Introduction
Cancer is among the most serious ailments
endangering human health, ranking second only to cardiovascular
disease (1). Ovarian cancer (OC) is
a heterogeneous group of malignancies of the fallopian tubes,
ovaries and abdominal cavity (2).
It is the fifth deadliest and eighth most common cancer affecting
females worldwide (3). Conventional
cancer treatments for OC include radiation, surgery, traditional
chemotherapy and invasive catheters (4). Epithelial OC (EOC) is the most common
type of gynecological malignancy (5). Despite great breakthroughs in EOC
therapy, patients frequently experience chemotherapeutic resistance
and disease relapse within five years, highlighting that improved
therapeutic options are necessary (6). Therefore, the accurate targeting of
treatment is important for improving patient prognosis.
Nanoparticles (NPs) typically range in diameter from
1 to 100 nm, with small NPs composed of only a few to several
hundred atoms (7,8). Nanomaterials (NMs) are widely utilized
in material science and nanotechnology due to their unique
properties, which differ from those of conventional materials. The
appeal of NPs for medical purposes lies in their special and
significant features, including a surface-to-mass proportion,
quantum nature and the capability of adsorbing and transporting
other compounds, including proteins, drugs and probes (9). The morphologies of NPs can be highly
varied and are indicative of their distinctive characteristics
(10). The nanoscale dimensions of
NPs render them suitable for biolabeling by enabling interaction
with biomolecules at both the surface and intracellular levels,
generating valuable signals and specific targets for diagnostic and
therapeutic applications (11). Due
to their unique features, NPs are highly valuable in various
applications, including tissue engineering, biomarker
identification and drug delivery systems (12). The value of NPs for medical purposes
may be attributed to various features, including their
surface-to-mass proportion, quantum nature and ability to adsorb
and transport other compounds, including proteins, drugs and
probes. As studies on NMs have become more prevalent, metal NPs
have been evaluated for a broad range of uses, including
electronics, catalysis and sensing (9,13,14).
Functionalized NPs, particularly those derived from metal NPs, have
the potential to serve as valuable biological probes for a range of
uses, including organic chemistry research tools, bioassays,
clinical diagnosis and cancer treatment (15). In addition, the use of NPs for drug
encapsulation is viewed as a promising and effective approach for
drug delivery (11).
Among different inorganic NPs, gold NPs (AuNPs) are
actively studied for their different biomedical applications. This
is mainly due to their stability, simple and easy synthesis,
low-cost preparation techniques, size-controllable synthesis,
biocompatibility, relatively easy surface modification and low
toxicity properties (16–18). In the present study, the features of
AuNPs, their potential in OC therapy and their contributions to
tumor treatment are reviewed.
AuNPs
Properties of AuNPs
AuNPs are ideal carriers as they can be
functionalized or modified with various chemical groups and are
inert to biological systems (19,20).
Owing to their biocompatibility and ability to be surface modified
with biocompatible molecules, AuNPs can be engineered to minimize
undesirable immune responses, such as antibody production (21–23).
Furthermore, AuNPs are resistant to oxidation and can be
synthesized by controlled crystallization methods, which provide
AuNPs with precise morphologies and advantageous size distributions
(14).
The electronic and optical properties of AuNPs can
be modulated by altering their shape, size, aggregation state and
surface chemistry (14). When AuNPs
are used as nanocarriers in various applications, chemical
modification is necessary. It is crucial that the surface
functionalization of the AuNPs is appropriate for the intended
usage, for example, to improve their stability and biocompatibility
while preventing aggregation (24).
The primary purposes of the surface modification of AuNPs include:
i) Stabilizing the AuNPs by the attachment of ligands to the AuNP
surface, ii) enabling additional functionalization reactions
through the bonding of linkers to the AuNP surface, and iii)
facilitating further functionalization or bioconjugation by
directly immobilizing functional ligands and biomolecules on the
AuNP surface, thereby expanding their application range (24–29)
(Table I).
 | Table I.Surface modification of AuNPs. |
Table I.
Surface modification of AuNPs.
| First author,
year | Surface
modification method | Mechanism | Function | (Refs.) |
|---|
| Ielo et al,
2021 | Secondary
modification | ‘Place exchange’ of
a thiol ligand | Introduces various
functionalities that may react via condensation | (24) |
| Xiao et al,
2018 | Physical
sorption | Physical sorption
of ligands or biomolecules on AuNP surfaces driven by electrostatic
and hydrophobic interactions | Modifies the AuNP
charge state and the degree of immobilization of functional
molecules | (26) |
| De Luca et
al, 2018 | Dative bonding and
formation of self-assembled monolayers | Thiolated ligands
densely bond to AuNP surfaces to produce self-assembled
monolayers | Stable capping of
the AuNPs prevents the coupling of other ligands and
biomolecules | (27) |
| Boyer et al,
2010 | Polymer
coating | Neutral polymers or
charged polymers are used to coat the AuNP surfaces | Steric repulsion or
repulsive electrostatic interactions, respectively, improve the
colloidal stability of the AuNPs | (28) |
| Presnova et
al, 2014 | Bioaffinity
immobilization of ligands | Synthesis of
chemically stable protein-@AuNP
conjugates by immobilization of affinity-bound biomolecules and
ligands | Allows biotinylated
nucleic acids, antibodies and aptamers to be immobilized | (29) |
Synthesis of AuNPs
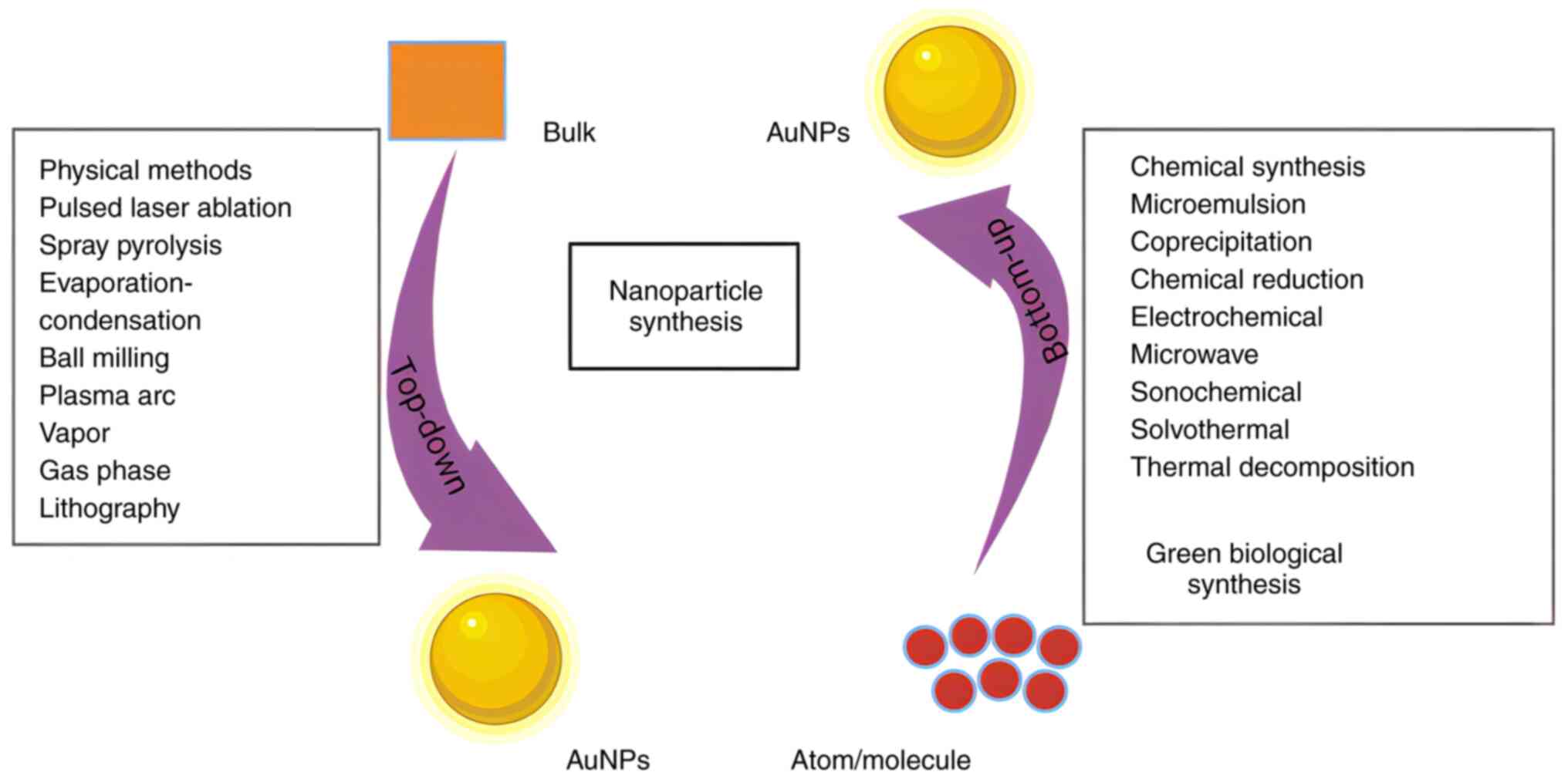
Approaches for synthesizing AuNPs can be categorized
as either ‘top-down’ or ‘bottom-up’ methods (30,31)
(Fig. 1). Top-down methods
typically involve producing NPs by reducing the size of bulk
materials. This serves as the basis for most physical approaches,
including pulsed laser ablation, plasma arc discharge,
evaporation-condensation, spray pyrolysis, ball milling, vapor and
gas phase processes, and lithographic techniques (32,33).
However, the unfinished surface structure of the resulting NPs
represents a disadvantage (34).
Another constraint of these top-down methods is their high costs,
as a substantial quantity of energy is needed to maintain
high-temperature and high-pressure conditions (24). Bottom-up methods are those that
generate NPs from smaller components, including atoms and
molecules, and include chemical synthesis techniques, including
microemulsion, coprecipitation, chemical reduction,
microwave-assisted synthesis, electrochemical, sonochemical,
solvothermal and thermal decomposition methods. Green biological
synthesis methods also fall into this category. Compared with
physical and chemical methods, the use of whole organisms or
biological molecules to synthesize NPs offers notable advantages.
