Introduction
Over the past few decades, rapid advances in genomic
technology and the accumulation of knowledge in cancer biology have
shifted the paradigm of cancer chemotherapy from conventional
cytotoxic small-molecule drugs to targeted and personalized
approaches (1). Sebaceous carcinoma
(SC) is a rare but aggressive malignancy arising from the adnexal
epithelium of the sebaceous glands and is primarily treated
surgically; however, the possibility of disease recurrence or
metastasis after resection is higher than that of other eyelid
malignancies. As SC frequently occurs on the eyelid, extensive
resection is often difficult for functional or cosmetic reasons.
However, there are no standardized protocols or highly effective
agents for the treatment of patients with advanced SC (2). The pathogenesis of SC remains poorly
understood, and studies investigating therapeutic targets for SC
are limited compared to those of recent therapeutic breakthroughs
for other cutaneous malignancies (3–6).
Recently, several researchers, including our group, have
investigated the genomic landscape of SC (7) and revealed candidates for potential
targetable alterations, such as PIK3CA, EGFR, and BRAF. However,
because these mutations are low in frequency and are not closely
associated with clinical outcomes, there is still a need to
identify more universal targets, such as hormonal receptors, in
breast cancer.
Sebocytes are metabolically active cells that
release numerous cytokines and chemokines under the influence of
hormones to maintain epidermal barrier and immune functions
(8,9). Steroid hormone receptors, such as
glucocorticoid receptors (GR), androgen receptors (AR), estrogen
receptors (ER), and progesterone receptors (PR), are nuclear
transcription factors that participate in cellular differentiation
and metabolic processes (10) and
are pathogenetically linked to solid tumors, most representatively
breast and prostate cancers (11–13).
Among the NRs, only the AR has been studied for its expression and
relationship with SC (14,15). For diagnostic purposes, AR is a
sensitive marker of sebaceous differentiation and is particularly
useful for identifying poorly differentiated sebaceous carcinoma
(16,17). However, the clinical significance of
AR expression in SC has not been clearly established, and its
relationship with other NRs remains unknown.
One of the most important functions of NRs is the
regulation of metabolism and inflammation, both of which are
involved in cancer pathogenesis. As cancer cells require more
energy than normal cells do, alterations in glucose metabolism,
called the Warburg effect or anaerobic glycolysis, occur in cancer
cells, resulting in excessive accumulation of lactate and
acidification of the extracellular pH in the tumor microenvironment
(18–21). These environmental changes caused by
NRs are associated with the aggressive biological behavior of
cancer cells by enhancing metastasis, angiogenesis, and
immunosuppression (22).
Additionally, a recent study has shown that lactate promotes the
expression of programmed cell death-1 (PD-1) in regulatory T cells
in the tumor microenvironment (23).
Given this background, we hypothesized that altered
NRs activity is associated with changes in glucose metabolism and
the immune microenvironment of SC. We investigated the expression
of four NRs and glucose metabolic pathway proteins, including
glucose transporter 1 (GLUT1), monocarboxylate transporters (MCT1
and MCT4), CD147, phosphorylated adenosine monophosphate-activated
protein kinase (pAMPK), and PD-L1 and correlated them with various
clinicopathological parameters. We sought to determine their
pathogenic role and clinical significance in SC.
Materials and methods
Patients
Patients diagnosed and treated for SC at Seoul
National University Hospital (Seoul, South Korea), Seoul
Metropolitan Government-Seoul National University Boramae Medical
Center (Seoul, South Korea), and Seoul National University Bundang
Hospital (Seongnam, South Korea) between January 2002 and December
2019 were included in this study. Clinical data were collected from
medical records, and pathological diagnoses were confirmed by an
experienced pathologist. Demographic information; histopathological
features; anatomical location; treatment details; outcomes, such as
local recurrence and nodal or distant metastases; and survival time
were reviewed. All tumors were restaged according to the American
Joint Committee on Cancer (AJCC) staging system, 8th edition.
This study was approved by the Institutional Review
Board of Seoul National University Hospital (H-1905-059-1032). This
study complied with the principles of the Declaration of
Helsinki.
Immunohistochemistry
Immunohistochemistry (IHC) was performed on
4-µm-thick serial sections of formalin-fixed, paraffin-embedded
tissue samples from patients with SC using an automated staining
platform (BenchMark ULTRA, Ventana, Tucson, AZ). The test items
included the GR (cat. no. 3660, Cell Signaling, Danvers, MA, USA),
ER (cat. no. M7047, Dako, Carpinteria, CA, USA), PR (cat. no.
M3569, Dako), AR (cat. no. MA5-13426, Thermo Fisher Scientific,
Carlsbad, CA, USA), MCT1 (cat. no. SC-365501, Santa Cruz
Biotechnology, Dallas, TX, USA), MCT4 (cat. no. SC-376140, Santa
Cruz Biotechnology), GLUT1 (cat. no. ab15309, Abcam, Cambridge,
UK), CD147 (cat. no. MA5-29060, Thermo Fisher Scientific), pAMPK
(cat. no. 2535, Cell Signaling), and PD-L1 (cat. no. 741-4905,
SP263, Ventana). A standardized protocol was used according to the
manufacturer's recommendations. Dried sections were deparaffinized
in xylene and rehydrated using a series of graded ethanol solutions
(95, 85, 70, and 55%) at room temperature for 10 min. Heat-induced
epitope retrieval was performed in a pressure cooker at 95°C for 2
min using 0.01 M citrate buffer. Slides were incubated overnight at
4°C for all the primary antibodies and washed with
phosphate-buffered saline four times. The UltraView Universal DAB
Detection Kit (cat. no. 760-500, Ventana) was used to visualize the
primary antibodies with 3,3′-diaminobenzidine tetrahydrochloride
chromogen. An experienced pathologist (JEK) performed
semi-quantitative interpretation using a BX51 light microscope
(magnification, ×200 and ×400) (Olympus Corporation, Tokyo, Japan)
blinded to the clinical data. For GR, ER, and PR, the result was
considered positive if ≥1% of the tumor cell nuclei were
immunoreactive. However, considering that AR is consistently found
in normal sebaceous glands, it was interpreted as high or low on
the basis of 10%. The PD-L1 test result was considered positive if
≥1% of tumor cells showed membrane staining, according to the
guidelines of the Ventana PD-L1 SP263 assay approved for non-small
cell lung cancer (https://diagnostics.roche.com/global/en/products/lab/pd-l1-sp263-ce-ivd-us-export-ventana-rtd001234.html).
For the metabolic markers, the H-score was generated by multiplying
the intensity (0–3+) by the percentage of positive tumor cells,
with scores ranging from 0 to 300; an H-score of 10 or higher was
considered positive (https://diagnostics.roche.com/global/en/products/lab/pd-l1-sp263-ce-ivd-us-export-ventana-rtd001234.html).
Statistical analyses
Fisher's exact and χ2 tests were
performed to determine the differences or associations among
categorical variables. Differences among the IHC expression
profiles and clinical data of the patients were examined using
non-parametric Mann-Whitney U tests. Correlations between the
expression of NRs, PD-L1 and glucose metabolic markers were
analyzed using the nonparametric Spearman correlation test.
