and Fottner C: Immune checkpoint
inhibitors in the treatment of patients with neuroendocrine
neoplasia. Oncol Res Treat. 41:306–312. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ferrara MG, Stefani A, Simbolo M, Pilotto
S, Martini M, Lococo F, Vita E, Chiappetta M, Cancellieri A,
D'Argento E, et al: Large Cell Neuro-Endocrine carcinoma of the
lung: Current treatment options and potential future opportunities.
Front Oncol. 11:6502932021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fan Y, Ma K, Wang C, Ning J, Hu Y, Dong D,
Dong X, Geng Q, Li E and Wu Y: Prognostic value of PD-L1 and PD-1
expression in pulmonary neuroendocrine tumors. Onco Targets Ther.
9:6075–6082. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim HS, Lee JH, Nam SJ, Ock CY, Moon JW,
Yoo CW, Lee GK and Han JY: Association of PD-L1 Expression with
Tumor-Infiltrating immune cells and mutation burden in high-grade
neuroendocrine carcinoma of the lung. J Thorac Oncol. 13:636–648.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tsuruoka K, Horinouchi H, Goto Y, Kanda S,
Fujiwara Y, Nokihara H, Yamamoto N, Asakura K, Nakagawa K, Sakurai
H, et al: PD-L1 expression in neuroendocrine tumors of the lung.
Lung Cancer. 108:115–120. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Karim NA, Sendilnathan A, Eldessouki I,
Orr-Asman MA, Xie C, Wang J and Elnakat H: PS06.06 immune
checkpoint markers in lung large cell neuroendocrine carcinomas
(L-LCNEC). J Thoracic Oncol. 12:1583–S1584. 2017. View Article : Google Scholar
|
|
46
|
Chauhan A, Arnold SM, Kolesar J, Thomas
HE, Evers M and Anthony L: Immune checkpoint inhibitors in large
cell neuroendocrine carcinoma: Current status. Oncotarget.
9:14738–14740. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Levra MG, Mazières J, Valette CA, Molinier
O, Planchard D, Frappat V, Ferrer L, Toffart AC and Moro-Sibilot D:
P1.07-012 efficacy of immune checkpoint inhibitors in large cell
neuroendocrine lung cancer: Results from a french retrospective
cohort. J Thoracic Oncol. S12 (Suppl):S702–S703. 2017. View Article : Google Scholar
|
|
48
|
Agar C, Geier M, Leveiller G, Lamy R,
Bizec JL, Tiercin M, Bernier C, Robinet G, Lena H, Ricordel C, et
al: Brief report on the efficacy of nivolumab in patients with
previously treated advanced Large-Cell neuroendocrine cancer of the
lung. JTO Clin Res Rep. 2:1001292021.PubMed/NCBI
|
|
49
|
Zhang X, Sun Y, Miao Y and Xu S: Immune
checkpoint inhibitor therapy achieved complete response for
drug-sensitive EGFR/ALK mutation-negative metastatic pulmonary
large-cell neuroendocrine carcinoma with high tumor mutation
burden: A case report. Onco Targets Ther. 13:8245–8250. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Takimoto Sato M, Ikezawa Y, Sato M, Suzuki
A and Kawai Y: Large cell neuroendocrine carcinoma of the lung that
responded to nivolumab: A case report. Mol Clin Oncol. 13:43–47.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sherman S, Rotem O, Shochat T, Zer A,
Moore A and Dudnik E: Efficacy of immune check-point inhibitors
(ICPi) in large cell neuroendocrine tumors of lung (LCNEC). Lung
Cancer. 143:40–46. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Komiya T, Ravindra N and Powell E: Role of
immunotherapy in stage IV large cell neuroendocrine carcinoma of
the lung. Asian Pac J Cancer Prev. 22:365–370. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Barroso-Sousa R, Barry WT, Garrido-Castro
AC, Hodi FS, Min L, Krop IE and Tolaney SM: Incidence of endocrine
dysfunction following the use of different immune checkpoint
inhibitor regimens: A systematic review and meta-analysis. JAMA
Oncol. 4:173–182. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lee H, Hodi FS, Giobbie-Hurder A, Ott PA,
Buchbinder EI, Haq R, Tolaney S, Barroso-Sousa R, Zhang K, Donahue
H, et al: Characterization of thyroid disorders in patients
receiving immune checkpoint inhibition therapy. Cancer Immunol Res.
5:1133–1140. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhan L, Feng HF, Liu HQ, Guo LT, Chen C,
Yao XL and Sun SR: Immune Checkpoint Inhibitors-Related thyroid
dysfunction: Epidemiology, clinical presentation, possible
pathogenesis, and management. Front Endocrinol (Lausanne).
12:6498632021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang Y, Wang X, Cheng L and Zhang G:
Correlation analysis between thyroid function abnormality and
efficacy in patients with advanced Non-small cell lung cancer after
immunotherapy. Zhongguo Fei Ai Za Zhi. 26:369–376. 2023.(In
Chinese). PubMed/NCBI
|