Biological synthesis methods are nontoxic and relatively
sustainable, providing a comparatively environmentally friendly
approach to NP synthesis (30,35).
The synthesis of AuNPs by biological methods can be
a relatively straightforward process that does not require high
temperature or pressure. The procedure generally involves the
dropwise addition of a biological extract, such as that from
bacteria, fungi and/or plants, into a solution of HAuCl4
salt with thorough mixing to initiate AuNP synthesis (36). Subsequent formulation of the AuNPs
consists of two primary phases: In the first phase, the gold
precursor, typically in the form of an aqueous gold salt solution,
is reduced to form AuNPs using a reducing agent, such as citrate.
In the second phase, the AuNPs are stabilized by the introduction
of a capping agent, which prevents the agglomeration of the
metallic NPs (37).
The use of toxic reducing agents and the gases
produced by the process of producing NPs are harmful to humans and
the environment. Therefore, safer, nontoxic and environmentally
friendly methods for the generation of NPs have been devised, with
the use of reducing agents obtained from plant materials, including
leaves, roots, flowers and seeds (38). Modification of the reaction time,
pH, reaction temperature and fungal biomass can improve the
efficiency of the fungal synthesis of AuNPs (39). Commonly employed methods for the
characterization of AuNPs include atomic force microscopy, X-ray
powder diffraction, scanning electron microscopy, dynamic light
scattering, high-resolution transmission electron microscopy, zeta
potential, energy dispersive spectroscopy, Fourier transform
infrared spectroscopy and ultraviolet (UV)-visible spectroscopy
(40). Fig. 2 schematically illustrates various
methods for the synthesis, optimization, characterization and
conjugation of therapeutic agents with AuNPs. The existing
synthesis approaches often involve costly and low-yield
purification processes, such as differential centrifugation, to
obtain NPs (41). Therefore, the
development of nonpoisonous, eco-friendly and clean sustainable
synthesis procedures with high yields and low cost is critical
(42).
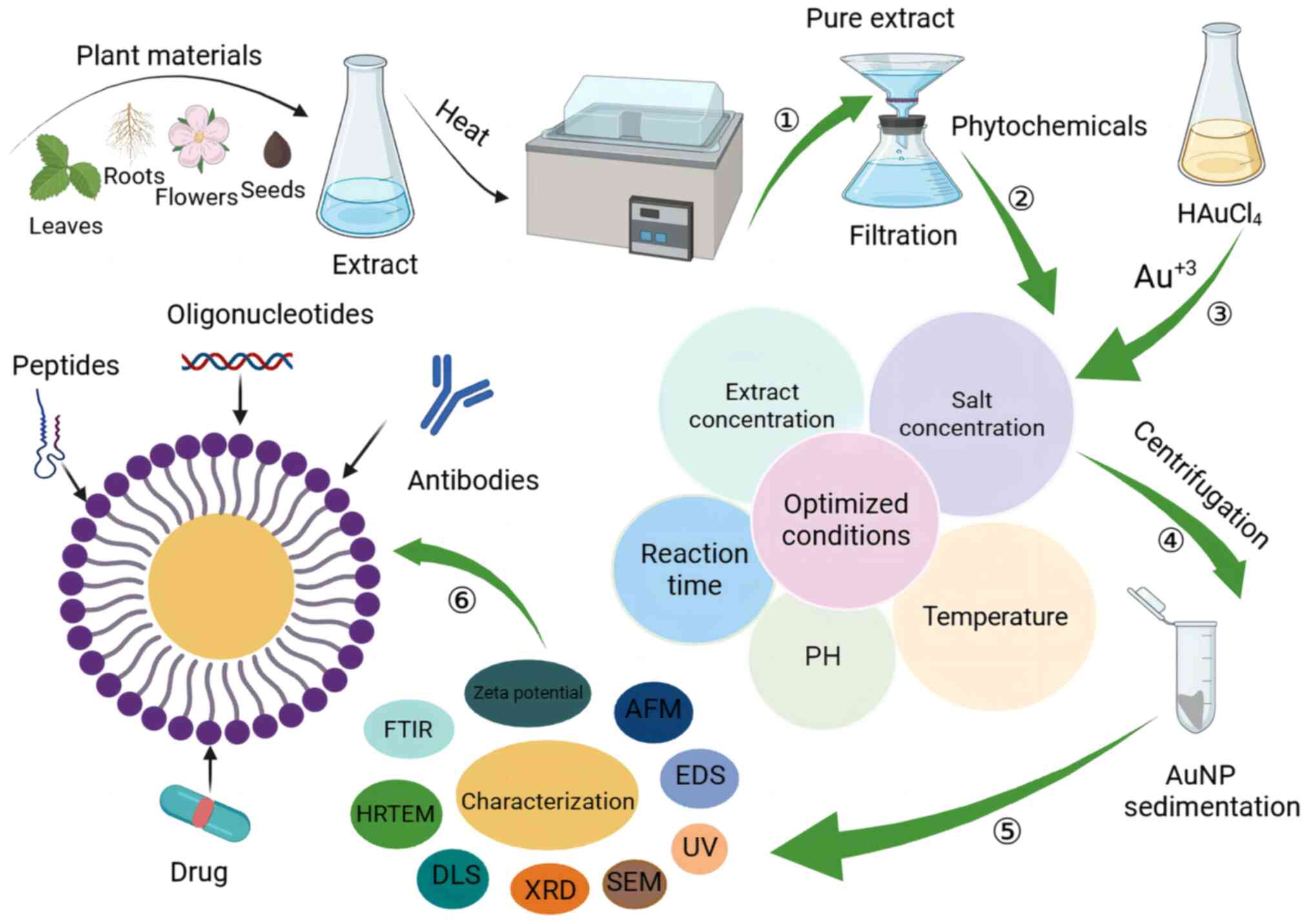 | Figure 2.Schematic illustration of the
synthesis, optimization and characterization of AuNPs and their
conjugation with therapeutic agents. In step 1, an extract of plant
material is obtained. Steps 2 and 3 involve optimizing the
synthesis of AuNPs by adjusting various reaction parameters. In
step 4, the reaction mixture is centrifuged to obtain AuNPs in the
form of a pellet. In step 5, thorough characterization and
elucidation of AuNP properties, including morphology and size, is
performed. In step 6, functional groups are attached. AuNPs with
appropriate characteristics and high stability can then be
conjugated with therapeutic agents, such as peptides, drugs,
antibodies and oligonucleotides. Figure created with BioRender
software (BioRender.com). AuNPs, gold nanoparticles; AFM, atomic
force microscopy; EDS, energy-dispersive X-ray spectroscopy; UV,
ultraviolet; SEM, scanning electron microscopy; XRD, X-ray
diffraction; DLS, dynamic light scattering; HRTEM, high-resolution
transmission electron microscopy; FTIR, Fourier-transform infrared
spectroscopy. |
Synthesis and stabilization procedures for precious
metal-based NPs using plant extracts are regarded to be safe,
economical, eco-friendly and green (43). In one study, AuNP formation was
realized at ambient temperature by mixing thyme extract with gold
salts. The reaction could be scaled by the adjustment of various
reaction conditions, particularly temperature (44). A number of other studies have also
synthesized AuNPs by methods using plant extracts or other
biological materials, such as fungi and bacteria, to reduce metal
salts and obtain bio-friendly, stable metal-based NPs (45,46).
For example, the microbial synthesis of AuNPs was first reported in
1980, with the use of Bacillus subtilis (47).
Table II presents
some other examples of AuNPs that have been synthesized (6,48–63).
 | Table II.Examples of AuNP synthesis and
characterization. |
Table II.
Examples of AuNP synthesis and
characterization.
| First author/s,
year | Name | Morphology | Size, nm | Ingredients |
Characterization | (Refs.) |
|---|
| Lee et al,
2022 | HA-AuDEN-Dox | Spherical | <2 | HAuCl4,
G6-NH2 dendrimer solution, NaBH4, HA,
Dox | UV-vis,
fluorescence spectroscopy, TEM, DLS and zeta potential | (49) |
| Lee et al,
2020 | Dox-DNA-AuNP | - | 13 | HAuCl4,
sodium citrate, DNA, sodium chloride, sodium phosphate buffer,
Dox | UV-vis, zeta
potential, DLS and fluorescence spectroscopy | (48) |
| Kotcherlakota et
al, 2017 | Au-TR-DX-si | Spherical | 105 | HAuCl4,
NaBH4, Dox, erbB2 siRNA, TR | UV-vis | (50) |
| Kotcherlakota et
al, 2019 | Au-C225-p53DNA | Spherical | 52.3±2.6 | HAuCl4,
C225, polyethylene imine, p53 DNA | UV-vis | (51) |
| Piktel et
al, 2021 | Peanut-shaped
AuNPs | Peanut-like |
60.00±4.24×30±3.49 | Cetrimonium
bromide, HAuCl4, AgNO3, NaBH4,
ascorbic acid | UV-vis | (6) |
| Piktel et
al, 2021 | AuP@CSA-131 | Peanut-like | 60±5×30±3.5 | CTAB,
HAuCl4, NaBH4, AgNO3, MHDA | HAADF-STEM,
Fourier-transform Raman spectroscopy and TGA | (52) |
| Jabir et al,
2020 | LG/LGC | - | 11/13 | HAuCl4,
NaBH4, GSH, linalool/CALNN | UV
spectrophotometry, SEM and TEM | (53) |
| Asl et al,
2023 | AuNPs | Spherical | 15.1±3.7 | HAuCl4,
NaOH, dimethyl sulfoxide, Satureja rechingeri Jamzad aqueous
leaf extract | XRD, FTIR, UV-vis,
TEM, SEM, EDX, DLS and zeta potential | (54) |
| Xiong et al,
2014 | AuNPs | - | 20 | HAuCl4
trihydrate, trisodium citrate, NaBH4 | DLS and zeta
potential | (55) |
| Kip et al,
2022 | AuNCs | Cone-shaped | 100 | HAuCl4,
o-phenetidine, hexane | UV-vis, DLS and
STEM | (56) |
| Patra et al,
2010 | Au-PSH-CP-FA | - | 5 | HAuCl4,
NaBH4, CP, FA, tritiated FA,
[3H]thymidine | UV-vis, TEM and
ICP | (57) |
| Borghei and
Hosseinkhani, 2022 | Wh@AuNPs | - | - | HAuCl4,
whey, 3,3′,5,5′-tetramethylbenzidine | UV-vis | (58) |
| Wang et al,
2014 | 15P-PPy-NPs | - | - | AuNPs, pyrrole
aqueous solution, SDS, acidic
(NH4)2S2O8 solution,
EDC | UV-vis and TEM | (59) |
| Shen et al,
2022 | RHMH18@AuD NPs | - | Varies with
reaction time and HAuCl4 concentration | RHMH18
protein, HAuCl4, NaOH, phosphate-buffered saline,
DTX | TEM | (60) |
| Van de Broek et
al, 2011 | Branched AuNPs | Branched | 60.4±9.7 | HAuCl4,
sodium citrate, BSPP, H2O2, NaOH, HCl,
HNO3, anti-HER2 | UV-vis | (61) |
| Geng et al,
2011 | Glu-AuNPs | - | 14.37±2.49 | HAuCl4,
NaBH4, sodium citrate, PEG, Glu | TEM and XPS | (62) |
| Cui et al,
2017 |
GNP-NHN=Dox-mPEG | - | 179.0±7.5 | HAuCl4,
hydrazine hydrate, DCC, DMAP, TFA, NPC, mPEG, Dox HCl,
NaBH4 | FTIR and NMR | (63) |
Application of AuNPs
The utilization of nanosized materials has
facilitated a number of advances in biological applications such as
biomedicine. These advances include antitumor activity and drug
delivery (64), fluorescent
biological labeling, gene delivery, tissue engineering, protein
detection, contrast enhancement magnetic resonance imaging, DNA
probing, hyperthermia treatment, phagokinetic research and cell or
molecular filtration leveraging biological interactions (11).