Univariate Kaplan-Meier analysis with a log-rank test was used to
evaluate post-operative metastasis-free survival between the groups
based on pathologic parameters. Cox proportional hazards regression
was used to identify the parameters associated with metastasis-free
survival. Statistical analyses were performed using the IBM SPSS
software version 25 (IBM, Armonk, NY, USA). All P-values reported
were two-sided, and P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient demographics and clinical
features
Table I lists the
baseline demographic characteristics of patients. A total of 39
cases of SC were included, of which 19 were periocular and 20 were
extraocular tumors. Based on the AJCC 8th edition criteria, 32
(82.1%) patients had tumors at the T2 level or lower, and seven
(17.9%) patients had tumors at the T3 level or higher. Lymph node
involvement or distant metastasis was detected in seven (17.9%)
patients at the time of treatment. During the follow-up period
(mean: 51.4 months; range, 5–258 months), five (12.8%) patients had
local recurrence, and 11 (28.2%) patients presented with nodal or
distant metastases.
 | Table I.Demographic data of 39 patients with
sebaceous carcinoma. |
Table I.
Demographic data of 39 patients with
sebaceous carcinoma.
| Clinicopathologic
features | Value |
|---|
| Mean ± SD age,
years (range) | 69.5±15.5
(26–97) |
| Sex, n (%) |
|
|
Male | 17 (43.6) |
|
Female | 22 (56.4) |
| Mean ± SD
follow-up, months (range) | 51.4±46.5
(5–258) |
| Primary site, n
(%) |
|
|
Periocular | 19 (48.7) |
|
Extraocular | 20 (51.3) |
| Initial stage, n
(%) |
|
|
Localized | 32 (82.1) |
|
Advanced (lymph node
involvement or distant metastasis) | 7 (17.9) |
| T category, n
(%) |
|
| T1 | 17 (43.6) |
| T2 | 15 (38.5) |
| T3 | 2 (5.1) |
| T4 | 5 (12.8) |
| Treatment outcome,
n (%) |
|
| No
recurrence | 23 (59.0) |
| Local
recurrence | 5 (12.8) |
| Nodal
or distant metastasis | 11 (28.2) |
Immunohistochemistry results
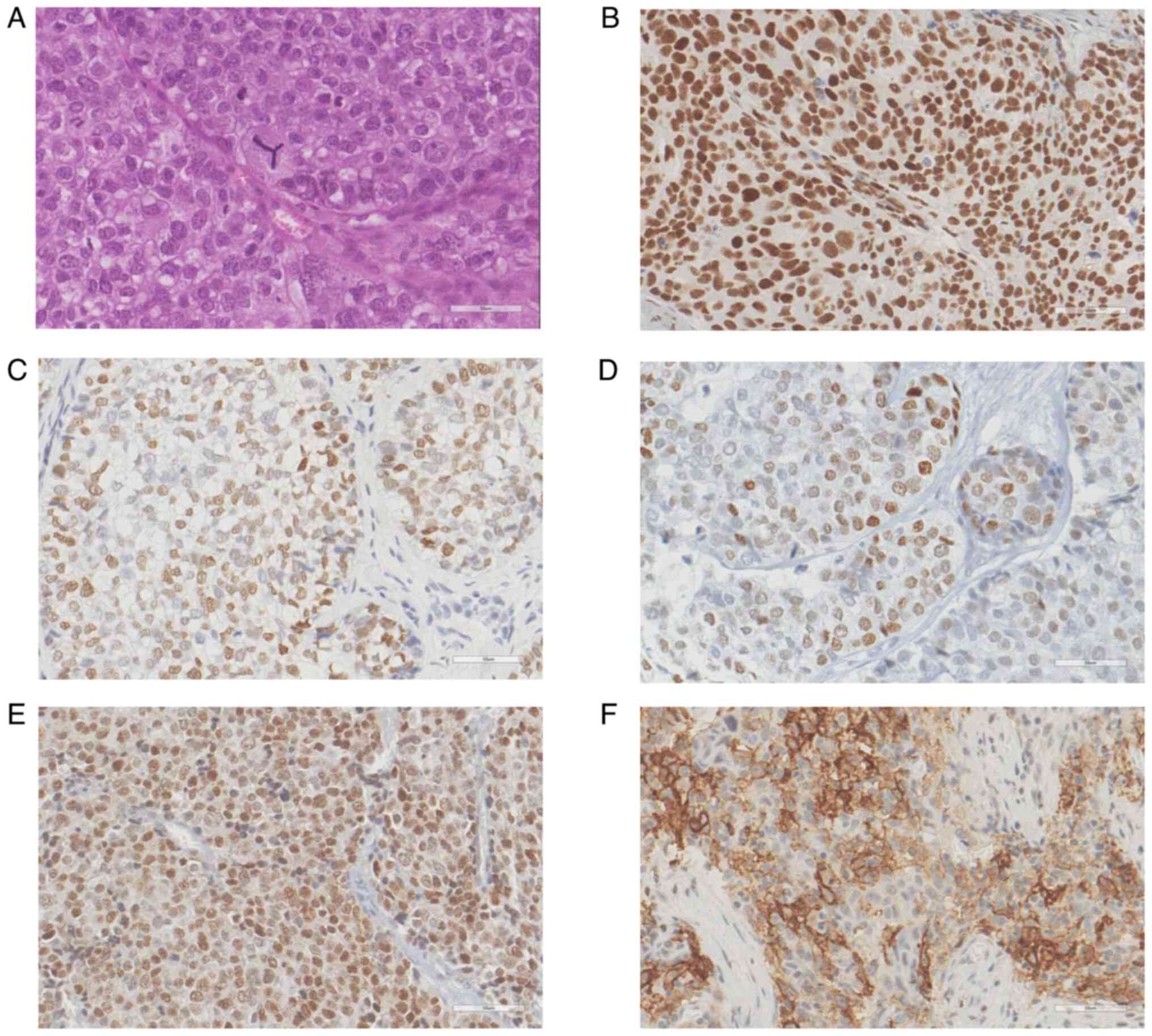
Representative images of positive immunoreactivity
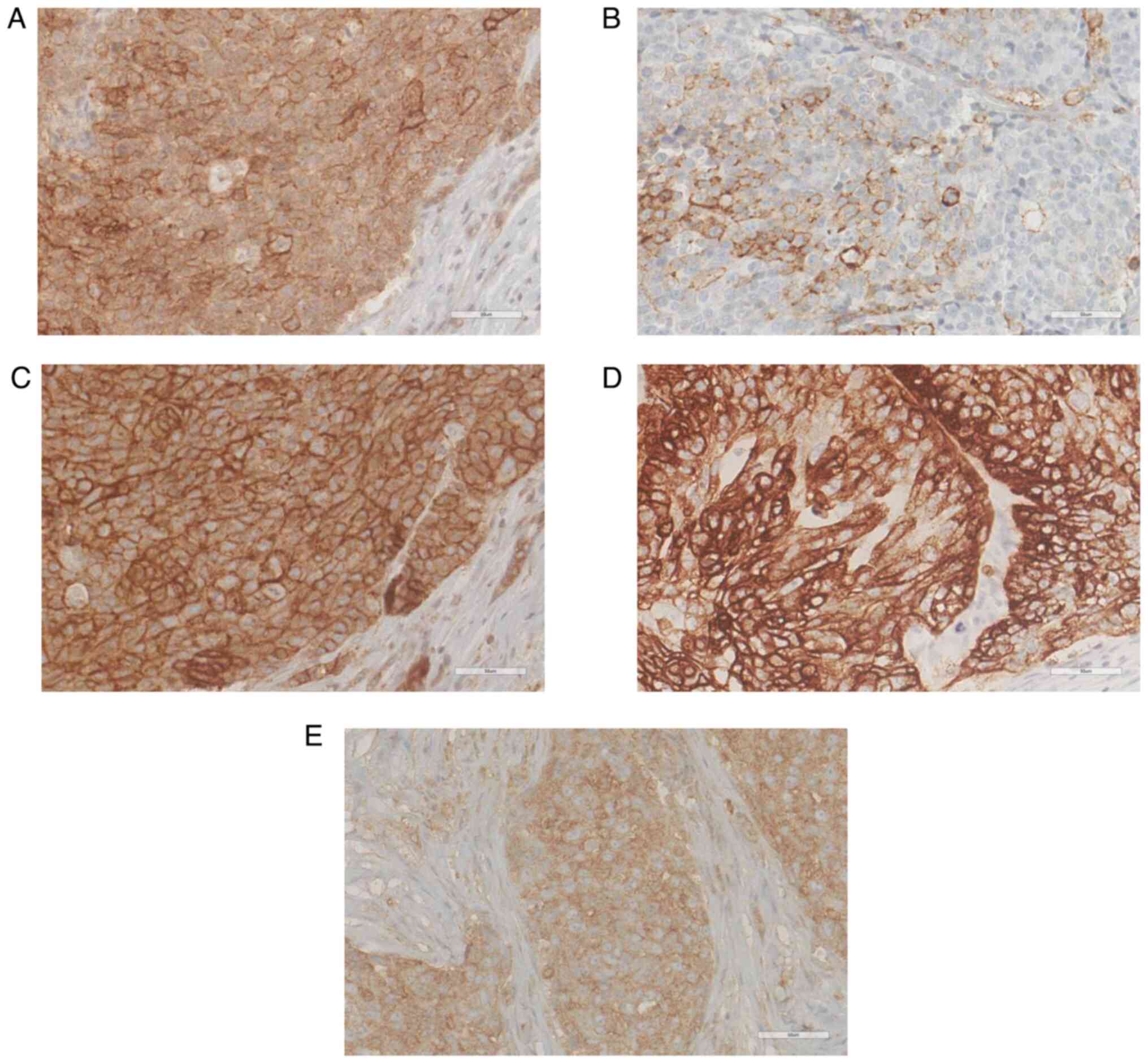
for NRs and PD-L1 in SC are shown in Fig. 1. Glucose metabolic pathway-related
proteins are shown in Fig. 2. In
all 39 SC cases, the NR positivity rate was 35 (89.7%) for GR, 20
(51.3%) for AR and ER, and 16 (41.0%) for PR. Membranous expression
of PD-L1 was found in five cases (12.8%). Regarding glucose
metabolism, CD147, GLUT1, and MCT1 were positively and highly
expressed in 39 (100%; median H-score:300), 35 (87.2%; median
H-score: 240), and 34 (87.2%; median H-score: 50) patients,
respectively. However, MCT4 and pAMPK cells showed low positivity
rates and relatively low expression levels (38.5%, median H-score:
0 and 35.9%, median H-score: 0, respectively).
To investigate the correlation between each IHC