AuNPs are among the most commonly used materials for
diagnostics, bioimaging and cancer therapy due to their inherent
stability and low cytotoxicity (65,66).
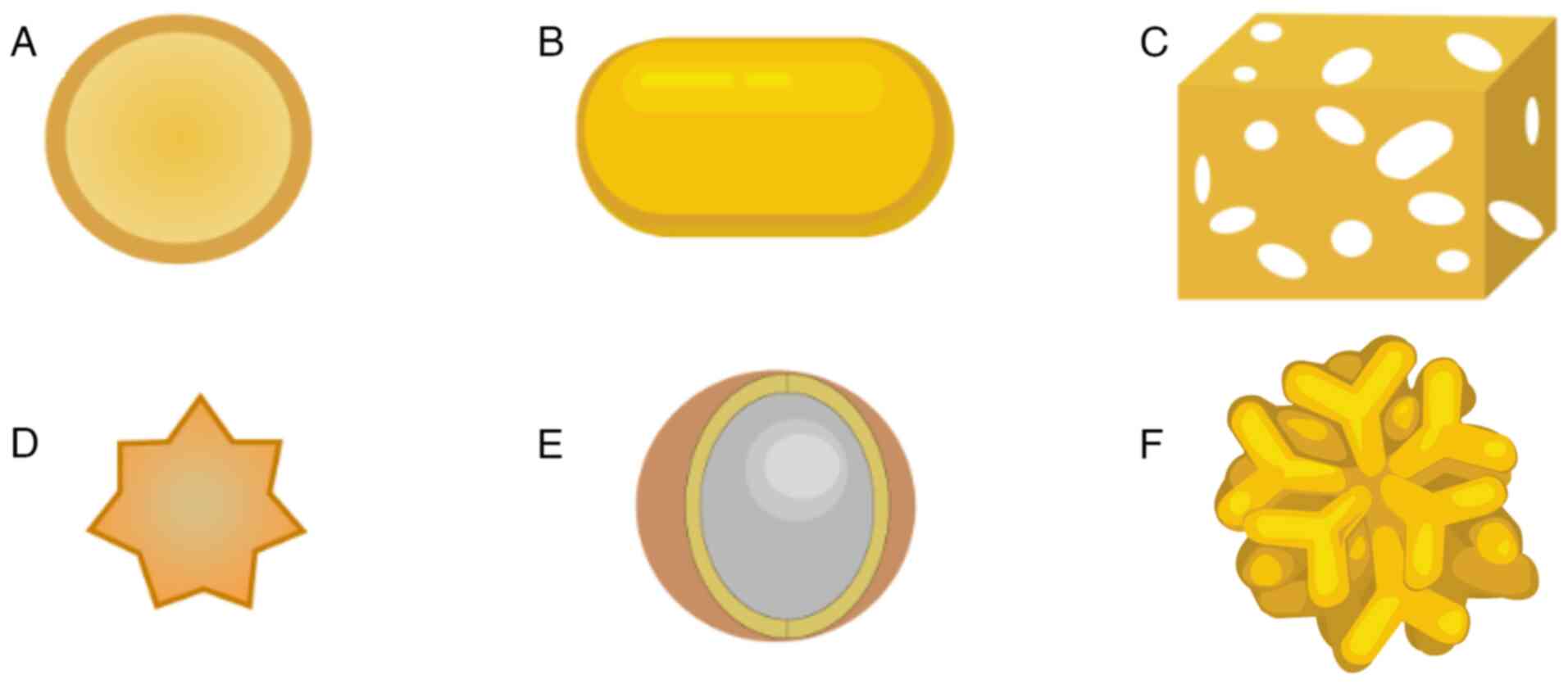
AuNPs of various shapes, such as nanoshells, nanorods, nanocages,
nanostars, nanospheres and branched AuNPs, have been manufactured
and investigated (67,68) (Fig.
3). For example, the hollow structure of gold nanocages
provides a high capacity for loading various types of payloads,
while the payload can be rapidly loaded and released through pores
in the walls. These features are particularly attractive for drug
delivery and controlled release (69,70).
Specific applications of AuNPs include their use as contrast agents
in medical imaging and as drug carriers for gene delivery (71). AuNPs have been extensively adopted
in drug delivery due to their chemical inertness, biocompatibility
and ease of functionalization (72,73).
In nanomedicine, AuNPs are non-toxic at the doses utilized for drug
delivery (74). The high affinity
of AuNPs for thiols, polymers and amines allows the introduction of
reactive molecules that can be employed for targeting, including
peptides, antibodies, carbohydrates and aptamers, and for the
conjugation of therapeutic agents, including radionuclides, drugs,
photosensitizers, genes and small interfering RNAs (75).
It has been suggested that AuNPs are redox active
and noncytotoxic, as they can reduce reactive oxygen and nitrite
species without inducing the secretion of tumor necrosis factor
(TNF)-α, interleukin (IL)-1β and other inflammatory factors, which
makes then ideal nanomedicine candidates (76). Ben Haddada et al (77) demonstrated that AuNPs prepared using
Hubertia ambavilla are nonpoisonous to human skin
fibroblasts and can scavenge free radicals and protect fibroblasts
and dermal cells against UV-A radiation-induced damage. In
addition, Taratummarat et al (78) reported that spherical AuNPs of
diameter 20–30 nm are nontoxic to mice, and exhibit
anti-inflammatory properties. It has been reported that positively
charged particles are more toxic to bacteria than are negatively
charged or neutral particles, indicating that surface charge
affects the toxicity of AuNPs (79). However, other studies have reported
conflicting findings, with one reporting that positively charged
AuNPs exhibited no toxicity to human epithelial cells (80), while another reported that both
positively and negatively charged AuNPs were toxic to human
keratinocytes (81). The reason for
such differences may be the various physicochemical features of
different NPs, and the lack of a standardized method to verify
toxicity (82). Moreover, Shukla
et al (76) suggested that
AuNPs do not elicit an initial immunological response or induce the
production of the proinflammatory cytokines IL-1β and TNF-α until a
high concentration of 100 µM is reached. In addition, Ghosh et
al (83) reported that AuNPs do
not trigger complement activation. Due to their lack of toxicity
and immunogenicity, AuNPs are an ideal choice for drug delivery
scaffolds. Moreover, the ability to functionalize AuNPs renders
them highly promising vehicles for drug delivery applications
(84–87). Currently, nanomedicine is advancing
the development of novel therapeutic and diagnostic tools,
including biosensors for biomolecule detection, tumor
chemotherapeutics, and gene or drug delivery. Owing to their good
biocompatibility and small particle size, AuNPs are promising
candidates for biological applications (88).
There are many methods for the treatment of OC,
which vary in their advantages and disadvantages (Fig. 4). Importantly, AuNPs can be used for
OC treatment. In one study, in vitro experiments
demonstrated that AuNPs successfully induced autophagy and
apoptosis in SK-OV-3 cells via reactive oxygen species
(ROS)-mediated pathways, indicating their potential as new
nanotherapeutics (6). AuNPs hold
great promise in diagnostic and therapeutic medicinal
applications.
Drug delivery systems targeting OC
The large surface-to-volume ratio and good
biocompatibility of AuNPs, together with the ability to synthesize
AuNPs with varied morphological characteristics and surface
chemistries, render AuNPs very suitable for use as drug delivery
vehicles (89). AuNPs coupled with
targeted molecules can precisely deliver tumor-targeting drugs via
both passive and active targeting mechanisms (90,91).
Passive targeting involves the nonspecific
accumulation of NPs in tumors due to the specific characteristics
of the tumor microenvironment (92,93).
In passive targeting, AuNPs primarily exploit the enhanced
permeability and retention effect. Drugs attached to AuNPs can
selectively accumulate in tumor tissues and persist for an extended
period due to vascular leakage and compromised lymphatic drainage,
respectively (94,95). Although a meta-analysis of 117
studies (96) on nanodrug delivery
found that only 0.7% of NPs successfully reached the tumor site,
indicating that although passive targeting often results in low
delivery efficacy, AuNPs have an improved ability to target tumor
tissue. In addition, AuNPs can be attached to a variety of ligands,
including drugs, peptides, antibodies and oligonucleotides, to
enhance their targeted delivery properties (97–99).
AuNP carriers can protect peptides, antibodies and oligonucleotides
from enzymatic degradation, thereby improving their effectiveness
in transporting drugs into solid tumors (100). Table
III presents some examples of targeted AuNPs and their
properties.
 | Table III.Properties of selected AuNPs
targeting OC. |
Table III.
Properties of selected AuNPs
targeting OC.
| First author,
year | Name | Properties | Passive/active
targeting | Drug/targeting
agent | Receptor | Drug release
system | (Refs.) |
|---|
| Lee et al,
2022 | HA-AuDEN-Dox | Multivalent
terminals of dendrimers can be functionalized with targeted ligands
for active targeting, inhibiting ovary tumor growth | Active | HA, Dox | CD44 | pH and GSH
stimuli-responsiveness | (49) |
| Lee et al,
2020 | Dox-DNA-AuNP | Excellent
anticancer activity for OC cells | Active | Dox | - | pH-dependent | (48) |
| Kotcherlakota et
al, 2017 | Au-TR-DX-si | Non-toxic,
target-specific uptake and significant OC tumor suppression | Active | Dox, TR, erbB2
siRNA | HER2 | - | (50) |
| Kotcherlakota et
al, 2019 | Au-C225-p53DNA | Delivers p53 DNA
and C225 specifically to OC cells that overexpress EGFR | Active | C225, p53 DNA | EGFR | - | (51) |
| Piktel et
al, 2021 | Peanut-shaped
AuNPs | Reduce the
viability and proliferation of OC cells by triggering ROS-mediated
apoptosis and autophagy | Passive | - | - | - | (6) |
| Piktel et
al, 2021 | AuP@CSA-131 | Improved anticancer
compared with CSA-131, enabling the effective dose to be
reduced | Active | CSA-131 | - | - | (52) |
| Jabir et al,
2020 | LG/LGC | Significant
antiproliferative effect on SK-OV-3 cells | Active | Linalool | - | - | (53) |
| Asl et al,
2023 | AuNPs | Potent anticancer
activity against CP-resistant OC cells | Passive | - | - | - | (54) |
| Xiong et al,
2014 | AuNPs | Sensitize OC cells
to CP by depleting stem cell pools and inhibiting key molecular
pathways | Active | CP | - | - | (55) |
| Patra et al,
2010 | Au-PSH-CP-FA | Enhanced cytotoxic
effect on OC cells and protective against cytotoxic damage in
normal cells | Active | CP, FA | Folate
receptor | pH and GSH
stimuli-responsiveness | (57) |
| Wang et al,
2014 | 15P-PPy-NPs | Target SK-OV-3
cells in vitro | Active | 15P | VEGFR3 | - | (59) |
| Shen et al,
2022 | RHMH18@AuDNPs | Good
biocompatibility and active chemotherapeutic photothermal
synergistic effect on human ovarian tumors | Active | DTX | - | pH-dependent | (60) |
| Van de Broek et
al, 2011 | Branched AuNPs | Actively target
HER2-expressing SK-OV-3 cells | Active | Anti-HER2 | HER2 | - | (61) |
| Geng et al,
2011 | Glu-AuNPs | Enhance the
effectiveness of radiotherapy on OC cells | Active | Glu | - | - | (62) |
Drugs
Drug-conjugated AuNPs are considered to be highly
promising and efficient nanoprodrugs. Such a conjugate may be
constructed, for example, by the attachment of multiple
thiol-terminated polyethylene glycol (PEG)-drug conjugates onto the
surface of AuNPs via thiol-Au covalent bonds (63). The attachment of drugs to the
surfaces of AuNPs offers several advantages while minimizing the
risk of severe systemic toxicity (101). For instance, due to their small
size, they can efficiently travel through capillaries to reach
target cells. Chemotherapeutic agents can be loaded or attached to
the AuNPs and can be passively or actively targeted to the tumor
site (82). In addition, the
incorporation of modifiers that are responsive to external stimuli,
including pH or enzymes, into the linking molecules facilitates
drug release (37).
The utilization of NP-based carriers for the
delivery of anticancer agents is a promising strategy for reducing
the dosages of antineoplastic compounds, as it minimizes their
systemic toxicity while simultaneously enhancing their therapeutic
efficacy (102). Piktel et
al (52) used nanotechnology to
manufacture a new nanosystem composed of AuNPs functionalized with
a shell comprising cationic steroid antibiotic-131. This nanosystem
exhibited marked activity against OC cells in vitro and
prevented the development of ovarian tumors in animals with minimal
toxicity. In another study, Dox-DNA-AuNPs exhibited an excellent
anticancer effect in an in vitro propagation test, and
efficacy in the prevention of tumor development in a xenograft
mouse model over a 16-day treatment period. Compared with free Dox,
Dox-DNA-AuNPs exhibited an ~2.5-fold greater inhibition of tumor
development, demonstrating their strong ability to inhibit cancer
development (48).
Cisplatin (CP) is a first-line chemotherapeutic drug
for OC. Although CP is very useful as a cancer treatment, it has
numerous side effects (103,104). Patra et al (57). described the manufacture and
functional characterization of an AuNP-based drug delivery system
for the potential treatment of OC. The system was fabricated by the
reaction of AuNPs with folic acid (FA), mercapto-PEG of molecular
weight 2,000 (PSH) and CP, to form an Au-PSH-CP-FA-based drug
delivery system. In vitro proliferation assays revealed that
the CP retained its cytotoxicity in this system, while normal cells
were protected against cytotoxicity. Asl et al (54) successfully synthesized AuNPs using
an extract derived from Satureja rechingeri Jamzad. The
obtained spherical AuNPs displayed potent anticancer activity
against CP-resistant OC cells, and low cytotoxicity to normal
cells, indicating their biocompatibility. These findings indicate
that AuNPs have strong potential for the treatment of OC.
By focusing on cancer cell markers that are more
highly expressed in tumor tissues than in normal cells and tissues,
active targeting agents can improve the precision of tumor tissue
targeting (105–107). Lee et al (49) created a targeted drug delivery
system for the treatment of OC that was responsive to changes in pH
and glutathione (GSH) levels. This was created by the attachment of
hyaluronic acid molecules to the surface of dendrimer-encapsulated
AuNPs via 1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide and
N-hydroxysuccinimide chemistry, and then loading Dox onto the Au
surface. This nanodrug demonstrated high biocompatibility,
excellent stability and effective targeting through the CD44
receptor. In addition, it effectively penetrated cancer cells,
where the release of Dox was induced in response to the acidic pH
and high GSH levels of the tumor microenvironment. This inhibited
tumor growth while causing fewer toxic side effects in mice.
Certain AuNPs have the ability to undergo photothermal
transformation, which generates heat, thereby promoting in
situ drug release and tumor ablation (108,109). For example, in one study,
ultrasmall NPs were incorporated into RHMH8 fusion protein via
biomimetic mineralization to form RHMH18@Au complexes which were further
loaded with docetaxel (DTX). The resulting RHMH18@AuDTX NPs contained AuNPs
clustered in the human serum albumin (HSA) portion of the fusion
protein and histidine-encapsulated DTX. These RHMH18@AuD NPs formed a uniform dispersion
in saline and exhibited chemo-photothermal therapeutic effects in
ovarian tumor tissue. In addition, in vitro experiments
demonstrated that under the influence of MMP-2, the RHMH18@AuDTX NPs decomposed into
arginine-glycine-aspartic acid (RGD)-HSA@Au and His@DTX NPs. It is likely that these two
components function in different areas of the tumor tissue, with
RGD-HSA@Au playing a photothermal
role in the extracellular matrix, and His@DTX NPs entering tumor cells due to their
nanoscale size and charge interactions with the cell surface. This
dual-targeting approach was demonstrated have good biocompatibility
and a favorable anti-OC effect in vivo, and presents a
promising novel strategy for tumor treatment (60).
Peptides
Various peptides have been employed for the specific
delivery of therapeutic AuNPs (110).
A previous study demonstrated that SK-OV-3 cells are
efficiently targeted by 15P (sequence, SHSWHWLPNLRHYAS) conjugated
to AuNPs via polypyrrole (PPy) linkers. These conjugates
demonstrated hyperthermic effects on the SK-OV-3 human OC cell line
when exposed to near-infrared laser irradiation, with high tumor
specificity. The hyperthermic effect of the PPy-conjugated AuNPs or
15P conjugates on tumor cells in vivo was investigated in
nude mice bearing subcutaneous SK-OV-3 tumors. Significant
inhibition of tumor growth was observed following near-infrared
laser-mediated treatment with both types of conjugates. These
findings indicate that 15P-PPy-AuNPs have excellent
biocompatibility, and the ability to effectively induce the
photothermal ablation of tumor cells in a tumor-targeted manner.
The study also found that while 15P-PPy-NPs effectively bind to and
ablate SK-OV-3 cells, they have no effect on HL-7702 or HepG2 cells
(59).
Linalool is a monoterpene compound that is active
against numerous cancer cell lines, but limited in its application
by its high toxicity. A novel peptide conjugate of AuNPs and
linalool was synthesized and characterized by Jabir et al
(53), with the aim of reducing the
general toxicity of linalool and improving its targeting ability.
Linalool was loaded onto AuNPs by reaction with GSH and linalool,
followed by the loading of CALNN peptide onto the surface of the
linalool-loaded AuNPs via a chemical reaction. The peptide
conjugate demonstrated strong antiproliferative effects on SK-OV-3
OC cells.
Antibodies
Drug-loaded AuNPs are able to actively target tumors
by strategies using antibody-modified ligands (110–112). Immunoglobulins and antibody
fragments are the most frequently employed molecules for antibody
targeting (104). HER2-positive OC
is recognized as being aggressive in nature, resistant to
chemotherapy and being associated with a high mortality rate
(113). Therefore, targeting HER2
receptors is considered as a potential approach for improving the
effectiveness of treatment and survival rates in patients with OC.
Van de Broek et al (61)
linked anti-HER2 nanobodies to branched AuNPs and demonstrated
their specific effect on HER2-positive SK-OV-3 cells. These authors
reported that the anti-HER2 conjugated AuNPs specifically bound to
the cells, indicating that the nanobodies retained their
specificity following conjugation to the AuNPs. In addition,
targeted photothermal damage of the tumor cells was achieved in
vitro by near-infrared laser irradiation of the branched AuNPs,
while exposure of the cells to either near-infrared light or AuNPs
alone did not affect cell viability; notably, when the two
components were combined, cell death was limited to the area of
laser/NP cotreatment. By contrast, AuNPs conjugated with anti-PSA
nanobodies did not induce cell death upon laser exposure,
underscoring the high specificity of these anti-HER2 AuNPs.