marker, a nonparametric Spearman's rank correlation analysis was
performed. PR expression positively correlated with MCT1 and pAMPK
(P=0.042 and P=0.001, respectively), but negatively correlated with
GLUT1 expression (P=0.001). GR levels were positively correlated
with pAMPK levels (P=0.015). However, the expression of AR, ER, and
PD-L1 was not significantly associated with that of the glucose
metabolic markers (Table II).
 | Table II.Correlation between the expression of
nuclear receptors, PD-L1, and glucose metabolic markers in
sebaceous carcinoma using nonparametric Spearman's rank correlation
analysis. |
Table II.
Correlation between the expression of
nuclear receptors, PD-L1, and glucose metabolic markers in
sebaceous carcinoma using nonparametric Spearman's rank correlation
analysis.
|
| Spearman's rank
correlation coefficients |
|---|
|
|
|
|---|
| Marker | MCT1 | MCT4 | GLUT1 | CD147 | pAMPK |
|---|
| GR | −0.002 | −0.264 | −0.117 | 0.047 | 0.388a |
| AR | 0.048 | −0.154 | 0.094 | 0.204 | −0.137 |
| ER | −0.017 | 0.023 | 0.252 | −0.228 | −0.285 |
| PR | 0.327a | −0.158 | −0.503a | −0.052 | 0.538a |
| PD-L1 | −0.003 | −0.170 | −0.231 | 0.058 | 0.118 |
We performed a stratified analysis to explore the
correlations between NR, PD-L1, and glucose metabolic markers in
the periocular and extraocular SC groups (Tables SI and SII). In the periocular SC group, a
significant negative correlation was observed between AR and pAMPK
(P=0.038) and between PR and GLUT1 (P=0.002). In the extraocular SC
group, PR expression was positively correlated with MCT1 and pAMPK
levels (P=0.010 and P=0.001, respectively). Additionally, a
significant positive correlation was observed between ER and GLUT1
expression (P=0.030). These findings indicate that the molecular
interactions between these biomarkers may differ depending on the
tumor origin, underscoring the potential influence of anatomical
sites on the biological behavior of SC.
Clinicopathologic correlation
We compared the clinical features and IHC results
between the 19 periocular and 20 extraocular SC groups (Table III). No significant differences
were found in the clinical characteristics or protein expression
levels between the two groups, except for the extent of the primary
tumors (higher T stage in periocular tumors). Notably, four of the
five cases showing PD-L1 expression were extraocular. However, no
significant relationships were identified between PD-L1 expression
and clinical variables, such as tumor origin, T grade, disease
stage, or clinical outcome.
 | Table III.Comparison of the clinicopathologic
findings in sebaceous carcinoma according to the primary site. |
Table III.
Comparison of the clinicopathologic
findings in sebaceous carcinoma according to the primary site.