Genes
Kotcherlakota et al (51) developed stable AuNPs, designated
Au-C225-p53DNA, for the specific delivery of p53 DNA to OC cells
with upregulated epidermal growth factor receptor (EGFR)
expression. The authors demonstrated that the targeted delivery of
the wild-type p53 gene using these NPs effectively inhibited the
growth of ovarian tumors in mice with SK-OV-3 ×enografts by the
restoration of gene function. The C225 component of these NPs, also
known as the EGFR-targeting antibody cetuximab, served as a
targeted delivery system for the efficient administration of the
p53 gene and the treatment of OC.
In another study, Kotcherlakota et al
(50) combined AuNPs with the
engineered bifunctional recombinant fusion protein TRAF(C) to
fabricate a drug delivery system. This system facilitated the
target-specific delivery of Dox and an erbB2-targeting
small-interfering RNA into SK-OV-3 cells, which have upregulated
expression levels of the HER2 receptor. These findings collectively
suggest that AuNP-mediated gene therapy is a promising therapeutic
approach for OC.
Others
In one study, thioglucose was used to modify the
surface of AuNPs. The rationale behind this approach was that
cancer cells have a greater metabolic rate and, therefore, a much
higher glucose uptake rate than normal cells. The selective glucose
uptake by cancer cells facilitated the specific internalization of
the thioglucose-coated AuNPs (Glu-AuNPs) (114). In vivo data demonstrated
that the accumulation of the Glu-GNPs in cancerous tissue was
10-fold greater than that in normal ovarian and uterine tissues. In
another study, Geng et al demonstrated the potential of
thioglucose-bound AuNPs as a sensitizer for the radiotherapy of OC.
When SK-OV-3 cells were treated with the AuNPs alone, irradiation
alone or the AuNPs in conjunction with irradiation, the
intracellular accumulation of AuNPs resulted in greater
antiproliferative activity compared with irradiation alone. The
interaction between X-ray radiation and AuNPs was shown to lead to
an increase in the production of ROS (62).
Combination therapies have garnered attention as a
strategy to overcome the limitations associated with traditional
cancer treatments. There has been an increasing interest in the use
of ultrasound (US) to increase the intracellular concentration of
chemotherapeutic agents, particularly in preclinical research. In
addition, research has shown that NPs can enhance the efficacy of
US therapy (115–117). Kip et al (56) exploited the US-active property of
nanocone-shaped AuNPs in a combined US and CP treatment strategy.
Triple-combination therapy comprising US, AuNPs and a low dose of
CP was found to effectively overcome drug resistance in OC cells
in vitro, indicating its potential for the reduction of
chemotherapy-induced side effects.
An economical, facile and eco-friendly method has
been devised for the fabrication of anisotropic AuNPs utilizing
whey proteins (Wh@AuNPs) (58). These Wh@AuNPs were found to exhibit potent
catalytic activity and the ability to emit strong red fluorescence
upon complexation by trypan blue, indicating their potential use in
optical sensors and live/dead cell imaging. In addition, the
Wh@AuNPs exhibited cytotoxic activity
against breast cancer and OC cells but no toxicity toward normal
cells, indicating that Wh@AuNPs may
be novel theranostic agents that do not harm normal cells. However,
further research is necessary to confirm the theranostic
effectiveness of these Wh@AuNPs in
vivo.
Therapeutic potential of AuNPs in OC
The poor biodegradability of AuNPs in vivo
poses a significant challenge for clinical applications.
Experiments in mice revealed that only 9% of 40-nm AuNPs
administered by intravenous injection were excreted from the liver
over 6 months (118). Other
preclinical experiments demonstrated that a year postinjection,
there was no detectible reduction in the quantity of 155-nm AuNPs
retained in vivo (119).
Higbee-Dempsey et al (120)
synthesized biodegradable AuNPs modified with thiolated dextran,
and introduced hydrophobic acetal groups onto the surface by the
covalent modification of dextran. The acetal groups cleaved when
exposed to an acidic environment, rendering the AuNPs highly
soluble and susceptible to degradation. This carrier system was
shown to facilitate the clearance of >85% of the AuNPs from the
livers of mice within a span of 3 months. Therefore, this study
resolves a key issue hindering the clinical translation of AuNPs
and their use as nanocarrier systems.
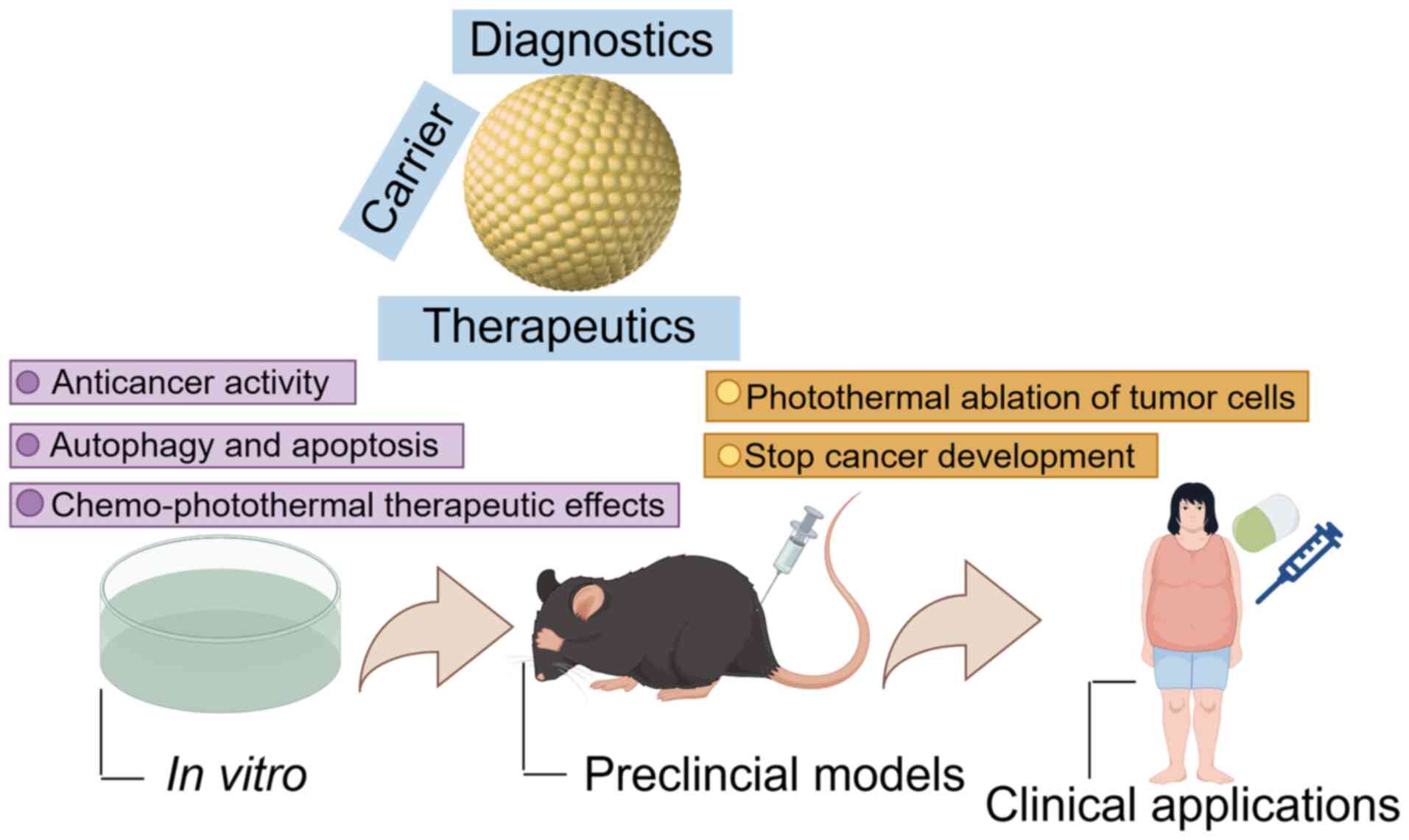
Conjugated AuNPs have garnered widespread
application as biomarkers and biodelivery vehicles within the
medical sphere, with potential utility in early and advanced cancer
diagnostics and therapeutics. This approach has demonstrated
promise in the early identification of cancer stem cells within
salivary gland tumors, as evidenced by a clinical trial using a
nanocomposite of AuNPs conjugated to CD24 (NCT04907422) (121). Therefore, it is anticipated that
clinical trials utilizing AuNP therapy for OC are likely to be
underway in the future (Fig.
5).
Conclusions and prospects
NPs are widely used for targeted drug delivery,
therapeutic purposes, catalysis, imaging and hyperthermia. AuNPs
are used for various medical purposes, for example, as targeted
therapeutic agents or drug delivery carriers, as well as in
electronics and sensing applications. In the treatment of OC, AuNPs
have been shown inhibit tumor growth, overcome drug resistance,
reduce the toxicity of anticancer drugs, and prevent cancer cell
invasion and migration. They can be combined with other therapies,
including chemotherapy and radiotherapy, to provide an improved
therapeutic effect. Despite these advances, certain constraints are
associated with the development of AuNPs. First, AuNPs may exhibit
toxicity at certain concentrations, particularly with long-term
exposure (122). Therefore,
further research is necessary to understand their toxicity and
ensure biocompatibility. Second, the methods for synthesizing AuNPs
are varied and often require strict experimental conditions and
technical expertise. Thus, the development of simpler and more
efficient preparation methods would be advantageous. Third, AuNPs
often exhibit nonuniform particle size distributions, which affects
their properties and application effectiveness (123). Therefore, improvements in their
preparation methods are required to achieve more uniform particle
size distributions. Fourth, AuNPs can aggregate and lose their
activity during storage and use, which impacts their stability and
long-term storage capability (124). Further research to develop more
stable AuNP materials is essential. Finally, the cost of preparing
AuNPs is high, limiting their potential for large-scale manufacture
and use. Therefore, it is important to focus on reducing synthesis
costs and improving scalability to enable the commercialization of
AuNPs.
In summary, the development of AuNPs faces
challenges and limitations that require further research and
improvement. By addressing these issues, the application prospects
of AuNPs can be further expanded.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
WH was responsible for writing the original draft of
the manuscript, and for visualization. FY reviewed and edited the
manuscript. QZ contributed to conceptualization of the study,
supervision, editing and manuscript revision. KC conceived the idea
of the study. All authors read and approved the final version of
the manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yang Y, Zheng X, Chen L, Gong X, Yang H,
Duan X and Zhu Y: Multifunctional gold nanoparticles in cancer
diagnosis and treatment. Int J Nanomedicine. 17:2041–2067. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schoutrop E, Moyano-Galceran L, Lheureux
S, Mattsson J, Lehti K, Dahlstrand H and Magalhaes I: Molecular,
cellular and systemic aspects of epithelial ovarian cancer and its
tumor microenvironment. Semin Cancer Biol. 86:207–223. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang R, Siu MKY, Ngan HYS and Chan KKL:
Molecular biomarkers for the early detection of ovarian cancer. Int
J Mol Sci. 23:120412022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bhardwaj BK, Thankachan S, Magesh P,
Venkatesh T, Tsutsumi R and Suresh PS: Current update on
nanotechnology-based approaches in ovarian cancer therapy. Reprod
Sci. 30:335–349. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jelovac D and Armstrong DK: Recent
progress in the diagnosis and treatment of ovarian cancer. CA
Cancer J Clin. 61:183–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Piktel E, Ościłowska I, Suprewicz Ł,
Depciuch J, Marcińczyk N, Chabielska E, Wolak P, Wollny T, Janion
M, Parlinska-Wojtan M and Bucki R: ROS-Mediated apoptosis and
autophagy in ovarian cancer cells treated with peanut-shaped gold
nanoparticles. Int J Nanomedicine. 16:1993–2011. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sperling RA and Parak WJ: Surface
modification, functionalization and bioconjugation of colloidal
inorganic nanoparticles. Philos Trans A Math Phys Eng Sci.
368:1333–1383. 2010.PubMed/NCBI
|
|
8
|
Kamal A, Saba M, Ullah K, Almutairi SM,
AlMunqedhi BM and Ragab abdelGawwad M: Mycosynthesis,
characterization of zinc oxide nanoparticles, and its assessment in
various biological activities. Crystals. 13:1712023. View Article : Google Scholar
|
|
9
|
Huang CC, Yang Z, Lee KH and Chang HT:
Synthesis of highly fluorescent gold nanoparticles for sensing
mercury(II). Angew Chem Int Ed Engl. 46:6824–6828. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huynh KH, Pham XH, Kim J, Lee SH, Chang H,
Rho WY and Jun BH: Synthesis, properties, and biological
applications of metallic alloy nanoparticles. Int J Mol Sci.
21:51742020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yaqoob AA, Ahmad H, Parveen T, Ahmad A,
Oves M, Ismail IMI, Qari HA, Umar K and Mohamad Ibrahim MN: Recent
advances in metal decorated nanomaterials and their various
biological applications: A review. Front Chem. 8:3412020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vargas-Molinero HY, Serrano-Medina A,
Palomino-Vizcaino K, López-Maldonado EA, Villarreal-Gómez LJ,
Pérez-González GL and Cornejo-Bravo JM: Hybrid systems of
nanofibers and polymeric nanoparticles for biological application
and delivery systems. Micromachines (Basel). 14:2082023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo R, Song Y, Wang G and Murray RW: Does
core size matter in the kinetics of ligand exchanges of
monolayer-protected Au clusters? J Am Chem Soc. 127:2752–2757.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Daniel MC and Astruc D: Gold
nanoparticles: Assembly, supramolecular chemistry,
quantum-size-related properties, and applications toward biology,
catalysis, and nanotechnology. Chem Rev. 104:293–346. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang S, Gnanasammandhan MK and Zhang Y:
Optical imaging-guided cancer therapy with fluorescent
nanoparticles. J R Soc Interface. 7:3–18. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aziz F, Ihsan A, Nazir A, Ahmad I, Bajwa
SZ, Rehman A, Diallo A and Khan WS: Novel route synthesis of porous
and solid gold nanoparticles for investigating their comparative
performance as contrast agent in computed tomography scan and
effect on liver and kidney function. Int J Nanomedicine.
12:1555–1563. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Spivak MY, Bubnov RV, Yemets IM, Lazarenko
LM, Tymoshok NO and Ulberg ZR: Development and testing of gold
nanoparticles for drug delivery and treatment of heart failure: a
theranostic potential for PPP cardiology. EPMA J. 4:202013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khan JA, Pillai B, Das TK, Singh Y and
Maiti S: Molecular effects of uptake of gold nanoparticles in HeLa
cells. Chembiochem. 8:1237–1240. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Scaletti F, Hardie J, Lee YW, Luther DC,
Ray M and Rotello VM: Protein delivery into cells using inorganic
nanoparticle-protein supramolecular assemblies. Chem Soc Rev.
47:3421–3432. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rosi NL, Giljohann DA, Thaxton CS,
Lytton-Jean AK, Han MS and Mirkin CA: Oligonucleotide-modified gold
nanoparticles for intracellular gene regulation. Science.
312:1027–1030. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dykman LA and Khlebtsov NG: Immunological
properties of gold nanoparticles. Chem Sci. 8:1719–1735. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen YS, Hung YC, Lin WH and Huang GS:
Assessment of gold nanoparticles as a size-dependent vaccine
carrier for enhancing the antibody response against synthetic
foot-and-mouth disease virus peptide. Nanotechnology.
21:1951012010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Connor EE, Mwamuka J, Gole A, Murphy CJ
and Wyatt MD: Gold nanoparticles are taken up by human cells but do
not cause acute cytotoxicity. Small. 1:325–327. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ielo I, Rando G, Giacobello F, Sfameni S,
Castellano A, Galletta M, Drommi D, Rosace G and Plutino MR:
Synthesis, chemical-physical characterization, and biomedical
applications of functional gold nanoparticles: A review. Molecules.
26:58232021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiong D, Chen M and Li H: Synthesis of
para-sulfonatocalix[4]arene-modified silver nanoparticles as
colorimetric histidine probes. Chem Commun (Camb). 880–882. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xiao W, Xiong J, Zhang S, Xiong Y, Zhang H
and Gao H: Influence of ligands property and particle size of gold
nanoparticles on the protein adsorption and corresponding targeting
ability. Int J Pharm. 538:105–111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
De Luca G, Bonaccorsi P, Trovato V,
Mancuso A, Papalia T, Pistone A, Casaletto MP, Mezzi A, Brunetti B,
Minuti L, et al: Tripodal tris-disulfides as capping agents for a
controlled mixed functionalization of gold nanoparticles. New J
Chem. 42:16436–16440. 2018. View Article : Google Scholar
|
|
28
|
Boyer C, Whittaker MR, Chuah K, Liu J and
Davis TP: Modulation of the surface charge on polymer-stabilized
gold nanoparticles by the application of an external stimulus.
Langmuir. 26:2721–2730. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Presnova GV, Rubtsova MY, Presnov DE,
Grigorenko VG, Yaminsky IV and Egorov AM: Conjugates of
Streptavidin conjugates with gold nanoparticles for the
visualization of DNA single interactions on the silicon surface.
Biomed Khim. 60:538–542. 2014.(In Russian). View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Das RK, Pachapur VL, Lonappan L, Naghdi M,
Pulicharla R, Maiti S, Cledon M, Dalila LMA, Sarma SJ and Brar SK:
Biological synthesis of metallic nanoparticles: Plants, animals and
microbial aspects. Nanotechnol. Environ. Eng. 2:182017.
|
|
31
|
Thakkar KN, Mhatre SS and Parikh RY:
Biological synthesis of metallic nanoparticles. Nanomedicine.
6:257–262. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shnoudeh AJ, Hamad I, Abdo RW, et al:
Synthesis, Characterization, and Applications of Metal
Nanoparticles. Biomaterials and Bionanotechnology. pp527–612. 2019.
View Article : Google Scholar
|
|
33
|
Kharissova OV, Kharisov BI, Oliva González
CM, Méndez YP and López I: Greener synthesis of chemical compounds
and materials. R Soc Open Sci. 6:1913782019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Medici S, Peana M, Nurchi VM, Lachowicz
JI, Crisponi G and Zoroddu MA: Noble metals in medicine: Latest
advances. Coord Chem Rev. 284:329–350. 2015. View Article : Google Scholar
|
|
35
|
Salem SS and Fouda A: Green synthesis of
metallic nanoparticles and their prospective biotechnological
applications: An overview. Biol Trace Elem Res. 199:344–370. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gu X, Xu Z, Gu L, Xu H, Han F, Chen B and
Pan X: Preparation and antibacterial properties of gold
nanoparticles: A review. Environ Chem Lett. 19:167–187. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Amina SJ and Guo B: A review on the
synthesis and functionalization of gold nanoparticles as a drug
delivery vehicle. Int J Nanomedicine. 15:9823–9857. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Omran BA, Whitehead KA and Baek KH:
One-pot bioinspired synthesis of fluorescent metal chalcogenide and
carbon quantum dots: Applications and potential biotoxicity.
Colloids Surf B Biointerfaces. 200:1115782021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu F, Li Y, Zhao X, Liu G, Pang B, Liao N,
Li H and Shi J: Diversity of fungus-mediated synthesis of gold
nanoparticles: Properties, mechanisms, challenges, and solving
methods. Crit Rev Biotechnol. 44:924–940. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Borse VB, Konwar AN, Jayant RD and Patil
PO: Perspectives of characterization and bioconjugation of gold
nanoparticles and their application in lateral flow immunosensing.
Drug Deliv Transl Res. 10:878–902. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Murphy CJ: Materials science. Nanocubes
and nanoboxes. Science. 298:2139–2141. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Das SK and Marsili E: A green chemical
approach for the synthesis of gold nanoparticles: Characterization
and mechanistic aspect. Rev Environ Sci Biotechnol. 9:199–204.