| Characteristic | Periocular
(n=19) | Extraocular
(n=20) |
P-valuec |
|---|
| Sex, n (%) |
|
|
|
|
Male | 6 (31.6) | 11 (55.0) | 0.200 |
|
Female | 13 (68.4) | 9 (45.0) |
|
| Mean ± SD age,
years | 72.8±15.0 | 66.4±15.7 | 0.122 |
| T category, n
(%) |
|
|
|
| T2 or
lesser | 13 (68.4) | 19 (95.0) | 0.044d |
| T3 or
higher | 6 (31.6) | 1 (5.0) |
|
| Initial stage, n
(%) |
|
|
|
|
Localized | 15 (78.9) | 17 (85.0) | 0.695 |
|
Advanceda | 4 (21.1) | 3 (15.0) |
|
| Disease
progression, n (%)b |
|
|
|
|
Absent | 11 (57.9) | 16 (80.0) | 0.176 |
|
Present | 8 (42.1) | 4 (20.0) |
|
| GR, n (%) |
|
|
|
|
Negative | 1 (5.3) | 3 (15.0) | 0.605 |
|
Positive | 18 (94.7) | 17 (85.0) |
|
| AR, n (%) |
|
|
|
|
Low | 12 (63.2) | 9 (45.0) | 0.341 |
|
High | 7 (36.8) | 11 (55.0) |
|
| ER, n (%) |
|
|
|
|
Negative | 8 (42.1) | 11 (55.0) | 0.421 |
|
Positive | 11 (57.9) | 9 (45.0) |
|
| PR, n (%) |
|
|
|
|
Negative | 10 (52.6) | 13 (65.0) | 0.433 |
|
Positive | 9 (47.4) | 7 (35.0) |
|
| PD-L1, n (%) |
|
|
|
|
Negative | 18 (94.7) | 16 (80.0) | 0.342 |
|
Positive | 1 (5.3) | 4 (20.0) |
|
| MCT1, n (%) |
|
|
|
|
Low | 18 (94.7) | 17 (85.0) | 0.605 |
|
High | 1 (5.3) | 3 (15.0) |
|
| MCT4, n (%) |
|
|
|
|
Low | 12 (63.2) | 12 (60.0) | >0.999 |
|
High | 7 (36.8) | 8 (40.0) |
|
| GLUT1, n (%) |
|
|
|
|
Low | 7 (36.8) | 11 (65.0) | 0.341 |
|
High | 12 (63.2) | 9 (35.0) |
|
| CD147, n (%) |
|
|
|
|
Low | 7 (36.8) | 9 (35.0) | 0.748 |
|
High | 12 (63.2) | 11 (65.0) |
|
| pAMPK, n (%) |
|
|
|
|
Low | 16 (84.2) | 13 (65.0) | 0.273 |
|
High | 3 (15.8) | 7 (35.0) |
|
The clinicopathological features and IHC results
according to the disease progression (postoperative metastasis)
status are shown in Table IV.
Significant differences were found in the T category and stage,
with the disease progression group exhibiting higher T and
advanced-stage tumors (P=0.012 and P=0.001, respectively). Among
the NRs, AR was the only one whose expression was significantly
higher in the group without disease progression than in that with
disease progression (P=0.005). No significant differences were
observed between the two groups in the expression levels of glucose
metabolism markers.
 | Table IV.Comparison of clinicopathologic
findings based on disease progression. |
Table IV.
Comparison of clinicopathologic
findings based on disease progression.
| Characteristic | Sebaceous carcinoma
without progressiona
(n=28) | Sebaceous carcinoma
with progression (n=11) |
P-valueb |
|---|
| Sex, n (%) |
|
|
|
|
Male | 11 (39.3) | 6 (54.5) | 0.387 |
|
Female | 17 (60.7) | 5 (45.5) |
|
| Mean ± SD age,
years (range) | 70.7±15.7
(26–97) | 66.6±15.2
(36–89) | 0.357 |
| Mean ± SD
follow-up, months (range) | 50.3±33.4
(5–136) | 54.2±72.2
(8–258) | 0.318 |
| Primary site, n
(%) |
|
|
|
|
Periocular | 12 (42.9) | 7 (63.6) | 0.301 |
|
Extraocular | 16 (57.1) | 4 (51.3) |
|
| T category, n
(%) |
|
|
|
| T2 or
lesser | 26 (92.9) | 26 (54.5) | 0.012c |
| T3 or
higher | 2 (7.1) | 2 (45.5) |
|
| Initial stage, n
(%) |
|
|
|
|
Localized | 27 (96.4) | 5 (45.5) | 0.001c |
| Advanced (lymph
node involvement or distant metastasis) | 1 (3.6) | 6 (54.5) |
|
| GR, n (%) |
|
|
|
|
Negative | 2 (7.1) | 2 (18.2) | 0.562 |
|
Positive | 26 (92.9) | 9 (81.8) |
|
| AR, n (%) |
|
|
|
|
Low | 11 (39.3) | 10 (90.9) | 0.005c |
|
High | 17 (60.7) | 1 (9.1) |
|
| ER, n (%) |
|
|
|
|
Negative | 12 (42.9) | 7 (63.6) | 0.301 |
|
Positive | 16 (57.1) | 4 (36.4) |
|
| PR, n (%) |
|
|
|
|
Negative | 17 (60.7) | 6 (54.5) | 0.725 |
|
Positive | 11 (39.3) | 5 (45.5 |
|
| PD-L1, n (%) |
|
|
|
|
Negative | 24 (85.7) | 10 (90.9) | >0.999 |
|
Positive | 4 (14.3) | 1 (9.1) |
|
| MCT1, n (%) |
|
|
|
|
Low | 26 (92.9) | 9 (81.8) | 0.562 |
|
High | 2 (7.1) | 2 (18.2) |
|
| MCT4, n (%) |
|
|
|
|
Low | 15 (53.6) | 9 (81.8) | 0.150 |
|
High | 13 (46.4) | 2 (18.2) |
|
| GLUT1, n (%) |
|
|
|
|
Low | 14 (50) | 4 (36.4) | 0.497 |
|
High | 14 (50) | 7 (63.6) |
|
| CD147, n (%) |
|
|
|
|
Low | 13 (46.4) | 3 (27.3) | 0.471 |
|
High | 15 (53.6) | 8 (72.7) |
|
| pAMPK, n (%) |
|
|
|
|
Low | 22 (78.6) | 7 (63.6) | 0.424 |
|
High | 6 (21.4) | 4 (36.4) |
|
Additionally, we stratified the patients into
periocular and extraocular groups to assess differences in the
expression of various markers between those with and without
disease progression (Tables SIII
and SIV). This analysis mirrored
the trends observed in the entire cohort (Table IV), particularly regarding the
association between low AR expression and disease progression.
Specifically, low AR expression was observed in 87.5 and 75.0% of
patients in the periocular and extraocular groups, respectively.
However, the statistical significance of these findings could not
be established.
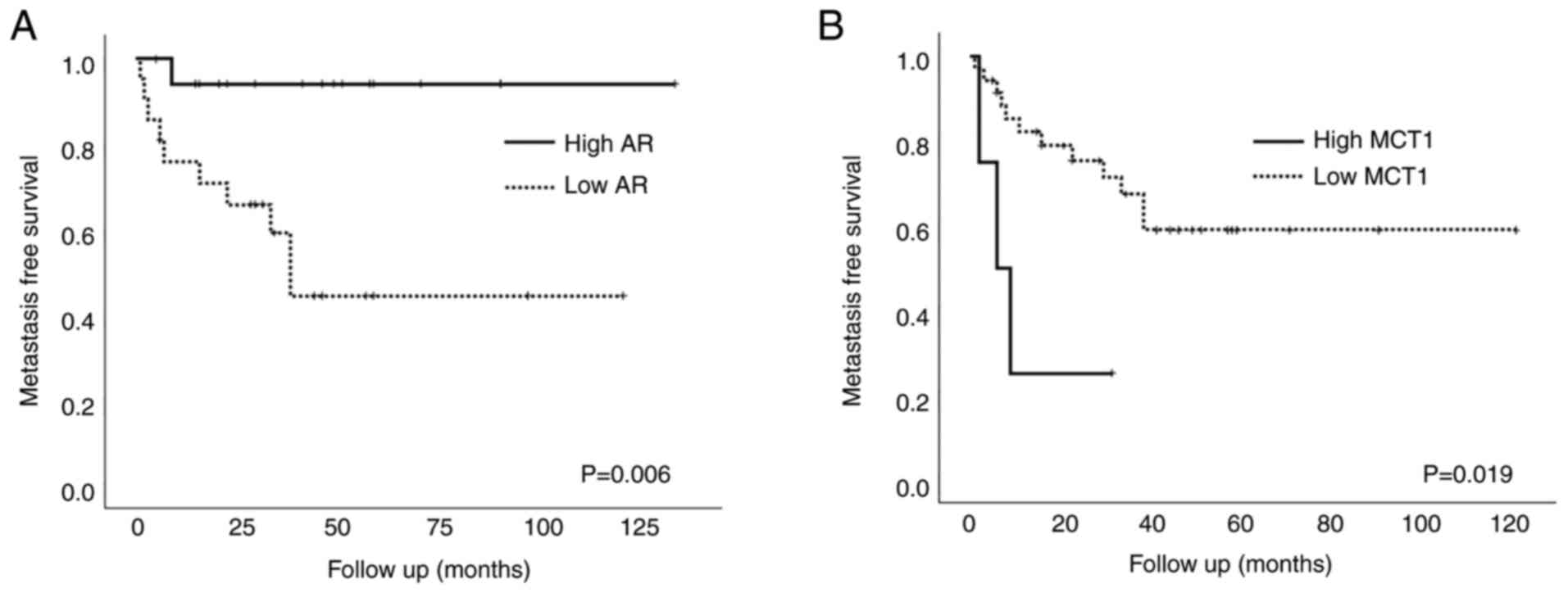
In univariate survival analysis, patients who
exhibited high AR expression had significantly longer
metastasis-free survival compared to those who did not (P=0.006)
(Fig. 3A), whereas other NRs did
not affect patient outcomes. High MCT1 expression was negatively
associated with patient survival (P=0.019; Fig. 3B). Expression of other glucose
metabolic markers or PD-L1 was not associated with patient
prognosis.
Multivariate analysis revealed that the advanced
stage of the initial tumor presentation, low AR, and high MCT1
expression levels were independent poor prognostic factors for
metastasis-free survival (P=0.039, P=0.034, and P=0.021,
respectively; Table V).
 | Table V.Multivariable Cox proportional
regression analysis of metastasis-free survival. |
Table V.
Multivariable Cox proportional
regression analysis of metastasis-free survival.
|
|
| 95% confidence
interval |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Hazard ratio | Lower | Upper | P-value |
|---|
| Age | 1.04 | 0.99 | 1.09 | 0.168 |
| Primary site
(Periocular: Extraocular) | 0.24 | 0.02 | 2.72 | 0.246 |
| T category (T2 or
lesser: T3 or higher) | 0.20 | 0.02 | 2.27 | 0.194 |
| Initial stage
(Localized: Advanceda) | 4.15 | 1.08 | 15.98 | 0.039b |
| GR | 0.07 | 0.00 | 1.44 | 0.085 |
| AR | 0.04 | 0.00 | 0.78 | 0.034b |
| PD-L1 | 7.27 | 0.20 | 26.67 | 0.281 |
| MCT1 | 41.90 | 1.77 | 99.07 | 0.021b |
| MCT4 | 0.02 | 0.00 | 1.10 | 0.056 |
Discussion
This study comprehensively investigated the
prognostic and therapeutic significance of NRs, glucose metabolic
alterations, and PD-L1 expression in SC. Most SC cases in our
cohort exhibited relatively high levels of all four NR expressions
and significant levels of glucose metabolism-related proteins.
Specifically, this study highlighted that low AR and high MCT1
expression levels were independent poor prognostic factors.
Research on the role of NRs, which are important
regulators of various transcriptional pathways involved in the
development or progression of cancer, as diagnostic markers and
targets for hormonal therapy has recently attracted increasing
attention (24). Most published
studies investigating NRs in SC have focused primarily on the
expression status of AR, a sensitive marker of sebaceous
differentiation (14,16,25).
Findings regarding the significance of AR as a prognostic marker
for SC are conflicting (25–27).
The current study revealed that AR expression is a potential
prognostic indicator and provides a new perspective for therapeutic
interventions. Breast and prostate cancers are the most common
types of cancers treated with anti-hormonal agents. AR is an
emerging and promising therapeutic target in a subset of
triple-negative breast cancer (TNBC), an aggressive subtype that
lacks ER, PR, or human epidermal growth factor receptor 2 (HER-2)
(28). Recent studies have shown
that patients with AR-positive TNBC may benefit from treatment with
AR inhibitors, such as bicalutamide, enzalutamide, and abiraterone,
with tolerable toxicity (28–32).
Because AR expression indicates less chemosensitivity and a
favorable prognosis in TNBC, the introduction of AR inhibitors may
lead to a change in treatment modalities. However, the role of AR
in SC is expected to be different from that in breast cancer
because AR is activated in normal sebocytes and downregulation of
AR indicates a lack of differentiation or even dedifferentiation.
However, for some patients with high AR expression who fail initial
treatment, AR inhibitors may be an alternative.
To the best of our knowledge, this is the first
study examining GR expression in SC. The function of the GR in
various cancer cells is well understood, both experimentally and
clinically. Latorre et al (33) demonstrated that GR deficiency
accelerates epidermal tumor growth and skin cancer growth in
knockout mouse models. In hormone-dependent solid tumors, GR
performs diverse functions that regulate cellular differentiation,
apoptosis, and proliferation (34–42).
GR expression has been demonstrated in various types of cancer
cells and serves as a favorable prognostic indicator and predictor
of anti-GR agents (40). The
majority of SC cases in our study showed relatively high levels of
GR expression, suggesting a pivotal role for GR in the pathogenesis
of SC. Only four of 39 (10.3%) patients had GR-negative tumors, but
two (50%) developed distant metastases during follow-up. Although
statistical significance regarding patient survival could not be
confirmed owing to the small number of GR-negative cases, the loss
of GR appears to be closely related to disease progression or
invasive potential. Furthermore, GR and AR share a canonical
hormone-responsive element, and these two NRs regulate overlapping
sets of genes. Therefore, it is unclear whether the GR plays an
independent or AR-dependent role in SC pathogenesis (43,44).
Changes in the metabolic environment caused by the
upregulation of NRs are common during cancer progression (18–21).
Glucose is transported by membrane-associated GLUT family proteins
that are carefully controlled under normal circumstances; however,
increased glucose uptake and the switch to aerobic glycolysis,
known as the Warburg effect, are prominent metabolic alterations
observed in most types of cancer (18–21).
This results in the rapid generation of ATP along with increased
glucose uptake and lactate formation. Furthermore, to facilitate
lactate transport, the activities of MCT1 and MCT4, along with
those of CD147, a chaperone for both proteins, are increased in
cancer cells. More specifically, MCT1 is responsible for
accumulating lactate in cells, whereas MCT4 contributes to the
transporting lactate out of the cells (45). Although MCT1 and MCT4 appear to act
in opposite directions, their dysregulation occurs in many types of
cancer, resulting in the activation of both proteins (46). These metabolic markers may not only
be poor prognostic factors (47–54)
but may also be potential therapeutic targets (55–58).