2010. View Article : Google Scholar
|
|
43
|
Hamelian M, Varmira K and Veisi H: Green
synthesis and characterizations of gold nanoparticles using Thyme
and survey cytotoxic effect, antibacterial and antioxidant
potential. J Photochem Photobiol B. 184:71–79. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Patil MP and Kim GD: Eco-friendly approach
for nanoparticles synthesis and mechanism behind antibacterial
activity of silver and anticancer activity of gold nanoparticles.
Appl Microbiol Biotechnol. 101:79–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Patil MP and Kim GD: Marine microorganisms
for synthesis of metallic nanoparticles and their biomedical
applications. Colloids Surf B Biointerfaces. 172:487–495. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shedbalkar U, Singh R, Wadhwani S,
Gaidhani S and Chopade BA: Microbial synthesis of gold
nanoparticles: Current status and future prospects. Adv Colloid
Interface Sci. 209:40–48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Beveridge TJ and Murray RG: Sites of metal
deposition in the cell wall of Bacillus subtilis. J Bacteriol.
141:876–887. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lee CS, Kim TW, Oh DE, Bae SO, Ryu J, Kong
H, Jeon H, Seo HK, Jeon S and Kim TH: In vivo and in vitro
anticancer activity of doxorubicin-loaded DNA-AuNP nanocarrier for
the ovarian cancer treatment. Cancers (Basel). 12:6342020.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lee CS, Kim TW, Kang Y, Ju Y, Ryu J, Kong
H, Jang YS, Oh DE, Jang SJ, Cho H, et al: Targeted drug delivery
nanocarriers based on hyaluronic acid-decorated dendrimer
encapsulating gold nanoparticles for ovarian cancer therapy. Mater
Today Chem. 26:1010832022. View Article : Google Scholar
|
|
50
|
Kotcherlakota R, Srinivasan DJ, Mukherjee
S, Haroon MM, Dar GH, Venkatraman U, Patra CR and Gopal V:
Engineered fusion protein-loaded gold nanocarriers for targeted
co-delivery of doxorubicin and erbB2-siRNA in human epidermal
growth factor receptor-2+ ovarian cancer. J Mater Chem B.
5:7082–7098. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kotcherlakota R, Vydiam K, Jeyalakshmi
Srinivasan D, Mukherjee S, Roy A, Kuncha M, Rao TN, Sistla R, Gopal
V and Patra CR: Restoration of p53 function in ovarian cancer
mediated by gold nanoparticle-based EGFR targeted gene delivery
system. ACS Biomater Sci Eng. 5:3631–3644. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Piktel E, Oscilowska I, Suprewicz Ł,
Depciuch J, Marcińczyk N, Chabielska E, Wolak P, Głuszek K, Klimek
J, Zieliński PM, et al: Peanut-Shaped gold nanoparticles with
shells of ceragenin CSA-131 display the ability to inhibit ovarian
cancer growth in vitro and in a tumor xenograft model. Cancers
(Basel). 13:54242021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jabir M, Sahib UI, Taqi Z, Taha A,
Sulaiman G, Albukhaty S, Al-Shammari A, Alwahibi M, Soliman D,
Dewir YH and Rizwana H: Linalool-Loaded glutathione-modified gold
nanoparticles conjugated with CALNN peptide as apoptosis inducer
and NF-κB translocation inhibitor in SKOV-3 cell line. Int J
Nanomedicine. 15:9025–9047. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Asl SS, Tafvizi F and Noorbazargan H:
Biogenic synthesis of gold nanoparticles using Satureja rechingeri
Jamzad: A potential anticancer agent against cisplatin-resistant
A2780CP ovarian cancer cells. Environ Sci Pollut Res Int.
30:20168–20184. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xiong X, Arvizo RR, Saha S, Robertson DJ,
McMeekin S, Bhattacharya R and Mukherjee P: Sensitization of
ovarian cancer cells to cisplatin by gold nanoparticles.
Oncotarget. 5:6453–6465. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kip B, Tunc CU and Aydin O:
Triple-combination therapy assisted with ultrasound-active gold
nanoparticles and ultrasound therapy against 3D cisplatin-resistant
ovarian cancer model. Ultrason Sonochem. 82:1059032022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Patra CR, Bhattacharya R and Mukherjee P:
Fabrication and functional characterization of goldnanoconjugates
for potential application in ovarian cancer. J Mater Chem.
20:547–554. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Borghei YS and Hosseinkhani S:
Bio-synthesis of a functionalized whey proteins theranostic
nanoprobe with cancer-specific cytotoxicity and as a live/dead cell
imaging probe. Journal of Photochemistry and Photobiology A:
Chemistry. 431:1140252022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang L, Wang L, Xu T, Guo C, Liu C, Zhang
H, Li J and Liang Z: Synthesis of 15P-conjugated PPy-modified gold
nanoparticles and their application to photothermal therapy of
ovarian cancer. Chem Res Chin Univ. 30:959–964. 2014. View Article : Google Scholar
|
|
60
|
Shen Y, Wang M, Wang H, Zhou J and Chen J:
Multifunctional human serum albumin fusion protein as a docetaxel
nanocarrier for chemo-photothermal synergetic therapy of ovarian
cancer. ACS Appl Mater Interfaces. 14:19907–19917. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Van de Broek B, Devoogdt N, D'Hollander A,
Gijs HL, Jans K, Lagae L, Muyldermans S, Maes G and Borghs G:
Specific cell targeting with nanobody conjugated branched gold
nanoparticles for photothermal therapy. ACS Nano. 5:4319–4328.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Geng F, Song K, Xing JZ, Yuan C, Yan S,
Yang Q, Chen J and Kong B: Thio-glucose bound gold nanoparticles
enhance radio-cytotoxic targeting of ovarian cancer.
Nanotechnology. 22:2851012011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Cui T, Liang JJ, Chen H, Geng DD, Jiao L,
Yang JY, Qian H, Zhang C and Ding Y: Performance of
doxorubicin-conjugated gold nanoparticles: Regulation of drug
location. ACS Appl Mater Interfaces. 9:8569–8580. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wani WA, Baig U, Shreaz S, Shiekh RA,
Iqbal PF, Jameel E, Ahmad A, Mohd-Setapar SH, Mushtaque M and Hun
LT: Recent advances in iron complexes as potential anticancer
agents. New J Chem. 40:1063–1090. 2016. View Article : Google Scholar
|
|
65
|
Baetke SC, Lammers T and Kiessling F:
Applications of nanoparticles for diagnosis and therapy of cancer.
Br J Radiol. 88:201502072015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Fan M, Han Y, Gao S, Yan H, Cao L, Li Z,
Liang XJ and Zhang J: Ultrasmall gold nanoparticles in cancer
diagnosis and therapy. Theranostics. 10:4944–4957. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang J, Potocny AM, Rosenthal J and Day
ES: Gold nanoshell-linear tetrapyrrole conjugates for near
infrared-activated dual photodynamic and photothermal therapies.
ACS Omega. 5:926–940. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhao X, Campbell S, Wallace GQ, Claing A,
Bazuin CG and Masson JF: Branched Au nanoparticles on nanofibers
for surface-enhanced raman scattering sensing of intracellular pH
and extracellular pH gradients. ACS Sens. 5:2155–2167. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Skrabalak SE, Chen J, Sun Y, Lu X, Au L,
Cobley CM and Xia Y: Gold nanocages: Synthesis, properties, and
applications. Acc Chem Res. 41:1587–1595. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yang M, Wang W, Qiu J, Bai MY and Xia Y:
Direct visualization and semi-quantitative analysis of payload
loading in the case of gold nanocages. Angew Chem Int Ed Engl.
58:17671–17674. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Murthy SK: Nanoparticles in modern
medicine: State of the art and future challenges. Int J
Nanomedicine. 2:129–141. 2007.PubMed/NCBI
|
|
72
|
Ghosh P, Yang X, Arvizo R, Zhu ZJ, Agasti
SS, Mo Z and Rotello VM: Intracellular delivery of a
membrane-impermeable enzyme in active form using functionalized
gold nanoparticles. J Am Chem Soc. 132:2642–2645. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhou T, Du Y and Wei T: Transcriptomic
analysis of human breast cancer cells reveals differentially
expressed genes and related cellular functions and pathways in
response to gold nanorods. Biophys Rep. 1:106–114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hu X, Zhang Y, Ding T, Liu J and Zhao H:
Multifunctional gold nanoparticles: A novel nanomaterial for
various medical applications and biological activities. Front
Bioeng Biotechnol. 8:9902020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhang J, Mou L and Jiang X: Surface
chemistry of gold nanoparticles for health-related applications.
Chem Sci. 11:923–936. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Shukla R, Bansal V, Chaudhary M, Basu A,
Bhonde RR and Sastry M: Biocompatibility of gold nanoparticles and
their endocytotic fate inside the cellular compartment: A
microscopic overview. Langmuir. 21:10644–10654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ben Haddada M, Gerometta E, Chawech R,
Sorres J, Bialecki A, Pesnel S, Spadavecchia J and Morel AL:
Assessment of antioxidant and dermoprotective activities of gold
nanoparticles as safe cosmetic ingredient. Colloids Surf B
Biointerfaces. 189:1108552020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Taratummarat S, Sangphech N, Vu CTB,
Palaga T, Ondee T, Surawut S, Sereemaspun A, Ritprajak P and
Leelahavanichkul A: Gold nanoparticles attenuates bacterial sepsis
in cecal ligation and puncture mouse model through the induction of
M2 macrophage polarization. BMC Microbiol. 18:852018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Feng ZV, Gunsolus IL, Qiu TA, Hurley KR,
Nyberg LH, Frew H, Johnson KP, Vartanian AM, Jacob LM, Lohse SE, et
al: Impacts of gold nanoparticle charge and ligand type on surface
binding and toxicity to Gram-negative and Gram-positive bacteria.
Chem Sci. 6:5186–5196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Cho TJ, MacCuspie RI, Gigault J, Gorham
JM, Elliott JT and Hackley VA: Highly stable positively charged
dendron-encapsulated gold nanoparticles. Langmuir. 30:3883–3893.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Schaeublin NM, Braydich-Stolle LK, Schrand
AM, Miller JM, Hutchison J, Schlager JJ and Hussain SM: Surface
charge of gold nanoparticles mediates mechanism of toxicity.