To date, studies on metabolic changes in SC are limited. Only one
study has proposed GLUT1 as a diagnostic marker for differentiating
SC from benign sebaceous lesions (59). In our study, glucose metabolic
markers were expressed to varying degrees across the cases, with
the most frequently expressed being GLUT1 and pAMPK, suggesting
that these two indicators may also be used for diagnostic purposes.
However, the only metabolic indicator related to patient outcome
was MCT1, although its expression rate was not high. Our results
provide evidence that MCT1 plays a pivotal role in tumor
progression and that metabolic transporters could serve as
potential therapeutic targets in SC. Because MCT1 inhibitors such
as AZD3965 have entered clinical trials for several types of cancer
(NCT01791595), it is expected that patients with refractory SC will
also benefit in the future (60).
The role of NRs, glucose metabolism, and the microenvironment in
tumor initiation and development are presented in Fig. S1.
Immunotherapy, which has recently attracted the most
attention in cancer treatment, was developed based on an
understanding of the interactions between tumor evasion and
microenvironmental changes (6,61).
Among cutaneous tumors, immunotherapy using anti-PD-L1 has
exhibited the most significant results in malignant melanoma;
however, data regarding the efficacy of this treatment in SC are
insufficient (62–65). The SP263 assay was selected because
it exhibits superiority in many cancer types (66), and counting tumor cells alone was
reasonable because most patients with SC present fewer immune cells
around the tumor. In this study, the positivity rate of PD-L1 and
SP263 was approximately 13% (5/39), including four extraocular
tumors. This positivity rate is generally lower than that observed
in breast cancer, non-small cell lung cancer, or malignant melanoma
(67). This can be explained as
follows: First, most SC cases in our cohort were of a limited
stage, and second, SC may not be a highly immunogenic tumor.
Although no significant relationships were identified between PD-L1
expression and the clinical variables or outcomes, our findings are
meaningful because some patients, especially those with extraocular
SC, may benefit from anti-PD-L1 treatment.
SC exhibits significant variations in clinical
presentation and prognosis depending on its location. Periocular SC
is particularly susceptible to diagnostic delays, potential spread
into the conjunctiva, and poorer prognosis due to its distinct
anatomy. This leads to different approaches in staging, treatment
strategies, and surveillance protocols compared to extraocular SC
(7). Building on these findings,
our current study focused on analyzing whether there are
differences in the correlations and expression patterns of various
markers based on tumor location. However, as indicated in Table III, our analysis did not reveal
any statistically significant differences in the expression of NR,
PD-L1, or glucose metabolic markers between the two groups. This
lack of significant findings can be attributed to several factors.
Firstly, the complexity of the involved molecular pathways may not
have been fully captured by the assessed markers. It is possible
that other unmeasured molecular factors play a role in
differentiating the periocular SC from the extraocular SC,
potentially explaining the observed differences in correlation
patterns rather than in overall expression levels. Secondly, the
initial tumor stages between the two groups varied notably, with
the periocular group including a higher proportion of advanced
T-category tumors (32%) compared to the extraocular group, where
only 6% of the cases were classified as T3 or higher. This
disparity in clinical severity may have influenced the expression
of these markers, complicating the detection of significant
differences between the groups. Given these considerations, we
believe that while our study did not find statistically significant
differences in marker expression between the periocular and
extraocular SC groups, these results should be interpreted with
caution. This study focused primarily on the expression of nuclear
receptors and glucose metabolic pathway proteins in SC. Although we
identified AR and MCT1 as potential biomarkers, we did not perform
functional experiments to elucidate the mechanisms by which these
proteins influence SC progression. This represents a notable
shortcoming, as these functional studies are critical for
validating the roles of AR and MCT1 in tumorigenesis. To further
explore these mechanisms, several research methods can be suggested
as follows: One approach involves using RNA interference (siRNA) or
short hairpin RNA (shRNA) to knockdown AR and MCT1 genes to observe
their effects on SC cell proliferation, migration, and invasion.
Another method would be to overexpress AR and MCT1 by using
plasmids or viral vectors, allowing for the evaluation of
functional changes in SC cells. Additionally, conducting
immunoprecipitation (Co-IP) experiments could be valuable in
studying the interactions between AR, MCT1, and other proteins.
Finally, using quantitative PCR (qPCR) and Western Blot analyses
could help detect changes in gene and protein expression levels
following the knockdown or overexpression of AR and MCT1. These
approaches will provide a more comprehensive understanding of the
molecular mechanisms underlying SC and help to validate the
potential of AR and MCT1 as therapeutic targets.
In conclusion, we explored the expression of NRs,
PD-L1, and glucose metabolic pathway proteins in SC and found that
low AR and high MCT1 expression were poor prognostic markers. Our
results provide a rationale for the use of anti-AR or immune
checkpoint inhibitors in patients with advanced SC, particularly in
cases where complete surgical resection is not feasible or the
tumor has metastasized. Additionally, investigating the crosstalk
between NR and metabolic dysregulation in SC will be crucial for
developing more effective therapeutic strategies in future.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Seoul National University
Research Fund (grant nos. 800-2021-0006 and 800-2021-0007).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
Conceptualization and supervision was performed by
HC and JEK. Research was performed by YJC, NK and SIK. YJC, MKY, HC
and JEK analyzed the data. MKY and YJC confirm the authenticity of
all the raw data. YJC wrote the manuscript. Reviewing and editing
was performed by MKY, HC and JEK. Funding acquisition was performed
by HC. All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Board of Seoul National University Hospital (H-1905-059-1032). The
requirement for written informed consent was waived due to the
retrospective nature of this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu Z, Delavan B, Roberts R and Tong W:
Lessons learned from two decades of anticancer drugs. Trends
Pharmacol Sci. 38:852–872. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Owen JL, Kibbi N, Worley B, Kelm RC, Wang
JV, Barker CA, Behshad R, Bichakjian CK, Bolotin D, Bordeaux JS, et
al: Sebaceous carcinoma: Evidence-based clinical practice
guidelines. Lancet Oncol. 20:e699–e714. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Migden MR, Rischin D, Schmults CD,
Guminski A, Hauschild A, Lewis KD, Chung CH, Hernandez-Aya L, Lim
AM, Chang ALS, et al: PD-1 blockade with cemiplimab in advanced
cutaneous squamous-cell carcinoma. N Engl J Med. 379:341–351. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Steren B, Burtness B, Bhatia A, Demirci H,
Shinder R, Yoo D, Tse B and Pointdujour-Lim R: Cemiplimab for
orbital squamous cell carcinoma in 11 cases. Ophthalmic Plast
Reconstr Surg. 38:496–502. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martel A, Lassalle S, Picard-Gauci A,
Gastaud L, Montaudie H, Bertolotto C, Nahon-Esteve S, Poissonnet G,
Hofman P and Baillif S: New targeted therapies and immunotherapies
for locally advanced periocular malignant tumours: Towards a new
‘eye-sparing’ paradigm? Cancers (Basel). 13:28222021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Habib LA, Wolkow N, Freitag SK and Yoon
MK: Advances in immunotherapy and periocular malignancy. Semin
Ophthalmol. 34:327–333. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Na HY, Park JH, Shin SA, Lee S, Lee H,
Chae H, Choung H, Kim N, Chung JH and Kim JE: Targeted sequencing
revealed distinct mutational profiles of ocular and extraocular
sebaceous carcinomas. Cancers (Basel). 13:48102021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zouboulis CC: Sebaceous gland receptors.
Dermatoendocrinol. 1:77–80. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schneider MR and Paus R: Sebocytes,
multifaceted epithelial cells: Lipid production and holocrine
secretion. Int J Biochem Cell Biol. 42:181–185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sherman MH, Downes M and Evans RM: Nuclear
receptors as modulators of the tumor microenvironment. Cancer Prev
Res (Phila). 5:3–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu K, Zou C and Qin B: The association
between nuclear receptors and ocular diseases. Oncotarget.
8:27603–27615. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shiota M, Fujimoto N, Kashiwagi E and Eto
M: The role of nuclear receptors in prostate cancer. Cells.
8:6022019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang L, Lonard DM and O'Malley BW: The
role of steroid receptor coactivators in hormone dependent cancers
and their potential as therapeutic targets. Horm Cancer. 7:229–235.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Azmahani A, Nakamura Y, McNamara KM and
Sasano H: The role of androgen under normal and pathological
conditions in sebaceous glands: The possibility of target therapy.
Curr Mol Pharmacol. 9:311–319. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kretzschmar K, Cottle DL, Schweiger PJ and
Watt FM: The androgen receptor antagonizes Wnt/β-Catenin signaling
in epidermal stem cells. J Invest Dermatol. 135:2753–2763. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jakobiec FA and Werdich X: Androgen
receptor identification in the diagnosis of eyelid sebaceous
carcinomas. Am J Ophthalmol. 157:687–696.e1-2. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Boecker W, Reusch M, Mielke V, Reusch U,
Hallermann C, Loening T, Tiemann M and Buchwalow I: Twenty-Eight
cases of extraocular sebaceous carcinoma: A correlative
clinicopathological and immunohistochemical analysis of extraocular
sebaceous carcinomas and benign sebaceous gland tumors. Am J
Dermatopathol. 43:93–102. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
DeBerardinis RJ and Chandel NS:
Fundamentals of cancer metabolism. Sci Adv. 2:e16002002016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jang M, Kim SS and Lee J: Cancer cell
metabolism: Implications for therapeutic targets. Exp Mol Med.
45:e452013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Danhier P, Bański P, Payen VL, Grasso D,
Ippolito L, Sonveaux P and Porporato PE: Cancer metabolism in space
and time: Beyond the Warburg effect. Biochim Biophys Acta Bioenerg.
1858:556–572. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thorne JL and Campbell MJ: Nuclear
receptors and the Warburg effect in cancer. Int J Cancer.
137:1519–1527. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kumagai S, Koyama S, Itahashi K,
Tanegashima T, Lin YT, Togashi Y, Kamada T, Irie T, Okumura G, Kono
H, et al: Lactic acid promotes PD-1 expression in regulatory T
cells in highly glycolytic tumor microenvironments. Cancer Cell.
40:201–218.e9. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dhiman VK, Bolt MJ and White KP: Nuclear
receptors in cancer-uncovering new and evolving roles through
genomic analysis. Nat Rev Genet. 19:160–174. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mulay K, Shah SJ, Aggarwal E, White VA and
Honavar SG: Periocular sebaceous gland carcinoma: Do androgen
receptor (NR3C4) and nuclear survivin (BIRC5) have a prognostic
significance? Acta Ophthalmol. 92:e681–e687. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yunoki T, Miyakoshi A, Otsuka M and
Hayashi A: Clinicopathological features of considerable reduction
in androgen receptor expression in sebaceous gland carcinoma of the
eyelid. Int Ophthalmol. 39:1703–1708. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Na HY, Choe JY, Shin SA, Choung HK, Oh S,
Chung JH, Park M and Kim JE: Proposal of a provisional
classification of sebaceous carcinoma based on hormone receptor
expression and HER2 Status. Am J Surg Pathol. 40:1622–1630. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gerratana L, Basile D, Buono G, De Placido
S, Giuliano M, Minichillo S, Coinu A, Martorana F, De Santo I, Del
Mastro L, et al: Androgen receptor in triple negative breast
cancer: A potential target for the targetless subtype. Cancer Treat
Rev. 68:102–110. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Traina TA, Miller K, Yardley DA, Eakle J,
Schwartzberg LS, O'Shaughnessy J, Gradishar W, Schmid P, Winer E,
Kelly C, et al: Enzalutamide for the treatment of androgen
receptor-expressing triple-negative breast cancer. J Clin Oncol.
36:884–890. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang R, Han J, Liang X, Sun S, Jiang Y,
Xia B, Niu M, Li D, Zhang J, Wang S, et al: Androgen receptor
expression and bicalutamide antagonize androgen receptor inhibit
β-catenin transcription complex in estrogen receptor-negative
breast cancer. Cell Physiol Biochem. 43:2212–2225. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu M, Yuan Y, Yan P, Jiang J, Ma P, Niu X,
Ma S, Cai H and Yang K: Prognostic significance of androgen
receptor expression in triple negative breast cancer: A systematic
review and meta-analysis. Clin Breast Cancer. 20:e385–e396. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sutton LM, Cao D, Sarode V, Molberg KH,
Torgbe K, Haley B and Peng Y: Decreased androgen receptor
expression is associated with distant metastases in patients with
androgen receptor-expressing triple-negative breast carcinoma. Am J
Clin Pathol. 138:511–516. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Latorre V, Sevilla LM, Sanchis A and Perez
P: Selective ablation of glucocorticoid receptor in mouse
keratinocytes increases susceptibility to skin tumorigenesis. J
Invest Dermatol. 133:2771–2779. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Conzen SD: Recent advances in
understanding glucocorticoid receptor function in cancer. Clin Adv
Hematol Oncol. 15:338–340. 2017.PubMed/NCBI
|
|
35
|
Abduljabbar R, Negm OH, Lai CF, Jerjees
DA, Al-Kaabi M, Hamed MR, Tighe PJ, Buluwela L, Mukherjee A, Green
AR, et al: Clinical and biological significance of glucocorticoid
receptor (GR) expression in breast cancer. Breast Cancer Res Treat.