Nanoscale. 3:410–420. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Singh P, Pandit S, Mokkapati VRSS, Garg A,
Ravikumar V and Mijakovic I: Gold nanoparticles in diagnostics and
therapeutics for human cancer. Int J Mol Sci. 19:19792018.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ghosh C, Priegue P, Leelayuwapan H,
Fuchsberger FF, Rademacher C and Seeberger PH: Synthetic
Glyconanoparticles Modulate Innate Immunity but Not the Complement
System. ACS Appl Bio Mater. 5:2185–2192. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Nath D and Banerjee P: Green
nanotechnology-a new hope for medical biology. Environ Toxicol
Pharmacol. 36:997–1014. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Pan Y, Leifert A, Ruau D, Neuss S,
Bornemann J, Schmid G, Brandau W, Simon U and Jahnen-Dechent W:
Gold nanoparticles of diameter 1.4 nm trigger necrosis by oxidative
stress and mitochondrial damage. Small. 5:2067–2076. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Yasinska IM, Calzolai L, Raap U, Hussain
R, Siligardi G, Sumbayev VV and Gibbs BF: Targeting of basophil and
mast cell pro-allergic reactivity using functionalised gold
nanoparticles. Front Pharmacol. 10:3332019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Weaver JL, Tobin GA, Ingle T, Bancos S,
Stevens D, Rouse R, Howard KE, Goodwin D, Knapton A, Li X, et al:
Evaluating the potential of gold, silver, and silica nanoparticles
to saturate mononuclear phagocytic system tissues under repeat
dosing conditions. Part Fibre Toxicol. 14:252017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Golchin K, Golchin J, Ghaderi S,
Alidadiani N, Eslamkhah S, Eslamkhah M, Davaran S and Akbarzadeh A:
Gold nanoparticles applications: From artificial enzyme till drug
delivery. Artif Cells Nanomed Biotechnol. 46:250–254. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Parveen S, Misra R and Sahoo SK:
Nanoparticles: A boon to drug delivery, therapeutics, diagnostics
and imaging. Nanomedicine. 8:147–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Byrne JD, Betancourt T and Brannon-Peppas
L: Active targeting schemes for nanoparticle systems in cancer
therapeutics. Adv Drug Deliv Rev. 60:1615–1626. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Attia MF, Anton N, Wallyn J, Omran Z and
Vandamme TF: An overview of active and passive targeting strategies
to improve the nanocarriers efficiency to tumour sites. J Pharm
Pharmacol. 71:1185–1198. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Blanco MD, Teijon C, Olmo RM and Teijo JM:
Targeted Nanoparticles for Cancer Therapy. In: Recent Advances in
Novel Drug Carrier Systems. InTech; 2012
|
|
93
|
Melancon M, Lu W and Li C: Gold-Based
magneto/optical nanostructures: Challenges for in vivo applications
in cancer diagnostics and therapy. Mater Res Bull. 34:415–421.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Wu J: The enhanced permeability and
retention (EPR) Effect: The significance of the concept and methods
to enhance its application. J Pers Med. 11:7712021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Matsumura Y and Maeda H: A new concept for
macromolecular therapeutics in cancer chemotherapy: Mechanism of
tumoritropic accumulation of proteins and the antitumor agent
smancs. Cancer Res. 46((12 Pt 1)): 6387–6392. 1986.PubMed/NCBI
|
|
96
|
Wilhelm S, Tavares AJ, Dai Q, Ohta S,
Audet J, Dvorak HF and Chan WCW: Analysis of nanoparticle delivery
to tumours. Nat Rev Mater. 1:160142016. View Article : Google Scholar
|
|
97
|
Daraee H, Eatemadi A, Abbasi E, Fekri Aval
S, Kouhi M and Akbarzadeh A: Application of gold nanoparticles in
biomedical and drug delivery. Artif Cells Nanomed Biotechnol.
44:410–422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Bai X, Wang Y, Song Z, Feng Y, Chen Y,
Zhang D and Feng L: The basic properties of gold nanoparticles and
their applications in tumor diagnosis and treatment. Int J Mol Sci.
21:24802020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wang X and Guo Z: Targeting and delivery
of platinum-based anticancer drugs. Chem Soc Rev. 42:202–224. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ruan S, Xiao W, Hu C, Zhang H, Rao J, Wang
S, Wang X, He Q and Gao H: Ligand-Mediated and enzyme-directed
precise targeting and retention for the enhanced treatment of
glioblastoma. ACS Appl Mater Interfaces. 9:20348–20360. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Bao QY, Geng DD, Xue JW, Zhou G, Gu SY,
Ding Y and Zhang C: Glutathione-mediated drug release from
Tiopronin-conjugated gold nanoparticles for acute liver injury
therapy. Int J Pharm. 446:112–118. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Piktel E, Niemirowicz K, Wątek M, Wollny
T, Deptuła P and Bucki R: Recent insights in nanotechnology-based
drugs and formulations designed for effective anti-cancer therapy.
J Nanobiotechnology. 14:392016. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Hartmann JT, Kollmannsberger C, Kanz L and
Bokemeyer C: Platinum organ toxicity and possible prevention in
patients with testicular cancer. Int J Cancer. 83:866–869. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Thompson SW, Davis LE, Kornfeld M, Hilgers
RD and Standefer JC: Cisplatin neuropathy. Clinical,
electrophysiologic, morphologic, and toxicologic studies. Cancer.
54:1269–1275. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Yoo J, Park C, Yi G, Lee D and Koo H:
Active targeting strategies using biological ligands for
nanoparticle drug delivery systems. Cancers. 11:6402019. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Wang Z, Qiao R, Tang N, Lu Z, Wang H,
Zhang Z, Xue X, Huang Z, Zhang S, Zhang G and Li Y: Active
targeting theranostic iron oxide nanoparticles for MRI and magnetic
resonance-guided focused ultrasound ablation of lung cancer.
Biomaterials. 127:25–35. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Suh MS, Shen J, Kuhn LT and Burgess DJ:
Layer-by-layer nanoparticle platform for cancer active targeting.
Int J Pharm. 517:58–66. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Slovak R, Ludwig JM, Gettinger SN, Herbst
RS and Kim HS: Immuno-thermal ablations-boosting the anticancer
immune response. J Immunother Cancer. 5:782017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Kroemer G, Galassi C, Zitvogel L and
Galluzzi L: Immunogenic cell stress and death. Nat Immunol.
23:487–500. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Goddard ZR, Marín MJ, Russell DA and
Searcey M: Active targeting of gold nanoparticles as cancer
therapeutics. Chem Soc Rev. 49:8774–8789. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Emami F, Banstola A, Vatanara A, Lee S,
Kim JO, Jeong JH and Yook S: Doxorubicin and Anti-PD-L1 antibody
conjugated gold nanoparticles for colorectal cancer
photochemotherapy. Mol Pharm. 16:1184–1199. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Huang N, Liu Y, Fang Y, Zheng S, Wu J,
Wang M, Zhong W, Shi M, Xing M and Liao W: Gold nanoparticles
induce tumor vessel normalization and impair metastasis by
inhibiting endothelial smad2/3 signaling. ACS Nano. 14:7940–7958.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Berchuck A, Kamel A, Whitaker R, Kerns B,
Olt G, Kinney R, Soper JT, Dodge R, Clarke-Pearson DL, Marks P, et
al: Overexpression of HER-2/neu is associated with poor survival in
advanced epithelial ovarian cancer. Cancer Res. 50:4087–4091.
1990.PubMed/NCBI
|
|
114
|
Kong T, Zeng J, Wang X, Yang X, Yang J,
McQuarrie S, McEwan A, Roa W, Chen J and Xing JZ: Enhancement of
radiation cytotoxicity in breast-cancer cells by localized
attachment of gold nanoparticles. Small. 4:1537–1543. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Zhang Y, Yu J, Bomba HN, Zhu Y and Gu Z:
Mechanical force-triggered drug delivery. Chem Rev.
116:12536–12563. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Wood AK and Sehgal CM: A review of
low-intensity ultrasound for cancer therapy. Ultrasound Med Biol.
41:905–928. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Zhang Y, Wan Y, Chen Y, Blum NT, Lin J and
Huang P: Ultrasound-Enhanced chemo-photodynamic combination therapy
by using albumin ‘Nanoglue’-Based Nanotheranostics. ACS Nano.
14:5560–5569. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Sadauskas E, Danscher G, Stoltenberg M,
Vogel U, Larsen A and Wallin H: Protracted elimination of gold
nanoparticles from mouse liver. Nanomedicine. 5:162–169. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Gad SC, Sharp KL, Montgomery C, Payne JD
and Goodrich GP: Evaluation of the toxicity of intravenous delivery
of auroshell particles (gold-silica nanoshells). Int J Toxicol.
31:584–594. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Higbee-Dempsey EM, Amirshaghaghi A, Case
MJ, Bouché M, Kim J, Cormode DP and Tsourkas A: Biodegradable Gold
nanoclusters with improved excretion due to pH-Triggered
hydrophobic-to-hydrophilic transition. J Am Chem Soc.
142:7783–7794. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Kesharwani P, Ma R, Sang L, Fatima M,
Sheikh A, Abourehab MAS, Gupta N, Chen ZS and Zhou Y: Gold
nanoparticles and gold nanorods in the landscape of cancer therapy.
Mol Cancer. 22:982023. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Mironava T, Hadjiargyrou M, Simon M,
Jurukovski V and Rafailovich MH: Gold nanoparticles cellular
toxicity and recovery: Effect of size, concentration and exposure
time. Nanotoxicology. 4:120–137. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Mikhailova EO: Gold nanoparticles:
Biosynthesis and potential of biomedical application. J Funct
Biomater. 12:2021. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Epanchintseva AV, Poletaeva JE, Pyshnyi
DV, Ryabchikova EI and Pyshnaya IA: Long-term stability and
scale-up of noncovalently bound gold nanoparticle-siRNA
suspensions. Beilstein J Nanotechnol. 10:2568–2578. 2019.
View Article : Google Scholar : PubMed/NCBI
|