150:335–346. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Skor MN, Wonder EL, Kocherginsky M, Goyal
A, Hall BA, Cai Y and Conzen SD: Glucocorticoid receptor antagonism
as a novel therapy for triple-negative breast cancer. Clin Cancer
Res. 19:6163–6172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Noureddine LM, Trédan O, Hussein N, Badran
B, Le Romancer M and Poulard C: Glucocorticoid Receptor: A
multifaceted actor in breast cancer. Int J Mol Sci. 22:44462021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Puhr M, Hoefer J, Eigentler A, Ploner C,
Handle F, Schaefer G, Kroon J, Leo A, Heidegger I, Eder I, et al:
The glucocorticoid receptor is a key player for prostate cancer
cell survival and a target for improved antiandrogen therapy. Clin
Cancer Res. 24:927–938. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
McNamara KM, Kannai A and Sasano H:
Possible roles for glucocorticoid signalling in breast cancer. Mol
Cell Endocrinol. 466:38–50. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Block TS, Murphy TI, Munster PN, Nguyen DP
and Lynch FJ: Glucocorticoid receptor expression in 20 solid tumor
types using immunohistochemistry assay. Cancer Manag Res. 9:65–72.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kost BP, Beyer S, Schröder L, Zhou J, Mayr
D, Kuhn C, Schulze S, Hofmann S, Mahner S, Jeschke U and Heidegger
H: Glucocorticoid receptor in cervical cancer: An
immunhistochemical analysis. Arch Gynecol Obstet. 299:203–209.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gandhi S, Elkhanany A, Oshi M, Dai T,
Opyrchal M, Mohammadpour H, Repasky EA and Takabe K: Contribution
of immune cells to glucocorticoid receptor expression in breast
cancer. Int J Mol Sci. 21:46352020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kanai A, McNamara KM, Iwabuchi E, Miki Y,
Onodera Y, Guestini F, Khalid F, Sagara Y, Ohi Y, Rai Y, et al:
Significance of glucocorticoid signaling in triple-negative breast
cancer patients: A newly revealed interaction with androgen
signaling. Breast Cancer Res Treat. 180:97–110. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mitani Y, Lin SH, Pytynia KB, Ferrarotto R
and El-Naggar AK: Reciprocal and autonomous glucocorticoid and
androgen receptor activation in salivary duct carcinoma. Clin
Cancer Res. 26:1175–1184. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Petersen C, Nielsen MD, Andersen ES, Basse
AL, Isidor MS, Markussen LK, Viuff BM, Lambert IH, Hansen JB and
Pedersen SF: MCT1 and MCT4 expression and lactate flux activity
increase during white and brown adipogenesis and impact adipocyte
metabolism. Sci Rep. 7:131012017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fiaschi T, Marini A, Giannoni E, Taddei
ML, Gandellini P, De Donatis A, Lanciotti M, Serni S, Cirri P and
Chiarugi P: Reciprocal metabolic reprogramming through lactate
shuttle coordinately influences tumor-stroma interplay. Cancer Res.
72:5130–5140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Payen VL, Mina E, Van Hée VF, Porporato PE
and Sonveaux P: Monocarboxylate transporters in cancer. Mol Metab.
33:48–66. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
de la Cruz-Lopez KG, Castro-Munoz LJ,
Reyes-Hernandez DO, Garcia-Carranca A and Manzo-Merino J: Lactate
in the regulation of tumor microenvironment and therapeutic
approaches. Front Oncol. 9:11432019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ufuk A, Garner T, Stevens A and Latif A:
Monocarboxylate transporters are involved in extracellular matrix
remodelling in pancreatic ductal adenocarcinoma. Cancers (Basel).
14:12982022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kobayashi M, Narumi K, Furugen A and Iseki
K: Transport function, regulation, and biology of human
monocarboxylate transporter 1 (hMCT1) and 4 (hMCT4). Pharmacol
Ther. 226:1078622021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Choi JW, Lee Y, Kim H, Cho HY, Min SK and
Kim YS: Coexpression of MCT1 and MCT4 in ALK-positive anaplastic
large cell lymphoma: Diagnostic and therapeutic implications. Am J
Surg Pathol. 46:241–248. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yuan C, Zhang J, Lou J and Wang S, Jiang
Y, Wu F and Wang S: Comprehensive Analysis of Monocarboxylate
Transporter 4 (MCT4) expression in breast cancer prognosis and
immune infiltration via integrated bioinformatics analysis.
Bioengineered. 12:3850–3863. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tong YH, Hu XP, Xiang XP and Fang L: High
expression of monocarboxylate transporter 4 (MCT 4), but not MCT 1,
predicts poor prognosis in patients with non-small cell lung
cancer. Transl Cancer Res. 10:1336–1345. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
de Carvalho PA, Bonatelli M, Cordeiro MD,
Coelho RF, Reis S, Srougi M, Nahas WC, Pinheiro C and Leite KRM:
MCT1 expression is independently related to shorter cancer-specific
survival in clear cell renal cell carcinoma. Carcinogenesis.
42:1420–1427. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang Y, Qin L, Chen W, Chen Q, Sun J and
Wang G: Novel strategies to improve tumour therapy by targeting the
proteins MCT1, MCT4 and LAT1. Eur J Med Chem. 226:1138062021.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Takenaga K, Koshikawa N, Akimoto M,
Tatsumi Y, Lin J, Itami M and Nagase H: MCT4 is induced by
metastasis-enhancing pathogenic mitochondrial NADH dehydrogenase
gene mutations and can be a therapeutic target. Sci Rep.
11:133022021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Miranda-Gonçalves V, Gonçalves CS, Granja
S, Vieira de Castro J, Reis RM, Costa BM and Baltazar F: MCT1 Is a
new prognostic biomarker and its therapeutic inhibition boosts
response to temozolomide in human glioblastoma. Cancers (Basel).
13:34682021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chandel V, Maru S, Kumar A, Kumar A,
Sharma A, Rathi B and Kumar D: Role of monocarboxylate transporters
in head and neck squamous cell carcinoma. Life Sci. 279:1197092021.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Barron CR and Smoller BR: GLUT1 expression
in cutaneous sebaceous lesions determined by immunohistochemical
staining patterns. Dermatopathology (Basel). 8:258–264. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Halford S, Veal GJ, Wedge SR, Payne GS,
Bacon CM, Sloan P, Dragoni I, Heinzmann K, Potter S, Salisbury BM,
et al: A Phase I dose-escalation study of AZD3965, an oral
monocarboxylate transporter 1 inhibitor, in patients with advanced
cancer. Clin Cancer Res. 29:1429–1439. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Swaika A, Hammond WA and Joseph RW:
Current state of anti-PD-L1 and anti-PD-1 agents in cancer therapy.
Mol Immunol. 67((2 Pt A)): 4–17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Saliba M, Shaheen M, Hajj RE, Abbas F,
Bashir S, Sheikh UN, Mahfouz R, Loya A and Khalifeh I: PD-L1
expression in sebaceous carcinomas. Cancer Immunol Immunother.
70:1907–1915. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wolkow N, Jakobiec FA, Afrogheh AH, Pai SI
and Faquin WC: High expression of programmed death ligand 1 and
programmed death ligand 2 in ophthalmic sebaceous carcinoma: The
case for a clinical trial of checkpoint inhibitors. Am J
Ophthalmol. 220:128–139. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jayaraj P and Sen S: Evaluation of PD-L1
and PD-1 expression in aggressive eyelid sebaceous gland carcinoma
and its clinical significance. Indian J Ophthalmol. 67:1983–1987.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kandl TJ, Sagiv O, Curry JL, Ning J, Ma J,
Hudgens CW, Van Arnam J, Wargo JA, Esmaeli B and Tetzlaff MT: High
expression of PD-1 and PD-L1 in ocular adnexal sebaceous carcinoma.
Oncoimmunology. 7:e14758742018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Smith J, Robida MD, Acosta K, Vennapusa B,
Mistry A, Martin G, Yates A and Hnatyszyn HJ: Quantitative and
qualitative characterization of Two PD-L1 clones: SP263 and E1L3N.
Diagn Pathol. 11:442016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Patel SP and Kurzrock R: PD-L1 expression
as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther.
14:847–856. 2015. View Article : Google Scholar : PubMed/NCBI
|